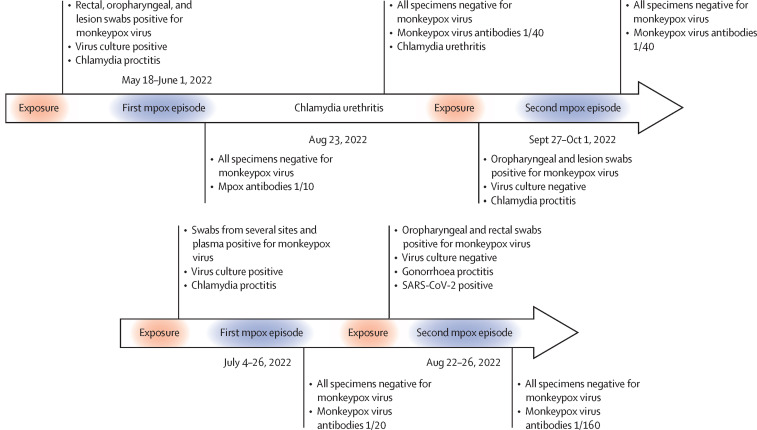
Over 80 000 mpox (formerly known as monkeypox) cases have been confirmed worldwide and recovered individuals are considered protected against reinfection.1, 2, 3 However, an individual with apparent reinfection has been recently reported.4 In this Comment we describe two individuals with potential monkeypox virus reinfection at San Raffaele Hospital, Milan, Italy (figure ; see appendix for details on testing and results).
Figure.
Timeline of monkeypox virus infection among two men who have sex with men
Most relevant virological and serological testing is described. Second mpox episodes were potential reinfections, although recrudescence or residual virus from exposure cannot be excluded.
The first individual is a 36-year-old man who has sex with men (MSM) who presented with asthenia, pharyngodynia, and fever with tenesmus and mucorrhoea on May 24, 2022, with symptom onset a week earlier, after attending a large gathering in Spain in early May, during which he had condomless oral intercourse and condomless anal intercourse with several partners. He is on antiretroviral therapy for a known HIV infection (1099 CD4+ cells per μL; HIV-RNA <20 copies per mL since 2015) and had no other medications or comorbidities.
He presented with a perianal ulceration, a pharyngeal lesion, and lymphadenopathy. Monkeypox virus PCR was positive for lesion, rectal, and oropharyngeal swabs with cycle threshold values ranging between 18 and 32, with concurrent chlamydia proctitis. Symptoms and lesions resolved by June 1, 2022, and a monkeypox PCR was negative for plasma, oropharyngeal, and anal swabs.
On Aug 23, 2022, he presented with oral and cutaneous non-ulcerated erythematous lesions. Mpox was excluded with oropharyngeal, anal, and lesion swabs, and chlamydia urethritis was detected, which resolved after doxycycline treatment (100 mg twice per day for 7 days).
On Sept 27, 2022, he presented with a single, umbilicated vesicular lesion on the glans penis following condomless oral intercourse and condomless anal intercourse with several individuals who were later diagnosed with mpox. Lesion swabs and oropharyngeal swabs were positive for monkeypox virus with cycle threshold values of 37 and 34, with concurrent chlamydia proctitis. The lesion resolved by Oct 1, 2022, and all subsequent swabs from several locations were negative for the monkeypox virus. He had not received mpox vaccinations and had antibody titre of 1/40 after the first episode. This titre remained stable after the second episode.
The second individual is a 33-year-old MSM who uses pre-exposure prophylaxis for HIV with no comorbidities or comedications. He presented with proctitis, a single vesicular lesion on the lower lip, and lymphadenopathy on July 7, 2022, after condomless oral intercourse and condomless anal intercourse with several partners during the previous month. Anoscopy revealed mucosal congestion with erosions and ulcerations, and a rectal swab was positive for both monkeypox virus with a cycle threshold value of 37, and for chlamydia. All other swabs were negative. On July 14, 2022, the proctitis and lesion resolved but he developed pharyngodynia with a positive oropharyngeal swab for monkeypox virus with a cycle threshold value of 25, and a negative rectal swab. On July 20, an oropharyngeal swab, a lesion swab from the right shoulder, and plasma sample were positive for monkeypox virus with cycle threshold values between 22 and 37. Symptoms and lesions resolved by July 26, and swabs from multiple body sites were negative on Aug 2, 2022.
On Aug 22, 2022, he presented with proctitis after a large gathering in Spain (Aug 5–13, 2022), where he had condomless oral intercourse and condomless anal intercourse with several partners. Rectal and oropharyngeal swabs were positive for monkeypox virus at cycle threshold values of 29 and 32, with concurrent gonorrhoea proctitis and SARS-CoV-2 infection of the upper respiratory tract.
Proctitis resolved and only the oropharyngeal swab remained positive on Aug 26. Swabs taken between Aug 31 and Sept 7, 2022 from different body sites were all negative. He was not vaccinated for mpox; after resolution of the first episode he had monkeypox antibody titres of 1/20, which increased to 1/160 after the second episode.
These cases represent two potential monkeypox virus reinfections. Following clinical and virological healing of the first episodes, we observed high cycle threshold values and short-lasting symptoms for both second episodes, with clinical characteristics consistent with mpox, and detectable neutralising antibodies. The cycle threshold values were high for new-onset mpox and suggest low viral loads. Possible alternative explanations to reinfection include relapse from tissue reservoirs or sexual contamination.5, 6 Indeed, potential relapse of infection and persistent monkeypox virus detection have been described previously.7, 8 The second patient, in particular, had episodes relatively close together, which could indicate recrudescence. However, high cycle threshold values and fast healing could also be linked to presence of neutralising antibodies. Furthermore, samples from different sites and different time-points (in the second patient) were positive for monkeypox virus, which makes environmental contamination less likely. Both patients had co-infections, which could have caused or exacerbated symptoms, or could have eased reinfection. We isolated and sequenced monkeypox virus from both patients from samples collected during the first episodes. We could not isolate the virus from samples from the second episodes, probably due to low viral loads as indicated by the high cycle threshold values. SARS-CoV-2 could also have had a negative influence.9, 10 Although genomic data cannot confirm the presence of two distinct viruses, and thus reinfection (in contrast to relapse, which would have presented with the same virus), clinicians need to be aware of potential monkeypox virus reinfections and should investigate with viral culture and sequencing. Furthermore, the potential of monkeypox reinfection has implications for transmission and vaccination policies.
DC has received research grants from Gilead Sciences and GlaxoSmithKline and received payment for educational events and support for attending meetings from Merck Sharp & Dohme and ViiV Healthcare. AC has received personal fees for advisory boards, speaker panels, and educational materials from Gilead Sciences, ViiV Healthcare, Janssen-Cilag, Merck Sharp & Dohme, and Theratechnologies. SN has received personal fees for advisory boards, speaker panels, and educational materials from Gilead Sciences, ViiV Healthcare, Janssen-Cilag, and Merck Sharp & Dohme. All other authors declare no competing interests. Individuals included in the study signed written informed consent to include case details, personal information, and images in the published version of the manuscript in all formats.
Supplementary Material
References
- 1.Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 2.Gilchuk I, Gilchuk P, Sapparapu G, et al. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell. 2016;167:684. doi: 10.1016/j.cell.2016.09.049. 94.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 4.Golden J, Harryman L, Crofts M, et al. Case of apparent mpox reinfection. Sex Transm Infect. 2023 doi: 10.1136/sextrans-2022-055736. published online Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palich R, Burrel S, Monsel G, et al. Viral loads in clinical samples of men with monkeypox virus infection: a French case series. Lancet Infect Dis. 2023;23:74–80. doi: 10.1016/S1473-3099(22)00586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suñer C, Ubals M, Tarín-Vicente EJ, et al. Viral dynamics in patients with monkeypox infection: a prospective cohort study in Spain. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00794-0. published online Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pettke A, Filén F, Widgren K, et al. Ten-week follow-up of monkeypox case-patient, Sweden, 2022. Emerg Infect Dis. 2022;28:2074–2077. doi: 10.3201/eid2810.221107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paran N, Yahalom-Ronen Y, Shifman O, et al. Monkeypox DNA levels correlate with virus infectivity in clinical samples, Israel, 2022. Euro Surveill. 2022;27 doi: 10.2807/1560-7917.ES.2022.27.35.2200636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UK Health Security Agency Investigation into monkeypox outbreak in England: technical briefing 1. Sept 23, 2022. https://www.gov.uk/government/publications/monkeypox-outbreak-technical-briefings/investigation-into-monkeypox-outbreak-in-england-technical-briefing-1
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.