Abstract
Background:
Patterns in location of death among children with life-threatening conditions (e.g., cancer, genetic disorders, neurologic conditions) may reveal important inequities in access to hospital and community support services. We aimed to identify demographic, socioeconomic and geographic factors associated with variations in location of death for children across Canada with life-threatening conditions.
Methods:
We used a retrospective observational cohort design and the Canadian Vital Statistics Database to identify children aged 19 years or younger who died from a life-threatening condition between Jan. 1, 2008, and Dec. 31, 2014. We used multivariable logistic regression to determine predictors of in-hospital death for children aged 1 month to 19 years, and for neonates younger than 1 month.
Results:
Overall, 13 115 decedents younger than 19 years had life-threatening conditions. Of 5250 children and 7865 neonates, 74.2% and 98.1%, respectively, died in hospital. Among children, we found a higher proportion of hospital deaths in the lowest (v. highest) income quintile (odds ratio [OR] 1.59, 95% confidence interval [CI] 1.28–1.97), and a lower proportion among children living more than 400 km (v. < 50 km) from a pediatric hospital (OR 0.73, 95% CI 0.65–0.86). Compared with Ontario, hospital death was most common in Quebec (OR 1.38, 95% CI 1.14–1.67) and least common in British Columbia (OR 0.43, 95% CI 0.34–0.53). Compared with an oncologic cause of death, all causes except neurologic and metabolic conditions had significantly higher odds of dying in hospital.
Interpretation:
In addition to demographics, we identified socioeconomic and geographic differences in location of death, suggesting potential inequities in access to high-quality care at the end of life. Health care policies and practices must ensure equitable access to services for children across Canada, particularly at the end of their life.
A child’s death has a long-lasting and potentially traumatic impact on families, communities and health professionals providing care.1,2 Thus, when death in childhood is anticipated — such as when a child is living with a life-threatening condition (e.g., cancer, genetic disorders, neurologic conditions)3 — it is important to provide high-quality care to maximize quality of life and facilitate end-of-life care and death in the preferred location.4 In Canada, provincial studies focused on children highlight the high proportion who die in hospitals.5–7 Variations in this proportion may reflect variation in child and family preference, but may also be influenced by availability of community services such as pediatric hospice or palliative home care, and specialized care through tertiary pediatric hospitals.4,8,9 Although there are some conflicting findings, geography and level of income have been associated with location of death among children.10–14 From a health equity lens, it is important to identify factors associated with location of death for children with life-threatening conditions.
We sought to identify demographic, socioeconomic and geographic factors associated with location of death in children who died from life-threatening conditions. Our goal was to identify potential health inequities and opportunities to optimize care across care settings.
Methods
Population and cohort
This national observational, retrospective cohort study drew on the population of Canadian residents who died at 19 years of age or younger from Jan. 1, 2008, through Dec. 31, 2014. Analysts at Statistic Canada created the initial cohort using the Canadian Vital Statistics Database, a yearly census of all deaths occurring in Canada with relevant demographic information and causes of death coded using the International Classification of Diseases, 10th Revision (ICD-10).15 To identify children who died from a life-threatening condition (i.e., conditions that have no cure and from which the child is expected to die or for which curative treatment may be feasible but can fail),3 we first excluded those whose primary cause of death was listed as external, such as death by accidents, assault, suicide or drowning (ICD-10 codes from V01 to Y36), or sudden infant death syndrome (R95). Next, we combined classifications developed in the United Kingdom16 and the United States17 to create a list of specific ICD-10 codes within 11 categories signifying life-threatening conditions in children (Appendix 1, available at www.cmajopen.ca/content/11/2/E298/suppl/DC1). Children in the final cohort had at least 1 relevant ICD-10 code listed as a primary or contributing cause of death. Based on previous research showing that most neonates die in hospital,4,7 we stratified the cohort to facilitate separate analysis of children (aged 29 d–19 yr) and neonates (< 29 d of age).
Outcome
We classified location of death as in a hospital (i.e., in locations licensed to operate as hospital under provincial, territorial or federal government legislation) or out of hospital (e.g., private home, freestanding birthing centre, other facility, other specified location).18
Predictors
We chose predictors based on previous research and available data.6,10,13 For the child group, we categorized age as 29–364 days, 1–4 years, 5–9 years, 10–14 years and 15–19 years. For the neonates, we categorized age as younger than 24 hours and 24 hours to 28 days. We assigned decedents into 11 categories of life-threatening conditions, namely neurologic, hematologic, oncologic, metabolic, respiratory, circulatory, gastrointestinal, genitourinary, perinatal, congenital and other (e.g., systemic lupus) conditions.16,17 For those with more than 1 relevant primary or contributing cause of death, we based assignment on the primary cause of death. In about 5% of the sample, there was no relevant primary cause and several relevant contributing causes. We developed an a priori hierarchy (Appendix 2, available at www.cmajopen.ca/content/11/2/E298/suppl/DC1) to prioritize diagnoses based on the likelihood they were a unifying cause of death (e.g., oncologic diagnoses were highest priority). We combined categories as needed to avoid small cell sizes (< 6) and preserve anonymity. Similarly, we collapsed residential provinces by region into Atlantic (Newfoundland and Labrador, Prince Edward Island, Nova Scotia and New Brunswick), Quebec, Ontario, Prairies (Manitoba and Saskatchewan), Alberta, British Columbia and the North (Northwest Territories, Yukon and Nunavut). We used the first 3 characters of postal codes to assign income quintiles according to residing neighbourhood and rurality (with a population < 10 000 classified as rural).19 We calculated distance from a tertiary pediatric hospital using longitude and latitude data derived from the decedent’s postal code and location of the nearest of 16 tertiary pediatric hospitals in Canada. We categorized distance (< 50 km, 50–199 km, 200–400 km, > 400 km) to represent increasingly complex trips (i.e., easy day trip both ways, substantial day trip both ways, trip likely involving overnight stay, overnight trip possibly involving a plane ride).20
Statistical analysis
We conducted all analyses using SAS (version 9.4). We summarized demographic characteristics and locations of death. We used multivariable logistic regression to model the odds of dying in hospital for each group (children and neonates). We selected model predictors a priori as described above. As we desired full model fit, we left variables in each model regardless of p value.21 Missing data were minimal (about 2%); thus, we used complete case analysis. We undertook model diagnostics, including assessment of multicollinearity, before selecting final models for each outcome. The maximum variance inflation factor was less than 5 in all cases. All statistical tests were 2-sided; p values less than 0.05 were considered significant.
Ethics approval
The study was approved by the Health Sciences Research Ethics Board at the University of Toronto (no. 34554).
Results
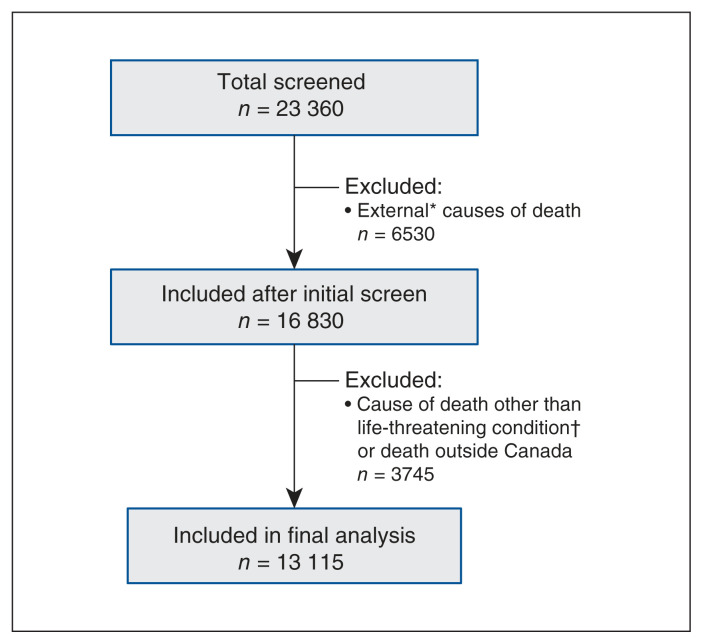
Of the 23 360 children in Canada who died over the 7-year study period, 13 115 (56.1%) had a life-threatening condition (5250 children and 7865 neonates) (Figure 1). Among children aged 29 days to 19 years, 3895 (74.2%) died in hospital and 845 (16.1%) died at home. In the neonate group, 7715 (98.1%) died in hospital (Table 1). The most common causes of death in children were congenital conditions (27.7%) followed closely by oncologic conditions (25.6%). Most neonates (67.2%) died within 24 hours of birth and most (61.9%) died from a perinatal condition. Demographics are summarized in Table 1.
Figure 1:
Flow diagram. *External causes of death included accidents, assault, suicide or drowning (International Classification of Diseases, 10th Revision [ICD-10] codes from V01 to Y36), or sudden infant death syndrome (R95). †Examples of causes of death other than life-threatening conditions included ill-defined or unknown causes (R99.9), depressive episodes (F32), influenza and pneumonia (J09–J18), asthma (J45–46) and infectious and parasitic diseases (A00–B99).
Table 1:
Characteristics of study cohort
| Characteristic | No. (%) of decedents* | |
|---|---|---|
| Older children (aged 29 d–19 yr) n = 5250 |
Neonates (aged < 29 d) n = 7865 |
|
| Age | ||
| < 24 h | – | 5285 (67.2) |
| 24 h–28 d | – | 2580 (32.8) |
| 29–364 d | 1700 (32.4) | – |
| 1–4 yr | 980 (18.7) | – |
| 5–9 yr | 665 (12.7) | – |
| 10–14 yr | 745 (14.2) | – |
| 15–19 yr | 1150 (22.0) | – |
| Sex | ||
| Male | 2440 (53.5) | 4270 (54.3) |
| Female | 2810 (46.5) | 3595 (45.7) |
| Cause of death† | ||
| Perinatal | 305 (5.8) | 4865 (61.9) |
| Congenital | 1455 (27.7) | 2690 (34.2) |
| Oncology | 1345 (25.6) | 60 (0.8)† |
| Hematology | 120 (2.3) | |
| Neurology | 980 (18.7) | 45 (0.6) |
| Metabolic | 345 (6.6) | 65 (0.8) |
| Circulatory | 330 (6.3) | 40 (0.5) |
| Respiratory | 195 (3.7) | 100 (1.3)† |
| Gastrointestinal | 95 (1.8) | |
| Genitourinary | 55 (1.1) | |
| Other | 30 (0.6) | |
| Province or region‡ | ||
| Ontario | 2055 (39.1) | 3240 (41.2) |
| Quebec | 1095 (20.9) | 2035 (25.9) |
| Alberta | 675 (12.9) | 1015 (12.9) |
| Prairies | 520 (9.9) | 600 (7.6) |
| British Columbia | 540 (10.3) | 570 (7.3) |
| Atlantic | 320 (6.1) | 350 (4.5) |
| North | 45 (0.9) | 55 (1.5) |
| Income quintile | ||
| 1 (lowest) | 1210 (23.4) | 2055 (26.7) |
| 2 | 1025 (19.8) | 1530 (19.9) |
| 3 | 1000 (19.3) | 1490 (19.3) |
| 4 | 1050 (20.3) | 1520 (19.7) |
| 5 (highest) | 890 (17.2) | 1110 (14.1) |
| Rurality | ||
| Urban | 4085 (78.3) | 6330 (81.7) |
| Rural§ | 1135 (21.7) | 1420 (18.3) |
| Distance from pediatric hospital, km | ||
| < 50 | 2855 (54.8) | 4700 (60.7) |
| 50–199 | 1460 (28.0) | 1910 (24.7) |
| 200–400 | 575 (11.0) | 645 (8.3) |
| > 400 | 325 (6.2) | 485 (6.3) |
| Location of death¶ | ||
| Hospital | 3895 (74.2) | 7715 (98.1) |
| Home | 845 (16.1) | 60 (0.8) |
| Other health care facility | 150 (2.9) | 30 (0.4) |
| Other location | 360 (6.9) | 60 (0.8) |
Note: ICD-10 = International Classification of Diseases, 10th Revision.
Numbers may not add to total cohort size because of missing data. Some categories combined to avoid small cell sizes.
Please see Appendix 1 for a complete list of included conditions for each category.
Prairies included Manitoba and Saskatchewan. Atlantic included Newfoundland and Labrador, Prince Edward Island, Nova Scotia and New Brunswick. North included Northwest Territories, Yukon and Nunavut.
Population < 10 000.
Hospitals included facilities licensed to operate as a hospital under provincial, territorial or federal government legislation. Other health care facilities included community health centres, freestanding birthing centres, and nursing and residential care facilities. Other locations might include a school, outdoors, at a park or on route to a hospital.
Predictors of dying in hospital
Results of univariate analyses are presented in Appendix 3, available at www.cmajopen.ca/content/11/2/E298/suppl/DC1. Based on multivariable logistic regression, among the child cohort (Table 2), those younger than a year of age had higher odds of in-hospital death than those aged 15–19 years (OR 1.73, 95% CI 1.40–2.15), while those aged 5–9 years had lower odds of dying in hospital (OR 0.66, 95% CI 0.54–0.82). All causes of death, other than neurologic and metabolic, had significantly higher odds of dying in hospital than cancer. The increased odds ranged from nearly double for congenital causes (OR 1.74, 95% CI 1.43–2.11) to more than 5 times higher for gastrointestinal causes of death (OR 5.36, 95% CI 2.54–11.30). Compared with Ontario, those residing in BC had lower odds (OR 0.43, 95% CI 0.34–0.53), and those from Quebec had higher odds (OR 1.38, 95% CI 1.14–1.67) of dying in hospital. Children in the lowest income quintile had higher odds of dying in hospital (OR 1.59, 95% CI 1.28–1.97), compared with those in the highest quintile. Finally, those living 50–199 km (OR 0.73, 95% CI 0.62–0.86) or more than 400 km (OR 0.73, 95% CI 0.65–0.86) from the nearest tertiary pediatric hospital had lower odds of dying in hospital than those living less than 50 km away.
Table 2:
Multivariable logistic regression of factors associated with death in hospital among children (aged 29 d–19 yr), n = 5250*
| Characteristic | OR (95% CI) |
|---|---|
| Age | |
| 15–19 yr | Ref. |
| 10–14 yr | 0.95 (0.77–1.17) |
| 5–9 yr | 0.66 (0.54–0.82) |
| 1–4 yr | 0.88 (0.72–1.07) |
| 29–364 d | 1.73 (1.40–2.15) |
| Sex | |
| Male | Ref. |
| Female | 1.06 (0.93–1.21) |
| Cause of death | |
| Oncology | Ref. |
| Congenital | 1.74 (1.43–2.11) |
| Neurology | 1.07 (0.90–1.28) |
| Metabolic | 1.12 (0.86–1.46) |
| Circulatory | 2.73 (1.96–3.79) |
| Perinatal | 2.78 (1.84–4.21) |
| Respiratory | 3.37 (2.15–5.31) |
| Hematology | 2.52 (1.49–4.26) |
| Gastrointestinal | 5.36 (2.54–11.30) |
| Genitourinary | 3.44 (1.43–8.26) |
| Other | 3.34 (1.15–9.68) |
| Region of residence | |
| Ontario | Ref. |
| Quebec | 1.38 (1.14–1.67) |
| Alberta | 1.03 (0.83–1.27) |
| Prairies | 1.27 (0.98–1.63) |
| British Columbia | 0.43 (0.34–0.53) |
| Atlantic | 1.02 (0.76–1.37) |
| North | 0.49 (0.23–1.05) |
| Income quintile | |
| 5 (highest) | Ref. |
| 4 | 1.08 (0.88–1.33) |
| 3 | 1.07 (0.87–1.32) |
| 2 | 1.23 (0.99–1.52) |
| 1 (lowest) | 1.59 (1.28–1.97) |
| Rurality | |
| Urban | Ref. |
| Rural | 0.98 (0.81–1.18) |
| Distance from pediatric hospital, km | |
| < 50 | Ref. |
| 50–199 | 0.73 (0.62–0.86) |
| 200–400 | 0.87 (0.68–1.11) |
| > 400 | 0.73 (0.65–0.86) |
Note: CI = confidence interval, OR = odds ratio, Ref. = reference.
‐< 2% missing data, degrees of freedom = 29, C-statistic = 0.69.
Among neonate decedents (Table 3), those younger than 24 hours had 13 times higher odds of dying in hospital (OR 13.0, 95% CI 7.94–21.32) than older neonates. Compared with neonates with perinatal conditions, those with congenital (OR 0.25, 95% CI 0.17–0.36) or other causes of death (OR 0.47, 95% CI 0.24–0.92) had lower odds of dying in hospital. Finally, those residing in BC had substantially lower odds of dying in hospital (OR 0.3, 95% CI 0.19–0.49) than those in Ontario.
Table 3:
Multivariable logistic regression examining factors associated with death in hospital among neonates (aged < 29 d), n = 7865*
| Characteristic | OR (95% CI) |
|---|---|
| Age | |
| < 24 hr | 13.01 (7.94–21.32) |
| 24 hr–28 d | Ref. |
| Sex | |
| Male | Ref. |
| Female | 0.75 (0.53–1.05) |
| Cause of death | |
| Perinatal | Ref. |
| Congenital | 0.25 (0.17–0.36) |
| All other causes | 0.47 (0.24–0.92) |
| Region of residence | |
| Ontario | Ref. |
| Quebec | 1.36 (0.79–2.34) |
| Alberta | 0.53 (0.31–0.88) |
| Prairies | 0.97 (0.49–1.92) |
| British Columbia | 0.30 (0.19–0.49) |
| Atlantic | 1.54 (0.53–4.45) |
| North | 0.27 (0.05–1.42) |
| Income quintile | |
| 5 (highest) | Ref. |
| 4 | 1.24 (0.70–2.18) |
| 3 | 1.33 (0.75–2.35) |
| 2 | 1.57 (0.88–2.83) |
| 1 (lowest) | 1.16 (0.69–1.94) |
| Rurality | |
| Urban | Ref. |
| Rural | 0.73 (0.45–1.19) |
| Distance from pediatric hospital, km | |
| < 50 | Ref. |
| 50–199 | 0.66 (0.43–1.01) |
| 200–400 | 1.08 (0.56–2.12) |
| > 400 | 0.89 (0.41–1.94) |
Note: CI: confidence interval, OR = odds ratio, Ref. = reference.
< 2% missing data, degrees of freedom = 18, C-statistic = 0.87.
Interpretation
Our study highlights the high proportion of children in Canada who died from a life-threatening condition in a hospital setting. Although it is not surprising that age and cause of death are significant predictors of location of death, variability based on province, income and distance from a tertiary pediatric hospital that persist after adjustment for other variables suggest potential inequities in care across the country.
In our study, the proportion of children aged 29 days to 19 years who died in hospital was 74.2%; national studies in England and New Zealand with children of similar age ranges and diagnoses found that 65.7%22 and 53.6% of children,10 respectively, died in hospital. This difference may be related to availability of resources like children’s hospices, which are prevalent in England. Although death in hospice occurred in only 7.7% of the English cohort,22 hospices provide supports throughout the illness, which may better enable the family to provide end-of-life care in the home. In the New Zealand study, 21% of the sample received palliative care, which was associated with a decreased risk of death in hospital.10 The authors noted a well-established system of community supports and outreach, particularly for children with cancer.10 Geography may also play a role; it may be easier to provide home support to children over a smaller geographical area.
In our study, almost all neonates (98%) died in hospital — many within the first 24 hours of life from a perinatal condition such as birth trauma, infection or asphyxia — leaving little opportunity to facilitate end-of-life care outside the hospital. Neonates with congenital conditions were more likely to die at home, suggesting that antenatal diagnosis and clearer prognosis may facilitate advanced care planning and out-of-hospital care. Studies have described a link between home death and improved bereavement outcomes, such as reduced depression, anxiety and complicated grief,4 highlighting the importance of improving access to end-of-life care at home even for families of neonates. Increased community support, including availability of freestanding hospices, may offer families additional options for location of care and death.23
As described in other research,10,11,22 children with cancer were more likely to die outside the hospital, possibly because of the more predictable illness trajectory, with more opportunities to plan and provide supports to facilitate a home death. Other diagnoses (e.g., congenital illnesses) may have a more unpredictable disease course. Challenges in identifying the terminal phase of an illness may be associated with less opportunity or desire for a home death.4,11
Although Canada has publicly funded health care systems that are meant to be accessible to all people in Canada regardless of where they live, we noted differences in location of death based on province and distance from a tertiary pediatric hospital. The decreased odds of a hospital death in BC may reflect the presence of Canuck Place Children’s Hospice, North America’s first freestanding children’s hospice, which opened in 1995 and provides residential palliative care and respite, as well as consultation and outreach across the province.24 Other research has noted a trend toward an increased number of home deaths for children when a well-developed system of pediatric palliative care services was available, both in hospitals and within community settings.10,25–27 Palliative care, other specialty services and family supports such as Ronald McDonald Houses are concentrated in the 16 tertiary pediatric hospitals across Canada. As in other studies, we found that those living further from these tertiary hospitals were less likely to die in hospital.6,12 Living very close (e.g., < 50 km) may facilitate relatively easy returns to the hospital, where care is provided by health care professionals who are well known to the family, possibly resulting in reluctance to develop new relationships with community-based providers. Given the challenges of travelling long distances when a child is nearing death, it is possible that those living furthest (e.g., > 400 km) from a tertiary hospital may be more likely to remain home if community supports are in place. Further research is needed to examine distance from hospital as a factor in decision-making about location of death.
Findings in previous research about the impact of socioeconomic status on location of death are conflicting.10,13 However, consistent with our study, a recent meta-analysis found that those living in neighbourhoods with the lowest income quintiles were more likely to die in hospital.11 Across studies, it is unclear what mechanisms may underlie this disparity; however, patient and family preference, system issues, provider biases or some combination of these factors may be at play.14 In one Canadian study of 75 children with cancer, lower income was associated with parent preference for death in hospital.28 Bona and Wolfe29 suggested that underserved populations may have differential access to palliative care supports, both in the community and in the hospital, and when support is provided there may be differences in the degree of benefit they experience from advanced care planning and efforts to improve quality of life. More research is needed to examine factors underlying socioeconomic status and their contribution to care inequities.
Limitations
Our data do not reflect the growth and development of pediatric palliative care in the last 8 years and its potential impact on supports available to children and families in their chosen location of care.30 However, this study provides an important baseline examination of location of death that can be used to study changes in the future.
Only death record data for decedents were available nationally. Our analysis was limited to variables available in this data set and did not include potentially relevant factors such as race, ethnicity or religion. Potential inequities based on income and geography should be explored in provincial samples to provide a more fulsome description of other factors influencing end-of-life care and location of death. Death records are also limited in the specificity of location of death outside of a hospital. For example, hospices provide an important alternative to both hospital and home but cannot be examined separately with current data.9 Administrative data do not allow for evaluation of the preferences of children or parents about location of death or the suitability of a nonhospital death. Unknown details of illness or preference could potentially confound the association between hospital death and income or location. Finally, a very small number of neonates died outside of a hospital, thus limiting the power and stability of the logistic regression model for this cohort.
Conclusion
Location of death is a common marker of quality of end-of-life care.31–33 Not all children or their parents prefer to be at home;4,8 however, given the link to potentially improved bereavement outcomes both for parents and siblings,4 it is important that families of children with life-threatening conditions are given the opportunity to be home if they so choose. Although the Canada Health Act34 includes the principles of universality, comprehensiveness and accessibility, our study highlighted concerning differences in the likelihood of children’s deaths occurring in hospital across measures of income, province of residence and distance from tertiary pediatric hospital. These differences may signify a lack of systematic access to both hospital and community-based services, including specialized pediatric palliative care teams, pediatric hospices and palliative home care. The geographically dispersed population of Canada requires greater efforts to ensure health care principles are applied to all people in Canada and, particularly, vulnerable children and their families.
Supplementary Material
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to study conception and design, and to acquisition, analysis and interpretation of data. Kimberley Widger, Sarah Brennenstuhl and Sumit Gupta drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by the Canadian Institutes of Health Research (CIHR) – Institute of Human Development and Child and Youth Health through an Operating Grant: Analyses of Existing Cohorts & Databases (no. 383565). Kimberley Widger is supported by a Tier 2 Canada Research Chair in Pediatric Palliative Care. Peter Tanuseputro is supported by a PSI Graham Farquharson Knowledge Translation Fellowship.
Data sharing: The study protocol and programing code are available from the corresponding author upon request. Access to The Canadian Vital Statistic Database is available through Statistics Canada (https://www.statcan.gc.ca/en/about/relevant/vscc/access).
Disclaimer: The analysis presented in this paper was conducted at the Toronto Research Data Centre (RDC), which is part of the Canadian Research Data Centre Network (CRDCN). The services and activities provided by the Toronto RDC are made possible by the financial or in-kind support of the Social Sciences and Humanities Research Council of Canada, the Canadian Institutes of Health Research, the Canada Foundation for Innovation, Statistics Canada, University of Toronto, Ryerson University and York University. The views expressed in this paper do not necessarily represent those of the CRDCN or its partners.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/11/2/E298/suppl/DC1.
References
- 1.Rosenberg AR, Postier A, Osenga K, et al. Long-term psychosocial outcomes among bereaved siblings of children with cancer. J Pain Symptom Manage. 2015;49:55–65. doi: 10.1016/j.jpainsymman.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patterson JM, Holm KE, Gurney JG. The impact of childhood cancer on the family: a qualitative analysis of strains, resources, and coping behaviors. Psychooncology. 2004;13:390–407. doi: 10.1002/pon.761. [DOI] [PubMed] [Google Scholar]
- 3.Spicer S, Macdonald ME, Davies D, et al. Introducing a lexicon of terms for paediatric palliative care. Paediatr Child Health. 2015;20:155–6. doi: 10.1093/pch/20.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston EE, Martinez I, Currie E, et al. Hospital or home? Where should children die and how do we make that a reality? J Pain Symptom Manage. 2020;60:106–15. doi: 10.1016/j.jpainsymman.2019.12.370. [DOI] [PubMed] [Google Scholar]
- 5.Chavoshi N, Miller T, Siden H. Resource utilization among individuals dying of pediatric life-threatening diseases. J Palliat Med. 2013;16:1210–4. doi: 10.1089/jpm.2013.0110. [DOI] [PubMed] [Google Scholar]
- 6.Kassam A, Sutradhar R, Widger K, et al. Predictors of and trends in high-intensity end-of-life care among children with cancer: a population-based study using health services data. J Clin Oncol. 2017;35:236–42. doi: 10.1200/JCO.2016.68.8283. [DOI] [PubMed] [Google Scholar]
- 7.Widger K, Seow H, Rapoport A, et al. Children’s end-of-life health care use and cost. Pediatrics. 2017;139:e20162956. doi: 10.1542/peds.2016-2956. [DOI] [PubMed] [Google Scholar]
- 8.Bluebond-Langner M, Beecham E, Candy B, et al. Preferred place of death for children and young people with life-limiting and life-threatening conditions: a systematic review of the literature and recommendations for future inquiry and policy. Palliat Med. 2013;27:705–13. doi: 10.1177/0269216313483186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siden H, Miller M, Straatman L, et al. A report on location of death in paediatric palliative care between home, hospice, and hospital. Palliat Med. 2008;22:831–4. doi: 10.1177/0269216308096527. [DOI] [PubMed] [Google Scholar]
- 10.Chang E, MacLeod R, Drake R. Characteristics influencing location of death for children with life-limiting illness. Arch Dis Child. 2013;98:419–24. doi: 10.1136/archdischild-2012-301893. [DOI] [PubMed] [Google Scholar]
- 11.Wolff SL, Christiansen CF, Nielsen MK, et al. Predictors for place of death among children: a systematic review and meta-analyses of recent literature. Eur J Pediatr. 2020;179:1227–38. doi: 10.1007/s00431-020-03689-2. [DOI] [PubMed] [Google Scholar]
- 12.Johnston EE, Alvarez E, Saynina O, et al. Disparities in the intensity of end-of-life care for children with cancer. Pediatrics. 2017;140:e20170671. doi: 10.1542/peds.2017-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardenas-Turanzas M, Tovalin-Ahumada H, Carrillo MT, et al. The place of death of children with cancer in the metropolitan areas of Mexico. J Palliat Med. 2008;11:973–9. doi: 10.1089/jpm.2008.0042. [DOI] [PubMed] [Google Scholar]
- 14.Johnston EE, Bogetz J, Saynina O, et al. Disparities in inpatient intensity of end-of-life care for complex chronic conditions. Pediatrics. 2019;143:e20182228. doi: 10.1542/peds.2018-2228. [DOI] [PubMed] [Google Scholar]
- 15.International Statistical Classification of Diseases and Related Health Problems, 10th rev (2: instruction manual) Geneva: World Health Organization; 2010. [Google Scholar]
- 16.Fraser LK, Miller M, Aldridge J, et al. Life-limiting and life-threatening conditions in children and young people in the United Kingdom; National and regional prevalence in relation to socioeconomic status and ethnicity: final report for Children’s Hospice UK 2011. Leeds (UK): Division of Epidemiology, University of Leeds; 2011. [Google Scholar]
- 17.Feudtner C, Feinstein JA, Zhong W, et al. Pediatric complex chronic conditions classification system version 2: Updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canadian Vital Statistics Death Database: data dictionary and user guide. Ottawa: Statistics Canada; 2013. [Google Scholar]
- 19.Plessis V, Beshiri R, Bollman RD, et al. Definitions of “rural”. [21-601-MIE] Ottawa: Statistics Canada; 2002. [Google Scholar]
- 20.Stephenson A, Hux J, Tullis E, et al. Socioeconomic status and risk of hospitalization among individuals with cystic fibrosis in Ontario, Canada. Pediatr Pulmonol. 2011;46:376–84. doi: 10.1002/ppul.21368. [DOI] [PubMed] [Google Scholar]
- 21.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, et al. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–81. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 22.Gibson-Smith D, Jarvis S, Fraser L. Place of death of children and young adults with a life-limiting condition in England: a retrospective cohort study. Arch Dis Child. 2020;106:780–5. doi: 10.1136/archdischild-2020-319700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Craig F, Mancini A. Can we truly offer a choice of place of death in neonatal palliative care? Semin Fetal Neonatal Med. 2013;18:93–8. doi: 10.1016/j.siny.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 24.About us [website] Vancouver: Canuck Place Children’s Hospice; [accessed 2022 July 19]. Available: https://www.canuckplace.org/about-us/ [Google Scholar]
- 25.Schmidt P, Otto M, Hechler T, et al. Did increased availability of pediatric palliative care lead to improved palliative care outcomes in children with cancer? J Palliat Med. 2013;16:1034–9. doi: 10.1089/jpm.2013.0014. [DOI] [PubMed] [Google Scholar]
- 26.Håkanson C, Öhlén J, Kreicbergs U, et al. Place of death of children with complex chronic conditions: cross-national study of 11 countries. Eur J Pediatr. 2017;176:327–35. doi: 10.1007/s00431-016-2837-0. [DOI] [PubMed] [Google Scholar]
- 27.Lysecki DL, Gupta S, Rapoport A, et al. Children’s health care utilization and cost in the last year of life: a cohort comparison with and without regional specialist pediatric palliative care. J Palliat Med. 2022;25:1031–40. doi: 10.1089/jpm.2021.0175. [DOI] [PubMed] [Google Scholar]
- 28.Kassam A, Skiadaresis J, Alexander S, et al. Parent and clinician preferences for location of end-of-life care: Home, hospital or freestanding hospice? Pediatr Blood Cancer. 2014;61:859–64. doi: 10.1002/pbc.24872. [DOI] [PubMed] [Google Scholar]
- 29.Bona K, Wolfe J. Disparities in pediatric palliative care: an opportunity to strive for equity. Pediatrics. 2017;140:e20171662. doi: 10.1542/peds.2017-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widger K, Davies D, Rapoport A, et al. Pediatric palliative care in Canada in 2012: a cross-sectional descriptive study. CMAJ Open. 2016;4:E562. doi: 10.9778/cmajo.20160054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widger K, Medeiros C, Trenholm M, et al. Indicators used to assess the impact of specialized pediatric palliative care: a scoping review. J Palliat Med. 2019;22:199–219. doi: 10.1089/jpm.2018.0420. [DOI] [PubMed] [Google Scholar]
- 32.Marcus KL, Santos G, Ciapponi A, et al. Impact of specialized pediatric palliative care: a systematic review. J Pain Symptom Manage. 2020;59:339–364e10. doi: 10.1016/j.jpainsymman.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor J, Booth A, Beresford B, et al. Specialist paediatric palliative care for children and young people with cancer: a mixed-methods systematic review. Palliat Med. 2020;34:731–75. doi: 10.1177/0269216320908490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canada Health Act, R.S.C., 1985 c.6, s.1. [accessed 2022 July 19]. Available: https://laws-lois.justice.gc.ca/eng/acts/c-6/page-1.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.