Abstract
Background and Objectives
Individuals with subjective cognitive decline (SCD) who perform normally on objective cognitive tests may have an increased risk of pathological cognitive decline and progression to Alzheimer’s disease (AD) and related dementia (ADRD). Working memory is widely regarded as an early sign of pathological cognitive decline. We tested the hypothesis that older adults with SCD already exhibit aberrant neurocognitive processing underlying working memory.
Research Design and Methods
Electroencephalography (EEG) was recorded during a delayed match-to-sample (DMS) task and an eyes-closed resting condition in cognitively healthy community-dwelling older adults who were assigned to the SCD or Control group.
Results
The SCD and Control groups showed comparable performance on the neuropsychological tests and DMS task. The SCD group showed an enhanced right frontal target-related P300 effect during working memory retrieval and higher frontal theta power during rest. Higher theta power was associated with worse working memory performance and greater left frontal nontarget-related positivity across all older adults.
Discussion and Implications
Our findings suggest that older people with SCD have subtle pathophysiological changes in working memory–related potentials and intrinsic theta power, which has important implications for predicting risks and early interventions in older adults in the preclinical stage of ADRD.
Keywords: Delayed match-to-sample, Event-related potentials, Resting-state EEG
Translational Significance: Older adults with subjective cognitive decline (SCD) are at an elevated risk of future pathological cognitive decline, including dementia, relative to healthy aging controls. As objective cognitive measures cannot differentiate between older adults with and without SCD, it is necessary to use more sensitive measures to explore early brain changes in older adults with SCD. The present study indicated subtle pathophysiological deficits in working memory (a core cognitive function) at the earliest preclinical stage of dementia, which is critical for the implementation of early-stage interventions to prevent the progression of the disease.
Background and Objectives
Subjective cognitive decline (SCD) is characterized by a self-experienced decline in cognitive function in the absence of objective cognitive deficits (Jessen et al., 2014). SCD is regarded as the first clinical manifestation of Alzheimer’s disease (AD) and related dementia (ADRD) (Jessen et al., 2020; Rabin et al., 2017), and older adults with SCD have an increased risk of developing mild cognitive impairment (MCI) and dementia compared with those without SCD (Mitchell et al., 2014; Slot et al., 2019). Accordingly, it is imperative to investigate subtle alterations in the brains of older adults with SCD to better understand the earliest pathological mechanisms of AD, which are critical for the timely implementation of interventions to slow the progression of abnormal aging.
Given that most standardized and objective neuropsychological or computerized tests cannot effectively differentiate between older adults with and without SCD, it would be preferable to use potentially more sensitive measures to identify subtle changes in brain function in older adults with SCD. Electroencephalography (EEG) might be a particularly suitable technique for detecting the early brain changes underlying pathological cognitive aging owing to its accessibility and affordability with high temporal resolution (Jackson & Snyder, 2008). For example, event-related potentials (ERP) measuring synchronized postsynaptic neural activity evoked by cognitive events have shown altered neurocognitive processing (e.g., reduced ERP amplitudes) during higher order cognitive tasks (e.g., memory or language) in patients with MCI and AD compared to healthy older adults (Paitel et al., 2021). Unfortunately, studies examining the early neurocognitive changes associated with cognitive tasks in older adults with SCD remain scarce.
Here, we emphasize working memory, one of the most common early symptoms of dementia, which is highly vulnerable to normal and pathological aging (Kirova et al., 2015). Working memory refers to a limited capacity system that involves intentionally holding task-relevant information in the mind in the service of complex cognitive tasks (Baddeley, 1986). Using a delayed match-to-sample (DMS) task, we previously examined ERP responses during working memory retrieval in cognitively normal controls and patients with MCI and AD (Li et al., 2017). Participants were required to hold a sample object in their minds and then indicate whether each of the following test objects was a matching target or nonmatching distractor (i.e., nontarget). We reported a similar right frontal P300 match versus nonmatch ERP effect during working memory retrieval in all three groups (Li et al., 2017). P300 refers to a positive deflection related to the allocation of attentional resources during stimulus evaluation and categorization (Morrison et al., 2019; Polich, 2007), with a more frontal scalp distribution in older adults (Saliasi et al., 2013). Accordingly, the right frontal match-related positivity reflects that all three groups allocated more attentional resources to the target stimuli during working memory retrieval. Interestingly, there was also an enhanced left frontal P300 nonmatch versus match ERP effect in patients with MCI and AD compared to normal controls, suggesting that MCI and AD recruited more neural resources to reject the nontarget stimuli from working memory because of their impaired distractor inhibition (Li et al., 2017). These findings indicate that the working memory retrieval-related frontal P300 effect is a promising EEG indicator of altered neurocognitive processing during pathological cognitive decline. If SCD truly represents the preclinical stage of the AD continuum and is more likely to progress to MCI and AD, individuals with SCD may already have aberrant patterns of neural activity during working memory retrieval (i.e., the frontal P300 effect), even if they behaviorally perform at equivalent levels to healthy controls. As a result, the first purpose of this study was to investigate whether older adults with SCD have altered electrophysiological signals regarding working memory retrieval compared with older adults without SCD.
In addition, intrinsic EEG oscillations during the eyes-closed resting state have been shown to act as a sensitive tool for evaluating the neural dynamics of the AD brain (Babiloni et al., 2021). Extended evidence has demonstrated the slowing of oscillatory brain activity in patients with MCI or AD, reflected by increased spectral power in low-frequency oscillations (e.g., delta band: 1–4 Hz, theta band: 4–8 Hz) and decreased power density in high-frequency oscillations (e.g., alpha band: 8–13 Hz, beta band: 13–28 Hz; Babiloni et al., 2016; Babiloni, Blinowska, et al., 2020; van Straaten et al., 2014). Concerning SCD in the preclinical stage of AD, cross-sectional studies have reported mixed EEG results, probably because of the differences in the research criteria for SCD or participant characteristics (e.g., Alexander et al., 2006; Babiloni, Lizio, et al., 2016; Gaubert et al., 2019). Fortunately, two longitudinal studies provided converging evidence that higher theta power during baseline in SCD was more likely related to subsequent clinical progression to MCI over time, particularly at the frontal sites, suggesting that the slowing of resting cortical EEG theta rhythms in SCD can predict future cognitive decline (Gouw et al., 2017; Prichep et al., 2006). Synchronous oscillations during the resting state in different frequency bands may reflect various aspects of cognitive functioning. Specifically, theta band oscillations in the lateral prefrontal regions have been proposed to be responsible for integrating task-relevant and suppressing task-irrelevant information in working memory (Sauseng et al., 2010; de Vries et al., 2020). Considering the close relationship between resting EEG theta rhythms and working memory, the second purpose of this study was to compare and analyze the power spectral differences in the theta band between older adults with SCD and normal controls. Moreover, we investigated the associations between the EEG measures and working memory performance, and the possible associations between the EEG and ERP measures, to indicate the pathophysiological dysfunctions underlying the EEG rhythm changes in older adults with SCD.
In summary, evidence regarding neurocognitive processing underlying working memory in older adults with SCD is still limited, especially when both working memory–related ERP and resting EEG are considered. To this end, we tested the hypothesis that cognitively intact older adults with SCD will show a subtle decline in working memory–related potentials and intrinsic oscillations in the current study. Specifically, working memory was investigated using a DMS task in a sample of cognitively healthy community-dwelling older adults divided into SCD and Control groups. EEG was recorded during the test phase of the DMS task and an eyes-closed resting condition.
First, working memory retrieval-related frontal P300 effects were examined by comparing correctly classified matched and nonmatched objects. If older adults with SCD had an increased risk of developing MCI and dementia, they would probably share a similar pattern of brain alterations with patients with MCI and AD (Li et al., 2017; Paitel et al., 2021) by displaying a similar or even reduced right frontal match-related P300 effect during working memory retrieval compared to the Control group. However, there may be another possibility. A previous functional magnetic resonance imaging (fMRI) study has demonstrated that individuals with SCD are associated with greater brain activity in the right dorsolateral prefrontal cortex (dlPFC) during memory retrieval, reflecting successful neural compensation for their behavioral performance (Erk et al., 2011). Therefore, in the present study, the SCD group was also likely to show an enhanced right frontal match-related P300 effect, due to neural compensation in the brain, to maintain their working memory performance at a level similar to that of the Control group. We tend to prefer the second one, given that this hypothesis is based on neuroimaging evidence directly derived from SCD. In addition, the SCD group was likely to show an enhanced left frontal nonmatch-related P300 effect as indicated in MCI (Li et al., 2017), as it is difficult for them to reject the nonmatching distractors from working memory due to their impaired cognitive control (Cespón et al., 2018; Smart et al., 2014; Susana et al., 2021).
Second, given that higher theta power at the prefrontal sites during baseline in SCD can predict future cognitive decline (Gouw et al., 2017; Prichep et al., 2006) and that patients with MCI or AD show EEG shifts to slow waves (Babiloni et al., 2016, 2020; van Straaten et al., 2014), we expected that the SCD group would show higher frontal theta power than the controls. Moreover, we expected a significant association between theta band power and working memory performance, and between theta band power and working memory–related ERP effects.
Finally, EEG signals were recorded using a portable EEG headset with more electrode sites placed in the bilateral frontal regions in the present study. As noted, the working memory–related ERP effects in the DMS task were mainly distributed in the bilateral prefrontal regions (Li et al., 2017), and SCD showed different patterns of functional activity in the dlPFC (Erk et al., 2011). Moreover, bilateral prefrontal EEG theta oscillations play an important role in working memory maintenance and retrieval (Helfrich & Knight, 2016; Sauseng et al., 2010). As a result, we believed that the present EEG equipment could effectively capture the ERP or EEG patterns during the task and rest in older adults with SCD.
Research Design and Methods
Participants
Older adults were recruited from communities near the Institute of Psychology in the Chaoyang District, Beijing, China. The inclusion criteria were as follows: (a) age ≥60 years; (b) education ≥6 years; (c) a score >21 on the Montreal Cognitive Assessment Beijing Version (MoCA-BJ) to rule out the older adults with cognitive impairment (Yu et al., 2012); (c) a score <8 on the 15-item Geriatric Depression Scale to exclude the effects of depression on SCD (Yesavage & Sheikh, 1986); (e) Functional Activity Questionnaire score <9 to ensure normal functional activities of daily living (Pfeffer et al., 1982); (f) free of neurological deficits or traumatic brain injury; and (g) free of visual and hearing difficulties.
Of the initial sample of 67 participants, 14 did not meet the inclusion criteria, resulting in a sample of 53 participants who were eligible to enroll in the present study (25 females, age range: 60–75 years). A battery of neuropsychological tests was administered to ensure that they were cognitively healthy. The battery comprised the Digit Span Forward (DSF) and Digit Span Backward (DSB) tests (Gong, 1992), Trail Making Tests A and B (TMT; Reitan, 1958), Category Fluency Test (CFT; Strauss et al., 2006), WHO/UCLA Auditory Verbal Learning Test (AVLT; Maj et al., 1993), and Paired-Association Learning Test (PALT) from the Clinical Memory Scale (Xu & Wu, 1986). These tests were used to assess short-term memory (DSF), working memory (DSB), executive function (TMT and CFT), and episodic memory (AVLT and PALT). All participants showed normal performance on each test, adjusted for age, sex, and education, according to our Chinese normative database.
Participants were divided into two groups according to their scores on the Chinese adaptation (Hao et al., 2019) of the nine-item Subjective Cognitive Decline Questionnaire (SCD-Q9; Gifford et al., 2015). The SCD-Q9 is a brief standardized tool for the screening and quantification of SCD. The total score on this questionnaire ranges from 0 to 9, with higher scores representing worse subjective cognition. In the present study, the total SCD-Q9 score ranged from 0 to 7.5. According to the cutoff value recommended by Hao et al. (2022) and the median SCD score (3 points) in this study, participants who scored equal to or below 3 were assigned to the Control group (n = 28), whereas those who scored above 3 were assigned to the SCD group (n = 25).
To further ensure the validity of the group division, we calculated the proportion of participants who gave affirmative answers to two mutually confirmed questions—“Do you think you have problems with your memory?” and “Do you think that your memory is worse than 5 years ago?”—in the SCD-Q9 for each group. These two questions are used to examine whether older adults have a self-experienced global memory decline compared with a previous status, which is thought to be more predictive of pathological cognitive decline (Jessen et al., 2020). Twenty-four out of 25 older adults in the SCD group versus 1 out of 28 older adults in the Control group gave affirmative answers to both questions, suggesting that there were qualitative differences between the SCD and Control groups.
The demographic characteristics and neuropsychological performance of the SCD and Control groups are presented in Table 1. As expected, the SCD group had a higher SCD-Q9 score than the Control group (p < .001). No other significant differences were observed between the SCD and Control groups. Each participant provided informed consent and was paid for their participation. This study was approved by the Ethics Committee of the Institute of Psychology, Chinese Academy of Sciences.
Table 1.
Demographic Characteristics and Neuropsychological Performance of the Control and SCD Groups
| Variable | Alla participants (n = 53) | Control (n = 28) a | SCD (n = 25) a | p Value b |
|---|---|---|---|---|
| Age (y) | 66.87 ± 4.07 | 66.29 ± 3.73 | 67.52 ± 4.40 | .274 |
| Sex (male/female) | 28/25 | 18/10 | 10/15 | .077 |
| Education (y) | 11.45 ± 2.35 | 11.93 ± 2.48 | 10.92 ± 2.12 | .120 |
| SCD-Q9 | 3.33 ± 2.42 | 1.36 ± 1.17 | 5.54 ± 1.24 | <.001 |
| GDS-15 | 1.96 ± 1.81 | 1.64 ± 1.75 | 2.32 ± 1.84 | .176 |
| Functional Activity Questionnaire | 0.3 ± 0.82 | 0.14 ± 0.45 | 0.48 ± 1.09 | .158 |
| MoCA-BJ | 24.96 ± 2.16 | 25.32 ± 2.29 | 24.56 ± 1.96 | .202 |
| Digit Span Forward | 7.43 ± 1.26 | 7.57 ± 1.32 | 7.28 ± 1.21 | .407 |
| Digit Span Backward | 4.64 ± 1.21 | 4.71 ± 1.36 | 4.56 ± 1.04 | .648 |
| Trail Making Test (s)c | 43.93 ± 27.54 | 43.61 ± 29.57 | 44.28 ± 25.67 | .930 |
| Verbal Fluency Test | 18.38 ± 4.03 | 18.57 ± 3.63 | 18.16 ± 4.50 | .714 |
| AVLT (total learning)d | 50.08 ± 7.12 | 51.04 ± 6.88 | 49.00 ± 7.37 | .303 |
| AVLT (delayed recall) | 10.85 ± 1.95 | 11.18 ± 1.85 | 10.48 ± 2.02 | .195 |
| Paired Associative Learning Test | 8.77 ± 3.23 | 8.61 ± 3.20 | 8.96 ± 3.31 | .695 |
Notes: AVLT = Auditory Verbal Learning Test; GDS = 15-item Geriatric Depression Scale; MoCA-BJ = Montreal Cognitive Assessment Beijing Version; SCD = Subjective Cognitive Decline; SCD-Q9 = 9-item Subjective Cognitive Decline Questionnaire.
aData are shown as mean and standard deviation except for Sex (male/female), which is number of participants.
bIndependent samples t test or Pearson chi-square test.
cTrail-making scores were obtained using Trail Making B minus Trail Making A.
dAVLT total learning refers to the total number of words immediately recalled during the five learning trials.
Materials
The stimuli consisted of 64 two-dimensional black and white pictures of common objects taken from Snodgrass and Vanderwart (1980). The DMS task consisted of 16 trials, separated into two blocks of 8 trials. Each trial began with two sample objects, followed by a test phase with repeated memory targets and distractors (see Figure 1). Each test item was one of the two target objects (i.e., match to either of the sample objects) or one of the two distractor objects (i.e., nonmatch to either of the sample objects). The target objects were presented two, three, or four times, and the distractor objects were presented two, three, or four times, for a total of 12 test pictures per trial. A total of 192 test pictures were used, with 96 serving as matching targets and 96 serving as nonmatching distractors.
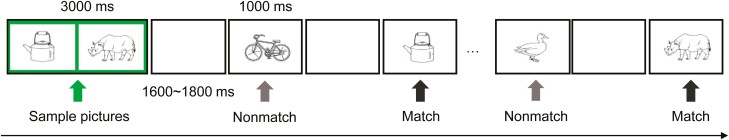
Figure 1.
Schematic illustration of the delayed match-to-sample task. Notes: For each memory trial in this task, two sample pictures were initially presented, followed by 12 successive test pictures (target or distractor objects).
Each test picture was presented with a black background in a rectangular area of 8.3 × 5.8 cm2. The sample pictures were also presented with a 6.5 mm green border. Test pictures were normalized across retrieval conditions (i.e., match or nonmatch) for image familiarity and complexity (Snodgrass & Vanderwart, 1980).
Procedure
Each participant first completed a 90-second resting session with eyes open, followed by a 90-second resting session with eyes closed, during which they were requested to stay relaxed and avoid falling asleep. Next, the participants were instructed to prepare for a working memory DMS task. The resting EEG and task procedures were designed using PsychoPy (Borhani et al., 2021; Peirce et al., 2019).
Figure 1 illustrates the procedures of the DMS task. Each trial began with two sample objects that were distinguished by a green border presented side by side for 3,000 milliseconds. The sample pictures were followed by 12 successive test pictures with a stimulus duration of 1,000 milliseconds each in a pseudo-randomized order. All test pictures were divided by an inter-stimulus interval of 1,600–1,800 milliseconds. Each trial lasted approximately 40 seconds.
Participants were told to hold the sample objects in their minds and indicate whether each of the following 12 test objects matched one of the sample objects by pressing either the “A” or “L” key. During the first block, participants made their responses of “match” by pressing the key “A” with their left index finger, and of “nonmatch” by pressing the key “L” with their right index finger. The assignment of hands was reversed during the second block. All participants performed two practice trials before data collection, and all the earlier tasks lasted approximately 15 minutes.
EEG Recording and Preprocessing
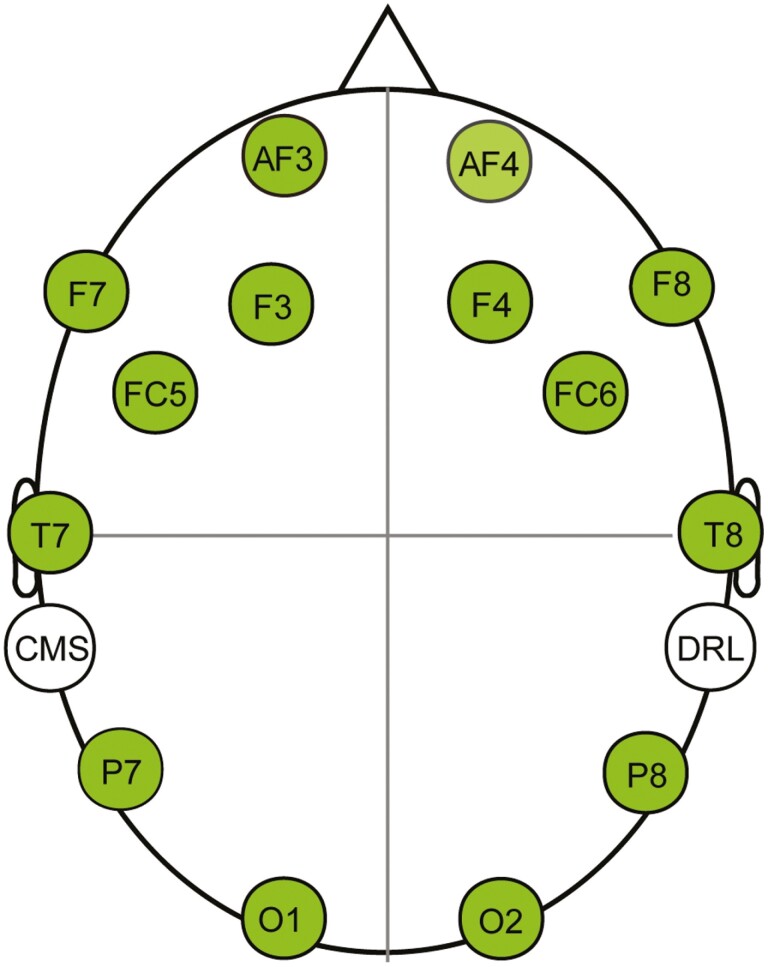
A portable and wireless EEG headset, Emotiv EPOC X (Emotiv Systems, Inc., San Francisco, CA), was used to record all EEG signals (https://www.emotiv.com/epoc-x/). The headset has 14 electrodes, aligned with the international 10–20 system: AF3/AF4, F7/F8, F3/F4, FC5/FC6, T7/T8, P7/P8, and O1/O2. In addition, there is a common mode sense and a driven right leg electrode at the left and right mastoids, respectively, serving as online reference sites (see Figure 2). These electrodes were connected to the participant’s scalp using saline-soaked felt pads. Data were digitally sampled at a rate of 128 Hz with a resolution of 14 bits. All electrode impedances were maintained below 10 kΩ during the experiments.
Figure 2.
Schematic maps of 14 electrode sites. Notes: CMS and DRL are online reference sites. CMS = common mode sense; DRL = driven right leg.
Offline EEG data were preprocessed in MATLAB (MathWorks, Inc., Natick, MA, USA) using the open-source toolbox of EEGLAB (Delorme & Makeig, 2004) and ERPLAB (Lopez-Calderon & Luck, 2014). The preprocessing procedure for the ERP data is presented in the Supplementary Material. Epochs with correct responses for the match and nonmatch conditions were used to average ERPs. A minimum of 15 artifact-free epochs were required for each participant to ensure an acceptable signal-to-noise ratio. One participant in the SCD group was excluded because of the limited number of valid epochs. Thus, 52 participants remained in the data set for the subsequent statistical analyses of the ERP data of the DMS task.
The preprocessing procedure for the resting EEG data is presented in the Supplementary Material. We conducted a spectral analysis of eyes-closed resting EEG data, which are widely used to measure the pathophysiological processes of disease progression (Babiloni et al., 2021). All 53 participants were included in the data set for subsequent statistical analyses of the resting EEG data.
Statistical Analyses
Behavioral and ERP data of the DMS task
The mean accuracy and response times (RT) data of correct responses in the DMS task were subjected to repeated-measures analysis of covariance (ANCOVA) with the between-subjects factor of Group (Control, SCD) and the within-subjects factor of Condition (match, nonmatch), and age, sex, and education as covariates. Furthermore, we reported the discrimination accuracy (Pr), which was computed by subtracting false alarm rates to distractor objects (i.e., “match” to a distractor object) from hit rates to target objects (i.e., “match” to a target object). Pr was analyzed using a one-way ANCOVA with the between-subjects factor of Group (Control, SCD) and age, sex, and education as covariates.
Based on visual inspection of the grand average waveforms and our previous study (Li et al., 2017), the P300 match/nonmatch ERP effect was quantified by calculating the mean amplitudes over the time window of 500–800 milliseconds. We calculated the mean amplitudes from two frontal scalp regions, each composed of an average of data from three electrode sites: the left frontal region (F3, F7, and FC5) and the right frontal region (F4, F8, and FC6), to obtain reliable frontal ERP effects related to working memory retrieval.
Initial repeated-measures ANCOVA with the within-subjects factors of Condition (match, nonmatch) and Region (left frontal, right frontal) and the between-subjects factor of Group (Control, SCD) were performed on the average amplitudes while controlling for age, sex, and education. Follow-up analyses were conducted separately for the Control and SCD groups when there were significant interactions involving group factors. Then, for the significant Condition × Region interaction, pairwise comparisons were used to quantify the P300 match/nonmatch effects in the left and right frontal regions. Topographic maps depicting the match/nonmatch effects were formed for each group. For the statistically significant match/nonmatch effects, between-group comparisons were directly conducted on the match/nonmatch difference waveforms, as necessary. The mean number of trials contributing to the grand average ERPs consisted of the Control group—match (71) and nonmatch (69)—and the SCD group—match (70) and nonmatch (69).
Eyes-closed resting EEG data
We primarily focused on the group differences in power spectra of the theta band in the frontal region. The absolute and relative EEG spectral powers of theta band data from six frontal sites (i.e., F3, F7, FC5, F4, F8, and FC6) were subjected to one-way ANCOVAs with the between-subjects factor of Group (Control, SCD) while controlling for age, sex, and education to examine the altered EEG characteristics in older adults with SCD. One-tailed tests of significance were employed because we expected the SCD group to show higher frontal theta power than the Control group. The spectral power of other frequency bands (i.e., delta: 1–4 Hz, alpha: 8–13 Hz, beta: 13–28 Hz, and gamma: 28–46 Hz) was analyzed in the same way to provide additional evidence for the early alterations in brain oscillatory activity in SCD.
Correlation analyses
Pearson correlation analyses were performed between the ERP/EEG measures and working memory performance (i.e., Pr, RT of the match and nonmatch conditions), and between EEG spectral power and working memory–related ERP effects. The working memory–related ERP effects were qualified by the mean amplitudes of the difference waveforms between the match and nonmatch conditions in the left or right frontal region. All correlation analyses were conducted using age, sex, and education as covariates. P-values were corrected for multiple testing with the Benjamini-Hochberg false discovery rate (BH-FDR) procedure.
The Greenhouse–Geisser correction for the nonsphericity of the data was applied as necessary. The uncorrected degrees of freedom, corrected p values, and effect size (partial η2) were reported for the repeated-measures ANOVAs. For all analyses, the significance level was set at .05.
Results
Behavioral Performance
The mean accuracy, RT, and Pr for the DMS task as a function of Group and/or Condition are presented in Table 2. For both accuracy and RT, ANCOVAs revealed no significant main effects or interactions. The Pr also revealed no significant group differences. In summary, the results showed that older adults with SCD were able to maintain a level of behavioral performance comparable to that of the controls.
Table 2.
Mean Accuracy and Response Times For Each Response Category, and Pr in the Control and SCD Groups
| Group | Accuracy | Response times (ms) | Pr | ||
|---|---|---|---|---|---|
| Match | Nonmatch | Match | Nonmatch | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Control | 0.85 (0.17) | 0.84 (0.16) | 711 (79) | 708 (79) | 0.82 (0.17) |
| SCD | 0.89 (0.08) | 0.86 (0.12) | 724 (50) | 731 (51) | 0.87 (0.09) |
Notes: SCD = subjective cognitive decline; SD = standard deviation.
ERP Results
The grand average ERPs evoked by correct responses to the match and nonmatch conditions are shown for the Control group in Supplementary Figure 1 and the SCD group in Supplementary Figure 2. Group comparisons of grand average waveforms and mean amplitudes of match/nonmatch effects in the frontal region are shown in Figure 3. The ERP data for the time window of 500–800 milliseconds were used to quantify the match/nonmatch effects during the working memory retrieval.
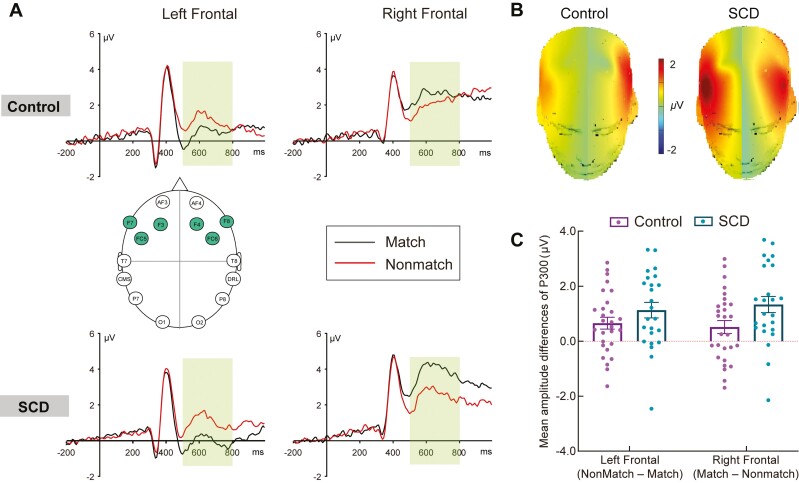
Figure 3.
Working memory–related potentials in the Control and SCD groups. Notes: Panel A shows the grand average waveforms elicited by correctly classified match and nonmatch objects in the left and right frontal regions in the Control and SCD groups, showing from −200 to 1,000 milliseconds. The left frontal region represents collapsed activity across F3, F7, and FC5, and the right frontal region represents collapsed activity across F4, F8, and FC6. The selected electrode sites are indicated by the inserted montage. The scale bars indicate the time windows used for the statistical analyses (500–800 milliseconds). The positive voltages are plotted upwards. Panel B shows the topographic maps of P300 match/nonmatch ERP effects (left: nonmatch minus match; right: match minus nonmatch) for the Control and SCD groups. The scale bar shows the amplitude range. Panel C shows the mean amplitudes of P300 match/nonmatch ERP effects (left: nonmatch minus match; right: match minus nonmatch) in the left and right frontal regions (500–800 milliseconds), which represent collapsed activity over three frontal electrodes separately for the Control and SCD groups. The error bars represent the standard error of the mean. ERP = event-related potentials; SCD = subjective cognitive decline.
The initial ANCOVA revealed a significant Condition × Region × Group interaction (F(1,47) = 7.75, p = .008, partial η2 = 0.14). Follow-up analyses of the Control group revealed a significant Condition × Region interaction (F(1,27) = 10.06, p = .004, partial η2 = 0.27). Pairwise comparisons revealed a more positive P300 for the match condition than for the nonmatch condition in the right frontal region (p = .038), whereas the nonmatch condition was more positive than the match condition in the left frontal region (p = .005; see Figure 3A). In addition, the amplitudes of the right frontal region were greater than those of the left frontal region for both the match (p < .001) and nonmatch (p = .035) conditions. Follow-up analyses of the SCD group also revealed a significant Condition × Region interaction (F(1,23) = 27.98, p < .001, partial η2 = .55). Pairwise comparisons again revealed that the match condition evoked a more positive P300 than the nonmatch condition in the right frontal region (p < .001), whereas the nonmatch condition evoked a more positive P300 than the match condition in the left frontal region (p = .001; see Figure 3A). In addition, the amplitudes of the right frontal region were greater than those of the left frontal region for both the match (p < .001) and nonmatch (p = .038) conditions. Topographic maps of the P300 effects for both groups are shown in Figure 3B. To capture the match or nonmatch-related P300 effect, the difference waveforms in the left frontal region were formed by subtracting the ERP of match objects from the ERP of nonmatch objects, whereas the difference waveforms in the right frontal region were formed by subtracting the ERP of nonmatch objects from the ERP of match objects.
Planned between-group comparisons were conducted on the frontal P300 match/nonmatch difference waveforms to directly explore group differences in the size of the working memory retrieval-related ERP effects. On the left side (collapsed over F3, F7, and FC5), the results revealed a similar magnitude of nonmatch-related positivity for both groups after controlling for age, sex, and education (F(1,47) = 1.97, p = .167, partial η2 = 0.04). On the right side (collapsed over F4, F8, and FC6), the results revealed a greater magnitude of match-related positivity in the SCD group than in the Control group after controlling for age, sex, and education (F(1,47) = 8.41, p = .006, partial η2 = .15, see Figure 3C).
In summary, both groups exhibited a match-related P300 effect in the right frontal region and a nonmatch-related P300 effect in the left frontal region during working memory retrieval. Moreover, a greater right frontal match-related P300 effect was found in the SCD group than in the Control group, whereas a similar magnitude of left frontal nonmatch-related P300 effect was observed in both the SCD and Control groups.
EEG Results
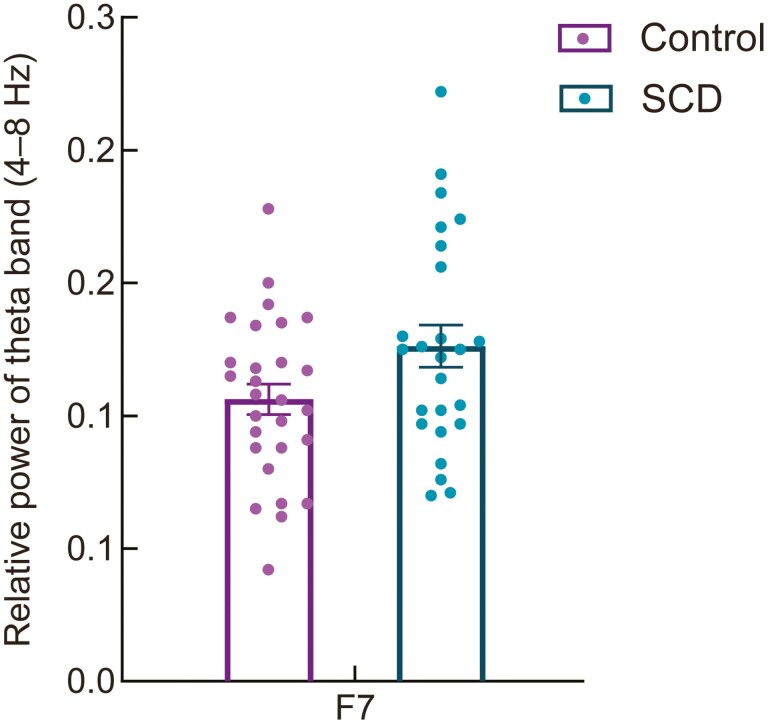
Resting-state eyes-closed EEG data were used to examine early alterations in oscillatory brain activity in older adults with SCD. Absolute theta power revealed no significant differences between the SCD and Control groups at any frontal site. Relative theta power was significantly higher in the SCD group than in the Control group at the left frontal site (F7, F(1,48) = 3.86, p = .028, partial η2 = 0.07) after controlling for age, sex, and education (see Figure 4). No other significant results were revealed for the remaining frequency bands for either the absolute or relative spectral power.
Figure 4.
The relative power of the theta band at the left frontal site (F7) was significantly higher in the SCD group compared to that in the Control group during the eyes-closed condition. Notes: The error bars represent the standard error of the mean. SCD = subjective cognitive decline.
Correlation Analyses
Correlation analyses between working memory–related ERP effects in the left or right frontal region and working memory performance (Pr, RT of the match and nonmatch conditions) revealed no significant results.
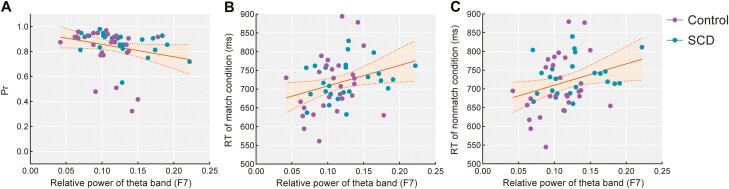
The electrode site showing significant between-group differences in theta power (i.e., F7) was used to calculate the correlations between eyes-closed EEG spectral power and working memory performance. For both older adults with and without SCD separately, no correlations reached statistical significance, but relative theta power was generally negatively correlated with Pr and positively correlated with the RT of the match and nonmatch conditions for both groups. Across the entire sample, as shown in Figure 5, relative theta power was significantly negatively correlated with Pr (r = −0.331, p = .028) and positively correlated with the RT of the match (r = 0.339, p = .048) and nonmatch (r = 0.323, p = .022) conditions.
Figure 5.
The relative power of the theta band at the left frontal site (F7) during the eyes-closed condition was significantly correlated with the accuracy index Pr (A), mean response times of correctly identified match (B), and nonmatch objects (C) across all older adults with age, sex, and education as covariates. Notes: Data of the Control group are colored in purple and those of the SCD group are colored in blue. The yellow fit line displays the relationship between the theta power and behavioral performance for all older adults. The shaded area represents a 95% confidence interval. RT = response times; SCD = subjective cognitive decline.
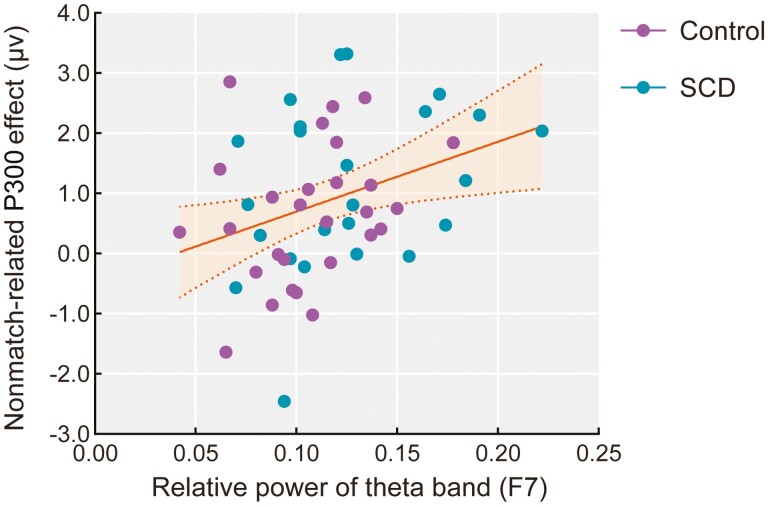
Correlation analyses were also conducted between the EEG spectral power and working memory–related ERP effects (left: nonmatch minus match; right: match minus nonmatch). For both older adults with and without SCD separately, no correlations reached statistical significance, but relative theta power was generally positively correlated with left frontal nonmatch-related positivity for both groups. Across the entire sample, the results revealed that relative theta power at F7 was significantly positively correlated with nonmatch-related positivity in the left frontal region during working memory retrieval (r = 0.326, p = .022, see Figure 6).
Figure 6.
The relative power of the theta band at the left frontal site (F7) during the eyes-closed resting state was positively correlated with the mean amplitudes of the nonmatch-related P300 effect (nonmatch minus match) in the left frontal region (500–800 milliseconds) during working memory retrieval across all older adults with age, sex, and education as covariates. Notes: Data of the Control group are colored in purple and those of the SCD group are colored in blue. The yellow fit line displays the relationship between the theta power and nonmatch-related P300 effect for all older adults. The shaded area represents a 95% confidence interval. SCD = subjective cognitive decline.
Taken together, correlation analyses revealed that higher eyes-closed theta power at the left frontal site was associated with worse working memory performance and greater nonmatch-related positivity on the left side across all older adults.
Discussion and Implications
In the present study, we investigated whether altered neurocognitive processing underlying working memory retrieval can be detected as early as the SCD stage by measuring working memory–related ERP and eyes-closed resting EEG rhythms, despite there being no evidence of objective cognitive decline in the SCD group. To the best of our knowledge, this is the first study to investigate the electrophysiological correlates of working memory retrieval in community-dwelling older adults with SCD.
Intentionally holding task-relevant information in mind for current use is an important function of working memory in daily life. In the present study, in the right scalp region, we found more positive-going waveforms for matching targets than for nonmatching distractors during the 500–800 milliseconds time window in both the SCD and Control groups. Consistent with the typical P300 component evoked in memory and attention tasks (Morrison et al., 2019; Polich, 2007), this match-related positivity could be due to the target stimuli being task relevant and consequently requiring more attentional resources than nontarget stimuli during working memory retrieval. This frontally distributed effect was consistent with our previous fMRI study using a similar DMS paradigm, which revealed that match-related responses predominantly engaged the prefrontal cortex (Jiang et al., 2000), and the ERP study showing that older adults were more likely to display frontally distributed P300 relative to young adults in working memory tasks (Saliasi et al., 2013).
Importantly, compared with the Control group, the SCD group was associated with an enhanced P300 match versus nonmatch effect at the right frontal sites during the working memory DMS task. These findings support the notion that older adults with SCD allocate more cognitive processing resources for stimulus evaluation and categorization of target objects to maintain their working memory performance at a level similar to that of controls. There are two possible explanations for the enhanced P300 effect observed in the SCD group. First, older adults with SCD may be associated with successful compensation to yield working memory performance comparable to that of older adults without SCD. According to the scaffolding theory of aging and cognition (STAC; Reuter-Lorenz & Park, 2014), older adults may adaptively show increased frontal activation as compensatory scaffolding in response to their neural degradation. Several neuroimaging studies have consistently indicated that individuals with SCD share a similar pattern of brain alterations with patients with MCI and AD by displaying brain atrophy or volume loss in the temporal and frontal lobes (Parker et al., 2022; Wang et al., 2020). In addition, fMRI studies have demonstrated increased brain activation in the dlPFC during episodic memory encoding or retrieval (Erk et al., 2011; Rodda et al., 2009) in individuals with SCD compared to controls. Based on these studies, the present finding of an enhanced right frontal P300 effect in the SCD group may reflect the existence of compensatory mechanisms during working memory retrieval in older adults with SCD.
An alternative explanation is that the greater P300 effect in older adults with SCD may reflect increased processing demands for maintaining and retrieving target objects in working memory owing to less efficient neural processing in the brain (see also Viviano & Damoiseaux, 2020). Shu et al. (2018) partially supported this possibility, as they revealed disrupted topologic efficiency of white matter structural connectome mainly in the bilateral prefrontal regions in older adults with SCD relative to controls, which may lead to impaired capacity of information transfer in the SCD group. In addition, using the exact memory paradigm of the current study, Jiang et al. (2016) reported that cognitively normal older adults with increased functional connectivity in the temporal, parietal, and frontal cortices were associated with higher levels of cerebrospinal fluid biomarkers for AD (i.e., pTau181). The exact interpretation of the enhanced right frontal P300 effect during working memory retrieval in the SCD group remains unclear; however, the current evidence indicates that the neurocognitive processes underlying working memory retrieval are affected in older people with SCD. In addition, considering that accumulating evidence has generally revealed reduced cognitive ERPs effects during higher order cognitive tasks in patients with MCI and AD relative to healthy older adult controls (Paitel et al., 2021), the present findings may reflect an early aberrant and differentiated pattern that precedes a pathological decline in behavioral performance in older adults with SCD.
For the ERP results, in addition to the right frontal match-related positivity, participants also exhibited a left frontal nonmatch-related P300 effect characterized by a more positive-going waveform for the nonmatch condition than for the match condition in the DMS task. In a previous study of the Kentucky cohort (Li et al., 2017), we used a similar DMS paradigm applied to normal older controls, MCI, and AD. A P300 component linked to the nonmatch condition relative to the match condition at the left frontal sites was reported in patients with MCI and AD, but not in normal older controls. We explained this left frontal cognitive ERP effect as reflecting that patients with MCI and AD would recruit more neural resources to reject nonmatching distractors because of their impaired ability to inhibit distracting information. In the present study, both groups displayed an indistinguishable nonmatch-related P300 effect during working memory retrieval, suggesting that both older adults with and without SCD may have difficulty rejecting nonmatching distractors during working memory retrieval. Nevertheless, the eyes-closed intrinsic resting EEG rhythms were more sensitive to neural processing and behavioral performance related to the rejection of distractor objects from working memory in older adults with SCD.
Specifically, higher relative power of the theta band in the left frontal region during the resting state was found in older adults with SCD than in controls in the present study. Importantly, correlational analyses indicated that increased relative power in the theta band was associated with slowing responses to nonmatching distractors and higher nonmatch-related P300 effect during the DMS task. On the one hand, these findings suggest that older adults with SCD share a similar pattern of alterations in resting cortical EEG rhythms with MCI and AD, who also show a slowing of oscillatory brain activity reflected by increased spectral power of the theta band (Babiloni et al., 2016; Babiloni, Blinowska, et al., 2020; van Straaten et al., 2014). Together with previous longitudinal evidence that higher theta power during baseline in SCD is related to subsequent clinical progression to MCI over time (Gouw et al., 2017; Prichep et al., 2006), the present findings support the notion that resting EEG rhythm changes in the theta band may also be sensitive to subtle pathophysiological changes in brain activity in older adults with SCD. On the other hand, frontal theta rhythms have been postulated to play an important role in the efficient maintenance and retrieval of information in working memory by engaging a key mechanism for top-down executive control to prevent distraction during working memory tasks (Helfrich & Knight, 2016; Sauseng et al., 2010; de Vries et al., 2020). Consequently, the results of the correlation analyses in the present study probably reflect that older adults with SCD have impaired neural control mechanisms for effective working memory retrieval, such as rejecting nonmatching distractors from working memory. These findings are consistent with those of previous ERP studies that reported impaired central executive function (e.g., inhibitory control) in older adults with SCD (Cespón et al., 2018; Smart et al., 2014; Susana et al., 2021). Taken together, the present findings provide new insights into the impaired mechanisms suppressing nonmatching distractors during working memory retrieval in older adults with SCD.
This study has two limitations. First, the relatively small sample of older adults in each group might have compromised the statistical power of the present study. Future studies should use a larger sample size. Second, individuals with SCD in preclinical AD often have mild symptoms of anxiety other than depression (Jessen et al., 2014, 2020). It is necessary to assess anxiety symptoms in future studies to rule out the effects of anxiety on SCD.
Conclusion
In summary, we observed aberrant neurocognitive processing underlying target retrieval from working memory and an impaired neural control mechanism related to the rejection of nonmatching distractors from working memory in older adults with SCD. These findings provide important evidence for the early signs of subtle pathophysiological deficits in working memory at the earliest stage of AD and important implications for predicting risks and early interventions in older adults with SCD.
Supplementary Material
Acknowledgments
We would like to thank Professor X. Zhao’s laboratory at the University of Tennessee for technical support.
Contributor Information
Zhiwei Zheng, Center on Aging Psychology, CAS Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Department of Psychology, University of Chinese Academy of Sciences, Beijing, China.
Xiaofeng Zhao, Center on Aging Psychology, CAS Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Department of Psychology, University of Chinese Academy of Sciences, Beijing, China.
Xiaoyu Cui, Center on Aging Psychology, CAS Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Department of Psychology, University of Chinese Academy of Sciences, Beijing, China.
Xiaomei Liu, Center on Aging Psychology, CAS Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Department of Psychology, University of Chinese Academy of Sciences, Beijing, China.
Xinyi Zhu, Center on Aging Psychology, CAS Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Department of Psychology, University of Chinese Academy of Sciences, Beijing, China.
Yang Jiang, Department of Behavioral Science, University of Kentucky College of Medicine, Lexington, Kentucky, USA.
Juan Li, Center on Aging Psychology, CAS Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, China; Department of Psychology, University of Chinese Academy of Sciences, Beijing, China.
Funding
This work was supported by the National Key Research and Development Program of China (2020YFC2003000, 2018YFC2000300, 2018YFC2001701, 2016YFC1305900, 2017YFB1401203); the National Natural Science Foundation of China (32271121, 32071079, 31861133011); the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2020089); the Scientific Foundation of Institute of Psychology, Chinese Academy of Sciences (E2CX3715CX); CAS Engineering Laboratory for Psychological Service (KFJ-PTXM-29); and the United States National Institute of Health (R56AG060608-01 to Y. Jiang).
Conflict of Interest
None declared.
References
- Alexander, D. M., Arns, M. W., Paul, R. H., Rowe, D. L., Cooper, N., Esser, A. H., Fallahpour, K., Stephan, B. C., Heesen, E., Breteler, R., Williams, L. M., & Gordon, E. (2006). EEG markers for cognitive decline in elderly subjects with subjective memory complaints. Journal of Integrative Neuroscience, 52(1), 49–74. doi: 10.1142/s0219635206001021 [DOI] [PubMed] [Google Scholar]
- Babiloni, C., Arakaki, X., Azami, H., Bennys, K., Blinowska, K., Bonanni, L., Bujan, A., Carrillo, M. C., Cichocki, A., de Frutos-Lucas, J., Del Percio, C., Dubois, B., Edelmayer, R., Egan, G., Epelbaum, S., Escudero, J., Evans, A., Farina, F., Fargo, K., … Guntekin, B. (2021). Measures of resting state EEG rhythms for clinical trials in Alzheimer’s disease: Recommendations of an expert panel. Alzheimer’s & Dementia, 172(9), 1528–1553. doi: 10.1002/alz.12311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni, C., Blinowska, K., Bonanni, L., Cichocki, A., de Haan, W., Del Percio, C., Dubois, B., Escudero, J., Fernández, A., Frisoni, G., Guntekin, B., Hajos, M., Hampel, H., Ifeachor, E., Kilborn, K., Kumar, S., Johnsen, K., Johannsson, M., Jeong, J., … Randall, F. (2020). What electrophysiology tells us about Alzheimer’s disease: A window into the synchronization and connectivity of brain neurons. Neurobiology of Aging, 852, 58–73. doi: 10.1016/j.neurobiolaging.2019.09.008 [DOI] [PubMed] [Google Scholar]
- Babiloni, C., Lizio, R., Marzano, N., Capotosto, P., Soricelli, A., Triggiani, A. I., Cordone, S., Gesualdo, L., & Del Percio, C. (2016). Brain neural synchronization and functional coupling in Alzheimer’s disease as revealed by resting state EEG rhythms. International Journal of Psychophysiology, 1032, 88–102. doi: 10.1016/j.ijpsycho.2015.02.008 [DOI] [PubMed] [Google Scholar]
- Babiloni, C., Lopez, S., Del Percio, C., Noce, G., Pascarelli, M. T., Lizio, R., Teipel, S. J., González-Escamilla, G., Bakardjian, H., George, N., Cavedo, E., Lista, S., Chiesa, P. A., Vergallo, A., Lemercier, P., Spinelli, G., Grothe, M. J., Potier, M. C., Stocchi, F., … INSIGHT-preAD Study GroupINSIGHT-preAD Study Group. (2020). Resting-state posterior alpha rhythms are abnormal in subjective memory complaint seniors with preclinical Alzheimer’s neuropathology and high education level: The INSIGHT-preAD study. Neurobiology of Aging, 902, 43–59. doi: 10.1016/j.neurobiolaging.2020.01.012 [DOI] [PubMed] [Google Scholar]
- Baddeley, A. D. (1986). Working memory. Oxford University Press. [Google Scholar]
- Borhani, S., Zhao, X., Kelly, M. R., Gottschalk, K. E., Yuan, F., Jicha, G. A., & Jiang, Y. (2021). Gauging working memory capacity from differential resting brain oscillations in older individuals with a wearable device. Frontiers in Aging Neuroscience, 132, 625006. doi: 10.3389/fnagi.2021.625006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cespón, J., Galdo-Álvarez, S., & Díaz, F. (2018). Event-related potentials reveal altered executive control activity in healthy elderly with subjective memory complaints. Frontiers in Human Neuroscience, 122, 445. doi: 10.3389/fnhum.2018.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme, A., & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 1342, 9–21. doi: 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Erk, S., Spottke, A., Meisen, A., Wagner, M., Walter, H., & Jessen, F. (2011). Evidence of neuronal compensation during episodic memory in subjective memory impairment. Archives of General Psychiatry, 682(8), 845–852. doi: 10.1001/archgenpsychiatry.2011.80 [DOI] [PubMed] [Google Scholar]
- Gaubert, S., Raimondo, F., Houot, M., Corsi, M. C., Naccache, L., Diego Sitt, J., Hermann, B., Oudiette, D., Gagliardi, G., Habert, M. O., Dubois, B., De Vico Fallani, F., Bakardjian, H., Epelbaum, S., & Alzheimer’s Disease Neuroimaging InitiativeAlzheimer’s Disease Neuroimaging Initiative. (2019). EEG evidence of compensatory mechanisms in preclinical Alzheimer’s disease. Brain, 1422(7), 2096–2112. doi: 10.1093/brain/awz150 [DOI] [PubMed] [Google Scholar]
- Gifford, K. A., Liu, D., Romano, R., 3rd., Jones, R. N., & Jefferson, A. L. (2015). Development of a subjective cognitive decline questionnaire using item response theory: A pilot study. Alzheimer’s & Dementia, 12(4), 429–439. doi: 10.1016/j.dadm.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, Y. X. (1992). Manual of Wechsler Adult Intelligence Scale—Chinese version. Chinese Map Press. [Google Scholar]
- Gouw, A. A., Alsema, A. M., Tijms, B. M., Borta, A., Scheltens, P., Stam, C. J., & van der Flier, W. M. (2017). EEG spectral analysis as a putative early prognostic biomarker in nondemented, amyloid positive subjects. Neurobiology of Aging, 572, 133–142. doi: 10.1016/j.neurobiolaging.2017.05.017 [DOI] [PubMed] [Google Scholar]
- Hao, L., Hu, X., Han, Y., & Jia, J. (2019). Localization of English version of SCD-Q9 and reliability and validity test. Chinese Journal of General Practice, 222(26), 3238–3245. doi: 10.12114/j.issn.1007-9572.2019.00.045 [DOI] [Google Scholar]
- Hao, L., Jia, J., Xing, Y., & Han, Y. (2022). An application study-subjective cognitive decline Questionnaire 9 in detecting mild cognitive impairment (MCI). Aging & Mental Health, 26(10), 2014–2021. doi: 10.1080/13607863.2021.1980860 [DOI] [PubMed] [Google Scholar]
- Helfrich, R. F., & Knight, R. T. (2016). Oscillatory dynamics of prefrontal cognitive control. Trends in Cognitive Sciences, 202(12), 916–930. doi: 10.1016/j.tics.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, C. E., & Snyder, P. J. (2008). Electroencephalography and event-related potentials as biomarkers of mild cognitive impairment and mild Alzheimer’s disease. Alzheimer’s & Dementia, 42(1 Suppl 1), S137–S143. doi: 10.1016/j.jalz.2007.10.008 [DOI] [PubMed] [Google Scholar]
- Jessen, F., Amariglio, R. E., van Boxtel, M., Breteler, M., Ceccaldi, M., Chételat, G., Dubois, B., Dufouil, C., Ellis, K. A., van der Flier, W. M., Glodzik, L., van Harten, A. C., de Leon, M. J., McHugh, P., Mielke, M. M., Molinuevo, J. L., Mosconi, L., Osorio, R. S., Perrotin, A., Petersen, R. C., & Subjective Cognitive Decline Initiative (SCD-I) Working Group. (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia, 102(6), 844–852. doi: 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen, F., Amariglio, R. E., Buckley, R. F., van der Flier, W. M., Han, Y., Molinuevo, J. L., Rabin, L., Rentz, D. M., Rodriguez-Gomez, O., Saykin, A. J., Sikkes, S., Smart, C. M., Wolfsgruber, S., & Wagner, M. (2020). The characterisation of subjective cognitive decline. Lancet Neurology, 192(3), 271–278. doi: 10.1016/S1474-4422(19)30368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y., Haxby, J. V., Martin, A., Ungerleider, L. G., & Parasuraman, R. (2000). Complementary neural mechanisms for tracking items in human working memory. Science, 2872(5453), 643–6. doi: 10.1126/science.287.5453.643 [DOI] [PubMed] [Google Scholar]
- Jiang, Y., Huang, H., Abner, E., Broster, L. S., Jicha, G. A., Schmitt, F. A., Kryscio, R., Andersen, A., Powell, D., Van Eldik, L., Gold, B. T., Nelson, P. T., Smith, C., & Ding, M. (2016). Alzheimer’s biomarkers are correlated with brain connectivity in older adults differentially during resting and task states. Frontiers in Aging Neuroscience, 82, 15. doi: 10.3389/fnagi.2016.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirova, A. M., Bays, R. B., & Lagalwar, S. (2015). Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed Research International, 20152, 748212. doi: 10.1155/2015/748212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Broster, L. S., Jicha, G. A., Munro, N. B., Schmitt, F. A., Abner, E., Kryscio, R., Smith, C. D., & Jiang, Y. (2017). A cognitive electrophysiological signature differentiates amnestic mild cognitive impairment from normal aging. Alzheimer’s Research & Therapy, 92(1), 3. doi: 10.1186/s13195-016-0229-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon, J., & Luck, S. J. (2014). ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 82, 213. doi: 10.3389/fnhum.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj, M., D’Elia, L., Satz, P., Janssen, R., Zaudig, M., & Uchiyama, C., et al. (1993). Evaluation of two new neuropsychological tests designed to minimize cultural bias in the assessment of HIV-1 seropositive persons: A WHO study. Archives of Clinical Neuropsychology, 82, 123–135. doi: 10.1016/0887-6177(93)90030-5 [DOI] [PubMed] [Google Scholar]
- Mitchell, A. J., Beaumont, H., Ferguson, D., Yadegarfar, M., & Stubbs, B. (2014). Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatrica Scandinavica, 1302(6), 439–451. doi: 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- Morrison, C., Kamal, F., & Taler, V. (2019). The influence of working memory performance on event-related potentials in young and older adults. Cognitive Neuroscience, 102(3), 117–128. doi: 10.1080/17588928.2019.1570104 [DOI] [PubMed] [Google Scholar]
- Paitel, E. R., Samii, M. R., & Nielson, K. A. (2021). A systematic review of cognitive event-related potentials in mild cognitive impairment and Alzheimer’s disease. Behavioural Brain Research, 3962, 112904. doi: 10.1016/j.bbr.2020.112904 [DOI] [PubMed] [Google Scholar]
- Parker, A. F., Ohlhauser, L., Scarapicchia, V., Smart, C. M., Szoeke, C., & Gawryluk, J. R. (2022). A systematic review of neuroimaging studies comparing individuals with subjective cognitive decline to healthy controls. Journal of Alzheimer’s Disease, 862(4), 1545–1567. doi: 10.3233/jad-215249 [DOI] [PubMed] [Google Scholar]
- Peirce, J., Gray, J. R., Simpson, S., MacAskill, M., Höchenberger, R., Sogo, H., Kastman, E., & Lindeløv, J. K. (2019). PsychoPy2: Experiments in behavior made easy. Behavior Research Methods, 512(1), 195–203. doi: 10.3758/s13428-018-01193-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer, R. I., Kurosaki, T. T., Harrah, C. H.Jr., Chance, J. M., & Filos, S. (1982). Measurement of functional activities in older adults in the community. Journal of Gerontology, 372(3), 323–329. doi: 10.1093/geronj/37.3.323 [DOI] [PubMed] [Google Scholar]
- Polich, J. (2007). Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology, 1182(10), 2128–2148. doi: 10.1016/j.clinph.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichep, L. S., John, E. R., Ferris, S. H., Rausch, L., Fang, Z., Cancro, R., Torossian, C., & Reisberg, B. (2006). Prediction of longitudinal cognitive decline in normal elderly with subjective complaints using electrophysiological imaging. Neurobiology of Aging, 272(3), 471–481. doi: 10.1016/j.neurobiolaging.2005.07.021 [DOI] [PubMed] [Google Scholar]
- Rabin, L. A., Smart, C. M., & Amariglio, R. E. (2017). Subjective cognitive decline in preclinical Alzheimer’s disease. Annual Review of Clinical Psychology, 132, 369–396. doi: 10.1146/annurev-clinpsy-032816-045136 [DOI] [PubMed] [Google Scholar]
- Reitan, R. M. (1958). Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills, 82(3), 271–276. doi: 10.2466/pms.8.7.271-276 [DOI] [Google Scholar]
- Reuter-Lorenz, P. A., & Park, D. C. (2014). How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychology Review, 242(3), 355–370. doi: 10.1007/s11065-014-9270-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda, J. E., Dannhauser, T. M., Cutinha, D. J., Shergill, S. S., & Walker, Z. (2009). Subjective cognitive impairment: Increased prefrontal cortex activation compared to controls during an encoding task. International Journal of Geriatric Psychiatry, 242(8), 865–874. doi: 10.1002/gps.2207 [DOI] [PubMed] [Google Scholar]
- Saliasi, E., Geerligs, L., Lorist, M. M., & Maurits, N. M. (2013). The relationship between P3 amplitude and working memory performance differs in young and older adults. PLoS One, 82(5), e63701. doi: 10.1371/journal.pone.0063701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng, P., Griesmayr, B., Freunberger, R., & Klimesch, W. (2010). Control mechanisms in working memory: A possible function of EEG theta oscillations. Neuroscience and Biobehavioral Reviews, 342(7), 1015–1022. doi: 10.1016/j.neubiorev.2009.12.006 [DOI] [PubMed] [Google Scholar]
- Shu, N., Wang, X., Bi, Q., Zhao, T., & Han, Y. (2018). Disrupted topologic efficiency of white matter structural connectome in individuals with subjective cognitive decline. Radiology, 2862(1), 229–238. doi: 10.1148/radiol.2017162696 [DOI] [PubMed] [Google Scholar]
- Slot, R., Sikkes, S., Berkhof, J., Brodaty, H., Buckley, R., Cavedo, E., Dardiotis, E., Guillo-Benarous, F., Hampel, H., Kochan, N. A., Lista, S., Luck, T., Maruff, P., Molinuevo, J. L., Kornhuber, J., Reisberg, B., Riedel-Heller, S. G., Risacher, S. L., Roehr, S., … van der Flier, W. M. (2019). Subjective cognitive decline and rates of incident Alzheimer’s disease and non-Alzheimer’s disease dementia. Alzheimer’s & Dementia, 152(3), 465–476. doi: 10.1016/j.jalz.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart, C. M., Segalowitz, S. J., Mulligan, B. P., & MacDonald, S. W. (2014). Attention capacity and self-report of subjective cognitive decline: A P3 ERP study. Biological Psychology, 1032, 144–151. doi: 10.1016/j.biopsycho.2014.08.016 [DOI] [PubMed] [Google Scholar]
- Snodgrass, J. G., & Vanderwart, M. (1980). A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory, 62(2), 174–215. doi: 10.1037//0278-7393.6.2.174 [DOI] [PubMed] [Google Scholar]
- Strauss, E., Sherman, E. M., & Spreen, O. (2006). A compendium of neuropsychological tests: Administration, norms, and commentary. American Chemical Society. [Google Scholar]
- Susana, C. F., Mónica, L., & Fernando, D. (2021). Event-related brain potential indexes provide evidence for some decline in healthy people with subjective memory complaints during target evaluation and response inhibition processing. Neurobiology of Learning and Memory, 1822, 107450. doi: 10.1016/j.nlm.2021.107450 [DOI] [PubMed] [Google Scholar]
- van Straaten, E. C., Scheltens, P., Gouw, A. A., & Stam, C. J. (2014). Eyes-closed task-free electroencephalography in clinical trials for Alzheimer’s disease: An emerging method based upon brain dynamics. Alzheimer’s Research & Therapy, 62(9), 86. doi: 10.1186/s13195-014-0086-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviano, R. P., & Damoiseaux, J. S. (2020). Functional neuroimaging in subjective cognitive decline: Current status and a research path forward. Alzheimer’s Research & Therapy, 122(1), 23. doi: 10.1186/s13195-020-00591-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries, I., Slagter, H. A., & Olivers, C. (2020). Oscillatory control over representational states in working memory. Trends in Cognitive Sciences, 242(2), 150–162. doi: 10.1016/j.tics.2019.11.006 [DOI] [PubMed] [Google Scholar]
- Wang, X., Huang, W., Su, L., Xing, Y., Jessen, F., Sun, Y., Shu, N., & Han, Y. (2020). Neuroimaging advances regarding subjective cognitive decline in preclinical Alzheimer’s disease. Molecular Neurodegeneration, 152(1), 55. doi: 10.1186/s13024-020-00395-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, S., & Wu, Z. (1986). The construction of “The Clinical Memory Test”. Acta Psychologica Sinica, 182, 100–108. Retrieved from https://journal.psych.ac.cn/xlxb/CN/Y1986/V18/I1/102 [Google Scholar]
- Yesavage, J. A., & Sheikh, J. I. (1986). Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontologist, 52(1–2), 165–173. doi: 10.1300/j018v05n01_09 [DOI] [Google Scholar]
- Yu, J., Li, J., & Huang, X. (2012). The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: A community-based study. BMC Psychiatry, 122, 156. doi: 10.1186/1471-244X-12-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.