Abstract
Background
Long-term effectiveness of COVID-19 mRNA boosters in populations with different previous infection histories and clinical vulnerability profiles is inadequately understood. We aimed to investigate the effectiveness of a booster (third dose) vaccination against SARS-CoV-2 infection and against severe, critical, or fatal COVID-19, relative to that of primary-series (two-dose) vaccination over a follow-up duration of 1 year.
Methods
This observational, matched, retrospective, cohort study was done on the population of Qatar in people with different immune histories and different clinical vulnerability to infection. The source of data are Qatar's national databases for COVID-19 laboratory testing, vaccination, hospitalisation, and death. Associations were estimated using inverse-probability-weighted Cox proportional-hazards regression models. The primary outcome of the study is the effectiveness of COVID-19 mRNA boosters against infection and against severe COVID-19.
Findings
Data were obtained for 2 228 686 people who had received at least two vaccine doses starting from Jan 5, 2021, of whom 658 947 (29·6%) went on to receive a third dose before data cutoff on Oct 12, 2022. There were 20 528 incident infections in the three-dose cohort and 30 771 infections in the two-dose cohort. Booster effectiveness relative to primary series was 26·2% (95% CI 23·6–28·6) against infection and 75·1% (40·2–89·6) against severe, critical, or fatal COVID-19, during 1-year follow-up after the booster. Among people clinically vulnerable to severe COVID-19, effectiveness was 34·2% (27·0–40·6) against infection and 76·6% (34·5–91·7) against severe, critical, or fatal COVID-19. Effectiveness against infection was highest at 61·4% (60·2–62·6) in the first month after the booster but waned thereafter and was modest at only 15·5% (8·3–22·2) by the sixth month. In the seventh month and thereafter, coincident with BA.4/BA.5 and BA.2·75* subvariant incidence, effectiveness was progressively negative albeit with wide CIs. Similar patterns of protection were observed irrespective of previous infection status, clinical vulnerability, or type of vaccine (BNT162b2 vs mRNA-1273).
Interpretation
Protection against omicron infection waned after the booster, and eventually suggested a possibility for negative immune imprinting. However, boosters substantially reduced infection and severe COVID-19, particularly among individuals who were clinically vulnerable, affirming the public health value of booster vaccination.
Funding
The Biomedical Research Program and the Biostatistics, Epidemiology, and the Biomathematics Research Core (both at Weill Cornell Medicine-Qatar), Ministry of Public Health, Hamad Medical Corporation, Sidra Medicine, Qatar Genome Programme, and Qatar University Biomedical Research Center.
Introduction
With waning of vaccine and previous infection protection against SARS-CoV-2 infection and against severe COVID-19,1, 2, 3 repeat booster vaccination could sustain immune protection against infection and disease.4, 5 However, the global population carries heterogeneous immune histories due to varying exposures to infection from different viral variants and vaccination.6 Booster effectiveness can vary by previous infection and vaccination history, previous variant exposure, and by age and clinical vulnerability to severe COVID-19. Immune imprinting, a phenomenon in which the specific sequence of immunological events (due to infection or vaccination, or both) can enhance or compromise a person's future immune protection, could affect the utility of booster vaccination.6, 7, 8 The optimal public health effect of boosters might not be achieved through a one size fits all approach.
We aimed to investigate the long-term real-world effectiveness of a booster (third dose) vaccination against SARS-CoV-2 infection and against severe,9 critical,9 or fatal10 COVID-19, relative to that of primary-series (two-dose) vaccination, in people with different immune histories and different clinical vulnerability to infection, over a follow-up duration of 1 year.
Research in context.
Evidence before this study
The long-term effectiveness of COVID-19 mRNA boosters against SARS-CoV-2 infection and against severe COVID-19 remains to be understood. Booster effectiveness can vary by previous infection and vaccination history, previous variant exposure, and by age and clinical vulnerability to severe COVID-19. The utility of the booster vaccination can also be theoretically compromised by immune imprinting. Immune imprinting is a phenomenon in which the specific sequence of immunological events (whether due to infection, or vaccination, or both) can affect a person's future immune protection. We searched PubMed and Google Scholar on Nov 14, 2022, using the search terms “third dose”, “booster vaccination”, “immunity”, “protection”, “immune imprinting”, “SARS-CoV-2”, and “COVID-19” with no language restrictions. We did not identify studies that provided a detailed characterisation of the long-term effectiveness of boosters by previous infection history and by clinical vulnerability profile.
Added value of this study
This study analysed the national federated databases for SARS-CoV-2 infection and COVID-19 vaccination in Qatar using an observational matched retrospective cohort study. Cohorts' outcomes were assessed for a duration of 1 year starting from 7 days after booster vaccination. A third mRNA booster dose was associated with 26% reduction in incidence of infection and 75% reduction in incidence of severe COVID-19 relative to the two-dose primary series. The protective effects of boosters were similar irrespective of previous infection status and whether previous infection was with pre-omicron or omicron viruses, although our study had relatively few people with previous omicron infections. Boosters particularly elicited strong protection against severe COVID-19 in individuals more clinically vulnerable. However, protection against infection waned gradually by month after the booster and was negligible by the sixth month. In the seventh month and thereafter, incidence of infection was higher among people who had the booster compared with those with only the primary series, suggesting a possibility for negative immune imprinting. There was no evidence that imprinting affected protection against severe COVID-19, which remained high after a year of follow-up.
Implications of all the available evidence
Boosters are effective in preventing infection in the first 6 months following vaccination even in individuals who recovered from previous pre-omicron or omicron infections. Although there was evidence suggesting negative immune imprinting after waning of short-term booster protection against infection, this imprinting finding does not negate the overall public health value of booster vaccination, as evidenced here by the booster's strong protection against severe COVID-19. Although negative imprinting has been observed for influenza immunity, this has not undermined the public health value of seasonal influenza vaccinations—an outcome that could also apply for COVID-19 boosters. Imprinting could suggest the need for updated booster strategies to blunt its effect. The findings accentuate the need for longer-term follow-up of boosted cohorts to better understand the effect of booster vaccination on both infection and severe disease.
Methods
Study design and procedures
We conducted an observational, matched, retrospective, cohort study that emulated a randomised target trial.4, 11 The outcomes assessed were effectiveness of vaccination with a third (booster) dose relative to two-dose (primary-series) vaccination against incidence of SARS-CoV-2 infection and of severe, critical, or fatal COVID-19. Incidence of breakthrough infection and associated severe, critical, or fatal COVID-19 were compared in the national cohort of people who received a booster vaccine dose (designated the three-dose cohort) to that in the national cohort of people who only received the primary series (designated the two-dose cohort). Incidence of infection was defined as the first PCR-positive or rapid antigen-positive test after the start of follow-up, regardless of symptoms. Infection severity classification followed WHO guidelines for COVID-19 case severity (acute-care hospitalisations),9 criticality (intensive care unit hospitalisations),9 and fatality10 (appendix pp 11–12).
This study was done on the population of Qatar including data between Jan 5, 2021, earliest record of second-dose vaccination, and Oct 12, 2022. We analysed the national, federated databases for COVID-19 laboratory testing, vaccination, hospitalisation, and death, which were retrieved from the integrated, nationwide, digital-health information platform (appendix pp 2–4). Databases include all SARS-CoV-2-related data with no missing information since the onset of the pandemic, including all PCR tests regardless of location or facility, and from Jan 5, 2022 onwards to the present date, all rapid antigen tests conducted at health-care facilities (appendix p 9). SARS-CoV-2 testing is widely available and performed extensively in Qatar, mostly for non-clinical reasons.1, 12 Most infections are diagnosed not because of symptoms, but because of routine testing (appendix p 3).1, 12 Qatar launched its COVID-19 vaccination programme in December, 2020, using BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) vaccines.13 These vaccines are accessible at multiple facilities throughout the country and are provided free of charge regardless of citizenship or residency status. Demographic information, such as sex and age, were extracted as registered in the national health registry. Further descriptions of Qatar's population and of the national databases have been reported previously.1, 4, 12
Participants
Cohorts were matched exactly one-to-one by sex, 10-year age group, nationality, number of coexisting conditions (0, 1, 2, 3, 4, 5, or ≥6 coexisting conditions), vaccine type (BNT162b2 or mRNA-1273), and previous infection status (no previous infection, or previous infection with either pre-omicron [B.1.1.529] or omicron viruses, or previous infections with both viruses) to balance observed confounders between exposure groups that are related to risk of infection.14, 15, 16 Matching by the considered factors was informed by results of previous studies that used matching to control for differences in infection exposure risk in Qatar.1, 13, 17, 18, 19
To control for time from second-dose vaccination, matching was also done by calendar week of the second dose (ie, matched pairs had to have second doses in the same calendar week). People receiving their third vaccine dose in a specific calendar week in the three-dose cohort were additionally matched to people in the two-dose cohort with records for SARS-CoV-2 testing in that same calendar week, ensuring that matched pairs were present in Qatar in the same period. Further details on matching are found in the appendix (pp 5–6).
People were eligible for inclusion in the three-dose cohort if they received three vaccine doses with the same mRNA vaccine and had no record for a SARS-CoV-2-positive test within 90 days before the start of follow-up. The second exclusion criterion applied to both groups of the study, which ensured that infections after the start of follow-up were incident infections and not prolonged SARS-CoV-2 positivity of earlier infections.20 People were eligible for inclusion in the two-dose cohort if they received two doses of the same mRNA vaccine. People receiving the paediatric BNT162b2 vaccine were excluded from both cohorts.
As in previous studies,4, 21 to ensure time for sufficient immunogenicity, both members of each matched pair were followed up starting 7 days after the calendar date in which the person in the three-dose cohort received the third dose. For exchangeability,4, 21 both members of each matched pair were censored at earliest occurrence of a person receiving a new vaccine dose. Accordingly, individuals were followed up until the first of any of the following events: a documented SARS-CoV-2 infection (regardless of symptoms), or fourth-dose vaccination for people in the three-dose cohort (with matched-pair censoring), or third-dose vaccination for people in the two-dose cohort (with matched-pair censoring), or death, or administrative end of follow-up on Oct 12, 2022.
The institutional review boards at Hamad Medical Corporation and Weill Cornell Medicine-Qatar approved this retrospective study with a waiver of informed consent. The study was reported according to STROBE guidelines (appendix pp 13–14). The authors vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol.
Statistical analysis
Eligible and matched cohorts were described using frequency distributions and measures of central tendency and were compared using standardised mean differences (SMDs). An SMD of 0·1 or less indicated adequate matching. Cumulative incidence of infection (defined as proportion of people at risk whose primary endpoint during follow-up was an infection) was estimated using the Kaplan-Meier estimator method. Incidence rate of infection in each cohort, defined as number of identified infections divided by number of person-weeks contributed by all individuals in the cohort was estimated with the corresponding 95% CI using a Poisson log-likelihood regression model with the Stata stptime command.
Overall hazard ratios (HRs), comparing incidence of infection in the cohorts and corresponding 95% CIs, were calculated using Cox regression adjusted for the matching factors with the Stata stcox command. The adjustment for the matching factors was done to ensure precise and unbiased standard variance.22 The overall HR provided a weighted average of the time-varying HRs.23 As the magnitude of the Cox-estimated HR in the presence of time variation in the HR depends on the scale and distribution of losses to follow-up (censoring) even if the losses occur at random,23 inverse-probability-weighted HRs were calculated to provide estimates that are representative for the entire time of follow-up. The presence of time variation in the HR could affect the Cox-regression standard variance estimator,23 but applying bootstrapping methods23 to all analyses proved computationally unfeasible given the large number of study outcomes. However, a sensitivity analysis was conducted in which bootstrapping with 100 replications was used to derive the 95% CI only in the main analysis.
Vaccine effectiveness was estimated as 1–adjusted hazard ratio (aHR) if the aHR was less than 1, and as 1/aHR–1 if the aHR was 1 or more.24 The latter was to ensure symmetric scale for both negative and positive effectiveness, ranging from –100% to 100%, leading to easier and meaningful interpretation of effectiveness, regardless of being positive or negative. For example, an effectiveness of 40% means that incidence of infection in the three-dose cohort was 40% less than that in the two-dose cohort. Meanwhile, an effectiveness of –40% means that incidence of infection in the two-dose cohort was 40% less than that in the three-dose cohort.
Waning of booster effectiveness over time was investigated. aHRs were estimated by month from the start of follow-up using separate Cox regressions with failures restricted to specific months. In each of these month-by-month analyses, the cohorts included people who were still at risk at the beginning of the month and not censored at earlier times.
Booster effectiveness was further estimated for specific subgroups in which both the three-dose cohort and the two-dose cohort had the same cofactor, such as a specific previous infection status, which was done by including an interaction term in the Cox regression between vaccine exposure status and the cofactor. The previous-infection subgroups included people with no previous infection, previous infection with either pre-omicron or omicron viruses, and previous infections with both viruses. Previous infections were classified as pre-omicron if they occurred before Dec 19, 2021, which is the date of onset of the omicron wave in Qatar,25 and as omicron otherwise.
Similarly, booster effectiveness was estimated for people who are less clinically vulnerable to severe COVID-19, defined as people who are aged below 50 years and with one or no coexisting conditions, and for people who are more clinically vulnerable to severe COVID-19, defined as people who are aged at least 50 years, or who are younger than 50 years but with two or more coexisting conditions (appendix pp 7–8). Effectiveness was also estimated for those vaccinated with BNT162b2 and mRNA-1273 vaccine types.
Sensitivity analyses were conducted by further adjusting the overall HR and the month-by-month HRs in the Cox regression for differences in testing rate (low testers defined as people having ≤2 tests per person-year, intermediate testers having 3–7 tests per person-year, and high testers having ≥8 tests per person-year during follow-up). These sensitivity analyses were done because most SARS-CoV-2 testing in Qatar is done for routine reasons and not because of symptoms, thereby potentially introducing differential ascertainment of infection across the cohorts if routine testing varied by cohort. Statistical analyses were performed using Stata (version 17.0).
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
The study population selection process is shown in the appendix (p 15), and the baseline characteristics of the full and matched cohorts are described in table 1 . Matched cohorts each included 304 091 people. Of the 304 091 people in the three-dose cohort, 39 203 (12·9%) entered the study first as people of the two-dose cohort. The uptake of second-dose and third-dose vaccinations in Qatar are shown in the appendix (p 16).
Table 1.
Baseline characteristics of eligible and matched cohorts
|
Full eligible cohorts |
Matched cohorts* |
||||||
|---|---|---|---|---|---|---|---|
| Three-dose cohort (n=658 947) | Two-dose cohort (n=2 228 686)† | SMD‡ | Three-dose cohort (n=304 091) | Two-dose cohort (n=304 091)† | SMD‡ | ||
| Median age, years | 39 (32–47) | 36 (30–44) | 0·23§ | 37 (31–44) | 37 (31–44) | 0·01§ | |
| Age, years | .. | .. | 0·26 | .. | .. | 0·00 | |
| 0–9 | 1 (<0·01%) | 11 (<0·01%) | .. | .. | .. | .. | |
| 10–19 | 39 663 (6·0%) | 132 021 (5·9%) | .. | 11 909 (3·9%) | 11 909 (3·9%) | .. | |
| 20–29 | 80 310 (12·2%) | 419 633 (18·8%) | .. | 44 296 (14·6%) | 44 296 (14·6%) | .. | |
| 30–39 | 227 636 (34·5%) | 840 553 (37·7%) | .. | 127 304 (41·9%) | 127 304 (41·9%) | .. | |
| 40–49 | 172 513 (26·2%) | 530 743 (23·8%) | .. | 80 707 (26·5%) | 80 707 (26·5%) | .. | |
| 50–59 | 93 428 (14·2%) | 219 088 (9·8%) | .. | 31 225 (10·3%) | 31 225 (10·3%) | .. | |
| 60–69 | 35 866 (5·4%) | 67 905 (3·0%) | .. | 7473 (2·5%) | 7473 (2·5%) | .. | |
| ≥70 | 9530 (1·4%) | 18 732 (0·8%) | .. | 1177 (0·4%) | 1177 (0·4%) | .. | |
| Sex | .. | .. | 0·18 | .. | .. | 0·00 | |
| Male | 432 830 (65·7%) | 1 644 730 (73·8%) | .. | 225 155 (74·0%) | 225 155 (74·0%) | .. | |
| Female | 226 117 (34·3%) | 583 956 (26·2%) | .. | 78 936 (26·0%) | 78 936 (26·0%) | .. | |
| Nationality¶ | .. | .. | 0·39 | .. | .. | 0·00 | |
| Bangladeshi | 57 949 (8·8%) | 312 144 (14·0%) | .. | 33 199 (10·9%) | 33 199 (10·9%) | .. | |
| Egyptian | 54 168 (8·2%) | 110 227 (4·9%) | .. | 17 473 (5·7%) | 17 473 (5·7%) | .. | |
| Filipino | 89 736 (13·6%) | 208 781 (9·4%) | .. | 34 734 (11·4%) | 34 734 (11·4%) | .. | |
| Indian | 207 941 (31·6%) | 549 301 (24·6%) | .. | 110 465 (36·3%) | 110 465 (36·3%) | .. | |
| Nepalese | 26 055 (4·0%) | 239 077 (10·7%) | .. | 18 474 (6·1%) | 18 474 (6·1%) | .. | |
| Pakistani | 31 132 (4·7%) | 106 388 (4·8%) | .. | 12 946 (4·3%) | 12 946 (4·3%) | .. | |
| Qatari | 39 301 (6·0%) | 199 432 (8·9%) | .. | 29 820 (9·8%) | 29 820 (9·8%) | .. | |
| Sri Lankan | 19 069 (2·9%) | 77 844 (3·5%) | .. | 9490 (3·1%) | 9490 (3·1%) | .. | |
| Sudanese | 11 777 (1·8%) | 46 267 (2·1%) | .. | 3800 (1·2%) | 3800 (1·2%) | .. | |
| Other nationalities‖ | 121 819 (18·5%) | 379 225 (17·0%) | .. | 33 690 (11·1%) | 33 690 (11·1%) | .. | |
| Number of coexisting conditions | .. | .. | 0·22 | .. | .. | 0·00 | |
| None | 494 154 (75·0%) | 1 857 593 (83·3%) | .. | 265 210 (87·2%) | 265 210 (87·2%) | .. | |
| 1 | 73 796 (11·2%) | 189 741 (8·5%) | .. | 21 078 (6·9%) | 21 078 (6·9%) | .. | |
| 2 | 42 121 (6·4%) | 89 898 (4·0%) | .. | 9 388 (3·1%) | 9388 (3·1%) | .. | |
| 3 | 21 633 (3·3%) | 41 405 (1·9%) | .. | 3905 (1·3%) | 3905 (1·3%) | .. | |
| 4 | 12 952 (2·0%) | 23 532 (1·1%) | .. | 2159 (0·7%) | 2159 (0·7%) | .. | |
| 5 | 7338 (1·1%) | 13 409 (0·6%) | .. | 1083 (0·4%) | 1083 (0·4%) | .. | |
| ≥6 | 6953 (1·1%) | 13 108 (0·6%) | .. | 1268 (0·4%) | 1268 (0·4%) | .. | |
| Vaccine type | .. | .. | 0·12 | .. | .. | 0·00 | |
| BNT162b2 | 429 788 (65·2%) | 1 322 503 (59·3%) | .. | 184 737 (60·8%) | 184 737 (60·8%) | .. | |
| mRNA-1273 | 229 159 (34·8%) | 906 183 (40·7%) | .. | 119 354 (39·2%) | 119 354 (39·2%) | .. | |
| Previous infection status** | .. | .. | .. | .. | .. | 0·00 | |
| No previous infection | 549 277 (83·4%) | .. | .. | 267 107 (87·8%) | 267 107 (87·8%) | .. | |
| Pre-omicron | 90 307 (13·7%) | .. | .. | 30 755 (10·1%) | 30 755 (10·1%) | .. | |
| Omicron | 17 500 (2·7%) | .. | .. | 6023 (2·0%) | 6023 (2·0%) | .. | |
| Pre-omicron and omicron | 1863 (0·3%) | .. | .. | 206 (0·1%) | 206 (0·1%) | .. | |
Data are n (%) or median (IQR). SMD=standardised mean difference.
Cohorts were matched exactly one-to-one by sex, 10-year age group, nationality, number of coexisting conditions, vaccine type, previous infection status, and calendar week of the second vaccine dose. People who received their third vaccine dose in a specific calendar week in the three-dose cohort were additionally matched to people who had a record for a SARS-CoV-2 test in that same calendar week in the two-dose cohort to ensure that matched pairs had presence in Qatar over the same time period.
All individuals with three doses had also two doses at some point and thus they are part of the two-dose cohort before receiving the third dose. Includes the 658 947 individuals in the three-dose cohort.
SMD is the difference in the mean of a covariate between groups divided by the pooled SD. An SMD of 0·1 or less indicates adequate matching.
SMD is the mean difference between groups divided by the pooled SD.
Nationalities were chosen to represent the most populous groups in Qatar.
These comprise up to 173 other nationalities in the unmatched cohorts, and 106 other nationalities in the matched cohorts.
Ascertained at the start of follow-up. Accordingly, distribution is not available for the unmatched two-dose cohort, as the start of follow-up for each person in the two-dose cohort is determined by that of their match in the three-dose cohort (7 days after the third dose) after the matching is done.
For the matched three-dose cohort, the median date of the first dose was April 15, 2021 (IQR March 16–May 24, 2021), of the second dose was May 12, 2021 (IQR April 6–June 19, 2021), and of the third dose was Jan 16, 2022 (IQR Dec 17, 2021–Feb 28, 2022). The median duration between the first and second doses was 22 days (IQR 21–28 days) and was 249 days between the second and third doses (IQR, 221–282 days). For the matched two-dose cohort, the median date of the first dose was April 15, 2021 (IQR March 16–May 23, 2021), and of the second dose was May 12, 2021 (IQR April 6–June 19, 2021). The median duration between the first and second doses was 22 days (IQR 21–28 days).
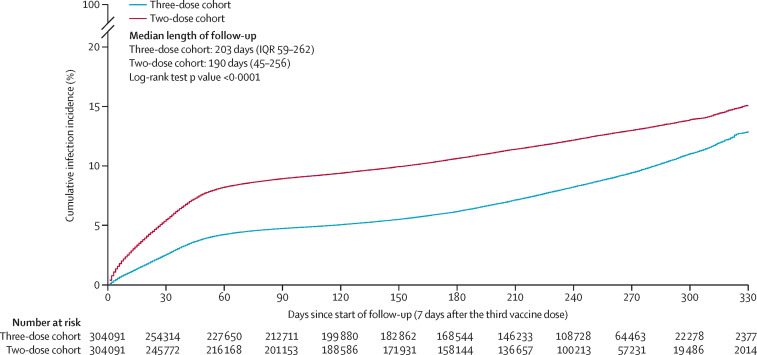
Median duration of follow-up was 203 days (IQR 59–262 days;) for the three-dose cohort and 190 days (45–256 days) for the two-dose cohort (figure 1 ). During follow-up, 20 528 infections were recorded in the three-dose cohort, of which seven infections progressed to severe COVID-19 (none progressed to critical or fatal infections; appendix p 15). Meanwhile, 30 771 infections were recorded in the two-dose cohort, of which 25 infections progressed to severe, three infections to critical, and three infections to fatal COVID-19.
Figure 1.
Cumulative incidence of SARS-CoV-2 infection in the matched three-dose and two-dose vaccination cohorts
Cumulative incidence of infection was 12·9% (95% CI 12·5–13·3) in the three-dose cohort and 15·1% (14·7–15·4) in the two-dose cohort 330 days after the start of follow-up (log-rank p<0·0001; figure 1). Incidence during follow-up was dominated by omicron subvariants including first a large BA.1 and BA.2 wave,26 and subsequently BA.4 and BA.527 and BA.2.75* (predominantly BA.2.75.2)28 waves (appendix p 17). A small proportion of the cohorts experienced low B.1.617.2 (delta) incidence, but only for a very short duration of follow-up.4
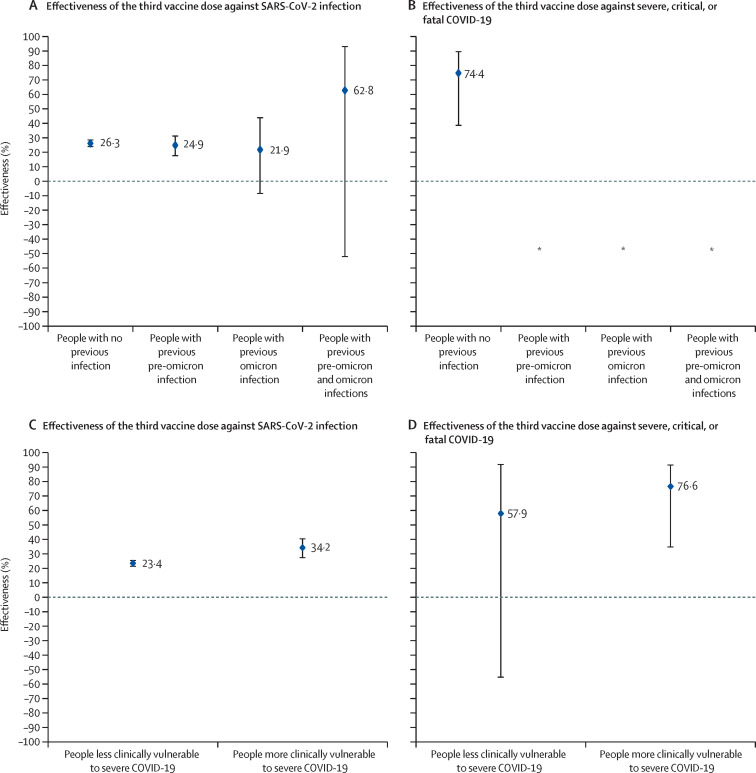
Booster effectiveness against infection was 26·2% (95% CI 23·6–28·6; table 2 ). A bootstrapping method with 100 replications yielded an identical 95% CI. Booster effectiveness against severe, critical, or fatal COVID-19 was 75·1% (40·2–89·6).
Table 2.
Effectiveness of booster relative to primary series against SARS-CoV-2 infection and against severe, critical, or fatal COVID-19 in the matched cohorts*
| Effectiveness against SARS-CoV-2 infection (% [95% CI])† | Effectiveness against severe, critical, or fatal COVID-19 (% [95% CI])† | ||
|---|---|---|---|
| Overall booster effectiveness | 26·2% (23·6 to 28·6) | 75·1% (40·2 to 89·6) | |
| Booster effectiveness by previous infection status | |||
| No previous infection | 26·3% (23·6 to 28·8) | 74·4% (38·3 to 89·4) | |
| Previous pre-omicron infection | 24·9% (17·4 to 31·6) | ..‡ | |
| Previous omicron infection | 21·9% (−8·7 to 44·3) | ..‡ | |
| Previous pre-omicron and omicron infections | 62·8% (−52·3 to 93·4) | ..‡ | |
| Booster effectiveness by clinical vulnerability to severe COVID-19 | |||
| People less clinically vulnerable to severe COVID-19 | 23·4% (21·1 to 25·7) | 57·9% (−55·6 to 92·1) | |
| People more clinically vulnerable to severe COVID-19 | 34·2% (27·0 to 40·6) | 76·6% (34·5 to 91·7) | |
| Booster effectiveness by vaccine type | |||
| Vaccinated with BNT162b2 | 30·4% (27·5 to 33·3) | 74·0% (37·2 to 89·2) | |
| Vaccinated with mRNA-1273 | 9·1% (5·0 to 13·0) | ..‡ | |
Cohorts were matched exactly one-to-one by sex, 10-year age group, nationality, number of coexisting conditions, vaccine type, previous infection status, and calendar week of the second vaccine dose. People who received their third vaccine dose in a specific calendar week in the three-dose cohort were additionally matched to people who had a record for a SARS-CoV-2 test in that same calendar week in the two-dose cohort to ensure that matched pairs had presence in Qatar over the same time period.
Adjusted for sex, 10-year age group, ten nationality groups, number of coexisting conditions, previous infection status, calendar week of second vaccine dose, and calendar week of third vaccine dose or SARS-CoV-2 test.
Could not be estimated or estimates were unstable because there were too few or no infections that progressed to severe, critical, or fatal COVID-19.
Of 304 091 matched individuals, 151 894 in the three-dose cohort (50·0%) and 137 709 (45·3%) in the two-dose cohort had a SARS-CoV-2 test during follow-up. The total number of tests was 344 855 in 142 836·6 person-years and 298 034 in 135 093·4 person-years corresponding to a testing frequency of 1·13 and 0·98 tests per person, and a testing rate of 2·4 and 2·2 tests per person-year, respectively. Among tests in which the reason for testing was available, the proportion of tests done because of symptoms was 5·1% (12 019 tests of 235 657) and 6·7% (15 247 tests of 226 442). Remaining tests were done for other reasons such as being travel related. Adjusting the aHR in a sensitivity analysis for differences in testing rate yielded a booster effectiveness against infection of 28·1% (95% CI 25·4–30·7).
Among people with no previous infection, booster effectiveness was 26·3% (95% CI 23·6 to 28·8) against infection (table 2, figure 2 ; appendix p 18) and 74·4% (38·3 to 89·4) against severe, critical, or fatal COVID-19 (table 2, figure 2). Booster effectiveness against infection was 24·9% (17·47 to 31·6%) among people with previous pre-omicron infection, 21·9% (–8·7 to 44·3) among people with previous omicron infection, and 62·8% (–52·3 to 93·4) among people with previous pre-omicron and omicron infections. Booster effectiveness against severe COVID-19 could not be estimated for each of these previous-infection subgroups because of too few severe COVID-19 cases.
Figure 2.
Booster effectiveness relative to the primary series against SARS-CoV-2 infection and severe, critical, or fatal COVID-19 by previous infection status (A, B) and by clinical vulnerability (C, D)
*Vaccine effectiveness could not be estimated because there were too few or no infections that progressed to severe, critical, or fatal COVID-19.
Among people more clinically vulnerable to severe COVID-19, booster effectiveness was 34·2% (95% CI 27·0 to 40·6) against infection (table 2, figure 2; appendix p 18) and 76·6% (34·5 to 91·7) against severe, critical, or fatal COVID-19 (figure 2). Among people less clinically vulnerable to severe COVID-19, booster effectiveness was 23·4% (21·1 to 25·7%) against infection and 57·9% (–55·6 to 92·1%) against severe, critical, or fatal COVID-19. The wide 95% CI was a consequence of too few severe COVID-19 cases among people less clinically vulnerable to severe COVID-19.
Among people vaccinated with BNT162b2, booster effectiveness was 30·4% (95% CI 27·5–33·3) against infection and 74·0% (37·2–89·2) against severe, critical, or fatal COVID-19 (table 2; appendix pp 18–19). Among people vaccinated with mRNA-1273, booster effectiveness was 9·1% (5·0–13·0%) against infection. Effectiveness against severe, critical, or fatal COVID-19 could not be estimated because of too few severe cases. The p values for the interaction terms for all subgroup analyses were <0·01.
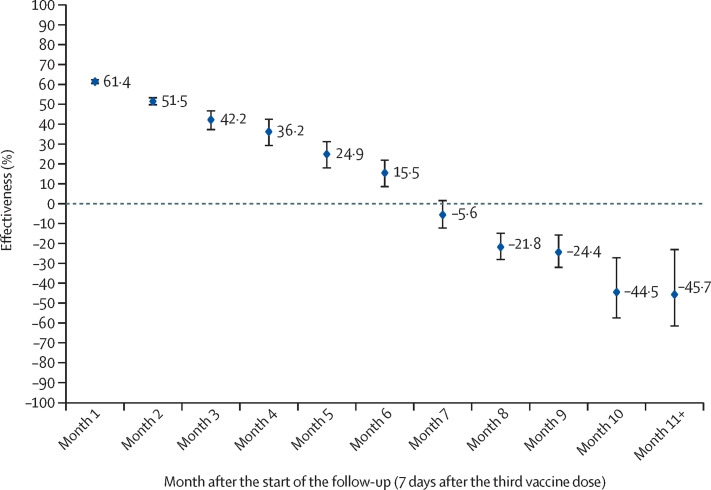
Booster effectiveness against infection was highest at 61·4% (95% CI 60·2–62·6) in the first month after the start of follow-up, but waned gradually thereafter and was modest at only 15·5% (8·3–22·2) by the sixth month of follow-up (figure 3 ). In the seventh month and thereafter, coincident with follow-up time during which BA.4/BA.527 and BA.2.75*28 dominated incidence, effectiveness was progressively negative although with relatively wide 95% CIs. Adjusting the month-by-month aHRs in a sensitivity analysis for differences in testing rate showed similar results (appendix p 20).
Figure 3.
Booster effectiveness relative to the primary series against SARS-CoV-2 infection by month since the start of the follow-up
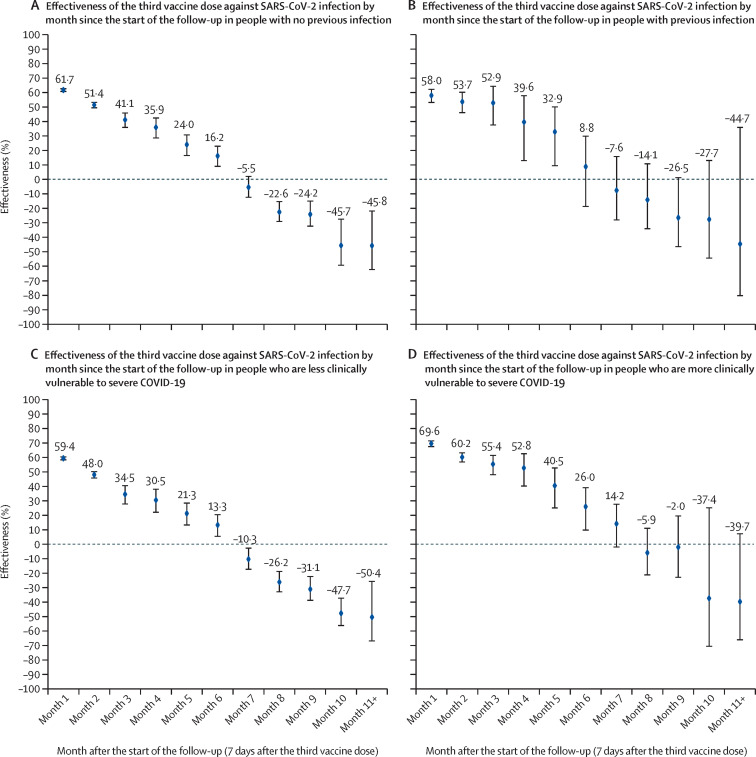
A similar pattern of waning of booster protection was observed irrespective of previous infection status, clinical vulnerability to severe COVID-19, or vaccine type (figure 4 ; appendix p 21). Effectiveness against severe, critical, or fatal COVID-19 could not be estimated by time interval of follow-up because of a small number of severe COVID-19 cases.
Figure 4.
Booster effectiveness relative to the primary series against SARS-CoV-2 infection over time after booster by previous infection status (A, B) and by clinical vulnerability (C, D)
Discussion
A third mRNA booster dose was associated with 26·2% reduction in incidence of infection and 75·1% reduction in incidence of severe COVID-19 over a year of follow-up. However, protection against infection waned gradually by month after the booster and was negligible by the sixth month. In the seventh month and thereafter, incidence of infection was higher among people who had the booster compared to those with only the primary series, suggesting a possibility for differential immune imprinting compromising protection in people who had the booster vaccination against the newer omicron sublineages. However, cohort size was much smaller at these late timepoints and CIs were wide. There was no evidence for imprinting compromising protection against severe COVID-19, but the number of severe COVID-19 cases was too small to allow concrete estimation.
Evidence for imprinting was observed only after complete waning of booster effectiveness, and coincident with infections with new omicron subvariants BA.4/BA.527 and BA.2.75*,28 consistent with a similar effect observed among cohorts who had a primary omicron infection, but different vaccination histories.8 The booster dose, a pre-omicron immunological event, that occurred several months after the primary-series vaccination, another pre-omicron immunological event, could have trained the immune response to expect a specific narrow pre-omicron challenge; thus, the response was suboptimal when the actual challenge was an immune-evasive omicron subvariant. Repeat immunological events of the same type (here pre-omicron challenge) could be associated with lower protection against a new kind of immunological event (here omicron challenge).
This effect appears related to the memory component of the immune response, perhaps explaining why the effect was observed only after waning of the antibody-mediated short-term booster protection. People with a booster might have had their immune memory geared towards expecting a pre-omicron challenge.29 The imprinting effect seems to arise from the mismatch between immune memory and actual immune challenge.29 The effect also seems consistent with emerging in-vitro laboratory data,6 and was observed irrespective of previous infection status, clinical vulnerability to severe COVID-19, or vaccine type.
Although imprinting is of concern when an antigenically divergent virus emerges, it does not negate the public health value of booster vaccinations. Imprinting affected protection against infection in the long term, but the booster was protective against infection in the short term, and overall infection incidence for the whole follow-up period was lower in the group with three doses than in the group with two doses. There was no evidence that imprinting affected protection against severe COVID-19, which remained high after a year of follow-up. Imprinting was observed for boosters based on index-virus (pre-omicron) design,30 but it might not be observed for bivalent boosters, or might only be observed after a longer duration, as bivalent boosters could produce more effective antibodies against currently circulating viruses.
Imprinting might influence the optimal type and timing of boosters in the future. Although imprinting has been observed for influenza,31, 32 this has not undermined the public health value of seasonal influenza vaccinations,33 an outcome that could also apply for COVID-19 boosters. The findings, however, do accentuate the need for longer-term follow-up of boosted cohorts to understand the full extent of the effects of booster vaccination on both infection and severe disease.
The protective effects of boosters, relative to primary series, were similar irrespective of previous infection status, highlighting the value of boosters even for those recovered from a previous infection, and irrespective of whether infections were due to pre-omicron or omicron viruses. This finding suggests that previous infection immunity and booster immunity could have acted independently of each other reflecting neither synergy nor redundancy of their individual biological effects, as suggested earlier for these two forms of protection.12 This finding is also consistent with neutralising antibodies being the correlate of protection against infection.34 However, the majority of our cohort had no documented previous infection and thus estimates for the relatively small number of people with hybrid immunity had wide CIs and the role of immune imprinting in people with previous infections will need to be confirmed in other studies.
The booster was associated with considerable protection against infection and high protection against severe COVID-19 among people more clinically vulnerable to severe COVID-19, underscoring the value of booster vaccinations for this population. Although severe COVID-19 was rare among people less clinically vulnerable, the booster elicited considerable temporary protection against infection. Booster protection was higher among those more clinically vulnerable and those BNT162b2 vaccinated. This higher protection could be a consequence of the more rapid waning of primary-series protection among those more clinically vulnerable (mostly the older population) and those BNT162b2 vaccinated, confirming earlier findings on this population and similar findings from other countries.1, 4, 13, 19
This study has limitations. There were too few severe COVID-19 cases among some subgroups to allow estimation of effectiveness against severe COVID-19 in subgroup analyses or by time interval since the booster. The 95% CIs were calculated using Cox regression in the presence of a time-varying HR, and thus could have been conservative.23 However, deriving the 95% CI in the main analysis using bootstrapping23 with 100 replications yielded a 95% CI that was identical to that of Cox regression. Although the reason for testing was available for all PCR tests, it was available for only a proportion of rapid antigen tests and this availability varied over time. This non-systematic availability of data for reason for testing precluded representative estimation of booster effectiveness against each of the symptomatic and asymptomatic infections.
Imprinting was observed among people with the longest time of follow-up, that is among those who first received the booster, but this population segment might not be representative of the wider population. A pattern of decreasing effectiveness over time could also arise from changes to the composition of the study population over time and thus might not reflect a genuine biological effect for booster vaccination. Yet, imprinting was observed among those who received the booster over several months and not only among those who became first eligible for the booster.
Individuals in the two-dose group were required to have been tested for SARS-CoV-2 on the week they were recruited. Such a requirement was not made for individuals in the three-dose group. Differing eligibility criteria between the two groups could bias the study if there was a correlation between testing and infection exposure. However, most of the testing in the study population was done for routine reasons, not because of infection exposure. Follow-up started 7 days after the test, and not immediately after the test. If bias existed, it would have particularly affected booster effectiveness in the first month of follow-up. However, booster effectiveness in the first month of follow-up was similar to that found in the global literature of studies in different countries and populations.35 This bias therefore is not likely to have affected our results.
Qatar has unusually diverse demographics in that 89% of the population are expatriates from over 150 countries.14 Data on travel history of the study population were not available. As most of the population is an expatriate population, it is plausible that the rate of travel is higher than in other countries. Accordingly, a testing requirement was added in the matching to ensure presence in Qatar in the same period. Possibility of travel is also one of the reasons for matching by nationality, age, and sex, to balance rates of travel across the cohorts. These demographic factors provide a powerful proxy for socioeconomic status and occupation in Qatar,14, 15, 16 and thus of the rate of travel outside the country.
Testing frequency differed between cohorts, suggesting the possibility of bias due to differential outcome ascertainment. Receiving a booster dose could be correlated with health-seeking behaviour that would result in more frequent testing. Such bias could affect the estimated effectiveness and could explain the negative effectiveness observed late in the study, if the two-dose cohort was affected by a proportionally higher level of undocumented infections. However, the sensitivity analysis adjusting for the differences in testing rates showed similar findings to the main analysis findings. The study also matched observable confounders across cohorts to control for any potential effects of differentials in testing across confounder values. If bias due to differential outcome ascertainment appreciably affected the results, the effect of bias should have been observed throughout the time of follow-up, as testing guidelines did not materially change during follow-up. However, booster effectiveness in the first months of follow-up was similar to that found in the global literature of studies in different countries and populations.35 This bias therefore might not explain the negative effectiveness observed late in the study.
Home-based rapid-antigen testing is not documented (appendix p 4) and is not factored in these analyses. However, there is no reason to believe that home-based testing could have differentially affected the followed study cohorts to alter study estimates. Matching was done while factoring key sociodemographic characteristics of the population,14, 15, 16 and this might also have controlled or reduced differences in home-based testing between cohorts.
Effectiveness was estimated by previous infection status, but some infections might have never been documented, thereby introducing the possibility of misclassification bias in defining some of the previous-infection subgroups, particularly the no-previous infection subgroup. However, the protective effects of boosters were similar irrespective of previous infection status, suggesting that such misclassification bias is not likely to have affected the study results. The variant and subvariant status of previous infections was determined by time of infection on the basis of the variant and subvariant that was dominant at the time (appendix p 17), and not based on viral genome sequencing of every infection. This approach in ascertaining variants and subvariants might have introduced misclassification bias in the variant and subvariant status of previous infections.
As an observational study, investigated cohorts were neither blinded or randomised, so effects for unmeasured or uncontrolled confounding factors cannot be excluded. Although matching covered key factors affecting infection exposure,14, 15, 16 it was not possible for other factors such as geography or occupation, for which data were unavailable. However, Qatar is essentially a city state and infection incidence was broadly distributed across neighbourhoods. Nationality, age, and sex (factors that were used in the matching) provide a powerful proxy for socioeconomic status and occupation in Qatar.14, 15, 16
The matching procedure used in this study was investigated in previous studies of different epidemiological designs, and using control groups to test for null effects.1, 13, 17, 18, 19 These control groups have included unvaccinated cohorts versus vaccinated cohorts within 2 weeks of the first dose1, 17, 18, 19 (when vaccine protection is negligible30), and mRNA-1273-vaccinated versus BNT162b2-vaccinated cohorts, also in the first 2 weeks after the first dose.13 These previous studies showed at different times during the pandemic that this procedure resulted in similar infection exposure levels across groups,1, 13, 17, 18, 19 suggesting that the matching strategy might also have controlled for differences in infection exposure in this study. Analyses were implemented on Qatar's total population and large samples, perhaps minimising the likelihood of bias. As this study emulated a target trial,4, 11 the matching algorithm was developed and exact matching was used to ensure that both cohorts are similar in terms of all factors known, or that have any potential to affect the risk of infection, other than the booster effect. Although this approach maximises the study's internal validity, it reduces the size of the study cohorts relative to the full cohorts, which could have affected the study generalisability.
In conclusion, mRNA boosters reduced incidence of infection and severe COVID-19 11 months of follow-up after vaccination, particularly among those clinically vulnerable to severe COVID-19. However, protection against infection waned after the booster, and eventually suggested a possibility for negative immune imprinting. Both patterns of protection and imprinting were observed irrespective of previous infection status, clinical vulnerability to severe COVID-19, and vaccine type. Although the imprinting is a theoretical concern, it is unlikely to negate the public health value of the booster vaccination. There is need for longer-term follow-up of boosted cohorts to understand the full extent of the effects of the booster vaccination on both infection and severe disease, particularly in the face of new emerging omicron subvariants of SARS-CoV-2.
Data sharing
The dataset of this study is a property of the Qatar Ministry of Public Health that was provided to the researchers through a restricted-access agreement that prevents sharing the dataset with a third party or publicly. The data are available under restricted access for preservation of confidentiality of patient data. Access can be obtained through a direct application for data access to Her Excellency the Minister of Public Health (https://www.moph.gov.qa/english/OurServices/eservices/Pages/Governmental-HealthCommunication-Center.aspx). The raw data are protected and are not available due to data privacy laws. Aggregate data are available within the paper and its supplementary information.
Declaration of interests
AAB has received institutional grant funding from Gilead Sciences unrelated to the work presented in this paper. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We acknowledge the many dedicated individuals at Hamad Medical Corporation, the Ministry of Public Health, the Primary Health Care Corporation, Qatar Biobank, Sidra Medicine, and Weill Cornell Medicine-Qatar for their diligent efforts and contributions to make this study possible. The authors are grateful for institutional salary support from the Biomedical Research Program and the Biostatistics, Epidemiology, and Biomathematics Research Core, both at Weill Cornell Medicine-Qatar, and for institutional salary support provided by the Ministry of Public Health, Hamad Medical Corporation, and Sidra Medicine. The authors are also grateful to the Qatar Genome Programme and Qatar University Biomedical Research Center for institutional support for the reagents needed for the viral genome sequencing. Statements made herein are solely the responsibility of the authors.
Contributors
HC, JSF, and LJA-R conceived and co-designed the study analyses. HC performed the statistical analyses and co-wrote the first draft of the article. LJA-R led the statistical analyses and co-wrote the first draft of the article. HC and LJA-R accessed and verified all the data. PT and MRH designed and conducted multiplex, real-time reverse-transcription PCR variant screening and viral genome sequencing. PC designed mass PCR testing to allow routine capture of S-gene target failure variants and conducted viral genome sequencing. HMY, AAAT, and HAA-K conducted viral genome sequencing. All authors contributed to data collection and acquisition, database development, discussion and interpretation of the results, and to the writing of the Article. All authors have read and approved the final manuscript. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. Decision to publish the paper was by consensus among all authors.
Supplementary Material
References
- 1.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chemaitelly H, Nagelkerke N, Ayoub HH, et al. Duration of immune protection of SARS-CoV-2 natural infection against reinfection. J Travel Med. 2022;29 doi: 10.1093/jtm/taac109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tartof SY, Slezak JM, Puzniak L, et al. Effectiveness and durability of BNT162b2 vaccine against hospital and emergency department admissions due to SARS-CoV-2 omicron sub-lineages BA.1 and BA.2 in a large health system in the USA: a test-negative, case-control study. Lancet Respir Med. 2023;11:176–187. doi: 10.1016/S2213-2600(22)00354-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N Engl J Med. 2022;386:1804–1816. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chemaitelly H, Ayoub HH, AlMukdad S, et al. Duration of mRNA vaccine protection against SARS-CoV-2 omicron BA.1 and BA.2 subvariants in Qatar. Nat Commun. 2022;13 doi: 10.1038/s41467-022-30895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reynolds CJ, Pade C, Gibbons JM, et al. Immune boosting by B.1·1.529 (omicron) depends on previous SARS-CoV-2 exposure. Science. 2022;377 doi: 10.1126/science.abq1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chemaitelly H, Ayoub HH, Tang P, et al. Immune imprinting and protection against repeat reinfection with SARS-CoV-2. N Engl J Med. 2022;387:1716–1718. doi: 10.1056/NEJMc2211055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chemaitelly H, Ayoub HH, Tang P, et al. COVID-19 primary series and booster vaccination and immune imprinting. medRxiv. 2022 doi: 10.1101/2022.10.31.22281756. published online Nov 1. (preprint). [DOI] [Google Scholar]
- 9.WHO Living guidance for clinical management of COVID-19. 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2
- 10.WHO International guidelines for certification and classification (coding) of COVID-19 as cause of death. 2020. https://www.who.int/publications/m/item/international-guidelines-for-certification-and-classification-(coding)-of-covid-19-as-cause-of-death
- 11.Hernán MA, Robins JM. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183:758–764. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Effectiveness of mRNA-1273 and BNT162b2 vaccines in Qatar. N Engl J Med. 2022;386:799–800.14. doi: 10.1056/NEJMc2117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. Sci Rep. 2021;11 doi: 10.1038/s41598-021-85428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coyle PV, Chemaitelly H, Ben Hadj Kacem MA, et al. SARS-CoV-2 seroprevalence in the urban population of Qatar: an analysis of antibody testing on a sample of 112,941 individuals. iScience. 2021;24 doi: 10.1016/j.isci.2021.102646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Thani MH, Farag E, Bertollini R, et al. SARS-CoV-2 infection is at herd immunity in the majority segment of the population of Qatar. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abu-Raddad LJ, Chemaitelly H, Yassine HM, et al. Pfizer-BioNTech mRNA BNT162b2 covid-19 vaccine protection against variants of concern after one versus two doses. J Travel Med. 2021;28 doi: 10.1093/jtm/taab083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27:1614–1621. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Raddad LJ, Chemaitelly H, Bertollini R. Waning mRNA-1273 vaccine effectiveness against SARS-CoV-2 infection in Qatar. N Engl J Med. 2022;386:1091–1093. doi: 10.1056/NEJMc2119432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojima N, Shrestha NK, Klausner JD. A systematic review of the protective effect of prior SARS-CoV-2 infection on repeat infection. Eval Health Prof. 2021;44:327–332. doi: 10.1177/01632787211047932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sjölander A, Greenland S. Ignoring the matching variables in cohort studies—when is it valid and why? Stat Med. 2013;32:4696–4708. doi: 10.1002/sim.5879. [DOI] [PubMed] [Google Scholar]
- 23.Stensrud MJ, Hernán MA. Why test for proportional hazards? JAMA. 2020;323:1401–1402. doi: 10.1001/jama.2020.1267. [DOI] [PubMed] [Google Scholar]
- 24.Tseng HF, Ackerson BK, Bruxvoort KJ, et al. Effectiveness of mRNA-1273 vaccination against SARS-CoV-2 omicron subvariants BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. Nat Commun. 2023;14:189. doi: 10.1038/s41467-023-35815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altarawneh HN, Chemaitelly H, Hasan MR, et al. Protection against the omicron variant from previous SARS-CoV-2 infection. N Engl J Med. 2022;386:1288–1290. doi: 10.1056/NEJMc2200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chemaitelly H, Ayoub HH, Coyle P, et al. Protection of omicron sub-lineage infection against reinfection with another omicron sub-lineage. Nat Commun. 2022;13 doi: 10.1038/s41467-022-32363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Protective effect of previous SARS-CoV-2 infection against omicron BA.4 and BA.5 subvariants. N Engl J Med. 2022;387:1620–1622. doi: 10.1056/NEJMc2209306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chemaitelly H, Tang P, Coyle P, et al. Protection against reinfection with the omicron BA.2.75 subvariant. N Engl J Med. 2023;388:665–667. doi: 10.1056/NEJMc2214114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheatley AK, Fox A, Tan HX, et al. Immune imprinting and SARS-CoV-2 vaccine design. Trends Immunol. 2021;42:956–959. doi: 10.1016/j.it.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janjua NZ, Skowronski DM, Hottes TS, et al. Seasonal influenza vaccine and increased risk of pandemic A/H1N1-related illness: first detection of the association in British Columbia, Canada. Clin Infect Dis. 2010;51:1017–1027. doi: 10.1086/656586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skowronski DM, Sabaiduc S, Leir S, et al. Paradoxical clade- and age-specific vaccine effectiveness during the 2018/19 influenza A(H3N2) epidemic in Canada: potential imprint-regulated effect of vaccine (I-REV) Euro Surveill. 2019;24 doi: 10.2807/1560-7917.ES.2019.24.46.1900585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung H, Buchan SA, Campigotto A, et al. Influenza vaccine effectiveness against all-cause mortality following laboratory-confirmed influenza in older adults, 2010-2011 to 2015–2016 seasons in Ontario, Canada. Clin Infect Dis. 2021;73:e1191–e1199. doi: 10.1093/cid/ciaa1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilbert PB, Donis RO, Koup RA, Fong Y, Plotkin SA, Follmann D. A covid-19 milestone attained - a correlate of protection for vaccines. N Engl J Med. 2022;387:2203–2206. doi: 10.1056/NEJMp2211314. [DOI] [PubMed] [Google Scholar]
- 35.VIEW-hub by IVAC Vaccine effectiveness studies. COVID-19 data. 2023. https://view-hub.org/covid-19/effectiveness-studies
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset of this study is a property of the Qatar Ministry of Public Health that was provided to the researchers through a restricted-access agreement that prevents sharing the dataset with a third party or publicly. The data are available under restricted access for preservation of confidentiality of patient data. Access can be obtained through a direct application for data access to Her Excellency the Minister of Public Health (https://www.moph.gov.qa/english/OurServices/eservices/Pages/Governmental-HealthCommunication-Center.aspx). The raw data are protected and are not available due to data privacy laws. Aggregate data are available within the paper and its supplementary information.