Case Presentation
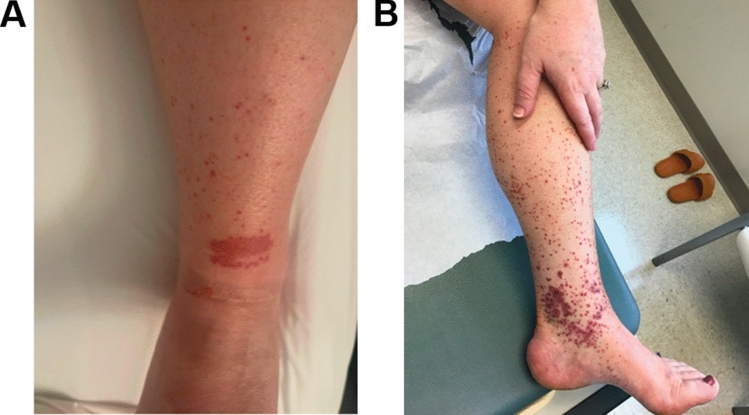
A 37-year-old female with a 26-year history of stricturing ileocolonic Crohn’s disease (CD) with a prior ileocecal resection was evaluated by our multidisciplinary inflammatory bowel disease (IBD) clinic with concern for a rash on her lower extremities. She identified the rash on both lower extremities, particularly near her ankles and calves (Fig. 1a). In addition to the rash, she also complained of significant bilateral ankle pain. At the time of presentation, she had been in clinical remission for at least 6 years while receiving infliximab monotherapy, with continuing endoscopic remission (Rutgeerts score i0). The rash and ankle pain began one month prior to the clinic visit and two months after being diagnosed with COVID-19. She denied any concurrent GI symptoms that were new or worsened from her baseline; specifically, she had no abdominal pain or change in her bowel habits, and no episodes of hematochezia. Though she was initially treated with topical hydrocortisone 2.5% cream which provided temporary improvement of her lower extremity lesions, they returned quickly after its discontinuation.
Fig. 1.
Palpable purpura on lower extremity in the setting of leukocytoclastic vasculitis. A Initial appearance of left lower extremity. B Return of skin lesions after initial response to prednisone, preceding start of ustekinumab
She was referred to the dermatology service for further evaluation. The differential diagnosis included leukocytoclastic vasculitis (LCV) secondary to idiopathic adult immunoglobulin (Ig) A vasculitis (formerly known as Henoch-Schönlein purpura), viral-induced small vessel vasculitis in the setting of SARS-CoV2 infection, or drug-induced vasculitis secondary to infliximab therapy. Laboratory evaluation was notable for antinuclear antibodies (ANA), elevated at 1:160. She had normal anti-neutrophil cytoplasm antibodies (ANCA), anti-myeloperoxidase antibody, anti-double stranded DNA (dsDNA) antibody, total IgG, total IgM, and rheumatoid factor (RF). Though urinalysis was positive on two separate occasions for microscopic hematuria, there was no proteinuria. The rash spread beyond her lower shins and calves to include her upper thighs and became more painful. Some of the purpuric papules on her lower shins developed hemorrhagic bullae (Fig. 1b).
Pathology of a representative lesion obtained by punch biopsy showed fibrinoid necrosis of capillary walls within the dermis with surrounding neutrophils and karyorrhectic debris consistent with a diagnosis of LCV (Supplemental Figure). Direct immunofluorescence demonstrated deposition of IgA and fibrinogen in the small vessels of the superficial dermis, revealing concurrent IgA vasculitis. Colonoscopy to assess the inflammatory activity of Crohn’s disease showed complete endoscopic remission (Rutgeerts i0, simple endoscopic score [SES-CD] of 0). Taken together, her diagnosis was most consistent with IgA vasculitis, with histopathological findings of LCV.
She was prescribed prednisone 40 mg by mouth daily, which temporarily improved the distribution of purpuric lesions; urinalysis showed resolution of microscopic hematuria. Dapsone (50 mg daily) was added as adjunctive therapy. Nevertheless, symptoms began to recur during the prednisone taper. Due to suspicion of infliximab-associated LCV, infliximab was discontinued and ustekinumab therapy was initiated as replacement maintenance therapy. Ultimately, with the combination of dapsone and ustekinumab, she was able to taper off corticosteroids with complete resolution of the rash and joint pain. She remains in clinical remission on ustekinumab, now 9 months after induction.
Discussion
LCV has been described in association with a variety of chronic infections, autoimmune disorders, and medications. In the population with IBD, LCV has been reported as a rare cutaneous, extraintestinal disease manifestation or complication, which may be related to the disease itself or associated with the use of biologic medications, particularly anti-tumor necrosis factor-α (anti-TNF) therapies. Systemic illness such as infection or malignancy are also associated with LCV.
Although LCV was formerly often referred to as small vessel vasculitis of the skin, it is not a homogenous diagnosis or disease; rather, it refers to microscopic features that are common to several diseases [1]. As detailed in a dermatologic addendum to the 2012 International Chapel Hill Consensus Conference, LCV represents “the (1) skin manifestations of systemic vasculitis, (2) a skin limited or skin dominant expression or variant of a systemic vasculitis, or (3) a single organ vasculitis that differs from recognized systemic vasculitides”[2]. As such, LCV may be present among numerous localized or systemic conditions [2] .
Histologic findings pathognomonic for LCV include fragmentation of the nuclei within neutrophils to “nuclear dust” (referred to as “leukocytoclasia”) within the walls of arterioles, capillaries, and post-capillary venules. Downstream effects of this process include extravasation of red blood cells and fibrinoid necrosis of vessel walls [1, 3]. These histologic features are present in a variety of immune-mediated vasculitides, including IgA vasculitis as seen in this case, ANCA vasculitis, or immune complex vasculitis, among others. LCV is also present in vasculitis associated with systemic diseases, including both CD and ulcerative colitis (UC), or with vasculitis associated with malignancy, infections, or medications [1, 2, 4]. Despite a variety of conditions associated with LCV, this type of vasculitis remains rare. Whereas the precise frequency of LCV in patients with IBD is unknown, the incidence of biopsy-proven LCV was recently estimated to be 4.5 (95% CI 3.5–5.4) per 100,000 person-years in the US [5].
Patients with cutaneous LCV typically have non-blanchable palpable purpura that accumulate on the lower extremities or on dependent areas. These lesions are at times associated with pain or urticaria, and a burning sensation in some cases. Depending on the severity and duration of symptoms, some patients may develop vesicles, pustules, or ulcers [6, 7]. Less specific systemic symptoms related to concurrent disease processes may also be present including fever or arthralgias [1]. In the setting of IgA vasculitis for example, patients may have concurrent abdominal pain or new onset hypertension as a consequence of gastrointestinal or renal vessel involvement.
LCV in patients with IBD does not necessarily track with intestinal disease activity, having been reported prior to diagnosis, during treatment of steady state disease, or during flares of active IBD. Approximately half of cases of LCV are attributed to ongoing systemic illnesses or associated findings such as malignancy, infections, or medications, or have ultimately been deemed idiopathic [1]. As such, important factors to evaluate include recent medication changes, concurrent infections, or systemic illnesses (Table 1). Among medications most relevant to IBD patients, anti-TNF therapies and other biologics are implicated in LCV pathogenesis. The pathophysiology related to anti-TNF use is believed to be a potential type III hypersensitivity reaction mediated by release of anti-drug antibodies within capillaries. Some also postulate that a cytokine imbalance and shifts in normal activity of T and B cells may be contributory [8]. When studied in a large retrospective cohort, skin findings attributed to TNF use in IBD patients do not generally correlate with anti-TNF trough drug or antibody levels [9]. It remains to be seen whether LCV is more frequently observed with anti-TNF biologics versus other biologic therapies currently used for IBD treatment. Indeed, there have been several cases of LCV associated with secukinumab and ustekinumab, among others [10] .
Table 1.
Overview of causes, workup and treatment of leukocytoclastic vasculitis in patients with inflammatory bowel disease
| Causes | Relevant evaluations | Treatment options |
|---|---|---|
| Primary cutaneous vasculitis | Complete Blood Count | Corticosteroids |
| Concurrent systemic vasculitis | Comprehensive Metabolic Panel | Immunosuppresants |
| Drug induced | Urinalysis with sediment | Azathioprine |
| Antibiotics (e.g., beta lactams) | ANCA panel | Methotrexate |
| NSAIDs | ANA panel | Cyclophosphamide |
| Biologics (e.g., anti-TNFs) | ENA panel | Biologics |
| Infection or malignancy related | Cryoglobulins | Infliximaba |
| Rheumatoid Factor | Adalimumaba | |
| Anti-myeloperoxidase antibodies | Abatacept | |
| Anti-double stranded DNA antibodies | Vedolizumab | |
| Complement (C3 and C4) | Plasma Exchange | |
| Immunoglobulins (Total IgG, IgM) | Intravenous immunoglobulin | |
| Skin biopsy with direct immunofluorescence | Adjuvants | |
| ± Cross-sectional imaging | Colchicine | |
| Dapsone |
aCan be considered if not suspected as the cause of leukocytoclastic vasculitis
There are a number of known causes of LCV including cryoglobulinemia, infection, collagen vascular disease, and drugs, though up to 30% of cases may be idiopathic. In mild cases, basic laboratory evaluation including a complete blood count, comprehensive metabolic panel, and urinalysis may be sufficient. If the presentation is more severe, as in this case, infectious and autoimmune evaluations are indicated. Ultimately, biopsy of the involved organs is essential to confirm the diagnosis. A skin biopsy should include the superficial dermis and hypodermis. Though typically a punch biopsy will be satisfactory for this evaluation, in some cases a wedge biopsy can be considered, especially if there is concern for disease involving medium-sized vessels [1]. In addition to the pathognomonic findings, namely leukocytoclasia and fibrinoid necrosis of vessel walls, eosinophilic infiltration may be observed [1]. Direct immunofluorescence microscopy may also establish the presence of LCV and thus should also be obtained when possible. Notably, IgA stains can diagnose IgA vasculitis as in the case, whereas the presence of IgG for example can be suggestive of an alternative diagnosis (such as systemic lupus erythematosus) as the underlying etiology [11] .
The management of LCV depends on the severity of presentation and the underlying cause. Since this is a rare entity, no randomized controlled trials have been performed to evaluate safety or efficacy of specific therapies. Fortunately, overall mortality and long-term morbidity is generally low, with prognosis often dependent on the severity of any underlying illness [1]. In mild cases limited only to skin, rest with elevation of the lower extremities and compression stockings can resolve symptoms. In more severe or refractory cases, topical or systemic corticosteroids are commonly used, concurrent with removal of the inciting medication or treating the underlying infection. Adjunctive therapies previously reported include colchicine and dapsone [12]; NSAIDs have been used anecdotally although may not be preferred in patients with IBD. When an ongoing systemic illness is present, immunosuppression and biologics may be indicated. Among general immunosuppressants, azathioprine and methotrexate, among others, are described as efficacious [1]. Rituximab has been used in cases associated with systemic vasculitides or malignancies, particularly hematologic [12]. In severe refractory cases of COVID-related vasculitis and cutaneous vasculitis, plasma exchange or intravenous immunoglobulin (IVIG) have also been beneficial [13, 14]. For maintenance therapy, if an anti-TNF agent is considered the primary cause of the vasculitis, switching to a treatment not directed at TNF is typically recommended, although there have been reports of small subsets of patients experiencing resolution of their LCV even while continuing the same anti-TNF therapy [13]. Often multiple factors may be present, complicating isolation of the exact cause of LCV. As such, potential alternative maintenance therapies not directed at TNF have been reported including vedolizumab [15] and in this case, ustekinumab.
Among patients with CD who have purpuric rash, a closely related, but distinct pathology, granulomatous vasculitis should also be considered. Granulomatous vasculitis is often referred to as “Extraintestinal CD” [16]. Though the presentation of granulomatous vasculitis varies, patients can manifest purpura in the lower extremities similar to LCV if small vessels are involved. In contrast to LCV, however, lesions can also be commonly found on the genitalia, trunk, and face. Cutaneous histologic findings include macrophages and lymphocytes surrounding dermal blood vessels with sometimes-seen fibrin in the walls of the vessels. Lichenoid infiltrate, necrosis and dermal edema have been observed as well, though less common [16]. Fortunately, while a distinct entity, management of granulomatous vasculitis is similar to that of LCV, and is similarly dependent on severity. Options include steroids, immunosuppressants such azathioprine, biologics including infliximab, as well as adjuvant therapy with dapsone [16] .
LCV is considered to be an immune complex-mediated hypersensitivity reaction. Immunoglobulins may be involved, as in this patient, whereby antigen-bound IgA is incorporated into immune complexes that are deposited in arterioles, capillaries, and post-capillary venules triggering complement-mediated injury to the vessel wall with extravasation of red blood cells and diapedesis of neutrophils. Antigens may be viral, bacterial, drugs or their metabolites, another antibody, or an unknown substance. Since the patient in this case improved after discontinuation of infliximab and her CD was in complete endoscopic and histologic remission, we hypothesize that although infliximab may have been a contributing factor to the development of LCV and IgA vasculitis, it is also possible that her recent COVID infection contributed to the altered immunogenicity that led to her skin findings.
Ultimately, while rare, LCV is an important pathologic condition that should be recognizable to providers who care for patients with IBD. As demonstrated in this case, patients with IBD who develop LCV require careful clinical and laboratory evaluation to assess potential causes and complications. Furthermore, clinicians should be aware that a diagnosis of LCV in a patient with IBD will likely involve change in maintenance therapy, since medications are often implicated in its etiology.
Key Points
LCV is a histopathologic diagnosis common to several illnesses, which is defined by microscopic features of leukocytoclasia (fragmentation of neutrophil nuclei) and fibrinoid necrosis of small vessel walls, including arterioles, capillaries, and post-capillary venules.
Cases of LCV in patients with IBD can be limited to skin involvement, or can occur simultaneously with other systemic vasculitides, such as IgA vasculitis.
Inciting causes of LCV include infections, medications (including anti-TNF therapy), and concurrent autoimmune diseases or malignancy.
Though treatment varies based on severity, most cases improve with removal of the inciting agent. IBD patients should switch maintenance medical therapy if it is implicated as a potential cause. Since anti-TNF therapies have been implicated in LCV, a switch to a different class of therapy is typically recommended in these instances.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This research was supported by Grants from the National Institutes of Health [K23DK127157-01 ELB].
Declarations
Conflict of interest
Hans H. Herfarth has served as a consultant for Alivio, AMAG, BMS, Boehringer, ExeGI, Finch, Fresenius Kabi, Galapagos, Janssen, Gilead, Lycera, Merck, Otsuka, Pfizer, PureTech, Seres, Ventyx and research support from Artizan Biosciences, Allakos, NovoNordisk, and Pfizer. Millie D. Long has served as a consultant for AbbVie, Takeda, Janssen, Pfizer, Lilly, Prometheus, Target Real World Evidence and has received research support from Pfizer, Takeda and Lilly. Edward L. Barnes has served as a consultant for AbbVie, Eli Lilly, Bristol-Meyers Squibb, and Target RWE. Kimberly Darlington, Animesh Jain, Priyanka Vedak and Paul Googe have no relevant disclosures for this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fraticelli P, Benfaremo D, Gabrielli A. Diagnosis and management of leukocytoclastic vasculitis. Intern Emerg Med. 2021;16:831–841. doi: 10.1007/s11739-021-02688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sunderkotter CH, Zelger B, Chen KR, et al. Nomenclature of Cutaneous Vasculitis: Dermatologic Addendum to the 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheumatol. 2018;70:171–184. doi: 10.1002/art.40375. [DOI] [PubMed] [Google Scholar]
- 3.Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3–23. doi: 10.1111/j.1365-2559.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- 4.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 5.Arora A, Wetter DA, Gonzalez-Santiago TM, et al. Incidence of leukocytoclastic vasculitis, 1996 to 2010: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2014;89:1515–1524. doi: 10.1016/j.mayocp.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iraji F, Galehdari H, Siadat AH, Bokaei Jazi S. Cutaneous leukocytoclastic vasculitis secondary to COVID-19 infection: a case report. Clin Case Rep. 2021;9:830–834. doi: 10.1002/ccr3.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camprodon Gomez M, Gonzalez-Cruz C, Ferrer B, Barbera MJ. Leucocytoclastic vasculitis in a patient with COVID-19 with positive SARS-CoV-2 PCR in skin biopsy. BMJ Case Rep. 2020;13:e238039. doi: 10.1136/bcr-2020-238039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song Y, Shi YH, He C, et al. Severe Henoch-Schonlein purpura with infliximab for ulcerative colitis. World J Gastroenterol. 2015;21:6082–6087. doi: 10.3748/wjg.v21.i19.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cleynen I, Van Moerkercke W, Billiet T, et al. Characteristics of skin lesions associated with anti-tumor necrosis factor therapy in patients with inflammatory bowel disease: a cohort study. Ann Intern Med. 2016;164:10–22. doi: 10.7326/M15-0729. [DOI] [PubMed] [Google Scholar]
- 10.da Silva Cendon Duran C, da Paz AS, Barreto Santiago M. Vasculitis induced by biological agents used in rheumatology practice: a systematic review. Arch Rheumatol. 2022;37:300–310. doi: 10.46497/ArchRheumatol.2022.9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takatu CM, Heringer APR, Aoki V, et al. Clinicopathologic correlation of 282 leukocytoclastic vasculitis cases in a tertiary hospital: a focus on direct immunofluorescence findings at the blood vessel wall. Immunol Res. 2017;65:395–401. doi: 10.1007/s12026-016-8850-6. [DOI] [PubMed] [Google Scholar]
- 12.Ak T, Durmus RB, Onel M. Cutaneous vasculitis associated with molecular tergeted therapies: systematic review of the literature. Clin Rheumatol. 2023;42:339–357. doi: 10.1007/s10067-022-06406-6. [DOI] [PubMed] [Google Scholar]
- 13.Mohan N, Edwards ET, Cupps TR, et al. Leukocytoclastic vasculitis associated with tumor necrosis factor-alpha blocking agents. J Rheumatol. 2004;31:1955–1958. [PubMed] [Google Scholar]
- 14.Nassani N, Sweiss N, Berry JT, et al. Leukocytoclastic vasculitis in cutaneous crohn disease in the setting of COVID-19. Inflamm Bowel Dis. 2021;27:e74–e75. doi: 10.1093/ibd/izab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancado GGL, Nardelli MJ, Gouthier MAD. Adalimumab-induced leukocytoclastic vasculitis in a Crohn's disease patient. Eur J Gastroenterol Hepatol. 2021;33:453. doi: 10.1097/MEG.0000000000001945. [DOI] [PubMed] [Google Scholar]
- 16.Burns AM, Walsh N, Green PJ. Granulomatous vasculitis in Crohn's disease: a clinicopathologic correlate of two unusual cases. J Cutan Pathol. 2010;37:1077–1083. doi: 10.1111/j.1600-0560.2010.01546.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.