Abstract
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) is a rare entity whose neoplastic cells retain a B-cell phenotype with expression of CD20. Radiotherapy is recommended for favorable stage IA disease while for other stages guidelines suggest therapeutic strategies similar to those used for classic HL. The role of rituximab, although quite widespread, is not completely elucidated. We retrospectively analyzed baseline characteristics of 308 consecutive patients with NLPHL diagnosed in 19 Italian centers from 2000 to 2018. With a median follow-up of 8.4 years (interquartile range: 4.5–12.4) for treated patients, median overall survival (OS) was not reached and estimated 5-year OS was 97.8% and 5-year progression-free survival (PFS) was 84.5%. Five-year cumulative incidence of histological transformation was 1.4%, 95% confidence interval (CI), 0.5%-3.8%. After adjusting for lymphocyte count, splenic involvement, bulky disease and B symptoms (fever, drenching night sweats, unintentional loss >10% of body weight within the preceding 6 months), patients with stage II or more showed superior PFS with immunochemotherapy in comparison to chemotherapy alone (hazard ratio = 0.4, 95% CI, 0.2-0.8; P = 0.015). Our data suggest an advantage of the use of rituximab combined with chemotherapy ± radiotherapy in the treatment of stage II–III–IV NLPHL.
INTRODUCTION
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) is a rare entity, accounting for approximately 5% of all Hodgkin lymphoma (HL) cases. The histological hallmark of NLPHL is the presence of atypical large malignant cells, known as lymphocyte-predominant cells (“popcorn cells”), staining consistently positive for the B-cell marker CD20 and negative for CD15 and CD30.1
NLPHL is often diagnosed in early stage and shows a tendency to relapse multiple times but with a long survival expectancy. Despite its generally indolent course, a subset experiences histological transformation (HT) into aggressive non-Hodgkin lymphoma (NHL).2–4 The rate of transformation varies significantly across studies (from nearly 1% to 17%)2–8; splenic involvement and tumor size > 5 cm have been reported as risk factor for HT.9
European Society Medical Oncology (ESMO) guidelines recommend the use of radiotherapy (RT) alone for stage IA patients without German Hodgkin Study Group (GHSG) clinical risk factors (large mediastinal mass, extranodal lesions, elevated erythrocyte sedimentation rate, and ≥3 nodal areas), whereas National Comprehensive Cancer Network (NCCN) guidelines support the role of RT in stage IA and IIA.10–12
Evidence regarding the treatment of advanced cases is less strong and these patients usually receive the same treatment adopted for classical HL.
Commonly used chemotherapy regimens include doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) and cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). Notably, the expression of CD20 by neoplastic cells provides a rationale for the use of rituximab (R) in NLPHL but its use in combination with chemotherapy has been investigated by only a few studies.13–15 These findings assessed the activity and tolerability of rituximab in NLPHL but retrospective series provided conflicting results on the impact of immunochemotherapy on progression-free survival (PFS) and overall survival (OS) in comparison to chemotherapy alone.13,16–18
The ESMO guidelines for advanced stage NLPHL recommend classic HL (cHL)–directed chemotherapy, whereas NCCN guidelines suggest either cHL or B-cell NHL-directed chemotherapy regimens, including R-CHOP.10,11
Based on these premises, we collected a large series of consecutive cases of NLPHL to identify clinical features and treatment strategies with a focus on the impact of the addition of rituximab to the chemotherapeutic backbone.
METHODS
Patient selection
We conducted a multi-institutional retrospective analysis on behalf of the Fondazione Italiana Linfomi (FIL): consecutive NLPHL subjects aged 18 years or older and diagnosed between the years 2000 and 2018 in 19 centers of FIL were enrolled.
Histological diagnosis of NLPHL was established according to the World Health Organization criteria1 by expert hemopathologists belonging to the FIL pathologists’ panel.
We collected clinical and laboratory data at diagnosis, management, response evaluation to treatment, and outcome. We registered progression of disease, transformation into high-grade lymphoma or relapse, and long-term toxicities.
Initial staging followed Cotswolds-modified Ann Arbor classification for those patients in whom only computed tomography (CT) scan and bone marrow biopsy were performed, otherwise the Lugano classification of 2014 was applied for those patients staged with fluorodeoxyglucose-positron emission tomography (FDG-PET) scan.19,20 Based on the previous study by Xing et al,9 bulky disease was defined as a mass of more than 5 cm at diagnosis; splenic involvement was defined as splenomegaly more than 13 cm in vertical length or by the presence of nodules at CT and/or PET-CT evaluation.
Response evaluation was assessed following the 2007 Revised Response Criteria or the 2014 Lugano Classification Criteria, according to the availability of FDG-PET/CT scan at the end of the treatment.20,21
The local Ethic Committee of participating Centers approved this study and research was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
The primary endpoint of the study was to assess the outcome of NLPHL patients in terms of progression-free survival.
Quantitative variables were summarized as median and interquartile range (IQR). Qualitative variables were described as counts and percentages of each category.
PFS was defined as the time since the start of therapy until disease progression, relapse, initiation of further therapy, histologic transformation into B-NHL, or death from any cause. If none of these events had occurred, PFS was censored at the date of the last follow-up. OS was measured from the start of treatment until death from any cause and was censored at the last date of survival status; both OS and PFS were referred only to the cohort of treated patients.
OS and PFS were estimated by Kaplan-Meier product limit method and the comparison of outcome between groups of patients was evaluated via the log-rank test. In patients with stage II/III/IV who received chemotherapy, the effect of treatment with rituximab and baseline characteristics on PFS was tested by univariable proportional hazard Cox model. Clustered sandwich estimator was applied in the standard errors estimation, to allow for intracenter correlation. Variables that in univariable analysis presented with a P value lower than 0.1 were inserted in a multivariable model, provided they were not too correlated with each other. Harrell’s C index was used to compare predictive power of survival models. P values lower than 0.05 were considered significant. Statistical analysis was performed using Stata 16 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC).
RESULTS
Baseline clinical characteristics and treatment details
Baseline characteristics of 308 patients are summarized in Table 1. Median age at diagnosis was 44 years (IQR: 33–57) with male predominance (70%). Disease was in stage I in 29% of patients and in stage II in 41% while only one-third of patients presented with stage III–IV disease (19% stage III and 11% stage IV). Bulky disease was detected in 20 patients (7%). Extranodal disease was infrequent (11%): skeleton, lung, liver, pharynx, muscle tissue, subcutaneous tissue, pancreas, and bowel; 10 cases had more than 1 extranodal site involvement. Bone marrow involvement was quite rare (1%): splenic involvement was identified in 35 patients (11%).
Table 1.
Baseline Features of 308 Patients With Nodular Lymphocyte-predominant Hodgkin Lymphoma
| Feature | N. (%) |
|---|---|
| Age (y) | |
| Median | 44 |
| Range, IQR | 33–57 |
| ≤45 y | 162 |
| Male/female | 216/92 (70/30) |
| Stage | |
| I | 89 (29) |
| II | 127 (41) |
| III | 59 (19) |
| IV | 33 (11) |
| B symptoms | 27/306 (9) |
| Extranodal disease | 35/308 (11) |
| Spleen involvement | 35/308 (11) |
| Bone marrow involvement | 4/305 (1) |
| Hemoglobin <10.5 g/dL | 7/279 (3) |
| Lymphocytes <8% | 4/272 (1) |
| Albumin <4 g/dL | 40/235 (17) |
| LDH > UNL | 32/261 (12) |
| Bulky mass (>5 cm) | 20/303 (7) |
| Elevated ESRa | 16/225 (7) |
| β2-microglobuline > UNL | 32/186 (17) |
≥50 mm/h if not B symptoms; ≥30 if B symptoms.
ESR = erythrocyte sedimentation rate; IQR = interquartile range; LDH = lactate dehydrogenase; UNL = upper normal limit.
Overall, only 14 patients (4%) were managed with a watch and wait approach (9%, 4%, 2%, and 0% among stages I, II, III, and IV, respectively), defined as the intention not to treat and no treatment administration within 6 months from diagnosis. Treatment details are provided in Table 2. Median time from diagnosis to treatment was 1.4 months (IQR: 0.9–2.3).
Table 2.
Details of Treatment Adopted for 294 Patients With Nodular Lymphocyte-predominant Hodgkin Lymphoma Who Have Undergone a Therapeutic Regimen According to Disease Stage
| Stage I (n = 81) | Stage II–III–IV (n = 213) | Total (n = 294) | |
|---|---|---|---|
| No rituximab | 63 (77.8%) | 90 (42.3%) | 153 (52.0%) |
| RT alone | 29 (35.8%) | 9 (4.2%) | 38 (12.9%) |
| Chemotherapy alone | 6 (7.4%) | 31 (14.6%) | 37 (12.6%) |
| ABVD alone | 6 | 30 | 36 |
| CHOP alone | 0 | 1 | 1 |
| Chemotherapy + RT | 28 (34.5%) | 50 (23.5%) | 78 (26.5%) |
| ABVD + RT | 28 | 50 | 78 |
| CHOP + RT | 0 | 0 | 0 |
| With rituximab | 18 (22.2%) | 123 (57.7%) | 141 (48.0%) |
| R alone | 3 (3.7%) | 8 (3.7%) | 11 (3.8%) |
| R + RT | 2 (2.5%) | 3 (1.4%) | 5 (1.7%) |
| R-chemotherapy | 2 (2.5%) | 80 (37.6%) | 82 (27.9%) |
| R-ABVD | 2 | 41 | 43 |
| R-CHOP | 0 | 39 | 39 |
| R-chemotherapy + RT | 11 (13.6%) | 32 (15%) | 43 (14.6%) |
| R-ABVD + RT | 9 | 25 | 34 |
| R-CHOP + RT | 2 | 7 | 9 |
ABVD = doxorubicin, bleomycin, vinblastine, and dacarbazine; CHOP = cyclophosphamide, doxorubicin, vincristine, and prednisone; R = rituximab; RT = radiotherapy.
Eighty-six percent of treated patients presenting with stage I (n = 70) received RT alone or combined to chemo- and immunochemotherapy. Ninety-one percent of patients with stage II/III/IV (n = 193) were treated with chemotherapy, combined with RT in 38%.
Of 193 patients in stage II/III/IV treated with chemotherapy, 112 (58%) patients received rituximab as part of induction; among these 112 patients, 66 (59%) received R-ABVD and 46 (41%) R-CHOP. Almost all patients treated without rituximab received ABVD or ABVD-like regimen (80/81, 99%).
Outcome and impact of rituximab
Response to treatment was evaluable in 294 intentionally treated patients; patients managed with a watch and wait approach were excluded from this analysis (details of responses are reported in Suppl. Tables S1 and S2).
At a median follow-up of 8.4 years (IQR: 4.5–12.4) for treated patients, median OS was not reached and estimated 5-year OS (95% confidence interval [CI]) was 97.8% (95.1%–99.0%) and 5-year PFS (95% CI) was 84.5% (79.7%–88.3%).
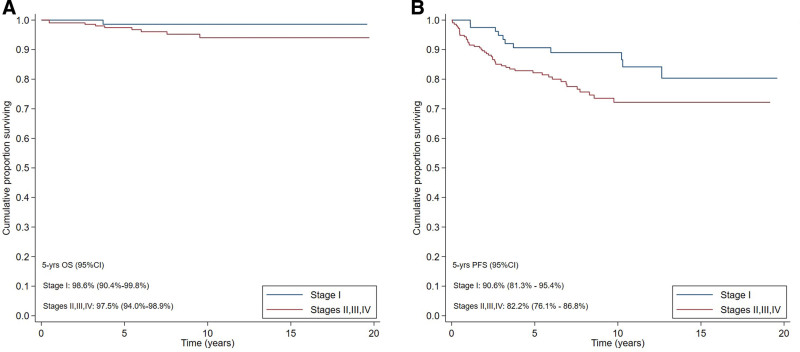
In stage I NLPHL, 5-year OS was 98.6% and 5-year PFS was 90.6%. In stage II–III–IV NLPHL, 5-year OS was 97.5% and 5-year PFS was 82.2% (Figure 1).
Figure 1.
Survival outcome. OS (A) and PFS (B) of 294 patients with nodular lymphocyte-predominant Hodgkin lymphoma according to stage. CI = confidence interval; OS = overall survival; PFS = progression-free survival.
Among 10 reported deaths, none was due to lymphoma; we registered 5 secondary cancers (1 prostatic, 2 gastric, 1 nasopharyngeal, and 1 acute myeloid leukemia) in our cohort occurring after a median time of 5 years from diagnosis (range 3–7 years).
In 193 stage II, III, and IV patients treated with a chemotherapy-based approach (only for 1 patient, the response data were not available), the median follow-up time for the immunochemotherapy cohort was 73 months (IQR: 44–103), whereas for patients treated without rituximab, the median follow-up time was 134 months (IQR: 104–169). This difference is due to the progressive introduction of rituximab in first-line regimens. In particular, we observed a gradual increase in the incidence of patients treated with immunochemotherapy over the years: 1 of 18 (5.6%) between 2000 and 2005; 21 of 58 (36.2%) between 2005 and 2010; 42 of 63 (66.7%) between 2010 and 2015; 48 of 54 (88.9%) between 2015 and 2020.
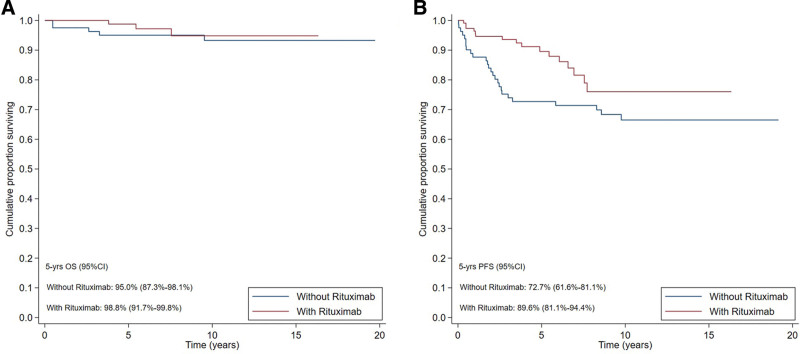
The 5-year PFS was 89.6% in the former group and 72.7% in the latter (P = 0.034), while no difference was found in terms of OS (P = 0.509) (Figure 2). In an explorative analysis limited to patients treated with immunochemotherapy, no significant difference was found in terms of OS and PFS according to the adopted chemotherapy regimen (P = 0.198 and P = 0.477, respectively). Further analysis is available in supplemental digital content and reports the outcome in terms of OS and PFS distinguishing early stage (II) versus advanced stage (III–IV).
Figure 2.
Survival outcome. OS (A) and PFS (B) of 193 chemotreated patients with stage II, III, IV nodular lymphocyte-predominant Hodgkin lymphoma according to the use of rituximab. CI = confidence interval; OS = overall survival; PFS = progression-free survival.
In univariate analysis conducted only in patients with stages II–IV treated with chemotherapy, the no-use of rituximab, splenic involvement, Ann Arbor stage III and IV, lymphopenia (<8%), presence of B symptoms and bulky disease were associated to a shorter PFS. In multivariate analysis, no-use of rituximab, presence of bulky disease, and splenic involvement were associated to a worse PFS (Table 3). At multivariate analysis, we excluded stage as a variable (III–IV versus II) because it was closely related to spleen involvement. An alternative model with stage instead of spleen involvement was carried out but this model presented a lower Harrel’s C (0.71 versus 0.73).
Table 3.
Crude (Univariate Analysis) and Adjusted (Multivariate Model) Effect of Rituximab and Baseline Characteristics on Progression-free Survival in 193 Patients With Stage II/III/IV Nodular Lymphocyte-predominant Hodgkin Lymphoma Who Received Chemotherapy
| Univariable Analysis | Multivariable Model | ||||||
|---|---|---|---|---|---|---|---|
| CHEMO (n = 81) | R-CHEMO (n = 112) | P value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age at diagnosis (y), median (IQR) | 42 (35–53) | 49 (39–59) | 0.014 | 1.0 (1.0-1.0) | 0.915 | - | |
| Stage, n (%) | 0.028 | ||||||
| II | 53 (65.4%) | 55 (49.1%) | Ref | Ref | - | - | |
| III–IV | 28 (34.6%) | 57 (50.9%) | 2.0 (1.1-3.6) | 0.030 | - | - | |
| Hemoglobin (g/dL), median (IQR) | 14.4 (13.2–15.4) | 14.3 (13.1–15.2) | 0.648 | 0.9 (0.7-1.1) | 0.217 | - | - |
| Lymphocytes, n (%) | >0.90 | ||||||
| ≥8% | 73 (98.7%) | 100 (98.0%) | Ref | Ref | Ref | Ref | |
| <8% | 1 (1.3%) | 2 (2.0%) | 4.5 (1.1-18.7) | 0.041 | 2.8 (0.6-13.3) | 0.184 | |
| Spleen involvement, n (%) | 0.239 | ||||||
| No | 71 (87.7%) | 90 (80.4%) | Ref | Ref | Ref | Ref | |
| Yes | 10 (12.3%) | 22 (19.6%) | 3.9 (2.0-7.6) | <0.001 | 3.2 (1.4-7.6) | 0.007 | |
| Bulky, n (%) | 0.386 | ||||||
| No | 72 (94.7%) | 80 (90.9%) | Ref | Ref | Ref | Ref | |
| Yes | 4 (5.3%) | 8 (9.1%) | 2.5 (0.9-7.1) | 0.085 | 3.4 (1.1-10.7) | 0.034 | |
| B symptoms, n (%) | 0.193 | ||||||
| No | 74 (91.4%) | 95 (84.8%) | Ref | Ref | Ref | Ref | |
| Yes | 7 (8.6%) | 17 (15.2%) | 2.2 (1.1-4.6) | 0.034 | 1.9 (0.8-4.7) | 0.155 | |
| Albumin, n (%) | 0.411 | - | |||||
| ≥4 g/dL | 56 (84.9%) | 72 (78.3%) | Ref | Ref | - | - | |
| <4 g/dL | 10 (15.1%) | 20 (21.7%) | 0.9 (0.3-2.3) | 0.804 | - | - | |
| Rituximab, n (%) | |||||||
| No | - | - | Ref | Ref | Ref | Ref | |
| Yes | - | - | 0.5 (0.3-1.0) | 0.037 | 0.4 (0.2-0.8) | 0.015 | |
CI = confidence interval; HR = hazard ratio; IQR = interquartile range.
Relapse and HT
Forty-seven patients experienced at least 1 relapse during follow-up; 38 patients relapsed as NLPHL (78%) and 5 patients underwent HT to diffuse large B-cell lymphoma (12%). Median time to HT was 25.1 months (IQR: 20.2–31.8) after primary management, and 5-year cumulative incidence of HT was 1.4% (95% CI: 0.5-3.8). Two of the 5 patients who experienced HT initially presented in stage IIA as NLPHL and both received ABVD and RT as first-line treatment; they showed PD at the final evaluation and, after a rebiopsy showing DLBCL transformation, they were both treated with rituximab, dexamethasone, cytarabine, and cisplatin (R-DHAP) and autologous stem cell transplantation (ASCT), achieving a complete response (CR). One patient was diagnosed with stage IIIA NLPHL, received 6 cycles of R-ABVD obtaining a CR, relapsed as DLBCL with infradiaphragmatic nodal diseases and was successfully treated with bendamustine and rituximab. The other 2 HT cases initially presented in stage IVA NLPHL: the former received only rituximab as first-line therapy with a final CR, relapsed in stage III and was treated with R-CHOP regimen, obtaining a stable CR; the latter was initially treated with combined modality therapy (R-CHOP and RT), showing PD and was subsequently treated with immunochemotherapy and ASCT.
First relapse as NLPHL occurred after a median time of 27.6 months (IQR: 10.7–70.2 months) from the start of therapy. Management at first relapse was heterogeneous; in 16 cases consolidation with autologous stem cell transplantation (SCT) was performed; 1 patient with multiple relapses underwent allogeneic SCT. All patients who underwent autologous SCT obtained a complete remission confirmed at last follow-up.
Among the 22 patients who relapsed as NLPHL and did not received consolidation with ASCT, we observed that all of them, irrespectively of their onset stage and first-line regimen, received rituximab as salvage treatment (alone or combined to chemotherapy or RT) and obtained a durable CR. One patient experienced a second relapse and was lost to follow-up after refusing ASCT; 1 patient died of pharyngeal cancer.
DISCUSSION
In the present study, we report clinical features, treatment, and outcome of patients with NLPHL diagnosed in 19 centers belonging to the Italian lymphoma cooperative group FIL; we decided to start collection from 2000 due to the implementation of a homogenous characterization of the disease by the panel of FIL pathologists and due to the availability of rituximab.
Population characteristics are superimposable with previous studies: higher incidence in males, a median age at onset higher than that of cHL, an early stage in many cases, a rare extranodal or bone marrow involvement, a low percentage of patients with systemic symptoms, and a sporadic presentation with bulky disease.16,18,22,23
Regarding treatment, NLPHL management is well defined only for stage IA. Our study stems from the lack of consensus on the best therapeutic strategy for stages II, III, and IV. ESMO guidelines recommend treating these patients with the same approach as with cHL. However, the advantage offered by the introduction of rituximab in association with chemotherapy is currently still under debate.8,10,15,24
In reference to a cHL oriented strategy, a recent retrospective study published by GHSG showed a good outcome in patients with NLPHL treated as cHL (from HD7 to HD15) with 10-year PFS and 10-year OS of around 75% and 92%.23 Overall, these data seem to be in line with our results that show a 5-year PFS of around 73% and a 5-year OS of 97% in patients undergoing chemotherapy alone or combined treatment with chemotherapy and RT.
Regarding the role of rituximab combined with chemotherapy, feasibility and efficacy of this approach has been previously described. Fanale et al13 reported an excellent outcome in 27 patients with NLPHL treated with R-CHOP (5-year PFS 88.5% and 5-year OS of 100%) and Mocikova et al15 reported a 5-year PFS of 90% and a 5-year OS of 100% in 23 patients treated with immunochemotherapy.
The lack of consensus for patients with stage disease other than IA13,25–27 prompted us to consider the impact of rituximab in the wide subgroup of patients with stage II–III and IV from our series.
Our data show a significantly better PFS in patients with stage II or higher, who underwent treatment with rituximab, after adjusting for clinical parameters.
Considering that patients receiving chemotherapy without rituximab had ABVD or ABVD-like regimens in the majority of cases, we cannot draw final conclusions on the role of rituximab in this setting, it is noteworthy that patients with advanced NLPHL receiving the intensive BEACOPP regimen do not appear to benefit from the addition of rituximab at least in case of PET-2 positivity.28
In comparison with other prognostic parameters previously reported in the NLPHL,9,29,30 albeit with the limit of the lack of characterization of variant patterns by Fan classification, we did not find correlation between PFS and albumin, gender, and hemoglobin level.
In addition, we took the opportunity to analyze the role of R-ABVD already described in previous studies with a limited number of cases. The first experience was based on 6 patients and concluded that R-ABVD is less toxic than R-CHOP and reported an estimated 6-year PFS of 75% and OS of 100%.14 A second study, based on 24 patients, confirmed the safety of this regimen but suggested to avoid the use of bleomycin in elderly patients as it reported a worse outcome with a 5-year PFS of 80% and a 5-year OS of 100%.31 Interestingly, in our series, outcome in terms of PFS and OS after R-ABVD and R-CHOP appears to be similar. Moreover, adjusting for stage (stage III–IV versus II) did not show any significant difference in terms of PFS between the 2 treatment groups (R-CHOP versus R-ABVD; P = 0.303). Unfortunately, the small number of events did not allow us to adjust this effect for clinical parameters.
A key point in NLPHL treatment is to delay relapse and to avoid or minimize HT: in our series, we observed a relapse in 17% of patients and most of them relapsed as NLPHL. In the study by Fanale et al,13 9 of 59 patients relapsed (15%), histology was still NLPHL in 7 patients, 2 patients showed HT, and no patient treated with R-CHOP transformed.
In our study, we observed a low rate of HT into DLBCL (5 cases with a 5-year cumulative incidence of HT of around 1.4%).
The risk of HT varies across different studies. A French registry of NLPHL patients, followed for nearly 10 years, indicated a cumulative HT risk of 12%, with a median time to transformation of 4.7 years from NLPHL diagnosis,3 while British Columbia Cancer Agency observed a 14% risk of transformation at a median follow-up of 8 years and none of the transformed cases received rituximab.2 Finally, the Mayo Clinic reported 220 NLPHL patients with a median follow-up of 16 years and described a transformation rate of 7.7%, with a median time to transformation of 35 months.6 Our data do not allow a detailed analysis on refractory/relapsed disease due to the heterogeneity of second-line approach. This limit is described in other papers focused on this theme due to a lack of consensus on the role of autologous SCT or other regimens.32,33
To acknowledge the limitations of our study, we underline its retrospective design and a consequent inhomogeneity of follow-up times across the adopted treatments. Furthermore, the lack of formal histological revision prevented the analysis of histological patterns.
In conclusion, our data demonstrate an advantage in terms of outcome by the addition of rituximab to the standard therapeutic strategy (chemotherapy ± RT) in patients with stage II or higher. The introduction of rituximab appears to improve outcome in terms of progression, irrespective of the associated chemotherapeutic regimen. Indeed, no significant differences in terms of OS and PFS were observed between ABVD and CHOP regimens in association to rituximab. Both these approaches can be considered as equally valuable alternatives for treatment of patients with NLPHL, while ABVD alone showed a poorer outcome when administered alone.9
Despite the rarity of the disease and the widespread use of rituximab, prospective studies in NLPHL are eagerly awaited to clarify the role of anti-CD20 therapy in this peculiar entity.
AUTHOR CONTRIBUTIONS
MG conceived and designed the study; AlP, FM, SL, CZ, LT, AlR, CR, AnR, FC, BB, EM, AnP, ID, GG, BP, FR, LN, AF, AML, MM, ARF, MB, VZ, GT, GA, GMD, IDG, IA, AV, SP, DD, CP, SV, SF, CC, VVF, UR; VVF analyzed data; VVF, RS, and CZ created the tables and figures; MG, RS, CZ, and LA wrote the first draft of the manuscript; all the authors critically reviewed and approved the final manuscript.
DISCLOSURES
LA received advisory honoraria from Roche, Celgene, Janssen-Cilag, Verastem, Eusa Pharma, and Incyte, research support from Gilead, and travel expenses from Roche, Celgene, Janssen-Cilag, and Eusa Pharma, speakers bureau from Novartis. AP declares honoraria from Roche. All the other authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This work was supported by the Italian Ministry of Education, University and Research (MIUR) to the Departments of Molecular Medicine (DMM) of the University of Pavia under the initiative “Dipartimenti di Eccellenza (2018–2022)” and by grants of the Fondazione Regionale Ricerca Biomedica (FRRB), Milan, Italy (FRRB project no. 2015-0042, genomic profiling of rare hematologic malignancies, development of personalized medicine strategies, and their implementation into the Rete Ematologica Lombarda clinical network).
Supplementary Material
Footnotes
Current address for S. Viviani: Division of Hemato-Oncology, IEO- Istituto Europeo di Oncologia IRCCS, Milan, Italy; I. Dogliotti: Stem Cell Transplant Unit, University Hospital AOU Città della Salute e della Scienza, Torino, Italy; L. Nassi: Hematology Department, Careggi Hospital and University of Florence, Italy.
Supplemental digital content is available for this article.
REFERENCES
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Mansour M, Connors JM, Gascoyne RD, et al. Transformation to aggressive lymphoma in nodular lymphocyte-predominant Hodgkin’s lymphoma. J Clin Oncol. 2010;28:793–799. [DOI] [PubMed] [Google Scholar]
- 3.Biasoli I, Stamatoullas A, Meignin V, et al. Nodular, lymphocyte-predominant Hodgkin lymphoma: a long-term study and analysis of transformation to diffuse large B-cell lymphoma in a cohort of 164 patients from the adult lymphoma study group. Cancer. 2010;116:631–639. [DOI] [PubMed] [Google Scholar]
- 4.Eyre TA, Gatter K, Collins GP, et al. Incidence, management, and outcome of high-grade transformation of nodular lymphocyte predominant Hodgkin lymphoma: long-term outcomes from a 30-year experience. Am J Hematol. 2015;90:E103–E110. [DOI] [PubMed] [Google Scholar]
- 5.Diehl V, Sextro M, Franklin J, et al. Clinical presentation, course and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease: report from the European task force on lymphoma project on lymphocyte-predominant Hodgkin’s disease. J Clin Oncol. 2019;17:776–783. [DOI] [PubMed] [Google Scholar]
- 6.Kenderian SS, Habermann TM, Macon WR, et al. Large b-cell transformation in nodular lymphocyte-predominant Hodgkin lymphoma: 40-year experience from a single institution. Blood. 2016;127:1960–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orlandi E, Lazzarino M, Brusamolino E, et al. Nodular lymphocyte predominance Hodgkin’s disease: long-term observation reveals a continuous pattern of recurrence. Leuk Lymphoma. 1997;26:359–368. [DOI] [PubMed] [Google Scholar]
- 8.Advani RH, Horning SJ, Hoppe RT, et al. Mature results of a phase II study of rituximab therapy for nodular lymphocyte-predominant Hodgkin lymphoma. J Clin Oncol. 2014;32:912–918. [DOI] [PubMed] [Google Scholar]
- 9.Xing KH, Connors JM, Lai A, et al. Advanced-stage nodular lymphocyte predominant Hodgkin lymphoma compared with classical Hodgkin lymphoma: a matched pair outcome analysis. Blood. 2014;123:3567–3573. [DOI] [PubMed] [Google Scholar]
- 10.Eichenauer DA, Aleman BMP, André M, et al. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:70–75. [DOI] [PubMed] [Google Scholar]
- 11.Hoppe RT, Advani RH, Ai WZ, et al. Hodgkin lymphoma, version 2.2012: featured updates to the NCCN guidelines. JNCCN J Natl Compr Cancer Netw. 2012;10:589–597. [DOI] [PubMed] [Google Scholar]
- 12.Eichenauer DA, Plütschow A, Fuchs M, et al. Long-term course of patients with stage IA nodular lymphocyte-predominant Hodgkin lymphoma: a report from the German Hodgkin study group. J Clin Oncol. 2015;33:2857–2862. [DOI] [PubMed] [Google Scholar]
- 13.Fanale MA, Cheah CY, Rich A, et al. Encouraging activity for R-CHOP in advanced stage nodular lymphocyte–predominant Hodgkin lymphoma. Blood. 2017;130:472–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cencini E, Fabbri A, Bocchia M. Rituximab plus ABVD in newly diagnosed nodular lymphocyte-predominant Hodgkin lymphoma. Br J Haematol. 2017;176:831–833. [DOI] [PubMed] [Google Scholar]
- 15.Mocikova H, Pytlik R, Stepankova P, et al. Can rituximab improve the outcome of patients with nodular lymphocyte-predominant Hodgkin’s lymphoma? Acta Haematol. 2015;134:187–192. [DOI] [PubMed] [Google Scholar]
- 16.Molin D, Linderoth J, Wahlin BE. Nodular lymphocyte predominant Hodgkin lymphoma in Sweden between 2000 and 2014: an analysis of the Swedish Lymphoma Registry. Br J Haematol. 2017;177:449–456. [DOI] [PubMed] [Google Scholar]
- 17.Shivarov V, Ivanova M. Nodular lymphocyte predominant Hodgkin lymphoma in USA between 2000 and 2014: an updated analysis based on the SEER data. Br J Haematol. 2018;182:727–730. [DOI] [PubMed] [Google Scholar]
- 18.Posthuma HLA, Zijlstra JM, Visser O, et al. Primary therapy and survival among patients with nodular lymphocyte-predominant Hodgkin lymphoma: a population-based analysis in the Netherlands, 1993–2016. Br J Haematol. 2020;189:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7:1630–1636. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- 22.Eichenauer DA, Engert A. Nodular lymphocyte-predominant Hodgkin lymphoma: a unique disease deserving unique management. Hematology. 2017;1:324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eichenauer DA, Plütschow A, Fuchs M, et al. Long-term follow-up of patients with nodular lymphocyte-predominant Hodgkin lymphoma treated in the HD7 to HD15 trials: a report from the German Hodgkin study group. J Clin Oncol. 2020;38:698–705. [DOI] [PubMed] [Google Scholar]
- 24.Binkley MS, Shahzad Rauf M, Milgrom SA, et al. Stage I-II nodular lymphocyte-predominant Hodgkin lymphoma: a multi-institutional study of adult patients by ILROG. Blood. 2020;135:2365–2374. [DOI] [PubMed] [Google Scholar]
- 25.Savage KJ, Skinnider B, Al-Mansour M, et al. Treating limited-stage nodular lymphocyte predominant Hodgkin lymphoma similarly to classical Hodgkin lymphoma with ABVD may improve outcome. Blood. 2011;118:4585–4590. [DOI] [PubMed] [Google Scholar]
- 26.Lazarovici J, Dartigues P, Brice P, et al. Nodular lymphocyte predominant Hodgkin lymphoma: a lymphoma study association retrospective study. Haematologica. 2015;100:1579–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nogová L, Reineke T, Brillant C, et al. Lymphocyte-predominant and classical Hodgkin’s lymphoma: a comprehensive analysis from the German Hodgkin study group. J Clin Oncol. 2008;26:434–439. [DOI] [PubMed] [Google Scholar]
- 28.Eichenauer DA, Kreissl S, Bühnen I, et al. PET-2-guided escalated BEACOPP for advanced nodular lymphocyte-predominant Hodgkin lymphoma: a subgroup analysis of the randomized German Hodgkin Study Group HD18 study. Ann Oncol. 2021;32:807–810. [DOI] [PubMed] [Google Scholar]
- 29.Fan Z, Natkunam Y, Bair E, et al. Characterization of variant patterns of nodular lymphocyte predominant Hodgkin lymphoma with immunohistologic and clinical correlation. Am J Surg Pathol. 2003;27:1346–1356. [DOI] [PubMed] [Google Scholar]
- 30.Hartmann S, Eichenauer DA, Plütschow A, et al. The prognostic impact of variant histology in nodular lymphocyte- predominant Hodgkin lymphoma: a report from the German Hodgkin Study Group (GHSG). Blood. 2013;122:4246–4252; quiz 4292. [DOI] [PubMed] [Google Scholar]
- 31.Garciaz S, Harel S, Amorim S, et al. Rituximab-ABV (D) for patients with nodular lymphocyte predominant Hodgkin lymphoma ineligible for radiation therapy. Br J Haematol. 2016;175:735–737. [DOI] [PubMed] [Google Scholar]
- 32.Eichenauer DA, Plütschow A, Schröder L, et al. Relapsed and refractory nodular lymphocyte-predominant Hodgkin lymphoma: an analysis from the German Hodgkin Study Group. Blood. 2018;132:1519–1525. [DOI] [PubMed] [Google Scholar]
- 33.Strati P, Cheng PTM, Steiner RE, et al. Outcome of relapsed and refractory nodular lymphocyte-predominant Hodgkin lymphoma: a North American analysis. Br J Haematol. 2021;192:560–567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.