Abstract
Background
Monotherapy immune checkpoint inhibitor (ICI) used in second- or later-line settings has been reported to induce hyperprogression. This study evaluated hyperprogression risk with ICI (atezolizumab) in the first-, second-, or later-line treatment of advanced non–small cell lung cancer (NSCLC), and provides insights into hyperprogression risk with contemporary first-line ICI treatment.
Methods
Hyperprogression was identified using Response Evaluation Criteria in Solid Tumours (RECIST)-based criteria in a dataset of pooled individual-participant level data from BIRCH, FIR, IMpower130, IMpower131, IMpower150, OAK, and POPLAR trials. Odds ratios were computed to compare hyperprogression risks between groups. Landmark Cox proportional-hazard regression was used to evaluate the association between hyperprogression and progression-free survival/overall survival. Secondarily, putative risk factors for hyperprogression among second- or later-line atezolizumab-treated patients were evaluated using univariate logistic regression models.
Results
Of the included 4644 patients, 119 of the atezolizumab-treated patients (n = 3129) experienced hyperprogression. Hyperprogression risk was markedly lower with first-line atezolizumab—either chemoimmunotherapy or monotherapy—compared to second/later-line atezolizumab monotherapy (0.7% vs. 8.8%, OR = 0.07, 95% CI, 0.04-0.13). Further, there was no statistically significant difference in hyperprogression risk with first-line atezolizumab-chemoimmunotherapy versus chemotherapy alone (0.6% vs. 1.0%, OR = 0.55, 95% CI, 0.22-1.36). Sensitivity analyses using an extended RECIST-based criteria including early death supported these findings. Hyperprogression was associated with worsened overall survival (HR = 3.4, 95% CI, 2.7-4.2, P < .001); elevated neutrophil-to-lymphocyte ratio was the strongest risk factor for hyperprogression (C-statistic = 0.62, P < .001).
Conclusions
This study presents first evidence for a markedly lower hyperprogression risk in advanced NSCLC patients treated with first-line ICI, particularly with chemoimmunotherapy, as compared to second- or later-line ICI treatment.
Keywords: immune checkpoint inhibitor, chemoimmunotherapy, hyperprogression, hyperprogressive disease, non–small cell lung cancer
This study evaluated hyperprogression risk with the use of immune checkpoint inhibitor (ICI) in the first-, second-, or later-line treatment of advanced non-small cell lung cancer, providing insight into hyperprogression risk with contemporary first-line ICI treatment.
Implications for Practice.
Hyperprogression is a phenomenon of marked acceleration of tumor growth associated with poor survival and has been studied among previously treated patients receiving monotherapy immune checkpoint inhibitors (ICI). However, there are limited insights to hyperprogression risk with contemporary ICI use as first-line agent either in combination with chemotherapy (chemoimmunotherapy) or as monotherapy. Our study presents the first evidence that the risk of hyperprogression with ICI treatment, particularly with chemoimmunotherapy, is markedly lower in treatment naïve patients (less than 1%) compared to previously treated patients (over 8%) with advanced non-small cell lung cancer.
Introduction
Immune checkpoint inhibitors (ICIs) have transformed the treatment landscape in many cancers, including non-small cell lung cancers (NSCLC).1 Initially approved and used as salvage therapies in the second- or later-line settings, ICIs have now become the standard of care for first-line treatment—as monotherapy or in combination with chemotherapy (chemoimmunotherapy)—of many patients with advanced NSCLC.1,2
While ICIs facilitate durable treatment response and survival for many patients, atypical response patterns have been observed.3 In particular, a subset of patients have been reported to experience hyperprogressive disease—a marked acceleration of tumor growth associated with very poor survival.3 Hyperprogression is of major clinical importance as it highlights that ICIs may paradoxically be harmful to a subset of patients. Toward this end, there is an urgent need for a better understanding of hyperprogression phenomenology, epidemiology, risk factors, and underlying mechanisms.4
Importantly, prior studies have investigated hyperprogression in single-agent, second/later-line ICI settings,5-12 which provides limited insights into the phenomenon’s occurrence with contemporary first-line and/or chemoimmunotherapy treatment. Second, prior studies examining clinicopathological features associated with hyperprogression are limited by small sample size and minimal investigation of key biomarkers (eg, tumor mutation burden or immunophenotypes).5-8,12-19
The present study aimed to evaluate the risk of hyperprogression observed across 7 clinical trials evaluating the ICI atezolizumab in the first-, second-, or later-line treatment of locally advanced or metastatic NSCLC, either as monotherapy or chemoimmunotherapy. A secondary aim was to identify biomarkers predictive of hyperprogression.
Materials and Methods
Patient Population
This study was a pooled post hoc analysis of individual-participant data from 7 clinical trials investigating atezolizumab. Specifically, BIRCH (NCT02031458, data cutoff 28/5/2015) and FIR (NCT01846416, data cutoff 7/1/2015) were phase II, single-arm studies of atezolizumab monotherapy in the first-, second-, or later-line treatment of locally advanced or metastatic NSCLC.20,21 POPLAR (phase II, NCT01903993, data cutoff 8/5/2015) and OAK (phase III, NCT02008227, data cutoff 7/7/2016) were randomized studies comparing atezolizumab monotherapy against docetaxel in the second- or later-line treatment of advanced or metastatic NSCLC.22,23 IMpower130 (phase III, NCT02367781, data cutoff 15/3/2018) was a randomized study comparing atezolizumab-immunochemotherapy (atezolizumab/carboplantin/nab-paclitaxel (ACnP)) against platinum-based chemotherapy for first-line treatment of metastatic non-squamous NSCLC.24 IMpower131 (phase III, NCT02367794, data cutoff 20/4/2018) a randomised, 3-arm study compared two atezolizumab-immunochemotherapy regimens (atezolizumab/carboplantin/paclitaxel (ACP) and ACnP) against platinum-based chemotherapy for first-line treatment of metastatic squamous NSCLC.25 Lastly, IMpower150 (phase III, NCT02366143, data cutoff 15/9/2017) was a randomized, 3-arm study comparing bevacizumab/carboplatin/paclitaxel (BCP) therapy against 2 atezolizumab-immunochemotherapy regimens (atezolizumab/BCP (ABCP) and ACP) in the first-line treatment of metastatic non-squamous NSCLC.26 To ensure consistency with current standard of care, a sensitivity analysis was carried out excluding patients with EGFR mutations and treated with first-line atezolizumab monotherapy.
Secondary analysis of anonymized data was deemed as minimal risk research by the Southern Adelaide Local Health Network, Officer for Research and Ethics, and was exempted from review.
Definition of Hyperprogression
Hyperprogression was determined using the Response Evaluation Criteria in Solid Tumors v1.1 (RECIST)-based criteria outlined by Matos et al.27 Hyperprogression was defined as an increase of ≥10 mm in the sum of the longest diameters (SLD) calculated at the first scheduled assessment compared to baseline SLD, plus either a ≥40% increase in SLD compared to baseline, or a ≥20% SLD increase accompanied with 2 or more new lesions in different organs. The first tumor assessment was scheduled at 6 weeks post-treatment initiation or randomization in all 7 studies.20-26 Patients without a week 6 tumor assessment were excluded from the primary analysis. As a sensitivity analysis, the RECIST-based hyperprogression criteria were extended to include patients who died prior to the week 6 tumor assessment.
Putative Risk Factors for Hyperprogression
Based on prior evidence and data availability the following biomarkers were assessed as potential risk factors for hyperprogression: blood-based tumor mutation burden (TMB), PD-L1 tumor-infiltrating immune cell expression, PD-L1 tumor cell expression, epidermal growth factor receptor (EGFR) mutation, or EML4-ALK rearrangement, CD3, CD4, CD8 peripheral blood cell count, C-reactive protein, neutrophil lymphocyte ratio, platelet count, albumin, and lactate dehydrogenase. In addition, the demographic and clinical characteristics age, sex, race, ECOG performance status (ECOGPS), smoking history (never, previous, current), body mass index (BMI), liver metastasis status, line of therapy, disease status (locally advanced or metastatic), histology (squamous or non-squamous), metastatic site count, and prior radiotherapy treatment status were also investigated.
Statistical Analysis
Hyperprogression risks between groups (ie, treatment arms and line of therapy) were evaluated using crude odds ratios with Wald CIs. The association between hyperprogression occurrence and progression-free survival (PFS)/overall survival (OS) was evaluated using a landmark Cox proportional-hazard regression approach, with findings presented as hazard ratios (HR) with 95% CIs. A landmark at 42 days (6 weeks) was applied to the Cox proportional-hazard survival analysis to address the immortal-time bias introduced by identifying hyperprogression using week 6 tumor assessment information.28 Univariable associations between biomarkers/clinical characteristics and hyperprogression incidence were evaluated using univariate logistic regression, with predictive performances measured by the C-statistic, and associations reported as odds ratios (OR) with 95% CIs. For the logistic regressions, continuous variables were tested for normality and log-transformed if skewed. For comparison of hyperprogression risks with and without atezolizumab treatment, analysis was limited to the 5 randomized controlled trials (RCTs) (IMpower130, IMpower131, IMpower150, OAK, and POPLAR). Median follow-up was estimated using the reverse Kaplan-Meier method. All statistical tests were 2-sided, and P-values of <.05 were considered statistically significant. Statistical analyses were performed using R (V3.6.2).
Data Availability
The data is not directly shareable by our research team. This publication is based on research using data from Roche that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication.
Results
Patient Population
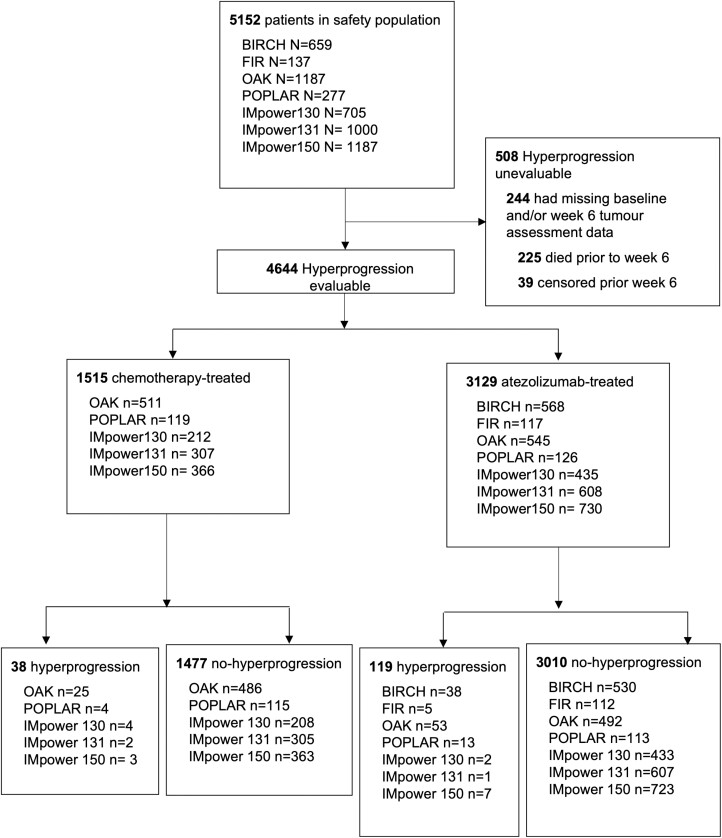
Of the 5152 patients’ data pooled from the safety populations of the 7 studies, 4644 had evaluable baseline and week 6 tumor assessments—3129 received therapy containing atezolizumab and 1515 did not receive atezolizumab (Fig. 1). Median follow-up in the evaluable cohort was 17.3 months (95% CI, 16.9-17.5 months). The demographic and baseline clinical characteristics of the evaluable cohort are listed in Supplementary Table S1. Supplementary Table S2 presents the demographic and baseline clinical characteristics of the 508 patients not evaluable for hyperprogression—225 deceased and 39 censored before week 6, while 244 had missing baseline and/or week 6 tumor assessment information.
Figure 1.
Flow diagram of study populations from BIRCH, FIR, OAK, POPLAR, IMpower130, IMpower131, and IMpower150.
Of the 3129 evaluable patients who received atezolizumab, 1919 (61%) received atezolizumab in the first-line setting and 1210 (39%) in the second or later line. Among the first-line treatment cohort, a majority (n = 1773, 92%) were treated with chemoimmunotherapy, and the remainder (n = 146, 8%) atezolizumab monotherapy. Supplementary Table S3 presents the demographic and baseline clinical characteristics of the first-line atezolizumab-treated cohort by intervention type (atezolizumab monotherapy and atezolizumab chemoimmunotherapy). All the second/later-line ICI treatment was atezolizumab monotherapy.
Risk of Hyperprogression with Atezolizumab
Within the pooled cohort of 3129 patients treated with atezolizumab, 119 experienced hyperprogression (Fig. 1, Supplementary Table S4). The hyperprogression incident risks by line-of-treatment, intervention arm, and cancer histotype are listed in Table 1. The risk of hyperprogression was markedly lower for patients treated with atezolizumab in the first-line setting—either chemoimmunotherapy or monotherapy—compared to second or later-line atezolizumab monotherapy treated patients (0.7% vs. 8.8%, OR = 0.07, 95% CI, 0.04-0.13). Supplementary Table S5 presents hyperprogression risks stratified by clinical trial among atezolizumab-treated patients.
Table 1.
Hyperprogession incidence among atezolizumab-treated cohorts by line of treatment, histotype, and treatment-arm.
| Line of treatment | Cancer histotype | Treatment arm | HPD/total no. (%) |
|---|---|---|---|
| First-line | 13/1919 (0.7) | ||
| Squamous | 2/646 (0.3) | ||
| Non-squamous | 11/1273 (0.9) | ||
| ABCP | 2/356 (0.6) | ||
| ACP | 5/674 (0.7) | ||
| ACnP | 3/743 (0.4) | ||
| Atez | 3/146 (2.1) | ||
| Second-line | 75/807 (9.3) | ||
| Squamous | 23/229 (10.0) | ||
| Non-squamous | 52/578 (9.0) | ||
| Atez | 75/807 (9.3) | ||
| Third- and later-later | 31/403 (7.7) | ||
| Squamous | 7/99 (7.1) | ||
| Non-squamous | 24/304 (7.9) | ||
| Atez | 31/403 (7.7) |
Abbreviations: ABCP: atezolizumab + bevacizumab +carboplatin + paclitaxel; ACP: atezolizumab + carboplatin + paclitaxel; ACnP: atezolizumab + carboplatin + nab-paclitaxel; Atez: atezolizumab monotherapy; HPD: hyperprogression.
Hyperprogression risk with first-line atezolizumab monotherapy was higher than that of first-line chemoimmunotherapy (2.1% vs. 0.6%, OR = 3.70, 95% CI, 1.01-13.59). This higher hyperprogression risk with first-line atezolizumab monotherapy was nonetheless much lower than that with atezolizumab monotherapy in the second or later-line settings (2.1% vs. 8.8%, OR = 0.22, 95% CI, 0.07-0.70). Further, among the 146 treatment naïve patients who received atezolizumab monotherapy, EGFR mutations occurred in 9 individuals, of whom none experienced hyperprogression. Thus, the sensitivity analysis with exclusion of these 9 participants only minimally altered abovementioned results (2.2% vs. 2.1% from the original analysis).
No clear difference in the risk of hyperprogression was observed between second versus later line treatment (9.3% vs. 7.7%, OR = 1.23, 95% CI, 0.79-1.90).
Sensitivity analysis with the definition of hyperprogression including death due to disease progression prior to 6 weeks (n = 81 deaths) similarly demonstrated that hyperprogression risk was markedly lower for patients treated with atezolizumab in the first-line setting—either chemoimmunotherapy or monotherapy—as compared to second or later-line treated patients (2.4% vs. 12.2%, OR = 0.18, 95% CI, 0.13-0.25). Supplementary Table S6 presents risks of hyperprogression defined with the extended RECIST-based criteria (including early death due to progressive disease) by line of treatment, intervention arm, and cancer histotype.
Clinical Outcomes of Hyperprogression
In the cohort of 3129 patients treated with atezolizumab, the occurrence of hyperprogression was associated with worsened OS (HR = 3.4, 95% CI, 2.7-4.2, P < .001) and PFS (HR = 10.9, 95% CI, 8.9-13.3, P < .001). Table 2 presents median OS and PFS estimates according to hyperprogression status and line of therapy.
Table 2.
Median overall survival and progression-free survival estimates according to hyperprogression status and line of therapy.
| N a | Median overall survival months (95% CI) |
Median PFS months (95% CI) |
|
|---|---|---|---|
| First-line | |||
| With hyperprogression | 13 | 4.3 (2.5-n/a) | 1.5 (1.4-n/a) |
| Without hyperprogression | 1901 | 18.6 (17.1-19.8) | 7.2 (7.0-7.5) |
| Second- or later-line | |||
| With hyperprogression | 106 | 6.1 (4.4-8.6) | 1.4 (1.4-1.4) |
| Without hyperprogression | 1101 | 16.2 (15.5-18.0) | 4.1 (3.5-4.2) |
a N at the day 42 landmark.
Abbreviation: PFS, progression-free survival.
Among the 119 patients identified as having hyperprogression in the atezolizumab-treated cohort, 43 (36%) continued atezolizumab treatment beyond the week 6 scan date—7 had one additional cycle, 14 had 2 additional cycles, and 21 had 3 or more cycles of treatment. Week 12 tumor assessment was undertaken for 31 individuals—6 (19%) had over 20% increase in tumor size relative to week 6, 23 (75%) had stable tumor size relative to week 6 (within ± 20% change), and 2 (6%) had over 20% reduction in tumor size relative to week 6. Longitudinal changes in SLD over time (Supplementary Fig. S1) indicate that a small subset of individuals had stabilized tumor size following week 6 hyperprogression, but no individuals subsequently achieved substantial tumor size reduction relative to baseline.
Predictors of Hyperprogression
Given the very low hyperprogression incidence in the first-line treatment setting, predictors of hyperprogression were explored using the second- or later-line atezolizumab-treated population (N = 1210). Biomarkers associated with an increased risk of hyperprogression included higher neutrophil-to-lymphocyte ratio, platelet count, and C-reactive protein levels (P < .05, Supplementary Table S7). Notably, TMB and PDL1 expression were not meaningfully associated with risk of hyperprogression. Clinical factors associated with an increased risk of hyperprogression included lower age, presence of liver metastases, and higher metastatic site count (P < .05, Supplementary Table S8). Neutrophil lymphocyte ratio was the single strongest predictor of hyperprogression within the second- or later-line atezolizumab-treated cohort (C-statistic = 0.62, P < .001).
Hyperprogression Risk With and Without Atezolizumab
Hyperprogression was also observed in the chemotherapy-treated cohort as summarized in Supplementary Table S9. In RCTs evaluating first-line treatment (IMpower130, IMpower131, and IMpower150), there was no significant difference in risk of hyperprogression between chemoimmunotherapy and chemotherapy alone (0.6% vs. 1.0%, OR = 0.55, 95% CI, 0.22-1.36). However, in RCTs of previously treated patients (OAK and POPLAR), the risk of hyperprogression was significantly higher with atezolizumab monotherapy compared to docetaxel (9.8% vs. 4.6%, OR = 2.26, 95% CI, 1.44-3.55).
Further, a higher hyperprogression risk was observed in the second- or later-line cohort compared to the first-line cohort in both atezolizumab and control groups. However, this risk increase was 2-fold higher among the atezolizumab-treated compared to chemotherapy-treated patients (Supplementary Table S10).
Discussion
For the first time, this study presents evidence of a markedly reduced risk of hyperprogression with first-line ICI treatment, particularly with chemoimmunotherapy, as compared to second- or later-line ICI treatment in patients with advanced NSCLC.
Findings that hyperprogression risk with first-line chemoimmunotherapy use is substantially lower than with ICI monotherapy, particularly in the second/later lines, are consistent with emerging evidence on ICI efficacies and mechanisms in patients with advanced NSCLC. First, recent pooled analyses suggest that chemoimmunotherapy achieves superior survival outcomes for patients with advanced NSCLC (including across PD-L1 expression levels) as compared to ICI monotherapies.2,29 Second, systemic chemotherapies are known to alter the cancer-clone dynamic and promote resistant tumor cells7,30—thus treatment-naïve patients may harbor an advantageous immune environment for anti-tumor immune responses. Finally, chemotherapies promote immunogenic cell death and neoantigen release, which in turn boosts tumor immunogenicity31—thus combining chemotherapy with ICIs potentially addresses key issues related to ICI resistance.
Our findings of an 8.8% incidence of hyperprogression for second/later-line atezolizumab monotherapy use in patients with advanced NSCLC is consistent with recent meta-analyses which ranged the incidence of hyperprogression for ICIs to be between 5.9 and 43.1%—notably, these meta-analyses contained cohorts treated with ICIs primarily in the second- or later-line.32 Matos et al,27 who proposed the RECIST-based hyperprogression criteria, also reported an overall hyperprogression rate of 10.7% across a range of cancer types among previously treated single agent ICI patients. Importantly, to the best of our knowledge, this study presents the first report on the incidence of hyperprogression in the first-line ICI-treatment setting, where the incidence was observed to be markedly reduced (0.7% in the first-line cohort-treated with ateozolizumab as either monotherapy or chemoimmunotherapy, and 0.6% in the first-line cohort-treated with atezolizumab chemoimmunotherapy only).
To our knowledge, this is the largest study to evaluate risk factors of ICI hyperprogression (N > 1000), and one of the most comprehensive evaluations of contemporary biomarkers (eg, first meaningful evaluation of tumor mutation burden) and clinical markers for its occurrence. A recent meta-analysis of 9 small retrospective studies (the largest including 406 patients) reported lactate dehydrogenase, Royal Marsden Hospital prognostic score, PD-L1 expression, number of metastatic sites, and the presence of liver metastases as risk factors for hyperprogression.5,7,8,12,14-19 This meta-analysis was limited by marked heterogeneity in risk factors available for each study and evidence of publication bias (ie, small-study effects) for the PD-L1 expression finding.19 Notably, in our very large cohort tumor mutation burden, PD-L1 immune cell and tumor cell expression, EGFR or EML4-ALK rearrangement, and CD3, CD4, and CD8 peripheral blood cell counts were all not significantly associated with hyperprogression risk. Opposingly, neutrophil-to-lymphocyte ratio was identified as the single strongest feature associated with hyperprogression risk.
With respect to limitations, the present analyses only examined patients with advanced NSCLC-treated with atezolizumab. Future research should examine hyperprogression risk in the first-line and combination therapy settings with other ICIs and cancer types. Herein, the hyperprogression risk comparisons between patients treated with and without ICIs used data from RCT cohorts only. Nonetheless, these comparisons may still be influenced by unaccounted heterogeneity, while findings from RCT/trial data have limitations in its generalizability to all patients treated in clinical practice. With respect to investigated markers to predict hyperprogression, it is acknowledged that despite the relatively large sample size, all evaluated markers displayed moderate to poor performance in predicting hyperprogression—highlighting a need for continued research to identify novel biomarkers informing atypical response patterns to ICIs. Lastly, the RECIST-based hyperprogression criteria were selected for this study as it is simple to use, applicable to the first-line treatment setting, shown to have good concordance with tumor growth rate-based definition, and it captures data on emerging new lesions.27,33,34 While many prior studies utilized tumor growth kinetics/rate-based definition of hyperprogression, such an approach is often not clinically applicable to contemporary first-line treatment of patients, and the hyperprogression risks reported here are comparable to prior studies.5,13,27,32,35
Conclusion
The risk of hyperprogression with ICI treatment was markedly lower for treatment naïve patients, particularly when treated with chemoimmunotherapy. Elevated neutrophil-to-lymphocyte ratio was the strongest risk factor for hyperprogression in patients with advanced NSCLC initiating second- or later-line atezolizumab monotherapy.
Supplementary Material
Contributor Information
Lee X Li, Department of Clinical Pharmacology, College of Medicine and Public Health, Flinders University, Adelaide, Australia.
Federico Cappuzzo, Oncology Department, Istituto Nazionale Tumori IRCCS “Regina Elena”, Rome, Italy.
Ignacio Matos, Department of Oncology, Clinica Universidad de Navarra, Madrid, Spain; Research Department of Haematology, Cancer Immunology Unit, University College London Cancer Institute, London, UK.
Mark A Socinski, Thoracic Oncology, AdventHealth Cancer Institute, Orlando, FL, USA.
Ashley M Hopkins, Department of Clinical Pharmacology, College of Medicine and Public Health, Flinders University, Adelaide, Australia.
Michael J Sorich, Department of Clinical Pharmacology, College of Medicine and Public Health, Flinders University, Adelaide, Australia.
Funding
This work was supported by Beat Cancer Research Fellowship from the Cancer Council South Australia to M.J.S., and Emerging Leader Investigator Grant from the National Health and Medical Research Council, Australia (APP2008119) to A.M.H.
Conflict of Interest
Federico Cappuzzo has received fees for membership on an advisory board or lectures from Roche, AstraZeneca, BMS, Pfizer, Takeda, Lilly, Bayer, Amgen, Sanofi, Pharmamar, Novocure, Mirati, Galecto, OSE, and MSD. Ignacio Matos reports investigator-initiated project grants from Beigene and ESMO (sponsored by Roche). Mark A. Socinski has received research grants from Genentech, Spectrum, AstraZeneca, Novartis, and Daichii Sankyo and has received personal fees for advisory roles for Genentech, AstraZeneca, Bayer, Norvartis, Guardant, and Amgen. Michael J. Sorich reports investigator-initiated project grants from Pfizer, outside the submitted work. The other authors indicated no financial relationships.
Author Contributions
Conception/design: L.X.L., A.M.H., M.J.S. Provision of study material or patients: A.M.H., M.J.S. Collection and/or assembly of data: L.X.L., A.M.H., M.J.S. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors.
Data Availability
The data underlying this study were accessed through Vivli Inc. according to Roche’s policy and process for clinical study data sharing and are available for request at vivli.org. As such, the data is not directly shareable by our research team. Vivli has not contributed to or approved and is not in any way responsible for the contents of this publication.
References
- 1. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801. https://doi.org/ 10.1038/s41467-020-17670-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dafni U, Tsourti Z, Vervita K, Peters S.. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer. 2019;134:127-140. https://doi.org/ 10.1016/j.lungcan.2019.05.029 [DOI] [PubMed] [Google Scholar]
- 3. Ferrara R, Matos I.. Atypical patterns of response and progression in the era of immunotherapy combinations. Future Oncol. 2020;16(23):1707-1713. https://doi.org/ 10.2217/fon-2020-0186 [DOI] [PubMed] [Google Scholar]
- 4. Adashek JJ, Kato S, Ferrara R, Lo Russo G, Kurzrock R.. Hyperprogression and immune checkpoint inhibitors: hype or progress? Oncologist. 2020;25(2):94-98. https://doi.org/ 10.1634/theoncologist.2019-0636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543-1552. https://doi.org/ 10.1001/jamaoncol.2018.3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim Y, Kim CH, Lee HY, et al. Comprehensive clinical and genetic characterization of hyperprogression based on volumetry in advanced non-small cell lung cancer treated with immune checkpoint inhibitor. J Thorac Oncol. 2019;14(9):1608-1618. https://doi.org/ 10.1016/j.jtho.2019.05.033 [DOI] [PubMed] [Google Scholar]
- 7. Tunali I, Gray JE, Qi J, et al. Novel clinical and radiomic predictors of rapid disease progression phenotypes among lung cancer patients treated with immunotherapy: an early report. Lung Cancer. 2019;129:75-79. https://doi.org/ 10.1016/j.lungcan.2019.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim CG, Kim KH, Pyo KH, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small cell lung cancer. Ann Oncol. 2019;30(7):1104-1113. https://doi.org/ 10.1093/annonc/mdz123 [DOI] [PubMed] [Google Scholar]
- 9. Arasanz H, Zuazo M, Bocanegra A, et al. Early detection of hyperprogressive disease in non-small cell lung cancer by monitoring of systemic t cell dynamics. Cancers. 2020;12(2):344. https://doi.org/ 10.3390/cancers12020344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruiz-Patiño A, Arrieta O, Cardona AF, et al. Immunotherapy at any line of treatment improves survival in patients with advanced metastatic non-small cell lung cancer (NSCLC) compared with chemotherapy (Quijote-CLICaP). Thorac Cancer. 2020;11(2):353-361. https://doi.org/ 10.1111/1759-7714.13272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berge D, Hurkmans D, den Besten I, et al. Tumour growth rate as a tool for response evaluation during PD-1 treatment for non-small cell lung cancer: a retrospective analysis. ERJ Open Res. 2019;5(4):179. https://doi.org/ 10.1183/23120541.00179-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lo Russo G, Moro M, Sommariva M, et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res. 2019;25(3):989-999. https://doi.org/ 10.1158/1078-0432.CCR-18-1390 [DOI] [PubMed] [Google Scholar]
- 13. Abbar B, De Castelbajac V, Gougis P, et al. Definitions, outcomes, and management of hyperprogression in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Lung Cancer. 2021;152:109-118. https://doi.org/ 10.1016/j.lungcan.2020.12.026 [DOI] [PubMed] [Google Scholar]
- 14. Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920-1928. https://doi.org/ 10.1158/1078-0432.CCR-16-1741 [DOI] [PubMed] [Google Scholar]
- 15. Sasaki A, Nakamura Y, Mishima S, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. 2019;22(4):793-802. https://doi.org/ 10.1007/s10120-018-00922-8 [DOI] [PubMed] [Google Scholar]
- 16. Kanjanapan Y, Day D, Wang L, et al. Hyperprogressive disease in early-phase immunotherapy trials: clinical predictors and association with immune-related toxicities. Cancer. 2019;125(8):1341-1349. https://doi.org/ 10.1002/cncr.31999 [DOI] [PubMed] [Google Scholar]
- 17. Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28(7):1605-1611. https://doi.org/ 10.1093/annonc/mdx178 [DOI] [PubMed] [Google Scholar]
- 18. Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23(15):4242-4250. https://doi.org/ 10.1158/1078-0432.CCR-16-3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim JY, Lee KH, Kang J, et al. Hyperprogressive disease during anti-PD-1 (PDCD1)/PD-L1 (CD274) therapy: a systematic review and meta-analysis. Cancers. 2019;11(11). https://doi.org/ 10.3390/cancers11111699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small cell lung cancer (BIRCH). J Clin Oncol. 2017;35(24):2781-2789. https://doi.org/ 10.1200/JCO.2016.71.9476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spigel DR, Chaft JE, Gettinger S, et al. FIR: efficacy, safety, and biomarker analysis of a phase II open-label study of atezolizumab in PD-L1–selected patients with NSCLC. J Thorac Oncol. 2018;13(11):1733-1742. https://doi.org/ 10.1016/j.jtho.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fehrenbacher LD, Spira AMD, Ballinger MP, et al. Atezolizumab versus docetaxel for patients with previously treated non-small cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846. https://doi.org/ 10.1016/s0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 23. Rittmeyer AMD, Barlesi FP, Waterkamp DMD, et al. Atezolizumab versus docetaxel in patients with previously treated non-small cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2016;389(10066):255-265. https://doi.org/ 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924-937. https://doi.org/ 10.1016/S1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- 25. Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol. 2020;15(8):1351-1360. https://doi.org/ 10.1016/j.jtho.2020.03.028 [DOI] [PubMed] [Google Scholar]
- 26. Socinski MA, Jotte RM, Cappuzzo F, et al. ; IMpower150 Study Group. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. https://doi.org/ 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 27. Matos I, Martin-Liberal J, Garcia-Ruiz A, et al. Capturing hyperprogressive disease with immune-checkpoint inhibitors using RECIST 1.1 criteria. Clin Cancer Res. 2020;26(8):1846-1855. https://doi.org/ 10.1158/1078-0432.ccr-19-2226 [DOI] [PubMed] [Google Scholar]
- 28. Yadav K, Lewis RJ.. Immortal time bias in observational studies. JAMA. 2021;325(7):686-687. https://doi.org/ 10.1001/jama.2020.9151 [DOI] [PubMed] [Google Scholar]
- 29. Akinboro O, Vallejo JJ, Mishra-Kalyani PS, et al. Outcomes of anti-PD-(L1) therapy in combination with chemotherapy versus immunotherapy (IO) alone for first-line (1L) treatment of advanced non-small cell lung cancer (NSCLC) with PD-L1 score 1-49%: FDA pooled analysis. J Clin Oncol. 2021;39(15_suppl):9001. https://doi.org/ 10.1200/JCO.2021.39.15_suppl.9001 [DOI] [Google Scholar]
- 30. Greaves M, Maley CC.. Clonal evolution in cancer. Nature. 2012;481(7381):306-313. https://doi.org/ 10.1038/nature10762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuo CHS, Wang CC, Huang YC, et al. Comparison of a combination of chemotherapy and immune checkpoint inhibitors and immune checkpoint inhibitors alone for the treatment of advanced and metastatic non-small cell lung cancer. Thorac Cancer. 2019;10(5):1158-1166. https://doi.org/ 10.1111/1759-7714.13057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park HJ, Kim KW, Won SE, et al. Definition, incidence, and challenges for assessment of hyperprogressive disease during cancer treatment with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Netw Open. 2021;4(3):e211136-e. https://doi.org/ 10.1001/jamanetworkopen.2021.1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramon-Patino JL, Schmid S, Lau S, et al. iRECIST and atypical patterns of response to immuno-oncology drugs. J Immunother Cancer. 2022;10(6). https://doi.org/ 10.1136/jitc-2022-004849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lo Russo G, Facchinetti F, Tiseo M, Garassino MC, Ferrara R.. Hyperprogressive disease upon immune checkpoint blockade: focus on non-small cell lung cancer. Curr Oncol Rep. 2020;22(5):41. https://doi.org/ 10.1007/s11912-020-00908-9 [DOI] [PubMed] [Google Scholar]
- 35. Kas B, Talbot H, Ferrara R, et al. Clarification of definitions of hyperprogressive disease during immunotherapy for non-small cell lung cancer. JAMA Oncol. 2020;6(7):1039-1046. https://doi.org/ 10.1001/jamaoncol.2020.1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is not directly shareable by our research team. This publication is based on research using data from Roche that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication.
The data underlying this study were accessed through Vivli Inc. according to Roche’s policy and process for clinical study data sharing and are available for request at vivli.org. As such, the data is not directly shareable by our research team. Vivli has not contributed to or approved and is not in any way responsible for the contents of this publication.