Abstract
Akkermansia muciniphila (A. muciniphila) has drawn much attention as an important gut microbe strain in recent years. A. muciniphila can influence the occurrence and development of diseases of the endocrine, nervous, digestive, musculoskeletal, and respiratory systems and other diseases. It can also improve immunotherapy for some cancers. A. muciniphila is expected to become a new probiotic in addition to Lactobacillus and Bifidobacterium. An increase in A. muciniphila abundance through direct or indirect A. muciniphila supplementation may inhibit or even reverse disease progression. However, some contrary findings are found in type 2 diabetes mellitus and neurodegenerative diseases, where increased A. muciniphila abundance may aggravate the diseases. To enable a more comprehensive understanding of the role of A. muciniphila in diseases, we summarize the relevant information on A. muciniphila in different systemic diseases and introduce regulators of A. muciniphila abundance to promote the clinical transformation of A. muciniphila research.
Introduction
The gut is the main digestive organ of the human body and absorbs the most nutrients [1–3]. It is also the largest immune and detoxification organ [4]. The gut is also called the “gut brain” or “second brain” owing to its complex neural network of approximately 100 billion scattered nerve cells [5]. The function of the gut is closely related to human health. The gut harbors a vast microbial community, which plays key roles in gut function [6,7]. Gut microbes include bacteria, phages, viruses, protists, worms, and fungi. Bacteria are the main inhabitants of the gut and participate in physiological activities through intermediate metabolites or surface antigens [8,9]. The gut flora plays an important role in substance metabolism and immune defense and is necessary to maintain human health [10,11]. The gut flora communicates with the central nervous system through neural, immune, and endocrine pathways, affecting mood, behavior, and other brain functions [12]. The gut flora is associated with sleep, memory, anxiety, and depression [13–15]. Normally, the gut flora composition is relatively stable. Changes in the microbial abundance, composition, and levels of metabolites of gut flora lead to a variety of diseases, including cancer.
Recently, Akkermansia muciniphila has garnered attention for its potential role in inducing weight loss and reducing lipid levels and its age-delaying effects. A. muciniphila belongs to the mucin-degrading bacterial family and can generate energy by decomposing mucin secreted by the gut mucosa [16]. A reduced abundance of A. muciniphila can disrupt the gut barrier, leading to increased plasma endotoxin levels, abnormal inflammatory responses, and metabolic disorders. A. muciniphila has an important role in the occurrence and development of obesity, diabetes, inflammatory bowel disease, psychiatric diseases, aging, and other diseases. It could also improve the efficacy of immunotherapy for the treatment of some cancers. A. muciniphila has enormous potential to become a next-generation probiotic drug and therapeutic target. We aim to provide a systematic and comprehensive overview of the role of A. muciniphila in human health and disease.
Shining Star: A. muciniphila
The discovery and biological characteristics of A. muciniphila
In 2004, Derrien et al. identified a new mucus-degrading bacterium from human feces through anaerobic culture: A. muciniphila. It was named after Dr. Antoon Akkermans (1941 to 2006), a renowned microbial ecologist in Wageningen [17,18]. A. muciniphila is an oval, anaerobic Gram-negative bacterium. It has limited tolerance to oxygen, but preliminary laboratory operations can be carried out under aerobic conditions [19–21]. Amuc_1100 is a heat-stable outer membrane protein from A. muciniphila. It can withstand a high temperature of 70 °C [22]. In addition, A. muciniphila has a wide range of host adaptations and phenotypic diversity [23–27]. Geerlings et al. [24] isolated 10 A. muciniphila strains from the feces of 15 species of mammals. The genomes of these strains are highly similar, with a nucleotide sequence homology of 93.9% to 99.7%. A highly conserved mucin degradation gene ensures the mucin degradation ability of A. muciniphila. A. muciniphila is present in humans in breast milk and many digestive organs, such as the mouth, pancreas, gall bladder, small intestine, appendix, and colon. Dubourg et al. [28] also isolated A. muciniphila from blood culture samples.
A. muciniphila is originally obtained from the mother [29,30]. A. muciniphila makes use of oligosaccharides in breast milk by synthesizing key glycan-degrading enzymes, which contributes to the early colonization and survival of A. muciniphila in the gut [31,32]. The abundance of A. muciniphila in the gut increases rapidly during infancy and nearly reaches the abundance of A. muciniphila in adults by the age of 1 year [33,34]. Intestinal mucus is synthesized and secreted by goblet cells and forms an inner layer without bacteria and an outer layer containing symbiotic bacteria. A. muciniphila mainly colonizes the outer mucin layer of the gut and maintains the stability of the mucin layer by decomposing mucin [35]. The genome of A. muciniphila ATCC BAA-835T encodes more than 61 proteins related to mucin degradation, such as sialidases, β-galactosidases, and α-L-fucosidases [36,37]. β-Galactosidase (Amuc_0771, Amuc_0824, and Amuc_1666) can effectively degrade mucin-derived sugar chains by selecting different glycosidic bonds [37]. The highly active sulfatase of A. muciniphila promotes its gut colonization [38,39]. A. muciniphila can be grown on nonmucin sugar glucose medium, but its growth efficiency is significantly lower than when grown on mucin medium [17]. The 30 hydrolases involved in mucin degradation are significantly upregulated under mucin conditions [40]. N-acetylglucosamine transporters Amuc_0502 and Amuc_0516 are the most highly upregulated genes when A. muciniphila is grown on mucin medium. A. muciniphila produces a variety of metabolites such as oligosaccharides and short-chain fatty acids (SCFAs). Oligosaccharides can regulate metabolism by interacting with other bacteria. SCFAs, including acetic acid, propionic acid, and butyric acid, are important metabolites of A. muciniphila produced through anaerobic fermentation [41–43].
The function of A. muciniphila
In addition to degrading mucin, A. muciniphila could also positively regulate mucin, thereby enhancing the barrier of the gut mucosa. Administration of A. muciniphila to mice increases the proliferation rate of intestinal stem cells, the number of goblet cells, and the number of Paneth cells [44,45]. A. muciniphila plays an important role in maintaining mucin homeostasis. In addition, mucin deficiency could result in the increased activation of glycolysis and other energy-generating metabolic pathways [46].
In vitro, A. muciniphila could increase enterocyte monolayer integrity and transepithelial electrical resistance to enhance the gut barrier [20]. Amuc_1100 activates Toll-like receptor 2 (TLR2) and its downstream NF-κB pathway [47]. Amuc_1100 can regulate metabolism and immunity and maintain the intestinal barrier [48]. Both butyric acid and propionic acid bind to G protein-coupled receptors to stimulate the secretion of intestinal peptides that regulate diet and glucose metabolism [49,50]. Acetate stimulates the growth of bacteria colonizing the mucus layer [51]. Mucin degradation products can also serve as nutritional resources for other bacteria [52]. A. muciniphila induces a specific T-cell response that increases the production of IgG1 antibodies in vivo [53]. However, there are significant individual differences in T-cell responses caused by A. muciniphila. Furthermore, microbial antigens from A. muciniphila induce the transformation of naive CD4+ CD44-Foxp3-T (Tn) cells into Treg lines [54]. A. muciniphila could also promote the biosynthesis of vitamin B12 [55].
A. muciniphila not only plays a role in maintaining normal physiological functions of the body but also is involved in the occurrence and development of endocrine system diseases, nervous system diseases, digestive system diseases, musculoskeletal system diseases, respiratory system diseases, and other diseases (Fig. 1) [56]. A. muciniphila has great potential to become one of the next generations of probiotics, to be applied to the diagnosis and treatment of human diseases, and to trigger a wave of intestinal microbial research.
Fig. 1.
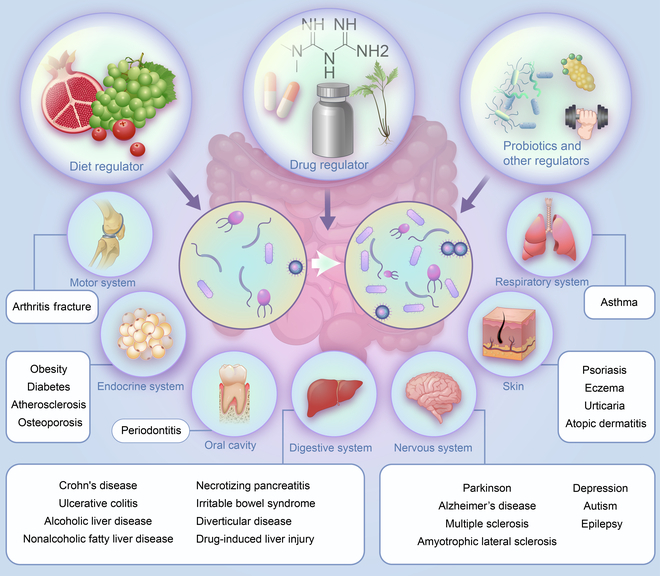
Changes in A. muciniphila abundance and regulators of A. muciniphila. Abnormal abundance of A. muciniphila can occur in the endocrine, nervous, digestive, respiratory, and motor systems. Under the influence of various factors, A. muciniphila abundance changes and affects the progression of these diseases.
The Role of A. muciniphila in Disease
Nonneoplastic disease
Endocrine system diseases
Obesity
In 2013, Everard et al. [57] found that the abundance of A. muciniphila decreases significantly in high-fat diet-induced and leptin-deficient obese mice. This study was the beginning of research on A. muciniphila and obesity. Increasing evidence has demonstrated an association between A. muciniphila and obesity. A study at Arizona State University found that healthy adolescent college freshmen with increased body mass index (BMI) and waist circumference (WC) have a lower abundance of A. muciniphila [58]. This phenomenon can also be observed in preschool children and pregnant women [59–61]. The abundance of A. muciniphila is correlated with the degree of obesity, history of bariatric surgery, and a variety of obesity markers [62–64]. In vivo results showed that the abundance of A. muciniphila is negatively correlated with body fat content, fat mass gain, and abdominal fat [65]. The A. muciniphila genome has a 3-gene operon in which tryptophanyl-tRNA synthase is related to waist-hip ratio and fat distribution [66,67]. Functionally, A. muciniphila inhibits the expression of PPARγ, C/EBPα, CD36, and other lipid marker genes and lipase genes in 3T3-L1 cells [68]. Moreover, pasteurized A. muciniphila reduces body weight, calorie intake, serum total cholesterol (TC) concentration, and blood glucose levels in high-fat diet-fed mice [68–71]. Pasteurized A. muciniphila increases energy expenditure and oxygen consumption in high-fat diet-fed mice. The fecal energy content of mice also increases significantly by the administration of pasteurized A. muciniphila [69]. In 2019, the results from a human trial confirmed the safety of A. muciniphila supplementation [72]. Oral supplementation with A. muciniphila significantly increased insulin sensitivity and reduced plasma TC levels, but the effect on obesity was not statistically significant.
In 2016, researchers isolated the outer membrane protein (Amuc_1100) of A. muciniphila. The function of Amuc_1100 is not affected by pasteurization. Amuc_1100 specifically induces cellular immunity by activating TLR2 [73]. It also increases p-Aktthr and p-Aktser levels to improve insulin sensitivity (Fig. 2). Additionally, Amuc_1100 activates Cnr1 and promotes CB1 expression to improve intestinal permeability in vivo. Cldn3 expression regulated by Amuc_1100 also contributes to the improvement in intestinal permeability [73]. Recently, scientists extracted an 84-kDa protein, named P9, from the secretions of A. muciniphila. P9 has a stronger effect than Amuc_1100 on improving obesity and glucose homeostasis [74]. The gastrointestinal hormone glucagon-like peptide 1 (GLP-1) can regulate energy balance and improve glucose homeostasis. The effect of P9 on GLP-1 secretion is 1,000 times stronger than that of Amuc_1100. After binding with its ligand intercellular adhesion molecule 2 (ICAM-2), P9 promotes thermogenesis and improves obesity and glucose homeostasis by activating the GLP-1R signaling pathway and interleukin-6 (IL-6). The mechanism associated with A. muciniphila has attracted the attention of many researchers investigating endocrine system diseases. The expression of hepatic lipogenesis markers (sterol regulatory element-binding protein 1c [SREBP-1c], acetyl CoA carboxylase [ACC], and fatty acid synthase [FAS]) is decreased after A. muciniphila administration [71]. A. muciniphila reduces carbohydrate absorption by decreasing the expression of glucose transporters such as glucose transporter 2 (GLUT2) and sodium-glucose linked transporter 1 (SGLT1) [69]. It also increases the levels of 2-PG and 1-PG and activates peroxisome proliferator-activated receptor alpha (PPARα) [75]. The cell lysate of A. muciniphila increases serine protease inhibitor A3G (SERPINA3G) expression and reduces lipogenesis in adipocytes [76]. Cathepsin B (CTSB) is a key molecule by which SERPINA3 inhibits adipogenesis. In addition, A. muciniphila improves the inflammatory response of brown adipose tissue, alleviates endotoxemia, and improves the barrier of the gut mucosa [70]. A higher abundance of A. muciniphila is associated with higher expression of sCD14 and IL-10 [77]. Both live A. muciniphila and pasteurized A. muciniphila significantly reduce the number of CD4+ Foxp3+ cells in vivo [71].
Fig. 2.
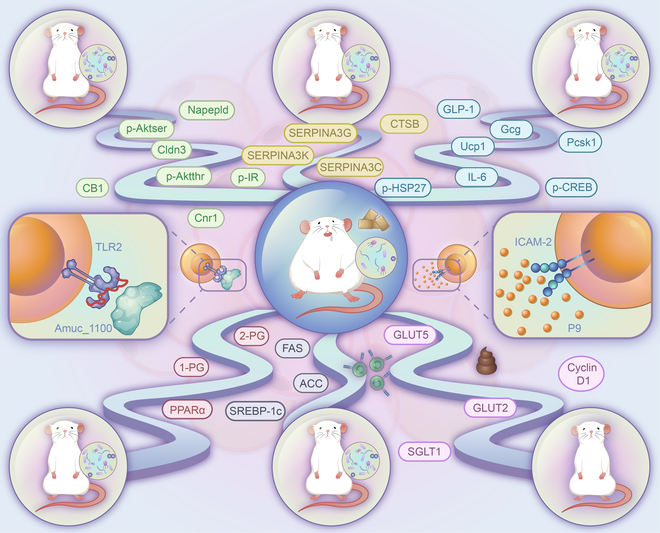
The regulatory mechanism of A. muciniphila in obesity. Amuc_1100 promotes p-Aktthr and Aktser expression to enhance insulin sensitivity. Amuc_1100 improves intestinal permeability activation by regulating Cldn3 expression and the Cnr1/CB1 axis in vivo. Cldn3 expression regulated by Amuc_1100 also contributes to the improvement of intestinal permeability. P9 recognizes and binds to the ligand ICAM-2. P9 then induces GLP-1 secretion by promoting p-CREB and p-HSP27 expression levels. P9 promotes thermogenesis and improves obesity and glucose homeostasis in high-fat diet-fed mice by activating the GLP-1R signaling pathway and IL-6. A. muciniphila administration inhibits adipogenesis in adipocytes via the SERPINA3/CTSB axis. It also increases 2-pg and 1-pg levels in circulating blood and activates peroxisome proliferator-activated receptor alpha (PPARα) to regulate lipid metabolism. A. muciniphila reduces carbohydrate absorption by reducing GLUT2, GLUT5, and SGLT1 expression. A. muciniphila reduces the expression levels of the hepatic lipogenesis markers SREBP-1c, ACC, and FAS.
Diabetes
A. muciniphila abundance decreases in obese diabetic mice and obese patients with type 2 diabetes mellitus (T2DM) [57,66,78–83]. The abundance of A. muciniphila is negatively correlated with fasting blood glucose and glycosylated hemoglobin levels [84]. Oral administration of A. muciniphila decreases levels of liver glycogen and increases serum insulin levels and glucose tolerance [74,85]. However, increased A. muciniphila abundance after bariatric surgery is not associated with glucose homeostasis [62,64]. However, Amuc_1100 and P9 play important roles in the regulation of glucose homeostasis [73,74]. P9 improves GLP-1 secretion and glucose tolerance [74]. Interferon γ (IFNγ) could modulate the role of A. muciniphila in glucose tolerance [86]. Glucose tolerance is significantly improved in IFNγ-knockout mice. IFNγ decreases the abundance of A. muciniphila in the gut mucosa by promoting the expression of Irgm1 in vivo. Pasteurized A. muciniphila reduces carbohydrate absorption by downregulating GLUT2 and SGLT1 expression [69]. A. muciniphila also enhances FGF15/19 to stimulate glycogen synthesis by inhibiting βCDCA [83]. However, the findings by Qin et al. are contrary to all the above results regarding the variation of A. muciniphila abundance in T2DM. The results showed that increased A. muciniphila abundance is significantly upregulated in T2DM. The enrichment of A. muciniphila is related to mucin degradation, but the regulation of A. muciniphila on T2DM was not evaluated in the study [87].
The abundance of A. muciniphila in the gut is significantly reduced in patients with type 1 diabetes (T1D) [88]. A. muciniphila could decrease serum endotoxin levels and promote mucus secretion and the expression of Reg3γ in non-obese diabetic (NOD) mice [89,90]. A. muciniphila inhibits T1D development by decreasing TLR expression, reducing monocyte infiltration of islet cells, and increasing the number of regulatory Foxp3+ Treg cells.
Atherosclerosis
In cases of obesity or diabetes, lipid metabolism is usually abnormal. Li et al. [91] found that A. muciniphila abundance is negatively correlated with the severity of atherosclerosis. A higher abundance of A. muciniphila is associated with lower levels of soluble tumor necrosis factor receptor II (sTNFR II). Camp-responsive element-binding protein H (CREBH) is a lipid regulatory transcription factor that binds to the endoplasmic reticulum. Increased intestinal A. muciniphila abundance reduces the production of chylomicron and very-low-density lipoprotein in CREBH-null mice [92]. A. muciniphila significantly reduces apoB100 and plasma triglyceride (TG) levels in CREBH-null mice but does not affect wild-type mice. A. muciniphila also promotes the clearance of plasma TGs and chylomicrons by increasing the levels of LDL receptors in CREBH-null mice. Apoe−/− mice and Apoe*3Leiden mice. CETP mice are commonly selected models for studying lipid metabolism and atherosclerosis. Fasting plasma TC and TG levels decrease in hyperlipidemic E3L CETP mice after oral administration of A. muciniphila for 4 weeks [93]. However, oral administration of A. muciniphila has no significant effect on serum TC, total TG, low-density lipoprotein, or high-density lipoprotein levels in Apoe−/− mice [91]. Whether A. muciniphila regulates the progression of atherosclerosis by regulating lipid metabolism remains to be further studied. Additionally, A. muciniphila regulates local and systemic immune responses. A. muciniphila affects the composition of immune cells in mesenteric lymph nodes and promotes the expression of CD86 on dendritic MHCII and B cells. The levels of macrophages, macrophage markers (F4/80), and inflammatory factors (IL-1β, tumor necrosis factor α [TNF-α], and monocyte chemoattractant protein 1 [MCP-1]) in the aorta and plasma of Apoe−/− mice decreased after oral administration of A. muciniphila. A. muciniphila protects against atherosclerosis by regulating systemic and vascular inflammation [91,93].
Osteoporosis
Most studies suggest that A. muciniphila is beneficial, but some have found that A. muciniphila promotes osteoporosis [94–96]. A. muciniphila is positively correlated with bone formation markers, 25-OH-D, and bone resorption markers [94]. A. muciniphila promotes bone resorption by increasing serum parathyroid hormone levels and serum amyloid A3 levels. Treg cells have anti-inflammatory effects that reduce bone loss. The number of Treg cells in the bone marrow and mesenteric lymph nodes decreased after pasteurized A. muciniphila administration.
Nervous system diseases
Neurodegenerative diseases
Neurodegenerative diseases such as Parkinson’s disease (PD), Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS) have become major public health problems, particularly for elderly individuals. Changes in A. muciniphila abundance can contribute to the occurrence and development of a variety of neurodegenerative diseases. Unlike the concordant role of A. muciniphila in most diseases, the role of A. muciniphila is changing in neurodegenerative diseases. A. muciniphila abundance is significantly downregulated in AD and ALS, while the abundance of A. muciniphila is upregulated in MS and PD.
Studies linking gut microbes to AD date back to 2016. Changes in the gut flora promote the progression of brain inflammation and amyloid-β (Aβ) protein deposition. A. muciniphila abundance is obviously downregulated in AD [97]. AD mice and APP/PS1 mice are common mouse models in AD research. A. muciniphila administration improves spatial learning and memory by reducing Aβ plaque deposition and Aβ40–42 levels in APP/PS1 mice [98]. A. muciniphila administration also promotes hippocampal glial cell proliferation and the expression of proinflammatory cytokines to improve neural development and synaptic plasticity. Early feeding of a high-fat diet impairs the learning and memory ability of AD mice and reduces the abundance of A. muciniphila. A. muciniphila administration improves glucose metabolism, the intestinal barrier, and lipid metabolism in mouse models of AD [98]. A. muciniphila also promotes the expression of UCP1 and increases thermogenesis in brown adipose tissue of AD mice. Although most studies suggest that A. muciniphila is a protective factor against AD, Khedr et al. observed the upregulation of A. muciniphila in AD patients. Further research is needed to explore the reasons for this refutation [99].
The development of ALS is also regulated by the gut microbiome. In vivo experiments showed that motor function was significantly impaired by treatment with antibiotics. The decreased A. muciniphila abundance is found in ALS. A. muciniphila administration can significantly improve the symptoms of ALS. Nicotinamide, a metabolite of A. muciniphila, plays a key role in the treatment of ALS. Nicotinamide supplementation improves motor function in vivo. ALS patients have lower levels of nicotinamide in the blood and cerebrospinal fluid than control individuals [100].
MS is an autoimmune-related neurodegenerative disease. Gut microbes regulate immune responses to cause neurodegenerative changes and the development of MS [101]. Experimental autoimmune encephalomyelitis is exacerbated in mice after stool transplantation from MS patients [102]. Increased A. muciniphila abundance was also found in MS patients compared with paired household healthy controls [103]. 16S rRNA sequencing analysis showed that A. muciniphila abundance is significantly higher in twins with MS than in healthy twins [101,102]. A. muciniphila stimulates peripheral blood mononuclear cells to differentiate into TH1 cells, promoting an inflammatory T-lymphocyte response [102].
The aggregation of α-synuclein is a key process in the pathogenesis of PD. Although this toxic aggregation occurs almost throughout the central system, increasing evidence showed that α-synuclein aggregation is found in enteric nerves before intracerebral aggregation of α-synuclein. These findings suggest that PD may originate within the gut. Some studies suggest that A. muciniphila abundance is significantly increased in PD, which contrasts with previous indications that A. muciniphila has beneficial effects in most diseases [104,105]. A. muciniphila-conditioned medium (CM) leads to ROS production and α-synuclein aggregation in enteroendocrine cells by increasing intracellular Ca+ levels (Fig. 3) [105]. However, the methods of these studies are limited. More complex models should be adopted in the study of A. muciniphila’s role in PD.
Fig. 3.
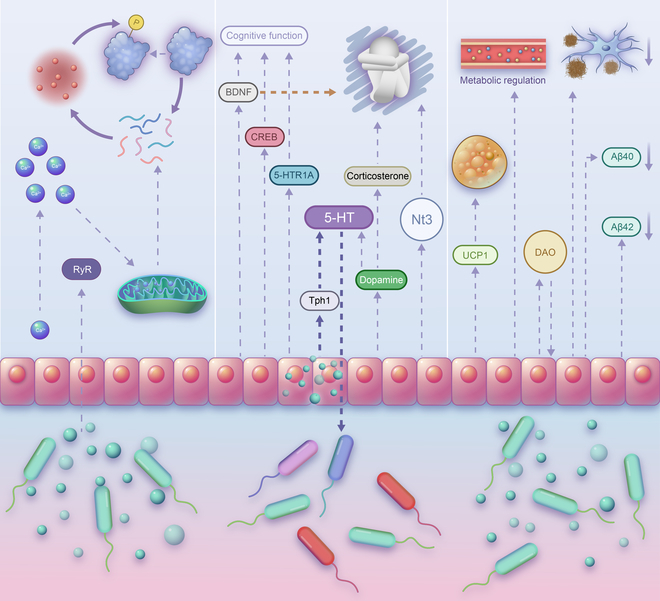
The regulatory mechanism of A. muciniphila in neurodegenerative diseases. Activation of RyR by A. muciniphila-conditioned medium increases the intracellular Ca+ concentration and promotes the misfolding of α-synuclein via ROS in Parkinson’s disease. In Alzheimer’s disease, A. muciniphila inhibits Aβ plaque deposition and reduces the expression levels of Aβ40 and Aβ42 to improve learning and memory in APP/PS1 mice. A. muciniphila also activates UCP1 to promote brown adipose tissue thermogenesis in AD mice. Glucose metabolism and the gut barrier in APP/PS1 mice are also regulated by A. muciniphila. A. muciniphila exerts an antidepressant effect by regulating serum corticosterone, dopamine, BDNF, CREB, NT3, and other depression-related molecules. A. muciniphila increases intestinal 5-HT levels in CUMS mice by activating the rate-limiting enzyme TPH1 for 5-HT synthesis.
Depression
The abundance of A. muciniphila decreases in depressed mice and Wistar-Kyoto (WKY) rats [106–108]. A. muciniphila abundance is positively correlated with the expression of MUC2 [106]. Oral administration of A. muciniphila significantly improves depression-like behavior in vivo [108,109]. A. muciniphila promotes the repair of the gut barrier and reduces depression-related colitis damage [106]. A. muciniphila decreases serum concentrations of corticosterone and increases dopamine and brain-derived neurotrophic factor (BDNF) levels in the hippocampus [109,110]. Amuc_1100 exerts antidepressant effects by regulating the gut flora, serum metabolites, and BDNF (Fig. 3). Extracellular vesicles (EVs) from A. muciniphila promote neurotrophic factor expression [108].
Autism
Wang et al. [111] found that the abundance of Bifidobacterium and A. muciniphila in autistic children is significantly reduced. In children with autism, the intestinal mucus layer becomes thinner. Reduced A. muciniphila abundance may contribute to the development of autism by reducing mucus barrier function and increasing the permeability of the gut mucosa. Fecal bacteria transplantation increases A. muciniphila abundance in FMR1-knockout mice with autism and improves cognitive dysfunction and social novelty preference [112]. However, fecal bacteria transplantation entails transplantation of the whole microbiome, and the role of A. muciniphila in the treatment of autism still needs further study.
Epilepsy
Ketogenic diets (KDs) mediated by gut microbes can be used to treat refractory epilepsy. A. muciniphila abundance in the gut of mice with 6-Hz-induced seizures increases significantly due to KDs [113,114]. The antiepileptic effect of KDs disappears after either aseptic feeding or antibiotic treatment. Simultaneous supplementation with A. muciniphila and Parabacteroides increases the threshold of epileptic seizures and rescues the antiepileptic effect of KDs in vivo. A. muciniphila and Parabacteroides increase GABA levels and the GABA/glutamate ratio in the hippocampus. A. muciniphila also decreases the activity of γ-glutamyl transpeptidase and inhibits γ-glutamylation. The combination of Bifidobacterium and A. muciniphila administration reduces the cytotoxicity induced by carbamazepine and lamotrigine in vitro [115].
Digestive system diseases
Inflammatory bowel disease (IBD)
A. muciniphila abundance is decreased in patients with Crohn’s disease or ulcerative colitis and multiple animal models of colitis [45,116–121]. A. muciniphila abundance is negatively correlated with colitis activity and inflammation score but positively correlated with the percentage of sulfated colonic mucins in the mucus gel layer [122]. Pasteurized A. muciniphila, A. muciniphila-derived EVs, Amuc_1100, and Amuc_2109 can significantly reduce colitis activity; alleviate colonic tissue damage and splenomegaly; and alleviate colonic infiltration by macrophages, CD8+ cytotoxic T lymphocytes (CTLs), inflammatory factors, TNF-α, IFNγ, and other infiltrating factors [22,106,123,124]. However, the components of EVs derived from A. muciniphila are complex, and future research should confirm the role of EVs in the pathogenesis of colitis. Amuc_2109 significantly decreases malondialdehyde (MDA) levels induced by dextran sulfate sodium (DSS) and increases glutathione (GSH) and superoxide dismutase (SOD) activities [124]. In the ipEC-J2 cell inflammatory model, A. muciniphila administration can also alleviate the inflammatory response of intestinal epithelial cells by regulating the expression of the phosphatidylinositol 3-kinase (PI3K) upstream receptor genes [125]. A. muciniphila inhibits apoptosis by inhibiting the expression of key genes in calcium signaling pathways and cell cycle signaling pathways [125]. NOD-like receptor family pyrin domain-containing 3 (NLRP3) is a relatively well-studied member of the NLR family and is a key molecule in pathogen recognition and cellular immunomodulation. In vitro and in vivo studies showed that NLRP3 mRNA and protein expression increases with increasing A. muciniphila abundance [45]. The anticolitis effect of A. muciniphila weakens after NLRP3 knockout. A. muciniphila alleviates DSS-induced colitis by activating NLRP3.
Tryptophan and its metabolites (5-hydroxytryptamine [5-HT], kynurenine, and indole derivatives) also play an important role in the development of colitis. A. muciniphila is significantly correlated with tryptophan metabolites. Pasteurized A. muciniphila promotes the expression of IA and IAA to activate AhR signaling and reduce colon inflammation [126]. A. muciniphila can increase serotonin transporter expression and increase the bioavailability of 5-HT in the gut [127]. The inhibitory effect of A. muciniphila on colitis is strain specific. A. muciniphila FSDLZ36M5-treated mice are significantly smaller in body weight and have a shorter colon length, while the levels of proinflammatory cytokines (TNF-α, IL-1β, and IL-6) increase and the levels of anti-inflammatory cytokines (IL-10) decrease [128]. However, A. muciniphila FSDLZ39M14, ATCC BAA-835, and FSDLZ20M4 did not significantly alleviate colitis in mice, and A. muciniphila FSDLZ20M4 even aggravated gut injury in mice. Both strain ATCC and strain 139 improve chronic colitis in mice, and strain ATCC has a stronger effect [129].
Nonalcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD)
A. muciniphila affects liver fat metabolism by regulating the expression of genes related to fat synthesis and different inflammatory factors, thereby preventing nonalcoholic fatty liver disease (NAFLD). A. muciniphila also mediates the effect of a variety of drugs used for the treatment of NAFLD, such as Si Miao Formula [130], MDG [131], and Bofutsushosan [132]. MDG alleviates obesity-induced liver damage and steatosis by increasing A. muciniphila abundance [131]. A. muciniphila abundance decreases in both mice and patients with NAFLD [132,133]. Higher A. muciniphila abundance is associated with lower concentrations of aspartate aminotransferase (AST), alanine aminotransferase (ALT), TC, and TG in serum [131]. The combination of quercetin and A. muciniphila can improve NAFLD [134]. A. muciniphila alleviates hepatic steatosis by regulating lipid oxidation and bile acid metabolism [135]. L-aspartic acid, a key component of the activator of the liver kinase B1 (LKB1)–AMP-activated protein kinase (AMPK) axis, promotes the expression of energy metabolism regulators and mitochondrial complexes in the liver [136]. A. muciniphila activates the LKB1–AMPK axis by increasing liver L-aspartic acid levels. Morrison et al. [135], however, found that although pasteurized A. muciniphila can improve intestinal permeability and mesenteric adipose tissue expansion, it has no significant effect on the development of NAFLD.
The abundance of A. muciniphila in ASH patients is significantly lower than that in nonobese healthy individuals [137]. Lower A. muciniphila abundance is associated with more serious liver lesions. Oral administration of A. muciniphila can alleviate alcoholic liver disease (ALD) [137]. Wild-type mice were fed the Lieber–DeCarli diet for 15 days, and then A. muciniphila was administered on day 10. A. muciniphila administration led to decreases in ALT, IL-1β, and TNF-α levels and MPO+ neutrophil infiltration. KRQKYD enhances the gut barrier and inhibits the inflammatory response mediated by A. muciniphila, thereby reducing susceptibility to ALD [137–139]. SREBP1c is a key component of liver adipogenesis. KRQKYD reduces SREBP1c expression by activating the NRF2/HO-1 antioxidant defense system and thereby inhibits lipid synthesis in the liver [140].
Other digestive system diseases
A. muciniphila abundance is also associated with necrotizing pancreatitis, irritable bowel syndrome (IBS), diverticular disease, and drug-induced liver injury. The abundance of A. muciniphila is significantly higher in patients with necrotizing pancreatitis than in healthy controls [141]. A. muciniphila abundance in symptomatic uncomplicated diverticular disease (SUDD) and asymptomatic diverticulosis is significantly higher than that in healthy controls [142]. Increased abundance of A. muciniphila can significantly improve the pain intensity of IBS [143]. A. muciniphila can significantly improve APAP-induced liver injury and reduce serum ALT and AST levels [144]. Mechanistically, A. muciniphila can significantly reduce GSH and SOD levels; inhibit liver infiltration by macrophages and neutrophils; and contribute to the release of IL-1β, IL-2, IL-6, and TNF-α. A. muciniphila regulates Bcl-2 and Bax expression to reduce apoptosis by activating the PI3K/Akt signaling pathway [144]. A. muciniphila also has protective effects on liver injury induced by HFD/CCl4 and concanavalin A, but these studies focused only on mice [145–147]. Owing to differences in the immune system and microbial composition between mice and humans, further studies are needed.
Diseases of the musculoskeletal system
Arthritis
A. muciniphila abundance is significantly lower in arthritis patients and arthritis animal models than in healthy controls [148–151]. In HLA-B27/β2 m+ rats, higher A. muciniphila abundance is associated with higher expression levels of inflammatory cytokines (IFNγ, IL-17A, and IL-23) and a higher incidence of spinal arthritis [148]. Ankle arthritis is aggravated when fecal microbes from spinal arthritis are transplanted into germ-free KRN/B6xNOD mice. A. muciniphila abundance is also negatively correlated with ankle swelling [150]. These studies have some limitations, such as inaccurate selection of the control group, and their results need further experimental verification. The role of chondroitin sulfate (CS) in the treatment of arthritis is controversial. Wang et al. [151] found that changes in gut flora play a decisive role in the treatment of osteoarthritis by CS. If the abundance of sulfatase-secreting bacteria (SSB) and sulfate-reducing bacteria (SRB) decreases while the abundance of A. muciniphila increases, then CS can improve OA. If A. muciniphila has a disadvantage in competition with SSB and SRB, CS can aggravate OA. This finding has stimulated the development of CS-related cocktails. The combination of CS with probiotics might inhibit the progression of arthritis.
Fracture
A. muciniphila can also promote revascularization and thus contribute to fracture healing. Administration of A. muciniphila reduces the amount of mineralized bone volume and total callus volume in a fractured segment [152]. Although A. muciniphila administration also changes the gut microbial community, such as reducing the abundance of γ-proteobacteria, the above effects of A. muciniphila still exist after pretreatment with antibiotics. A. muciniphila can improve intestinal barrier function in mice, inhibit the systemic inflammatory response, and promote the formation of type H vessels to promote fracture healing [152].
Respiratory system diseases
Asthma
A. muciniphila and Faecalibacterium prausnitzii abundances decrease significantly in asthmatic patients [153]. Lower abundances of A. muciniphila and F. prausnitzii are associated with greater severity of asthma [154]. In vivo studies show that A. muciniphila significantly reduces airway hyperresponsiveness and airway inflammation. A. muciniphila administration significantly decreased the number of BAL eosinophils and IFNγ+ CD4 T cells, while the number of IL-10+Foxp3+ lymphocytes was increased by A. muciniphila administration. After oral administration of A. muciniphila, airway hyperreactivity to acetylcholine is significantly reduced in vivo [153]. Resveratrol (RES) attenuates allergic asthma and increases pulmonary A. muciniphila abundance in ovalbumin-exposed mice [155]. However, whether RES plays a role in lung A. muciniphila mediation needs to be confirmed.
Other diseases
Cutaneous disorders
Recent studies on the skin microbiome show that skin-related diseases, such as psoriasis, eczema, urticaria, and atopic dermatitis in children, are associated with changes in A. muciniphila abundance. A. muciniphila abundance decreases in chronic urticaria but increases in infantile eczema [156–159]. The changes in A. muciniphila abundance in psoriasis are controversial [156,160]. A significant increase in A. muciniphila abundance in patients with psoriasis is contrary to the findings of some research teams [156,158,160]. However, A. muciniphila abundance is also a marker of psoriasis biotherapy. Changes in A. muciniphila abundance are most significant in patients who receive systemic biotherapy compared to controls [161]. However, this study did not sample individual patients at different time points, and the experimental results are susceptible to many potentially confounding factors, such as the effects of age, sex, and severity of disease. The combination of Bifidobacterium breve, Bifidobacterium pseudocatenulatum, Bifidobacterium adolescentis, Escherichia coli, F. prausnitzii, and A. muciniphila can be used to identify whether pediatric atopic dermatitis is related to food allergy [162].
Oral diseases
A. muciniphila abundance decreases in patients with periodontitis [163]. A. muciniphila is also present in the oral cavity of healthy individuals and disappears completely in the progression of periodontitis [164]. A. muciniphila is effective in treating periodontitis caused by Porphyromonas gingivalis. In the experimental periodontitis model, A. muciniphila or Amuc_1100 reduces the inflammatory response and osteoclast activity in the interdental spaces, thereby reducing alveolar bone loss [165,166]. A. muciniphila antagonizes the elevation of IL-12 levels and the decrease in IL-10 expression induced by P. gingivalis in bone marrow-derived macrophages. A. muciniphila or Amuc_1100 increases the ratio of M2 macrophages/M1 macrophages during P. gingivalis infection and recruits Th1 cells into gingival tissue by upregulating CXCL10 [167]. A. muciniphila administration promotes the expression of intercellular adhesion molecule 1 (ICAM-1) and tight binding molecules (ZO-1) in gingival epithelial cells (TIGK). However, only oral administration, not gastric gavage, prevents periodontitis caused by P. gingivalis [166]. Pasteurized A. muciniphila is a very promising drug for the treatment of periodontitis.
Aging
A. muciniphila administration prolongs the lifespan and reduces the incidence of age-related complications in vivo [168,169]. After A. muciniphila supplementation, the expression of Reg3g and Tff3 in the ileum of Ercc1-/Δ7 mice increases, which promotes barrier repair of the mucosal layer and wound healing [170]. Studies on senescence-related metabolites show that A. muciniphila can increase polyamine, 2-hydroxybutyrate, SCFA, and bile acid levels in the gut [169].
Neoplastic disease
Differences in the fecal microbiota exist in many cancers and are risk factors for cancer development [171]. A. muciniphila can inhibit the progression of lung cancer and prostate cancer [172,173]. Alterations in A. muciniphila abundance are controversial in patients with colorectal cancer (CRC) [174–176]. Functionally, A. muciniphila administration can inhibit cell proliferation in CRC [22,174,177]. The inhibitory effect of Amuc_1434, a protein derived from A. muciniphila, on CRC cell proliferation and the cell cycle is enhanced with increasing Amuc_1434 concentration [177]. Amuc_1434 induces G0/G1 arrest by promoting p53 expression (Fig. 4). Muc2 is a key molecule by which Amuc_1434* inhibits proliferation. Amuc_1434 activates the death receptor and mitochondrial apoptotic pathways to promote apoptosis by regulating caspase 3, caspase 8, and the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Further in vivo experiments confirmed the inhibitory effect of Amuc_1434 on CRC. The immune system is the medium of the A. muciniphila–tumor interaction. A. muciniphila promotes the activation of M1-like macrophages and the expression of related cytokines (IL-23, TNF-α, and IL-27) [174]. The NLRP3 pathway is a key pathway for the polarization of macrophages. A. muciniphila abundance is negatively correlated with NLRP3 and TLR2 expression. A. muciniphila promotes the polarization of M1-like macrophages and inhibits the progression of CRC by regulating the TLR2/NLRP3 and NF-κB pathways. Activated NLRP3 promotes the recruitment of NK cells. CTLs are the main cellular mediators of anti-CRC immunity. A. muciniphila and Amuc_1100 recruit CTLs and increase the percentage of CTLs in the mesenteric lymph nodes and colon by promoting the secretion of chemokines [22]. However, the interaction between macrophages and CTLs in the progression of colon cancer remains to be studied. Similarly, in vitro and in vivo experiments show that A. muciniphila EVs increase the number of M1 macrophages in prostate cancer tissues. CM from A. muciniphila-EV-treated macrophages inhibits PCa cell proliferation and invasion [173]. A. muciniphila EVs induce a CTL anti-prostate cancer immune response by increasing the proportion of GZMB+ and IFNγ+CD8+ T cells [173] (Fig. 4). EVs carry several immunogens that may affect human physiological functions. Researchers further explored the safety of A. muciniphila EVs. A. muciniphila EVs have no obvious toxicity to vital organs, such as the kidney, liver, and normal prostate cell lines. A. muciniphila EVs are well tolerated in vitro and in vivo. A. muciniphila EVs are a promising treatment for prostate cancer.
Fig. 4.
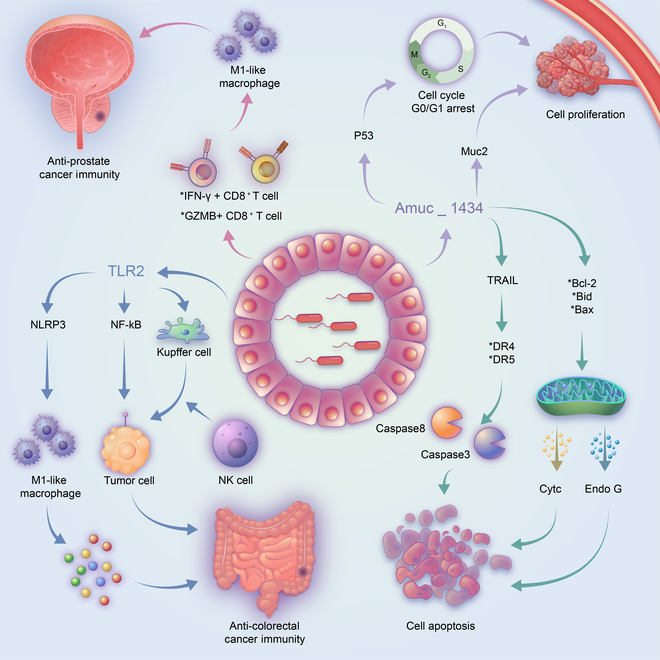
The regulatory role of A. muciniphila in neoplastic disease. Amuc_1434 induces G0/G1 cell cycle arrest by promoting p53 expression in colorectal cancer. Amuc_1434 inhibits the proliferation of LS174T cells through regulation of Muc2. Amuc_1434 activates death receptors and mitochondrial apoptosis pathways to promote apoptosis in LS174T cells. In colorectal cancer, A. muciniphila induces M1-like macrophage polarization by regulating the TLR2/NLRP3 and NF-κB pathways; activated NLRP3 in Kupffer cells promotes the recruitment of NK cells to kill tumor cells. A. muciniphila extracellular vesicles enhance the anti-prostate cancer immune response of cytotoxic T lymphocytes by increasing the proportion of GZMB+CD8+ T cells and IFNγ+CD8+ T cells.
A. muciniphila is a key regulator of many anticancer drugs and affects the efficacy of antitumor therapy. Oxaliplatin, fluorouracil, and FOLFOX (oxaliplatin, fluorouracil, and calcium folinate) are commonly used as first-line chemotherapies for colon cancer. Hou et al. [178] found a significant increase in A. muciniphila abundance in colon cancer patients treated with FOLFOX. Increased abundance of A. muciniphila is associated with better prognosis in colon cancer patients treated with FOLFOX. In lung cancer, A. muciniphila administration combined with cisplatin (CDDP) significantly inhibits tumor growth and improves lung cancer tissue morphology at the pathological level in Lewis lung cancer mice [179]. Mechanistically, A. muciniphila administration combined with CDDP downregulates the expression of Ki-67, p53, and FasL. Immune checkpoint inhibitors (ICIs) that target the PD-1/PD-L1 immune checkpoint significantly improve patient outcomes. However, ICI drug resistance is a major problem in cancer diagnosis and treatment. It is very important to explore the mechanism of ICI resistance and to identify biomarkers for predicting therapeutic effects. Tumor patients taking antibiotics are resistant to PD-1 inhibitors and have poor prognoses [180]. A. muciniphila abundance increases significantly in patients with epithelial tumors who have a good response to ICI treatment. A. muciniphila administration improves the therapeutic response to PD-1 inhibitors in vivo [180]. A. muciniphila abundance is positively associated with the objective response rate and overall survival rate in patients with advanced non-small cell lung cancer (NSCLC) who receive ICI treatment [181,182]. A. muciniphila abundance can be used as a marker to predict the response to ICI treatment in patients with NSCLC. HYR-2 may play an anti-lung cancer role by inhibiting the protein expression of PD-L1 and increasing A. muciniphila abundance [183]. Abiraterone acetate (AA) is an androgen biosynthesis inhibitor targeting CYP17, and it also has therapeutic effects on androgen-independent prostate cancer. The mechanism of this phenomenon was explored by Daisley et al. [184] They found a significant increase in A. muciniphila abundance in the gut flora of prostate cancer patients treated with ADT+ (androgen deprivation therapy) AA. Regulating the intestinal ecosystem to improve cancer treatment is a promising research direction.
Regulators of A. muciniphila Abundance
A. muciniphila is involved in the occurrence and development of a variety of human diseases, even cancer. It is a shining star among gut bacteria. Increasing A. muciniphila abundance may inhibit or even reverse disease progression. Though A. muciniphila uses mucin secreted by the intestinal mucosa as its sole source of energy, Dietary, drug-derived compounds and some gut microbes provide a microenvironment for A. muciniphila growth and could regulate A. muciniphila abundance. To obtain a more detailed and comprehensive understanding of A. muciniphila regulators and promote the clinical application of A. muciniphila, we summarize the relevant literature on A. muciniphila regulators (Fig. 1).
Dietary regulators
Dietary intervention is the main way to regulate the abundance of gut microbes. Numerous studies have demonstrated that dietary regulators play an important role in the regulation of A. muciniphila abundance. Polyphenols include flavonoids, phenolic acids, stilbenes, and lignans. Dietary polyphenols, such as grape polyphenols [185], tea polyphenols [186,187], cranberry extract [188], pomegranate ellagic tannins [189], and black raspberry [190], are the most commonly studied substances regulating A. muciniphila abundance. Pomegranate extract, grape procyanidins, cranberry extract, and green tea polyphenols all significantly increase A. muciniphila abundance, thereby improving metabolism and intestinal inflammation. Grape polyphenols can reduce obesity, glucose intolerance, and oxidative stress caused by increased A. muciniphila abundance [191–193]. Cranberry extract promotes liver lipid metabolism and reverses insulin resistance by upregulating A. muciniphila abundance [194]. Mechanically, cranberry extract improves the gut barrier by enhancing mucin secretion and promotes A. muciniphila growth [195]. The mechanism of other polyphenols in upregulating A. muciniphila abundance remains unclear. Further experiments are needed to investigate this. The cometabolism of epigallocatechin-3-gallate, mucin, and glucose effectively promotes the growth of A. muciniphila in vitro [187]. These studies suggest that the gut–liver axis is the main target of dietary polyphenols. The antimicrobial activity of polyphenols and A. muciniphila’s tolerance to polyphenols may give A. muciniphila a competitive advantage in the gut. However, not all polyphenols are effective or equally effective in humans and animals. Oligosaccharides are also important regulators of A. muciniphila. Stachyose is used by probiotics in the gut. Both low-dose and high-dose stachyose can improve the abundance of A. muciniphila in the gut. High-dose stachyose can also promote the proliferation of bifidobacteria [196]. By regulating the gut microbiota, stachyose increases goblet cell number and tight junction protein expression. KDs increase the threshold of epileptic seizures by increasing A. muciniphila abundance in the gut of mice [114]. Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) diets also affect A. muciniphila abundance [197]. A. muciniphila abundance in people who ate a high-FODMAP diet was found to be significantly higher than that in a low-FODMAP diet group. A. muciniphila and Lactobacillus abundance were higher in the cecal contents of mice fed fish oil than in those fed lard [198,199]. Capsaicin (CAP) is the main pungent ingredient in chili peppers. Shen et al. [200] found an association between CAP concentration and A. muciniphila abundance. High concentrations of CAP (20 or 200 μg/ml) inhibited the growth of A. muciniphila, but low concentrations did not.
Drug regulators
Metformin also plays an important role in the regulation of gut microbiota. Metformin can increase A. muciniphila abundance and decrease the abundance of Clostridiaceae 02d06 and Firmicutes [41,89,201]. The enhancement of gut barrier mediated by A. muciniphila may further promote the antidiabetic effect of metformin. Depletion of gut microbes using a combination of antibiotics (carbenicillin, metronidazole, neomycin, and vancomycin) eliminates metformin’s regulatory effects on glucose homeostasis [202]. The combination of metformin and mannan (MOS) improves the antidiabetic effect of metformin [203]. Metformin enhances the mucus barrier and anti-inflammatory effects by regulating the gut microbiota and increasing A. muciniphila abundance, thereby alleviating ulcerative colitis [204]. Early application of vancomycin can increase the abundance of A. muciniphila and Proteobacteria and reduce the incidence of diabetes [205]. OPS-2071 is a new quinolone antibacterial agent. The broad-spectrum antibacterial activity of OPS-2071 and its low bacteriostatic effect on A. muciniphila give A. muciniphila an advantage in gut colonization [206].
Long-term use of atorvastatin leads to gut microbiome disorders and a decrease in A. muciniphila abundance, thereby promoting weight gain and glucose tolerance [207]. Inulin and butyrate supplementation significantly increased A. muciniphila abundance and the expression levels of TNF-α, Hs-CRP, and MDA [208]. Hyaluronic acid–bilirubin nanomedicine (HABN) is a nanodrug targeting colitis via the HA–CD44 interaction. HABN regulates the distribution of gut flora and significantly increases the abundance of A. muciniphila [209]. A variety of herbs can also regulate A. muciniphila and inhibit disease progression. Berberine (BBR) is an extract from Coptis chinensis and Phellodendri chinensis that is used to treat various cardiovascular and metabolic diseases [210]. By stimulating the secretion of intestinal mucus, BBR indirectly changes the gut flora of mice in a dose-dependent and time-dependent manner and increases the abundance of A. muciniphila [211]. Supplementation with rhubarb extract can change the relative abundance of A. muciniphila, Parabacteroides, and Erysipelatoclostridium, among which A. muciniphila is the most affected [212,213]. Rhubarb extract maintains the gut barrier by promoting the interaction between A. muciniphila and Reg3y and alleviates alcohol-induced liver injury. Puerarin improves weight gain and glucose homeostasis by regulating the gut microbiota, particularly A. muciniphila [214]. In addition, other herbs, such as Panax notoginseng saponins, Bofutsushosan, the total flavone of Abelmoschus manihot, and Huoxue Yiqi Recipe-2 (HyR-2), modulate A. muciniphila abundance. They improve metabolism and even anticancer activity by affecting A. muciniphila [183,215–217].
Probiotic regulators
The gut microbes interact with each other to influence the distribution and composition of the community. Lactobacillus acidophilus LA5 increases A. muciniphila abundance in the colon of mice [218]. The regulatory effect of L. acidophilus LA5 on A. muciniphila has not been confirmed in vitro. The acidifying property of L. acidophilus LA5 may be one reason that it promotes A. muciniphila growth. Alard et al. [219] showed that the B. animalis subsp. lactis strain can increase A. muciniphila abundance, while L. rhamnosus has the opposite effect. The effect of B. animalis subsp. lactis on A. muciniphila may be related to the increase in SCFA levels [219]. Exogenous A. muciniphila supplementation is the most direct way to regulate A. muciniphila. Kriebs [220] demonstrated the safety and efficacy of injecting A. muciniphila into humans in the treatment of metabolic syndrome.
A. muciniphila is difficult to culture and preserve. It is necessary to study suitable and convenient storage methods for A. muciniphila. EGCG-modified sodium alginate microcapsules can significantly improve the survival rate of A. muciniphila [221]. Dark chocolate, as a microencapsulated A. muciniphila carrier, can also maintain the survival of A. muciniphila for a long time [222]. This protective effect persists even in an in vitro astrointestinal model. Dark chocolate-containing microcapsules are expected to be an alternative carrier for A. muciniphila administration. However, the effect of chocolate on A. muciniphila and the appropriate concentration of A. muciniphila still need to be further explored.
Other regulators
Secondary bile acids are produced by the biotransformation of gut microbes and are closely related to the abundance of A. muciniphila [223]. Most bile acids, including sodium glycocholate (GCA and GDCA), ethanol sodium deoxycholate (GCDCA), sodium taurodeoxycholate hydrate (TDCA), and sodium taurodeoxycholate (TCDCA and CA), are negatively correlated with A. muciniphila abundance and inhibit the growth of A. muciniphila. However, secondary bile DCA can increase the abundance of A. muciniphila. Ursodeoxycholic acid increases A. muciniphila abundance and improves colitis in mice [224]. A squalene synthase inhibitor (zaragozic acid A) increases the sensitivity of A. muciniphila to bile acid. Oleoylethanolamide (OEA), an endocannabinoid, is an important lipid signaling molecule in the gut–brain axis. Supplementation with OEA can increase A. muciniphila abundance and reduce energy and carbohydrate intake [225]. Physical exercise can regulate the gut flora in various diseases. Regular physical exercise increases intestinal Prevotella levels and decreases A. muciniphila abundance in patients with MS [226]. In the HF+DSS-induced mouse colitis model, a certain intensity of exercise training increases the diversity of gut flora (increases abundance of A. muciniphila) and reduces gut inflammation [227]. The gut flora may mediate the antidepressant effects of the C-terminal domain of the heavy chain of tetanus toxin (HC-TETX) [228].
Conclusions and Perspectives
The gut is the primary site of microbial settlement and contains trillions of microbes. Bacteria, as the main inhabitants of the gut, interact with the host in many ways and play an important role in regulating body functions and defending against diseases. The gut flora is involved in human digestion and absorption, energy generation, vitamin synthesis, and immune regulation. It can even regulate the central nervous system and affect the physiological functions of the human brain. However, if the gut flora is out of balance, an increase or decrease in a certain type of bacteria can lead to disease. A. muciniphila also promotes intestinal stem cell proliferation and increases the number of goblet cells. A. muciniphila and its metabolites play an important role in endocrine system diseases, nervous system diseases, digestive system diseases, musculoskeletal system diseases, respiratory system diseases, and other diseases.
A. muciniphila abundance is decreased in most diseases but increased in PD, MS, necrotizing pancreatitis, and infantile eczema. The reasons for this difference need further experimental investigation. In original studies of A. muciniphila and disease, only complete live bacteria produced a probiotic effect; pretreated A. muciniphila did not. However, subsequent studies found that A. muciniphila used after pasteurization has higher activity than live A. muciniphila [73]. The difference may be due to the tolerance of A. muciniphila within a certain range of temperatures. Heat treatment at 121 °C and 225 kPa denatured the active components of A. muciniphila. The better effect of pasteurized A. muciniphila compared with live A. muciniphila may be due to some other components of the bacteria that interfere with Amuc_1100 but cannot tolerate pasteurization temperatures. P9 has potential for use in lipid-reducing and hypoglycemic drugs. Breakthroughs in determining the structure of P9 and the complex structure of P9 and ICAM-2 will greatly promote the clinical application of P9. A. muciniphila is expected to become the next generation of probiotics in addition to Lactobacillus and bifidobacteria. In 2019, the results from the first human trial of A. muciniphila probiotic supplements confirmed the safety of A. muciniphila administration [72]. This study brings hope for the clinical application of A. muciniphila, although it has some limitations, such as the small sample size and the lack of an accurate method to measure visceral and subcutaneous fat. Indirect regulation of A. muciniphila abundance may inhibit or even reverse disease progression. A. muciniphila abundance is regulated by dietary, drug, and probiotic factors. Although most of these regulators cannot specifically target A. muciniphila abundance, prior studies provide new ideas for us to target A. muciniphila regulation.
Acknowledgments
Funding: This work was funded by the National Key Research and Development Program of China (2021YFC2301800) and the National Nature Science Foundation of China (82200673 and U20A20343). Author contributions: L.L. and J.L. designed the work, and reviewed and edited the manuscript; C.X., G.L., and X.G. participated in original draft preparation; Y.S., Q.Z., and X.Y. helped with reviewing the manuscript; Z.B. collected the references. All authors have read and approved the final manuscript. Competing interests: The authors declare that they have no competing interests.
References
- 1.Mercado-Perez A, Beyder A. Gut feelings: Mechanosensing in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2022;19(5):283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson AJ, Bourke CD, Robertson RC, Shivakumar N, Edwards CA, Preston T, Holmes E, Kelly P, Frost G, Morrison DJ, et al. Understanding the role of the gut in undernutrition: What can technology tell us? Gut. 2021;70(8):1580–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut. 2020;69(12):2232–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penny HA, Domingues RG, Krauss MZ, Melo-Gonzalez F, Lawson MAE, Dickson S, Parkinson J, Hurry M, Purse C, Jegham E, et al. Rhythmicity of intestinal IgA responses confers oscillatory commensal microbiota mutualism. Sci Immunol. 2022;7(75): Article eabk2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj JS, Ng SC, Schnabl B. Promises of microbiome-based therapies. J Hepatol. 2022;76(6):1379–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, Shah K. The potential of the gut microbiome to reshape the cancer therapy paradigm: A review. JAMA Oncol. 2022;8(7):1059–1067. [DOI] [PubMed] [Google Scholar]
- 8.Zheng X, Cai X, Hao H. Emerging targetome and signalome landscape of gut microbial metabolites. Cell Metab. 2022;34(1):35–58. [DOI] [PubMed] [Google Scholar]
- 9.Rollenske T, Burkhalter S, Muerner L, Gunten S, Lukasiewicz J, Wardemann H, Macpherson AJ. Parallelism of intestinal secretory IgA shapes functional microbial fitness. Nature. 2021;598(7882):657–661. [DOI] [PubMed] [Google Scholar]
- 10.Wypych TP, Pattaroni C, Perdijk O, Yap C, Trompette A, Anderson D, Creek DJ, Harris NL, Marsland BJ. Microbial metabolism of L-tyrosine protects against allergic airway inflammation. Nat Immunol. 2021;22(3):279–286. [DOI] [PubMed] [Google Scholar]
- 11.Brown EM, Sadarangani M, Finlay BB. The role of the immune system in governing host-microbe interactions in the intestine. Nat Immunol. 2013;14(7):660–667. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22(10):1079–1089. [DOI] [PubMed] [Google Scholar]
- 13.Teichman EM, O'Riordan KJ, Gahan CGM, Dinan TG, Cryan JF. When rhythms meet the blues: Circadian interactions with the microbiota-gut-brain axis. Cell Metab. 2020;31(3):448–471. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Mu X, Cao Q, Shi Y, Hu X, Zheng H. Honeybee gut Lactobacillus modulates host learning and memory behaviors via regulating tryptophan metabolism. Nat Commun. 2022;13(1): Article 2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant CV, Loman BR, Bailey MT, Pyter LM. Manipulations of the gut microbiome alter chemotherapy-induced inflammation and behavioral side effects in female mice. Brain Behav Immun. 2021;95:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trastoy B, Naegeli A, Anso I, Sjögren J, Guerin ME. Structural basis of mammalian mucin processing by the human gut O-glycopeptidase OgpA from Akkermansia muciniphila. Nat Commun. 2020;11(1): Article 4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derrien M, Vaughan EE, Plugge CM, Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54(Pt 5):1469–1476. [DOI] [PubMed] [Google Scholar]
- 18.Belzer C, Vos WM. Microbes inside—From diversity to function: The case of Akkermansia. ISME J. 2012;6(8):1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machado D, Almeida D, Seabra CL, Andrade JC, Gomes AM, Freitas AC. Uncovering Akkermansia muciniphila resilience or susceptibility to different temperatures, atmospheres and gastrointestinal conditions. Anaerobe. 2020;61: Article 102135. [DOI] [PubMed] [Google Scholar]
- 20.Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, Vos WM, Satokari R. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 2015;81(11):3655–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouwerkerk JP, van der Ark KCH, Davids M, Claassens NJ, Finestra TR, de Vos WM, Belzer C. Adaptation of Akkermansia muciniphila to the oxic-anoxic interface of the mucus layer. Appl Environ Microbiol. 2016;82(23):6983–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Tang L, Feng Y, Zhao S, Han M, Zhang C, Yuan G, Zhu J, Cao S, Wu Q, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut. 2020;69(11):1988–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogata Y, Sakamoto M, Ohkuma M, Hattori M, Suda W. Complete genome sequence of Akkermansia muciniphila JCM 30893, isolated from feces of a healthy Japanese male. Microbiol Resour Announc. 2020;9(7): Article e01543-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geerlings SY, Ouwerkerk JP, Koehorst JJ, Ritari J, Aalvink S, Stecher B, Schaap PJ, Paulin L, de Vos WM, Belzer C. Genomic convergence between Akkermansia muciniphila in different mammalian hosts. BMC Microbiol. 2021;21(1): Article 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arias-Jayo N, Alonso-Saez L, Ramirez-Garcia A, Pardo MA. Zebrafish axenic larvae colonization with human intestinal microbiota. Zebrafish. 2018;15(2):96–106. [DOI] [PubMed] [Google Scholar]
- 26.Ouwerkerk JP, Aalvink S, Belzer C, Vos WM. Akkermansia glycaniphila sp. nov., an anaerobic mucin-degrading bacterium isolated from reticulated python faeces. Int J Syst Evol Microbiol. 2016;66(11):4614–4620. [DOI] [PubMed] [Google Scholar]
- 27.Ouwerkerk JP, Koehorst JJ, Schaap PJ, Ritari J, Paulin L, Belzer C, de Vos WM. Complete genome sequence of Akkermansia glycaniphila strain PytT, a mucin-degrading specialist of the reticulated python gut. Genome Announc. 2017;5(1): Article e01098-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubourg G, Cornu F, Edouard S, Battaini A, Tsimaratos M, Raoult D. First isolation of Akkermansia muciniphila in a blood-culture sample. Clin Microbiol Infect. 2017;23(9):682–683. [DOI] [PubMed] [Google Scholar]
- 29.Collado MC, Isolauri E, Laitinen K, Salminen S. Effect of mother's weight on infant's microbiota acquisition, composition, and activity during early infancy: A prospective follow-up study initiated in early pregnancy. Am J Clin Nutr. 2010;92(5):1023–1030. [DOI] [PubMed] [Google Scholar]
- 30.Qi Y, Yu L, Tian F, Zhao J, Zhang H, Chen W, Zhai Q. A. Muciniphila supplementation in mice during pregnancy and lactation affects the maternal intestinal microenvironment. Nutrients. 2022;14(2): Article 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kostopoulos I, Elzinga J, Ottman N, Klievink JT, Blijenberg B, Aalvink S, Boeren S, Mank M, Knol J, de Vos WM, et al. Akkermansia muciniphila uses human milk oligosaccharides to thrive in the early life conditions in vitro. Sci Rep. 2020;10(1): Article 14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luna E, Parkar SG, Kirmiz N, Hartel S, Hearn E, Hossine M, Kurdian A, Mendoza C, Orr K, Padilla L, et al. Utilization efficiency of human milk oligosaccharides by human-associated Akkermansia is strain dependent. Appl Environ Microbiol. 2022;88(1): Article e0148721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derrien M, Collado MC, Ben-Amor K, Salminen S, Vos WM. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol. 2008;74(5):1646–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. 2007;73(23):7767–7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derrien M, Passel MW, Bovenkamp JH, Schipper RG, Vos WM, Dekker J. Mucin-bacterial interactions in the human oral cavity and digestive tract. Gut Microbes. 2010;1(4):254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xing J, Li X, Sun Y, Zhao J, Miao S, Xiong Q, Zhang Y, Zhang G. Comparative genomic and functional analysis of Akkermansia muciniphila and closely related species. Genes Genomics. 2019;41(11):1253–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosciow K, Deppenmeier U. Characterization of three novel β-galactosidases from Akkermansia muciniphila involved in mucin degradation. Int J Biol Macromol. 2020;149:331–340. [DOI] [PubMed] [Google Scholar]
- 38.Kim JS, Kang SW, Lee JH, Park SH, Lee JS. The evolution and competitive strategies of Akkermansia muciniphila in gut. Gut Microbes. 2022;14(1):2025017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li CC, Tang XY, Zhu YB, Song Y-J, Zhao N-L, Huang Q, Mou X-Y, Luo G-H, Liu T-G, Tong A-P, et al. Structural analysis of the sulfatase AmAS from Akkermansia muciniphila. Acta Crystallogr D Struct Biol. 2021;77(Pt 12):1614–1623. [DOI] [PubMed] [Google Scholar]
- 40.Ottman N, Davids M, Suarez-Diez M, Boeren S, Schaap PJ, Dos Santos VAPM, Smidt H, Belzer C, de Vos WM. Genome-scale model and omics analysis of metabolic capacities of Akkermansia muciniphila reveal a preferential mucin-degrading lifestyle. Appl Environ Microbiol. 2017;83(18): Article e01014-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, Escobar JS. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40(1):54–62. [DOI] [PubMed] [Google Scholar]
- 42.Huang S, Hu S, Liu S, Tang B, Liu Y, Tang L, Lei Y, Zhong L, Yang S, He S. Lithium carbonate alleviates colon inflammation through modulating gut microbiota and Treg cells in a GPR43-dependent manner. Pharmacol Res. 2022;175: Article 105992. [DOI] [PubMed] [Google Scholar]
- 43.Lee JY, Jin HS, Kim KS, Baek JH, Kim BS, Lee DW. Nutrient-specific proteomic analysis of the mucin degrading bacterium Akkermansia muciniphila. Proteomics. 2022;22(3): Article e2100125. [DOI] [PubMed] [Google Scholar]
- 44.Kim S, Shin YC, Kim TY, Kim Y, Lee Y-S, Lee S-H, Kim M-N, O E, Kim KS, Kweon M-N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes. 2021;13(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qu S, Fan L, Qi Y, Xu C, Hu Y, Chen S, Liu W, Liu W, Si J. Akkermansia muciniphila alleviates dextran sulfate sodium (DSS)-induced acute colitis by NLRP3 activation. Microbiol Spectr. 2021;9(2): Article e0073021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin J, Noh JR, Chang DH, Kim Y-H, Kim MH, Lee ES, Cho S, Ku BJ, Rhee M-S, Kim B-C, et al. Elucidation of Akkermansia muciniphila probiotic traits driven by mucin depletion. Front Microbiol. 2019;10: Article 1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Xiang R, Wang R, Zhang B, Gong W, Zhang J, Zhang M, Wang M. The variable oligomeric state of Amuc_1100 from Akkermansia muciniphila. J Struct Biol. 2020;212(1): Article 107593. [DOI] [PubMed] [Google Scholar]
- 48.Ottman N, Reunanen J, Meijerink M, Pietilä TE, Kainulainen V, Klievink J, Huuskonen L, Aalvink S, Skurnik M, Boeren S, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLOS ONE. 2017;12(3): Article e0173004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiao A, Yu B, He J, Yu J, Zheng P, Luo Y, Luo J, Yan H, Wang Q, Wang H, et al. Sodium acetate, propionate, and butyrate reduce fat accumulation in mice via modulating appetite and relevant genes. Nutrition. 2021;87–88: Article 111198. [DOI] [PubMed] [Google Scholar]
- 50.Heimann E, Nyman M, Degerman E. Propionic acid and butyric acid inhibit lipolysis and de novo lipogenesis and increase insulin-stimulated glucose uptake in primary rat adipocytes. Adipocytes. 2015;4(2):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daïen CI, Tan J, Audo R, Mielle J, Quek LE, Krycer JR, Angelatos A, Duraes M, Pinget G, Ni D, et al. Gut-derived acetate promotes B10 cells with antiinflammatory effects. JCI Insight. 2021;6(7): Article e144156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Herreweghen F, De Paepe K, Roume H, Kerckhof FM, Van de Wiele T. Mucin degradation niche as a driver of microbiome composition and Akkermansia muciniphila abundance in a dynamic gut model is donor independent. FEMS Microbiol Ecol. 2018;94(12): Article fiy186. [DOI] [PubMed] [Google Scholar]
- 53.Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, Brown EM, Graham DB, Xavier RJ, Moon JJ, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. 2019;364(6446):1179–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuczma MP, Szurek EA, Cebula A, Ngo VL, Pietrzak M, Kraj P, Denning TL, Ignatowicz L. Self and microbiota-derived epitopes induce CD4+ T cell anergy and conversion into CD4+Foxp3+ regulatory cells. Mucosal Immunol. 2021;14(2):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kirmiz N, Galindo K, Cross KL, Luna E, Rhoades N, Podar M, Flores GE. Comparative genomics guides elucidation of vitamin B(12) biosynthesis in novel human-associated Akkermansia strains. Appl Environ Microbiol. 2020;86(3): Article e02117-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopetuso LR, Quagliariello A, Schiavoni M, Petito V, Russo A, Reddel S, Chierico FD, Ianiro G, Scaldaferri F, Neri M, et al. Towards a disease-associated common trait of gut microbiota dysbiosis: The pivotal role of Akkermansia muciniphila. Dig Liver Dis. 2020;52(9):1002–1010. [DOI] [PubMed] [Google Scholar]
- 57.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Journey EK, Ortega-Santos CP, Bruening M, Whisner CM. Changes in weight status and the intestinal microbiota among college freshman, aged 18 years. J Adolesc Health. 2020;66(2):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karlsson CL, Onnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring). 2012;20(11):2257–2261. [DOI] [PubMed] [Google Scholar]
- 60.Collado MC, Laitinen K, Salminen S, Isolauri E. Maternal weight and excessive weight gain during pregnancy modify the immunomodulatory potential of breast milk. Pediatr Res. 2012;72(1):77–85. [DOI] [PubMed] [Google Scholar]
- 61.Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, Martí-Romero M, Lopez RM, Florido J, Campoy C, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104(1):83–92. [DOI] [PubMed] [Google Scholar]
- 62.Dao MC, Belda E, Prifti E, Everard A, Kayser BD, Bouillot J-L, Chevallier J-M, Pons N, Chatelier EL, Ehrlich SD, et al. Akkermansia muciniphila abundance is lower in severe obesity, but its increased level after bariatric surgery is not associated with metabolic health improvement. Am J Physiol Endocrinol Metab. 2019;317(3):E446–E459. [DOI] [PubMed] [Google Scholar]
- 63.Palmisano S, Campisciano G, Silvestri M, Guerra M, Giuricin M, Casagranda B, Comar M, Manzini N. Changes in gut microbiota composition after bariatric surgery: A new balance to decode. J Gastrointest Surg. 2020;24(8):1736–1746. [DOI] [PubMed] [Google Scholar]
- 64.Mabey JG, Chaston JM, Castro DG, Adams TD, Hunt SC, Davidson LE. Gut microbiota differs a decade after bariatric surgery relative to a nonsurgical comparison group. Surg Obes Relat Dis. 2020;16(9):1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee J, Jang JY, Kwon MS, Lim SK, Kim N, Lee J, Park HK, Yun M, Shin M-Y, Jo HE, et al. Mixture of two Lactobacillus plantarum strains modulates the gut microbiota structure and regulatory T cell response in diet-induced obese mice. Mol Nutr Food Res. 2018;62(24): Article e1800329. [DOI] [PubMed] [Google Scholar]
- 66.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut. 2016;65(3):426–436. [DOI] [PubMed] [Google Scholar]
- 67.Isokpehi RD, Simmons SS, Johnson MO, Payton M. Genomic evidence for bacterial determinants influencing obesity development. Int J Environ Res Public Health. 2017;14(4):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang M, Bose S, Lim S, Seo JG, Shin JH, Lee D, Chung W-H, Song E-J, Nam Y-D, Kim H. Beneficial effects of newly isolated Akkermansia muciniphila strains from the human gut on obesity and metabolic dysregulation. Microorganisms. 2020;8(9): Article 1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Depommier C, Van Hul M, Everard A, Delzenne NM, De Vos WM, Cani PD. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes. 2020;11(5):1231–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deng L, Ou Z, Huang D, Li C, Lu Z, Liu W, Wu F, Nong C, Gao J, Peng Y. Diverse effects of different Akkermansia muciniphila genotypes on Brown adipose tissue inflammation and whitening in a high-fat-diet murine model. Microb Pathog. 2020;147: Article 104353. [DOI] [PubMed] [Google Scholar]
- 71.Choi Y, Bose S, Seo J, Shin J-H, Lee D, Kim Y, Kang SG, Kim H. Effects of live and pasteurized forms of Akkermansia from the human gut on obesity and metabolic dysregulation. Microorganisms. 2021;9(10): Article 2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–113. [DOI] [PubMed] [Google Scholar]
- 74.Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, Kim J-h, Han D, Cha KH, Moon SH, Lee K, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol. 2021;6(5):563–573. [DOI] [PubMed] [Google Scholar]
- 75.Depommier C, Vitale RM, Iannotti FA, Silvestri C, Flamand N, Druart C, Everard A, Pelicaen R, Maiter D, Thissen J-P, et al. Beneficial effects of Akkermansia muciniphila are not associated with major changes in the circulating endocannabinoidome but linked to higher mono-palmitoyl-glycerol levels as new PPARα agonists. Cell. 2021;10(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee JS, Song WS, Lim JW, Choi T-R, Jo S-H, Jeon H-J, Kwon J-E, Park J-H, Kim Y-R, Yang Y-H, et al. An integrative multiomics approach to characterize anti-adipogenic and anti-lipogenic effects of Akkermansia muciniphila in adipocytes. Biotechnol J. 2022;17(2): Article e2100397. [DOI] [PubMed] [Google Scholar]
- 77.Mitsou EK, Detopoulou M, Kakali A, Fragopoulou E, Nomikos T, Antonopoulou S, Panagiotakos DB, Kyriacou A. Mining possible associations of faecal A. muciniphila colonisation patterns with host adiposity and cardiometabolic markers in an adult population. Benef Microbes. 2019;10(7):741–749. [DOI] [PubMed] [Google Scholar]
- 78.Zhou Q, Pang G, Zhang Z, Yuan H, Chen C, Zhang N, Yang Z, Sun L. Association between gut Akkermansia and metabolic syndrome is dose-dependent and affected by microbial interactions: A cross-sectional study. Diabetes Metab Syndr Obes. 2021;14:2177–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLOS ONE. 2013;8(8): Article e71108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Zhao W, Shi J, Wang J, Hao J, Pang X, Huang X, Chen X, Li Y, Jin R, et al. Intestinal microbiota contributes to altered glucose metabolism in simulated microgravity mouse model. FASEB J. 2019;33(9):10140–10151. [DOI] [PubMed] [Google Scholar]
- 81.Liu F, Ling Z, Xiao Y, Lv L, Yang Q, Wang B, Lu H, Zheng L, Jiang P, Wang W, et al. Dysbiosis of urinary microbiota is positively correlated with type 2 diabetes mellitus. Oncotarget. 2017;8(3):3798–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Allin KH, Tremaroli V, Caesar R, Jensen BAH, Damgaard MTF, Bahl MI, Licht TR, Hansen TH, Nielsen T, Dantoft TM, et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia. 2018;61(4):810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang J, Ni Y, Qian L, Fang Q, Zheng T, Zhang M, Gao Q, Zhang Y, Ni J, Hou X, et al. Decreased abundance of Akkermansia muciniphila leads to the impairment of insulin secretion and glucose homeostasis in lean type 2 diabetes. Adv Sci (Weinh). 2021;8(16): Article e2100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shih CT, Yeh YT, Lin CC, Yang LY, Chiang CP. Akkermansia muciniphila is negatively correlated with hemoglobin A1c in refractory diabetes. Microorganisms. 2020;8(9): Article 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang L, Qin Q, Liu M, Zhang X, He F, Wang G. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation, and normalize intestine microbiota in streptozotocin-induced diabetic rats. Pathog Dis. 2018;76(4): Article fty028. [DOI] [PubMed] [Google Scholar]
- 86.Greer RL, Dong X, Moraes AC, Zielke RA, Fernandes GR, Peremyslova E, Vasquez-Perez S, Schoenborn AA, Gomes EP, et al. Akkermansia muciniphila mediates negative effects of IFNγ on glucose metabolism. Nat Commun. 2016;7: Article 13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. [DOI] [PubMed] [Google Scholar]
- 88.Fassatoui M, Lopez-Siles M, Díaz-Rizzolo DA, Jmel H, Naouali C, Abdessalem G, Chikhaoui A, Nadal B, Jamoussi H, Abid A, et al. Gut microbiota imbalances in Tunisian participants with type 1 and type 2 diabetes mellitus. Biosci Rep. 2019;39(6): Article BSR20182348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou ZY, Ren LW, Zhan P, Yang HY, Chai DD, Yu ZW. Metformin exerts glucose-lowering action in high-fat fed mice via attenuating endotoxemia and enhancing insulin signaling. Acta Pharmacol Sin. 2016;37(8):1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hänninen A, Toivonen R, Pöysti S, Belzer C, Plovier H, Ouwerkerk JP, Emani R, Cani PD, De Vos WM. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut. 2018;67(8):1445–1453. [DOI] [PubMed] [Google Scholar]
- 91.Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe–/– mice. Circulation. 2016;133(24):2434–2446. [DOI] [PubMed] [Google Scholar]
- 92.Shen J, Tong X, Sud N, Khound R, Song Y, Maldonado-Gomez MX, Walter J, Su Q. Low-density lipoprotein receptor signaling mediates the triglyceride-lowering action of Akkermansia muciniphila in genetic-induced hyperlipidemia. Arterioscler Thromb Vasc Biol. 2016;36(7):1448–1456. [DOI] [PubMed] [Google Scholar]
- 93.Katiraei S, Vries MR, Costain AH, Thiem K, Hoving LR, Diepen JA, Smits HH, Bouter KE, Rensen PCN, Quax PHA, et al. Akkermansia muciniphila exerts lipid-lowering and immunomodulatory effects without affecting neointima formation in hyperlipidemic APOE*3-Leiden.CETP mice. Mol Nutr Food Res. 2020;64(15): Article e1900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qin Q, Yan S, Yang Y, Chen J, Yan H, Li T, Gao X, Wang Y, Li A, Wang S, et al. The relationship between osteoporosis and intestinal microbes in the Henan Province of China. Front Cell Dev Biol. 2021;9: Article 752990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lawenius L, Scheffler JM, Gustafsson KL, Henning P, Nilsson KH, Colldén H, Islander U, Plovier H, Cani PD, Vos WM, et al. Pasteurized Akkermansia muciniphila protects from fat mass gain but not from bone loss. Am J Physiol Endocrinol Metab. 2020;318(4):E480–E491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Keshavarz Azizi Raftar S, Hoseini Tavassol Z, Amiri M, Ejtahed H-S, Zangeneh M, Sadeghi S, Ashrafian F, Kariman A, Khatami S, Siadat SD. Assessment of fecal Akkermansia muciniphila in patients with osteoporosis and osteopenia: A pilot study. J Diabetes Metab Disord. 2021;20(1):279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harach T, Marungruang N, Duthilleul N, Cheatham V, McCoy KD, Frisoni G, Neher JJ, Fåk F, Jucker M, Lasser T, et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci Rep. 2017;7: Article 41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ou Z, Deng L, Lu Z, Wu F, Liu W, Huang D, Peng Y. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer's disease. Nutr Diabetes. 2020;10(1): Article 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khedr EM, Omeran N, Karam-Allah Ramadan H, Ahmed GK, Abdelwarith AM. Alteration of gut microbiota in Alzheimer's disease and their relation to the cognitive impairment. J Alzheimers Dis. 2022;88(3):1103–1114. [DOI] [PubMed] [Google Scholar]
- 100.Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, Kleimeyer C, Moresi C, Harnik Y, Zur M, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572(7770):474–480. [DOI] [PubMed] [Google Scholar]
- 101.Liu S, Rezende RM, Moreira TG, Tankou SK, Cox LM, Wu M, Song A, Dhang FH, Wei Z, Costamagna G, et al. Oral administration of miR-30d from feces of MS patients suppresses MS-like symptoms in mice by expanding Akkermansia muciniphila. Cell Host Microbe. 2019;26(6):779–794.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cekanaviciute E, Yoo BB, Runia TF, Debelius JW, Singh S, Nelson CA, Kanner R, Bencosme Y, Lee YK, Hause SL, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A. 2017;114(40):10713–10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.iMSMS Consortium. Gut microbiome of multiple sclerosis patients and paired household healthy controls reveal associations with disease risk and course. Cell. 2022;185(19):3467–3486.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zapała B, Stefura T, Wójcik-Pędziwiatr M, Kabut R, Bałajewicz-Nowak M, Milewicz T, Dudek A, Stój A, Rudzińska-Bar M. Differences in the composition of gut microbiota between patients with Parkinson's disease and healthy controls: A cohort study. J Clin Med. 2021;10(23): Article 5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Amorim Neto DP, Bosque BP, Pereira de Godoy JV, Rodrigues PV, Meneses DD, Tostes K, Costa Tonoli CC, Carvalho HF, González-Billault C, Castro Fonseca M. Akkermansia muciniphila induces mitochondrial calcium overload and α-synuclein aggregation in an enteroendocrine cell line. iScience. 2022;25(3): Article 103908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen T, Wang R, Duan Z, Yuan X, Ding Y, Feng Z, Bu F, Liu L, Wang Q, Zhou J, et al. Akkermansia muciniphila protects against psychological disorder-induced gut microbiota-mediated colonic mucosal barrier damage and aggravation of colitis. Front Cell Infect Microbiol. 2021;11: Article 723856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Getachew B, Tizabi Y. Effects of C-terminal domain of the heavy chain of tetanus toxin on gut microbiota in a rat model of depression. Clin Pharmacol Transl Med. 2019;3(2):152–159. [PMC free article] [PubMed] [Google Scholar]
- 108.Choi J, Kwon H, Kim YK, Han PL. Extracellular vesicles from gram-positive and gram-negative probiotics remediate stress-induced depressive behavior in mice. Mol Neurobiol. 2022;59(5):2715–2728. [DOI] [PubMed] [Google Scholar]
- 109.Ding Y, Bu F, Chen T, Shi G, Yuan X, Feng Z, Duan Z, Wang R, Zhang S, Wang Q, et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl Microbiol Biotechnol. 2021;105(21–22):8411–8426. [DOI] [PubMed] [Google Scholar]
- 110.Cheng R, Xu W, Wang J, Tang Z, Zhang M. The outer membrane protein Amuc_1100 of Akkermansia muciniphila alleviates the depression-like behavior of depressed mice induced by chronic stress. Biochem Biophys Res Commun. 2021;566:170–176. [DOI] [PubMed] [Google Scholar]
- 111.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol. 2011;77(18):6718–6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goo N, Bae HJ, Park K, Kim J, Jeong Y, Cai M, Cho K, Jung SY, Kim D-H, Ryu JH. The effect of fecal microbiota transplantation on autistic-like behaviors in Fmr1 KO mice. Life Sci. 2020;262: Article 118497. [DOI] [PubMed] [Google Scholar]
- 113.Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728–1741.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dooling SW, Costa-Mattioli M. Gut bacteria seize control of the brain to prevent epilepsy. Cell Host Microbe. 2018;24(1):3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ilhan ZE, Brochard V, Lapaque N, Auvin S, Lepage P. Exposure to anti-seizure medications impact growth of gut bacterial species and subsequent host response. Neurobiol Dis. 2022;167: Article 105664. [DOI] [PubMed] [Google Scholar]
- 116.Zhang T, Li P, Wu X, Lu G, Marcella C, Ji X, Ji G, Zhang F. Alterations of Akkermansia muciniphila in the inflammatory bowel disease patients with washed microbiota transplantation. Appl Microbiol Biotechnol. 2020;104(23):10203–10215. [DOI] [PubMed] [Google Scholar]
- 117.Png CW, Lindén SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin THJ. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105(11):2420–2428. [DOI] [PubMed] [Google Scholar]
- 118.Bajer L, Kverka M, Kostovcik M, Macinga P, Dvorak J, Stehlikova Z, Brezina J, Wohl P, Spicak J, Drastich P. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J Gastroenterol. 2017;23(25):4548–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vigsnæs LK, Brynskov J, Steenholdt C, Wilcks A, Licht TR. Gram-negative bacteria account for main differences between faecal microbiota from patients with ulcerative colitis and healthy controls. Benef Microbes. 2012;3(4):287–297. [DOI] [PubMed] [Google Scholar]
- 120.James SL, Christophersen CT, Bird AR, Conlon MA, Rosella O, Gibson PR, Muir JG. Abnormal fibre usage in UC in remission. Gut. 2015;64(4):562–570. [DOI] [PubMed] [Google Scholar]
- 121.Shang Q, Sun W, Shan X, Jiang H, Cai C, Hao J, Li G, Yu G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol Lett. 2017;279:87–95. [DOI] [PubMed] [Google Scholar]
- 122.Earley H, Lennon G, Balfe Á, Coffey JC, Winter DC, O'Connell PR. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci Rep. 2019;9(1):15683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kang CS, Ban M, Choi EJ, Moon H-G, Jeon J-S, Kim D-K, Park S-K, Jeon SG, Roh T-Y, Myung S-J, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLOS ONE. 2013;8(10): Article e76520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qian K, Chen S, Wang J, Sheng K, Wang Y, Zhang M. A β-N-acetylhexosaminidase Amuc_2109 from Akkermansia muciniphila protects against dextran sulfate sodium-induced colitis in mice by enhancing intestinal barrier and modulating gut microbiota. Food Funct. 2022;13(4):2216–2227. [DOI] [PubMed] [Google Scholar]
- 125.Luo Y, Lan C, Xie K, Li H, Devillard E, He J, Liu L, Cai J, Tian G, Wu A, et al. Active or autoclaved Akkermansia muciniphila relieves TNF-α-induced inflammation in intestinal epithelial cells through distinct pathways. Front Immunol. 2021;12: Article 788638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gu Z, Pei W, Shen Y, Wang L, Zhu J, Zhang Y, Fan S, Wu Q, Li L, Zhang Z. Akkermansia muciniphila and its outer protein Amuc_1100 regulates tryptophan metabolism in colitis. Food Funct. 2021;12(20):10184–10195. [DOI] [PubMed] [Google Scholar]
- 127.Bian X, Wu W, Yang L, Lv L, Wang Q, Li Y, Ye J, Fang D, Wu J, Jiang X, et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol. 2019;10: Article 2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu Q, Lu W, Tian F, Zhao J, Zhang H, Hong K, Yu L. Akkermansia muciniphila exerts strain-specific effects on DSS-induced ulcerative colitis in mice. Front Cell Infect Microbiol. 2021;11: Article 698914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhai R, Xue X, Zhang L, Yang X, Zhao L, Zhang C. Strain-specific anti-inflammatory properties of two Akkermansia muciniphila strains on chronic colitis in mice. Front Cell Infect Microbiol. 2019;9:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Han R, Qiu H, Zhong J, Zheng N, Li B, Hong Y, Ma J, Wu G, Chen L, Sheng L, et al. Si Miao formula attenuates non-alcoholic fatty liver disease by modulating hepatic lipid metabolism and gut microbiota. Phytomedicine. 2021;85: Article 153544. [DOI] [PubMed] [Google Scholar]
- 131.Zhang L, Wang Y, Wu F, Wang X, Feng Y, Wang Y. MDG, an Ophiopogon japonicus polysaccharide, inhibits non-alcoholic fatty liver disease by regulating the abundance of Akkermansia muciniphila. Int J Biol Macromol. 2022;196:23–34. [DOI] [PubMed] [Google Scholar]
- 132.Nishiyama M, Ohtake N, Kaneko A, Tsuchiya N, Imamura S, Iizuka S, Ishizawa S, Nishi A, Yamamoto M, Taketomi A, et al. Increase of Akkermansia muciniphila by a diet containing Japanese traditional medicine Bofutsushosan in a mouse model of non-alcoholic fatty liver disease. Nutrients. 2020;12(3):839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Özkul C, Yalınay M, Karakan T, Yılmaz G. Determination of certain bacterial groups in gut microbiota and endotoxin levels in patients with nonalcoholic steatohepatitis. Turk J Gastroenterol. 2017;28(5):361–369. [DOI] [PubMed] [Google Scholar]
- 134.Juárez-Fernández M, Porras D, Petrov P, Román-Sagüillo S, García-Mediavilla MV, Soluyanova P, Martínez-Flórez S, González-Gallego J, Nistal E, Jover R, et al. The synbiotic combination of Akkermansia muciniphila and quercetin ameliorates early obesity and NAFLD through gut microbiota reshaping and bile acid metabolism modulation. Antioxidants (Basel). 2021;10(12):2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morrison MC, Gart E, Duyvenvoorde WV, Snabel J, Nielsen MJ, Leeming DJ, Menke A, Kleemann R. Heat-inactivated Akkermansia muciniphila improves gut permeability but does not prevent development of non-alcoholic steatohepatitis in diet-induced obese Ldlr–/–.Leiden mice. Int J Mol Sci. 2022;23(4):2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rao Y, Kuang Z, Li C, Guo S, Xu Y, Zhao D, Hu Y, Song B, Jiang Z, Ge Z, et al. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes. 2021;13(1):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, Ward DV, Grabherr F, Gerner RR, Pfister A, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67(5):891–901. [DOI] [PubMed] [Google Scholar]
- 138.Grander C, Grabherr F, Spadoni I, Enrich B, Oberhuber G, Rescigno M, Tilg H. The role of gut vascular barrier in experimental alcoholic liver disease and A. muciniphila supplementation. Gut Microbes. 2020;12(1):1851986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nie W, Xu F, Zhou K, Yang X, Zhou H, Xu B. Stearic acid prevent alcohol-induced liver damage by regulating the gut microbiota. Food Res Int. 2022;155: Article 111095. [DOI] [PubMed] [Google Scholar]
- 140.Nie W, Du YY, Xu FR, Zhou K, Wang Z-m, Al-Dalali S, Wang Y, Li X-m, Ma Y-H, Xie Y, et al. Oligopeptides from Jinhua ham prevent alcohol-induced liver damage by regulating intestinal homeostasis and oxidative stress in mice. Food Funct. 2021;12(20):10053–10070. [DOI] [PubMed] [Google Scholar]
- 141.Berg FF, Hugenholtz F, Boermeester MA, Zaborina O, Alverdy JC. Spatioregional assessment of the gut microbiota in experimental necrotizing pancreatitis. BJS Open. 2021;5(5): Article zrab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tursi A, Mastromarino P, Capobianco D, Elisei W, Miccheli A, Capuani G, Tomassini A, Campagna G, Picchio M, Giorgett GM, et al. Assessment of fecal microbiota and fecal metabolome in symptomatic uncomplicated diverticular disease of the colon. J Clin Gastroenterol. 2016;50(Suppl 1):S9–S12. [DOI] [PubMed] [Google Scholar]
- 143.Cruz-Aguliar RM, Wantia N, Clavel T, Vehreschild MJGT, Buch T, Bajbouj M, Haller D, Busch D, Schmid RM, Stein-Thoeringer CK. An open-labeled study on fecal microbiota transfer in irritable bowel syndrome patients reveals improvement in abdominal pain associated with the relative abundance of Akkermansia muciniphila. Digestion. 2019;100(2):127–138. [DOI] [PubMed] [Google Scholar]
- 144.Xia J, Lv L, Liu B, Wang S, Zhang S, Wu Z, Yang L, Bian X, Wang Q, Wang K, et al. Akkermansia muciniphila ameliorates acetaminophen-induced liver injury by regulating gut microbial composition and metabolism. Microbiol Spectr. 2022;10(1): Article e0159621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Keshavarz Azizi Raftar S, Ashrafian F, Yadegar A, Lari A, Moradi HR, Shahriary A, Azimirad M, Alavifard H, Mohsenifar Z, Davari M, et al. The protective effects of live and pasteurized Akkermansia muciniphila and its extracellular vesicles against HFD/CCl4-induced liver injury. Microbiol Spectr. 2021;9(2): Article e0048421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Raftar SKA, Ashrafian F, Abdollahiyan S, Yadegar A, Moradi HR, Masoumi M, Vaziri F, Moshiri A, Siadat SD, Zali MR. The anti-inflammatory effects of Akkermansia muciniphila and its derivates in HFD/CCL4-induced murine model of liver injury. Sci Rep. 2022;12(1): Article 2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu W, Lv L, Shi D, Ye J, Fang D, Guo F, Li Y, He X, Li L. Protective effect of Akkermansia muciniphila against immune-mediated liver injury in a mouse model. Front Microbiol. 2017;8:1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Asquith MJ, Stauffer P, Davin S, Mitchell C, Lin P, Rosenbaum JT. Perturbed mucosal immunity and dysbiosis accompany clinical disease in a rat model of spondyloarthritis. Arthritis Rheumatol. 2016;68(9):2151–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stoll ML, Kumar R, Morrow CD, Lefkowitz EJ, Cui X, Genin A, Cron RQ, Elson CO. Altered microbiota associated with abnormal humoral immune responses to commensal organisms in enthesitis-related arthritis. Arthritis Res Ther. 2014;16(6):486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stoll ML, Pierce MK, Watkins JA, Zhang M, Weiss PF, Weiss JE, Elson CO, Cron RQ, Kumar R, Morrow CD, et al. Akkermansia muciniphila is permissive to arthritis in the K/BxN mouse model of arthritis. Genes Immun. 2019;20(2):158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Q, Huang SQ, Li CQ, Xu Q, Zeng QP. Akkermansia muciniphila may determine chondroitin sulfate ameliorating or aggravating osteoarthritis. Front Microbiol. 2017;8:1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu JH, Yue T, Luo ZW, Cao J, Yan Z-Q, Jin L, Wan T-F, Shuai C-J, Wang Z-G, Zhou Y, et al. Akkermansia muciniphila promotes type H vessel formation and bone fracture healing by reducing gut permeability and inflammation. Dis Model Mech. 2020;13(11): dmm043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Demirci M, Tokman HB, Uysal HK, Demiryas S, Karakullukcu A, Saribas S, Cokugras H, Kocazeybek BS. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol Immunopathol. 2019;47(4):365–371. [DOI] [PubMed] [Google Scholar]
- 154.Michalovich D, Rodriguez-Perez N, Smolinska S, Pirozynski M, Mayhew D, Uddin S, Van Horn S, Sokolowska M, Altunbulakli C, Eljaszewicz A, et al. Obesity and disease severity magnify disturbed microbiome-immune interactions in asthma patients. Nat Commun. 2019;10(1):5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Alharris E, Mohammed A, Alghetaa H, Zhou J, Nagarkatti M, Nagarkatti P. The ability of resveratrol to attenuate ovalbumin-mediated allergic asthma is associated with changes in microbiota involving the gut-lung axis, enhanced barrier function and decreased inflammation in the lungs. Front Immunol. 2022;13: 805770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tan L, Zhao S, Zhu W, Wu L, Li J, Shen MX, Lei L, Chen X, Peng C. The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exp Dermatol. 2018;27(2):144–149. [DOI] [PubMed] [Google Scholar]
- 157.Nabizadeh E, Jazani NH, Bagheri M, Shahabi S. Association of altered gut microbiota composition with chronic urticaria. Ann Allergy Asthma Immunol. 2017;119(1):48–53. [DOI] [PubMed] [Google Scholar]
- 158.Schade L, Mesa D, Faria AR, Santamaria JR, Xavier CA, Ribeiro D, Hajar FN, Azevedo VF. The gut microbiota profile in psoriasis: A Brazilian case-control study. Lett Appl Microbiol. 2022;74(4):498–504. [DOI] [PubMed] [Google Scholar]
- 159.Zheng H, Liang H, Wang Y, Miao M, Shi T, Yang F, Liu E, Yuan W, Ji Z-S, Li D-K. Altered gut microbiota composition associated with eczema in infants. PLOS ONE. 2016;11(11): e0166026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Codoñer FM, Ramírez-Bosca A, Climent E, Carrión-Gutierrez M, Guerrero M, Pérez-Orquín JM, Parte JH, Genovés S, Ramón D, Navarro-López V, et al. Gut microbial composition in patients with psoriasis. Sci Rep. 2018;8(1):3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Valentini V, Silvestri V, Marraffa F, Greco G, Bucalo A, Grassi S, Gagliardi A, Mazzotta A, Ottini L, Richetta AG. Gut microbiome profile in psoriatic patients treated and untreated with biologic therapy. J Dermatol. 2021;48(6):786–793. [DOI] [PubMed] [Google Scholar]
- 162.Fieten KB, Totté JEE, Levin E, Reyman M, Meijer Y, Knulst A, Schuren F, Pasmans SGMA. Fecal microbiome and food allergy in pediatric atopic dermatitis: A cross-sectional pilot study. Int Arch Allergy Immunol. 2018;175(1-2):77–84. [DOI] [PubMed] [Google Scholar]
- 163.Tavares CO, Rost FL, Silva RBM, Dagnino AP, Adami B, Schirmer H, de Figueiredo JAP, Souto AA, Maito FDM, Campos MM. Cross talk between apical periodontitis and metabolic disorders: Experimental evidence on the role of intestinal adipokines and Akkermansia muciniphila. J Endod. 2019;45(2):174–180. [DOI] [PubMed] [Google Scholar]
- 164.Coretti L, Cuomo M, Florio E, Palumbo D, Keller S, Pero R, Chiariotti L, Lembo F, Cafiero C. Subgingival dysbiosis in smoker and non-smoker patients with chronic periodontitis. Mol Med Rep. 2017;15(4):2007–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Huck O, Mulhall H, Rubin G, Kizelnik Z, Iyer R, Perpich JD, Haque N, Cani PD, Vos WM, Amar S. Akkermansia muciniphila reduces Porphyromonas gingivalis-induced inflammation and periodontal bone destruction. J Clin Periodontol. 2020;47(2):202–212. [DOI] [PubMed] [Google Scholar]
- 166.Mulhall H, DiChiara JM, Huck O, Amar S. Pasteurized Akkermansia muciniphila reduces periodontal and systemic inflammation induced by Porphyromonas gingivalis in lean and obese mice. J Clin Periodontol. 2022;49(7):717–729. [DOI] [PubMed] [Google Scholar]
- 167.Mulhall H, DiChiara JM, Deragon M, Iyer R, Huck O, Amar S. Akkermansia muciniphila and its pili-like protein Amuc_1100 modulate macrophage polarization in experimental periodontitis. Infect Immun. 2020;89(1): e00500-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cerro ED, Lambea M, Félix J, Salazar N, Gueimonde M, De la Fuente M. Daily ingestion of Akkermansia muciniphila for one month promotes healthy aging and increases lifespan in old female mice. Biogerontology. 2022;23(1):35–52. [DOI] [PubMed] [Google Scholar]
- 169.Grajeda-Iglesias C, Durand S, Daillère R, Iribarren K, Lemaitre F, Derosa L, Aprahamian F, Bossut N, Nirmalathasan N, Madeo F, et al. Oral administration of Akkermansia muciniphila elevates systemic antiaging and anticancer metabolites. Aging. 2021;13(5):6375–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lugt B, Beek AA, Aalvink S, Meijer B, Sovran B, Vermeij WP, Brandt RMC, Vos WM, Savelkoul HFJ, Steegenga WT, et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1-/Δ7 mice. Immun Ageing. 2019;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lapidot Y, Amir A, Nosenko R, Uzan-Yulzari A, Veitsman E, Cohen-Ezra O, Davidov Y, Weiss P, Bradichevski T, Segev S, et al. Alterations in the gut microbiome in the progression of cirrhosis to hepatocellular carcinoma. mSystems. 2020;5(3): e00153-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vernocchi P, Gili T, Conte F, Chierico FD, Conta G, Miccheli A, Botticelli A, Paci P, Caldarelli G, Nuti M, et al. Network analysis of gut microbiome and metabolome to discover microbiota-linked biomarkers in patients affected by non-small cell lung cancer. Int J Mol Sci. 2020;21(22):8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Luo ZW, Xia K, Liu YW, Liu J-H, Rao S-S, Hu X-K, Chen C-Y, Xu R, Wang Z-X, Xie H. Extracellular vesicles from Akkermansia muciniphila elicit antitumor immunity against prostate cancer via modulation of CD8+ T cells and macrophages. Int J Nanomedicine. 2021;16:2949–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fan L, Xu C, Ge Q, Lin Y, Wong CC, Qi Y, Ye B, Lian Q, Zhuo W, Si J, et al. A. muciniphila suppresses colorectal tumorigenesis by inducing TLR2/NLRP3-mediated M1-like TAMs. Cancer Immunol Res. 2021;9(10):1111–1124. [DOI] [PubMed] [Google Scholar]
- 175.Osman MA, Neoh HM, Ab Mutalib NS, Chin S-F, Mazlan L, Raja Ali RA, Zakaria AD, Ngiu CS, Ang MY, Jamal R. Parvimonas micra, Peptostreptococcus stomatis, Fusobacterium nucleatum and Akkermansia muciniphila as a four-bacteria biomarker panel of colorectal cancer. Sci Rep. 2021;11(1):2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang F, Cai K, Xiao Q, He L, Xie L, Liu Z. Akkermansia muciniphila administration exacerbated the development of colitis-associated colorectal cancer in mice. J Cancer. 2022;13(1):124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Meng X, Zhang J, Wu H, Yu D, Fang X. Akkermansia muciniphila aspartic protease Amuc_1434* inhibits human colorectal cancer LS174T cell viability via TRAIL-mediated apoptosis pathway. Int J Mol Sci. 2020;21(9): 3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hou X, Zhang P, Du H, Chu W, Sun R, Qin S, Tian Y, Zhang Z, Xu F. Akkermansia muciniphila potentiates the antitumor efficacy of FOLFOX in colon cancer. Front Pharmacol. 2021;12: 725583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen Z, Qian X, Chen S, Fu X, Ma G, Zhang A. Akkermansia muciniphila enhances the antitumor effect of cisplatin in Lewis lung cancer mice. J Immunol Res. 2020;2020: 2969287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. [DOI] [PubMed] [Google Scholar]
- 181.Derosa L, Routy B, Thomas AM, Iebba V, Zalcman G, Friard S, Mazieres J, Audigier-Valette C, Moro-Sibilot D, Goldwasser F, et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022;28(2):315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Akk abundance alters survival in patients with NSCLC on ICIs. Cancer Discov. 2022;12(4):OF8. [DOI] [PubMed] [Google Scholar]
- 183.Teng L, Wang K, Chen W, Wang YS, Bi L. HYR-2 plays an anti-lung cancer role by regulating PD-L1 and Akkermansia muciniphila. Pharmacol Res. 2020;160:105086. [DOI] [PubMed] [Google Scholar]
- 184.Daisley BA, Chanyi RM, Abdur-Rashid K, Al KF, Gibbons S, Chmiel JA, Wilcox H, Reid G, Anderson A, Dewar M, et al. Abiraterone acetate preferentially enriches for the gut commensal Akkermansia muciniphila in castrate-resistant prostate cancer patients. Nat Commun. 2020;11(1):6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang L, Carmody RN, Kalariya HM, Duran RM, Moskal K, Poulev A, Kuhn P, Tveter KM, Turnbaugh PJ, Raskin I, et al. Grape proanthocyanidin-induced intestinal bloom of Akkermansia muciniphila is dependent on its baseline abundance and precedes activation of host genes related to metabolic health. J Nutr Biochem. 2018;56:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jeong HW, Kim JK, Kim AY, Kim JK, Young KA, Cho D, Lee J-H, Choi JK, Park M, Kim W. Green tea encourages growth of Akkermansia muciniphila. J Med Food. 2020;23(8):841–851. [DOI] [PubMed] [Google Scholar]
- 187.Xia Y, Zhang X, Jiang M, Zhang H, Wang Y, Zhang Y, Seviour R, Kong Y. In vitro co-metabolism of epigallocatechin-3-gallate (EGCG) by the mucin-degrading bacterium Akkermansia muciniphila. PLOS ONE. 2021;16(12):e0260757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Neto CC, Mortzfeld BM, Turbitt JR, Bhattarai SK, Yeliseyev V, Benedetto ND, Bry L, Bucci V. Proanthocyanidin-enriched cranberry extract induces resilient bacterial community dynamics in a gnotobiotic mouse model. Microb Cell. 2021;8(6):131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Henning SM, Summanen PH, Lee R-P, Yang J, Finegold SM, Heber D, Li Z. Pomegranate ellagitannins stimulate the growth of Akkermansia muciniphila in vivo. Anaerobe. 2017;43:56–60. [DOI] [PubMed] [Google Scholar]
- 190.Tu P, Bian X, Chi L, Gao B, Ru H, Knobloch TJ, Weghorst CM, Lu K. Characterization of the functional changes in mouse gut microbiome associated with increased Akkermansia muciniphila population modulated by dietary black raspberries. ACS Omega. 2018;3(9):10927–10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, Raskin I. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes. 2015;64(8):2847–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Anonye BO. Commentary: Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Front Immunol. 2017;8:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64(6):872–883. [DOI] [PubMed] [Google Scholar]
- 194.Anhê FF, Nachbar RT, Varin TV, Vilela V, Dudonné S, Pilon G, Fournier M, Lecours M-A, Desjardins Y, Roy D, et al. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol Metab. 2017;6(12):1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pierre JF, Heneghan AF, Feliciano RP, Shanmuganayagam D, Roenneburg DA, Krueger CG, Reed JD, Kudsk KA. Cranberry proanthocyanidins improve the gut mucous layer morphology and function in mice receiving elemental enteral nutrition. JPEN J Parenter Enteral Nutr. 2013;37(3):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xi M, Li J, Hao G, An X, Song Y, Wei H, Ge W. Stachyose increases intestinal barrier through Akkermansia muciniphila and reduces gut inflammation in germ-free mice after human fecal transplantation. Food Res Int. 2020;137:109288. [DOI] [PubMed] [Google Scholar]
- 197.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64(1):93–100. [DOI] [PubMed] [Google Scholar]
- 198.Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 2015;22(4):658–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pal A, Sun S, Armstrong M, Manke J, Reisdorph N, Adams VR, Kennedy A, Zu Y, Moustaid-Moussa N, Carroll I, et al. Beneficial effects of eicosapentaenoic acid on the metabolic profile of obese female mice entails upregulation of HEPEs and increased abundance of enteric Akkermansia muciniphila. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867(1):159059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shen W, Shen M, Zhao X, Yang Y, Lu S, Tan Y, Li G, Li M, Wang J, et al. Anti-obesity effect of capsaicin in mice fed with high-fat diet is associated with an increase in population of the gut bacterium Akkermansia muciniphila. Front Microbiol. 2017;8:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Prattichizzo F, Giuliani A, Mensà E, Sabbatinelli J, De Nigris V, Rippo MR, La Sala L, Procopio AD, Olivieri F, Ceriello A. Pleiotropic effects of metformin: Shaping the microbiome to manage type 2 diabetes and postpone ageing. Ageing Res Rev. 2018;48:87–98. [DOI] [PubMed] [Google Scholar]
- 202.Shin NR, Lee JC, Lee HY, Kim M-S, Whon TW, Lee M-S, Bae J-W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–735. [DOI] [PubMed] [Google Scholar]
- 203.Zheng J, Li H, Zhang X, Jiang M, Luo C, Lu Z, Xu Z, Shi J. Prebiotic mannan-oligosaccharides augment the hypoglycemic effects of metformin in correlation with modulating gut microbiota. J Agric Food Chem. 2018;66(23):5821–5831. [DOI] [PubMed] [Google Scholar]
- 204.Ke H, Li F, Deng W, Li Z, Wang S, Lv P, Chen Y. Metformin exerts anti-inflammatory and mucus barrier protective effects by enriching Akkermansia muciniphila in mice with ulcerative colitis. Front Pharmacol. 2021;12:726707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hansen CH, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sørensen SJ, Buschard K, Hansen AK. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55(8):2285–2294. [DOI] [PubMed] [Google Scholar]
- 206.Nakashima T, Fujii K, Seki T, Aoyama M, Azuma A, Kawasome H. Novel gut microbiota modulator, which markedly increases Akkermansia muciniphila occupancy, ameliorates experimental colitis in rats. Dig Dis Sci. 2022;67(7):2899–2911. [DOI] [PubMed] [Google Scholar]
- 207.Cheng T, Li C, Shen L, Wang S, Li X, Fu C, Li T, Liu B, Gu Y, Wang W, et al. The intestinal effect of atorvastatin: Akkermansia muciniphila and barrier function. Front Microbiol. 2021;12:797062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Roshanravan N, Mahdavi R, Alizadeh E, Ghavami A, Saadat YR, Alamdari NM, Alipour S, Dastouri MR, Ostadrahimi A. The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; a randomized, double-blind, placebo-controlled trial. J Cardiovasc Thorac Res. 2017;9(4):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lee Y, Sugihara K, Gillilland MG III, Jon S, Kamada N, Moon JJ. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat Mater. 2020;19(1):118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fang Y, Zhang J, Zhu S, He M, Ma S, Jia Q, Sun Q, Song L, Wang Y, Duan L. Berberine ameliorates ovariectomy-induced anxiety-like behaviors by enrichment in equol generating gut microbiota. Pharmacol Res. 2021;165:105439. [DOI] [PubMed] [Google Scholar]
- 211.Dong C, Yu J, Yang Y, Zhang F, Su W, Fan Q, Wu C, Wu S. Berberine, a potential prebiotic to indirectly promote Akkermansia growth through stimulating gut mucin secretion. Biomed Pharmacother. 2021;139:111595. [DOI] [PubMed] [Google Scholar]
- 212.Régnier M, Rastelli M, Morissette A, Suriano F, Roy TL, Pilon G, Delzenne NM, Marette A, Van Hul M, Cani PD. Rhubarb supplementation prevents diet-induced obesity and diabetes in association with increased Akkermansia muciniphila in mice. Nutrients. 2020;12(10):2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Neyrinck AM, Etxeberria U, Taminiau B, Daube G, Van Hul M, Everard A, Cani PD, Bindels LB, Delzenne NM. Rhubarb extract prevents hepatic inflammation induced by acute alcohol intake, an effect related to the modulation of the gut microbiota. Mol Nutr Food Res. 2017;61(1):1500899. [DOI] [PubMed] [Google Scholar]
- 214.Wang L, Wu Y, Zhuang L, Chen X, Min H, Song S, Liang Q, Li A-D, Gao Q. Puerarin prevents high-fat diet-induced obesity by enriching Akkermansia muciniphila in the gut microbiota of mice. PLOS ONE. 2019;14(6):e0218490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xu Y, Wang N, Tan HY, Li S, Zhang C, Zhang Z, Feng Y. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKα/STAT3 signaling in diet-induced obesity. Theranostics. 2020;10(24):11302–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fujisaka S, Usui I, Nawaz A, Igarashi Y, Okabe K, Furusawa Y, Watanabe S, Yamamoto S, Sasahara M, Watanabe Y, et al. Bofutsushosan improves gut barrier function with a bloom of Akkermansia muciniphila and improves glucose metabolism in mice with diet-induced obesity. Sci Rep. 2020;10(1):5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bu F, Ding Y, Chen T, Wang Q, Wang R, Zhou J-Y, Jiang F, Zhang D, Xu M, Shi G, et al. Total flavone of Abelmoschus manihot improves colitis by promoting the growth of Akkermansia in mice. Sci Rep. 2021;11(1):20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ondee T, Pongpirul K, Visitchanakun P, Saisorn W, Kanacharoen S, Wongsaroj L, Kullapanich C, Ngamwongsatit N, Settachaimongkon S, Somboonna N, et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci Rep. 2021;11(1):6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Alard J, Lehrter V, Rhimi M, Mangin I, Peucelle V, Abraham A-L, Mariadassou M, Maguin E, Waligora-Dupriet A-J, Pot B, et al. Beneficial metabolic effects of selected probiotics on diet-induced obesity and insulin resistance in mice are associated with improvement of dysbiotic gut microbiota. Environ Microbiol. 2016;18(5):1484–1497. [DOI] [PubMed] [Google Scholar]
- 220.Kriebs A. Microbiota supplements to improve metabolic health. Nat Rev Endocrinol. 2019;15(9):500–501. [DOI] [PubMed] [Google Scholar]
- 221.Chang Y, Yang Y, Xu N, Mu H, Zhang H, Duan J. Improved viability of Akkermansia muciniphila by encapsulation in spray dried succinate-grafted alginate doped with epigallocatechin-3-gallate. Int J Biol Macromol. 2020;159:373–382. [DOI] [PubMed] [Google Scholar]
- 222.Marcial-Coba MS, Saaby L, Knøchel S, Nielsen DS. Dark chocolate as a stable carrier of microencapsulated Akkermansia muciniphila and Lactobacillus casei. FEMS Microbiol Lett. 2019;366(2):fny290. [DOI] [PubMed] [Google Scholar]
- 223.Pierre JF, Martinez KB, Ye H, Nadimpalli A, Morton TC, Yang J, Wang Q, Patno N, Chang EB, Yin DP. Activation of bile acid signaling improves metabolic phenotypes in high-fat diet-induced obese mice. Am J Physiol Gastrointest Liver Physiol. 2016;311(2):G286–G304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Van den Bossche L, Hindryckx P, Devisscher L, Devriese S, Van Welden S, Holvoet T, Vilchez-Vargas R, Vital M, Pieper DH, Bussche JV, et al. Ursodeoxycholic acid and its taurine- or glycine-conjugated species reduce colitogenic dysbiosis and equally suppress experimental colitis in mice. Appl Environ Microbiol. 2017;83(7):e02766-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Payahoo L, Khajebishak Y, Alivand MR, Soleimanzade H, Alipour S, Barzegari A, Ostadrahimi A. Investigation the effect of oleoylethanolamide supplementation on the abundance of Akkermansia muciniphila bacterium and the dietary intakes in people with obesity: A randomized clinical trial. Appetite. 2019;141:104301. [DOI] [PubMed] [Google Scholar]
- 226.Mokhtarzade M, Molanouri Shamsi M, Abolhasani M, Bakhshi B, Sahraian MA, Quinn LS, Negaresh R. Home-based exercise training influences gut bacterial levels in multiple sclerosis. Complement Ther Clin Pract. 2021;45:101463. [DOI] [PubMed] [Google Scholar]
- 227.Cho J, Kim D, Kang H. Exercise preconditioning attenuates the response to experimental colitis and modifies composition of gut microbiota in wild-type mice. Life. 2020;10(9):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Getachew B, Mendieta L, Csoka AB, Aguilera J, Tizabi Y. Antidepressant effects of C-terminal domain of the heavy chain of tetanus toxin in a rat model of depression. Behav Brain Res. 2019;370:111968. [DOI] [PMC free article] [PubMed] [Google Scholar]


