Abstract
Background
Anodyspareunia may be an adverse outcome of prostate cancer (PCa) treatment for gay, bisexual, and other men who have sex with men (GBM).
Aim
The aims of this study were to (1) describe the clinical symptoms of painful receptive anal intercourse (RAI) in GBM following PCa treatment, (2) estimate the prevalence of anodyspareunia, and (3) identify clinical and psychosocial correlates.
Methods
This was a secondary analysis of baseline and 24-month follow-up data from the Restore-2 randomized clinical trial of 401 GBM treated for PCa. The analytic sample included only those participants who attempted RAI during or since their PCa treatment (N = 195).
Outcomes
Anodyspareunia was operationalized as moderate to severe pain during RAI for ≥6 months that resulted in mild to severe distress. Additional quality-of-life outcomes included the Expanded Prostate Cancer Index Composite (bowel function and bother subscales), the Brief Symptom Inventory–18, and the Functional Assessment of Cancer Therapy–Prostate.
Results
Overall 82 (42.1%) participants reported pain during RAI since completing PCa treatment. Of these, 45.1% experienced painful RAI sometimes or frequently, and 63.0% indicated that the pain was persistent. The pain at its worst was moderate to very severe for 79.0%. The experience of pain was at least mildly distressing for 63.5%. Painful RAI worsened for a third (33.4%) of participants after completing PCa treatment. Of the 82 GBM, 15.4% were classified as meeting criteria for anodyspareunia. Antecedents of anodyspareunia included a lifelong history of painful RAI and bowel dysfunction following PCa treatment. Those reporting symptoms of anodyspareunia were more likely to avoid RAI due to pain (adjusted odds ratio, 4.37), which was negatively associated with sexual satisfaction (mean difference, −2.77) and self-esteem (mean difference, −3.33). The model explained 37.2% of the variance in overall quality of life.
Clinical Implications
Culturally responsive PCa care should include the assessment of anodyspareunia among GBM and explore treatment options.
Strengths and Limitations
This is the largest study to date focused on anodyspareunia among GBM treated for PCa. Anodyspareunia was assessed with multiple items characterizing the intensity, duration, and distress related to painful RAI. The external validity of the findings is limited by the nonprobability sample. Furthermore, the cause-and-effect relationships between the reported associations cannot be established by the research design.
Conclusions
Anodyspareunia should be considered a sexual dysfunction in GBM and investigated as an adverse outcome of PCa treatment.
Keywords: cancer survivor, prostatic neoplasms, nonheterosexual persons, GLBT persons, sexual minorities, sexual dysfunction, aged
Introduction
Sexual dysfunction is a common and well-known adverse outcome of prostate cancer (PCa) treatment.1,2 While an estimated 1 in 8 gay, bisexual, and other men who have sex with men (GBM) will be diagnosed with PCa in their lifetimes, little is known about how PCa treatment affects their sexual function.3,4 The reason is that the assessment of sexual dysfunction is largely focused on heteronormative assumptions of sex (ie, the ability to produce erections firm enough for vaginal intercourse).5,6 Therefore, the available evidence to inform clinical practice does not fully address the needs of GBM.
Culturally responsive PCa survivorship care should address the fact that, for many GBM, receptive anal intercourse (RAI) is a common and culturally important sexual behavior.7 Up to 90% of gay men and 66% of bisexual men have ever engaged in RAI.8 RAI is an important behavior to the sexual lives of many GBM across the life span and among GBM with PCa.3,8 Patient-reported outcome measures commonly used to identify adverse treatment effects among PCa survivors, such as the Expanded Prostate Cancer Index Composite (EPIC), do not address RAI as a sexual function. Given that RAI as a sexual and sociocultural practice is uniquely valued by GBM as a patient population, it should be fully considered in the diagnosis and treatment of PCa-related sexual dysfunction.4,9
PCa treatment may affect RAI functional status in multiple direct and indirect ways. First, the removal of the prostate can lead to changes in sensation and the loss of pleasure during RAI.10 Second, radiation treatments can result in bowel dysfunction, chronic proctitis, and rectal fibrosis.11 These conditions can make RAI painful. They can also cause performance anxiety related to RAI that may decrease pleasure and increase pain.12 Painful RAI has only recently been identified as an important component of sexual dysfunction in GBM requiring treatment.13
Anodyspareunia—defined as recurrent or persistent pain experienced during RAI—is a potential adverse outcome of PCa treatment among GBM who engage in RAI.3 In 1998, Rosser and colleagues coined the term “anodyspareunia” to define “recurrent or persistent pain experienced by the receptive partner during anal intercourse”.3 They proposed 3 diagnostic criteria. The pain needed to be (1) recurrent or persistent, (2) sufficient to cause marked distress or interpersonal difficulty, and (3) not caused by other medical conditions or exclusively by other behavioral determinants (eg, lack of lubrication). Anodyspareunia is conceptually related to genitopelvic pain/penetration disorders (GPPPDs).14 But unlike GPPPD in female cancer survivors, the prevalence, causes, and treatment options for painful RAI are severely understudied. Painful vaginal intercourse (ie, dyspareunia) has been widely researched, can be assessed with validated sexual function measures (eg, Female Sexual Function Index), and can be treated with a variety of modalities.15-17 Almost nothing is known about anodyspareunia in GBM treated for cancer.
The prevalence of anodyspareunia among GBM in the general population varies widely depending on the conceptual definition. When pain is defined as severe and reoccurring, prevalence estimates range between 10% and 14%.18 Only 1 study to date has characterized anodyspareunia in GBM with PCa.3 In that study, MASKED FOR REVIEW and colleagues defined anodyspareunia as “severe pain in the rectum during receptive anal sex, sufficient to stop having sex.”3 The estimated prevalence was 23% among GBM treated for PCa. However, their analysis was restricted to GBM with a recent RAI attempt (within the previous month). Thus, men with more severe sexual dysfunction may have been systematically excluded. The current study builds on this previous research by characterizing the experience of pain during RAI over a longer time frame (ie, since being treated for PCa).
The causes and consequences of anodyspareunia among GBM in general and PCa survivors in particular are unknown. Informed by the broader literature on sexual dysfunction, Grabski and Kasparek found that sexual performance anxiety was the strongest correlate of anodyspareunia among a sample of GBM in Poland.18 They also found that older men and those in long-term relationships experienced less anodyspareunia. MASKED FOR REVIEW and colleagues, in their study of GBM treated for PCa, identified worse mental health and bowel problems (ie, EPIC bowel bother) as significant correlates of anodyspareunia.3 Overall, the existing literature has not used theory to identify potential causes and consequences of anodyspareunia.
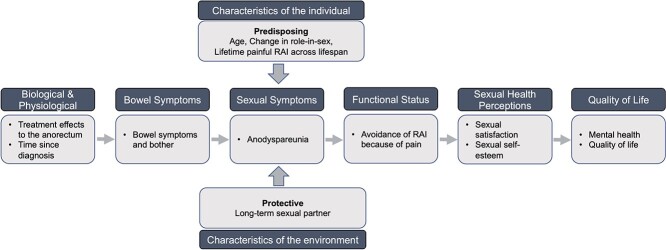
Wilson and Cleary’s model of health-related quality of life provides a useful framework to investigate the causes and consequences of anodyspareunia in patients with PCa. Their model emphasizes subjective function and health perceptions.19 In the case of PCa, treatments result in physiologic changes (eg, anal resting pressure, rectal distensibility, and chronic inflammation) and related symptoms (ie, perceptions about the state of the body).11,20 It is well established that PCa treatments can cause physiologic changes to the bowel that result in long-term symptoms (eg, rectal urgency, cramping).21 We propose that these physiologic changes and the experience of bowel symptoms can affect the occurrence of anodyspareunia directly (ie, through chronic inflammation and rectal fibrosis) and indirectly (eg, through performance anxiety from the experience of bowel symptoms) (Figure 1). Individual-level factors could be predisposing, such as experiences of painful RAI prior to treatment. Other individual-level factors could be protective, like being in a long-term relationship. Under this framework, the consequences of anodyspareunia are lower sexual satisfaction, lower sexual self-esteem (ie, functional changes), and ultimately poorer quality of life.
Figure 1.
Theoretical framework to understand the antecedents and consequences of anodyspareunia in gay and bisexual survivors of prostate cancer.
Research is needed to better understand anodyspareunia, which will inform equitable clinical care that addresses the rehabilitation needs of GBM with PCa. The purpose of this study was to (1) estimate the prevalence of anodyspareunia, (2) describe clinical symptoms of painful RAI in GBM following PCa treatment, and (3) identify clinical and psychosocial correlates of anodyspareunia.
Methods
Sample and recruitment
Data were from a clinical trial (MASKED FOR REVIEW) designed to test the effectiveness of an online rehabilitation program for GBM with PCa. Participants were recruited through online advertisements, gay community resources (eg, print media and service organizations), word of mouth, or other online sources and from clinics. Participants completed a brief screening survey. Inclusion criteria for the parent study were as follows: (1) identified as gay, bisexual, or a man who has sex with men; (2) lived in the United States; (3) had been diagnosed with PCa; (4) had completed treatment, were in active treatment, or were scheduled to receive treatment within 2 months of study enrollment; (5) reported current sexual and/or urinary problems; (6) were able to read English; and (7) were able to access intervention materials online. Transgender women were also welcome to participate, although none did. Participants who were deemed eligible by the screening survey received a confirmatory vetting telephone call to verify eligibility.
The current study utilized a subset of participants who had attempted or engaged in RAI since starting or completing their PCa treatment (195 of the 401 participants in the parent study). All demographic information was taken from the baseline survey. All other data in this analysis were taken from the 24-month survey. Each participant received $50 in compensation for the baseline survey and $25 for the 24-month survey. Detailed methods for the parent study are described elsewhere.3 All aspects of this study were approved by the Institutional Review Board of the MASKED FOR REVIEW.
Measures
Pain during RAI
The presence of pain during RAI was first assessed with the following item: “Thinking about the time period since you completed your prostate cancer treatment until now, have you experienced pain during receptive anal sex?” Response options included yes or no. Those responding yes were asked to describe the following: severity (intensity of worst pain, with 4 categories from mild to very severe), persistence (period lasting ≥6 months when painful receptive anal sex has been an issue, with 4 categories from not true to very true and an unsure option), and distress (with 5 categories from not at all distressing to severely distressing). Next, participants were asked about the frequency of pain (with 5 categories from never to always), if the pain got worse since PCa treatment (4 categories from not true to very true with an unsure option), perception that the pain was a direct result of PCs treatment (4 categories from not true to very true with an unsure option), and what they perceived to be the causes of the pain. The 5 most common causes of pain were identified by asking participants to choose ≥1 reason for their pain (eg, medications, general health, lack of lubrication, lack of foreplay, position of sex). All participants, regardless of their experience of painful RAI after PCa treatment, were asked if, throughout their lives, RAI has always or almost always been painful (with 4 categories from not true to very true).
Individual and environmental characteristics
Questions from the US Census and the 2018 American Community Survey were used to assess participant demographics such as race, ethnicity, age, and education.22 Questions related to sexual orientation, change in sex role identity, and relationship status were based on measures used in the MASKED FOR REVIEW-1 study.3 Participants were asked to report their role in sex in the year prior to PCa diagnosis and in the most recent 4 weeks to determine if there was a change (eg, from exclusive bottom to not applicable).
Clinical factors
Participants selected all applicable PCa treatments from a list of 9. For analysis, treatment was collapsed into 3 exclusive categories: radical prostatectomy only (ie, surgery only), radiation (external beam radiation or brachytherapy only), or combined/systemic. The latter category included prostatectomy with radiation, androgen deprivation therapy, or a combination of these treatments. Participants reported the month and year of their PCa diagnosis. They also reported the year of their radical prostatectomy and brachytherapy treatments as well as the month and year of their radiation and androgen deprivation therapy. Current treatment status was assessed by asking participants what their status was according to their most recent checkup.
Symptoms and function
The EPIC bowel function and bother subscales were used to measure PCa-specific symptoms.23 These assessed the frequency of bowel symptoms and the distress caused by those symptoms. Each domain is scored from 0 to 100, with 100 indicating better health. The overall summary score is the combination of its corresponding functional and bother domain scores. In general populations, the EPIC has high internal consistency (Cronbach α ≥ 0.82), test-retest reliability (r ≥ 0.80), and validity, with Pearson correlation coefficients ranging from 0.29 to 0.77.23
One item adapted from the Sexual Minorities and Prostate Cancer Scale was used to assess how frequently, in the past 4 weeks, participants had avoided RAI because of pain.3 Responses were recorded on a 5-point unipolar scale ranging from none of the time to all of the time.
Sexual health perceptions
Sexual health perceptions were assessed with the Multidimensional Sexuality Questionnaire, which measures psychological tendencies associated with human sexuality in 12 domains. We used the sexual satisfaction and sexual esteem (sexual self-esteem) domains in the current study.24 Each domain contains 5 items that are measured with a 5-point Likert scale from 0 to 4. Participants were also given an option of “I am not currently in a sexual relationship” for 2 items in the sexual satisfaction domain; as such, participants who chose this did not receive a total sexual satisfaction domain score. Responses to domain items are summed to create overall domain scores ranging from 0 to 20, with higher scores indicating better sexual satisfaction or self-esteem. The sexual satisfaction and self-esteem subscales demonstrated good internal consistency in previous studies (Cronbach α = 0.87 and 0.90, respectively).24
Quality of life
Mental health quality of life was assessed via the Brief Symptom Inventory–18 (BSI-18). The scale assesses mental health in 3 domains: somatization, anxiety, and depression. Each of the 3 domains has 6 items based on a 5-point Likert scale. Responses items are summed to create domain scores ranging from 0 to 24, and the 3 domain scores are summed to create the Global Severity Index (range, 0-72). Higher BSI-18 scores indicate worse mental health. The scale has high internal consistency, with Cronbach α coefficients ranging from 0.74 to 0.89.25
Overall quality of life related to cancer was assessed through the general summary score from the Functional Assessment of Cancer Therapy–Prostate (FACT-P). The FACT-P measures quality of life related to cancer and its treatment in 4 domains—physical, social/family, functional, and emotional well-being—with a PCa symptom-specific domain score.26 The FACT-General score is the sum of the physical, social/family, functional, and emotional well-being scores. Higher scores represent better overall quality of life. Cronbach α coefficients for this scale range from 0.65 (for the PCa domain) to 0.89 (for the sum of all 5 FACT-P domains).26
Data analysis
Participants were excluded from the analytic sample if they answered not applicable (n = 15) or refused to answer (n = 1) the survey item assessing whether they had experienced pain during receptive anal sex since completing treatment. The final analytic sample consisted of 195 participants who attempted RAI since PCa treatment.
The operationalization of anodyspareunia in the current study was based on symptoms of GPPPD and on a dynamic time frame (ie, since being treated for PCa) to be inclusive of men who may completely abstain from RAI due to pain. Participants were classified as having anodyspareunia if they met the following criteria: (1) they had experienced pain during RAI since completing treatment; (2) they experienced moderate, severe, or very severe pain during RAI; (3) they experienced mild, moderate, or severe distress from the pain; and (4) there had been periods lasting ≥6 months when painful RAI had been an issue for them. Participants who did not meet all criteria were classified as not having anodyspareunia.
Years since treatment was operationalized as a continuous measure ±6 months due to variability in reporting of PCa treatment dates. To increase the interpretation, the EPIC bowel summary score was reverse coded, with higher scores indicating worse overall bowel health (ie, “worse bowel symptoms/bother”). The frequency of participants avoiding RAI due to pain was collapsed into none of the time or at least some of the time (ie, a little, some, or most of the time). Participant demographic, clinical, and quality-of-life characteristics were summarized with means and standard deviations for continuous variables and counts and percentages for categorical variables. Participant characteristics were compared by anodyspareunia classification based on t-tests for continuous variables and chi-square tests or Fisher exact tests for categorical variables.
Multivariable linear regression was used to assess the unadjusted mean differences and adjusted mean differences (aMDs) for all quality-of-life measures (EPIC bowel summary domain, BSI-18, and FACT-General) and sexual health measures (sexual satisfaction and sexual self-esteem). Multivariable logistic regression was used to assess the unadjusted and adjusted odds ratios (aORs) for the outcomes of sexual symptoms (anodyspareunia classification) and functional status (avoiding RAI because of pain). Adjusted models included all antecedent factors as well as predisposing and protective factors for each outcome as outlined in the model (Figure 1).
Mean differences and odds ratios were considered to be statistically significant at P < .05. All reported P values were 2-sided. Analyses were conducted with Stata Statistical Software (release 17; StataCorp).
Results
Sample characteristics are presented in Table 1. Participants were on average 62.5 years of age (SD, 6.7), and most had completed active treatment for PCa (92.3%; average, 7.7 years prior). The most common treatment received was surgery (66.2%), followed by radiation (15.9%) and combined/systemic modalities (17.9%). Most participants were non-Hispanic White (87.7%), college educated (71.8%), and married or partnered (50.8%).
Table 1.
Sample characteristics and bivariate correlates of anodyspareunia among gay and bisexual men attempting RAI after prostate cancer treatment (N = 195).a
| Anodyspareunia clinical classification b | ||||
|---|---|---|---|---|
| Sample | No | Yes | P value c | |
| Total | 195 | 165 (84.6) | 30 (15.4) | |
| Individual characteristics | ||||
| Age | 64.5 ± 6.7 | 64.6 ± 6.8 | 64.2 ± 6.6 | .758 |
| Race/ethnicity | .476 | |||
| White and non-Hispanic | 171 (87.7) | 144 (87.3) | 27 (90.0) | |
| Non-White and/or Hispanic | 24 (12.3) | 21 (12.7) | 3 (10.0) | |
| Education | .968 | |||
| Less than bachelor degree | 55 (28.2) | 46 (27.9) | 9 (30.0) | |
| Bachelor degree | 69 (35.4) | 59 (35.8) | 10 (33.3) | |
| Graduate or professional degree | 71 (36.4) | 60 (36.4) | 11 (36.7) | |
| Change in sex role identity | .390 | |||
| No change/current bottom or versatile | 125 (67.2) | 104 (65.8) | 21 (75.0) | |
| Change from sex role to “not applicable” | 61 (32.8) | 54 (34.2) | 7 (25.0) | |
| Lifetime experience of painful RAI | <.001 | |||
| Mostly true/very true | 33 (16.9) | 19 (11.7) | 14 (48.3) | |
| Not true/somewhat true | 159 (81.5) | 144 (88.3) | 15 (51.7) | |
| Characteristics of the environment | ||||
| Relationship status | .038 | |||
| Single, dating, divorced, widowed | 96 (49.2) | 76 (46.1) | 20 (66.7) | |
| Partnered, married | 99 (50.8) | 89 (53.9) | 10 (33.3) | |
| Clinical factors | ||||
| Treatment | .119 | |||
| Surgery (only) | 129 (66.2) | 114 (69.1) | 15 (50.0) | |
| Radiation (only) | 31 (15.9) | 24 (14.6) | 7 (23.3) | |
| Combined/systemic | 35 (17.9) | 27 (16.4) | 8 (26.7) | |
| Current treatment status | .146 | |||
| Undergoing initial treatment | 2 (1.0) | 1 (0.6) | 1 (3.3) | |
| In treatment for advanced prostate cancer | 11 (5.6) | 10 (6.1) | 1 (3.3) | |
| Finished treatment | 180 (92.3) | 153 (92.7) | 27 (90.0) | |
| Other | 2 (1.0) | 1 (0.6) | 1 (3.3) | |
| Time since, y | ||||
| Last treatment | 6.1 ± 5.1 | 6.0 ± 5.0 | 6.6 ± 5.5 | .560 |
| Diagnosis | 7.7 ± 4.9 | 7.6 ± 4.8 | 8.2 ± 5.7 | .545 |
| Symptoms and function | ||||
| EPIC | ||||
| Bowel function | 88.5 ± 12.2 | 89.7 ± 9.8 | 81.9 ± 19.9 | .001 |
| Bowel bother | 89.2 ± 15.5 | 90.8 ± 12.8 | 80.4 ± 24.2 | .001 |
| Avoidance of RAI because of pain | <.001 | |||
| A little, some, most of the time | 54 (27.8) | 36 (22.0) | 18 (60.0) | |
| None of the time | 140 (72.2) | 128 (78.0) | 12 (40.0) | |
| Sexual health perceptions | ||||
| Sexual self-esteem | 11.2 ± 6.3 | 11.9 ± 6.2 | 7.6 ± 6.1 | .001 |
| Sexual satisfaction | 8.5 ± 6.8 | 9.1 ± 6.9 | 4.9 ± 4.9 | .009 |
| Quality of life | ||||
| Brief Symptom Inventory–18 | ||||
| Somatic | 2.4 ± 3.1 | 2.3 ± 3.0 | 3.0 ± 3.7 | .276 |
| Depression | 3.7 ± 4.7 | 3.6 ± 4.7 | 4.1 ± 4.6 | .568 |
| Anxiety | 2.3 ± 3.5 | 2.3 ± 3.6 | 2.0 ± 2.8 | .645 |
| Summary (Global Severity Index) | 8.3 ± 9.7 | 8.2 ± 9.8 | 9.1 ± 9.5 | .646 |
| Functional Assessment of Cancer Therapy–General | 2.52 ± 1.30 | 2.54 ± 1.29 | 2.43 ± 1.33 | .682 |
Abbreviations: EPIC, Expanded Prostate Cancer Index Composite; RAI, receptive anal intercourse.
aData are presented as No. (%) or mean ± SD.
Defined as moderate to severe pain during RAI lasting for ≥6 months with mild to severe distress.
Bold indicates statistically significant difference, P < .05.
All the participants in this analysis attempted RAI since completing their PCa treatment; however, the frequency of RAI varied. Nearly half indicated that they rarely had RAI (47.2%); others reported RAI sometimes (35.4%) or frequently (15.9%).
Characteristics of painful RAI
The experience of any pain during RAI since PCa treatment was reported by 82 (42.1%) participants. The frequency of RAI was not associated with posttreatment pain (P = .378). Out of the 82 participants who reported posttreatment painful RAI, 29 (35.4%) indicated lifetime painful RAI; however, 51 (62.2%) cited either infrequent lifetime painful RAI or none.
Characteristics of the pain experienced during RAI following PCa treatment is described for those 82 participants in Table 2. The pain was moderate to severe for 79%, persistent for ≥6 months for 64.4%, and mildly to severely distressing for 63.4%. The frequency of pain during RAI occurred frequently or always for 37.0% of participants. Another third (33.4%) indicated that painful RAI was at least somewhat worse since being treated for PCa; however, most (52.6%) did not attribute painful RAI directly to their PCa treatment. Approximately 1 in 8 (12.4%) believed that their pain was related to their PCa treatment. Other perceived causes of painful RAI were the size of the partner’s penis (17.3%), roughness/duration of RAI (12.4%), anal fissures/hemorrhoids (12.4%), and anxiety (11.1%).
Table 2.
Description of pain symptoms among participants who experienced painful RAI since being treated for prostate cancer (n = 82).
| No. (%) | |
|---|---|
| Severity, persistence, and distress related to painful RAI | |
| Intensity of the worst pain during RAI | |
| Mild | 17 (21.0) |
| Moderate | 38 (46.9) |
| Severe | 23 (28.4) |
| Very severe | 3 (3.7) |
| Periods lasting ≥6 mo when painful RAI has been an issue | |
| Not true for me | 26 (35.6) |
| Somewhat true for me | 26 (35.6) |
| Mostly true for me | 12 (16.4) |
| Very true for me | 8 (11.0) |
| Unsure | 10 (1.4) |
| Distress associated with painful RAI | |
| Not at all | 7 (8.5) |
| Minimal | 23 (28.1) |
| Mild | 25 (30.5) |
| Moderate | 18 (22.0) |
| Severe | 9 (11.0) |
| Consistency, progression, and perceived causes of painful RAI | |
| Frequency of pain during RAI | |
| Never | 1 (1.2) |
| Rarely | 12 (14.8) |
| Sometimes | 38 (46.9) |
| Frequently | 15 (18.5) |
| Always | 15 (18.5) |
| Pain during RAI got worse since my prostate cancer treatment | |
| Not true | 41 (54.7) |
| Somewhat true | 15 (20.0) |
| Mostly true | 5 (6.7) |
| Very true | 5 (6.7) |
| Unsure | 9 (12.0) |
| Perception that painful RAI as a direct result of prostate cancer treatment | |
| Not true | 40 (52.6) |
| Somewhat true | 9 (11.8) |
| Mostly true | 5 (6.6) |
| Very true | 3 (4.0) |
| Unsure | 19 (25.0) |
| Common perceived causes of painful RAIa | |
| Size of partner’s penis | 14 (17.3) |
| Prostate cancer treatment related | 10 (12.4) |
| Roughness or duration of the sex we had | 10 (12.4) |
| Anal fissures or hemorrhoids | 10 (12.4) |
| Anxiety (including engaging in RAI) | 9 (11.1) |
Abbreviation: RAI, receptive anal intercourse.
Participant could select >1 response.
Prevalence of anodyspareunia
Among those who experienced any pain during RAI since PCa treatment (n = 82), 30 met classification for anodyspareunia, defined as moderate to severe pain during RAI lasting for ≥6 months with mild to severe distress. This equates to 15.4% of the total sample of men who reengaged in RAI and 36.6% of the subset who experienced any painful RAI since their PCa treatment.
Clinical and psychosocial correlates of anodyspareunia
Bivariate differences among participants meeting classification for anodyspareunia are described in Table 1 across the theoretical domains. As predicted by the model, differences in bowel function and bother and avoidance of RAI were associated with anodyspareunia. Among the predisposing and protective characteristics, relationship status and lifetime painful RAI were also associated with anodyspareunia. Other characteristics, such as age and change in sex role identity, were not associated with anodyspareunia and thus not included in the regression models. Results from the regression models are presented in Table 3, and the final model results (aMDs and aORs) are summarized by theoretical domain.
Table 3.
Regression models identifying the antecedents and impact of anodyspareunia in gay and bisexual men treated for prostate cancer (N = 195).a
| Mean difference or odds ratio (95% CI) | ||
|---|---|---|
| Domain—outcome variable: predictor | Unadjusted b | Adjusted c |
| Bowel and sexual symptoms | ||
| Worse bowel symptoms/botherd | ||
| Radiation only treatment (ref: surgery only) | 3.14 (−1.96, 8.24) | 3.67 (−1.54, 8.88) |
| Combination/ADT (ref: surgery only) | 4.57 (−0.29, 9.42) | 4.72 (−0.21, 9.66) |
| Time since treatment, y | 0.04 (−0.32, 0.41) | 0.11 (−0.26, 0.48) |
| R 2 | 0.024 | |
| Anodyspareunia since PCa treatmente | ||
| Worse bowel symptoms/botherd | 1.04 (1.02, 1.07) b | 1.04 (1.01, 1.08) c |
| Lifetime painful RAI (ref: not true/somewhat true) | 7.09 (2.89, 17.38) b | 7.98 (2.94, 21.66) c |
| Single (ref: married/partnered) | 2.34 (1.03, 5.31) b | 1.89 (0.75, 4.76)c |
| Radiation only treatment (ref: surgery only) | 2.22 (0.82, 6.02)b | 1.53 (0.50, 4.67)c |
| Combination/hormone treatment (ref: surgery only) | 2.25 (0.87, 5.85)b | 2.49 (0.81, 7.65)c |
| Time since treatment, years | 1.02 (0.95, 1.10)b | 0.99 (0.91, 1.09)c |
| R 2 | 0.198 | |
| Functional status | ||
| Avoidance of RAI because of pain | ||
| Anodyspareunia since PCa treatmente | 5.33 (2.35, 12.09) b | 4.37 (1.65, 11.58) c |
| Lifetime painful RAI (ref: not true/somewhat true) | 1.21 (0.51, 2.87)b | 0.60 (0.20, 1.80)c |
| Single (ref: married/partnered) | 1.92 (1.01, 3.64) b | 1.67 (0.81, 3.43)c |
| Worse bowel symptoms/botherd | 1.05 (1.02, 1.08) b | 1.04 (1.01, 1.07) c |
| Radiation only treatment (ref: surgery only) | 3.08 (1.35, 7.03) b | 2.60 (1.03, 6.52) c |
| Combination/hormone treatment (ref: surgery only) | 2.21 (0.99, 4.95)b | 1.77 (0.71, 4.41)c |
| Time since treatment, y | 0.97 (0.91, 1.04)b | 0.96 (0.89, 1.03)c |
| R 2 | 0.148 | |
| Sexual health perceptions | ||
| Sexual satisfaction | ||
| Avoidance of RAI because of pain | −4.19 (−6.54, −1.84) | −2.77 (−5.33, −0.21) |
| Anodyspareunia since PCa treatmente | −4.28 (−7.48, −1.08) | −2.43 (−5.75, 0.89) |
| Worse bowel symptoms/botherd | −0.15 (−0.23, −0.06) | −0.11 (−0.20, −0.02) |
| Radiation only treatment (ref: surgery only) | 0.16 (−3.05, 3.38) | 0.87 (−2.27, 4.00) |
| Combination/hormone treatment (ref: surgery only) | −1.39 (−4.44, 1.67) | −0.07 (−3.09, 2.94) |
| Time since treatment, years | 0.04 (−0.20, 0.28) | −0.01 (−0.24, 0.22) |
| R 2 | 0.138 | |
| Sexual self-esteem | ||
| Avoidance of RAI because of pain | −4.00 (−5.93, −2.07) | −3.33 (−5.44, −1.23) |
| Anodyspareunia since PCa treatmente | −4.30 (−6.71, −1.88) | −3.14 (−5.69, −0.59) |
| Worse bowel symptoms/botherd | −0.06 (−0.12, 0.01) | −0.002 (−0.07, 0.07) |
| Radiation only treatment (ref: surgery only) | 0.14 (−2.38, 2.65) | 1.38 (−1.12, 3.89) |
| Combination/hormone treatment (ref: surgery only) | −1.32 (−3.72, 1.08) | −0.33 (−2.68, 2.02) |
| Time since treatment, y | 0.06 (−0.12, 0.24) | 0.06 (−0.12, 0.23) |
| R 2 | 0.118 | |
| Quality of life | ||
| Mental health (BSI-18/GSI) | ||
| Sexual satisfaction | −0.35 (−0.56, −0.15) | −0.20 (−0.48, 0.07) |
| Sexual self-esteem | −0.24 (−0.46, −0.02) | −0.05 (−0.36, 0.26) |
| Avoidance of RAI because of pain | 2.73 (−0.33, 5.80) | 1.15 (−2.28, 4.58) |
| Anodyspareunia since PCa treatmente | 0.89 (−2.93, 4.71) | −1.28 (−5.52, 2.95) |
| Worse bowel symptoms/botherd | 0.28 (0.19, 0.38) | 0.21 (0.09, 0.33) |
| Radiation only treatment (ref: surgery only) | 0.80 (−3.04, 4.63) | 1.14 (−2.84, 5.12) |
| Combination/hormone treatment (ref: surgery only) | 2.59 (−1.06, 6.25) | 1.31 (−2.51, 5.14) |
| Time since treatment, y | −0.19 (−0.46, 0.09) | −0.06 (−0.35, 0.24) |
| R 2 | 0.184 | |
| Overall quality of life (FACT-General) | ||
| Sexual satisfaction | 1.19 (0.83, 1.54) | 0.63 (0.17, 1.08) |
| Sexual self-esteem | 1.01 (0.66, 1.37) | 0.55 (0.05, 1.05) |
| Avoidance of RAI because of pain | −8.41 (−13.62, −3.19) | −2.67 (−8.31, 2.97) |
| Anodyspareunia since PCa treatmente | −4.86 (−11.44, 1.71) | 1.52 (−5.45, 8.48) |
| Worse bowel symptoms/botherd | −0.51 (−0.68, −0.34) | −0.38 (−0.57, −0.18) |
| Radiation only treatment (ref: surgery only) | −5.01 (−11.62, 1.59) | −5.56 (−12.11, 0.99) |
| Combination/hormone treatment (ref: surgery only) | −5.81 (−12.10, 0.49) | −2.42 (−8.71, 3.86) |
| Time since treatment, years | 0.31 (−0.16, 0.78) | −0.08 (−0.57, 0.41) |
| R 2 | 0.372 | |
Abbreviations: ADT, androgen deprivation therapy; BSI-18, Brief Symptom Inventory–18; GSI, Global Severity Index; FACT-General, Functional Assessment of Cancer Therapy–General; PCa, prostate cancer; RAI, receptive anal intercourse; ref, reference.
Bold indicates statistically significant difference, P < .05.
Crude odds ratio.
Adjusted odds ratio: adjusted models included all antecedent factors as well as predisposing and protective factors (if applicable) for each outcome.
Expanded Prostate Cancer Index Bowel Composite: higher values indicate worse bowel function.
Defined as moderate to severe pain during RAI lasting for ≥6 months with mild to severe distress.
Symptom status
Type or time since treatment was not associated with bowel symptoms/bother. However, those with worse bowel symptoms/bother had higher odds of meeting classification for anodyspareunia (aOR, 1.04; 95% CI, 1.01-1.08) after adjusting for predisposing and protective characteristics.
Functional status
Participants meeting classification for anodyspareunia had higher odds of avoiding RAI because of pain (aOR, 4.37; 95% CI, 1.65-11.58). Avoidance was also independently associated with worse bowel symptoms/bother (aOR, 1.05; 95% CI, 1.01-1.07) and receiving radiation treatment vs surgery (aOR, 2.60; 95% CI, 1.03-6.52). Models were adjusted for predisposing and protective characteristics.
Sexual health perceptions
Avoidance of RAI because of pain was independently associated with worse sexual satisfaction (aMD, −2.77; 95% CI, −5.33 to −0.21) and worse sexual self-esteem (aMD, −3.33; 95% CI, −5.44 to −1.23). In addition, sexual satisfaction was lower among those with worse bowel symptoms/bother (aMD, −0.11; 95% CI, −0.20 to −0.02), and sexual self-esteem was lower among those with anodyspareunia (aMD, −3.14; 95% CI, −5.69 to −0.59).
Quality of life
Worse bowel symptoms/bother were independently associated with worse mental health symptoms (aMD, 0.21; 95% CI, 0.09-0.33). Sexual satisfaction and self-esteem were not associated with mental health after adjusting for bowel symptoms/bother. However, overall quality of life was positively associated with sexual satisfaction (aMD, 0.63; 95% CI, 0.17-1.08) and sexual self-esteem (aMD, 0.55; 95% CI, 0.05-1.05). Conversely, overall quality of life was lower for those with worse bowel symptoms/bother (aMD, −0.38; 95% CI, −0.57 to −0.18). The model explained 37.2% of the variance in quality of life.
Discussion
This is the first study to examine clinical characteristics of anodyspareunia among GBM cancer survivors. Painful RAI was reported by a significant subset of participants. For most of these men, the experience of painful RAI was not disruptive to their sexual functioning. However, there was significant disruption among the men meeting classification for anodyspareunia. Men in this group described pain during RAI as moderate to severe, persistent, and resulting in psychological distress. Painful RAI was not a lifelong issue for most men meeting classification for anodyspareunia, suggesting that this issue emerged following PCa treatment. While a third of men in this study described worsening pain following PCa treatment, the perceived cause of the pain varied. Some men directly attributed the pain to their treatments while many were unsure.
In total, 1 in 7 GBM in this sample met the classification for anodyspareunia (~15.4% of our sample). This estimate is greater than that from samples of GBM in the general population (ie, without PCa) but lower than a previous study of GBM with PCa.3,18 The discrepancy among these estimates, which collectively range from 10% to 23%, likely results from methodological differences. The operationalization of anodyspareunia in the current study was based on criteria for GPPPD (ie, intensity, persistence, and distress) and used a dynamic time frame (ie, since being treated for PCa) to be inclusive of men who may completely abstain from RAI due to pain. This measurement approach differs from previous studies that only measured painful RAI from the past 30 days (eg, PROMIS Sexual Function and Satisfaction: Anal Discomfort; adapted items from the Female Sexual Function Index).27,28 Future studies are needed to establish validated measures of anodyspareunia.
Wilson and Cleary’s model provided a useful framework to understand the antecedents and consequences of anodyspareunia for GBM with PCa.19 As proposed in the framework (Figure 1), physiologic changes (eg, anal resting pressure, rectal distensibility, and inflammation) from treatment can result in a set of patient-reported symptoms that ultimately affect functional status and quality of life.11,20 The empirical findings show that GBM with worse bowel symptoms were more likely to meet classification of anodyspareunia, avoided RAI because of pain, and reported lower sexual satisfaction. Bowel symptoms were also independently associated with worse mental health and overall quality of life.
There are several biopsychosocial mechanisms to explain the connection among PCa treatment, bowel symptoms, and anodyspareunia. First, bowel symptoms are indicative of tissue damage and chronic inflammation (eg, radiation proctitis) that can result in painful RAI.11 In this study, bowel symptoms were independently associated with anodyspareunia and a decrease in sexual function (ie, avoidance of RAI due to pain). Furthermore, as found in other studies, bowel dysfunction was worse in men treated with radiation therapy than in those treated with surgery alone; however, we were unable to replicate these findings in the current study (likely due to the low sample size in the radiation and combined/systemic treatment groups).29
Second, painful RAI may result from increased sensitivity to painful stimuli (ie, hyperalgesia) that results from prolonged tissue damage caused by radiation therapy. This can be perceived as worsening pain over time. A subset of men in this study reported that pain during RAI worsened following PCa treatment. Last, fear and hypervigilance to pain may inhibit the relaxation of the anal sphincter muscles, resulting in painful RAI. Persistent bowel symptoms can also increase sexual performance anxiety, which can result in pain and discomfort during RAI. Identifying specific mechanisms leading to painful RAI, whether physiologic or psychological, will help to inform future sexual rehabilitation interventions.
A key finding of this study is that sexual function in RAI is important to the quality of life of GBM following PCa treatment. The final model explained approximately 40% of the variance in overall quality of life with sexual self-esteem as an independent correlate. Avoidance of RAI due to pain was associated with lower sexual satisfaction and self-esteem. As described in previous qualitative research, a loss of anal sex function and sex role identity can be “devastating.”3 Future research is needed to explore the ways in which GBM who experience anodyspareunia following PCa cope with loss of RAI sexual function.
As described in the DSM-5, sexual dysfunctions are a “heterogeneous group of disorders that are typically characterized by a clinically significant disturbance in a person’s ability to respond sexually or to experience sexual pleasure.”30 In this study, GBM described the severity and persistence of pain that caused psychological distress and reduced their sexual satisfaction and self-esteem. The occurrence of anodyspareunia worsened after PCa treatment for a subset of our sample, suggesting a possible causative role. These findings, with those from previous studies,3 suggest that anodyspareunia should be considered a sexual dysfunction in GBM and investigated as an adverse outcome of PCa treatment.
As predicted by the model, a history of painful RAI was an important predisposing factor to anodyspareunia. As in GPPPD, anodyspareunia may be lifelong or acquired. No existing research has sought to explore the history of painful RAI across the life course. Previous studies have found that older age was negatively associated with painful RAI,18,27 but age was not a significant predictor in the current analysis (possibly due to the low variability in age). Another possible predisposing factor not explored in the current study is internalized homophobia. Previous research based on samples of GBM without cancer suggested that painful RAI may be more likely among those with higher levels of internalized homophobia.3,18,28
Patient-centered approaches are needed to treat anodyspareunia and restore sexual function for GBM. Based on the findings reported here, underlying bowel issues are a key target for interventions. Proctitis should be evaluated in patients who received pelvic radiation, in addition to other potential causes (eg, inflammatory bowel disease, diverticular colitis). Proctitis can also result from untreated sexually transmitted infections (STIs).31 Recent evidence demonstrated that GBM PCa survivors are at high risk of STIs; therefore, routine STI testing should be a part of survivorship care for sexually active GBM.
Furthermore, GBM patients with PCa should be counseled on the potential impact of radiation treatment and bowel function on RAI (eg, anodyspareunia). The American Society of Clinical Oncology recommends that a member of the oncology team initiate a discussion with each patient about the sexual health and dysfunction that can result from cancer treatment. It states that such discussions should be initiated at the time of diagnosis and reassessed periodically. Given our findings, we recommend that for GBM, clinical consultations should include a discussion of their preferred role in anal sex (insertive and/or receptive, if applicable) and history of anodyspareunia. This information is vital to informed treatment decisions.
Psychological interventions focused on reducing anxiety during RAI may prove effective, as similar approaches have been successfully used to treat GPPPD in women.14 Pelvic floor physical therapy has also been used to treat bowel and sexual dysfunction in cancer survivors, but its role in the treatment of anodyspareunia has not been investigated.32-34 The use of therapeutic anal dilators to decrease pain and help with relaxation is one possible treatment approach that warrants exploration. Vaginal dilators have been used to treat stenosis and GPPPD in women following cancer treatments.35 Preliminary evidence suggests that the use of anal dilators for the treatment of sexual dysfunction is an acceptable approach among GBM with PCa.3
As this was a cross-sectional nonexperimental analysis, the cause-and-effect relationships between the constructs could not be determined. Some constructs (eg, experience of pain during RAI) were also retrospectively measured and could reflect recall bias. The results reported here were from a nonprobability sample of mostly college educated White GBM with PCa. Therefore, the findings may not be generalizable to the larger population of GBM PCa survivors. Prospective studies on ethnoracially diverse samples of GBM with PCa are needed to replicate findings and establish temporal relationships between the theoretical constructs.
Conclusions
RAI was a common sexual behavior in this sample of older GBM who were treated for PCa. Painful RAI was reported by 42.1%. For a subset of these men, painful RAI was moderate to severe in intensity, persisted for ≥6 months, and resulted in psychological distress. We propose that this subset of participants are describing clinical characteristics of anodyspareunia. Antecedents of anodyspareunia included a lifelong history of painful RAI in addition to bowel dysfunction following PCa treatment. Those citing symptoms of anodyspareunia were more likely to avoid RAI due to pain, which was negatively associated with sexual satisfaction, self-esteem, and ultimately overall quality of life. It is essential that GBM are informed about the potential sexual impacts of PCa treatments on RAI as part of shared clinical decision making. Painful RAI should also be assessed and monitored as an adverse patient-reported outcome for GBM. Future research is needed to test treatment options for anodyspareunia.
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health under award R01CA218657. The National Cancer Institute had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. Research reported in this publication was also supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development under award T32HD095134. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This project also benefited from support provided by the Minnesota Population Center (P2CHD041023), which receives funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Conflicts of interest: None declared.
Contributor Information
Christopher W Wheldon, Department of Social and Behavioral Sciences, College of Public Health, Temple University, Philadelphia, PA United States.
Alex J Bates, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota, United States.
Elizabeth J Polter, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota, United States.
B R Simon Rosser, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota, United States.
Aditya Kapoor, Department of Radiology, Trinity Teleradiology Services, Vancouver, BC, Canada.
Kristine M C Talley, Adult and Gerontological Health, University of Minnesota School of Nursing School, Minneapolis, Minnesota, United States.
Ryan Haggart, Department of Urology, University of Minnesota, Minneapolis, Minnesota, United States.
Nidhi Kohli, Department of Educational Psychology, University of Minnesota, Minneapolis, Minnesota, United States.
Badrinath R Konety, Department of Urology, Rush Medical College, Chicago, Illinois.
Darryl Mitteldorf, Malecare Cancer Support, New York, New York, United States.
Michael W Ross, Department of Family Medicine and Community Health, University of Minnesota Medical School, Minneapolis, Minnesota, United States.
William West, Department of Writing Studies, University of Minnesota, Minneapolis, Minnesota, United States.
Morgan Wright, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota, United States.
References
- 1. Loi M, Wortel RC, Francolini G, et al. Sexual function in patients treated with stereotactic radiotherapy for prostate cancer: a systematic review of the current evidence. J Sex Med. 2019;16:1409–1420. 10.1016/j.jsxm.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 2. Vernooij RWM, Lancee M, Cleves A, et al. Radical prostatectomy versus deferred treatment for localised prostate cancer. Cochrane Database Syst Rev. 2020;6:CD006590. 10.1002/14651858.CD006590.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ussher J, Perz J, SimonRosser BR, Simon Rosser BR, Hunt S, Capistrant B, et al. Understanding prostatecancer in gay, bisexual, and other men who have sex with men and transgenderwomen: a review of the literature. Gay & Bisexual Men Living with ProstateCancer 2018:12–37. 10.17312/harringtonparkpress/2018.06.gbmlpc.001. [DOI]
- 4. Mitchell E, Ziegler E. Sexual dysfunction in gay and bisexual prostate cancer survivors: a concept analysis. J Homosex. 2022;69:1119–1139. 10.1080/00918369.2021.1905384. [DOI] [PubMed] [Google Scholar]
- 5. Lee TK, Breau RH, Mallick R, et al. A systematic review of Expanded Prostate Cancer Index Composite (EPIC) quality of life after surgery or radiation treatment. Can J Urol. 2015;22:7599–7606. [PubMed] [Google Scholar]
- 6. Rnic K, Linden W, Tudor I, et al. Measuring symptoms in localized prostate cancer: a systematic review of assessment instruments. Prostate Cancer Prostatic Dis. 2013;16:111–122. 10.1038/pcan.2013.1. [DOI] [PubMed] [Google Scholar]
- 7. Rosser BRS, Rider GN, Kapoor A, et al. Every urologist and oncologist should know about treating sexual and gender minority prostate cancer patients: translating research findings into clinical practice. Transl Androl Urol. 2021;10:3208–3225. 10.21037/tau-20-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dodge B, Herbenick D, Fu TC, et al. Sexual behaviors of US men by self-identified sexual orientation: results from the 2012 National Survey of Sexual Health and Behavior. J Sex Med. 2016;13:637–649. 10.1016/j.jsxm.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 9. Amarasekera C, Wong V, Jackson K, et al. A pilot study assessing aspects of sexual function predicted to be important after treatment for prostate cancer in gay men: an underserved domain highlighted. LGBT Health. 2020;7:271–276. 10.1089/lgbt.2018.0245. [DOI] [PubMed] [Google Scholar]
- 10. Levin RJ. Prostate-induced orgasms: a concise review illustrated with a highly relevant case study—prostate-induced orgasms. Clin Anat. 2018;31:81–85. 10.1002/ca.23006. [DOI] [PubMed] [Google Scholar]
- 11. Vanneste BGL, Van De Voorde L, de Ridder RJ, et al. Chronic radiation proctitis: tricks to prevent and treat. Int J Color Dis. 2015;30:1293–1303. 10.1007/s00384-015-2289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hollows K. Anodyspareunia: a novel sexual dysfunction? An exploration into anal sexuality. Sex Relatsh Ther. 2007;22:429–443. 10.1080/14681990701481409. [DOI] [Google Scholar]
- 13. Cheng PJ. Sexual dysfunction in men who have sex with men. Sex Med Rev. 2022;10:130–141. 10.1016/j.sxmr.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 14. Conforti C. Genito-pelvic pain/penetration disorder (GPPPD): an overview of current terminology, etiology, and treatment. UOJM. 2017;7:6. 10.18192/uojm.v7i2.2198. [DOI] [Google Scholar]
- 15. Baser RE, Li Y, Carter J. Psychometric validation of the Female Sexual Function Index (FSFI) in cancer survivors: FSFI validation in cancer survivors. Cancer. 2012;118:4606–4618. 10.1002/cncr.26739. [DOI] [PubMed] [Google Scholar]
- 16. Ramaseshan AS, Felton J, Roque D, et al. Pelvic floor disorders in women with gynecologic malignancies: a systematic review. Int Urogynecol J. 2018;29:459–476. 10.1007/s00192-017-3467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mendoza N, Carrión R, Mendoza-Huertas L, et al. Efficacy and safety of treatments to improve dyspareunia in breast cancer survivors: a systematic review. Breast Care (Basel). 2020;15:599–607. 10.1159/000506148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grabski B, Kasparek K. Sexual anal pain in gay and bisexual men: in search of explanatory factors. J Sex Med. 2020;17:716–730. 10.1016/j.jsxm.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 19. Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA 1995;273:59–65. 10.1001/jama.273.1.59. [DOI] [PubMed] [Google Scholar]
- 20. Krol R, Smeenk RJ, van Lin ENJT, et al. Systematic review: anal and rectal changes after radiotherapy for prostate cancer. Int J Color Dis. 2014;29:273–283. 10.1007/s00384-013-1784-8. [DOI] [PubMed] [Google Scholar]
- 21. Clark JA, Wray N, Brody B, et al. Dimensions of quality of life expressed by men treated for metastatic prostate cancer. Soc Sci Med. 1997;45:1299–1309. 10.1016/S0277-9536(97)00058-0. [DOI] [PubMed] [Google Scholar]
- 22. US Census Bureau . American Community Survey. US Census Bureau; 2018.
- 23. Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urol. 2000;56:899–905. 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 24. Snell WE, Fisher TD, Walters AS. The Multidimensional Sexuality Questionnaire: an objective self-report measure of psychological tendencies associated with human sexuality. Ann Sex Res. 1993;6:27–55. 10.1007/BF00849744. [DOI] [Google Scholar]
- 25. Petrowski K, Schmalbach B, Jagla M, et al. Norm values and psychometric properties of the Brief Symptom Inventory–18 regarding individuals between the ages of 60 and 95. BMC Med Res Methodol. 2018;18:164. 10.1186/s12874-018-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Esper P, Mo F, Chodak G, et al. Measuring quality of life in men with prostate cancer using the Functional Assessment of Cancer Therapy–Prostate instrument. Urol. 1997;50:920–928. 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 27. Vansintejan J, Vandevoorde J, Devroey D. The Gay Men Sex Studies: anodyspareunia among Belgian gay men. Sex Med. 2013;1:87–94. 10.1002/sm2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li DH, Remble TA, Macapagal K, et al. Stigma on the streets, dissatisfaction in the sheets: is minority stress associated with decreased sexual functioning among young men who have sex with men? J Sex Med. 2019;16:267-277. 10.1016/j.jsxm.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barocas DA, Alvarez J, Resnick MJ, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA. 2017;317:1126. 10.1001/jama.2017.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. American Psychiatric Association . Sexual dysfunctions. In: Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 31. Steed JM, Henry-Okafor Q, Pitts CJ. Proctitis in men who have sex with men. Nurs Clin North Am. 2020;55:325–335. 10.1016/j.cnur.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 32. Tchelebi LT, Eng C, Messick CA, et al. Current treatment and future directions in the management of anal cancer. CA A Cancer J Clin. 2022;72:183–195. 10.3322/caac.21712. [DOI] [PubMed] [Google Scholar]
- 33. Lin K-Y, Granger CL, Denehy L, et al. Pelvic floor muscle training for bowel dysfunction following colorectal cancer surgery: a systematic review—pelvic floor muscle training in bowel cancer. Neurourol Urodyn. 2015;34:703–712. 10.1002/nau.22654. [DOI] [PubMed] [Google Scholar]
- 34. Rosenbaum TY. Pelvic floor involvement in male and female sexual dysfunction and the role of pelvic floor rehabilitation in treatment: a literature review. J Sex Med. 2007;4:4–13. 10.1111/j.1743-6109.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 35. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:CD007291. 10.1002/14651858.CD007291.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]