Abstract
Indocyanine green (ICG)-based dynamic contrast-enhanced fluorescence imaging (DCE-FI) can objectively assess bone perfusion intraoperatively. However, it is susceptible to motion artifacts due to patient’s involuntary respiration during the 4.5-minute DCE-FI data acquisition. An automated motion correction approach based on mutual information (MI) frameby-frame was developed to overcome this problem. In this approach, MIs were calculated between the reference and the adjacent frame translated and the maximal MI corresponded to the optimal translation. The images obtained from eighteen amputation cases were utilized to validate the approach and the results show that this correction can significantly reduce the motion artifacts and can improve the accuracy of bone perfusion assessment.
Keywords: motion artifact, dynamic contrast-enhanced fluorescence imaging, indocyanine green, bone vascular perfusion, open orthopedic surgery, time-intensity curve
1. INTRODUCTION
Infection following trauma is one of the most prevalent and challenging complications faced by orthopedic surgeons. Inadequate vascular perfusion is a major cause of this complication since poorly perfused bone is a nidus for biofilm formation creating resistance to antibiotics. Because of this, management of open fractures and fracture-related infection is based on aggressive, thorough debridement in an effort to remove all poorly perfused bone. However, there is a critical lack of image tools to objectively inform bone perfusion and guiding debridement [1]. In contrast to other clinical imaging modalities, such as dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) [2, 3] and combined positron emission tomography/computed tomography (PET/CT) [4], which cannot be used in the operating room, indocyanine green (ICG)-based dynamic contrast-enhanced fluorescence imaging (DCE-FI) has shown an ability to objectively assess bone perfusion intraoperatively and provide surgical guidance for debriding poorly perfused tissues [5].
Although DCE-FI is feasible and easy to use in the operating room, the motion artifacts, due to patient’s involuntary expiration and other mechanical disturbance during the imaging data acquisition, lead to the errors in quantitative bone perfusion assessment. To overcome this problem to improve the accuracy of bone perfusion assessment, an automated imaging processing approach has been developed to correct motion artifacts in intraoperative DCE-FI.
2. MATERIALS AND METHODS
2.1. Patient imaging
Under an IRB-approved protocol, 18 receiving lower extremity amputation were enrolled into this study. For each patient, at each imaging session, ICG-based DCE-FI was acquired by a SPY Elite imaging system every 0.267 seconds for 4.5 minutes. Following 20 seconds of pre-injection imaging, 0.1 mg/kg of ICG was administered intravenously to the patient. During the surgery, three individual imaging sessions were recorded before and after two surgical procedures of cut and strip, leading to a total of 53 evaluable datasets.
2.2. Imaging processing
The proposed automatic motion correction method is based on mutual information (MI), a measure of the statistical dependence between the corresponding pixel intensities of two images [6]. The MI of two images would be maximal if they are best geometrically aligned, which serves as a robust criterion for medical image registration [7]. Because our datasets lack rigid and reliable reference objects whereas MI takes the global information of the image into account, the maximization of MI stands out to be a straightforward and effective solution to motion artifact removal for DCE-FI [8].
In our approach, MI was calculated frame-by-frame because the average intensity of DCE-FI was dynamically changing along the time. Corrections based on fixed reference frame have been tested and failed to produce the satisfied results.
The maximal MI represents the optimal translation, by which the target frame was adjusted accordingly. The corrected target frame was then set as the new reference, with the next frame as the new target. This process was repeated, with the reference and the target frame updated during each run, until the last frame was reached. Down-sampling and kernel search were also included to speed up the computation.
After applying MI-based motion correction, kinetic curves and bone blood perfusion-related parameters such as the maximal intensity (Imax), time-to-peak (TTP) and ingress slope (IS) [9] in the corresponding regions of interest (ROI) were estimated for validating the effectiveness of this approach. Other evaluation methods including dice score and kinetic map comparison were also applied [10].
3. RESULTS
To visually illustrate the results of motion correction, Figure 1 shows an example of a overlaid tibia ICG image of a 53 years old male patient. The images were taken after an osteotomy at the middle of the tibia. Two frames at separate time points (t1=50s, t2=100s) were extracted and overlaid to highlight the differences before (Fig.1a) and after (Fig.1b) the correction. Bright colors such as pink or green indicates the areas with substantial intensity differences, whereas grayscale represents a close agreement between the two frames. It can be seen that the edges between bone and skin areas before the correction were clearly mismatched, highlighted with the yellow arrow (Fig.1a), while those edges were mostly aligned or overlaid after the correction (Fig.1b).
Figure 1.
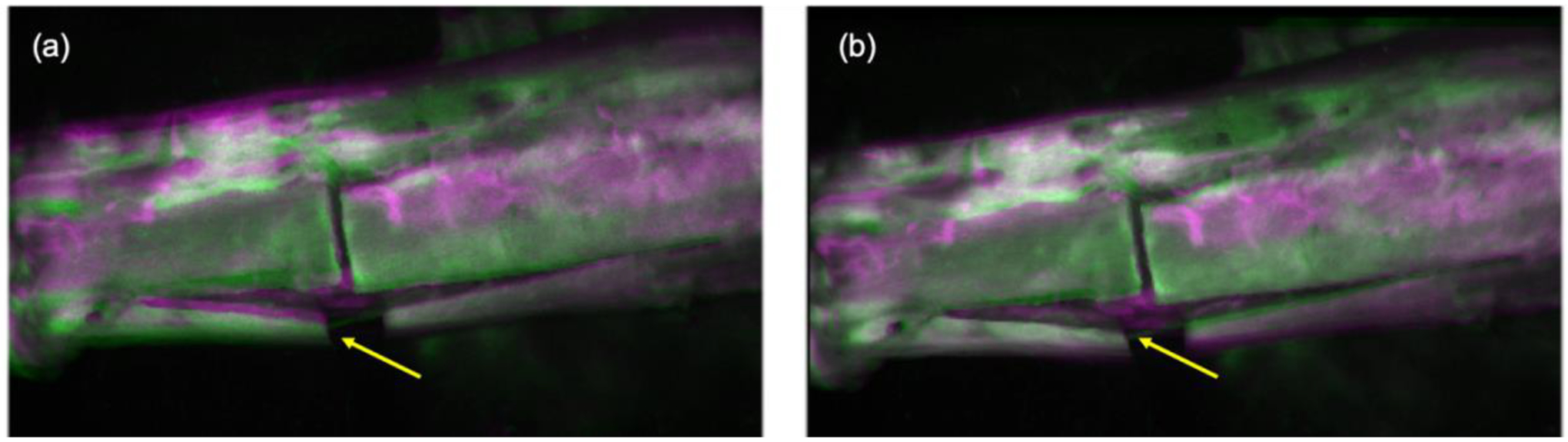
Overlay of two frames of a tibia ICG image at two separate time points (t1=50s, t2=100s) before (a) and after the motion correction (b).
Figure 2 (a)–(e) shows the kinetic curves before and after motion correction on DCE-FI of the same amputation case shown in Fig.1, with an osteotomy at the middle of the tibia. Fig.2(a) is a FI (imaged at 1 minute after ICG injection), overlaid on a white light image taken before ICG was injected. Four circular ROIs (d1, d2, p1, p2) were drawn in different areas of the bone. Kinetic curves are shown in Fig.2(b)–(e), with the same color as their corresponding ROIs. Solid and dash lines represent kinetic curves before and after the motion correction, respectively. The motion-induced intensity surges were smoothed out and the general trend of ICG bolus take-in and wash-out were restored.
Figure 2.
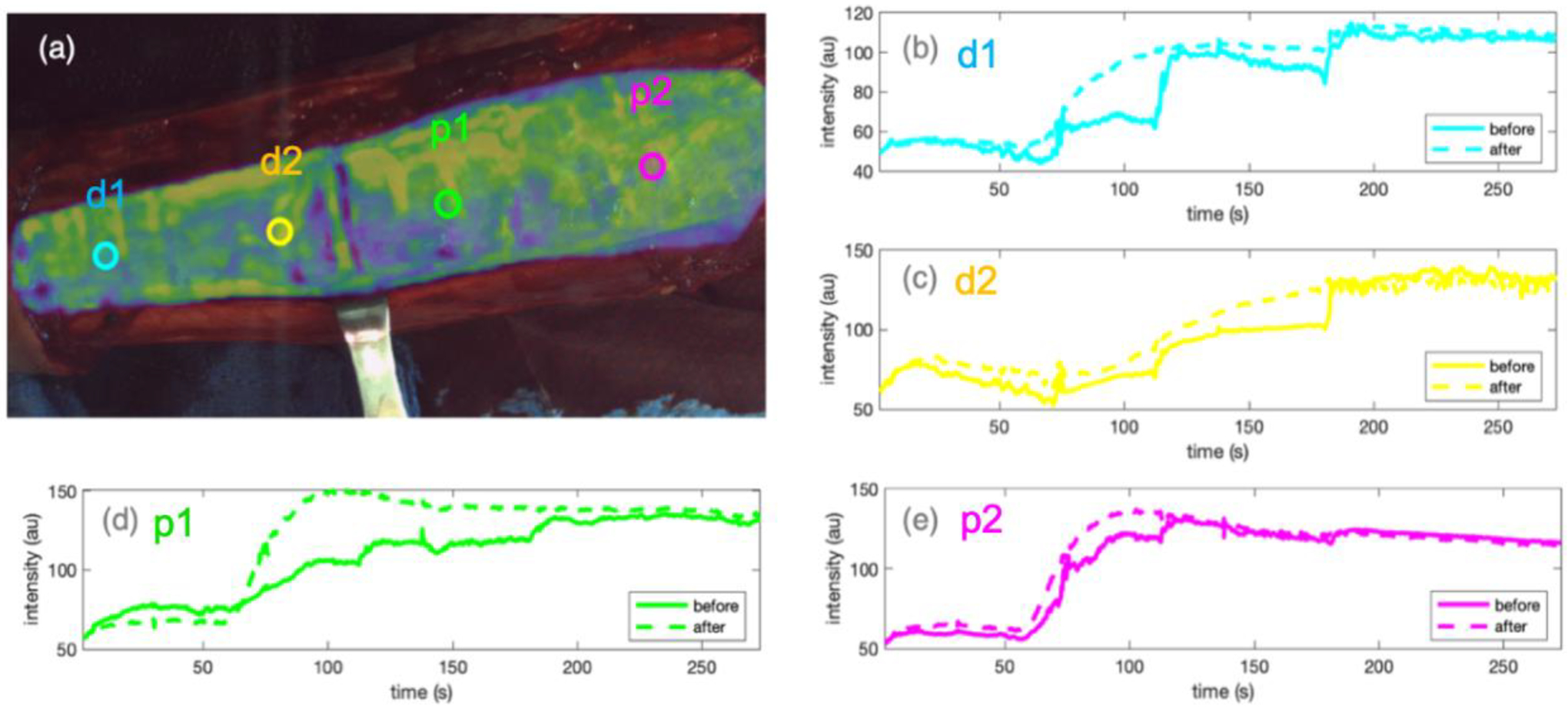
MI-based motion correction applied on an amputation case. (a) FI overlaid on the white light image of the surgical view, with 4 ROIs drawn on different areas of the bone, where kinetic curves were extracted. (b)-(e) kinetic curves of four ROIs in corresponding colors. Solid and dashed lines represent kinetic curves before and after the correction, respectively.
Figure 3 shows the kinetic parameters extracted from the four ROIs in Fig.2 (a) with corresponding colors, before and after the correction. As shown in Fig.2 (a), ROIs of d1 (blue) and d2 (yellow) were at the distal region (towards the foot), whereas p1 (green) and p2 (pink) were at the periosteal area (towards head). Since the osteotomy damaged endosteal blood supply of the ROIs in distal region, bone blood perfusion-related kinetic parameters such as Imax, TTP and IS in ROIs of d1 and d2 were expected to be obviously different than those in ROIs of p1 and p2. It can be seen in Fig.3 that there was no clear trend of Imax, TTP nor IS in d1 and d2, compared to those in p1 and p2, before the motion correction. However, after the correction, Imax and IS in p1 and p2 (dashed line) were obviously higher than those in d1 and d2 (solid line), whereas periosteal TTPs became much lower than that in distal ROIs. The trends of these perfusion-related parameters after the motion correction demonstrated lower perfusion in the distal ROIs as expected.
Figure 3.
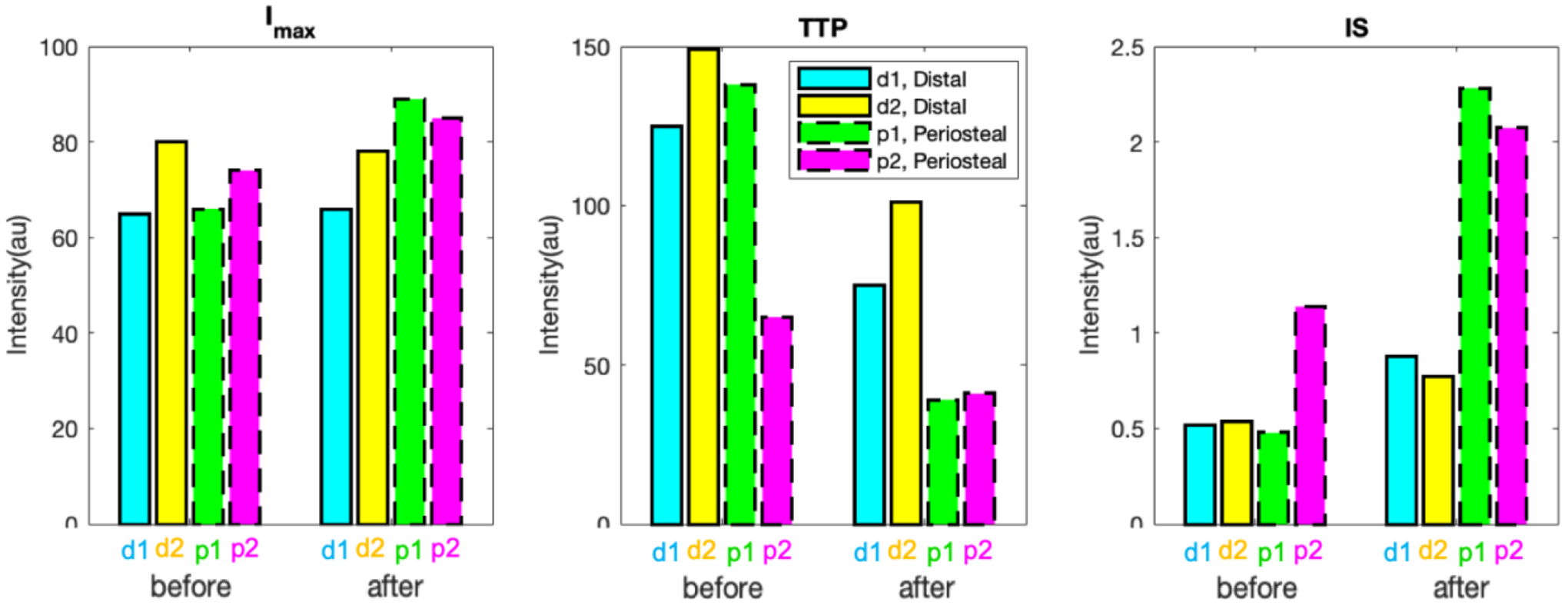
Bone blood perfusion-related kinetic parameters of four ROIs from Fig.2a in corresponding colors. Solid and dashed lines represent ROIs in distal and periosteal regions, respectively.
The motion correction has been applied to all images in 53 imaging sessions of 18 amputation cases. The statistical results shown that an average of 28.5 and 68.8 pixels were corrected for each case in the x-axis and y-axis, respectively.
To reduce the processing time, temporal down-sampling was applied, and maximum MI was searched by a limited size of kernel (instead of exhaustive grid-search). However, the processing time for each imaging data set is still about twenty minutes. Given that the perfusion assessment during open orthopedic surgeries requires the feedback from the imaging within a couple of minutes [11], further improvement such as using parallel computing is ongoing.
4. CONCLUSION
In conclusion, the results from this study demonstrated that automated MI-based correction is capable to remove the motion artifacts during the imaging data acquisition and to improve the accuracy of bone perfusion assessment at different ROIs over the bone.
ACKNOWLEGEMENTS
This work was funded by a NIH R01 AR077157, a DOD Clinical Translational Research award (OR190062) and a Gillian Reny Stepping Strong Center for Trauma Innovation award.
REFERENCES
- [1].Marenzana M, and Arnett TR, “The Key Role of the Blood Supply to Bone,” Bone Research 1, 203–215 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jodal L, Nielsen OL, Afzelius P, Alstrup AKO, and Hansen SB, “Blood perfusion in osteomyelitis studied with [(15)O]water PET in a juvenile porcine model,” EJNMMI Res 7, 4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Poot DHJ, van der Heijden RA, van Middelkoop M, Oei EHG, and Klein S, “Dynamic contrast-enhanced MRI of the patellar bone: How to quantify perfusion,” J Magn Reson Imaging 47, 848–858 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dyke JP, Synan M, Ezell P, Ballon D, Racine J, and Aaron RK, “Characterization of bone perfusion by dynamic contrast-enhanced magnetic resonance imaging and positron emission tomography in the Dunkin-Hartley guinea pig model of advanced osteoarthritis,” J Orthop Res 33, 366–372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Han X, Demidov V, Vaze VS, Jiang S, Gitajn IL, and Elliott JT, “Spatial and temporal patterns in dynamiccontrast enhanced intraoperative fluorescence imaging enable classification of bone perfusion in patients undergoing leg amputation,” Biomed Opt Express 13, 3171–3186 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Maes F, Collignon A, Vandermeulen D, Marchal G, and Suetens P, “Multimodality image registration by maximization of mutual information,” IEEE Trans Med Imaging 16, 187–198 (1997). [DOI] [PubMed] [Google Scholar]
- [7].Rohde GK, Dawant BM, and Lin SF, “Correction of motion artifact in cardiac optical mapping using image registration,” Ieee Transactions on Biomedical Engineering 52, 338–341 (2005). [DOI] [PubMed] [Google Scholar]
- [8].Maes F, Vandermeulen D, and Suetens P, “Medical image registration using mutual information,” Proceedings of the Ieee 91, 1699–1722 (2003). [Google Scholar]
- [9].Jiang S, Elliott JT, Gunn JR, Xu C, Ruiz AJ, Henderson ER, Pogue BW, and Gitajn IL, “Endosteal and periosteal blood flow quantified with dynamic contrast-enhanced fluorescence to guide open orthopaedic surgery,” Proc SPIE Int Soc Opt Eng 11222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Elliott JT, Jiang SD, Pogue BW, and Gitajn IL, “Bone-specific kinetic model to quantify periosteal and endosteal blood flow using indocyanine green in fluorescence guided orthopedic surgery,” Journal of Biophotonics 12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gitajn IL, Elliott JT, Gunn JR, Ruiz AJ, Henderson ER, Pogue BW, and Jiang SD, “Evaluation of bone perfusion during open orthopedic surgery using quantitative dynamic contrast-enhanced fluorescence imaging,” Biomedical Optics Express 11, 6458–6469 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]


