Abstract
We tested whether aspects of the childhood/adolescent home environment mediate genetic risk for alcohol problems within families across generations. Parental relationship discord and parental divorce were the focal environments examined. The sample included participants of European Ancestry (N = 4806, 51% female) and African Ancestry (N = 1960, 52% female) from the high-risk Collaborative Study on the Genetics of Alcoholism. Alcohol outcomes in the child generation included lifetime criterion counts for DSM-5 Alcohol Use Disorder (AUD), lifetime maximum drinks in 24 hours, age at initiation of regular drinking, and age at first alcohol intoxication. Predictors in the parent generation included relationship discord, divorce, alcohol measures parallel to those in the child generation, and polygenic scores for alcohol problems. Parental polygenic scores were partitioned into alleles that were transmitted and nontransmitted to the child. The results from structural equation models were consistent with genetic nurture effects in European ancestry families. Exposure to parental relationship discord and parental divorce mediated, in part, the transmission of genetic risk for alcohol problems from parents to children to predict earlier ages regular drinking (βindirect = −0.018 [−0.026, −0.011]) and intoxication (βindirect = −0.015 [−0.023, −0.008]), greater lifetime maximum drinks (βindirect = 0.006 [0.002, 0.01]) and more lifetime AUD criteria (βindirect = 0.011 [0.006, 0.016]). In contrast, there was no evidence that parental alleles had indirect effects on offspring alcohol outcomes via parental relationship discord or divorce in the smaller number of families of African ancestry. In conclusion, parents transmit genetic risk for alcohol problems to their children not only directly, but also indirectly via genetically influenced aspects of the home environment. Further investigation of genetic nurture in non-European samples is needed.
Introduction
Family-based studies were critical to demonstrating the role of genetic factors in the intergenerational transmission of alcohol use disorder (AUD)1 and provided the empirical foundation for subsequent gene identification efforts ranging from linkage to GWAS2. The conventional understanding is that genetic risk is passed in families from parents to children through allele sharing. Yet, allele sharing represents only one potential mode of transmission of genetic risk in families. Indeed, robust evidence from twin and adoption studies indicate that genetic influences also operate “beyond the skin” to shape the environment3. Thus, in addition to direct allele transmission, parental genotypes may influence children’s outcomes indirectly, via the family environment4. Here we examined two common family adversities, parental divorce and parental relationship discord, as mechanisms through which genetic risk for alcohol problems may be transmitted in families.
Parental divorce is common5, and 28.24% of American adults experience parental divorce or permanent non-marital parental separation prior to age 186. Moreover, it is well recognized that many offspring are exposed to parental relationship discord even in the absence of parental divorce7–10. Exposure to parental divorce and relationship discord are associated with AUD11–14 and related outcomes, including earlier age at first drink15–18 and higher substance involvement and misuse19–21. Divorce and relationship discord share genetic influences with alcohol problems22–24. Thus, offspring exposed to parental divorce and relationship discord also inherit a genetic predisposition towards alcohol problems, an example of how genetic inheritance is also associated with environmental exposures25.
In the present study, we used family-based data from an ancestrally diverse, high-risk sample to examine parental divorce and relationship discord as family adversities that mediate genetic risk for alcohol problems. We tested two pre-registered hypotheses26.
Parental divorce and parental relationship discord will be associated with a range of risky alcohol-related outcomes in children: (a) earlier age at alcohol initiation; (b) earlier age at first intoxication (c) a higher reported number of maximum drinks consumed in 24 hours; and (d) endorsement of more of lifetime clinical criteria for alcohol use disorder.
Parental divorce and parental relationship discord will mediate the transmission of genetic risk for alcohol problems from parents to children.
Parental divorce and relationship discord were examined separately in view of prior evidence that both parental marital status and the tenor of their relationship represent separate and unique influences on children’s outcomes27.
Methods
Sample
The sample for this study was drawn from the Collaborative Study on the Genetics of Alcoholism (COGA)17, 28, 29 (dbGaP Study Accession: phs000763.v1.p1). Probands (i.e., index individuals) were identified through alcohol treatment programs at seven U.S. sites. Probands and their families were invited to participate if the family was sufficiently large with two or more members in the COGA catchment areas. Comparison families were recruited from the same communities. The Institutional Review Boards at all data gathering sites approved this study, and written consent was obtained from all participants. Within the larger COGA sample, we selected parents and children (confirmed by genotyping) with relevant phenotypic and genome-wide genotypic data available. This was done separately by European ancestry (EA) and African ancestry (AA) groups, as defined by principal components in GWAS data calculated using SNPrelate30, to avoid population stratification in the downstream analyses incorporating genome-wide polygenic scores31. Analytic sample sizes differed across the phenotypes of interest and ranged from 4321 to 4806 in EAs (51% female, Mage (SD) = 30.72 (9.81) years) and 1616 to 1960 in AAs (52% female, Mage (SD) = 29.04 (8.76) years) and are specified in the results.
Measures
Alcohol use behaviors.
Measures in parents and their children were coded from the Semi-Structured Assessment for the Genetics of Alcoholism Interview (SSAGA)32. Age of initiation of regular drinking (Initiation) was the age at which participants reported first drinking regularly, defined as once a month for 6 months or more. Age of first intoxication (Intoxication) was the age at which participants first reported getting drunk, defined as slurred speech or being unsteady on one’s feet. Maximum drinks (MaxDrinks) was participants’ report of the largest number of drinks consumed in a 24-hour period. AUD lifetime criterion counts (AUDSx) were defined by the Diagnostic and Statistical Manual 5 (DSM-5)33. Those <23 years of age at their last assessment who did not endorse any AUD criteria were set to missing to ensure that participants had passed through the period of highest risk for onset of AUD before being classified as unaffected.
Parental divorce and offspring perceptions of parental relationship discord.
Participants’ retrospective reports of whether their parents were divorced/separated while growing up were coded from the SSAGA32. Participants also retrospectively reported on their perceptions of parental relationship discord34, which included questions about the quality of their parents’ marriage/relationship (a 4-point ordinal scale with response options of excellent, good, fair, or poor); whether their parents usually seemed to enjoy each other (yes or no); whether their parents often argued or fought in front of them (yes or no); whether either of their parents ever hit the other (yes or no); and how much conflict or tension there was in the household (an ordinal item with response options a lot, some, a little, or none). These questions were asked with respect to ages 6–13 for those administered the SSAGA-II (65% and 57% of the EA and AA samples, respectively), and ages 12–17 for those administered the SSAGA-IV (35% and 43% of the EA and AA samples, respectively).
Covariates.
Covariates included gender (coded male and female), age at last assessment, generational cohort (dummy coded using the following scheme from Bourdon et al.35: silent [b. prior to 1946], baby boomer [b. 1946 to 1964], generation X [b. 1965 to 1980], millennial [b. 1981 to 1996]); and the first ten within-ancestry principal components. Principal components are often used to address population stratification in PRS analyses36. Similar to other recent studies37–40 we used 10 ancestry principal components as covariates.
Alcohol problems polygenic scores.
Four different genotyping arrays were used: the Illumina 1M, Illumina OmniExpress 12VI, and Illumina 2.5M (Illumina, San Diego, CA), and Smokescreen (BioRealm LLC, Walnut, CA). Quality control and imputation procedures are described in Lai et al.29. Imputed genotypes for the COGA parent-offspring trios, in combination with summary statistics from independent GWAS discovery samples, were used to construct the genome-wide polygenic risk scores using PRS-CSx41. This procedure uses ancestry-specific discovery sample GWAS weights, paired with linkage disequilibrium information from an ancestry-matched external reference panel, to estimate the posterior effect size for each SNP. Reference panels from the 1000 Genomes Phase III European or African subsamples were used for the EA and AA groups, respectively. For participants of European ancestry, the discovery sample consisted of a meta-analysis of DSM-IV alcohol dependence from the Psychiatric Genomics Consortium (COGA sample removed)42, AUDIT-P from the UKBiobank43, and DSM-5 Alcohol Use Disorder from the Million Veteran Program in individuals of European ancestry44. For participants of African ancestry, GWAS summary statistics were drawn from the meta-analyzed European ancestry summary statistics in tandem with the African ancestry GWAS summary statistics from analyses of DSM-IV alcohol dependence from the Psychiatric Genomics Consortium (COGA sample removed)42 and the African ancestry GWAS summary statistics from analyses of Alcohol Use Disorder derived from ICD codes obtained from electronic health records from the Million Veteran Program44. This approach was informed by evidence that combining African ancestry specific GWAS summary statistics with GWAS summary statistics available from a larger-scale European ancestry sample improves polygenic prediction in an African-ancestry target sample41. Following the calculation of the posterior effect sizes, additive polygenic scores were calculated for transmitted and nontransmitted alleles for mothers and fathers, separately. SNPs where parental origin was ambiguous (e.g., an offspring C/T SNP for parents who are C/T heterozygotes) were removed from the PRS calculation.
Analytic Plan
Parental relationship discord composite.
An exploratory factor analysis (EFA) on the five items related to children’s perceptions of parental relationship discord was conducted to inform the calculation of the parental relationship discord composite. After establishing unidimensional factor structure (see Supplementary Information and Supplementary Tables 1 and 2), a composite measure was calculated by taking the prorated sum of the items for participants who responded to at least 3 items. Binary items were coded 0 or 1. Four-response ordinal items were rescaled to range between 0 and 1 (0, 0.33, 0.66, 1) so that ordinal and binary items were weighted similarly in the prorated sum.
Nature of nurture design.
We used an extended nature of nurture design45, 46, depicted in Figure 1, to examine the mechanisms through which parental genotypes influence offspring outcomes. In this model, paternal (P) and maternal (M) genotypes are partitioned into those that are shared with offspring (i.e., transmitted alleles, T) and those that are not shared with offspring (i.e., nontransmitted alleles, NT). This cross-generational allele sharing information can be used to construct separate genome-wide polygenic scores that represent the portion of each parent’s ‘genetic loading’ for a trait or disorder, such as AUDSx, that is directly transmitted to the offspring, and that which is indirectly transmitted to the offspring via the environment through a ‘genetic nurture’ pathway. We expanded this design to examine whether the parental divorce and relationship discord composite measures mediate both the effects of transmitted and nontransmitted alleles. In addition, we included matching parental alcohol use phenotypes as mediators of the intergenerational transmission of genetic risk.
Figure 1.
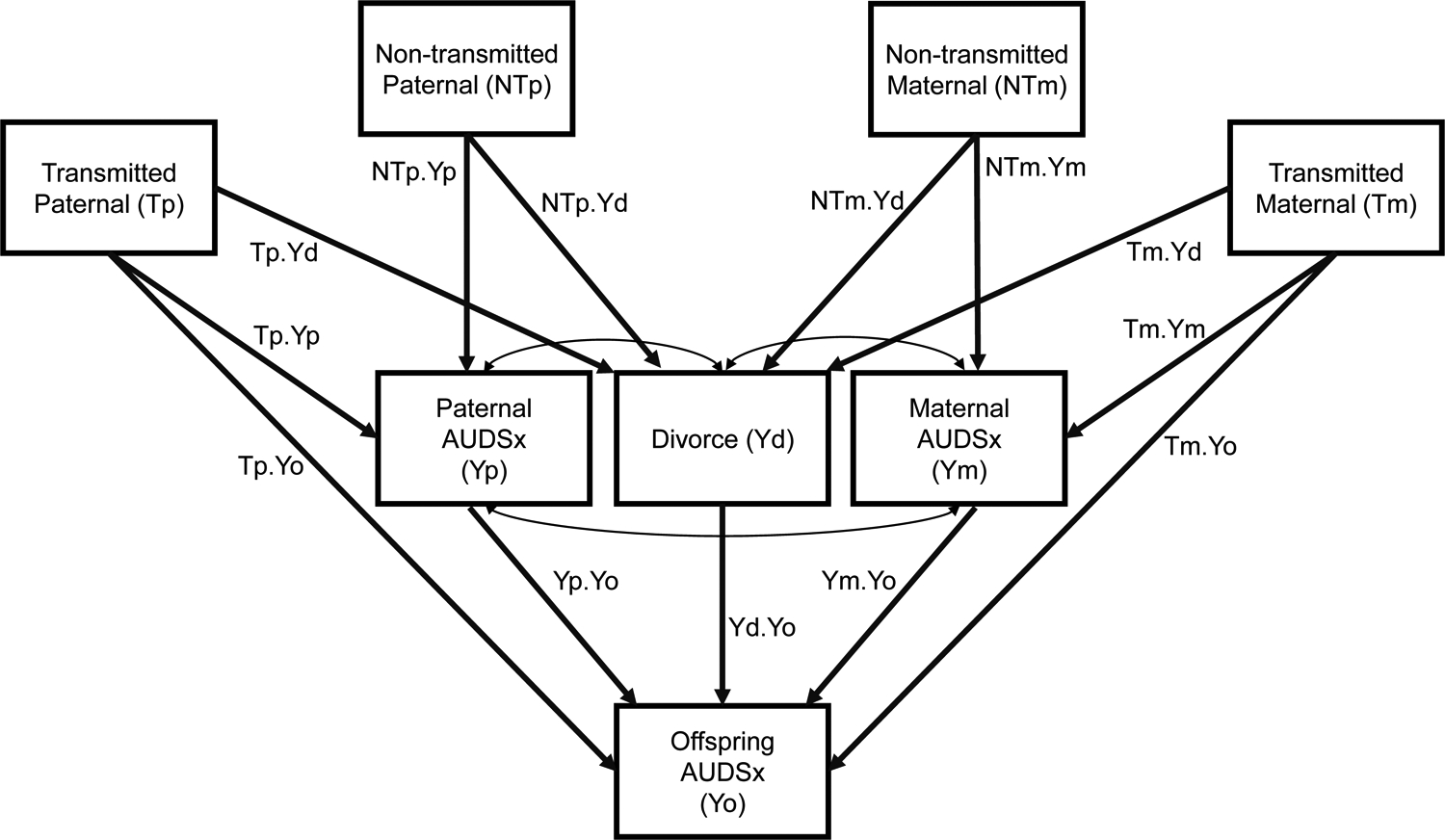
Illustrative extended nature of nurture model for AUDSx (DSM-5 Alcohol Use Disorder Criterion Count) depicting parental divorce and parental AUDSx as mediators of genetic risk across generations. Paternal and maternal genotypes are partitioned into those that are shared with the offspring (i.e., transmitted paternal and alleles, Tp and Tm, respectively) and those that are not shared with offspring (i.e., nontransmitted paternal and maternal alleles, NTp and NTm, respectively). Parental divorce (Yd), paternal AUDSx (Yp), and maternal AUDSx (Ym) are included as mediators, and the outcome is offspring AUDSx (Yo). Transmitted paternal alleles can have a direct effect on offspring AUDSx (Tp.Yo), or mediated (indirect) effects through paternal AUDSx (Tp.Yp × Yp.Yo) or parental divorce (Tp.Yd × Yd.Yo). Nontransmitted paternal alleles can have indirect effects through paternal AUDSx (NTp.Yp × Yp.Yo) or parental divorce (NTp.Yd × Yd.Yo). Corresponding paths exist for mothers, such that transmitted maternal alleles can have a direct effect on offspring AUDSx (Tm.Yo), or mediated (indirect) effects through maternal AUDSx (Tm.Ym × Ym.Yo) or parental divorce (Tm.Ym × Ym.Yd). Nontransmitted maternal alleles can have indirect effects through maternal AUDSx (NTm.Ym × Ym.Yo) or parental divorce (NTm.Yd × Yd.Yo). All paths are estimated simultaneously.
Models were fit using the structural equation modeling package OpenMx47 in R48. A total of 16 models were tested: all combinations of 4 alcohol use phenotypes (AUDSx, MaxDrinks, Initiation, and Intoxication), 2 parental relationship mediators (parental relationship discord and divorce), and 2 ancestry groups (EA and AA).
Offspring alcohol use phenotypes were regressed on mediator variables, the transmitted component of parental polygenic scores, and a series of exogenous covariates (offspring sex, offspring age at last assessment, and offspring 10 within-ancestry principal components). Mediator variables were regressed on the transmitted and nontransmitted components of the parental polygenic scores and the covariates, except offspring sex. Offspring birth cohort was included as a covariate in the models examining parental marital discord as a mediator, but excluded from models examining parental divorce because of problems with model convergence. Parental alcohol use phenotypes were regressed on the corresponding parental polygenic scores (maternal phenotype on maternal polygenic score and paternal phenotype on paternal polygenic score) and the exogenous covariates, except offspring sex. Parental relationship variables (i.e., divorce and discord in the respective models) were regressed on all parental polygenic scores.
Divorce was modeled using a single threshold, identified by fixing the mean to 0, variance to 1, and estimating the threshold. All other variables were treated as continuous and standardized to mean=0, SD=1. Indirect effects were calculated for all paths from parental polygenic scores to offspring alcohol use outcome through mediator variables, for a total of 8 indirect effects per model. Confidence intervals for indirect effects were calculated via bootstrapping with 1000 replications.
Results
Descriptive statistics and correlations for key study variables
Descriptive statistics for the key study variables are summarized in Table 1. EA offspring endorsed an average of 3.74 AUDSx and 20.47 MaxDrinks. Age in years at initiation of regular drinking was 17.14, and age at first intoxication was 16.15. Approximately 17% of the sample experienced parental divorce in childhood/adolescence. AA offspring endorsed an average of 2.61 AUDSx and 16.92 MaxDrinks. Average age at initiation of regular drinking was 18.05 and average age at first intoxication was 17.44 years. Approximately 33% of the sample experienced parental divorce in childhood/adolescence.
Table 1.
Descriptive statistics for key study variables in European and African ancestry participants, overall sample
| EA (Total N = 4846) | AA (Total N = 2005) | ||||||
|---|---|---|---|---|---|---|---|
| N | % or Mean (SD) | N | % or Mean (SD) | ||||
| Birth Cohort | |||||||
| Silent | 110 | 2.27% | 21 | 1.05% | |||
| Baby Boomer | 1495 | 30.85% | 447 | 22.29% | |||
| GenX | 1428 | 29.47% | 569 | 28.38% | |||
| Millenial | 1813 | 37.41% | 968 | 48.28% | |||
| Sex | |||||||
| Male | 2360 | 48.70% | 965 | 48.13% | |||
| Female | 2486 | 51.30% | 1040 | 51.87% | |||
| Age | 4846 | 30.72 | (9.81) | 2005 | 29.04 | (8.76) | |
| Parental Divorce | |||||||
| No | 3153 | 82.76% | 722 | 67.10% | |||
| Yes | 657 | 17.24% | 354 | 32.90% | |||
| Parental Discord | 3364 | 1.58 | (1.47) | 788 | 1.83 | (1.49) | |
| Offspring AUDSx | 4617 | 3.74 | (3.56) | 1919 | 2.61 | (3.23) | |
| Maternal AUDSx | 4549 | 1.94 | (2.98) | 1789 | 2.45 | (3.44) | |
| Paternal AUDSx | 3542 | 4.28 | (3.69) | 957 | 4.65 | (3.84) | |
| Offspring MaxDrinks | 4812 | 20.47 | (16.94) | 1990 | 16.92 | (17.93) | |
| Maternal MaxDrinks | 4490 | 9.59 | (9.88) | 1697 | 11.55 | (13.00) | |
| Paternal MaxDrinks | 3501 | 22.46 | (16.70) | 948 | 26.24 | (24.10) | |
| Offspring Initiation (years) | 4456 | 17.14 | (3.80) | 1695 | 18.05 | (4.02) | |
| Maternal Initiation (years) | 3555 | 22.6 | (9.15) | 1291 | 21.61 | (7.33) | |
| Paternal Initiation (years) | 3330 | 19.22 | (6.15) | 862 | 19.21 | (5.74) | |
| Offspring Intoxication (years) | 4560 | 16.15 | (3.11) | 1738 | 17.44 | (3.67) | |
| Maternal Intoxication (years) | 3720 | 20.58 | (8.00) | 1307 | 20.23 | (7.22) | |
| Paternal Intoxication (years) | 3334 | 17.62 | (4.82) | 870 | 17.87 | (5.69) | |
Note. Table includes participants included in any analysis. Available Ns for each measure are noted; percentages and means correspond to participants with non-missing values. Abbreviations: AUDSx = DSM-5 Alcohol Use Disorder Criterion Count, MaxDrinks = Maximum drinks consumed in 24 hours, Initiation = age at initiation of regular drinking, Intoxication = age at first intoxication.
Zero-order correlations among key study variables are summarized in Supplementary Table 3. Pearson and polyserial correlations are presented for pairs of continuous variables and pairs where one variable is continuous and the other is binary, respectively. Selected effects are summarized here. In the EA sample, parental relationship discord was associated with more AUDSx (r = .18, 95% CI [0.15, 0.22]), higher MaxDrinks (r = 0.09, 95% CI [0.05, 0.12]), earlier Initiation (r = −0.07, 95% CI [−0.10, −0.03]) and earlier Intoxication (r = −0.16, 95% CI [−0.19, −0.12]). Parental divorce was associated with earlier Initiation (r = −0.30, 95% CI [−0.35, −0.24]) and earlier Intoxication (r = −0.17, 95% CI [−0.22, −0.12]), but was not significantly associated with AUDSx (r = −0.02, 95% CI [−0.07, 0.04]) nor MaxDrinks (r = 0.01, 95% CI [−0.04, 0.06]). Parental relationship discord was associated with parental divorce (r = 0.30, 95% CI [0.25, 0.35]).
In the EA sample, both transmitted and nontransmitted maternal alleles were associated with higher offspring AUDSx (r = 0.10, 95% CI [0.07, 0.13] and r = 0.04, 95% CI [0.01, 0.07]) and MaxDrinks (r = 0.07, 95% CI [0.03, 0.10] and r = 0.03, 95% CI [0.00, 0.06]), and offspring earlier age at first intoxication (r = −0.06, 95% CI [−0.09, −0.03] and r = −0.05, 95% CI [−0.08, −0.02]). Transmitted maternal alleles, but not nontransmitted maternal alleles, were associated with earlier age of offspring initiation (r = −0.03, 95% CI [−0.07, 0.00]). Both transmitted and nontransmitted paternal alleles were associated with higher offspring AUDSx (r = 0.11, 95% CI [0.07, 0.15] and r = 0.05, 95% CI [0.01, 0.09]) and MaxDrinks (r = 0.07, 95% CI [0.03, 0.11] and r = 0.04, 95% CI [0.01, 0.08]). Transmitted paternal alleles, but not nontransmitted paternal alleles, were associated with earlier age at intoxication (r = −0.05, 95% CI [−0.09, −0.01]. Neither transmitted nor nontransmitted paternal alleles were associated with offspring age at initiation at a statistically significant level. Transmitted and nontransmitted maternal alleles (r = 0.07, 95% CI [0.03, 0.11] and r = 0.05, 95% CI [0.01, 0.09]) and paternal alleles (r = 0.07, 95% CI [0.03, 0.11] and r = 0.11, 95% CI [0.07, 0.15]) were associated with parental relationship discord. Nontransmitted maternal and paternal alleles were associated with divorce (r = 0.08, 95% CI [0.02, 0.13] and r = 0.13, 95% CI [0.07, 0.20]), but neither maternal nor paternal transmitted alleles were associated with divorce at a statistically significant level.
In the AA sample, parental relationship discord was associated with more AUDSx (r = .10, 95% CI [0.03, 0.17]), but was not significantly associated with MaxDrinks (r = 0.03, 95% CI [−0.04, 0.10]), age at Initiation (r = 0.06, 95% CI [−0.02, 0.14]) or age at Intoxication (r = −0.04, 95% CI [−0.11, 0.04]). Parental divorce was associated with earlier Initiation (r = −0.10, 95% CI [−0.19, −0.02]) and Intoxication (r = −0.10, 95% CI [−0.19, −0.01]), but was not significantly associated with AUDSx (r = −0.06, 95% CI [−0.15, 0.02]) nor MaxDrinks (r = 0.02, 95% CI [−0.05, 0.10]). Parental relationship discord was associated with parental divorce (r = 0.21, 95% CI [0.12, 0.31]).
In the AA sample, nontransmitted maternal alleles were associated with lower offspring AUDSx (r = −0.07, 95% CI [−0.13, −0.02]), and transmitted paternal alleles were associated with higher offspring AUDSx (r = 0.08, 95% CI [0.00, 0.16]) and earlier age at intoxication (r = −0.08, 95% CI [−0.16, 0.00]). No other associations were observed between transmitted or nontransmitted parental alleles and offspring AUDSx, MaxDrinks, age at initiation or age at intoxication. Neither maternal nor paternal transmitted nor nontransmitted alleles were associated with parental relationship discord nor divorce at a statistically significant level.
Nature of nurture modeling
We used an extended nature of nurture model to examine the pathways through which parental genotypes influenced offspring alcohol outcomes. All paths shown in Figure 1 were modeled simultaneously and the full results are presented in Supplementary Tables 4–7 for the AUDSx, MaxDrinks, Initiation, and Intoxication outcomes separately by ancestry. Descriptive statistics for each analytic sample are presented in Supplementary Tables 8–11. In what follows, we focus on the indirect pathways of primary interest: parental relationship discord and divorce as mediators of transmitted and nontransmitted parental and maternal alleles on offspring alcohol outcomes, and paternal and maternal alcohol use behaviors as mediators of the effects of transmitted and nontransmitted paternal and maternal alleles on offspring’s matched alcohol outcomes.
Indirect genetic effects through parental relationship discord and divorce.
Table 2 summarizes the indirect effects of transmitted and nontransmitted paternal and maternal alleles on offspring alcohol outcomes via parental relationship discord and divorce for each ancestry group, separately. These effects correspond to paths Tp.Yd × Yd.Yo, NTp.Yd × Yd.Yo, Tm.Ym × Ym.Yd, NTm.Yd × Yd.Yo in Figure 1. Among EA participants, there was evidence that nontransmitted paternal and transmitted maternal alleles had indirect genetic effects on offspring AUDSx, MaxDrinks, Initiation, and Intoxication through parental relationship discord. Nontransmitted paternal and nontransmitted maternal alleles had indirect effects on offspring Initiation and Intoxication through parental divorce.
Table 2.
Parental relationship discord and divorce as mediators of transmitted and nontransmitted paternal and maternal alleles on offspring alcohol outcomes in European and African ancestry families
| Parental relationship discord as mediator | |||||
|---|---|---|---|---|---|
| TransmittedPaternal via discord β [95% CI] |
NontransmittedPaternal via discord β [95% CI] |
TransmittedMaternal via discord β [95% CI] |
NontransmittedMaternal via discord β [95% CI] |
||
| European Ancestry | AUDSx | 0.002 [−0.002, 0.006] |
0.011
[0.006, 0.016] |
0.007
[0.003, 0.011] |
0.003 [−0.001, 0.006] |
| MaxDrinks | 0.002 [−0.001, 0.004] |
0.006
[0.002, 0.01] |
0.003
[0.00, 0.005] |
0.001 [−0.001, 0.003] |
|
| Initiation | −0.002 [−0.005, 0.001] |
−0.009
[−0.014, −0.005] |
−0.005
[−0.008, −0.002] |
−0.001 [−0.004, 0.002] |
|
| Intoxication | −0.005 [−0.011, 0.00] |
−0.016
[−0.022, −0.01] |
−0.009
[−0.014, −0.003] |
−0.004 [−0.008, 0.001] |
|
| African Ancestry | AUDSx | 0.005 [−0.004, 0.014] |
0.001 [−0.004, 0.007] |
−0.004 [−0.011, 0.003] |
−0.001 [−0.006, 0.004] |
| MaxDrinks | −0.001 [−0.007, 0.005] |
0.00 [−0.004, 0.004] |
0.00 [−0.004, 0.005] |
0.00 [−0.003, 0.003] |
|
| Initiation | 0.009 [−0.003, 0.021] |
0.003 [−0.005, 0.01] |
−0.002 [−0.008, 0.004] |
−0.001 [−0.008, 0.005] |
|
| Intoxication | −0.001 [−0.009, 0.007] |
0.00 [−0.005, 0.004] |
0.00 [−0.003, 0.004] |
0.00 [−0.003, 0.003] |
|
| Parental divorce as mediator | |||||
| TransmittedPaternal via divorce β [95% CI] |
NontransmittedPaternal Via divorce β [95% CI] |
TransmittedMaternal via divorce β [95% CI] |
NontransmittedMaternal via divorce β [95% CI] |
||
| European Ancestry | AUDSx | 0.00 [−0.002, 0.001] |
0.001 [−0.008, 0.01] |
0.00 [−0.002, 0.003] |
0.00 [−0.003, 0.003] |
| MaxDrinks | 0.00 [−0.001, 0.002] |
0.004 [−0.002, 0.01] |
0.001 [−0.001, 0.003] |
0.001 [−0.001, 0.004] |
|
| Initiation | −0.003 [−0.01, 0.004] |
−0.018
[−0.026, −0.011] |
−0.005 [−0.01, 0.001] |
−0.009
[−0.015, −0.002] |
|
| Intoxication | −0.004 [−0.01, 0.003] |
−0.015
[−0.023, −0.008] |
−0.002 [−0.007, 0.003] |
−0.008
[−0.013, −0.002] |
|
| African Ancestry | AUDSx | −0.006 [−0.019, 0.007] |
0.003 [−0.008, 0.013] |
−0.002 [−0.011, 0.007] |
−0.001 [−0.01, 0.008] |
| MaxDrinks | −0.005 [−0.02, 0.009] |
−0.004 [−0.017, 0.01] |
−0.006 [−0.018, 0.005] |
−0.003 [−0.013, 0.007] |
|
| Initiation | 0.00 [−0.005, 0.005] |
0.00 [−0.004, 0.004] |
0.00 [−0.003, 0.003] |
0.00 [−0.003, 0.003] |
|
| Intoxication | 0.005 [−0.006, 0.016] |
0 [−0.009, 0.01] |
0.001 [−0.007, 0.008] |
0.001 [−0.006, 0.008] |
|
Notes. β estimates represent the mediated (indirect) effects of transmitted and nontransmitted paternal and maternal genotypes on offspring alcohol outcomes through parental relationship discord (top panel) and parental divorce (bottom panel). These mediated effects correspond to paths Tp.Yd × Yd.Yo, NTp.Yd × Yd.Yo, Tm.Ym × Ym.Yd, NTm.Yd × Yd.Yo depicted in Figure 1. Bold indicates p < 0.05. Abbreviations: AUDSx = DSM-5 Alcohol Use Disorder Criterion Count, MaxDrinks = Maximum drinks consumed in 24 hours, Initiation = age at initiation of regular drinking, Intoxication = age at first intoxication.
Among AA participants, there was no evidence that transmitted or nontransmitted parental alleles had indirect effects on offspring alcohol outcomes via parental relationship discord or divorce.
Indirect genetic effects through parental phenotypes.
Table 3 summarizes the indirect effects of transmitted and nontransmitted paternal and maternal alleles on offspring alcohol outcomes via parental alcohol use behaviors for each ancestry group, separately. These indirect effects correspond to paths Tp.Yp × Yp.Yo, NTp.Yp × Yp.Yo, Tm.Ym × Ym.Yo, NTm.Ym × Ym.Yo in Figure 1 and were calculated for the parental relationship discord and parental divorce models separately. The pattern of results was largely the same across the parental discord and divorce models and are thus summarized holistically.
Table 3.
Paternal and maternal alcohol use behaviors as mediators of transmitted and nontransmitted paternal and maternal alleles on offspring alcohol outcomes in European and African ancestry families
| Genetic effects mediated through parental alcohol phenotype (from parental relationship discord model) | |||||||
|---|---|---|---|---|---|---|---|
| TransmittedPaternal via paternal ALC β [95% CI] |
NontransmittedPaternal via paternal ALC β [95% CI] |
TransmittedMaternal via maternal ALC β [95% CI] |
NontransmittedMaternal via maternal ALC β [95% CI] |
||||
| European Ancestry | AUDSx |
0.007
[0.004, 0.011] |
0.015
[0.009, 0.021] |
0.009
[0.006, 0.013] |
0.016
[0.011, 0.021] |
||
| MaxDrinks |
0.005
[0.002, 0.009] |
0.01
[0.006, 0.014] |
0.011
[0.007, 0.015] |
0.012
[0.008, 0.017] |
|||
| Initiation |
−0.004
[−0.007, −0.0001] |
−0.003
[−0.005, −0.0002] |
−0.003
[−0.005, −0.0001] |
−0.003
[−0.006, −0.0002] |
|||
| Intoxication | −0.001 [−0.002, 0.00] |
−0.002 [−0.004, 0.00] |
0.00 [−0.002, 0.003] |
−0.004
[−0.007, −0.001] |
|||
| African Ancestry | AUDSx | 0.001 [−0.002, 0.005] |
0.005 [−0.002, 0.011] |
−0.005
[−0.009, −0.001] |
0.009
[0.003, 0.015] |
||
| MaxDrinks | 0.001 [−0.005, 0.008] |
0.006 [−0.002, 0.014] |
−0.001 [−0.004, 0.002] |
0.008
[0.003, 0.013] |
|||
| Initiation | 0.00 [−0.002, 0.002] |
0.00 [−0.005, 0.005] |
−0.002 [−0.006, 0.002] |
0.002 [−0.002, 0.007] |
|||
| Intoxication | 0.00 [−0.003, 0.003] |
−0.002 [−0.012, 0.007] |
0.001 [−0.003, 0.006] |
−0.002 [−0.007, 0.003] |
|||
| Genetic effects mediated through parental alcohol phenotype (from parental divorce model) | |||||||
| TransmittedPaternal via paternal ALC β [95% CI] |
NontransmittedPaternal via paternal ALC β [95% CI] |
TransmittedMaternal via maternal ALC β [95% CI] |
NontransmittedMaternal via maternal ALC β [95% CI] |
||||
| European Ancestry | AUDSx |
0.011
[0.006, 0.016] |
0.023
[0.016, 0.03] |
0.009
[0.005, 0.012] |
0.015
[0.009, 0.02] |
||
| MaxDrinks |
0.006
[0.003, 0.01] |
0.012
[0.007, 0.016] |
0.01
[0.006, 0.013] |
0.011
[0.007, 0.015] |
|||
| Initiation | −0.003 [−0.006, 0.001] |
−0.002
[−0.005, 0.00] |
0.00 [−0.002, 0.002] |
0.00 [−0.002, 0.002] |
|||
| Intoxication | 0.00 [−0.002, 0.001] |
−0.001 [−0.003, 0.001] |
0.00 [−0.001, 0.002] |
−0.002
[−0.004, 0.00] |
|||
| African Ancestry | AUDSx | 0.001 [−0.002, 0.004] |
0.003 [−0.004, 0.009] |
−0.004
[−0.007, 0.00] |
0.007
[0.002, 0.012] |
||
| MaxDrinks | 0.001 [−0.004, 0.005] |
0.003 [−0.002, 0.009] |
−0.001 [−0.003, 0.002] |
0.005
[0.0005, 0.01] |
|||
| Initiation | 0.00 [−0.002, 0.002] |
−0.001 [−0.005, 0.004] |
−0.001 [−0.003, 0.002] |
0.001 [−0.002, 0.004] |
|||
| Intoxication | 0.001 [−0.003, 0.005] |
−0.004 [−0.013, 0.006] |
0.00 [−0.004, 0.004] |
0.00 [−0.005, 0.005] |
|||
Notes. β estimates represent the mediated (indirect) effects of transmitted and nontransmitted paternal and maternal genotypes on offspring alcohol outcomes through the corresponding parental alcohol use behavior. These mediated effects correspond to paths Tp.Yp × Yp.Yo, NTp.Yp × Yp.Yo, Tm.Ym × Ym.Yo, NTm.Ym × Ym.Yo depicted in Figure 1. Bold indicates p < 0.05. Abbreviations: AUDSx = DSM-5 Alcohol Use Disorder Criterion Count, MaxDrinks = Maximum drinks consumed in 24 hours, Initiation = age at initiation of regular drinking, Intoxication = age at first alcohol intoxication.
Among EA participants, there was evidence that both transmitted and nontransmitted alleles had indirect genetic effects on offspring AUDSx, Max Drinks, and Initiation through the corresponding parental alcohol phenotypes. For Intoxication, only maternal nontransmitted alleles had significant indirect effects on offspring Intoxication through maternal Intoxication.
Among AA participants, maternal (but not paternal) transmitted and nontransmitted alleles had significant indirect effects on offspring AUDSx mediated through maternal AUDSx. Maternal nontransmitted alleles had significant indirect effects on offspring MaxDrinks through maternal MaxDrinks. There was no evidence that parental genotypes had indirect effects on offspring Initiation or Intoxication through the corresponding parental alcohol phenotypes.
Discussion
Using extended nature of nurture methods, we examined parental relationship discord and parental divorce as mechanisms through which genetic risk for alcohol problems is transmitted in families. We comment on three key features of our findings. First, in European ancestry families, we found a pattern of effects consistent with our hypothesis that parental genotypes for alcohol problems impact a range of their children’s alcohol use behaviors indirectly through the environment. Specifically, nontransmitted paternal alleles and transmitted maternal alleles for alcohol problems were associated with greater parental relationship discord, which in turn was associated with increases in offspring alcohol use disorder clinical criteria, and other indicators of potential problem use, including greater maximum drinks, and an earlier age at initiation and first alcohol intoxication. Parental divorce also mediated the effects of paternal and maternal nontransmitted alleles for alcohol problems on children’s age at initiation and age at first intoxication, but not AUD clinical criterion counts nor maximum drinks.
This pattern of effects is consistent with prior evidence that there are overlapping sets of genetic influences that contribute to alcohol problems, relationship discord, and divorce22, and indicates that even nontransmitted alleles influence offspring alcohol outcomes via parental relationship discord. It is notable that indirect genetic effects mediated through parental relationship discord were observed across the range of offspring alcohol use behaviors ranging from initiation to meeting clinical criteria. In contrast, indirect genetic effects mediated through parental divorce were observed for initiation and intoxication only. This suggests that while both parental relationship discord and divorce are involved in the pathways to alcohol use and misuse, there may be important differences in these factors in terms of risk for clinically significant alcohol use problems. This may reflect the more acute disruptions to parenting associated with divorce (e.g., lower parental monitoring) that facilitate adolescents’ access to alcohol15 versus the potentially more pervasive effects of exposure to high levels of parental relationship discord7.
Second, our observations that parental relationship discord and divorce mediated genetic influences on a range of alcohol outcomes across generations were robust to another highly plausible mediational path through parental alcohol use behaviors. Extended twin family and adoption studies have repeatedly shown that parental alcohol use behaviors capture genetic and environmental risk for offspring49. Using molecular genetic data, which permits the decomposition of parental genotypes into alleles that are transmitted and nontransmitted, we built on prior evidence from latent genetic studies to demonstrate that parents’ own alcohol use behaviors represent a type of environmental inheritance. In European ancestry families, nontransmitted maternal and paternal alleles for alcohol problems were associated with parental AUD clinical criterion counts, which were in turn associated with a greater number of AUD criteria and maximum drinks in the child generation. Maternal age at first intoxication also mediated the effects of nontransmitted maternal alleles on children’s age at first intoxication.
Third, our study is the first (to our knowledge) to include families of African ancestry in analyses of the environmental mechanisms that transmit genetic risk for alcohol problems across generations. We found a pattern of effects consistent with environmental inheritance whereby maternal AUD clinical criterion counts mediated the effects of nontransmitted maternal alleles on AUD clinical criterion counts and maximum drinks in the child generation. However, we did not find support for the hypothesis that parental relationship discord nor divorce mediated genetic influences on offspring alcohol outcomes in our subsample of African ancestry families. These null effects may be attributable to reduced statistical power due the smaller number of families in this subsample, and/or the limited predictive power of polygenic risk scores in African ancestry populations due to historic underrepresentation in genome-wide association studies. Consistent with this possibility, zero-order correlations indicated very few statistically significant associations between parental alleles and offspring alcohol outcomes, and no statistically significant associations between parental alleles and relationship discord and divorce. In view of these weak polygenic associations, and as with any nonsignificant result, we caution against overinterpreting our null indirect effects as evidence that parental relationship discord and divorce are not mechanisms through which genetic risk for alcohol problems is transmitted across generations in African ancestry families. Rather, the pattern of evidence observed here highlights the challenges to partitioning already weak associations, and the need for larger sample sizes to establish a more robust genetic signal for alcohol problems in African ancestry populations. It also worth noting that others have documented racial/ethnic differences in the associations of alcohol use outcomes with a range of adverse childhood experiences19, 50, further underscoring the importance of considering racial/ethnic differences in efforts to understand how genetic and environmental factors come together to influence alcohol problems. Accordingly, our findings should be considered as initial evidence, and clearly additional research on the intergenerational transmission of alcohol problems in diverse populations is warranted.
It is worth commenting on the magnitude of the observed effects. The variance accounted for by genome-wide polygenic scores for alcohol problems and related substance use behaviors remains very modest (< 1% of the variance)39. We note that in genetic nurture analyses for tobacco use, Saunders et al.51 reported indirect effect sizes of a magnitude comparable to our findings for alcohol phenotypes. The modest predictive ability of the alcohol problems polygenic score also cautions against overinterpreting the parent-of-origin effects, particularly in view of the observation that the maternal and paternal effects were typically of the same magnitude and direction of effect.
Limitations
Our results should be interpreted within the context of the following limitations. First, COGA is a high-risk sample and findings may not generalize to other populations. Second, the measure of parental relationship discord is retrospective and reported from the child’s perspective. Others report moderate correlations between children’s and parents’ perceptions of relationship discord52. Additionally, and as shown in Supplementary Table 12, mean parental relationship discord was higher among subjects who provided retrospective reports in SSAGA-II (age 6–13) compared to subjects who provided reports in SSAGA-IV (age 12–17), although the differences were modest (e.g., 1.74 vs. 1.19 in EAs, and 2.02 vs. 1.44 in AAs). Third, strict interpretations of mediated effects require that the predictor precedes the mediator in time. In this study, we modeled lifetime phenotypes and our results are not meant to be interpreted causally. Fourth, our conceptual model emphasizes a direction of influence from parents→offspring; however, we recognize that there are offspring effects on parents53, 54. If the offspring phenotype directly influences the parental phenotype, transmitted parental alleles and non-transmitted parental alleles provide quantitatively different contributions to the parental phenotype. Transmitted and non-transmitted alleles (i.e., all parental alleles) influence the parental phenotype directly. Transmitted alleles would also influence the parental phenotype indirectly through the offspring phenotype (Tp.Yo × Yp.Yo and Tm.Yo × Ym.Yo in Figure 1). This indirect path is not accounted for in our model because parental phenotypes are not regressed on offspring phenotypes. As a result, any covariance between transmitted alleles and the parental phenotype that would be explained by this indirect relationship is represented in the direct path from transmitted parental alleles. This would inflate the path from transmitted parental alleles to the parental phenotype, without affecting the path from non-transmitted parental alleles to the parental phenotype. Thus, if children’s effects on parents were a major confound, we might expect larger indirect genetic effects for transmitted compared to nontransmitted alleles. However, the indirect genetic effects were largely of the same magnitude for transmitted and nontransmitted alleles, reducing this concern. Fifth, although we statistically controlled for the parents’ matched alcohol phenotype in all analyses, we recognize that other unmeasured parental characteristics may also impact children’s alcohol outcomes.
Conclusions
In a sample of families densely affected by alcohol use disorder, we found evidence for genetic nurture effects. Both transmitted and nontransmitted alleles were associated with increased risk of exposure to parental relationship discord and divorce, which were in turn associated with riskier alcohol outcomes ranging from earlier age at initiation to likelihood of developing alcohol use disorder symptoms. Genetic nurture effects were more pronounced in EA families than in AA families. The results underscore that the genetic risk for alcohol problems may be transmitted across generations “beyond the skin” through exposure to adverse family experiences.
Supplementary Material
Acknowledgements
The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators B. Porjesz, V. Hesselbrock, T. Foroud; Scientific Director, A. Agrawal; Translational Director, D. Dick, includes eleven different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, T. Foroud, Y. Liu, M. Plawecki); University of Iowa Carver College of Medicine (S. Kuperman, J. Kramer); SUNY Downstate Health Sciences University (B. Porjesz, J. Meyers, C. Kamarajan, A. Pandey); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield, R. Hart, J. Salvatore); The Children’s Hospital of Philadelphia, University of Pennsylvania (L. Almasy); Virginia Commonwealth University (D. Dick); Icahn School of Medicine at Mount Sinai (A. Goate, P. Slesinger); and Howard University (D. Scott). Other COGA collaborators include: L. Bauer (University of Connecticut); J. Nurnberger Jr., L. Wetherill, X., Xuei, D. Lai, S. O’Connor, (Indiana University); G. Chan (University of Iowa; University of Connecticut); D.B. Chorlian, J. Zhang, P. Barr, S. Kinreich, G. Pandey (SUNY Downstate); N. Mullins (Icahn School of Medicine at Mount Sinai); A. Anokhin, S. Hartz, E. Johnson, V. McCutcheon, S. Saccone (Washington University); J. Moore, Z. Pang, S. Kuo (Rutgers University); A. Merikangas (The Children’s Hospital of Philadelphia and University of Pennsylvania); F. Aliev (Virginia Commonwealth University); H. Chin and A. Parsian are the NIAAA Staff Collaborators. We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co-PI of COGA, and also owe a debt of gratitude to other past organizers of COGA, including Ting- Kai Li, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA). Additional support for this project came from NIAAA award R01AA028064 to JES. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
COGA Collaborators
Bernice Porjesz4, Victor Hesselbrock5, Tatiana Foroud10, Arpana Agrawal3, Danielle M. Dick2, Howard J. Edenberg7, Yunlong Liu11, Martin H. Plawecki8, Samuel Kuperman6, John R. Kramer6, Jacquelyn M. Meyers4, Chella Kamarajan4, Ashwini Pandey4, Laura Bierut3, John Rice3, Kathleen K. Bucholz3, Marc A. Schuckit9, Jay Tischfield12, Ronald Hart13, Jessica E. Salvatore2, Laura Almasy14, Alison Goate15, Paul Slesinger16, Denise Scott17
10. Department of Medical & Molecular Genetics, Indiana University
11. Departments of Medical & Molecular Genetics, Biostatistics, and BioHealth Informatics, Indiana University, Indianapolis, IN, USA
12. Department of Genetics, Rutgers University, Piscataway, NJ, USA
13. Department of Cell Biology and Neuroscience, Rutgers University, Piscataway, NJ, USA
14. The Children’s Hospital of Philadelphia, University of Pennsylvania, Philadelphia, PA, USA
15. Departments of Genetics and Genomic Sciences, Neuroscience, and Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
16. Departments of Neuroscience and Pharmacological Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA
17. Department of Pediatrics, Howard University, Washington DC, USA
Conflict of Interest
None
References
- 1.Merikangas KR, Leckman JF, Prusoff BA, Pauls DL, Weissman MM. Familial transmission of depression and alcoholism. Arch Gen Psychiat 1985; 42: 367–372. [DOI] [PubMed] [Google Scholar]
- 2.Hart AB, Kranzler HR. Alcohol dependence genetics: lessons learned from genome-wide association studies (GWAS) and post-GWAS analyses. Alcohol Clin Exp Res 2015; 39(8): 1312–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kendler KS, Baker JH. Genetic influences on measures of the environment: a systematic review. Psychol Med 2007; 37(5): 615–626. [DOI] [PubMed] [Google Scholar]
- 4.Hart SA, Little C, van Bergen E. Nurture might be nature: cautionary tales and proposed solutions. NPJ Sci Learn 2021; 6(1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green JG. Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication: associations with first onset of DSM-IV disorders. Arch Gen Psychiat 2010; 67(2): 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giano Z, Wheeler DL, Hubach RD. The frequencies and disparities of adverse childhood experiences in the U.S. BMC Public Health 2020; 20(1): 1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amato PR, Spencer Loomis L, Booth A. Parental divorce, marital conflict, and offspring well-being during early adulthood. Soc Forces 1995; 73(3): 895–915. [Google Scholar]
- 8.Emery RE. Interparental conflict and the children of discord and divorce. Psychol Bull 1982; 92(2): 310–330. [PubMed] [Google Scholar]
- 9.Musick K, Meier A. Are both parents always better than one? Parental conflict and young adult well-being. Soc Sci Res 2010: 814–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amato PR, Kane JB. Parents’ marital distress, divorce, and remarriage: Links with daughters’ early family formation transitions. J Fam Issues 2011; 32(8): 1073–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson RG Jr., Lizardi D, Keyes KM, Hasin DS. Childhood or adolescent parental divorce/separation, parental history of alcohol problems, and offspring lifetime alcohol dependence. Drug Alcohol Depend 2008; 98(3): 264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson RG, Alonzo D, Hasin DS. Parental divorce, maternal-paternal alcohol problems, and adult offspring lifetime alcohol dependence. J Soc Work Pract Addict 2013; 13(3): 295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med 1997; 27(5): 1101–1119. [DOI] [PubMed] [Google Scholar]
- 14.Meyers JL, Sartor CE, Werner KB, Koenen KC, Grant BF, Hasin D. Childhood interpersonal violence and adult alcohol, cannabis, and tobacco use disorders: variation by race/ethnicity? Psychol Med 2018; 48(9): 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson KM, Rogers ML, Sartor CE. Parental divorce and initiation of alcohol use in early adolescence. Psychol Addict Behav 2016; 30(4): 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waldron M, Watkins NK, Bucholz KK, Madden PAF, Heath AC. Interactive effects of maternal alcohol problems and parental separation on timing of daughter’s first drink. Alcohol Clin Exp Res 2018; 42(1): 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bucholz KK, McCutcheon VV, Agrawal A, Dick DM, Hesselbrock VM, Kramer JR et al. Comparison of parent, peer, psychiatric, and cannabis use influences across stages of offspring alcohol involvement: Evidence from the COGA Prospective Study. Alcohol Clin Exp Res 2017; 41(2): 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant JD, Waldron M, Sartor CE, Scherrer JF, Duncan AE, McCutcheon VV et al. Parental separation and offspring alcohol involvement: findings from offspring of alcoholic and drug dependent twin fathers. Alcohol Clin Exp Res 2015; 39(7): 1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waldron M, Vaughan EL, Bucholz KK, Lynskey MT, Sartor CE, Duncan AE et al. Risks for early substance involvement associated with parental alcoholism and parental separation in an adolescent female cohort. Drug Alcohol Depend 2014; 138: 130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aro H. Parental discord, divorce and adolescent development. Eur Arch Psychiatry Clin Neurosci 1988; 237: 106–1111. [DOI] [PubMed] [Google Scholar]
- 21.Huurre T, Lintonen T, Kaprio J, Pelkonen M, Marttunen M, Aro H. Adolescent risk factors for excessive alcohol use at age 32 years. A 16-year prospective follow-up study. Soc Psychiatry Psychiatr Epidemiol 2010; 45(1): 125–134. [DOI] [PubMed] [Google Scholar]
- 22.Salvatore JE, Larsson Lönn S, Sundquist J, Lichtenstein P, Sundquist K, Kendler K. Alcohol use disorder and divorce: evidence for a genetic correlation in a population-based Swedish sample. Addiction 2017; 112(4): 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvatore JE, Prom-Wormley E, Prescott C, Kendler KS. Overlapping genetic and environmental influences among men’s alcohol consumption and problems, romantic quality, and social support. Psychol Med 2015; 45(11): 2353–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salvatore JE, Larsson Lönn S, Sundquist J, Sundquist K, Kendler KS. Genetics, the rearing environment, and the intergenerational transmission of divorce: a Swedish national adoption study. Psychol Sci 2018; 29(3): 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarr S, McCartney K. How people make their own environments: a theory of genotype greater than environment effects. Child Dev 1983; 54(2): 424–435. [DOI] [PubMed] [Google Scholar]
- 26.Parental divorce and relationship discord as environmental mediators of genetic risk for alcohol problems in offspring [Open Science Framework pre-registration]. Retrieved from osf.io/m27uc, 2021, April 2, Accessed Date Accessed 2021, April 2 Accessed.
- 27.Gager CT, Yabiku ST, Linver MR. Conflict or Divorce? Does Parental Conflict and/or Divorce Increase the Likelihood of Adult Children’s Cohabiting and Marital Dissolution? Marriage & Family Review 2015; 52(3): 243–261. [Google Scholar]
- 28.Begleiter H, Reich T, Hesselbrock V, Porjesz B, Li TK, Schuckit MA et al. The Collaborative Study on the Genetics of Alcoholism. Alcohol Health Res W 1995; 19: 228–236. [PMC free article] [PubMed] [Google Scholar]
- 29.Lai D, Wetherill L, Bertelsen S, Carey CE, Kamarajan C, Kapoor M et al. Genome-wide association studies of alcohol dependence, DSM-IV criterion count and individual criteria. Genes Brain Behav 2019; 18(6): e12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng X, Levine D, Shen J, Gogarten SM, Laurie C, Weir BS. A high-performance computing toolset for relatedness and principal component analysis of SNP data. Bioinformatics 2012; 28(24): 3326–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet 2003; 361: 598–604. [DOI] [PubMed] [Google Scholar]
- 32.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JL et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: A report on the reliability of the SSAGA. J Stud Alcohol 1994; 55(2): 149–158. [DOI] [PubMed] [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fifth edition. 5th edn. American Psychiatric Publishing: Arlington, VA, 2013. [Google Scholar]
- 34.Holmes SJ, & Robins LN The role of parental disciplinary practices in the development of depression and alcoholism. Psychiatry: Interpersonal and Biological Processes 1988; 51(1): 24–36. [DOI] [PubMed] [Google Scholar]
- 35.Bourdon JL, Tillman R, Francis MW, Dick DM, Stephenson M, Kamarajan C et al. Characterization of Service Use for Alcohol Problems Across Generations and Sex in Adults With Alcohol Use Disorder. Alcohol Clin Exp Res 2020; 44(3): 746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi SW, Mak TS, O’Reilly PF. Tutorial: a guide to performing polygenic risk score analyses. Nature protocols 2020; 15(9): 2759–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barr PB, Ksinan A, Su J, Johnson EC, Meyers JL, Wetherill L et al. Using polygenic scores for identifying individuals at increased risk of substance use disorders in clinical and population samples. Transl Psychiatry 2020; 10(1): 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Easey KE, Wootton RE, Sallis HM, Haan E, Schellhas L, Munafò MR et al. Characterization of alcohol polygenic risk scores in the context of mental health outcomes: Within-individual and intergenerational analyses in the Avon Longitudinal Study of Parents and Children. Drug Alcohol Depend 2021; 221: 108654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnson EC, Sanchez-Roige S, Acion L, Adams MJ, Bucholz KK, Chan G et al. Polygenic contributions to alcohol use and alcohol use disorders across population-based and clinically ascertained samples. Psychol Med 2021; 51(7): 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai D, Johnson EC, Colbert S, Pandey G, Chan G, Bauer L et al. Evaluating risk for alcohol use disorder: Polygenic risk scores and family history. Alcohol Clin Exp Res 2022; 46(3): 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruan Y, Anne Feng Y-C, Chen C-Y, Lam M, Sawa A, Martin AR et al. Improving polygenic prediction in ancestrally diverse populations. Nat Genet 2022; 54(5): 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE et al. Trans-ancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nature Neuroscience 2018; 21(12): 1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez-Roige S, Fontanillas P, Elson SL, 23andMe Research Team, Gray JC, de Wit H et al. Genome-wide association study of Alcohol Use Disorder Identification Test (AUDIT) scores in 20 328 research participants of European ancestry. Addict Biol 2017; 24(1): 121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kranzler H, Zhou H, Kember R, Smith RV, Justice A, Damrauer S et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nature Commun 2019; 10(1): 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bates TC, Maher BS, Medland SE, McAloney K, Wright MJ, Hansell NK et al. The nature of nurture: using a virtual-parent design to test parenting effects on children’s educational attainment in genotyped families. Twin Res Hum Genet 2018; 21(2): 73–83. [DOI] [PubMed] [Google Scholar]
- 46.Kong A, Thorleifsson G, Frigge ML, Vilhjalmsson BJ, Young AI, Thorgeirsson TE et al. The nature of nurture: effects of parental genotypes. Science 2018; 359(6374): 424–428. [DOI] [PubMed] [Google Scholar]
- 47.Neale MC, Hunter MD, Pritikin JN, Zahery M, Brick TR, Kirkpatrick RM et al. OpenMx 2.0: extended structural equation and statistical modeling. Psychometrika 2016; 81(2): 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.R: A language and environment for statistical computing. https://www.R-project.org/, 2021, Accessed Date Accessed 2021 Accessed.
- 49.Kendler KS, Ji J, Edwards AC, Ohlsson H, Sundquist J, Sundquist K. An extended Swedish national adoption study of alcohol use disorder. JAMA Psychiatry 2015; 72(3): 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Werner KB, Grant JD, McCutcheon VV, Madden PA, Heath AC, Bucholz KK et al. Differences in childhood physical abuse reporting and the association between CPA and alcohol use disorder in European American and African American women. Psychol Addict Behav 2016; 30(4): 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saunders GRB, Liu M, Vrieze S, McGue M, Iacono WG, Gwas et al. Mechanisms of parent-child transmission of tobacco and alcohol use with polygenic risk scores: Evidence for a genetic nurture effect. Dev Psychol 2021; 57(5): 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grych JH, Seid M, Fincham FD. Assessing marital conflict from the child’s perspective: the children’s perception of interparental conflict scale. Child Dev 1992; 63(3): 558–572. [DOI] [PubMed] [Google Scholar]
- 53.Wymbs BT, Pelham WE. Child effects on communication between parents of youth with and without attention-deficit/hyperactivity disorder. J Abnorm Psychol 2010; 119(2): 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Emery RE, Binkoff JA, Houts AC, Carr EG. Children as independent variables: some clinical implications of child-effects. Behav Ther 1983; 14(3): 398–412. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


