Abstract
Background:
Insufficient data on the rate and distribution of SARS-CoV-2 infection in Canada has presented a substantial challenge to the public health response to the COVID-19 pandemic. Our objective was to assess SARS-CoV-2 seroprevalence in a representative sample of pregnant people throughout Canada, across multiple time points over 2 years of the pandemic, to describe the seroprevalence and show the ability of this process to provide prevalence estimates.
Methods:
This Canadian retrospective serological surveillance study used existing serological prenatal samples across 10 provinces over multiple time periods: Feb. 3–21, 2020; Aug. 24–Sept. 11, 2020; Nov. 16–Dec. 4, 2020; Nov. 15–Dec. 3, 2021; and results from the province of British Columbia during a period in which the SARS-CoV-2 B.1.1.529 (Omicron) variant was predominant, from Nov. 15, 2021, to June 11, 2022. Age and postal code administrative data allowed for comparison with concurrent polymerase chain reactivity (PCR)–positive results collected by Statistics Canada and the Canadian Surveillance of COVID-19 in Pregnancy (CANCOVID-Preg) project.
Results:
Seropositivity in antenatal serum as early as February 2020 indicates SARS-CoV-2 transmission before the World Health Organization’s declaration of the pandemic. Seroprevalence in our sample of pregnant people was 1.84 to 8.90 times higher than the recorded concurrent PCR-positive prevalence recorded among females aged 20–49 years in November–December 2020. Overall seropositivity in our sample of pregnant people was low at the end of 2020, increasing to 15% in 1 province by the end of 2021. Seroprevalence among pregnant people in BC during the Omicron period increased from 5.8% to 43% from November 2021 to June 2022.
Interpretation:
These results indicate widespread vulnerability to SARS-CoV-2 infection before vaccine availability in Canada. During the time periods sampled, public health tracking systems were under-reporting infections, and seroprevalence results during the Omicron period indicate extensive community spread of SARS-CoV-2 infection.
The COVID-19 pandemic has presented great challenges to health care systems and policy-makers worldwide since the World Health Organization (WHO) declaration in March 2020.1 It has been critical that public health networks collaborate to ensure the rapid dissemination of information and data to inform policies, yet obtaining accurate assessments of community spread of SARS-CoV-2 has been difficult. In Canada, case detection initially relied on polymerase chain reaction (PCR) assays on specimens obtained via symptomatic testing and contact tracing. Tracking using PCR alone underestimates cases, owing to missed asymptomatic or mild cases, individuals electing not to be tested and variable testing capacity.2–6 In other global settings using similar testing criteria, it is estimated that 33%–66% of all cases were undetected.2,3 Although various SARS-CoV-2 serosurveillance studies have been performed in Canada, including those involving recruitment via the postal service or using residual sera from blood donors and clinical samples, each study has challenges that limit generalizability to the broader Canadian population, particularly with regard to geographic location.7–12
Antenatal sera are collected as part of standard prenatal care across Canada to screen for infections and immunity to viruses such as HIV, rubella and hepatitis B, or for aneuploidy. In some jurisdictions, antenatal specimens are stored for later use or reference. With a very high uptake among all pregnant people — 93% to 96% in Canada13–15 — these sera are highly representative of the greater population of reproductive-age people, according to a meta-analysis that found no difference between sex groups in seroprevalence studies.16 In addition, these samples encompass the diversity of residents in Canada by geographic location, socioeconomic status, and race and ethnicity, thereby providing an opportunity to test and assess population seroprevalence among a minimally biased sample throughout the pandemic.
Our objective was to assess SARS-CoV-2 seroprevalence in adults by testing available antenatal serum samples across Canada. To capture the progression of the pandemic, we selected several sampling periods for assessment of seroprevalence, with comparison to concurrent PCR-positivity cumulative prevalence where possible. This serial, cross-sectional study represents a large, national, multi–time point study and presents Canadian data from periods spanning 2 years of the pandemic.16,17
Methods
Setting
As part of a national serological surveillance project, we present data from 10 provinces: British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, Newfoundland and Labrador, Nova Scotia, New Brunswick and Prince Edward Island. Where possible, we retrieved stored antenatal samples from 5 sampling periods between Feb. 3, 2020, and Dec. 3, 2021, as follows. Period A: Feb. 3 to Feb. 21, 2020, before the WHO declared the COVID-19 outbreak to be a pandemic and nationwide restrictions were implemented, with testing for SARS-CoV-2 limited to high-risk travellers. Period B: Aug. 24 to Sept. 11, 2020, which corresponds to seroprevalence after the first wave of infection. Period C: Nov. 16 to Dec. 4, 2020, corresponding to the start of the second period of SARS-CoV-2 infection and before vaccine introduction. Period D: Nov. 15 to Dec. 3, 2021, corresponding to the beginning of the Omicron wave in Canada. Finally, we carried out a provincial BC longitudinal analysis, sampling weekly batches from Nov. 15, 2021, to June 11, 2022, which spanned the period in which the SARS-CoV-2 B.1.1.529 (Omicron) variant was predominant.
Sample selection
British Columbia, Alberta and Saskatchewan participated in time period A, with the addition of data from Newfoundland and Labrador and Nova Scotia for time period B. All 10 provinces participated in time period C. Time period D consists of data from BC, Alberta, Newfoundland and Labrador and Prince Edward Island. Time periods A–C predated SARS-CoV-2 vaccine roll-out, and the results therefore uniformly represent response to infection and used a combination of anti-nucleocapsid and anti-spike antibody assays, whereas time period D reports anti-nucleocapsid antibodies to differentiate infection and vaccine-derived antibody response. Similarly, the BC Omicron analysis used a multiplex serology assay that was able to differentiate anti-nucleocapsid, anti-spike and anti-receptor-binding domain antibodies. Given the relatively continuous flow of samples from pregnant people, we selected all eligible samples (e.g., adequate volume and integrity) from a set time period to provide proportionate sampling based on population size in each jurisdiction for time periods A–D. To reduce bias, participating laboratory sites in all provinces included all samples with adequate residual sera within the selected time period.
During the longitudinal BC sampling period, we assayed randomly selected samples in batches of 100–200 per week. Antenatal sera are routinely stored for all pregnant people in Canada and most are collected after the initial presentation to a health care provider, regardless of whether the pregnancy is planned or continued or where the person resides.
Laboratory assays
Samples were assayed for SARS-CoV-2 antibodies by the most sensitive and specific assay platform available in each reference laboratory in each province, with assay specificities of 100% (95% confidence interval [CI] 99.1%–100%)18 and sensitivities ranging from 63% to 96% (Appendix 1, available at www.cmajopen.ca/content/11/2/E305/suppl/DC1).18 For time periods after the commencement of national vaccination programs, we used anti-nucleocapsid assays to assess infection-induced antibody presence. These were immunoglobulin G assays with sensitivity higher than 95% and specificity of 100%.18 Details of the time period and assays used are available in Appendix 1.
Statistical analysis
Sample results from each province were shared with the coordinating centre in Vancouver. We estimated raw seroprevalence as the number of positive samples over the total, and stratified by age within each province with exact 95% confidence intervals, except for Prince Edward Island, where age-linkage was not obtainable owing to strict confidentiality restrictions. We used the direct method to age-standardize the seroprevalence within each province using Statistics Canada (StatCan) data. We standardized estimates from each time period to the number and age distribution of deliveries in each province separately as a percentage of the annual total that reflected the number of months being considered. We calculated confidence intervals for the age-standardized estimates using the gamma method19 as implemented in the package dsrTest.20 We estimated the proportion of nucleocapsid-positive samples over time in BC from Nov. 15, 2021, to June 11, 2022, using a generalized additive model with a logit link and a smooth term for time. We carried out all analyses in R version 4.1.1 (2021–08–10).21
Comparison of PCR-confirmed cases
We obtained the cumulative number of PCR-positive pregnant cases per province from the Canadian Surveillance of COVID-19 in Pregnancy: Epidemiology, Maternal and Infant Outcomes (CANCOVID-Preg) project,22 and the number of cumulative PCR-positive cases for SARS-CoV-2 infection for females aged 20–49 years by region from StatCan.23 Province-specific data for females aged 20–49 years were not available for all provinces from StatCan; therefore, several are combined (with territories) to represent 5 regions: BC; Prairies, including Alberta, Saskatchewan, Manitoba and the Northwest Territories; Ontario and Nunavut; Quebec; and Atlantic, including New Brunswick, Nova Scotia, Prince Edward Island and Newfoundland and Labrador. To estimate the PCR-positive prevalence in each province for the pregnant population and the population of females aged 20–49 years, we used data from StatCan. We used the number of births in 2020 as a proxy for the number of pregnant people in each province, adjusted for the relevant time period (as a percentage of the year) to use as a denominator for PCR-positive prevalences in pregnancy,24 and we used the number of females aged 20–49 years in each region as the denominator for calculating regional PCR-confirmed-positive result prevalences among this age group more generally.23 The PCR prevalence is the cumulative total of PCR cases as an indicator of the number of people who have tested positive up to that point, whereas the seroprevalence at any given time point is an indicator of the number of people infected to that time point. We calculated prevalence rate ratios and confidence intervals of seroprevalence to PCR prevalence using a normal approximation and Wald confidence intervals. We used raw seroprevalence as a comparison for the pregnant population, and age-standardized for the female population aged 20–49 years.
Ethics approval
Ethics approval was granted for each site (listed in Appendix 1) to allow for laboratory testing and data transfer to the central coordination centre of the study. Coordination and data management were established through the Women’s Health Research Institute at BC Women’s Hospital and Health Centre (H20–02252).
Results
A total of 40 684 samples were tested from across Canada between February 2020 and June 2022. In period A, 7328 samples from BC, Alberta and Saskatchewan were tested; in period B, 6446 samples from BC, Saskatchewan, Alberta, Nova Scotia and Newfoundland and Labrador were tested; in period C, 15 804 samples from all 10 participating provinces were tested; in period D, 6407 samples from BC, Alberta, Newfoundland and Labrador and Prince Edward Island were tested; and in the longitudinal analysis from BC (Nov. 2021–June 2022), 4699 samples were tested. All areas of the participating provinces were represented and the age range of all samples reflects the age distribution of pregnant people.
Period A: Feb. 3–21, 2020
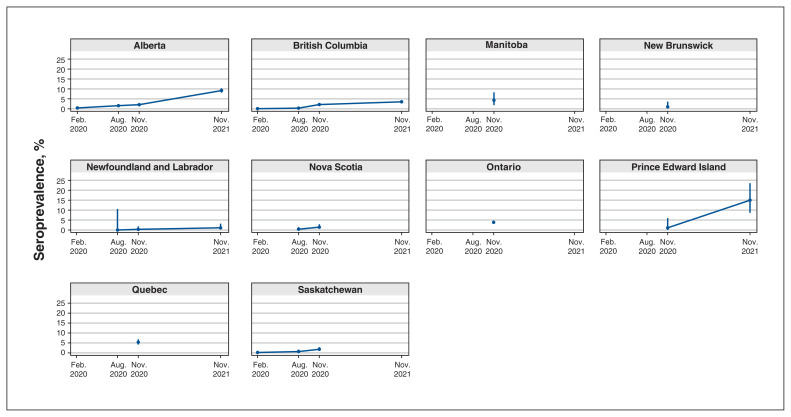
We selected sampling period A to determine whether there were any notable levels of SARS-CoV-2 circulating within the general population before the COVID-19 pandemic was declared by the WHO. A total of 7328 antenatal serum samples from BC, Alberta and Saskatchewan were available for this 3-week period. The median age was similar in BC and Saskatchewan, with values of 32 (range 15–60) years and 30 (range 14–41) years, respectively. The raw seroprevalence in BC was 0.07% (95% CI 0.01–0.24); in Alberta, 0.43% (95% CI 0.22–0.75); and in Saskatchewan, 0.18% (95% CI 0.01–1.02) (Appendix 2, available at www.cmajopen.ca/content/11/2/E305/suppl/DC1). No formal reporting of PCR-positive cases in pregnant people occurred during this sampling period. Figure 1 shows the range of raw seropositivity for all time periods and provinces.
Figure 1:
Raw seroprevalence by province for all time periods and provinces. Error bars indicate 95% confidence intervals. Data for this figure are available in Appendix 2 (available at www.cmajopen.ca/content/11/2/E305/suppl/DC1).
Period B: Aug. 24–Sept. 11, 2020
Testing of samples from period B was performed to determine the seropositivity prevalence after the first wave and to indicate susceptibility for the second wave after natural infection. For sampling period B, BC and Saskatchewan retrieved and centrally tested 2427 antenatal samples. Alberta tested 3497, Nova Scotia tested 489, and Newfoundland and Labrador tested 33. Raw seroprevalence was 0.34% (95% CI 0.12–0.73) in BC, 1.57% (95% CI 1.16–2.08) in Alberta, 0.66% (95% CI 0.18–1.68) in Saskatchewan and 0.41% (95% CI 0.05–1.47) in Nova Scotia. Newfoundland and Labrador tested 33 serological samples for this period and detected no confirmed seropositive samples.
Period C: Nov. 16–Dec. 4, 2020
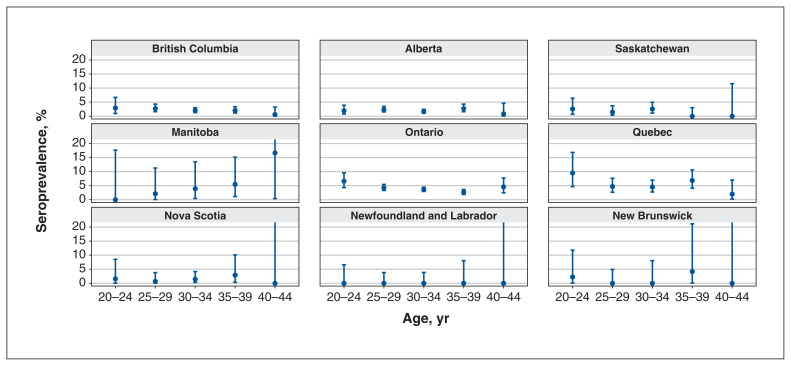
This period reflects the weeks just before public access to SARS-CoV-2 vaccines in Canada (initially, only at-risk groups were eligible, which did include pregnant people in high-risk occupations, such as health care workers).25,26 All 10 provinces retrieved and tested 15 804 antenatal samples for sampling period C. Age-adjusted data were available for 9 provinces (all except Prince Edward Island), and raw prevalence rates only for Prince Edward Island; therefore, age-adjusted prevalence rates and PCR comparisons were possible by province for all but Prince Edward Island. The highest raw seroprevalence rates were evident in Quebec at 5.38% (95% CI 4.19–6.80) and the lowest rates occurred in Newfoundland and Labrador, at 0.33% (95% CI 0.01–1.80).
Age-stratified seroprevalences are shown in Figure 2. The figure omits the youngest (< 20 yr) and oldest (≥ 45 yr) age groups, owing to low numbers, and are standardized to the age distribution of deliveries in each province for this time period.
Figure 2:
Age-stratified seroprevalence for time period C (Nov. 16–Dec. 4, 2020) for each province. Error bars indicate 95% confidence intervals, and those that extend past 20% (resulting from small numbers in this age group) have been cut off for ease of visual comparison among provinces.
Comparison of PCR versus seroprevalence
We compared the prevalence of PCR-confirmed positive SARS-CoV-2 infections in pregnant people and prevalence of PCR-confirmed positive SARS-CoV-2 infections in females aged 20–49 years by region, versus the seroprevalence for time period C; see Table 1. We found higher prevalence rate ratios for all provinces where direct comparison of age-adjusted seroprevalence and PCR prevalence can be made (BC, Ontario and Quebec). The seroprevalence rate ratio average is 4.3 times higher than the PCR-positive prevalence for the 10 provinces, with the median prevalence rate ratio of 4.42 (95% CI 3.97–4.87) for Ontario. All prevalence rate ratios are higher for seroprevalence than for PCR-positive prevalence in pregnant people for provinces where this comparison was possible.
Table 1:
Comparison of rates of positive infection confirmed by polymerase chain reaction testing among pregnant people from CANCOVID-Preg, and among females aged 20–49 years from StatCan region for time period C (Nov. 16–Dec. 4, 2020) and results of SARS-CoV-2 sero-screening adjusted for age (November and December 2020)*
| Region | Province | Raw seroprevalence (95% CI) | PCR-positive rate in pregnant people (95% CI) | Rate ratio (95% CI) | Age-adjusted seroprevalence (95% CI) | PCR-positive rate in region-specific females aged 20–49 yr (95% CI) | Rate ratio (95% CI) |
|---|---|---|---|---|---|---|---|
| 1 | British Columbia | 2.14 (1.63–2.74) | 0.62 (0.55–0.70) | 3.44 (2.57–4.48) | 1.87 (1.12–2.80) | 1.13 (1.12–1.16) | 1.84 (1.37–2.36) |
| 2 | Alberta | 2.07 (1.63–2.59) | 0.86 (0.78–0.95) | 2.40 (1.84–3.02) | 1.78 (1.20–2.47) | 2.11 (2.08–2.13) | 0.85 (0.65–1.06) |
| 2 | Saskatchewan | 1.81 (1.06–2.88) | – | – | 1.29 (0.62–2.19) | 2.11 (2.08–2.13) | 0.61 (0.30–0.96) |
| 2 | Manitoba | 4.28 (1.86–8.26) | 1.24 (1.08–1.44) | 3.44 (1.28–6.11) | 5.62 (1.42–12.42) | 2.11 (2.08–2.13) | 2.79 (1.27–4.55) |
| 3 | Ontario | 3.86 (3.39–4.38) | 0.47 (0.43–0.51) | 8.25 (7.13–9.48) | 5.67 (3.93–7.50) | 1.28 (1.27–1.29) | 4.42 (3.97–4.87) |
| 4 | Quebec | 5.38 (4.19–6.80) | 1.01 (0.94–1.08) | 5.33 (4.06–6.68) | 5.95 (2.66–10.44) | 2.45 (2.42–2.47) | 2.43 (1.90–2.99) |
| 5 | Nova Scotia | 1.41 (0.57–2.88) | 0.17 (0.09–0.30) | 8.27 (2.56–20.47) | 0.96 (0.33–1.90) | 0.11 (0.10–0.12) | 8.90 (1.80–17.28) |
| 5 | Newfoundland and Labrador | 0.33 (0.01–1.80) | 0.00 (0.00–0.13) | –† | 0.24 (0.01–0.87) | 0.11 (0.10–0.12) | 2.88 (0.00–9.02) |
| 5 | New Brunswick | 1.01 (0.12–2.59) | 0.05 (0.01–0.15) | 15.69 (2.64–93.37) | 0.91 (0.09–2.65) | 0.11 (0.10–0.12) | 8.93 (0.00–22.79) |
| 5 | Prince Edward Island | 1.11 (0.03–6.04) | 0.00 (0.00–0.36) | –† | 1.11 (0.03–6.04) | 0.11 (0.10–0.12) | 9.83 (0.00–30.97) |
Note: CANCOVID-Preg = COVID-19 in Pregnancy: Epidemiology, Maternal and Infant Outcomes, CI = confidence interval, PCR = polymerase chain reaction, StatCan = Statistics Canada.
Rate ratios shown for comparison with raw rates for the pregnant population, and age-adjusted seroprevalence estimates for the general female population. Rate for Prince Edward Island is not age adjusted. Shown as rates per 100. PCR-positive rates not available for pregnant people in Saskatchewan.
Rate ratio not possible to calculate.
Geographic seropositivity mapping
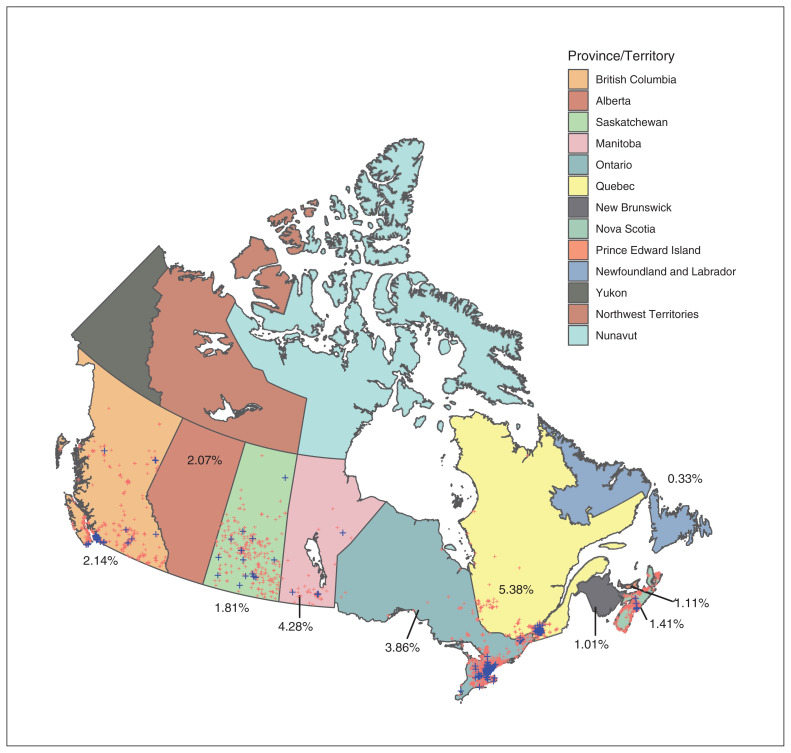
Figure 3 shows the positive and negative sera results geographically for time period C before the introduction of SARS-CoV-2 vaccines in Canada. Positive serum sampling is demonstrated in remote parts of BC, Saskatchewan and Manitoba as well as on the east coast in regions that may have underestimated the amount of community spread during this time period.
Figure 3:
Location of positive and negative antenatal serum results for SARS-CoV-2 for time period C (Nov. 16–Dec. 4, 2020). Blue crosses indicate seropositive samples and red crosses indicate seronegative samples. Regional mapping of samples was not possible for samples from Alberta, New Brunswick and Newfoundland and Labrador. Numbers indicate raw seroprevalence.
Period D: Nov. 15–Dec. 3, 2021
British Columbia, Alberta, Newfoundland and Labrador and Prince Edward Island tested a combined 6407 samples in this time period. Raw anti-nucleocapsid seroprevalence was 3.53% (95% CI 2.93–4.21) in BC, 9.18% (95% CI 8.11–10.35) in Alberta, 1.1% (95% CI 0.23–3.20) in Newfoundland and Labrador and 15.0% (95% CI 8.65–23.53) in Prince Edward Island. Age-adjusted anti-nucleocapsid seroprevalence was 5.78% (95% CI 3.76–8.21) in BC, 11.8% (95% CI 6.87–17.84) in Alberta and 0.60% (95% CI 0.11–1.51) in Newfoundland and Labrador.
Comparison over time was possible with BC and Alberta data for all 4 time periods, with Saskatchewan and Newfoundland and Labrador for 3 time periods (B, C and D), Nova Scotia for 2 time periods (B and C), and Prince Edward Island for 2 time periods (C and D), and is reflected in Figure 1, showing increases in seropositivity for time periods C and D.
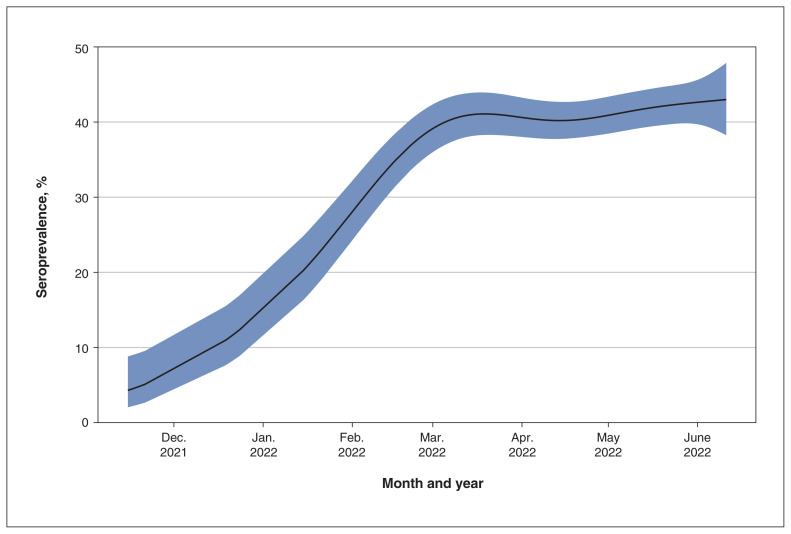
Longitudinal analysis of BC samples: Nov. 15, 2021–June 11, 2022
To assess seroprevalence after the emergence of the SARS-CoV-2 Omicron variant B.1.1.529, 4699 samples were tested in BC in weekly batches over 7 months, using a multiplex serology assay. Seroprevalence increased sharply from November 2021, before plateauing in March 2022 and gradually increasing between April and June 2022 (Figure 4). Estimated seroprevalence was 4.3% (95% CI 2.0%–8.8%) in November 2021, rising to 39.1% (95% CI 36.0%–42.4%) by the beginning of March 2022, and 43.0% (95% CI 38.3%–47.9%) by the middle of June 2022.
Figure 4:
Longitudinal assessment of seroprevalence over time for British Columbia during the Omicron period (Nov. 15, 2021–June 11, 2022). Generalized additive model estimated degrees of freedom = 4.2, p < 0.0001. Shaded area indicates 95% confidence intervals around the estimated seroprevalence.
Interpretation
Data from this national serosurveillance study show several key findings regarding SARS-CoV-2 spread in Canada. First, SARS-CoV-2 community spread before the declaration of the pandemic in March 2020 was more expansive than detected via PCR testing. During sampling period A, it was believed that there was no community spread in BC and Alberta, and no infections in Saskatchewan.27–29 Despite this, the raw seroprevalence results show early, low community spread among pregnant people within these provinces. During sampling period C, before the introduction of SARS-CoV-2 vaccines, seroprevalence among pregnant people varied widely across provinces, from 0.33% in Newfoundland and Labrador to 5.38% in Quebec. For the 4 provinces where comparison can be made between December 2020 and December 2021 (time periods C and D), we found a modest rise from 2.14% to 5.78% for BC, and more significant increases from 1%–2% to more than 10% in Alberta and Prince Edward Island. The evolution of the pandemic between provinces and within each province has shown distinctive differences, possibly a result of varying public health policy and population density. Nonetheless, data from all provinces showed significant vulnerability to subsequent waves of the pandemic.
Before this study, Canadian seroprevalence estimates from testing of residual serum by Canadian Blood Services7,10 showed results similar to ours for sampling periods A and B, but with some important differences within sampling period C. Canadian Blood Services samples from November 2020 showed a lower seroprevalence in Ontario (3.86% v. 0.77%), Nova Scotia (1.41% v. 0.19%) and Prince Edward Island (1.11% v. 0%), but higher seroprevalence in Saskatchewan (1.81% v. 4.17%) and Manitoba (4.28% v. 8.56%). These discrepancies may reflect the selection biases of blood donors in the Canadian Blood Services sampling methodology, which may be different from the potential biases inherent in antenatal serum screening. Although antenatal sera results may reflect variability in fertility rates in certain subpopulations that can affect generalizability of results, compared with other sampling they cover broad geographies, cultures and ethnicities, and socioeconomic groups, owing to high uptake rates of prenatal infection screening.13–15
Our findings also show important differences when compared with prevalence of positive PCR tests for SARS-CoV-2 for the same time periods, with seroprevalence found to be consistently higher.
Comparison between antenatal serum samples and PCR test results indicates the underdetection of SARS-CoV-2 infections in a comparable population.
Despite varying provincial PCR testing protocols and provisions, these findings of higher seroprevalence when compared with PCR test results in nearly all of the provinces show an important level of undetected SARS-CoV-2 spread. They also suggest a useful mechanism for public health monitoring of the pandemic that overcomes the substantial cost and variable availability of PCR testing. This is particularly evident in the BC provincial longitudinal analysis from November 2021 to June 2022. During this time period, PCR testing was no longer available in the community for contact tracing or mildly symptomatic cases. These current results are consistent with postal seroprevalence estimates from Alberta through March 202230 and confirm a finding of marked increases in seropositivity during the Omicron wave, reflective of widespread community exposure in reproductive-age adults.
Limitations
Although testing used antenatal sera with the possibility of some pregnant people being more cautious of SARS-CoV-2 transmission, these samples do not necessarily represent planned or continued pregnancies. Given the large variation of adjusted societal behaviour, samples were not available from many provinces for the sampling periods A and B, and were limited by laboratory capacity for time period D, resulting in a smaller sample size for these time periods as compared with sampling period C. Additionally, given that waning of SARS-CoV-2 antibodies remains an area of investigation, a lack of detectable antibody does not necessarily indicate a lack of previous infection. Seroprevalence was presumed to be cumulative for the purposes of comparison with PCR-confirmed cases and it is therefore possible, with waning antibody levels, that the percentage of individuals with previous SARS-CoV-2 infection is higher than our seroprevalence estimates. However, as the only accepted correlate of immunity is neutralization of viral strain cultures among convalescent patients, the seroprevalence estimates presented here likely represent the upper limit of individuals who were previously exposed to SARS-CoV-2 before vaccination.31 Of importance, the delay in approvals for doing the assays and sharing the data greatly limited the public health value of this information to inform real-time decisions. This is a concern that has been echoed by the Pan-Canadian Health Data Strategy expert advisory group32 and highlights how administrative delays in the context of a pandemic need to be improved upon for effective future public health responses in Canada.
Conclusion
The findings from this study provide data that may be of use to policy-makers and epidemiologists who now have the task of reviewing the various approaches during the COVID-19 pandemic, including effective and ineffective prevention strategies and public health responses, with a view to better global preparation in the future. Polymerase chain reaction testing, although used extensively for the first year of the pandemic, presented a costly and inaccurate measure of population prevalence, according to these seroprevalence results. Use of this approach in the future presents an opportunity to track infectious epidemiology throughout a subset of the population.
This national serosurveillance project using antenatal serum samples as a window into the general population shows more expansive spread of SARS-CoV-2 before the pandemic was declared than previously thought. We also document variability in how each province experienced waves of SARS-CoV-2 infection and show that seroprevalence was up to 8.90 (95% CI 1.80–17.28) times greater than reported prevalence of PCR-positive cases. Despite this undetected spread, seroprevalence in our sampling of pregnant people remained low in Canada until the end of 2021 and at a level suggesting widespread susceptibility to SARS-CoV-2 infection before the introduction of vaccination. Antenatal sero-samples represent a highly valuable window into the population health burden of this pandemic and, possibly, other infectious diseases of public health significance. In the future, they can be deployed in a timely and effective manner to inform and guide public health responses.
Supplementary Material
Acknowledgements
The authors thank Kathryn Bocking, Kaamel Hafizi, Marie-Ève Hamelin, Fergall Magee, Hasna Meddour, Phil Murphy, Donnette O’Brien, Glenn Patriquin, Tamara Pidduck, Sabrina Plitt and Suzanne Taillefer.
Footnotes
Competing interests: Lori Beach reports receiving funding from the Public Health Agency of Canada (PHAC) and from the Canadian Diagnostic Executive Forum, Toronto, for support for travel to speak at a meeting. Dr. Beach is also division head of Public Outreach and Communications for the Canadian Society of Clinical Chemists. Shelly Bolotin reports receiving support for the present manuscript from COVID-19 Immunity Task Force and Public Health Ontario. Dr. Bolotin is co-investigator on several COVID-19 grants funded by the Canadian Institutes of Health Research (CIHR), the Canadian Immunity Taskforce, the Canadian Immunization Research Network and PHAC. Dr. Bolotin is associate professor at the University of Toronto and, as part of that role, is director of the centre for Vaccine Preventable Diseases at the university. The centre is supported by the Dalla Lana School of Public Health (DLSPH), which receives funding from government, philanthropic, not-for-profit and private-sector organizations. Private-sector funding sources include vaccine manufacturers, specifically Merck, Sanofi and Pfizer. A set of governance processes are in place at the DLSPH to ensure independent operation of the centre. Isabelle Boucoiran reports receiving grants or contracts from Altona, the National Institutes of Health, CIHR and the Québec Ministère de la Santé et des Services Sociaux, and consulting fees from Pfizer. Dr. Boucoiran has also received equipment, materials, drugs, medical writing, gifts or other services from Altona. Mel Krajden reports receiving reagent support from Siemens to assess SARS-CoV-2 serology and had a contract with Hologic to support respiratory virus testing. Vanessa Poliquin reports receiving grants or contracts from GSK as the site principal investigator for a respiratory syncytial virus vaccine study. Dr. Poloquin has received an honorarium from Sanofi Pasteur for teaching at a continuing medical education event and payment for expert testimony from the Department of Justice Canada. George Zahariadis reports receiving support for the current manuscript from the Public Health Agency of Canada, as well as grants or contracts from Genome Atlantic and CIHR. Dr. Zahariadis also reports receiving unrestricted educational funding from Roche and Abbott to the Eastern Health Regional Health Authority to attend virtual meetings and in specific restricted circumstances, travel to educational, technical and research meetings. Dr. Zahariadis is the past president of the National Molecular Users Group. Jason Robinson reports receiving a Roche Ideas Grant including in-kind reagents for previous work on salivary antibody detection. Vanessa Tran reports receiving grants from CIHR, the COVID-19 Immunity Task Force, Canadian Immunization Research Network and PHAC, outside the submitted work. Dr. Tran is also a member of Canadian Immunization Research Network Management Committee and the COVID-19 Immunity Task Force Leadership Group. Deborah Money reports receiving support for the present manuscript from PHAC, paid to the institution on behalf of the COVID-19 Immunity Task Force. Dr. Money has also received past funding for unrelated work from Merck, GSK, Sanofi and Novartis, paid to the institution. No other competing interests were declared.
Contributors: Arianne Albert, Elisabeth McClymont, Lori Beach, Shelly Bolotin, Isabelle Boucoiran, Jared Bullard, Carmen Charlton, Joan Crane, Shelley Dougan, Jean-Claude Forest, Yves Giguère, Mel Krajden, Amanda Lang, Paul Levett, Jessica Minion, Cody Neudorf, Jason Robinson, Heather Scott, Derek Stein, Vanessa Tran, George Zahariadis and Deborah Money contributed to the conception and design of the work. All of the authors contributed to the acquisition and interpretation of the data. Arianne Albert contributed to the analysis of the data. Andrea Atkinson, Arianne Albert, Elisabeth McClymont, Janice Andrade and Deborah Money drafted the manuscript. All of the authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This research was funded by the following: the Public Health Agency of Canada through the COVID-19 Immunity Task Force; a Royal Australian and New Zealand College of Obstetricians and Gynaecologists Women’s Health Foundation Jean Murray Jones Scholarship to Andrea Atkinson; a Michael Smith Foundation for Health Research Trainee Award and Canadian Institutes of Health Research Canadian HIV Trials Network Postdoc to Elisabeth McClymont, and a Fonds de Recherche du Québec–Santé salary award to Isabelle Boucoiran.
Data sharing: The data that support the findings of this study are available on request from the corresponding author (deborah.money@ubc.ca). The data are not publicly available owing to confidentiality restrictions.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/11/2/E305/suppl/DC1.
References
- 1.WHO Director-General’s opening remarks at the media briefing on COVID-19 — 11 March 2020. Geneva: World Health Organization; 2020. [accessed 2021 Aug. 8]. Available https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020. [Google Scholar]
- 2.Oran DP, Topol EJ. The proportion of SARS-CoV-2 Infections that are asymptomatic: a systematic review. Ann Intern Med. 2021;174:655–62. doi: 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalish H, Klump-Thomas C, Hunsberger S, et al. Undiagnosed SARS-CoV-2 seropositivity during the first 6 months of the COVID-19 pandemic in the United States. Sci Transl Med. 2021 Jul 7;13:eabh3826.. doi: 10.1126/scitranslmed.abh3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vos ERA, den Hartog G, Schepp RM, et al. Nationwide seroprevalence of SARS-CoV-2 and identification of risk factors in the general population of the Netherlands during the first epidemic wave. J Epidemiol Community Health. 2021;75:489–95. doi: 10.1136/jech-2020-215678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess S, Ponsford MJ, Gill D. Are we underestimating seroprevalence of SARS-CoV-2? BMJ. 2020;370:m3364. doi: 10.1136/bmj.m3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng DL, Goldof GM, Shy BR, et al. SARS-CoV-2 seroprevalence and neutralizing activity in donor and patient blood. Nat Commun. 2020;11:4698. doi: 10.1038/s41467-020-18468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saeed S, Drews SJ, Pambrun C, et al. SARS-CoV-2 seroprevalence among blood donors after the first COVID-19 wave in Canada. Transfusion. 2021;61:862–72. doi: 10.1111/trf.16296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeBlanc JJ, Gubbay JB, Li Y, et al. Real-time PCR-based SARS-CoV-2 detection in Canadian laboratories. J Clin Virol. 2020 Jul;128:104433. doi: 10.1016/j.jcv.2020.104433.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlton CL, Nguyen LT, Bailey A, et al. Pre-vaccine positivity of SARS-CoV-2 antibodies in Alberta, Canada during the first two waves of the COVID-19 pandemic. Microbiol Spectr. 2021;9:e0029121. doi: 10.1128/spectrum.00291-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skowronski DM, Sekirov I, Sabaiduc S, et al. Low SARS-CoV-2 seroprevalence based on anonymized residual sero-survey before and after first wave measures in British Columbia, Canada, March–May 2020 [preprint] medRxiv. Jul 15, doi: 2020.07.13.20153148.
- 11.Lewin A, Therrien R, De Serres G, et al. SARS-CoV-2 seroprevalence among blood donors in Québec, and analysis of symptoms associated with seropositivity: a nested case-control study. Can J Public Health. 2021;112:576–86. doi: 10.17269/s41997-021-00531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang X, Sharma A, Pasic M, et al. SARS-CoV-2 seroprevalence during the first and second pandemic waves in Canada. SSRN. 2021. Aug 12, [accessed 2022 Jan. 12]. Available: [DOI]
- 13.Biondi MJ, Marchand-Austin A, Cronin K, et al. Prenatal hepatitis B screening, and hepatitis B burden among children, in Ontario: a descriptive study. CMAJ. 2020;192:E1299–305. doi: 10.1503/cmaj.200290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remis RS, Merid MF, Palmer RW, et al. High uptake of HIV testing in pregnant women in Ontario, Canada. PLoS One. 2012;7:e48077. doi: 10.1371/journal.pone.0048077.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plitt SS, Smith TR, Berry W, et al. Examination of a Canadian provincial prenatal HIV screening program: 2010 to 2014. Can J Public Health. 2020;111:555–61. doi: 10.17269/s41997-019-00281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bobrovitz N, Arora RK, Cao C, et al. Global seroprevalence of SARS-CoV-2 antibodies: a systematic review and meta-analysis. PLoS One. 2021;16:e0252617. doi: 10.1371/journal.pone.0252617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAuley A, Gousias P, Hasan T, et al. National population prevalence of antibodies to SARS-CoV-2 among pregnant women in Scotland during the second wave of the COVID-19 pandemic: a prospective national serosurvey. Public Health. 2021;199:17–9. doi: 10.1016/j.puhe.2021.08.005.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone M, Grebe E, Sulaeman H, et al. Evaluation of commercially available high-throughput SARS-CoV-2 serological assays for surveillance and related applications [preprint] medRxiv. 2021 Sept 15; doi: 10.1101/2021.09.04.21262414. [DOI] [Google Scholar]
- 19.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–69. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 20.Nelson M. dsrTest: tests and confidence intervals on directly standardized rates for several methods. 2017. [accessed 2022 Feb. 1]. R package version 0.2.1. Available: https://CRAN.R-project.org/package=dsrTest.
- 21.R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna: Austria: R Core Team; 2020. [accessed 2022 Feb. 1]. Available https://www.R-project.org/ [Google Scholar]
- 22.Money D. Maternal and infant outcomes (March 1, 2020 to March 31, 2021) from five Canadian provinces. Canadian surveillance of covid-19 in pregnancy: epidemiology, maternal and infant outcomes. Vancouver: University of British Columbia; 2021. [accessed 2022 Feb. 4]. Available: https://ridprogram.med.ubc.ca/cancovid-preg/ [Google Scholar]
- 23.Preliminary dataset on confirmed cases of COVID-19, Public Health Agency of Canada, 2020–2021. Ottawa: Statistics Canada; 2021. [accessed 2022 Feb. 4]. Available https://www150.statcan.gc.ca/n1/en/catalogue/132600032020001. [Google Scholar]
- 24.Table 13-10-0415-01 Live births, by month. Ottawa: Statistics Canada; 2022. [accessed 2022 Feb. 4]. Available. [DOI] [Google Scholar]
- 25.Archived: Guidance on the prioritization of key populations for COVID-19 immunization. Ottawa: Public Health Agency of Canada; 2021. [accessed 2022 Oct. 4]. Available: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/guidance-prioritization-key-populations-covid-19-vaccination.html. [Google Scholar]
- 26.COVID-19 vaccination during pregnancy in Ontario: Surveillance Report #2, covering December 14, 2020 to June 30, 2021. Ottawa: Better Outcomes Registry & Network (BORN) Ontario; 2021. [accessed 2023 Feb. 15]. Available: https://www.bornontario.ca/en/whats-happening/resources/Documents/BORN-COVID-19-Vaccination-During-Pregnancy-in-Ontario-Report-2---FINAL.pdf. [Google Scholar]
- 27.Timeline: Every case of COVID-19 identified in British Columbia. CTV Online. 2020. Mar 9, [accessed 2022 Oct. 7]. Available: https://bc.ctvnews.ca/timeline-every-case-of-covid-19-identified-in-british-columbia-1.4845820.
- 28.Alberta’s first presumptive case of COVID-19 is in the Calgary Zone. CTV Online. 2020. Mar 5, [accessed 2022 Oct. 7]. Available: https://calgary.ctvnews.ca/alberta-s-first-presumptive-case-of-covid-19-is-in-the-calgary-zone-1.4841008.
- 29.Saskatchewan confirms presumptive case of COVID-19. Regina: Saskatchewan Health Department; [accessed 2022 Oct. 7]. [updated 2020 Mar. 12]. Available: https://www.saskatchewan.ca/government/news-and-media/2020/march/12/confirmed-case-covid-19. [Google Scholar]
- 30.Brown PE, Fu SH, Bansal A, et al. Ab-C Study Collaborators. Ab-C Study Investigators. Omicron BA.1/1.1 SARS-CoV-2 infection among vaccinated Canadian adults. N Engl J Med. 2022;386:2337–9. doi: 10.1056/NEJMc2202879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–11. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 32.Expert Advisory Group-Report 2. Building Canada’s Health Data Foundation. Ottawa: Public Health Agency of Canada; 2021. [accessed 2022 Feb. 4]. Available: https://www.canada.ca/en/public-health/corporate/mandate/about-agency/external-advisory-bodies/list/pan-canadian-health-data-strategy-reports-summaries/expert-advisory-group-report-02-building-canada-health-data-foundation.html. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.