Abstract
Exposure to outdoor air pollution may affect incidence and severity of coronavirus disease 2019 (COVID-19). In this retrospective cohort based on patient records from the Greater Manchester Care Records, all first COVID-19 cases diagnosed between March 1, 2020 and May 31, 2022 were followed until COVID-19 related hospitalization or death within 28 days. Long-term exposure was estimated using mean annual concentrations of particulate matter with diameter ≤2.5 μm (PM2.5), ≤10 μm (PM10), nitrogen dioxide (NO2), ozone (O3), sulphur dioxide (SO2) and benzene (C6H6) in 2019 using a validated air pollution model developed by the Department for Environment, Food and Rural Affairs (DEFRA). The association of long-term exposure to air pollution with COVID-19 hospitalization and mortality were estimated using multivariate logistic regression models after adjusting for potential individual, temporal and spatial confounders. Significant positive associations were observed between PM2.5, PM10, NO2, SO2, benzene and COVID-19 hospital admissions with odds ratios (95% Confidence Intervals [CI]) of 1.27 (1.25–1.30), 1.15 (1.13–1.17), 1.12 (1.10–1.14), 1.16 (1.14–1.18), and 1.39 (1.36–1.42), (per interquartile range [IQR]), respectively. Significant positive associations were also observed between PM2.5, PM10, SO2, or benzene and COVID-19 mortality with odds ratios (95% CI) of 1.39 (1.31–1.48), 1.23 (1.17–1.30), 1.18 (1.12–1.24), and 1.62 (1.52–1.72), per IQR, respectively. Individuals who were older, overweight or obese, current smokers, or had underlying comorbidities showed greater associations between all pollutants of interest and hospital admission, compared to the corresponding groups. Long-term exposure to air pollution is associated with developing severe COVID-19 after a positive SARS-CoV-2 infection, resulting in hospitalization or death.
Keywords: Air pollution, COVID-19, SARS-CoV-2, Hospitalization, Benzene, Mortality
Graphical abstract
1. Introduction
Pollutants from human activities are detrimental to human health. Primary anthropogenic sources include combustion of fuels such as wood, coal, and the emissions from vehicle exhausts. Industry has been suggested to be the primary source of sulphur dioxide (SO2) and benzene (C6H6) in a number of studies, whilst nitrogen dioxide (NO2) is primarily from vehicle combustion emissions in a city environment (Jephcote & Mah, 2019; Restrepo, 2021; Tiwari et al., 2010). Particulate matter with diameter ≤2.5 μm (PM2.5) and ≤10 μm (PM10) will encompass both sources. Benzene may also be viewed as a proxy for other industrial pollutants such as polycyclic aromatic hydrocarbons (PAHs), and other volatile organic compounds, for which ambient levels are not monitored on a national scale (Jephcote & Mah, 2019).
Air pollution is a major environmental risk factor for morbidity and mortality, leading to 4.2 million deaths every year globally, primarily from heart disease, stroke, chronic obstructive pulmonary disorder (COPD), lung cancer and acute respiratory infections (WHO, 2021). Air pollution affects people in different ways, with the elderly, children and those with pre-existing health conditions being the most sensitive to the health impacts. In addition, the people living in the most deprived areas of cities are more likely to be breathing in higher concentrations of air pollution and have inadequate medical treatment (Brunt et al., 2017; Daras et al., 2021).
National and international studies have linked air pollution exposure to incidence, morbidity and mortality of coronavirus disease 2019 (COVID-19), which is caused by the SARS-CoV-2 virus that instigated a global pandemic, seriously challenging healthcare systems since 2020 (Heald et al., 2022). However, results have mostly been drawn from ecological analyses that use population-level data and are therefore prone to bias (Perone, 2022; Renard et al., 2022; Travaglio et al., 2021; Wu et al., 2020). Only a few studies have examined the association between long-term exposure to air pollution and COVID-19 morbidity and mortality, using individual-level data on COVID-19 outcomes in a cohort design (Bowe et al., 2021; Bozack et al., 2021; C. Chen et al., 2022; Z. Chen et al., 2022; English et al., 2022; Mendy et al., 2021). However, these studies used different definitions of COVID-19 severity, included fewer individual pollutants, and did not account for a wide range of individual-level determinants of COVID-19 spread or severity, such as body mass index (BMI), smoking status, or history of comorbidities.
In this study we investigated the association between long-term exposure to air pollutants (PM2.5, PM10, Ozone (O3), NO2, SO2, benzene), and COVID-19-related severity (hospitalization or death) in those infected with SARS-CoV-2 in Greater Manchester, United Kingdom (UK).
2. Methods
2.1. Study population
Data was extracted from the Greater Manchester Care Record (GMCR), which is a digital resource bringing together National Health Service (NHS) information of over 3.1 million residents, including general practitioners (GPs) and hospitals, across all 10 Greater Manchester boroughs (Greater Manchester Care Records, 2022). The cohort consisted of individual residents of Greater Manchester who were diagnosed with COVID-19 by a polymerase chain reaction (PCR) laboratory test result, between March 01, 2020 and May 31, 2022 (n = 456,510). PCR testing capacity in the UK varied over time but sufficiently high levels were established from October 26, 2020 until the end of this study (UKHSA, 2023). Diagnosis of COVID-19 by clinical examination was not recorded in the NHS database. COVID-19 hospitalizations or mortality were defined as any cause of hospital admission or death within 28 days after a first positive COVID-19 test, respectively, in line with the NHS definition and previous literature (Gao et al., 2022). Individuals with a positive PCR result within 48 h of admission were also included in the analysis to account for delays in reporting. Covariates were identified from prior literature, and depicted in a directed acyclic graph (see Supplementary Fig. S1). The final dataset included individual-level information on age, sex, ethnicity, BMI, smoking status, history of comorbidities (coronary heart disease (CHD), stroke, hypertension, asthma, chronic obstructive pulmonary disorder (COPD), and type 2 diabetes), as well as area-level socioeconomic status (SES), index of multiple deprivation (IMD) at lower super output area (LSOA) level. LSOAs are part of a geographic hierarchy and designed to be a similar size, containing approximately 1500 residents each. Population density was obtained from the Office for National Statistics (ONS, 2022). The study was reviewed and approved by the GMCR Secondary Uses and Research Group (reference number RQ-043). All data used was anonymised and was obtained after approval of a three-stage application reviewed by the GMCR Board. All clinical code sets and scripts used for the data preparation in this project can be found at: https://github.com/rw251/gm-idcr/tree/master/projects/043%20-%20Cruickshank.
2.2. Air pollution exposure
Annual mean concentrations of PM2.5, PM10, O3, NO2, SO2, and benzene were estimated by the Department for Environment, Food and Rural Affairs (DEFRA). As a secondary pollutant whose distribution is noticeably different to other pollutants, O3 is measured as the number of days on which the daily max 8-h O3 concentration is greater than 120 μg m−3 (DGT120), which is used in targets set by UK Air Quality Standards Regulations (DEFRA, 2022a). The metric for all other pollutants is annual mean in μg m−3. The modelled air pollution was estimated at a 1 × 1 km resolution, with the DEFRA model combining ground monitoring, location of industrial facilities and transport networks (DEFRA, 2022b). DEFRA models are continually updated and the underlying methodology often changes each year (DEFRA, 2022b; DEFRA, 2022c). Ambient exposure to air pollution in 2019, 2020 and an average of both years were linked to each individual's LSOA code level, as a measure of long-term exposure to air pollution. A 1-year exposure in 2019 was used as the main exposure in this study to reflect air pollution levels prior to the pandemic, which has also been used by previous literature (Bowe et al., 2021; Bozack et al., 2021; Z. Chen et al., 2022; Veronesi et al., 2022). The raw DEFRA data (grid of points) was converted to raster with 1 × 1 km cells and then a coverage weighted mean was calculated for each LSOA using the intersected raster cells, in line with previous literature (Daras et al., 2019). Figures were created to visualize these annual concentrations across Greater Manchester LSOAs (see Fig. 1 and Supplementary Fig. S2).
Fig. 1.
The annual mean concentration of air pollution (SO2, NO2, PM2.5 and benzene) in 2019 in Greater Manchester.
PM2·5 = particulate matter with diameter ≤2·5 μm. NO2 = nitrogen dioxide. SO2 = sulphur dioxide. 2019 annual DEFRA data converted to raster data producing a 1 × 1 km grid for each pollutant, then the average value of grid cells that intersect with each LSOA polygon was used to produce a coverage-weighted mean. There are 1673 LSOAs in Greater Manchester.
2.3. Statistical analysis
To visualize the relationship between air pollution exposure and COVID-19 severity we used generalized additive models with a regression spline function with 5 degrees of freedom for air pollution exposure. The association between long-term exposure to air pollution of PM2.5, PM10, O3, NO2, SO2, and benzene (2019 annual levels) and COVID-19 severity (hospitalizations and mortality in separate analyses) were assessed using multivariate logistic regression. As most pollutants showed linear associations, each pollutant was included separately in a linear model after adjusting for individual- and area-level covariates in three a priori defined steps: Model 1 (crude) included the temporal trends (month of diagnosis), sex, age (20-year-age band), and ethnicity (white, black, Asian, mixed, other); Model 2 (individual-level factors) further adjusted for BMI (underweight, normal, overweight, obese class 1, obese class 2, obese class 3) and smoking (current smoker, previous smoker, non-smoker); and Model 3 (main model) additionally adjusted for area-level factors, including IMD in 2019 decile score from 1 to 10, and population density (people per square km in each LSOA).
Potential effect modifications of the association of individual pollutants and COVID-19 severity by age, sex, BMI, and smoking were explored by further adding a multiplicative interaction between the covariates and exposure into the main model and examining the significance by likelihood ratio test. Additionally, we investigated the effect modifications by comorbidity with cardiovascular disease (CHD, stroke, hypertension), respiratory disease (asthma, COPD, and type 2 diabetes), as well as hypertension, type 2 diabetes, asthma and COPD.
To investigate the temporal effects on associations, we further stratified the analysis into different periods of the pandemic; before and after mass vaccination (March 1, 2020–December 31, 2020 and January 1, 2021–May 31, 2022), and corresponding waves (wave 1; March 1, 2020–June 30, 2020, wave 2; August 1, 2020–May 31, 2021, and wave 3; June 1, 2021–May 31, 2022), defined by previous literature (Feinmann, 2021; Kirwan et al., 2022; DHSC, 2022).
Multiple sensitivity analyses were performed to assess the robustness of our findings, including 1) assigning annual concentrations of air pollution in 2020 as an exposure matrix, 2) assigning a two-year exposure window with 2019 and 2020 as an exposure matrix, 3) further adjusting for cardiovascular or respiratory disease in the main model, 4) limiting the cohort to hospitalized patients and looking at mortality as outcome, 5) excluding different area-level variables (IMD score or population density), 6) using the test date with a natural cubic spline with 6–8 knots per year to control for the time trend, as well as, 7) exploring two-pollutant analysis by mutually adjusting any pollution-pairs with correlations ≤0.7, to distinguish the single pollutant effects.
The results are reported as odds ratios (ORs) and 95% confidence intervals (CI) per interquartile range (IQR) increase for each exposure. All the statistical analysis was conducted using R version 4.1.2.
3. Results
Of the 456,510 Individuals who tested positive for SARS-CoV-2 in Greater Manchester, United Kingdom between March 1, 2020 and May 31, 2022, 26 individuals were excluded due to an incorrect COVID-19 diagnosis date, 25 due to unknown sex, 131,903 due to incorrect BMI (missing or outside of an acceptable range: 0–80 kg/m2), and 10,899 who were registered to an LSOA outside Greater Manchester, leaving 313,657 individuals in final analyses (see Supplementary Fig. S3). The mean age and standard deviation (SD) of the cohort participants was 44 (17.9) years and the majority were women (58.8%), white (72.3%), overweight or obese (60.3%), non-smokers (52.1%), and lived in an area with an IMD decile of 5 or less (67.2%). Of the cohort participants, 27% had a history of at least one of the following comorbidities at the time of SARS-CoV-2 infection: asthma, CHD, stroke, type 2 diabetes, COPD, hypertension. Of the 313,657 people with SARS-CoV-2 infection, 43,301 (14.1%) were hospitalized and 4379 (1.4%) died within 28 days of their PCR test (see Table 1 ).
Table 1.
Characteristics of study cohort.
| Total COVID-19 Cases (N = 313,657) | |
| Age | |
| Mean y, (SD) | 44 (17.90) |
| By age group, n (%) | |
| 0-20 | 20,502 (6.54) |
| 21-40 | 113,944 (36.33) |
| 41-60 | 117,108 (37.34) |
| 61-80 | 50,637 (16.14) |
| 80+ | 11,466 (3.66) |
| Sex, n (%) | |
| Female | 184,378 (58.78) |
| Male | 129,279 (41.22) |
| Ethnicity, n (%) | |
| White | 227,754 (72.61) |
| Black | 7209 (2.30) |
| Asian | 30,960 (9.87) |
| Mixed | 4229 (1.35) |
| Other/Unclassified | 43,505 (13.87) |
| BMI Category, n (%) | |
| Underweight | 18,903 (6.03) |
| Normal | 105,568 (33.66) |
| Overweight | 97,775 (31.17) |
| Obese class 1 | 53,587 (17.08) |
| Obese class 2 | 23,140 (7.38) |
| Obese class 3 | 14,684 (4.68) |
| Tobacco Use, n (%) | |
| Non-smoker | 163,421 (52.10) |
| Previous-smoker | 38,138 (12.16) |
| Current Smoker | 112,098 (35.74) |
| IMD 2019 Deprivation Score (1 is most deprived), n (%) | |
| 1 | 71,803 (22.89) |
| 2 | 47,886 (15.27) |
| 3 | 39,117 (12.47) |
| 4 | 28,883 (9.21) |
| 5 | 23,226 (7.40) |
| 6 | 22,489 (7.17) |
| 7 | 21,397 (6.82) |
| 8 | 24,421 (7.79) |
| 9 | 19,933 (6.36) |
| 10 | 14,502 (4.62) |
| Comorbidities, n (%) | |
| Asthma | 62,593 (19.96) |
| CHD | 11,740 (3.74) |
| Stroke | 4099 (1.31) |
| Type 2 Diabetes | 23,644 (7.54) |
| COPD | 8208 (2.62) |
| Hypertension | 52,043 (16.59) |
| Air pollution, mean (IQR) | |
| PM2.5 | 8.20 (1.90) |
| PM10 | 13.10 (2.85) |
| O3 | 7.40 (2.03) |
| NO2 | 10.99 (4.35) |
| SO2 | 1.16 (0.38) |
| Benzene | 0.35 (0.13) |
| Outcome, n (%) | |
| Hospitalization* | 44,301 (14.12) |
| Death* | 4379 (1.40) |
Definitions: BMI = body mass index; CHD = coronary heart disease; COPD = chronic obstructive pulmonary disorder; COVID-19 = coronavirus disease; IMD = index of multiple deprivation.
PM2·5 and PM10 = particulate matter with diameter ≤2·5 and 10 μm, respectively. NO2 = nitrogen dioxide. O3 = ozone. SO2 = sulphur dioxide.*Within 28 days of a first positive SARS-CoV-2 test.
Mean levels (IQR) of PM2.5, PM10, NO2, SO2, and benzene across Greater Manchester were 9.12 (1.05), 13.81 (1.45), 17.25 (4.45), 2.05 (0.47), and 0.44 (0.09) μg/m3, respectively. Mean (IQR) 2019 annual levels of O3 was 6.15 (0.74) days with 8 h exceeding 120 μg/m3. A strong correlation is observed among all pollutants in the LSOAs, for example, PM2.5 was correlated 0.81, 0.70, 0.83 with NO2, SO2, and benzene, respectively (see Supplementary Fig. S4). Pollutant levels in 2019 and 2020 were highly correlated (see Supplementary Fig. S5).
We observed no evidence that associations departed from linearity for most pollutants, while NO2 and O3 showed inverse U-shaped relationships with COVID-19 hospitalization but linearity with COVID-19 mortality (see Supplementary Figs. S6 and S7).
In the fully adjusted model, significant positive associations were observed between most pollutants and COVID-19 severity, for both hospitalization and mortality after COVID-19 infection (see Table 2 ). An IQR increase in PM2.5, PM10, NO2, SO2 or benzene was associated with COVID-19 hospitalization with ORs (95% CI) of 1.27 (1.25–1.30), 1.15 (1.13–1.17), 1.12 (1.10–1.14), 1.16 (1.14–1.18), and 1.39 (1.36–1.42), respectively. However, O3 was inversely associated with COVID-19 hospitalizations with an OR (95% CI) of 0.96 (0.95–0.98). An IQR increase in PM2.5, PM10, SO2 or benzene were associated with COVID-19 mortality with ORs (95% CI) of 1.39 (1.31–1.48), 1.23 (1.17–1.30), 1.18 (1.12–1.24), and 1.62 (1.52–1.72) (see Table 2). While there was little confounding by the individual-level variables, the association attenuated the most after adjusting for the area-level confounders in model 3.
Table 2.
The association between long-term exposure to air pollution and hospitalization and mortality from COVID-19 in Greater Manchester.
| Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) | |
|---|---|---|---|
| COVID-19 hospitalization (N=44,301) | |||
| PM2.5 | 1.31 (1.28–1.33) | 1.30 (1.27–1.32) | 1.27 (1.25–1.30) |
| PM10 | 1.20 (1.18–1.22) | 1.19 (1.17–1.21) | 1.15 (1.13–1.17) |
| NO2 | 1.16 (1.15–1.18) | 1.16 (1.14–1.18) | 1.12 (1.10–1.14) |
| O3 | 0.92 (0.91–0.93) | 0.93 (0.91–0.94) | 0.96 (0.95–0.98) |
| SO2 | 1.21 (1.20–1.23) | 1.20 (1.18–1.22) | 1.16 (1.14–1.18) |
| Benzene | 1.36 (1.34–1.39) | 1.35 (1.33–1.37) | 1.39 (1.36–1.42) |
| COVID-19 mortality (N=4379) | |||
| PM2.5 | 1.58 (1.50–1.66) | 1.56 (1.48–1.65) | 1.39 (1.31–1.48) |
| PM10 | 1.41 (1.34–1.48) | 1.40 (1.33–1.47) | 1.23 (1.17–1.30) |
| NO2 | 1.19 (1.14–1.24) | 1.18 (1.12–1.23) | 1.02 (0.97–1.07) |
| O3 | 0.89 (0.85–0.93) | 0.90 (0.86–0.94) | 1.02 (0.97–1.07) |
| SO2 | 1.35 (1.29–1.40) | 1.34 (1.28–1.40) | 1.18 (1.12–1.24) |
| Benzene | 1.72 (1.64–1.81) | 1.70 (1.62–1.79) | 1.62 (1.52–1.72) |
OR = odds ratio. CI = confidence interval. PM2·5 and PM10= particulate matter with diameter ≤2·5 and 10 μm, respectively. NO2 = nitrogen dioxide. O3 = ozone. SO2 = sulphur dioxide. Results are presented for interquartile range increase.
Model 1 adjusted for temporal trends (month of diagnosis), sex, and age (each 20-year-age band); Model 2 additionally adjusted for, ethnicity (white, black, Asian, mixed, other), BMI (underweight, normal, overweight, obese class 1, obese class 2, obese class 3) and smoking (smoker, previous smoker, non-smoker); Model 3 further adjusted for deprivation score (IMD) and population density.
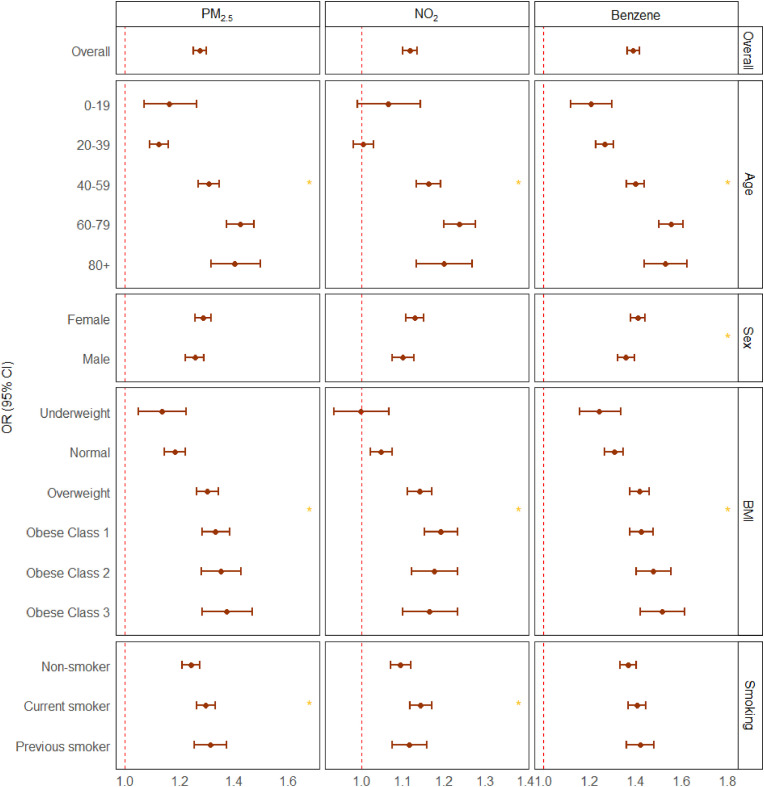
The associations between exposure to air pollution and COVID-19 hospitalization were significantly modified by age, BMI, smoking habits, and historical comorbidities (P-value for interaction <0.05). Individuals in age group 40–59 years, overweight or obese, current smokers, or patients with pre-existing diseases such as type 2 diabetes, hypertension, or COPD showed stronger association between exposure to air pollution and COVID-19 hospitalization (see Fig. 2, Fig. 3 ). The associations between exposure to air pollution and COVID-19 mortality were significantly modified by age and historical COPD. Individuals in age group 40–59, or patients with pre-exisiting COPD showed stronger association between exposure to air pollution and COVID-19 mortality (see Supplementary Fig. S8 and Supplementary Fig. S9).
Fig. 2.
Effect modification of the association between long-term exposure to air pollution and hospitalization from COVID-19 in Greater Manchester by age, sex, BMI, and smoking status.
OR = odds ratio. CI = confidence interval. PM2·5 = particulate matter with diameter ≤2·5 μm. NO2 = nitrogen dioxide. Main model adjusted for temporal trends (month of diagnosis), sex, and age (each 20-year-age band), ethnicity (white, black, Asian, mixed, other), BMI (underweight, normal, overweight, obese class 1, obese class 2, obese class 3), smoking (smoker, previous smoker, non-smoker), deprivation score (IMD) and population density. Results are presented for per interquartile range increase for each pollutant. Model differences are examined using likelihood ratio test and the significant difference with a global-p value < 0.05 are highlighted with *.
Fig. 3.
Effect modification of the association between long-term exposure to air pollution and hospitalization from COVID-19 in Greater Manchester by comorbidity.
OR = odds ratio. CI = confidence interval. PM2·5 = particulate matter with diameter ≤2·5 μm. NO2 = nitrogen dioxide. CVD = cardiovascular diseases, RD = respiratory diseases, COPD = chronic obstructive pulmonary disease. Main model adjusted for temporal trends (month of diagnosis), sex, and age (each 20-year-age band), ethnicity (white, black, Asian, mixed, other), BMI (underweight, normal, overweight, obese class 1, obese class 2, obese class 3), smoking (smoker, previous smoker, non-smoker), deprivation score (IMD) and population density. Results are presented for per interquartile range increase for each pollutant. Model differences are examined using likelihood ratio test and the significant difference with a global-p value < 0.05 are highlighted with *.
Time-stratification analysis showed the association of COVID-19 hospitalization persisted in all periods, and the association of COVID-19 mortality became stronger after mass vaccination (see Supplementary Fig. S10) and in the later stages of the pandemic (see Supplementary Fig. S11).
Replacing 2019 annual mean concentrations of air pollution with 2020 levels and using a 2-year exposure model combining years 2019 and 2020 as an exposure matrix showed the same trends as our main results but with generally stronger associations (see Supplementary Fig. S12 and Supplementary Fig. S13, respectively). Further adjustment for preexisting cardiovascular and respiratory disease did not change the main associations (see Supplementary Fig. S14). Limiting the study population to hospitalized patients mildly attenuated associations with COVID-19 mortality (see Supplementary Fig. S15). Exclusion of IMD score in the model mildly strengthened associations, whilst exclusion of population density attenuated associations (see Supplementary Fig. S16). Using test date to control for temporal trends did not alter our results (see Supplementary Table S1). Finally, two-pollutant models showed that significant positive associations generally persisted with almost all pollutant combinations. Additionally, O3 showed negative or non-significant associations in single pollutant models but showed significant positive associations with COVID-19 severity when adjusted with all other pollutants in two-pollutant models (see Supplementary Table S2). Results from our two-pollutant model should be taken with caution due to the high correlations that exist between most pollutants in our study.
4. Discussion
This study, using individual-level data on 313,657 residents of Greater Manchester, United Kingdom, who tested positive for SARS-CoV-2, showed significant positive associations between residential exposure to air pollution and severe COVID-19 requiring hospitalization or resulting in death. We found that older people, obese individuals, current smokers, and individuals with historic cardiovascular disease (hypertension, CHD, stroke), type 2 diabetes, and COPD had greater risk of developing severe COVID-19 related to air pollution.
There are currently five published cohort studies that have investigated long-term exposure to air pollution and COVID-19 severity and the findings are mostly in line with the results in this study. Chen Z et al. (2022) examined air pollution exposure and COVID-19 severity in California, USA. They found that a 1-SD increase in 1-year averaged PM2.5 exposure was associated with increased risk of COVID-19-related hospital admission and death, respectively for PM2.5; [OR (95% CI): 1.23 (1.17–1.30), 1.11 (1.02–1.21)], whilst NO2 was only associated with hospitalization; [OR (95% CI): 1.13 (1.07–1.18)] (Z. Chen et al., 2022). Chen C et al. (2022) performed a prospective cohort study using residents of Ontario, Canada with confirmed COVID-19 diagnosis and found an IQR increase in exposure to O3 was associated with increased risk of COVID-19-related hospital admission and death, respectively; [OR (95% CI):1.15 (1.06–1.23), 1.18 (1.02–1.36)], whilst PM2.5 was only associated with hospitalization; [OR CI (95%) PM2.5: 1.06 (1.01–1.12)] (C. Chen et al., 2022). A number of smaller cohort studies also found higher long-term PM2.5 exposure was associated with COVID-19 hospitalization and/or mortality (Bowe et al., 2021; Bozack et al., 2021; Mendy et al., 2021).
This study found that pollutants from industrial sources may have played a key role in COVID-19 severity during the pandemic, with SO2 and benzene having some of the strongest associations with COVID-19 hospitalization and mortality. This has not been investigated by similar cohort studies (Bowe et al., 2021; Bozack et al., 2021; C. Chen et al., 2022; Z. Chen et al., 2022; Mendy et al., 2021), although an ecological study also found that benzene was positively correlated with the spread of COVID-19, and mortality in 107 Italian provinces (Perone, 2022).
A unique finding in our study is a significant negative (protective) association between O3 and COVID-19 hospitalization. This is most likely due to the fact that ground-level O3 concentration depends on other pollutants (e.g. nitrogen oxides (NOX)) and solar light (Higham et al., 2021). Urban settings such as Greater Manchester are expected to have low relative O3 concentrations because it reacts with NO, forming NO2 (Kolluru et al., 2022). This hypothesis is supported by our results, where O3 shows significant positive associations with COVID-19 severity when adjusted with all other pollutants in two-pollutant models. An ecological study looking at O3 and COVID-19 transmissibility in China also found a protective effect and indicates that another explanation for this may arise from the virucidal activity and possible stimulatory effects of O3 on host innate defences (Ran et al., 2020).
Whilst the exact molecular mechanisms by which air pollution affects COVID-19 severity remain unknown, there is evidence that exposure to pollutants found in ambient air can cause chronic inflammation and hyperactivation of the immune system at sustained low doses (Tripathy et al., 2021). This may contribute to reaching thresholds to trigger an extreme inflammatory state called a cytokine storm, leading to acute respiratory distress syndrome (Lavigne et al., 2022). Air pollution may also cause this indirectly by affecting the macrophage response to viral infection, allowing them to persist for longer and exacerbate the cytokine storm (Pieters, 2011). Another possible mechanism could be that air pollution causes ACE2 (receptor for SARS-CoV-2 entry into cells) overexpression increasing viral load during invasion and leading to increased risk of severe disease (Wang et al., 2020).
Strengths of this study include access to data on a city-wide cohort of 313,657 individuals who were diagnosed with COVID-19 from a PCR test, with detailed individual-level data on lifestyle and co-morbidities. The study population was defined from NHS records, which includes the entire population of Greater Manchester except those who opted out of data sharing, which was 5.4% in 2022 (NHS Digital, 2022). The study period covered more than two-years of the pandemic from March 1, 2020 to May 31, 2022, which included several SARS-CoV-2 variants, and showed robust associations over the entire period. A key strength of this study is the inclusion of all major pollutants PM2.5, PM10, NO2, O3, as well as SO2 and benzene.
There are certain limitations of this study, which mostly arise from working with restricted NHS data. Firstly, severity was considered based on two levels (hospitalization and death) as more detailed information regarding disease severity was not available. Other studies have included further levels of COVID-19 severity, such as if a patient required an intensive care unit, and date of death. Information on the cause of death was not available, preventing the exclusion of deaths that may not have been COVID-19-related. Secondly, due to data security it was only possible to link individual exposure to air pollution in LSOA levels. The variation of exposure among individuals within the same LSOA is not captured. Additionally, PM2.5 spatial distribution has been shown to be greater than expected in big city environments (Renard and Marchand, 2021). However, each LSOA in Greater Manchester has a very fine resolution with a mean area of 4.345 km2. Previous epidemiology research has validated the credibility of using small area level exposure to air pollution to estimate individual exposure (Z. Chen et al., 2022; Di et al., 2017). Thirdly, total exposure may also be linked to levels of pollutants in indoor microenvironments (transport, home, office), and emerging research presents a complex mix of mitigating factors such as building ventilation (DEFRA, 2022d). However, to include this information would require personal monitoring and it is not feasible for our large cohort. Fourthly, historical migration of individuals over our 27-month study period was not included as this information (e.g., change of address) was not systematically recorded in our NHS dataset. This may lead to some non-differential misclassification and is it likely to be a key reason that we generally see stronger associations with COVID-19 severity when including 2020 air pollution exposure data in our model. Finally, information was not available on individual SES factors, such as income, education and occupation, although exposure and health outcomes are associated with individual SES (C. Chen et al., 2022; Z. Chen et al., 2022). To account for this, we included area-level deprivation index, IMD decile, in the model to adjust for the SES inequality. Additionally, there were other key covariates that we did not have information on, such as being part of an outbreak, being an essential worker, health care access, care home status, and detailed vaccination information.
Based on the observed associations between air pollution exposure and COVID-19 severity, it may be beneficial to prioritize individuals for vaccination not only on their risk factors but on their estimated outdoor pollution exposure too. Better communication to the general public, especially if in a vulnerable group, of methods to mitigate exposure may help to reduce their exposure and risk. This could further be supported by the implementation of local council and/or government schemes such as clean air zones, speed limits on roads or restriction by vehicle type, industrial regulations, and investment in public transport to meet WHO recommended air standards (Isphording and Pestel, 2021).
COVID-19 remains a highly infectious disease with the capacity to cause severe and long-term damage to health, as well as to overwhelm health systems (WHO, 2023). Further, evidence shows that air pollution may affect severity in other respiratory diseases, such as influenza (Su et al., 2019) and allergic respiratory disease (Gledson et al., 2023), with SO2 having some of the strongest associations in both studies. It is therefore imperative that we learn as much as possible from the COVID-19 pandemic, utilizing the unprecedented scale of testing data and advances in digitalization of health data observed during the last few years.
5. Conclusions
This is the first cohort study investigating COVID-19 severity associated with long-term air pollution exposure in a UK city (Greater Manchester). Long-term exposure to air pollution, specifically, PM2.5, PM10, NO2, SO2, and benzene, was associated with increased risk of hospital admission and death within 28 days of a positive SARS-CoV-2 test. The study included NHS data, allowing for observations of greater associations among those in a certain age group (40–59), current smokers, and those with historical obesity, hypertension, COPD and type 2 diabetes, compared to the corresponding groups.
These findings highlight the need to mitigate exposure for the general public in city environments, especially if they are in a vulnerable group. It may be beneficial to prioritize individuals for vaccination not only on their risk factors but on their estimated outdoor pollution exposure too.
This study found that SO2 and benzene had some of the strongest associations with severe COVID-19 outcomes. Previous literature has focused primarily on PM2.5, and there is a need for future studies to include exposure to a wider range of pollutants, and to quantify exposure to source-specific emissions such as those from industry.
Additionally, this study was not able to include covariates such as vaccination, SARS-CoV-2 variants, and SES factors, and further research is needed to assess the impact they could have on these results.
Credit author statement
Samuel Hyman: Conceptualization, Methodology, Software, Formal Analysis, Investigation, Data Curation, Writing – Original Draft, Visualisation, Project Administration. Jiawei Zhang: Conceptualization, Methodology, Software, Formal Analysis, Investigation, Data Curation, Writing – Original Draft, Visualisation. Zorana Jovanovic Andersen: Conceptualization, Formal Analysis, Writing – Review & Editing, Visualisation, Supervision. Sheena Cruickshank: Conceptualization, Writing – Review & Editing, Visualisation, Supervision. Peter Møller: Conceptualization, Formal Analysis, Writing – Review & Editing, Supervision, Visualisation. Konstantinos Daras: Software, Writing – Review & Editing, Visualisation. Richard Williams: Conceptualization, Data Curation, Writing – Review & Editing, Visualisation. David Topping: Conceptualization, Writing – Review & Editing, Visualisation, Supervision. Youn-Hee Lim: Conceptualization, Methodology, Software, Formal Analysis, Investigation, Writing – Original Draft, Writing – Review & Editing, Visualisation, Supervision.
Data statement
The study was also reviewed and approved by the Greater Manchester Care Record Secondary Uses and Research Group (reference number RQ-043). The data used in the analyses presented was obtained with the permission of the Greater Manchester Care Record Board and was fully anonymised prior to being made available to the investigators. R code and study protocol are available upon request. All clinical code sets and scripts used for the data preparation in this project can be found at: https://github.com/rw251/gm-idcr/tree/master/projects/043%20-%20Cruickshank.
Funding
No funding was available for this research. SH acknowledges the EPSRC Centre for Doctoral Training in Aerosol Science (EP/S023593/1) for financial support. KD is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration North West Coast (ARC NWC, NIHR200182). The time of RW was supported by the NIHR Applied Research Collaboration Greater Manchester (NIHR200174) and the National Institute for Health and Care Research (NIHR) Greater Manchester Patient Safety Translational Research Centre (award number: PSTRC-2016-003). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence (where permitted by UKRI, ‘Open Government Licence’ or ‘Creative Commons Attribution No-derivatives (CC BY-ND) licence may be stated instead) to any Author Accepted Manuscript version arising.
Transparency
The lead author SH affirms that the manuscript is an honest, accurate, and transparent account of the study reported; no important aspects of the study have been omitted.
Dissemination to participants and related patient and public communities
It is anticipated to disseminate the results of this research to wider community via press release and social media platforms.
Provenance and peer review
Not commissioned; externally peer reviewed.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
SH would like to thank all co-authors for their time and patience, especially the Copenhagen University group for welcoming him back into their wonderful department. A special thanks to Charlotte Elston, Megan Griffiths and Paul Hyman for their support and feedback on the final drafts.
Footnotes
This paper has been recommended for acceptance by Payam Dadvand.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envpol.2023.121594.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Bowe B., Xie Y., Gibson A.K., Cai M., van Donkelaar A., Martin R.V., Burnett R., Al-Aly Z. Ambient fine particulate matter air pollution and the risk of hospitalization among COVID-19 positive individuals: cohort study. Environ. Int. 2021;154 doi: 10.1016/j.envint.2021.106564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozack A., Pierre S., DeFelice N., Colicino E., Jack D., Chillrud S.N., Rundle A., Astua A., Quinn J.W., McGuinn L., Yang Q., Johnson K., Masci J., Lukban L., Maru D., Lee A.G. Long-term air pollution exposure and COVID-19 mortality: a patient-level analysis from New York city. Am. J. Respir. Crit. Care Med. 2021;205(6):651–662. doi: 10.1164/rccm.202104-0845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt H., Barnes J., Jones S.J., Longhurst J.W.S., Scally G., Hayes E. Air pollution, deprivation and health: understanding relationships to add value to local air quality management policy and practice in Wales, UK. Journal of public health (Oxford, England) 2017;39(3):485–497. doi: 10.1093/pubmed/fdw084. [DOI] [PubMed] [Google Scholar]
- Chen C., Wang J., Kwong J., Kim J., van Donkelaar A., Martin R.V., Hystad P., Su Y., Lavigne E., Kirby-McGregor M., Kaufman J.S., Benmarhnia T., Chen H. Association between long-term exposure to ambient air pollution and COVID-19 severity: a prospective cohort study. Can. Med. Assoc. J. 2022;194(20):E693. doi: 10.1503/cmaj.220068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Sidell M.A., Huang B.Z., Chow T., Eckel S.P., Martinez M.P., Gheissari R., Lurmann F., Thomas D.C., Gilliland F.D., Xiang A.H. Ambient air pollutant exposures and COVID-19 severity and mortality in a cohort of patients with COVID-19 in southern California. Am. J. Respir. Crit. Care Med. 2022;206(4):440–448. doi: 10.1164/rccm.202108-1909OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daras K., Alexiou A., Rose T.C., Buchan I., Taylor-Robinson D., Barr B. How does vulnerability to COVID-19 vary between communities in england? Developing a small area vulnerability index (SAVI) J. Epidemiol. Community Health. 2021;75(8):729. doi: 10.1136/jech-2020-215227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daras K., Green M.A., Davies A., Barr B., Singleton A. Open data on health-related neighbourhood features in Great Britain. Sci. Data. 2019;6(1):107. doi: 10.1038/s41597-019-0114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEFRA . Department for Environment Food and Rural Affairs; 2022. Concentrations of Ozone.https://www.gov.uk/government/statistics/air-quality-statistics/concentrations-of-ozone Accessed. [Google Scholar]
- DEFRA . Department for Environmental Food and Rural Affairs; 2022. Modelled Background Pollution Data.https://uk-air.defra.gov.uk/data/pcm-data Accessed 13/11/2022. [Google Scholar]
- DEFRA . Department for Environmental Food and Rural Affairs; 2022. Technical report on UK supplementary modelling assessment under the air quality standards regulations 2010 for 2020; pp. 22–113.https://uk-air.defra.gov.uk/assets/documents/reports/cat09/2203150935_2020_PCM_technical_report.pdf 1. Last updated 15/03/2022. [Google Scholar]
- DEFRA . Department for Environmental Food and Rural Affairs; 2022. Report: indoor air quality; pp. 8–16.https://uk-air.defra.gov.uk/library/reports?report_id=1101 [Google Scholar]
- DHSC . Department of Health and Social Care; 2022. 'Direct and Indirect Health Impacts of COVID-19 in England: Emerging Omicron Impacts.https://www.gov.uk/government/publications/direct-and-indirect-health-impacts-of-covid-19-in-england-emerging-omicron-impacts/direct-and-indirect-health-impacts-of-covid-19-in-england-emerging-omicron-impacts Accessed. [Google Scholar]
- Di Q., Wang Y., Zanobetti A., Wang Y., Koutrakis P., Choirat C., Dominici F., Schwartz J.D. Air pollution and mortality in the medicare population. N. Engl. J. Med. 2017;376(26):2513–2522. doi: 10.1056/NEJMoa1702747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English P.B., Von Behren J., Balmes J.R., Boscardin J., Carpenter C., Goldberg D.E., Horiuchi S., Richardson M., Solomon G., Valle J., Reynolds P. Association between long-term exposure to particulate air pollution with SARS-CoV-2 infections and COVID-19 deaths in California, U.S.A. Environmental Advances. 2022;9 doi: 10.1016/j.envadv.2022.100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinmann J. Covid-19: global vaccine production is a mess and shortages are down to more than just hoarding. BMJ. 2021;375:n2375. doi: 10.1136/bmj.n2375. [DOI] [PubMed] [Google Scholar]
- Gao M., Aveyard P., Lindson N., Hartmann-Boyce J., Watkinson P., Young D., Coupland C., Clift A.K., Harrison D., Gould D., Pavord I.D., Smith M., Hippisley-Cox J. Association between smoking, e-cigarette use and severe COVID-19: a cohort study. Int. J. Epidemiol. 2022;51(4):1062–1072. doi: 10.1093/ije/dyac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gledson A., Lowe D., Reani M., Topping D., Hall I., Cruickshank S., Harwood A., Woodcock J., Jay C. A comparison of experience sampled hay fever symptom severity across rural and urban areas of the UK. Sci. Rep. 2023;13(1):3060. doi: 10.1038/s41598-023-30027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greater Manchester Care Records . 2022. Introducing the GM Care Record.https://gmwearebettertogether.com/gm-care-record/ Retrieved 23/10/2022 from. [Google Scholar]
- Heald A.H., Jenkins D.A., Williams R., Sperrin M., Mudaliar R.N., Syed A., Naseem A., Bowden Davies K.A., Peng Y., Peek N., Ollier W., Anderson S.G., Delanerolle G., Gibson J.M. Mortality in People with Type 2 Diabetes Following SARS-CoV-2 Infection: A Population Level Analysis of Potential Risk Factors. Diabetes Therapy. 2022;13(5):1037–1051. doi: 10.1007/s13300-022-01259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham J.E., Ramírez C.A., Green M.A., Morse A.P. UK COVID-19 lockdown: 100 days of air pollution reduction? Air Qual Atmos Health. 2021;14(3):325–332. doi: 10.1007/s11869-020-00937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isphording I.E., Pestel N. Pandemic meets pollution: Poor air quality increases deaths by COVID-19. J. Environ. Econ. Manag. 2021;108 doi: 10.1016/j.jeem.2021.102448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jephcote C., Mah A. Regional inequalities in benzene exposures across the European petrochemical industry: A Bayesian multilevel modelling approach. Environ. Int. 2019;132 doi: 10.1016/j.envint.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan P.D., Charlett A., Birrell P., Elgohari S., Hope R., Mandal S., De Angelis D., Presanis A.M. Trends in COVID-19 hospital outcomes in England before and after vaccine introduction, a cohort study. Nat. Commun. 2022;13:4834. doi: 10.1038/s41467-022-32458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluru S.S.R., Nagendra S.M.S., Patra A.K., Gautam S., Alshetty V.D., Kumar P. Did unprecedented air pollution levels cause spike in Delhi's COVID cases during second wave? Stoch. Environ. Res. Risk Assess. 2022 doi: 10.1007/s00477-022-02308-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne E., Ryti N., Gasparrini A., Sera F., Weichenthal S., Chen H., To T., Evans G.J., Sun L., Dheri A., Lemogo L., Kotchi S.O., Stieb D. Short-term exposure to ambient air pollution and individual emergency department visits for COVID-19: a case-crossover study in Canada. Thorax. 2022 doi: 10.1136/thoraxjnl-2021-217602. thoraxjnl-2021-217602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendy A., Wu X., Keller J.L., Fassler C.S., Apewokin S., Mersha T.B., Xie C., Pinney S.M. Air pollution and the pandemic: Long-term PM(2.5) exposure and disease severity in COVID-19 patients. Respirology. 2021;26(12):1181–1187. doi: 10.1111/resp.14140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS Digital . National Health Service; 2022. [MI] National Data Opt-out, April 2022.https://digital.nhs.uk/data-and-information/publications/statistical/national-data-opt-out/april-2022 [Google Scholar]
- ONS . Office for National Statistics; 2022. Lower layer Super Output Area population density (National Statistics)https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/lowersuperoutputareapopulationdensity [Google Scholar]
- Perone G. Assessing the impact of long-term exposure to nine outdoor air pollutants on COVID-19 spatial spread and related mortality in 107 Italian provinces. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-17215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters R. In: Allergens and Respiratory Pollutants. Williams M.A., editor. Woodhead Publishing; 2011. 9 - Role of macrophages in adverse pulmonary effects of particulate pollutants; pp. 201–212. [DOI] [Google Scholar]
- Ran J., Zhao S., Han L., Chen D., Yang Z., Yang L., Wang M.H., He D. 'The ambient ozone and COVID-19 transmissibility in China: A data-driven ecological study of 154 cities. J. Infect. 2020;81:e9–e11. doi: 10.1016/j.jinf.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J.B., Surcin J., Annesi-Maesano I., Delaunay G., Poincelet E., Dixsaut G. Relation between PM2.5 pollution and Covid-19 mortality in Western Europe for the 2020-2022 period. Sci. Total Environ. 2022;848 doi: 10.1016/j.scitotenv.2022.157579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J.B., Marchand C. Atmosphere. 2021. High Resolution Mapping of PM2.5 Concentrations in Paris (France) Using Mobile Pollutrack Sensors Network. 2020. [DOI] [Google Scholar]
- Restrepo C.E. Nitrogen Dioxide, Greenhouse Gas Emissions and Transportation in Urban Areas: Lessons From the Covid-19 Pandemic [Policy Brief] Front. Environ. Sci. 2021;9 doi: 10.3389/fenvs.2021.689985. [DOI] [Google Scholar]
- Su W., Wu X., Geng X., Zhao X., Liu Q., Liu T. The short-term effects of air pollutants on influenza- like illness in Jinan, China. BMC Publ. Health. 2019;19:1319. doi: 10.1186/s12889-019-7607-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V., Hanai Y., Masunaga S. Ambient levels of volatile organic compounds in the vicinity of petrochemical industrial area of Yokohama, Japan. Air Quality, Atmosphere & Health. 2010;3(2):65–75. doi: 10.1007/s11869-009-0052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travaglio M., Yu Y., Popovic R., Selley L., Leal N.S., Martins L.M. Links between air pollution and COVID-19 in England. Environ. Pollut. 2021;268 doi: 10.1016/j.envpol.2020.115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S., Marsland A.L., Kinnee E.J., Tunno B.J., Manuck S.B., Gianaros P.J., Clougherty J.E. Long-Term Ambient Air Pollution Exposures and Circulating and Stimulated Inflammatory Mediators in a Cohort of Midlife Adults. Environ. Health Perspect. 2021;129(5) doi: 10.1289/ehp7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UKHSA . UK Government; 2023. 'Coronavirus (COVID-19) in the UK.https://coronavirus.data.gov.uk/details/testing Accessed. [Google Scholar]
- Veronesi G., Matteis S.D., Calori G., Pepe N., Ferrario M.M. Long-term exposure to air pollution and COVID-19 incidence: a prospective study of residents in the city of Varese, Northern Italy. Occup. Environ. Med. 2022;79:192. doi: 10.1136/oemed-2021-107833. [DOI] [PubMed] [Google Scholar]
- Wang B., Chen H., Chan Y.L., Oliver B.G. Is there an association between the level of ambient air pollution and COVID-19? Am. J. Physiol. Lung Cell Mol. Physiol. 2020;319(3):L416–l421. doi: 10.1152/ajplung.00244.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO European Centre for Environment and Health; 2021. WHO Global Air Quality Guidelines. Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulphur Dioxide and Carbon Monoxide.https://www.who.int/publications/i/item/9789240034228 World Health Organisation. World Health Organisation. [PubMed] [Google Scholar]
- WHO, 2023. Statement on the Fourteenth Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Coronavirus Disease (COVID-19) Pandemic. The World Health Organization. https://www.who.int/news/item/30-01-2023-statement-on-the-fourteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic. (Accessed 28 February 2023) Accessed.
- Wu X., Nethery R.C., Sabath M.B., Braun D., Dominici F. Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Sci. Adv. 2020;6(45) doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.