Abstract
Introduction
ERAS pathway has been proposed as the standard of care in elective abdominal surgery. Guidelines on ERAS in emergency surgery have been recently published; however, few evidences are still available in the literature. The aim of this study was to evaluate the feasibility of an enhanced recovery protocol in a large cohort of patients undergoing emergency surgery and to identify possible factors impacting postoperative protocol compliance.
Methods
This is a prospective multicenter observational study including patients who underwent major emergency general surgery for either intra-abdominal infection or intestinal obstruction. The primary endpoint of the study is the adherence to ERAS postoperative protocol. Secondary endpoints are 30-day mortality and morbidity rates, and length of hospital stay.
Results
A total of 589 patients were enrolled in the study, 256 (43.5%) of them underwent intestinal resection with anastomosis. Major complications occurred in 92 (15.6%) patients and 30-day mortality was 6.3%. Median adherence occurred on postoperative day (POD) 1 for naso-gastric tube removal, on POD 2 for mobilization and urinary catheter removal, and on POD 3 for oral intake and i.v. fluid suspension. Laparoscopy was significantly associated with adherence to postoperative protocol, whereas operative fluid infusion > 12 mL/Kg/h, preoperative hyperglycemia, presence of a drain, duration of surgery and major complications showed a negative association.
Conclusions
The present study supports that an enhanced recovery protocol in emergency surgery is feasible and safe. Laparoscopy was associated with an earlier recovery, whereas preoperative hyperglycemia, fluid overload, and abdominal drain were associated with a delayed recovery.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00268-023-06984-9.
Introduction
Enhanced recovery after surgery (ERAS®) is an evidence-based multimodal approach to optimize perioperative pathway which allowed to reduce postoperative morbidity and shortened length of hospital stay. Therefore, enhanced recovery protocols have been proposed as the standard of care in elective colorectal surgery [1, 2].
Despite the publication of proper guidelines [3], the spread of enhanced recovery protocols in patients undergoing emergency surgery is still limited. Preliminary results have been published in patients who underwent surgery for obstructing colorectal cancer or perforated peptic ulcers [4–8]. However, systematic reviews and meta-analysis showed that enhanced recovery protocols were different across the studies, especially concerning the intraoperative items [9, 10]. Limiting factors to the widespread of enhanced recovery protocols in emergency surgery are the non-applicability of preoperative items and the presence of acute stressful diseases often requiring tailored care rather than standardized protocols [11, 12].
The aim of this study was to evaluate the feasibility of an enhanced recovery protocol in a large cohort of patients undergoing emergency general surgery and to identify possible factors impacting postoperative protocol compliance.
Methods
This is a prospective, observational, multicenter cohort study promoted by the Italian Society of Emergency Surgery and Trauma and the Perioperative Italian Society. Eight Italian high-volume hospitals with previous experience in enhanced recovery protocols in major elective surgery were involved. The study protocol was shared during a multidisciplinary meeting involving surgeons and anesthesiologists from each center. Supplementary Table reports the study protocol which was approved by the Ethical Committee of the promoting center (n. 0,012,747 08/10/2020) and was registered on clinicaltrial.gov (identifier NCT04648644).
Patients aged > 18 years undergoing unscheduled intestinal resections with or without anastomosis, hollow viscus injury repair, enteric bypass or adhesiolysis in presence of either peritoneal contamination or intestinal obstruction were included in the study. Exclusion criteria were refused to participate, septic shock, and emergency surgery for complications following elective surgery, operative endoscopy or diagnostic procedures. Patients presenting with multiple organ failure who required damage control surgery with open abdomen and/or immediate postoperative ICU stay longer than 72 h were dropped out from the study because they had no chance to adhere to the enhanced recovery protocol.
Demographics, body mass index, Charlson Comorbidity Index, primary diagnosis, type of surgery, adherence to each ERAS item and short-term outcome parameters were anonymously collected from all patients. Major complications have been classified according to the Clavien-Dindo scale [13]. Patients’ follow-up was carried out by office visits or telephone calls.
The primary endpoint of the study was the adherence to the enhanced recovery protocol. Secondary endpoints are 30-day mortality and morbidity rates, and length of hospital stay.
Statistical analysis
Continuous variables were described as median and interquartile range (IQR). Categorical variables were described as percentages. The cumulative adherence was recorded daily for each postoperative item. Univariate and multiple ordinal regression models were calculated using the number of achieved postoperative items as outcome. For each postoperative item, patients’ compliance was defined using the median value as the threshold. Variables with a significant association at the univariate analysis (p < 0.05) were adopted in the multiple ordinal regression model. Pearson’s linear correlation was calculated between the number of achieved postoperative items and length of stay. Statistical analysis was performed using the IBM SPSS Statistics 27 software (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp).
Results
Between November 2020 and November 2021 among the eight participating centers 909 patients fulfilled the inclusion criteria. Figure 1 reports the flow diagram of the study. A total number of 589 patients were included in the analysis.
Fig. 1.
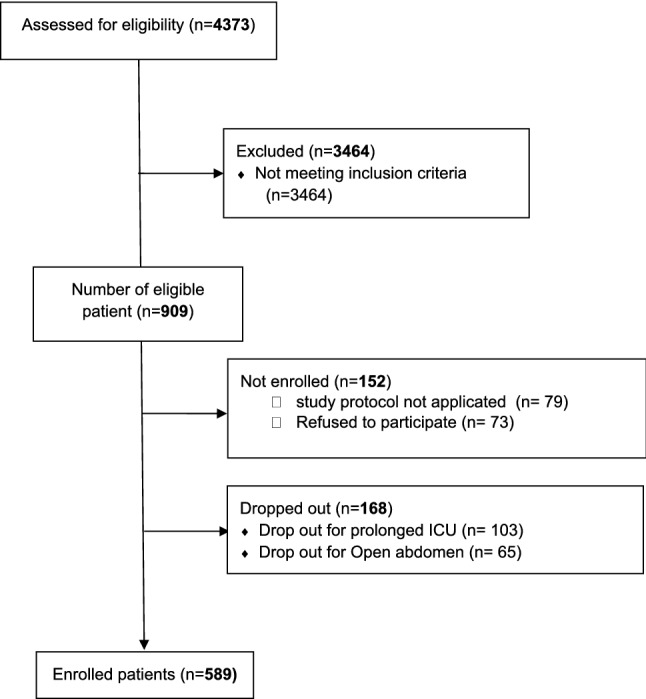
Study flow diagram
Table 1 shows that 242 (41.1%) patients were over 75 yrs., 318 (54.4%) had ASA 3–4, while body temperature was below 36.0 °C in 177 (33.5%) patients. Three hundred and twenty-seven (55.5%) patients had intestinal obstruction, while 262 (44.6%) had intra-abdominal infections. The median time from hospital admission to operation was 8 h (3–25). Two hundred and fifty-six (43.5%) patients underwent intestinal resection with anastomosis and 93 (15.8%) underwent intestinal resection without anastomosis. One hundred and forty-four (24.6%) patients were treated with minimally invasive techniques.
Table 1.
Patients’ characteristics
| Median (IQR) | N | % | ||
|---|---|---|---|---|
| Age | 72 (57–81) | |||
| Age range | 19–40 | 40 | 6.8% | |
| 41–65 | 192 | 32.6% | ||
| 66–75 | 115 | 19.5% | ||
| 76–85 | 167 | 28.4% | ||
| > 85 | 75 | 12.7% | ||
| Sex | F | 311 | 52.8% | |
| M | 278 | 47.2% | ||
| BMI | 24.22 (22–26) | |||
| BMI range | < 18 | 27 | 5.4% | |
| 18–24 | 246 | 48.9% | ||
| 25–30 | 182 | 36.2% | ||
| > 30 | 48 | 9.5% | ||
| ASA class | 1 | 57 | 9.8% | |
| 2 | 209 | 35.8% | ||
| 3 | 253 | 43.3% | ||
| 4 | 65 | 11.1% | ||
| Charlson comorbidity index | 4 (2–7) | |||
| Time from admission to surgery (hours) | 8 (3–25) | |||
| Time from admission to surgery (hours) | < 24 h | 433 | 73.5% | |
| > 24 h | 156 | 26.5% | ||
| pH | 7.4 (7.2–7.4) | |||
| Hb (mg/dL) | 12 (11–14) | |||
| Lac (mmol/L) | 1.5 (1–2.4) | |||
| Body temperature at admission | 36.4 (36–36.9) | |||
| Body temperature | (36.0–37.5) | 318 | 60.2% | |
| (< 36.0) | 177 | 33.5% | ||
| (> 37.5) | 33 | 6.3% | ||
| Preop. Glycemia (mg/dL) | 120 (100–145) | |||
| SARS-CoV2 positive nasal swab | 4 | 0.7% | ||
| Diagnosis | Obstruction | 300 | 51% | |
| Lower GI perforation | 172 | 29% | ||
| Ischemia | 32 | 12% | ||
| Incarcerated hernia | 27 | 5% | ||
| Upper GI perforation | 48 | 8% | ||
| Others | 10 | 2% | ||
| Surgical procedure | Resection with anastomosis | 256 | 43.5% | |
| Lysis of adhesion | 168 | 28.5% | ||
| Resection without anastomosis | 93 | 15.8% | ||
| Perforated peptic ulcer repair | 38 | 6.5% | ||
| By-pass | 20 | 3.4% | ||
| Hollow viscus perforation repair | 14 | 2.4% | ||
| Surgery duration (min) | 120 (80–180) | |||
| Surgical technique | Open surgery | 348 | 59.4% | |
| Laparoscopy | 144 | 24.6% | ||
| Laparoscopy converted to open | 94 | 16.0% | ||
Table 2 reports adherence to the enhanced recovery items. The highest adherence was obtained for operative warming and postoperative nausea and vomiting prophylaxis. The median operative fluid infusion was 12 (8.33–17.14) mL/Kg/h. Drains were placed in 92.7% of patients with intra-abdominal sepsis and in 55.7% of patients with intestinal obstruction.
Table 2.
Adherence to enhanced recovery items
| Median (IQR) | N | % | ||
|---|---|---|---|---|
| Depth of anesthesia monitoring (entropy) | 302 | 51.3% | ||
| Neuromuscular blockade monitoring | 230 | 39.0% | ||
| PONV prevention | 516 | 87.6% | ||
| General + locoregional anesthesia | 142 | 24.1% | ||
| Active warming | 551 | 95.8% | ||
| Invasive arterial pressure monitoring | 102 | 17.3% | ||
| Opioid used | Fentanyl | 269 | 47.7% | |
| Morphine | 140 | 24.8% | ||
| Remifentanil | 129 | 22.9% | ||
| Disufen | 8 | 1.4% | ||
| Ketamine | 8 | 1.4% | ||
| Other | 10 | 1.8% | ||
| Intraoperative transfusion | 71 | 12.2% | ||
| Inotropes/Vasopressors | 70 | 12.0% | ||
| Intravenous fluids ml/kg/h | 12 (8.33–17.14) | |||
| Intravenous fluids | 3–6 ml/Kg/h | 125 | 21.2% | |
| 7–12 ml/Kg/h | 183 | 31.1% | ||
| > 20 ml/Kg/h | 281 | 47.7% | ||
| Minimally invasive surgery | 144 | 24.6% | ||
| Drain | All patients | 422 | 72.1% | |
| obstruction | 181 | 55.7% | ||
| Intra-abdominal sepsis | 241 | 92.7% | ||
PONV: Postoperative nausea and vomiting
Table 3 summarizes the postoperative short-term outcome. Overall morbidity was 60.4%, major complications occurred in 92 (15.6%) patients, and 30-day mortality was 6.3%. Median length of hospital stay was 8 (6–12) days.
Table 3.
Short-term postoperative outcome
| Median (IQR) | N | % | ||
|---|---|---|---|---|
| Overall morbidity | 356 | 60.44% | ||
| 30-day mortality | 37 | 6.28% | ||
| Complication grade | 0 | 233 | 39.56% | |
| I | 135 | 22.92% | ||
| II | 129 | 21.90% | ||
| IIIa | 13 | 2.21% | ||
| IIIb | 27 | 4.58% | ||
| IVa | 10 | 1.70% | ||
| IVb | 5 | 0.85% | ||
| V | 37 | 6.28% | ||
| Surgical site infection | 82 | 14.16% | ||
| Anastomotic leak | 23 | 6.97% | ||
| Respiratory infection | 46 | 7.94% | ||
| Urinary tract infection | 20 | 3.45% | ||
| Cardiovascular complications | 63 | 10.90% | ||
| Readmission within 30 days | 32 | 5.48% | ||
| Length of stay ( days) | 8 (6–12) |
Table 4 shows the cumulative compliance to postoperative items. The median adherence was reached on postoperative day (POD) 1 for naso-gastric tube removal (55.3%), on POD 2 for mobilization (68.8%) and urinary catheter removal (57%), and on POD 3 for oral intake (56.4%) i.v. fluid stop (52.3%).
Table 4.
Cumulative compliance to postoperative items
| POD | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|
| Naso-gastric tube removal | 31.1 | 55.3 | 77.9 | 88.3 | 92.9 | 95.3 | 97.4 | 98.6 |
| Oral fluid intake | 0 | 29.0 | 57.2 | 72.7 | 81.6 | 86.5 | 89.2 | 90.6 |
| Mobilization > 4 h | 0 | 36.1 | 68.8 | 85.1 | 92.5 | 96.0 | 97.2 | 97.9 |
| Urinary catheter removal | 0 | 29.2 | 57.0 | 71.1 | 78.3 | 83.6 | 86.4 | 88.2 |
| Solid food intake | 0 | 6.1 | 28.5 | 56.4 | 78.1 | 86.5 | 91.8 | 94.4 |
| i.v. fluids stop | 0 | 9.6 | 31.6 | 52.3 | 65.6 | 75.2 | 80.8 | 84.3 |
POD: postoperative day. i.v.: intravenous
Table 5 shows that laparoscopy was positively associated with an increasing postoperative compliance at the ordinal logistic regression analysis. A negative association with postoperative compliance was found for preoperative hyperglycemia, operative fluid infusion > 12 mL/Kg/h, presence of abdominal drain, perforated peptic ulcer repair, duration of surgery and major complications. There was a linear correlation between the increasing postoperative items compliance and the length of hospital stay (r = −0.552, p < 0.001) as shown in Fig. 2.
Table 5.
Univariate and multiple ordinal regression analyses for postoperative recovery items
| Univariate ordinal regression | Multiple ordinal regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | CI 95% | .p-value | OR | CI 95%25 | p-value | ||||
| Lower | Higher | Lower | Higher | ||||||
| Patients characteristics | |||||||||
| Sex (men) | 0.957 | 0.714 | 1.283 | 0.768 | |||||
| Age > 70 | 0.445 | 0.330 | 0.599 | < 0.001 | 0.655 | 0.424 | 1.011 | 0.056 | |
| BMI | 1.005 | 0.968 | 1.043 | 0.795 | |||||
| ASA III–IV | 0.393 | 0.290 | 0.531 | < 0.001 | 0.675 | 0.421 | 1.079 | 0.101 | |
| Charlson | 0.839 | 0.796 | 0.885 | < 0.001 | 0.922 | 0.844 | 1.008 | 0.076 | |
| Intra-abdominal sepsis | 0.535 | 0.397 | 0.721 | < 0.001 | 0.899 | 0.544 | 1.485 | 0.678 | |
| time from admission to surgery | < 24 h | 1 | |||||||
| > 24 h | 0.731 | 0.524 | 1.021 | 0.066 | |||||
| Preop. Glycemia | 0.992 | 0.989 | 0.996 | < 0.001 | 0.992 | 0.988 | 0.996 | < 0.001 | |
| Postop. Glycemia | 0.997 | 0.992 | 1.001 | 0.149 | |||||
| pH | 3.895 | 0.406 | 37.401 | 0.239 | |||||
| Hb | 1.102 | 1.038 | 1.170 | 0.001 | 1.019 | 0.933 | 1.113 | 0.675 | |
| Lactate | 0.995 | 0.971 | 1.019 | 0.662 | |||||
| Body temperature | 0.886 | 0.753 | 1.042 | 0.143 | |||||
| intraoperative blood transfusion | 0.365 | 0.229 | 0.584 | < 0.001 | 1.128 | 0.561 | 2.269 | 0.735 | |
| Inotropes/vasopressors | 0.435 | 0.270 | 0.700 | 0.001 | 0.718 | 0.386 | 1.336 | 0.296 | |
| Duration of surgery (min) | 0.994 | 0.992 | 0.996 | < 0.001 | 0.991 | 0.988 | 0.995 | < 0.001 | |
| Procedure | Lysys of adhesions | 1 | 1 | ||||||
| Bypass | 0.218 | 0.088 | 0.540 | 0.001 | 0.260 | 0.091 | 1.094 | 0.057 | |
| Hollow viscus perforation repair | 0.479 | 0.191 | 1.202 | 0.117 | 0.507 | 0.159 | 1.619 | 0.252 | |
| Resection with anastomosis | 0.376 | 0.261 | 0.541 | < 0.001 | 0.915 | 0.505 | 1.658 | 0.770 | |
| Resection without anastomosis | 0.308 | 0.191 | 0.495 | < 0.001 | 1.004 | 0.488 | 2.068 | 0.990 | |
| Perforated peptic ulcer repair | 0.173 | 0.094 | 0.321 | < 0.001 | 0.072 | 0.026 | 0.195 | < 0.001 | |
| Major morbidity | 0.292 | 0.185 | 0.460 | < 0.001 | 0.564 | 0.319 | 0.997 | 0.049 | |
| Enhanced recovery interventions | |||||||||
| Depth of anesthesia monitoring (entropy) | 0.956 | 0.714 | 1.282 | 0.765 | |||||
| Neuromuscular blockade monitoring | 1.649 | 1.217 | 2.236 | 0.001 | 1.240 | 0.830 | 1.852 | 0.293 | |
| PONV prevention | 1.328 | 0.857 | 2.058 | 0.204 | |||||
| Multimodal anesthesia | 0.898 | 0.639 | 1.262 | 0.534 | |||||
| Active warming | 0.569 | 0.274 | 1.183 | 0.131 | |||||
| Infusions ml/kg/h (continuous variable) | 0.968 | 0.950 | 0.985 | < 0.001 | |||||
| Infusions (categorical) | [infusions 3–6 ml/Kg/h] | 1 | 1 | ||||||
| [infusions 7–12 ml/Kg/h] | 0.817 | 0.511 | 1.306 | 0.398 | 0.634 | 0.367 | 1.095 | 0.102 | |
| [infusions > 12 ml/Kg/h] | 0.558 | 0.363 | 0.857 | 0.008 | 0.288 | 0.164 | 0.508 | < 0.001 | |
| Minimally invasive surgery | 3.132 | 2.198 | 4.463 | < 0.001 | 2.222 | 1.395 | 3.538 | 0.001 | |
| Drain | 0.277 | 0.195 | 0.393 | < 0.001 | 0.561 | 0.334 | 0.942 | 0.029 | |
| Use of opioid after surgery | 0.681 | 0.499 | 0.930 | 0.016 | 0.906 | 0.605 | 1.357 | 0.632 | |
Fig. 2.
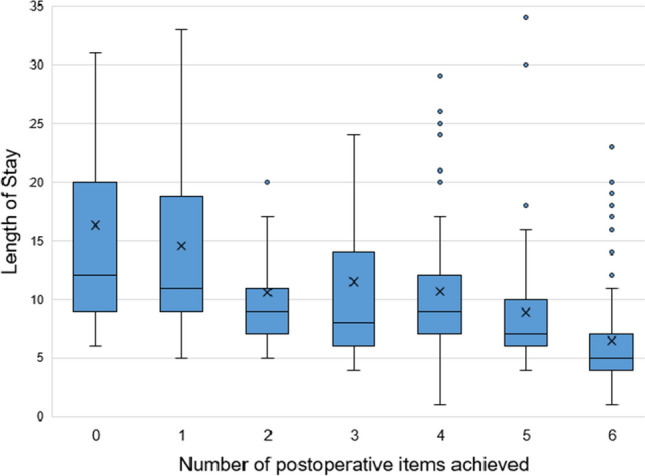
Linear correlation between number of postoperative items achieved and length of hospital stay
Discussion
The present study supports the implementation of enhanced recovery protocols in patients undergoing emergency gastrointestinal surgery. The median adherence to postoperative recovery outcomes was reached on postoperative day 1 for naso-gastric tube removal, on day 2 for mobilization and urinary catheter removal and on day 3 for oral feeding and i.v. fluid suspension. Laparoscopy was associated with an earlier recovery, whereas preoperative hyperglycemia, fluid overload, abdominal drain, duration of surgery, and major morbidity were associated to a delayed recovery.
Few studies have been published on patients who underwent emergency surgery, the majority focused on patients with obstructing colorectal cancer or perforated peptic ulcers [4–8]. Enhanced recovery protocols were associated with shorter length of stay and lower postoperative complications when compared to traditional care. Roulin and coll. reported a lower compliance to postoperative protocol in patients who underwent urgent colectomy when compared to patients who underwent elective colectomy [6].
The present study enrolled consecutive patients without any selection bias. In fact, the majority of patients were elderly with ASA score > 2 and the median time from admission to surgery was short, thus reflecting the daily practice of emergency surgery. Patients’ compliance to postoperative items was satisfactory with all targets reached one-day later in comparison with what has been reported following elective colorectal surgery [14].
The multiple regression analysis showed that minimally invasive surgery positively impacted on postoperative recovery (p = 0.004). Despite a positive impact of minimally invasive technique on postoperative outcomes has been widely demonstrated in elective colorectal surgery [15, 16], the implementation of laparoscopy in emergency surgery is still a matter of debate [17, 18]. In our cohort about 40% of patients had a minimally invasive approach and laparoscopic surgery was successfully completed in two-thirds of them. A national UK study showed that laparoscopy was adopted in less than 20% of patients who underwent emergency surgery [17]. The present data should contribute to a wider use of minimally invasive approach in emergency surgery.
Intraoperative fluid management is a cornerstone of enhanced recovery protocols. In elective major noncardiac surgery a goal-directed fluid management strategy reduced postoperative complications [19, 20]. In the present study, a fluid overload negatively impacted on postoperative recovery delaying mobilization and oral feeding (p < 0.001). To prevent the risk of fluid overload, operative hemodynamic monitoring should be implemented to yield a proper goal-directed fluid therapy [21]. An abdominal drain was placed in 55.7% of patients operated for an intestinal obstruction, suggesting an over-indication in absence of a macroscopic peritoneal contamination. Similarly to the elective setting [22], the abdominal drain was negatively associated with postoperative recovery (p = 0.007), therefore, it should be placed in selected cases and removed as early as possible.
Preoperative hyperglycemia has been strongly correlated with morbidity and mortality in both elective [23–27] and critically ill surgical patients [28–30]. Hyperglycemia could also be considered a marker of severity of disease and organ dysfunction. Our data confirm the importance of a tight glycemic control in patients undergoing emergency surgery (p = 0.002). The repair of perforated peptic ulcer was associated with delayed postoperative recovery, too (p < 0.001). Despite randomized trials reported both feasibility and safety of an enhanced recovery protocol after peptic ulcer repair [4, 5], our results probably reflect some reluctance of surgeons to early feed these patients.
The present study has some limitations. Patients were recruited during the second pandemic wave with all the well-known restrictions and changes in hospital admissions and clinical practice. Pandemic affected hospitals’ organizational models making more difficult to have a dedicated equipe for emergency patients management. Moreover, this study is burdened by the intrinsic limits of the emergency setting not allowing a fixed and dedicated team with possible incomplete protocol application. On the other hand, strengths of the study are the large number of patients recruited in one-year period and the wide experience of the participating centers in enhanced recovery practice. There are several areas of possible improvement, such as preoperative hyperglycemia correction, operative fluids optimization, and implementation of minimally-invasive approach.
In conclusion, the present study supports the implementation of enhanced recovery protocols in patients undergoing emergency gastro-intestinal surgery. Multiple regression analysis showed that laparoscopy was associated with an earlier recovery, whereas preoperative hyperglycemia, fluid overload, and abdominal drain were associated with a delayed recovery.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
ERAS-Emergency general surgery collaborative group Francesca Teodora Armao, Michele Ballabio, Marco Longhi, Vincenzo Tripodi: General and Emergency Surgery, ASST Lodi, Lodi, Italy; Andrea Bottari, Sara Riccadonna, Riccardo Somigli: General Surgery, Ospedale S. Jacopo, Pistoia, Italy; Luigi Beretta: Anesthesia and Intensive Care Department, IRCCS Ospedale San Raffaele, Milano, Italy; Chiara Bondi: Anesthesia and Intensive Care, Ospedale S. Jacopo, Pistoia, Italy; Serena Calcinati: Anesthesia and Intensive Care Department, Milano-Bicocca University, School of Medicine and Surgery, Monza, Italy; Michele Carlucci, Arianna Libera Ciravegna, Valentina Tomajer: General and Emergency Surgery, IRCCS Ospedale San Raffaele, Milano, Italy; Massimo Chiarugi, Federico Coccolini, Camilla Cremonini: General, Emergency and Trauma Surgery Unit, University of Pisa, Pisa, Italy; Valerio Cozza, Valeria Fico: Emergency Surgery and Trauma, Fondazione Policlinico Universitario A. Gemelli IRCCS Roma - Universita’ Cattolica del Sacro Cuore, Rome, Italy; Federica Ferraina, Michele Fogliata, Luca Gianotti: General and Emergency Surgery Department, Milano-Bicocca University, School of Medicine and Surgery, Monza, Italy; Paola Germani, Lucia Paiano: General Surgery Department, Cattinara Hospital, ASUGI, Strada di Fiume, Trieste, Italy; Samuele Grandi, Alessia Malagnino, Giovanni Pesenti: Emergency and Robotic Surgery Department, Emergency and General Surgery Unit, A. Manzoni Hospital - ASST Lecco, Italy; Lorenzo Guiotto: Emergency Department, Anesthesiology and Intensive Care Unit, A. Manzoni Hospital - ASST Lecco, Italy; Enrico Lena, Marco Montino: Department of Anesthesia and Intensive Care, Cattinara Hospital, ASUGI, Strada di Fiume, Trieste, Italy; Irene Lorenzi: Emergency Intensive Care Unit, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy; Bruno Romanò, Andrea Russo: Department of Anesthesiology and Intensive Care Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Funding
Open access funding provided by Università degli Studi di Milano - Bicocca within the CRUI-CARE Agreement.
Declarations
Conflict of interest
All the authors have no conflict of interest to declare.
Ethical approval
The study protocol was approved by the Ethical Committee of the promoting center (n. 0012747 08/10/2020) and was registered on clinicaltrial.gov (identifier NCT04648644). Informed consent was obtained from all patients.
Footnotes
The ERAS-emergency surgery collaborative group members are listed in “Acknowledgements” section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marco Ceresoli, Email: marco.ceresoli@unimib.it.
the ERAS-emergency surgery collaborative group:
Francesca Teodora Armao, Andrea Bottari, Michele Ballabio, Luigi Beretta, Chiara Bondi, Serena Calcinati, Michele Carlucci, Massimo Chiarugi, Arianna Libera Ciravegna, Federico Coccolini, Valerio Cozza, Camilla Cremonini, Federica Ferraina, Valeria Fico, Michele Fogliata, Paola Germani, Luca Gianotti, Samuele Grandi, Lorenzo Guiotto, Enrico Lena, Marco Longhi, Irene Lorenzi, Alessia Malagnino, Marco Montino, Lucia Paiano, Giovanni Pesenti, Sara Riccadonna, Bruno Romanò, Andrea Russo, Riccardo Somigli, Valentina Tomajer, and Vincenzo Tripodi
References
- 1.Greco M, Capretti G, Beretta L, et al. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38:1531–1541. doi: 10.1007/s00268-013-2416-8. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Yang J, Chen X, et al. Enhanced recovery after surgery on multiple clinical outcomes. Medicine. 2020;99:e20983. doi: 10.1097/MD.0000000000020983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peden CJ, Aggarwal G, Aitken RJ, et al. Guidelines for perioperative care for emergency laparotomy enhanced recovery after surgery (ERAS) society recommendations: part 1—preoperative: diagnosis, rapid assessment and optimization. World J Surg. 2021;45:1272–1290. doi: 10.1007/s00268-021-05994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonenc M, Dural AC, Celik F, et al. Enhanced postoperative recovery pathways in emergency surgery: a randomised controlled clinical trial. Am J Surg. 2014;207:807–814. doi: 10.1016/j.amjsurg.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Mohsina S, Shanmugam D, Sureshkumar S, et al. Adapted ERAS pathway vs. standard care in patients with perforated duodenal ulcer—a randomized controlled trial. J Gastrointest Surg. 2018;22:107–116. doi: 10.1007/s11605-017-3474-2. [DOI] [PubMed] [Google Scholar]
- 6.Roulin D, Blanc C, Muradbegovic M, et al. Enhanced recovery pathway for urgent colectomy. World J Surg. 2014;38:2153–2159. doi: 10.1007/S00268-014-2518-Y/TABLES/4. [DOI] [PubMed] [Google Scholar]
- 7.Lohsiriwat V. Enhanced recovery after surgery vs conventional care in emergency colorectal surgery. World J Gastroenterol. 2014;20:13950. doi: 10.3748/wjg.v20.i38.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang Y, Guo C, Zhang D. Modified enhanced recovery after surgery protocols are beneficial for postoperative recovery for patients undergoing emergency surgery for obstructive colorectal cancer. Medicine. 2018;97:e12348. doi: 10.1097/MD.0000000000012348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajibandeh S, Hajibandeh S, Bill V, Satyadas T. Meta-analysis of enhanced recovery after surgery (ERAS) protocols in emergency abdominal surgery. World J Surg. 2020;44:1336–1348. doi: 10.1007/s00268-019-05357-5. [DOI] [PubMed] [Google Scholar]
- 10.Paduraru M, Ponchietti L, Casas IM, et al. Enhanced recovery after emergency surgery: a systematic review. Bull Emerg Trauma. 2017;5:70–78. [PMC free article] [PubMed] [Google Scholar]
- 11.Havens JM, Peetz AB, Do WS, et al. The excess morbidity and mortality of emergency general surgery. J Trauma Acute Care Surg. 2015;78:306–311. doi: 10.1097/TA.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 12.Lee KC, Sturgeon D, Lipsitz S, et al. Mortality and health care utilization among medicare patients undergoing emergency general surgery vs those with acute medical conditions. JAMA Surg. 2020 doi: 10.1001/jamasurg.2019.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braga M, Pecorelli N, Scatizzi M, et al. Enhanced recovery program in high-risk patients undergoing colorectal surgery: results from the perioperative italian society registry. World J Surg. 2017;41:860–867. doi: 10.1007/s00268-016-3766-919. [DOI] [PubMed] [Google Scholar]
- 15.Lourenco T, Murray A, Grant A, et al. Laparoscopic surgery for colorectal cancer: safe and effective a systematic review. Surg Endosc Interventional Tech. 2008;22(5):1146–1160. doi: 10.1007/s00464-007-9686-x. [DOI] [PubMed] [Google Scholar]
- 16.King PM, Blazeby JM, Ewings P, et al. Randomized clinical trial comparing laparoscopic and open surgery for colorectal cancer within an enhanced recovery programme. Br J Surg. 2006 doi: 10.1002/bjs.5216. [DOI] [PubMed] [Google Scholar]
- 17.Pucher PH, Mackenzie H, Tucker V, Mercer SJ. A national propensity score-matched analysis of emergency laparoscopic versus open abdominal surgery. Br J Surg. 2021;108:934–940. doi: 10.1093/bjs/znab048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ceresoli M, Pisano M, Abu-Zidan F, et al. Minimally invasive surgery in emergency surgery: a WSES survey. World J Emerg Surg. 2022 doi: 10.1186/s13017-022-00419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messina A, Robba C, Calabrò L, et al. Association between perioperative fluid administration and postoperative outcomes: a 20-year systematic review and a meta-analysis of randomized goal-directed trials in major visceral/noncardiac surgery. Crit Care. 2021;25:43. doi: 10.1186/s13054-021-03464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benes J, Giglio M, Brienza N, Michard F. The effects of goal-directed fluid therapy based on dynamic parameters on post-surgical outcome: a meta-analysis of randomized controlled trials. Crit Care. 2014;18:1–11. doi: 10.1186/s13054-014-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feldheiser A, Aziz O, Baldini G, et al. Enhanced recovery after surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand. 2016;60:289–334. doi: 10.1111/aas.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gemma M, Pennoni F, Braga M. Studying enhanced recovery after surgery (ERAS®) core items in colorectal surgery: a causal model with latent variables. World J Surg. 2021;45:928–939. doi: 10.1007/s00268-020-05940-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon S, Thompson R, Dellinger P, et al. Importance of perioperative glycemic control in general surgery. Ann Surg. 2013;257:8–14. doi: 10.1097/SLA.0b013e31827b6bbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ata A, Lee J, Bestle SL, et al. Postoperative hyperglycemia and surgical site infection in general surgery patients. Arch Surg. 2010;145:858–864. doi: 10.1001/ARCHSURG.2010.179. [DOI] [PubMed] [Google Scholar]
- 25.Rogers SO, Ramos M, Khalpey Z, et al. Relationship of perioperative hyperglycemia and postoperative infections in patients who undergo general and vascular surgery. Ann Surg. 2008;248:585–590. doi: 10.1097/SLA.0B013E31818990D1. [DOI] [PubMed] [Google Scholar]
- 26.Ambiru S, Kato A, Kimura F, et al. Poor postoperative blood glucose control increases surgical site infections after surgery for hepato-biliary-pancreatic cancer: a prospective study in a high-volume institute in Japan. J Hosp Infect. 2008;68:230–233. doi: 10.1016/J.JHIN.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 27.McConnell YJ, Johnson PM, Porter GA. Surgical site infections following colorectal surgery in patients with diabetes: association with postoperative hyperglycemia. J Gastrointest Surg. 2009;13:508–515. doi: 10.1007/S11605-008-0734-1. [DOI] [PubMed] [Google Scholar]
- 28.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78:1471–1478. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 29.Falciglia M, Freyberg RW, Almenoff PL, et al. Hyperglycemia-related mortality in critically ill patients varies with admission diagnosis. Crit Care Med. 2009;37:3001–3009. doi: 10.1097/CCM.0B013E3181B083F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NICE-SUGAR Study Investigators. Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


