Abstract
Purpose of review:
Transition from acute kidney injury (AKI) to chronic kidney disease (CKD) is increasingly accepted. Less well recognized, but supported by very similar data, is development of disease of other organ systems after AKI. Awareness of other-organ sequelae of AKI may inform efforts to improve the care of patients after AKI.
Recent findings:
Stroke, Hypertension, Reproductive risk, Dementia, and Death (SHReDD) are sequelae which occur with increased risk relative to that of non-AKI within 6 months-3 years after AKI diagnosis, and which are supported by preclinical/mechanistic study. Adjusted hazard ratios for these sequelae are strikingly similar to that of AKI-CKD, ranging from 1.2–3.0. Mechanistic studies suggest kidney-centric mechanisms including sodium regulation, volume status regulation, and the renin-angiotensin system are drivers of long-term, extra-renal, change.
Summary:
Further clinical characterization and mechanistic insight is necessary, and may have considerable translational impact. Programs which screen or follow post-AKI patients may increase clinical utility if focus is expanded to include the SHReDD complications.
Keywords: AKI sequelae, reproductive sequelae of AKI, cardiovascular sequelae of AKI, Post-AKI screening
Introduction:
The doctrine “no harm, no foul” directs basketball referees not to penalize players for transgressions which are unlikely to affect the game’s outcome. Derived from 17th-century English legal doctrine de minimis non curat lex—“the law is not troubled by trifles” [1, 2], “no harm, no foul” also became a medical aphorism applied to transient rise in serum creatinine. However, in the last 15 years, observational studies and mechanistic basic science have refuted this aphoristic approach. Strong mechanistic studies have increased recognition of acute kidney injury to chronic kidney disease (AKI-CKD) transition. Although it may no longer be surprising that AKI initiates chronic disease in the kidney, an increasing body of literature suggests we may yet be surprised to find that AKI can initiate long-term disease beyond the kidney. Although in some cases data are sparse, cardiovascular disease, all-cause mortality, reproductive, and cognitive function may be impacted after AKI. Because of the potential for causality, identification of AKI represents a critical moment at which it may be possible to act to prevent or at least identify the burden of future disease. Thus, as with AKI-CKD transition, enumerating and characterizing the transition between AKI and chronic other-organ disease, AKI-CxD, is a first step, which could greatly improve human health. Critical questions include temporality, comorbid risks including concomitant CKD, and impact. New data prior to the COVID-19 pandemic indicates that AKI incidence is high and increasing, perhaps as high as 2 per 1000 population [3]. Since COVID-19 caused worldwide increase in AKI, it is likely the case that long-term outcomes of AKI will have high impact on population health.
Here, we review evidence for AKI-CxD with significant potential human health impact: Stroke, Hypertension, Reproductive risk, Dementia, Death (SHReDD). We summarize mechanisms, estimate impact, propose observational and preclinical investigational strategies, and provide rationale for screening. Our hypothesis is that by viewing AKI-CxD as a single entity with diverse manifestations, the importance of kidney-centered investigation, and thus the probability of successful intervention, may be increased.
Stroke
Stroke is the third leading cause of death due to cardiovascular disease [4]. The annual cost of stroke in the United State is about $103 billion [5]. Multiple reports indicate AKI as a predisposing factor for stroke. Wu et al [6] reported that patients recovered from AKI have a higher incidence of stroke than the patients without AKI. They studied 4,315 propensity-matched recovered, dialysis-requiring AKI and non-AKI patients. After mean follow-up of 3.36 years, the rate of incident stroke was significantly higher in the AKI-recovery patients than in the non-AKI patients (15.6 vs 11.5 per 1,000 person-years). Regarding mechanism, Liu et al reported inflammation and functional changes in the brain after ischemic AKI in mice [7]. They revealed that AKI increased the microvascular permeability in the brain, which resulted in leaky blood–brain barrier. They also reported that ischemic AKI led to increased levels of the proinflammatory chemokines including granulocyte colony stimulating factor which have been implicated in the pathogenesis of stroke [8]. Experiments in a severe rodent renal IRI model (60 minutes bilateral IRI) and in vitro modeling confirm that AKI dysregulates the blood brain barrier, induces neuronal transcriptional change, and induces inflammation, including activation of microglia [9, 10]. A hopeful preclinical insight is provided by Zhao et al; using IRI as a model, they demonstrated direct brain microvascular effect of AKI, which persists at least a week. Brain arterioles of IRI-subjected animals lose angiotensin II (AngII) responsiveness and alter expression of fibroblast growth factor 2, suggesting direct AKI-induced change in vascular physiology which could potentially be amenable to renin-angiotensin system-targeted treatment [11]. The suggestion that AKI induces intracranial inflammation and vascular changes implicated in stroke pathogenesis may prove a starting point toward further clinical investigation and screening. Significant advances in assessment of intracranial inflammation such as novel-contrast MRI, molecular imaging, and cerebrospinal fluid biomarkers may provide avenues for translational study [12, 13].
Hypertension
Hypertension is among the most common and serious health problems; worldwide it is the most common cause of CKD, and an initiator of additional cardiovascular disease [4]. A bidirectional relationship between hypertension and kidney disease has long been suspected; accordingly, clinical and animal studies strongly suggest that AKI causes hypertension. Using retrospective design, Hsu et al studied 2,451 AKI patients without history of hypertension [14]. Blood pressure elevation was observed in 30.6% of AKI patients at 180 days, and 46.1% at 730 days after AKI; this was a significantly greater proportion than in non-AKI patients. AKI was independently associated with elevated blood pressure during follow-up, with more severe AKI associated with higher adjusted risk. Based on this data, it is estimated that AKI potentially causes 22.1 incident hypertension events per 100 person/years [14, 15]. Of greater impact is the increase in hypertension caused by pediatric AKI, reported by Askenazi et al [16]. 6 of 29 (20.6%) hospitalized children developed hypertension within 3–5 years after AKI, highlighting the increased burden of chronic disease initiated in childhood.
Animal studies that demonstrate hypertension developing after AKI suggest potential pathophysiologic mechanisms. Soranno et al reported ischemic AKI-induced hypertension in mice [17–19]. AKI induced by 25-minute bilateral ischemia-reperfusion injury (IRI), caused elevated systolic blood pressure and left ventricular diastolic dysfunction at 1 year in males, but not females. Histone deacetylase (HDAC) inhibition ameliorated the male-only blood pressure elevation and cardiac dysfunction, without changing long-term loss of glomerular filtration rate (GFR). It is unclear whether other regulatory effects of HDAC inhibition might be involved but the lack of change in long-term GFR loss, despite prevention of adverse cardiovascular consequence, argues that cardiovascular disease initiated by AKI may be GFR-independent. Similarly, Spurgeon-Pechman et al reported development of salt sensitive hypertension in rats subjected to IRI [20]; this did not occur in sham-treated rats. Salt-sensitive hypertension and then albuminuria developed a week after initiation of the high-salt diet, suggesting that albuminuria was driven by hypertension; high-salt induced glomerular damage was noted on histologic sections. A subsequent study demonstrated loss of pressure natriuresis and renal medullary autoregulation; it is suggested these together result in the observed salt-sensitive hypertension [21].
Overall, preclinical and clinical data provide strong evidence for AKI-induced hypertension. Additional preclinical investigation in translational models of AKI such as cecal ligation and puncture (a sepsis model) and cardiac arrest/cardiopulmonary resuscitation (a model of acute cardiorenal syndrome) would be expected to inform further clinical investigation [22, 23]. As IRI is considered a translational model of renal transplant, investigation of the clinical course of postoperative hypertension and natriuresis in renal transplant patients may also provide important data.
Reproductive Risk
Evidence suggests that even mild changes in renal function such as that in living kidney donors and those with mild CKD may increase risk for pregnancy complications [24]. Two recent studies by Tangren et al strongly support that AKI may worsen future pregnancy outcomes. In the first, investigators identified that recovered AKI conferred increased risk of preeclampsia (adjusted odds ratio 2.9) and adverse fetal outcomes (adjusted odds ratio 2.4) including fetal growth restriction, even after matching for demographics, BMI, diastolic blood pressure, and other comorbidities. The mean time from AKI to pregnancy was 32 months. Sensitivity analysis restricted to patients with documented normal prepartum creatinine demonstrated similar result, as did subgroup analysis in nulliparous people, suggesting the association was specific to AKI. Interrogation of the cohort after accrual of additional parturients revealed additional risk of preterm birth, and further demonstrated a dose-response relationship between severity of AKI and subsequent risk of preeclampsia for KDIGO stages 2–3 [24–26].
The mechanism of AKI-reproductive risk is unclear but strong support for the phenomenon and suggestion of mechanism is provided by Gillis et al. Rats with a history of recovered ischemia-reperfusion-induced AKI demonstrated complicated pregnancies similar to those observed by Tangren. Recovered AKI-exposed dams had fewer, smaller litters with fetal growth restriction and increased preterm death. Involvement of the renin-angiotensin system was suggested by impaired response to sodium loading during pregnancy, reduced plasma volume, and increased uterine artery resistance index in AKI-exposed dams [27]. Observational studies support direct and indirect mechanisms. A direct AKI-preeclampsia mechanism could be inferred from studies of preeclampsia and CKD, in which a linkage is better understood [28]. Molina-Pérez et al. found that women with CKD and angiogenic imbalance were more likely to have preeclampsia [29]. The study enrolled 171 pregnant patients with CKD and assessed the balance of soluble Fms-Like Tyrosine Kinase-1 (sFlt-1) to placental growth factor (PlGF), which associates with preeclampsia risk [30]. Risk of preeclampsia associated with increasing sFLT-1 to PlGF ratio. Overexpression of sFlt1 results in parallel rise of AngII [31]; since increased AngII sensitivity is suggested in mechanistic studies of AKI [32], the potential for connection to pregnancy risk should be investigated. AKI-induced hypertension could also indirectly lead to reproductive risk, as hypertension has a well-described role in complications of pregnancy, including preeclampsia [33].
To our knowledge, no investigation addresses effects of AKI on fertility. However, CKD decreases fertility in both sexes: impaired spermatogenesis, erectile dysfunction, and reduced ovulation. As these effects are negated by renal transplant [34, 35], post-AKI infertility deserves further investigation.
In 2012, the total cost burden of preeclampsia in the United States was $2.1 billion in the first year following birth alone [36]. The potential for lifelong disease greatly increases the impact of adverse maternal health. Although studies on AKI parental effects after birth have largely centered on AKI that occurs during pregnancy, as recovered AKI increases the rate of preeclampsia, patients with recovered AKI who give birth are likely at higher risk for future renal, metabolic, and cardiovascular diseases [37]. Studies expanding knowledge on the impact of recovered AKI on parental and offspring outcomes enable longitudinal risk assessment and provide mechanistic avenues for treatment, potentially reducing long-term disease in AKI survivors and their offspring. Prospective study of AKI survivors for fertility disorders and reproductive risk are likely to have high impact given the risk of lifelong disease in offspring.
Dementia
The most common cause of dementia in the elderly is Alzheimer’s disease, which affects 5.8 million people in the United States and more than 55 million people worldwide [38, 39]. Global costs of dementia were estimated as approximately $1.3 trillion in 2019 and rising rapidly [38, 40]. Several population studies demonstrate association between recovered AKI (as determined from coding data or dialysis requirement) and later onset of dementia, with surprisingly consistent hazard ratios of 1.88–3.04. Because CKD also associates with dementia, all three studies controlled for CKD using exclusion, sensitivity analysis, and/or propensity scoring; AKI associates with dementia independently of preexisting or subsequent CKD, although subsequent CKD increases risk. The transition to dementia may occur quickly – mean time to onset of dementia was reduced in AKI patients compared with controls, and Kaplan-Meier curves diverge within two years. One study reported mean time to diagnosis of dementia in the AKI group as 1.9 [0.2–4.1] years (median [interquartile range]), quite similar to that reported for AKI-CKD transition [41–43]. Mechanisms are poorly characterized but studies describe cognitive and behavioral changes induced by AKI, supporting interaction with the neural circuits affected in dementia. Cognitive/behavioral tests in rodents are altered in the acute phase of several AKI models, although changes do not appear to persist [44, 45]. Vanderlinden et al. found that AKI patients assessed nearly 7 months after AKI demonstrated greater evidence of neurocognitive impairment than hospitalized, non-AKI controls [46]. Together, growing evidence connects a history of AKI and early cognitive impairment. Important questions remain regarding the influence of comorbid organ failure and mechanism. However, early diagnosis of dementia may improve quality of life and slow progression; since all current medical therapy targets slowed progression, earlier diagnosis may also improve benefit from therapy [47].
Mortality
AKI impacts both short- and long-term mortality. Numerous clinical reports have suggested that AKI complicating cardiovascular events [48, 49], progression of CKD [50, 51], and recurrence of AKI [52, 53], increase long-term mortality. Odutayo et al reported AKI associated with an 86% increased risk of cardiovascular disease-associated mortality [48]. The seminal AKI-CKD meta-analysis of 13 cohorts by Coca et al which identified the incidence of CKD and ESRD after AKI also demonstrated doubled mortality risk in AKI patients with compared with non-AKI patients [51]. Siew et al demonstrated that patients with recurrent AKI had a higher 1-year mortality (35%) than patients without recurrent AKI (18%)[53]. Increased severity of AKI itself also increases long-term risk of mortality [54, 55]; together these suggest that the vector of recovery may impact long-term mortality. Investigating this hypothesis. Kellum et al identified five recovery patterns. In particular patients with early reversal of recovery (37.3% of the cohort) demonstrated elevated risk of 1 year mortality compared with patients with sustained recovery [56]. Mechanistic studies of mortality after AKI are lacking. While it seems reasonable to hypothesize that early mortality is due to AKI-induced systemic disease, this relationship may be either more straightforward or more complicated. Observation of perimortem comorbidities in clinical cohorts may be partly revealing and suggest a path for preclinical study.
Surveillance of AKI Survivors:
Characterization of AKI-CKD recently led to the development of guidelines and even clinics for post-hospital surveillance of AKI survivors; however, this practice is the subject of considerable current controversy (reviewed in Vanmassenhove, Vanholder and Lameire [57]). First, AKI is not always recorded as a diagnosis, or coded in the electronic medical record, making it challenging to identify the right patients for follow-up. Second given the incidence of AKI, a requirement for specialist follow-up likely exceeds the capacity of nephrology specialists. Third, AKI survivors suffer urgent comorbidities which often require follow-up and thus may not have capacity for or access to additional medical visits. Fourth, physiologic changes during critical care (for example a fall in serum creatinine due to lost muscle mass) may confound posthospital assessments. Most importantly, two recent studies have demonstrated lack of renal benefit to specialist care following AKI. Silver et al found that randomization to nephrology specialist follow-up after AKI resulted in a clinically insignificant (although statistically significant) increase in major adverse kidney events (MAKE), and Thanapongsatorn et al found no difference in eGFR or MAKE with nephrologist-inclusive multidisciplinary follow-up after AKI [58, 59]. The latter study, however, offers a tantalizing hint at success: specialist care resulted in better urinary albumin:creatinine ratio, and better blood pressure control. Rather than impacting overall renal function, post-AKI surveillance may offer the chance to better manage – or possibly prevent – new disease including but not limited to CKD. Figure 1 summarizes the AKI-related cost, incidence, and time-to-appearance of AKI-CxD complications.
Figure 1:
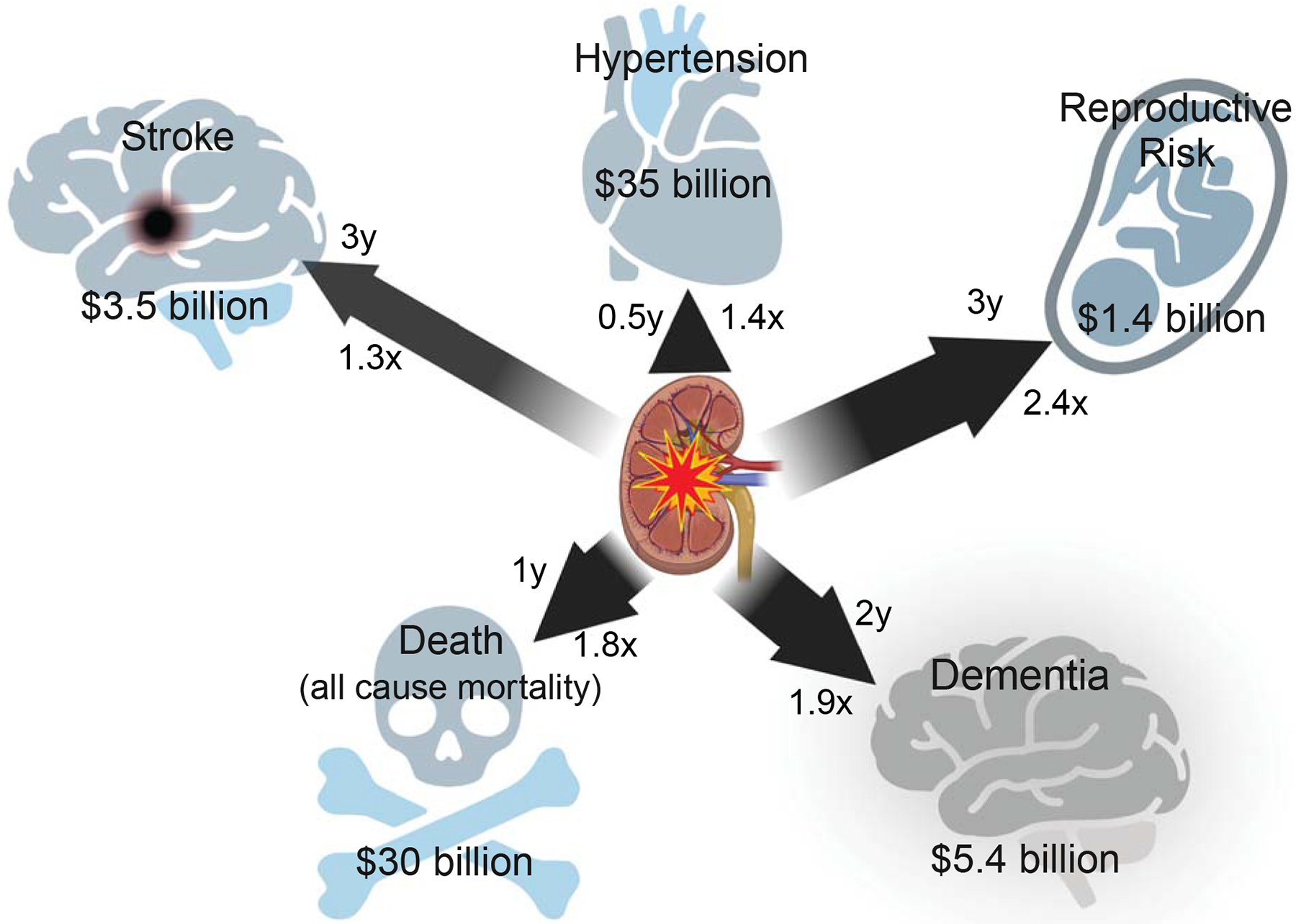
SHReDD (stroke, hypertension, reproductive risk, dementia, and death) complications of AKI occur rapidly and may have high health care impact. Time-to-discovery, adjusted hazard ratio, and estimated AKI-related cost are depicted. Arrow length is proportional to time-to-discovery, and arrow width to hazard ratio. For reproductive risk, attributable cost is due to preeclampsia only, and only represents one year, not lifetime cost. Attributable cost was estimated using CDC Disease Facts and the most conservative adjusted hazard ratio from sources referenced in the text.
Conclusion
Together, disease initiated by AKI represents tremendous suffering and cost; AKI is not a trifle. The doctrine should not be “de minimus” but rather, “de magnis curat medicinus” – “Physicians care about great things”. Preclinical and clinical studies should focus and inform each other on mechanisms of transition to chronic disease in multiple organs after AKI (AKI-CxD). Investigation of post-AKI screening, seems likely to succeed by focusing on risk of the composite outcome: Stroke, Hypertension, Reproductive risk, Death, and Dementia (“SHReDD”), as each of these has modifiable risk factors and early interventions. With this perspective, the identification of AKI represents a tremendous opportunity to reduce the burden of future disease.
Key Points.
AKI associates with future Stroke, Hypertension, Reproductive risk, Dementia, and Death. These risks are likely independent of incident CKD.
Preclinical studies support mechanistic connection between AKI and subsequent disease of nonrenal systems, so-called AKI-CxD transition.
Post-AKI screening programs have thus far demonstrated mixed results. Impact would likely be increased by broad screening for kidney-induced disease of the brain, cardiovascular, and reproductive systems
Acknowledgements:
The authors acknowledge Tahnee Groat, MPH, for careful editing and critique of the manuscript. Figure 1 was prepared with BioRender.
Financial support and sponsorship
This work was supported by the United States Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (VA Merit Award #101BX004288 to MPH), and the United States Department of Defense (W81XWH2010196 to MPH). JFH is supported by the Oregon Students Learn and Experience Research (OSLER) TL1 grant TL1TR002371. The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Abbreviations:
- AKI
Acute kidney injury
- ANG II
Angiotensin II
- CKD
Chronic kidney disease
- COVID-19
Coronavirus Disease 2019
- CxD
Chronic other organ disease
- eGFR
Estimated glomerular filtration rate
- ESRD
End-stage renal disease
- GFR
Glomerular filtration rate
- HDAC
Histone deacetylase
- IRI
Ischemia reperfusion injury
- KDIGO
Kidney Disease Improving Global Outcomes
- MAKE
Major Adverse Kidney Events
- mmHg
Millimeters of Mercury
- MRI
Magnetic resonance imaging
- PlGF
Placental growth factor
- sFlt-1
Fms-like tyrosine kinase-1
- SHReDD
Stroke, Hypertension, Reproductive Risk, Death, Dementia
Footnotes
Conflicts of Interest
All authors report no conflicts of interest to disclose.
References
- [1].O.E. Dictionary, “de minimis, adv., n., and adj”, Oxford University Press. [Google Scholar]
- [2].Mirriam-Webster, De minimis non curat lex., 2022 https://www.merriam-webster.com/dictionary/de%20minimis%20non%20curat%20lex. (Accessed September 28, 2022 2022).
- [3].Rewa O, Bagshaw SM, Acute kidney injury—epidemiology, outcomes and economics, Nature Reviews Nephrology 10(4) (2014) 193–207. [DOI] [PubMed] [Google Scholar]
- [4].Tsao CW, Aday AW, Almarzooq ZI, et al. , Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association, Circulation 145(8) (2022) e153–e639. [DOI] [PubMed] [Google Scholar]
- [5].Girotra T, Lekoubou A, Bishu KG, et al. , A contemporary and comprehensive analysis of the costs of stroke in the United States, J. Neurol. Sci 410 (2020) 116643. [DOI] [PubMed] [Google Scholar]
- [6].Wu VC, Wu PC, Wu CH, et al. , The Impact of Acute Kidney Injury on the Long-term Risk of Stroke, Journal of the American Heart Association 3(4) (2014) e000933–e000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu M, Liang Y, Chigurupati S, et al. , Acute kidney injury leads to inflammation and functional changes in the brain, J Am Soc Nephrol 19(7) (2008) 1360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hasselblatt M, Jeibmann A, Riesmeier B, et al. , Granulocyte-colony stimulating factor (G-CSF) and G-CSF receptor expression in human ischemic stroke, Acta Neuropathol. 113(1) (2007) 45–51. [DOI] [PubMed] [Google Scholar]
- [9].Chou AH, Lee CM, Chen CY, et al. , Hippocampal transcriptional dysregulation after renal ischemia and reperfusion, Brain Res. 1582 (2014) 197–210. [DOI] [PubMed] [Google Scholar]
- [10].Burek M, Burmester S, Salvador E, et al. , Kidney Ischemia/Reperfusion Injury Induces Changes in the Drug Transporter Expression at the Blood–Brain Barrier in vivo and in vitro, Frontiers in Physiology 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao L, Cao X, Li L, et al. , Acute Kidney Injury Sensitizes the Brain Vasculature to Ang II (Angiotensin II) Constriction via FGFBP1 (Fibroblast Growth Factor Binding Protein 1), Hypertension (2020) 1924–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Woo A, Botta A, Shi SSW, et al. , Obesity-Related Neuroinflammation: Magnetic Resonance and Microscopy Imaging of the Brain, Int. J. Mol. Sci 23(15) (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cerami C, Iaccarino L, Perani D, Molecular Imaging of Neuroinflammation in Neurodegenerative Dementias: The Role of In Vivo PET Imaging, Int. J. Mol. Sci 18(5) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hsu CY, Hsu RK, Yang J, et al. , Elevated BP after AKI, J Am Soc Nephrol 27(3) (2016) 914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Noble RA, Lucas BJ, Selby NM, Long-Term Outcomes in Patients with Acute Kidney Injury, Clin J Am Soc Nephrol 15(3) (2020) 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Askenazi DJ, Feig DI, Graham NM, et al. , 3–5 year longitudinal follow-up of pediatric patients after acute renal failure, Kidney Int 69(1) (2006) 184–9. [DOI] [PubMed] [Google Scholar]
- [17].Soranno DE, Gist KM, Acute Kidney Injury and Diastolic Dysfunction: Opportunity for Targeted Intervention?, Nephron (2022) 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Mini-review summarizing clinical relevance report of sexually dimorphic diastolic dysfunction in 1-year cohort of IRI-exposed mice and suggesting translational implications.
- [18].Soranno DE, Baker P 2nd, Kirkbride-Romeo L, et al. , Female and male mice have differential longterm cardiorenal outcomes following a matched degree of ischemia-reperfusion acute kidney injury, Sci. Rep 12(1) (2022) 643. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Elegant, detailed, 1-year cohorted characterization of cardiorenal outcomes including hypertension and diastolic dysfunction in male and female mice after ischemia-reperfusion injury.
- [19].Soranno DE, Kirkbride-Romeo L, Wennersten SA, et al. , Acute Kidney Injury Results in Long-Term Diastolic Dysfunction That Is Prevented by Histone Deacetylase Inhibition, JACC Basic Transl Sci 6(2) (2021) 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Initial description of 1-year followup of ischemia-reperfusion injury-exposed mice, which developed hypertension. Treatment with the HDAC inhibitor ITF2357 ameliorated hypertension, likely through a cardiac, non renal mechanism.
- [20].Spurgeon-Pechman KR, Donohoe DL, Mattson DL, et al. , Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium, Am J Physiol Renal Physiol 293(1) (2007) F269–78. [DOI] [PubMed] [Google Scholar]
- [21].Pechman KR, Miguel CD, Lund H, et al. , Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension, American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 297(5) (2009) R1358–R1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matsushita K, Saritas T, Eiwaz MB, et al. , The acute kidney injury to chronic kidney disease transition in a mouse model of acute cardiorenal syndrome emphasizes the role of inflammation, Kidney Int 97(1) (2020) 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hukriede NA, Soranno DE, Sander V, et al. , Experimental models of acute kidney injury for translational research, Nat Rev Nephrol 18(5) (2022) 277–293. [DOI] [PubMed] [Google Scholar]; *Comprehensive, recent review of translational models of AKI with high level of detail.
- [24].Tangren JS, Thadhani R, Animal Model of Pregnancy after Acute Kidney Injury Mirrors the Human Observations, J Am Soc Nephrol 32(2) (2021) 259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Editorial by Tangren evaluating the impact of the Gillis et al paper (below). Well-summarized background on relationship between AKI and reproductive risk.
- [25].Tangren JS, Wan Md Adnan WAH, Powe CE, et al. , Risk of Preeclampsia and Pregnancy Complications in Women With a History of Acute Kidney Injury, Hypertension 72(2) (2018) 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tangren JS, Powe CE, Ankers E, et al. , Pregnancy Outcomes after Clinical Recovery from AKI, Journal of the American Society of Nephrology 28(5) (2017) 1566–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gillis EE, Brands MW, Sullivan JC, Adverse Maternal and Fetal Outcomes in a Novel Experimental Model of Pregnancy after Recovery from Renal Ischemia-Reperfusion Injury, Journal of the American Society of Nephrology 32(2) (2021) 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Groundbreaking work describing pregnancy outcome in rats with recovered AKI.
- [28].Kattah A, Preeclampsia and Kidney Disease: Deciphering Cause and Effect, Curr. Hypertens. Rep 22(11) (2020) 91. [DOI] [PubMed] [Google Scholar]
- [29].Molina-Pérez CJ, Nolasco-Leaños AG, Carrillo-Juárez RI, et al. , Clinical usefulness of angiogenic factors in women with chronic kidney disease and suspected superimposed preeclampsia, Journal of Nephrology 35(6) (2022) 1699–1708. [DOI] [PubMed] [Google Scholar]
- [30].Zeisler H, Llurba E, Chantraine F, et al. , Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia, New England Journal of Medicine 374(1) (2016) 13–22. [DOI] [PubMed] [Google Scholar]
- [31].Burke SD, Zsengellér ZK, Khankin EV, et al. , Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia, J Clin Invest 126(7) (2016) 2561–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Phillips SA, Pechman KR, Leonard EC, et al. , Increased ANG II sensitivity following recovery from acute kidney injury: role of oxidant stress in skeletal muscle resistance arteries, American journal of physiology. Regulatory, integrative and comparative physiology 298(6) (2010) R1682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fox R, Kitt J, Leeson P, et al. , Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring, J Clin Med 8(10) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lundy SD, Vij SC, Male infertility in renal failure and transplantation, Transl Androl Urol 8(2) (2019) 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Holley JL, Schmidt RJ, Changes in fertility and hormone replacement therapy in kidney disease, Adv Chronic Kidney Dis 20(3) (2013) 240–5. [DOI] [PubMed] [Google Scholar]
- [36].Stevens W, Shih T, Incerti D, et al. , Short-term costs of preeclampsia to the United States health care system, Am. J. Obstet. Gynecol 217(3) (2017) 237–248.e16. [DOI] [PubMed] [Google Scholar]
- [37].Carty DM, Delles C, Dominiczak AF, Preeclampsia and future maternal health, J. Hypertens 28(7) (2010) 1349–55. [DOI] [PubMed] [Google Scholar]
- [38].Nichols E, Steinmetz JD, Vollset SE, et al. , Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019, The Lancet Public Health 7(2) (2022) e105–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Arvanitakis Z, Shah RC, Bennett DA, Diagnosis and Management of Dementia: Review, JAMA 322(16) (2019) 1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Shin JH, Dementia Epidemiology Fact Sheet 2022, Ann Rehabil Med 46(2) (2022) 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kao C-C, Wu C-H, Lai C-F, et al. , Long-term risk of dementia following acute kidney injury: A population-based study, Tzu Chi Medical Journal 29(4) (2017) 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kendrick J, Holmen J, Srinivas T, et al. , Acute Kidney Injury Is Associated With an Increased Risk of Dementia, Kidney International Reports 4(10) (2019) 1491–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tsai H-H, Yen R-F, Lin C-L, et al. , Increased risk of dementia in patients hospitalized with acute kidney injury: A nationwide population-based cohort study, PLoS One 12(2) (2017) e0171671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ali BH, Ramkumar A, Madanagopal TT, et al. , Motor and behavioral changes in mice with cisplatin-induced acute renal failure, Physiol. Res 63(1) (2014) 35–45. [DOI] [PubMed] [Google Scholar]
- [45].Al Banchaabouchi M, D’Hooge R, Marescau B, et al. , Behavioural deficits during the acute phase of mild renal failure in mice, Metab. Brain Dis 14(3) (1999) 173–87. [DOI] [PubMed] [Google Scholar]
- [46].Vanderlinden JA, Semrau JS, Silver SA, et al. , Acute kidney injury is associated with subtle but quantifiable neurocognitive impairments, Nephrology Dialysis Transplantation 37(2) (2021) 285–297. [DOI] [PubMed] [Google Scholar]; *Unique use of novel device to quantfy neurocognitive impairments reveals long-term deficits in patients with recovered AKI.
- [47].Rasmussen J, Langerman H, Alzheimer’s Disease - Why We Need Early Diagnosis, Degener Neurol Neuromuscul Dis 9 (2019) 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Odutayo A, Wong CX, Farkouh M, et al. , AKI and Long-Term Risk for Cardiovascular Events and Mortality, J Am Soc Nephrol 28(1) (2017) 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Saratzis A, Harrison S, Barratt J, et al. , Intervention Associated Acute Kidney Injury and Long-Term Cardiovascular Outcomes, Am J Nephrol 42(4) (2015) 285–94. [DOI] [PubMed] [Google Scholar]
- [50].Cerdá J, Lameire N, Eggers P, et al. , Epidemiology of acute kidney injury, Clin. J. Am. Soc. Nephrol 3(3) (2008) 881–6. [DOI] [PubMed] [Google Scholar]
- [51].Coca SG, Singanamala S, Parikh CR, Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis, Kidney international 81(5) (2012) 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hounkpatin HO, Fraser SDS, Glidewell L, et al. , Predicting Risk of Recurrent Acute Kidney Injury: A Systematic Review, Nephron 142(2) (2019) 83–90. [DOI] [PubMed] [Google Scholar]
- [53].Siew ED, Parr SK, Abdel-Kader K, et al. , Predictors of Recurrent AKI, J Am Soc Nephrol 27(4) (2016) 1190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].See EJ, Jayasinghe K, Glassford N, et al. , Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure, Kidney Int 95(1) (2019) 160–172. [DOI] [PubMed] [Google Scholar]
- [55].Coca SG, Yusuf B, Shlipak MG, et al. , Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis, Am J Kidney Dis 53(6) (2009) 961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kellum JA, Sileanu FE, Bihorac A, et al. , Recovery after Acute Kidney Injury, Am J Respir Crit Care Med 195(6) (2017) 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Vanmassenhove J, Vanholder R, Lameire N, Points of Concern in Post Acute Kidney Injury Management, Nephron 138(2) (2018) 92–103. [DOI] [PubMed] [Google Scholar]
- [58].Thanapongsatorn P, Chaikomon K, Lumlertgul N, et al. , Comprehensive versus standard care in post-severe acute kidney injury survivors, a randomized controlled trial, Critical Care 25(1) (2021) 322. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Randomized, controlled trial of comprehensive, multidisciplinary care after AKI. Comprehensive care did not alter mean eGFR at 12 months, but did result in improved blood pressure control.
- [59].Silver SA, Adhikari NK, Bell CM, et al. , Nephrologist Follow-Up versus Usual Care after an Acute Kidney Injury Hospitalization (FUSION): A Randomized Controlled Trial, Clin. J. Am. Soc. Nephrol 16(7) (2021) 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Randomized controlled trial of 1-year nephrologist followup for AKI patients. Followup was challenging for patients and did not alter major adverse kidney events.


