Abstract
Purpose:
This descriptive study aimed to assess the characteristics of pelvic pain and explore predictive factors for pelvic pain in transgender (trans) individuals using testosterone therapy.
Methods:
An online cross-sectional survey was open between August 28, 2020, and December 31, 2020, to trans people presumed female at birth, using testosterone for gender affirmation, living in Australia, and >16 years of age. The survey explored characteristics of pelvic pain following initiation of testosterone therapy, type and length of testosterone therapy, menstruation history, and relevant sexual, gynecological, and mental health experiences. Logistic regression was applied to estimate the effect size of possible factors contributing to pain after starting testosterone.
Results:
Among 486 participants (median age = 27 years), 351 (72.2%)* reported experiencing pelvic pain following initiation of testosterone therapy, described most commonly as in the suprapubic region and as “cramping.” Median duration of testosterone therapy was 32 months. Persistent menstruation, current or previous history of post-traumatic stress disorder, and experiences of pain with orgasm were associated with higher odds of pelvic pain after testosterone therapy. No association was observed with genital dryness, intrauterine device use, previous pregnancy, penetrative sexual activities, touching external genitalia, or known diagnoses of endometriosis, vulvodynia, vaginismus, depression, anxiety, or obesity.
Conclusions:
Pelvic pain is frequently reported in trans people following initiation of testosterone therapy. Given the association with persistent menstruation and orgasm, as well as the known androgen sensitivity of the pelvic floor musculature, further research into pelvic floor muscle dysfunction as a contributor is warranted.
Keywords: menstruation disturbances, pelvic pain, sexual activity, sexual function, testosterone, transgender persons
Introduction
Pelvic pain in transgender and gender diverse (herein referred to as trans) people presumed female at birth, who are using testosterone as gender-affirming hormone therapy (GAHT), is poorly understood.1 Understanding adverse effects of testosterone therapy is important, given that trans people comprise an estimated 0.5%–4.5% of the adult population.2–4 Furthermore, there is an increasing demand for gender-affirming health care globally.5–7
Testosterone GAHT is very effective at inducing masculinizing physical changes, including significant genital and reproductive system effects, an increase in body and facial hair, deepening of the voice, increase in muscle mass, and a decrease in fat mass.8 Menstrual cessation, one of the most desired aspects of testosterone GAHT, typically occurs within the first 6 months of therapy, although breakthrough bleeding is not uncommon.9 Clitoral enlargement, vulvovaginal atrophy, and increase in libido are also frequently observed.10,11 Endometrial changes can be varied regardless of menstrual cessation with either proliferative (in 40%) or atrophic endometrium (in 50%).12 No significant histopathological change appears to occur in the ovaries.13
Pelvic pain in people with a uterus and ovaries is extremely common in the general population with 17%–81% reporting dysmenorrhea, 8%–22% reporting dyspareunia, and 2%–24% reporting noncyclical pain.14 Chronic pelvic pain persisting beyond 6 months affects 1 in 7.15 Causes are multifactorial and rarely reflect a single pathological process.16 There is a considerable economic burden on people experiencing chronic pelvic pain and on health care systems worldwide.17 Diagnosis and management can be challenging and require an individualized approach.18
As clinicians (gynecologists, endocrinologists, and physiotherapists), we have seen increasing numbers of trans individuals on testosterone seeking assistance to relieve symptoms of pelvic pain. However, there is a paucity of data regarding pelvic pain in trans individuals using testosterone GAHT.1,19 Given that estradiol deficiency may lead to atrophic vaginitis, we hypothesized that pelvic pain in trans people using testosterone would be predominantly lower abdominal and would worsen with symptoms of genital dryness or penetrative sexual activities. Furthermore, we hypothesized that pre-existing endometriosis, vulvodynia, or vaginismus would be risk factors. Given the limited research on the prevalence and/or the characteristics of pelvic pain experienced by individuals using testosterone GAHT, this was an exploratory study aiming to identify the characteristics of pelvic pain in trans people using testosterone GAHT and to explore potential factors associated with experiencing pelvic pain after commencing testosterone GAHT.
Materials and Methods
Participant recruitment
Participants in this study were recruited from a larger longitudinal Australian trans health study known as TRANSform. Inclusion criteria for TRANSform were assessed by three screening questions: (a) currently living in Australia; (b) identification as trans (“is your gender different to what was presumed for you at birth?”); and (c) 16 years of age or older. Participants were recruited using a nonprobability snowball sampling approach with recruitment calls posted on social media (Facebook and Instagram) and shared widely by trans and gender diverse community support groups and organizations in Australia.
A total of 670 TRANSform participants who indicated that they were using testosterone therapy for gender affirmation were emailed an individualized link to a survey titled “Pain experiences in trans men and trans masculine people using testosterone survey.” This online cross-sectional survey was open between August 28, 2020, and December 31, 2020. Written informed consent was not obtained; however, the survey preamble outlined that completing the survey implied consent.
Survey design and ethical approval
The survey was designed collaboratively by our core team of researchers (S.Z., A.F.Q.W., T.C., and K.E.), who are members of the Australian trans community, and clinicians specialized in trans health care. Survey data were collected and managed using REDCap electronic data capture tools hosted at The University of Melbourne. The study was completed in accordance with the Declaration of Helsinki as revised in 2013 and received ethical and governance approval by the Austin Health Human Research Ethics Committee (reference No. HREC/57155/Austin-2019), ACON Research Ethics Review Committee (reference No. 2020/03), and the Thorne Harbour Health Community Research Endorsement Panel (reference No. THH/CREP 20-006). A small participation incentive (AUD$5 gift card) was provided for completion.
Survey questions are outlined in detail in the Appendix. In brief, demographic data, testosterone formulation, dosage, duration of use, and self-reported testosterone concentrations were obtained. Participants were asked to describe characteristics and location of pelvic pain and rate severity, as well as compare the presence of pelvic pain before and after commencing testosterone therapy for gender affirmation. Potential associated factors were explored, including persistent menstruation; presence of genital dryness; history of hysterectomy or oophorectomy; presence of pain with sexual activities; use of intrauterine device; and known diagnoses of depression, anxiety, post-traumatic stress disorder (PTSD), endometriosis, vulvodynia (pain in the area around the vulva, not necessarily with touch), or vaginismus (involuntary tightening of the muscles around the vagina, not necessarily with penetration). The number of pregnancies (including miscarriages and terminations) and number of live births were also determined.
Data analysis
Participant characteristics are reported as frequency (percentage) for categorical variables, and median (interquartile range) as appropriate for not normally distributed data. Logistic regression was used to estimate the effects of possible factors contributing to pain on the odds of experiencing pain after starting testosterone. The factors considered in the regression were selected before performing the analysis based on potential risk factors for pelvic pain from expert opinion (given the lack of published research in this field). Results are reported as odds ratios (OR) with corresponding 95% confidence intervals. This is a complete case analysis with an alpha level of 5% (p < 0.05) considered statistically significant. Statistical analyses were performed using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Of the 670 trans people presumed female at birth and using testosterone GAHT, who were invited to complete the survey, a total of 506 responded. After removing duplicates and incomplete responses, 486 valid responses remained.
Characteristics of participants
Detailed characteristics are outlined in Table 1. The median age of the 486 respondents was 27 years (23–34 years). Twenty respondents were between 50 and 67 years of age (presumed postmenopausal). Twenty (4.1%) of the respondents identified as Aboriginal or Torres Strait Islander, 7 (1.4%) reported having a variation of sex characteristics (intersex), and a further 45 (9.3%) indicated they did not know if they had a variation of sex characteristics. The median duration of testosterone GAHT use was 32 months (14.0–60.5 months) with intramuscular testosterone undecanoate injections, the most used formulation.
Table 1.
Characteristics of the Study Sample
| Characteristic | N | % |
|---|---|---|
| Age (years) (N = 479) | ||
| 16–25 | 192 | 40.3 |
| 26–35 | 190 | 39.7 |
| 36–45 | 62 | 12.9 |
| 46–55 | 25 | 5.2 |
| 56–65 | 9 | 1.9 |
| 66–75 | 1 | 0.2 |
| Limited gender category (N = 486) | ||
| Man/trans man | 373 | 76.8 |
| Nonbinary | 113 | 23.3 |
| Testosterone formulation (N = 486)a | ||
| Testosterone undecanoate (Reandron) | 358 | 73.7 |
| Testosterone enanthate (Primoteston depot) | 38 | 7.8 |
| Testosterone esters (Sustanon 250) | 10 | 2.1 |
| Testosterone 1% gel (Testogel) | 62 | 12.8 |
| Testosterone 2% gel (Testavan) | 10 | 2.1 |
| Testosterone 5% cream (Androforte 5) | 11 | 2.3 |
| Testosterone 2% cream (Androforte 2) | 1 | 0.2 |
| Other | 3 | 0.6 |
| Consistency of testosterone use (N = 485) | ||
| Consistent use since starting | 426 | 87.8 |
| Regularly stop and start use | 10 | 2.1 |
| Other (e.g., change in dosage or formulation) | 46 | 9.5 |
| Unsure/prefer not to say | 3 | 0.6 |
Multiple responses allowed for this question so total responses do not sum to 100%.
Characteristics of pain in people experiencing pain after commencing testosterone therapy
A total of 351 (72.2%) of the study sample experienced pelvic pain after starting testosterone therapy. Of those 351 respondents, 316 (90%) reported pelvic pain “sometimes” and 35 (10%) reported pelvic pain “always or almost always.” Of the 20 respondents older than 50 years and presumed postmenopausal, 9 (45%) reported pelvic pain “sometimes” and 2 (10%) reported pelvic pain “always or almost always.” A majority of the 351 participants (N = 345, 98.3%) reported some form of pelvic pain before starting testosterone therapy. This included 190 (65.5%) who “always or almost always” and 89 (30.7%) who “sometimes” experienced pain around menstruation, 48 (14.3%) who “always or almost always” and 102 (30.5%) who “sometimes” experienced assumed ovulation pain, and 31 (9.3%) who “always or almost always” and 140 (41.9%) who “sometimes” experienced pain between menstrual periods.
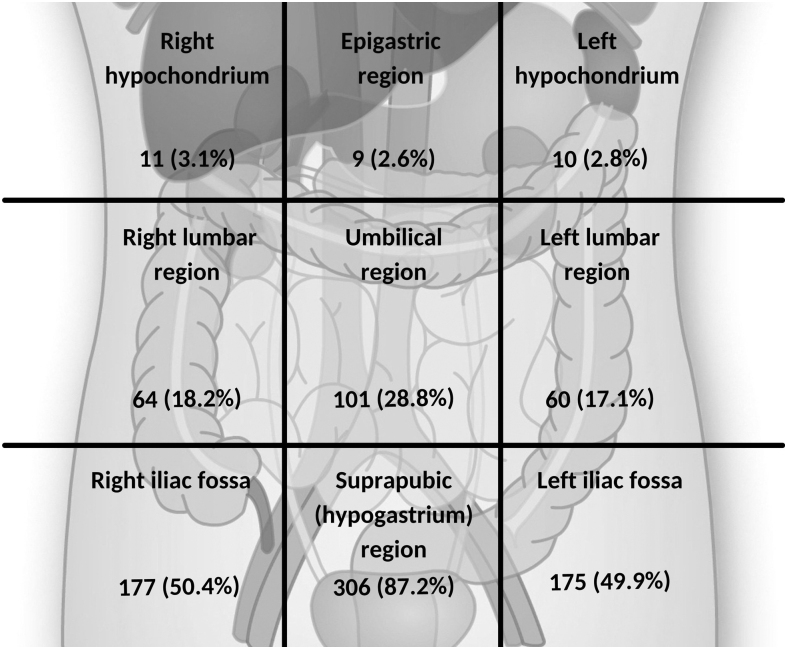
The most common description of pain was cramping (described by 72.6%), followed by aching (58.1%), stabbing (39.9%), and sharp (33.9%). The pain was most commonly located in the hypogastric region (described by 87.2% of respondents) (Fig. 1). The median score for pain severity after commencement of testosterone GAHT on a scale from 0 to 10 (most severe pain) was 6.2 (4.0–7.7). This was similar to the median score of 6.7 (5.5–7.8) reported for pain severity around menstruation before commencement of testosterone GAHT. Consistent with this, the median score in response to the question “How does the pelvic pain you experience using testosterone compare to the pelvic pain you experienced before starting testosterone?” (0 = much less severe, 5 = about the same to 10 = much more severe), was 4.3 (2.2–6.8).
FIG. 1.
Diagram of abdominal and pelvic regions (N = 351). Location of pelvic pain selected by number of respondents describing pelvic pain since starting testosterone therapy. Multiple responses allowed for this question so total responses do not sum to 100%.
Associated factors
Multivariable regression demonstrated that there were higher odds of reporting pelvic pain after starting testosterone in people also experiencing persistent menstruation (OR = 4.46 [1.33–14.97]), or who had a current or previous diagnosis of PTSD (OR = 2.50 [1.07–5.85]) (Table 2). Pelvic pain was also positively associated with pain with orgasm (OR = 32.72 [10.65–100.52]). A total of 11.3% (N = 39) reported that pelvic pain “always or almost always” resulted in them stopping sexual activity or made them consider alternative ways/methods of being sexually active, and 39.2% (N = 135) reported this was “sometimes” the case.
Table 2.
Factors Associated with Pain After Starting Testosterone Therapy
| Factors | Pelvic pain since commencing testosterone (N = 351) N (%) | No pelvic pain since commencing testosterone (N = 122) N (%) | Odds ratio (95% CI)a | p Valuea |
|---|---|---|---|---|
| Persistent menstruation | ||||
| Yes | 53 (16.0%) | 12 (11.0%) | ||
| No | 266 (80.1%) | 94 (86.2%) | ||
| Unsure/prefer not to say | 13 (3.9%) | 3 (2.8%) | 4.46 (1.33–14.97) | 0.015 |
| Genital dryness | ||||
| Sometimes/always | 192 (54.7%) | 50 (41.3%) | ||
| Never | 140 (39.9%) | 65 (53.7%) | ||
| Unsure/prefer not to say | 19 (5.4%) | 6 (5.0%) | 1.82 (0.89–3.74) | 0.103 |
| Intrauterine device in situ | ||||
| Yes | 38 (10.8%) | 9 (7.4%) | ||
| No | 312 (88.9%) | 112 (92.6%) | ||
| Unsure/prefer not to say | 1 (0.3%) | 0 (0.0%) | 1.74 (0.37–8.11) | 0.480 |
| Duration of testosterone therapy (months) | ||||
| Median (IQR) | 34 (17–61) | 24 (10–57) | 1.00 (1.00–1.01) | 0.524 |
| Diagnosis of endometriosis | ||||
| Yes | 39 (11.1%) | 3 (2.5%) | ||
| No | 302 (86.3%) | 117 (96.7%) | ||
| Unsure/prefer not to say | 9 (2.6%) | 1 (0.8%) | 4.41 (0.41–47.54) | 0.221 |
| Diagnosis of vulvodynia or vaginismus | ||||
| Yes | 67 (19.1%) | 8 (6.6%) | ||
| No | 284 (80.9%) | 114 (93.4%) | 1.95 (0.66–5.78) | 0.230 |
| Previous pregnancy | ||||
| None | 311 (88.6%) | 109 (90.1%) | ||
| One or more | 39 (11.1%) | 12 (9.9%) | ||
| Unsure/prefer not to say | 1 (0.3%) | 0 (0.0%) | 1.17 (0.31–4.45) | 0.821 |
| Current or previous PTSD | ||||
| Current/previous | 122 (35.1%) | 17 (14.0%) | ||
| Never | 194 (55.7%) | 92 (76.0%) | ||
| Unsure/prefer not to say | 32 (9.2%) | 12 (9.9%) | 2.50 (1.07–5.85) | 0.035 |
| Current or previous depression | ||||
| Current/previous | 292 (83.2%) | 82 (67.2%) | ||
| Never | 50 (14.2%) | 34 (27.9%) | ||
| Unsure/prefer not to say | 9 (2.6%) | 6 (4.9%) | 2.62 (0.86–7.98) | 0.091 |
| Current or previous anxiety | ||||
| Current/previous | 280 (80.0%) | 78 (65.0%) | ||
| Never | 59 (16.9%) | 35 (29.2%) | ||
| Unsure/prefer not to say | 11 (3.1%) | 7 (5.8%) | 0.97 (0.32–2.95) | 0.961 |
| Obesity | ||||
| Body mass index <30 kg/m2 | 232 (66.7%) | 96 (80.0%) | ||
| Body mass index ≥30 kg/m2 | 116 (33.3%) | 24 (20.0%) | 1.38 (0.62–3.07) | 0.425 |
| Pain with orgasm | ||||
| Sometimes/always | 208 (60.1%) | 10 (8.6%) | ||
| Never | 125 (36.1%) | 99 (85.3%) | ||
| Unsure/prefer not to say | 13 (3.8%) | 7 (6.0%) | 32.72 (10.65–100.52) | <0.0001 |
| Pain with touching of external genitalia | ||||
| Sometimes/always | 127 (36.9%) | 23 (19.8%) | ||
| Never | 212 (61.6%) | 93 (80.2%) | ||
| Unsure/prefer not to say | 5 (1.5%) | 0 (0.0%) | 0.84 (0.32–2.16) | 0.715 |
| Pain with penetrative sexual activities | ||||
| Sometime/always | 230 (66.5%) | 50 (43.1%) | ||
| Never | 60 (17.3%) | 29 (25.0%) | ||
| Unsure/prefer not to say | 9 (2.6%) | 2 (1.7%) | ||
| Do not do penetrative activities | 47 (13.6%) | 35 (30.2%) | 1.18 (0.58–2.40) | 0.640 |
“Unsure/Prefer not to say” was treated as missing and excluded from analysis. Bold values denote statistical significance at the p < 0.05 level.
Odds ratio (95% CI) and p-values from logistic regression mutually adjusted for all factors presented in the table.
CI, confidence interval; IQR, interquartile range; PTSD, post-traumatic stress disorder.
Treatments for pelvic pain in people using testosterone
The most reported treatments used for relieving pelvic pain included analgesic medication “pain killers” (56.7%, N = 199), with nonsteroidal anti-inflammatory drugs (NSAIDs; Ibuprofen, Diclofenac and Aspirin) and paracetamol reported most as being helpful (Table 3). Of the small number of participants (n = 26) who had undergone hysterectomy, pelvic pain was the most common indicator for surgery (61.5%), followed by gender dysphoria (46.2%). A majority (72%) reported reduction in pain, while 20% reported little to no change in their pelvic pain and two reported an increase in pelvic pain following hysterectomy. However, given the small sample, inferences are limited.
Table 3.
Treatments for Pelvic Pain in People Using Testosterone
| Characteristic | N | % |
|---|---|---|
| Types of treatment reported as helpful (N = 351)a | ||
| Pain killers | 199 | 56.7 |
| Heat | 160 | 45.6 |
| Testosterone therapy | 77 | 21.9 |
| Other (e.g., massage, progesterone implant, IUD) | 63 | 17.9 |
| Cannabis | 40 | 11.4 |
| Exercise | 39 | 11.1 |
| Unsure/prefer not to say | 22 | 6.3 |
| Estrogen containing vaginal cream or pessary | 16 | 4.6 |
| Progesterone tablet | 9 | 2.6 |
| Danazol tablets | 0 | 0.0 |
| GnRH agonists | 0 | 0.0 |
| Types of painkillers reported as helpful (N = 351)a | ||
| Nonsteroidal anti-inflammatory drugs (Ibuprofen, Diclofenac and Aspirin) | 163 | 46.4 |
| Paracetamol | 108 | 30.8 |
| Combination of paracetamol and Ibuprofen | 47 | 13.4 |
| Morphine | 34 | 9.7 |
| Tramadol | 10 | 2.8 |
| Types of treatment reported as unhelpful (N = 351)a | ||
| Pain killers | 64 | 18.2 |
| Exercise | 61 | 17.4 |
| Unsure/prefer not to say | 52 | 14.8 |
| Heat | 48 | 13.7 |
| Testosterone therapy | 32 | 9.1 |
| Progesterone tablet | 21 | 6.0 |
| Other (e.g., progesterone implant, IUD) | 16 | 4.6 |
| Estrogen-containing vaginal cream or pessary | 12 | 3.4 |
| Cannabis | 12 | 3.4 |
| GnRH agonists | 2 | 0.6 |
| Danazol tablets | 1 | 0.3 |
| Impact of hysterectomy on pain (N = 25)* | ||
| (0 to 29) Pain is worse since hysterectomy | 2 | 8.0 |
| (30 to 69) Pain is about the same | 5 | 20.0 |
| (70 to 100) Pain is better since hysterectomy | 18 | 72.0 |
| Reasons for hysterectomy (N = 26)a | ||
| Pelvic pain | 16 | 61.5 |
| Gender dysphoria | 12 | 46.2 |
| Ongoing bleeding | 5 | 19.2 |
| Other (e.g., cancer diagnosis, phalloplasty, endometriosis) | 11 | 42.3 |
| Impact of oophorectomy on pain (N = 21) | ||
| (score 0 to 29) Pain is worse since oophorectomy | 3 | 14.3 |
| (score 30 to 69) Pain is about the same | 4 | 19.0 |
| (score 70 to 100) Pain is better since oophorectomy | 14 | 66.7 |
Multiple responses allowed for this question so total responses do not sum to 100%.
IUD, intrauterine device.
Discussion
Main findings
Pelvic pain after initiation of testosterone therapy was reported by 72.2% of trans people responding to this online survey. Cramping pain in the suprapubic (hypogastric) regions were the most common descriptors. Factors associated with increased odds of reporting pain with testosterone were persistent menstruation, pain with orgasm, and current or previous diagnoses of PTSD. NSAIDs were most commonly used to relieve pain. Of the small number of participants who had undergone hysterectomy, a majority reported reduction in pain. Consistent with our hypothesis and the only other published study describing pelvic pain in trans people using testosterone GAHT, ∼70% of respondents reported pelvic pain with the most common description as cramping, most commonly in the hypogastrium or suprapubic region, followed by right and left iliac regions.1
Although our exploratory study cannot determine causation or mechanisms of pain, the increased likelihood of reporting pain in people with persistent menstruation and orgasm may suggest increased pelvic floor muscle dysfunction.20 It is known that levator ani, the collective group of pelvic floor muscles consisting of the deeper layer of pubococcygeus, the iliococcygeus, and the puborectalis, as well as superficial perineal muscles such as the bulbospongiosus, are enriched with androgen receptors and exquisitely androgen sensitive in male humans, and in male rodents.21 Androgen-receptor knockout mice, and men who have androgen deprivation therapy for prostate cancer have a marked reduction in the size of the levator ani muscle.22,23 Androgens have been shown in female humans and rats to have anabolic effects on pelvic floor muscles.24–26 As such, it is plausible that testosterone GAHT may affect the pelvic floor and perineal muscle, and therefore may contribute to the experience of pain.
While pain from endometriosis has been described to worsen with orgasm and penetrative sexual activity27 and could explain pelvic pain after commencing testosterone therapy, previous research has suggested that this is unlikely.19 A review of 67 people who had gender-affirming hysterectomy, among whom 51% had reported pelvic pain, found intraoperative endometriosis in 32% of those who reported pain as well as in 22% of those without pain.19 Our analysis did not show an association between having a diagnosis of endometriosis, vaginismus, vulvodynia, or genital dryness and pelvic pain.
Participants who reported persistent menstruation had significantly higher odds (OR = 4.46) of reporting pain after starting testosterone. Individuals who do have persistent vaginal bleeding would generally be continuing to have typical rises and falls in estradiol and progesterone concentrations over the course of a menstrual cycle. Pelvic pain in people with persistent menstruation may arise from factors such as dysmenorrhea from myometrial contractions, the release of inflammatory mediators that cause endometrial shedding, or pelvic floor muscle dysfunction. Menstruation with fluctuations in estradiol and testosterone levels have been demonstrated to affect pelvic floor muscle activity with significantly higher levels of muscle tone in the luteal phase relative to the follicular and ovulatory phases.28
Sexual dysfunction among trans people has been reported to be very common, likely compounded by increased sexual desire after commencing testosterone therapy.29 However, there is little published research.30 Those who had pain with orgasm had significantly elevated odds (OR = 32.72) of reporting pelvic pain after starting testosterone GAHT. Pain with orgasm or dysorgasmia is one of the least understood and poorly studied areas in sexual medicine, involving a complex interplay of psychological, neural, vascular, and endocrine factors. Dysorgasmia may be the result of bladder neck contractions, uterine neuroinflammation, uterine contractions, and/or pelvic floor musculature dystonia.31,32 As such, treatment for sexual pain would be best tailored to the individual with a multidisciplinary approach involving gynecology, physical therapy, pain management, sexual therapy, and mental health professionals who specialize in chronic pain.33 Further research is needed on etiologies and evaluating multimodal approaches.
There is a known clear association between chronic pain and PTSD in people of all genders, explained by both genetic and environmental factors.34,35 We observed that people who had a current or previous diagnosis of PTSD had higher odds (OR = 2.50) of reporting pelvic pain after starting testosterone GAHT. Research has previously shown that in cisgender women with chronic pelvic pain, there is an increased prevalence of abuse experiences, high number of major life events, and diagnosis of PTSD (but not depression).36,37
One study in 107 cisgender women suggests that temporally, PTSD appears to precede the diagnosis of chronic pelvic pain supporting a partial role for PTSD or its trauma-related trigger in the pathophysiology of chronic pelvic pain.38 Notably, previous studies in people presumed to be female have suggested an independent association between chronic pelvic pain and anxiety, depression and mixed anxiety, and depressive disorder.39 This was not observed in our analyses, which potentially may be related to the high prevalence of depression and anxiety among trans people overall.40
Clinical implications
Treatment of pelvic pain can be challenging in the general population.18 A multidisciplinary biopsychosocial approach that addresses contribution of various factors to the individual is needed.41 This may include medical therapies, pelvic floor physical therapy, addressing sexual function, hypersensitivity to pain, and psychological factors such as PTSD.41 Respondents to this survey had reported various self-management strategies. Over-the-counter pain-relieving medications in the form of paracetamol, NSAIDs, and heat were the most frequent strategies reported to manage pelvic pain. In a Cochrane review of management of dysmenorrhea, NSAIDs and heat were recommended as first-line treatment to alleviate pain symptoms produced by the release of prostaglandins from the endometrial lining. It is recommended that these strategies are initiated 48 hours before onset of menses.42 Irregular bleeding and amenorrhea may explain the ineffectiveness of these strategies in this population, with limited warning of the onset of breakthrough bleeding episodes.9
Many trans people seek hysterectomy and/or oophorectomy as part of gender affirmation, or due to pelvic pain or ongoing or abnormal bleeding. Of the individuals in this study who had a hysterectomy, 72% reported relief of pelvic pain symptoms after hysterectomy. While the overall number of respondents undergoing a hysterectomy and/or oophorectomy was too small for meaningful statistical analyses, surgery would indeed cure persistent menstruation, which was much more likely in people reporting pain after commencing testosterone therapy. Moreover, cisgender women have also reported resolution or decrease in pelvic pain following hysterectomy.43 It must be noted that some individuals in this study reported little to no change, or an increase in pelvic pain following hysterectomy. Further research is warranted.
While further studies need to evaluate the possibility of high pelvic floor muscle tone as a causative factor for pelvic pain in trans people after starting testosterone for gender affirmation, a recent systematic review of pelvic floor physical therapy to release myofascial trigger points found positive beneficial effects, particularly in people with chronic pelvic pain and dyspareunia.44 This systematic review did not specifically include studies involving trans people, but did include both men and women with a range of conditions, including dyspareunia, provoked vulvodynia, interstitial cystitis, painful bladder syndrome, chronic prostatitis, or chronic pelvic pain syndrome.44
Given the lack of current treatments available to alleviate often debilitating pelvic pain in trans people on testosterone therapy, pelvic floor physical therapy may be a low-risk treatment strategy.41 A pelvic floor muscle down-training program, which focuses on the quality of the muscle function and relaxation phase of the contraction can be particularly helpful in this clinical setting.
Strengths and limitations
This study has multiple limitations. As this was an online survey recruited through nonprobability sampling, this may have encouraged a greater proportion of responders who were younger individuals and may not be representative of the broader trans community. Of the 670 people participating in the larger TRANSform study, who indicated they were using testosterone therapy and were invited to participate in this testosterone and pain study, 486 responded, corresponding to a response rate of 72.5%.
Given that potential participants were invited to participate in a study titled “Pain experiences in trans men and trans masculine people using testosterone survey,” there may well have been responder bias, with individuals experiencing pain syndromes more likely to respond and overrepresenting the proportion of individuals on testosterone experiencing pelvic pain. Furthermore, as participants were asked to recall experiences from before commencing testosterone, there is the possibility of recall bias. Medical conditions were self-reported, and we were unable to confirm diagnoses.
We also acknowledge that, although a number of participants indicated that they had or were unsure about whether they had a variation of sex characteristics (intersex), participants were not asked whether they had a uterus and ovaries as part of their birth anatomy. In addition, questions regarding penetrative sexual activities did not specify vaginal or anal penetration. Standardized questionnaires for sexual dysfunction were not included, as these are not validated for trans and gender diverse populations. The impact of testosterone therapy on sexual function warrants further investigation. Despite the limitations, this survey is the largest study to date exploring pelvic pain in trans people using testosterone therapy and is hypothesis generating for future studies examining the pathophysiology of, or the effectiveness of interventions on pelvic pain.
Conclusions
Pelvic pain occurring after commencing testosterone GAHT is frequently reported by trans people. The increased likelihood of reporting pain in people with persistent menstruation and orgasm, as well as the known androgen sensitivity of the pelvic floor musculature, warrant further research on pelvic floor muscle dysfunction as a contributor.20 Until further evidence is available, a tailored multidisciplinary trauma-informed approach addressing the needs of the individual with pelvic pain should be provided, which may encompass pain management, sexual function, addressing persistent menstruation, and mental health.
Data Availability Statement
Deidentified participant data are available upon reasonable request from the corresponding author through email (adac@unimelb.edu.au), provided that the related research is deemed to be of benefit to the trans and gender diverse community, and has undergone Austin Health Human Research Ethics Committee approval in the form of an amendment.
Acknowledgments
The authorship team includes trans people of diverse genders, including male and nonbinary. Authors would like to thank MCATS (Melbourne Clinical and Translational Sciences research platform), for the administrative and technical support that greatly facilitated this research.
Appendix
Online Survey: Pain Experiences in Trans Men and Trans Masculine People Using Testosterone
The following survey will be asking directly about experiences of pain related to genitals, ovaries, menstruation, and testosterone. Thank you for participating in this research and please seek support if you become distressed. All questions are optional and you can return to this survey if you need some time.
Testosterone Use
This first set of questions will ask you about your testosterone use and levels.
-
1.
What date (approximately) did you start testosterone therapy? [dd/mm/yyyy]
-
2.
What type of testosterone are you currently using? (please select all that apply)
Injection - Reandron
Injection – Primoteston
Injection – Sustanon
Gel/cream – Testogel
Gel/Cream – Androforte
Implant
Other
-
Unsure/Prefer not to say
You selected “other.” Can you please elaborate? [free-text]
-
3.
What were your testosterone levels on your most recent blood test? [nmol/L]
-
4.
How have you been using testosterone?
I have used testosterone consistently since I started
I regularly stop and start testosterone
Other
-
Unsure/prefer not to say
You selected “other.” Can you please elaborate? [free-text]
-
5.
What dose of testosterone are you on?
Full dose
Half dose
Quarter dose
Other (e.g., change between full dose and lower dose)
-
Unsure/prefer not to say
You selected “other.” Can you please elaborate? [free-text]
Pelvic Pain Before Testosterone
The next set of questions will ask you about your experiences of pelvic pain before starting testosterone, including questions related to menstruation. We understand that some of the terminology or subject matter may make some people uncomfortable. Please skip any question you do not want to answer.
-
6.
Did you ever experience pelvic pain before starting testosterone therapy?
Yes
No
Unsure/prefer not to say
-
7.
Did you have periods/bleeding before starting testosterone?
Yes, monthly
Yes, more often than monthly
Sometimes/occasionally (less often than monthly)
Never
-
Unsure/prefer not to say
If yes or sometimes to periods, before starting testosterone, on average, how many days did your periods/bleeding go on for?
1–2 days
3–4 days
5–6 days
6–7 days
-
8+ days
If yes or sometimes to periods, before starting testosterone, how would you describe your average period/bleeding?
Very light
Light to medium
Medium to heavy
-
Very heavy
If yes or sometimes to periods, did you experience pain around the time of bleeding/periods? (i.e., in the couple of days before or during bleeding/periods)
Always or almost always
Sometimes
Never
-
Unsure/Prefer not to say
If always or sometimes to pain around periods, on a scale of 0 to 10 (10 = most severe pain), how severe was this pain when it occurred?
If yes or sometimes to periods, did you experience pelvic pain between bleeding/periods?
Always or almost always
Sometimes
Never
-
Unsure/prefer not to say
If always or sometimes, on a scale of 0 to 10 (10 = most severe pain) how severe was this pain when it occurred?
If yes or sometimes to periods, did you experience pelvic pain at or around the time of ovulation?
Always or almost always
Sometimes
Never
-
Unsure/prefer not to say
If always or sometimes, on a scale of 0 to 10 (10 = most severe pain) how severe was this pain when it occurred?
If yes or sometimes to pain before testosterone, did you use medications to suppress periods or reduce pelvic pain?
Yes
No
-
Unsure/prefer not to say
If yes to medication, what pain relief or medications did you use? (select all that apply)
Hormonal
Pain killers
Cannabis
Other
-
Unsure/prefer not to say
You selected “other.” Can you please elaborate? [free text]
If hormonal, what sort of hormonal therapy? [free text]
If hormonal, did the hormonal therapy work?
Yes
Sometimes
No
-
Unsure/prefer not to say
If pain killers, what sort of pain killers? [free-text]
If pain killers, did the pain killers work?
Yes
Sometimes
No
-
Unsure/prefer not to say
If cannabis, did cannabis work? Yes
Sometimes
No
-
Unsure/prefer not to say
If pain killers or cannabis, where did you source this pain relief? (please select all that apply)
Prescription from a doctor
Over the counter
Friends
The internet
A dealer
Other
-
Unsure/prefer not to say
You selected “other.” Can you please elaborate? [free-text]
Pelvic Pain Since Starting Testosterone
The next set of questions will ask you about your experiences of pelvic pain since starting testosterone, including questions related to menstruation. We understand that some of the terminology or subject matter may make some people uncomfortable. Please skip any question you do not want to answer.
-
8.
Have you experienced pelvic pain since commencing testosterone therapy?
Always or almost always
Sometimes
Never
-
Unsure/prefer not to say
If always or sometimes pain on testosterone, on a scale of 0 to 10 (10 = most severe pain), how severe is your current pelvic pain?
If always or sometimes pain on testosterone, is there any trigger for your pelvic pain? (e.g., bleeding, exercise) [free-text]
If always or sometimes pain on testosterone, is there a time relationship to last dose of testosterone? [free-text]
If always or sometimes pain on testosterone, what treatment or strategies (if any) have eased your pelvic pain? (please select all that apply)
Hormonal medication
Pain killers
Cannabis
Heat
Exercise
Other
-
Unsure/prefer not to say
If always or sometimes pain on testosterone, what treatments or strategies have you tried that have not worked? (please select all that apply)
Hormonal medication
Pain killers
Cannabis
Heat
Exercise
Other
Unsure/prefer not to say
-
9.
Have your periods/bleeding stopped since starting testosterone?
Yes
No
-
Unsure/prefer not to say
If yes periods stopped, how long did it take for your periods/bleeding to stop after starting testosterone?
1–3 months
3–6 months
6–9 months
9–12 months
-
Longer than 12 months
If no to periods stopping, how would you best describe the bleeding/periods you have experienced since starting testosterone?
Occasional spotting
Regular spotting
Occasional bleeding
Regular bleeding (similar to a monthly period)
-
Bleeding more often than not/ongoing bleeding
If no to periods stopping, have you been offered any option to stop the bleeding/periods?
Yes
No
-
Unsure/prefer not to say
If yes, can you describe the option/s you have been offered to stop the bleeding/periods? [free-text]
-
10.
Do you experience genital dryness?
Always
Sometimes
Never
-
Unsure/prefer not to say
If pain before and on T, how does the pelvic pain you experience using testosterone compare to the pelvic pain you experienced before starting testosterone? (0 = much less severe and 10 = much more severe)
Sexual Activity and Pelvic Pain
This next set of questions asks you about your sexual activity since starting on testosterone. We understand that some of the terminology or subject matter may make some people uncomfortable. Please skip any question you do not want to answer.
-
11.
Are you sexually active (including masturbation)?
Yes
No
-
Unsure/prefer not to say
If yes to sexual activity, does touching of your external genitalia cause pain?
Always or almost always
Sometimes
Never
-
Unsure/prefer not to say
If always or sometimes to pain with external touch, is this pain the same as the pelvic pain?
Yes
Sometimes
No
-
Unsure/prefer not to say
If yes to sexual activity, do penetrative sexual activities, provoke pain?
Always or almost always
Sometimes
Never
Unsure/prefer not to say
-
Do not do penetrative sexual activities
If always or sometimes to pain with penetration, is this pain the same as the pelvic pain?
Yes
Sometimes
No
-
Unsure/prefer not to say
If yes to sexual activity, does orgasm cause pain?
Always or almost always
Sometimes
Never
-
Unsure/prefer not to say
If yes to pain with orgasm, is this pain the same as the pelvic pain?
Yes
Sometimes
No
-
Unsure/prefer not to say
If yes to sexual activity and pelvic pain on testosterone, does the pelvic pain stop you or make you consider alternative ways/methods of being sexually active?
Always or almost always
Sometimes
Never
Unsure/prefer not to say
Pelvic Surgeries and Diagnoses
The next set of questions asks you about pelvic surgeries and diagnoses. We understand that some of the terminology or subject matter may make some people uncomfortable. Please skip any question you do not want to answer.
-
12.
Have you ever had an intrauterine device (IUD)?
Yes
No
-
Unsure/prefer not to say
If yes to IUD, did the IUD cause/provoke physical pain?
Always or almost always
Sometimes
Never
-
Unsure/prefer not to say
If yes to pain with IUD, is this pain the same as the pelvic pain?
Yes
No
Unsure/prefer not to say
-
13.
Have you ever been diagnosed with endometriosis?
Yes
No
-
Unsure/prefer not to say
If yes to endometriosis, how was this diagnosed?
No test, just my pain history
By ultrasound
By laparoscopy
-
Unsure/prefer not to say
If yes to endometriosis, what date (approximately) was this diagnosed? [dd/mm/yy]
-
14.
Have you had an ultrasound of your pelvis to look for the cause of the pelvic pain?
Yes
No
-
Unsure/prefer not to say
If yes to ultrasound, was it reported as normal?
Yes
No
-
Unsure/prefer not to say
If not normal, what did the ultrasound show? [free-text]
-
15.
Have you had a hysterectomy (removal of uterus)?
Yes
No
-
Unsure/prefer not to say
If yes to hysterectomy, what was the reason for the hysterectomy? (please select all that apply)
Gender dysphoria
Pain
Ongoing bleeding
Other
-
Unsure/prefer not to say
If yes to hysterectomy, what date (approximately) did you have this done? [dd/mm/yy]
If yes to hysterectomy, did hysterectomy improve your pelvic pain?
Yes
No
Unsure/prefer not to say
-
16.
Have you had an oophorectomy (removal of ovaries)?
Yes
No
-
Unsure/prefer not to say
If yes to oophorectomy, what date (approximately) did you have this done? [dd/mm/yy]
If yes to oophorectomy, did oophorectomy improve your pelvic pain?
Yes
No
Unsure/prefer not to say
-
17.
Have you ever been diagnosed with any of the following? (please select all that apply)
Vulvodynia
Vaginismus
Polycystic ovary syndrome
-
Genital or pelvic cancer
If cancer, what type/s of cancer did/do you have? [free-text]
-
18.
Do you experience regular/recurrent occurrences of any of the following? (please select all that apply)
Vaginal thrush
Bacterial vaginosis
Urinary tract infection
-
19.
How many pregnancies have you ever had (including miscarriages and terminations)?
None
One or more
-
Unsure/prefer not to say
If one or more pregnancies, how many live births have you had?
None
1
2
3
4
5 or more
Other Pain and Mental Health
The next set of questions will ask you about other pain problems and mental health diagnoses. We understand that some of the terminology or subject matter may make some people uncomfortable. Please skip any question you do not want to answer.
-
20.
Do you have any other pain problems?
Yes
No
-
Unsure/prefer not to say
If yes, please select all that apply.
Migraines
Back pain
Chronic fatigue
Joint pain
Fibromyalgia
Other
-
21.
Have you previously had or currently have a diagnosis of post-traumatic stress disorder?
Current
Previous
Never
Unsure/prefer not to say
-
22.
Have you previously had or currently have a diagnosis of depression?
Current
Previous
Never
Unsure/prefer not to say
-
23.
Have you previously had or currently have a diagnosis of anxiety?
Current
Previous
Never
Unsure/prefer not to say
-
24.
What is your approximate weight in kg? [30–200 kg]
-
25.
What is your approximate height in cm? [100–250 cm]
-
26.
Do you have any other comments about your pelvic pain? [free text]
Footnotes
Correction added on March 28, 2023 after first online publication January 4, 2023: In Table 3, data were mistakenly noted as “Impact of hysterectomy on pain (N = 26)”. The text has been corrected to (N = 25).*
Correction added on March 28, 2023 after first online publication January 4, 2023: In the opening sentence of the Results section beginning with “Among 486 participants (median age = 27 years), 351 (72.42%)”. The text has been corrected to (72.2%).
Authors' Contributions
S.Z.: conceptualization (equal), data curation (lead), formal analysis (lead), methodology (equal), project administration (lead), writing - original draft (lead), and writing - review and editing (lead). L.B.: conceptualization (equal), methodology (equal), writing - original draft (lead), and writing - review and editing (supporting). A.F.Q.W.: conceptualization (equal), formal analysis (lead), methodology (equal), and writing - review and editing (supporting). S.Y.L.: formal analysis (lead) and writing - review and editing (supporting). T.C.: conceptualization (equal), methodology (equal), and writing - review and editing (supporting). L.M.A.: conceptualization (equal), methodology (equal), and writing - review and editing (supporting).
K.E.: conceptualization (equal), methodology (equal), and writing - review and editing (supporting). C.V.E.: conceptualization (equal), methodology (equal), and writing - review and editing (supporting). S.R.G.: conceptualization (equal), methodology (equal), and writing - review and editing (supporting). J.D.Z.: conceptualization (equal), methodology (equal), supervision (lead), and writing - review and editing (supporting). A.S.C.: conceptualization (equal), methodology (equal), project administration (lead), funding acquisition (lead), supervision (lead), writing - original draft (lead), and writing - review and editing (lead).
Author Disclosure Statement
The authors report no conflicts of interest.
Funding Information
A.S.C. is supported by an Australian Government National Health and Medical Research Council Early Career Fellowship (No. 1143333), Investigator Grant (No. 2008956), and The University of Melbourne Dame Kate Campbell Fellowship. L.M.A. is supported by the Research Training Program Scholarship from the Australian Commonwealth Government.
References
- 1. Grimstad FW, Boskey E, Grey M. New-onset abdominopelvic pain after initiation of testosterone therapy among trans-masculine persons: A community-based exploratory survey. LGBT Health 2020;7(5):248–253; doi: 10.1089/lgbt.2019.0258 [DOI] [PubMed] [Google Scholar]
- 2. Crissman HP, Berger MB, Graham LF, et al. Transgender demographics: A household probability sample of US adults, 2014. Am J Public Health 2017;107(2):213–215; doi: 10.2105/AJPH.2016.303571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Åhs JW, Dhejne C, Magnusson C, et al. Proportion of adults in the general population of Stockholm County who want gender-affirming medical treatment. PLoS One 2018;13(10):e0204606; doi: 10.1371/journal.pone.0204606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lai MC, Chiu Y-N, Gadow KD, et al. Correlates of gender dysphoria in Taiwanese university students. Arch Sex Behav 2010;39(6):1415–1428; doi: 10.1007/s10508-009-9570-y [DOI] [PubMed] [Google Scholar]
- 5. Cheung AS, Ooi O, Leemaqz S, et al. Sociodemographic and clinical characteristics of transgender adults in Australia. Transgend Health 2018;3(1):229–238; doi: 10.1089/trgh.2018.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delahunt JW, Denison HJ, Sim DA, et al. Increasing rates of people identifying as transgender presenting to Endocrine Services in the Wellington region. N Z Med J 2018;131(1468):33–42. [PubMed] [Google Scholar]
- 7. Telfer MM, Tollit MA, Feldman D. Transformation of health-care and legal systems for the transgender population: The need for change in Australia. J Paediatr Child Health 2015;51(11):1051–1053; doi: 10.1111/jpc.12994 [DOI] [PubMed] [Google Scholar]
- 8. Cheung AS, Wynne K, Erasmus J, et al. Position statement on the hormonal management of adult transgender and gender diverse individuals. Med J Aust 2019;211(3):127–133; doi: 10.5694/mja2.50259 [DOI] [PubMed] [Google Scholar]
- 9. Grimstad F, Kremen J, Shim J, et al. Breakthrough bleeding in transgender and gender diverse adolescents and young adults on long-term testosterone. J Pediatr Adolesc Gynecol 2021;34(5):706–716; doi: 10.1016/j.jpag.2021.04.004 [DOI] [PubMed] [Google Scholar]
- 10. Defreyne J, Vanwonterghem Y, Collet S, et al. Vaginal bleeding and spotting in transgender men after initiation of testosterone therapy: A prospective cohort study (ENIGI). Int J Transgend Health 2020;21(2):163–175; doi: 10.1080/26895269.2020.1719951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Defreyne J, Elaut E, Kreukels B, et al. Sexual desire changes in transgender individuals upon initiation of hormone treatment: Results from the longitudinal European Network for the Investigation of Gender Incongruence. J Sex Med 2020;17(4):812–825; doi: 10.1016/j.jsxm.2019.12.020 [DOI] [PubMed] [Google Scholar]
- 12. Hawkins M, Deutsch MB, Obedin-Maliver J, et al. Endometrial findings among transgender and gender nonbinary people using testosterone at the time of gender-affirming hysterectomy. Fertil Steril 2021;115(5):1312–1317; doi: 10.1016/j.fertnstert.2020.11.008 [DOI] [PubMed] [Google Scholar]
- 13. Grimstad FW, Fowler KG, New EP, et al. Ovarian histopathology in transmasculine persons on testosterone: A multicenter case series. J Sex Med 2020;17(9):1807–1818; doi: 10.1016/j.jsxm.2020.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: A neglected reproductive health morbidity. BMC Public Health 2006;6(1):177–183; doi: 10.1186/1471-2458-6-177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mathias SD, Kuppermann M, Liberman RF, et al. Chronic pelvic pain: Prevalence, health-related quality of life, and economic correlates. Obstet Gynecol 1996;87(3):321–327; doi: 10.1016/0029-7844(95)00458-0 [DOI] [PubMed] [Google Scholar]
- 16. Vercellini P, Somigliana E, Viganò P, et al. Chronic pelvic pain in women: Etiology, pathogenesis and diagnostic approach. Gynecol Endocrinol 2009;25(3):149–158; doi: 10.1080/09513590802549858 [DOI] [PubMed] [Google Scholar]
- 17. Huang G, Le AL, Goddard Y, et al. A systematic review of the cost of chronic pelvic pain in women. J Obstet Gynaecol Can 2022;44(3):286–293.e283; doi: 10.1016/j.jogc.2021.08.011 [DOI] [PubMed] [Google Scholar]
- 18. Passavanti MB, Pota V, Sansone P, et al. Chronic pelvic pain: Assessment, evaluation, and objectivation. Pain Res Treat 2017;2017:1–15; doi: 10.1155/2017/9472925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferrando CA, Chapman G, Pollard R. Preoperative pain symptoms and the incidence of endometriosis in transgender men undergoing hysterectomy for gender affirmation. J Minim Invasive Gynecol 2021;28(9):1579–1584; doi: 10.1016/j.jmig.2021.01.018 [DOI] [PubMed] [Google Scholar]
- 20. Faubion SS, Shuster LT, Bharucha AE. Recognition and management of nonrelaxing pelvic floor dysfunction. Mayo Clin Proc 2012;87(2):187–193; doi: 10.1016/j.mayocp.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johansen JA, Breedlove SM, Jordan CL. Androgen receptor expression in the levator ani muscle of male mice. J Neuroendocrinol 2007;19(10):823–826; doi: 10.1111/j.1365-2826.2007.01592.x [DOI] [PubMed] [Google Scholar]
- 22. Davey RA, Clarke MV, Russell PK, et al. Androgen action via the androgen receptor in neurons within the brain positively regulates muscle mass in male mice. Endocrinology 2017;158(10):3684–3695; doi: 10.1210/en.2017-00470 [DOI] [PubMed] [Google Scholar]
- 23. Cheung AS, Cunningham C, Ko DK, et al. Selective loss of levator ani and leg muscle volumes in men undergoing androgen deprivation therapy. J Clin Endocrinol Metab 2018;104(6):2229–2238; doi: 10.1210/jc.2018-01954 [DOI] [PubMed] [Google Scholar]
- 24. Ho MH, Bhatia NN, Bhasin S. Anabolic effects of androgens on muscles of female pelvic floor and lower urinary tract. Curr Opin Obstet Gynecol 2004;16(5):405–409; doi: 10.1097/00001703-200410000-00009 [DOI] [PubMed] [Google Scholar]
- 25. Mammadov R, Simsir A, Tuglu I, et al. The effect of testosterone treatment on urodynamic findings and histopathomorphology of pelvic floor muscles in female rats with experimentally induced stress urinary incontinence. Int Urol Nephrol 2011;43(4):1003–1008; doi: 10.1007/s11255-011-9938-5 [DOI] [PubMed] [Google Scholar]
- 26. Ponnusamy S, Sullivan RD, Thiyagarajan T, et al. Tissue selective androgen receptor modulators (SARMs) increase pelvic floor muscle mass in ovariectomized mice. J Cell Biochem 2017;118(3):640–646; doi: 10.1002/jcb.25751 [DOI] [PubMed] [Google Scholar]
- 27. Fairbanks F, Abdo CH, Baracat EC, et al. Endometriosis doubles the risk of sexual dysfunction: A cross-sectional study in a large amount of patients. Gynecol Endocrinol 2017;33(7):544–547; doi: 10.1080/09513590.2017.1302421 [DOI] [PubMed] [Google Scholar]
- 28. Micussi MT, Freitas RP, Angelo PH, et al. Is there a difference in the electromyographic activity of the pelvic floor muscles across the phases of the menstrual cycle? J Phys Ther Sci 2015;27(7):2233–2237; doi: 10.1589/jpts.27.2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kerckhof ME, Kreukels BPC, Nieder TO, et al. Prevalence of sexual dysfunctions in transgender persons: Results from the ENIGI follow-up study. J Sex Med 2019;16(12):2018–2029; doi: 10.1016/j.jsxm.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 30. Sorensen J, Bautista KE, Lamvu G, et al. Evaluation and treatment of female sexual pain: A clinical review. Cureus 2018;10(3):e2379; doi: 10.7759/cureus.2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barnas JL, Pierpaoli S, Ladd P, et al. The prevalence and nature of orgasmic dysfunction after radical prostatectomy. BJU Int 2004;94(4):603–605; doi: 10.1111/j.1464-410X.2004.05009.x [DOI] [PubMed] [Google Scholar]
- 32. Yong PJ. Clinical conundrum: A 33-year-old with pain post-orgasm and a history of endometriosis. J Obstet Gynaecol Can 2020;42(5):625–628; doi: 10.1016/j.jogc.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 33. Committee opinion no. 673: Persistent vulvar pain. Obstet Gynecol 2016;128(3):e78–e84; doi: 10.1097/aog.0000000000001645 [DOI] [PubMed] [Google Scholar]
- 34. Fishbain DA, Pulikal A, Lewis JE, et al. Chronic pain types differ in their reported prevalence of post-traumatic stress disorder (PTSD) and there is consistent evidence that chronic pain is associated with PTSD: An evidence-based structured systematic review. Pain Med 2017;18(4):711–735; doi: 10.1093/pm/pnw065 [DOI] [PubMed] [Google Scholar]
- 35. Gasperi M, Panizzon M, Goldberg J, et al. Posttraumatic stress disorder and chronic pain conditions in men: A twin study. Psychosom Med 2021;83(2):109–117; doi: 10.1097/psy.0000000000000899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heim C, Ehlert U, Hanker JP, et al. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosom Med 1998;60(3):309–318; doi: 10.1097/00006842-199805000-00017 [DOI] [PubMed] [Google Scholar]
- 37. Meltzer-Brody S, Leserman J, Zolnoun D, et al. Trauma and posttraumatic stress disorder in women with chronic pelvic pain. Obstet Gynecol 2007;109(4):902–908; doi: 10.1097/01.Aog.0000258296.35538.88 [DOI] [PubMed] [Google Scholar]
- 38. Chelimsky GG, Yang S, Sanses T, et al. Autonomic neurophysiologic implications of disorders comorbid with bladder pain syndrome vs myofascial pelvic pain. Neurourol Urodyn 2019;38(5):1370–1377; doi: 10.1002/nau.23995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Siqueira-Campos VME, Da Luz RA, de Deus JM, et al. Anxiety and depression in women with and without chronic pelvic pain: Prevalence and associated factors. J Pain Res 2019;12:1223–1233; doi: 10.2147/jpr.S195317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bretherton I, Thrower E, Zwickl S, et al. The health and well-being of transgender Australians: A national community survey. LGBT Health 2021;8(1):42–49; doi: 10.1089/lgbt.2020.0178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grinberg K, Sela Y, Nissanholtz-Gannot R. New insights about chronic pelvic pain syndrome (CPPS). Int J Environ Res Public Health 2020;17(9):3005–3016; doi: 10.3390/ijerph17093005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marjoribanks J, Ayeleke RO, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev 2015;2015(7):CD001751; doi: 10.1002/14651858.CD001751.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hillis SD, Marchbanks PA, Peterson HB. The effectiveness of hysterectomy for chronic pelvic pain. Obstet Gynecol 1995;86(6):941–945; doi: 10.1016/0029-7844(95)00304-A [DOI] [PubMed] [Google Scholar]
- 44. van Reijn-Baggen DA, Han-Geurts IJM, Voorham-van der Zalm PJ, et al. Pelvic floor physical therapy for pelvic floor hypertonicity: A systematic review of treatment efficacy. Sex Med Rev 2022;10(2):209–230; doi: 10.1016/j.sxmr.2021.03.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified participant data are available upon reasonable request from the corresponding author through email (adac@unimelb.edu.au), provided that the related research is deemed to be of benefit to the trans and gender diverse community, and has undergone Austin Health Human Research Ethics Committee approval in the form of an amendment.