Introduction
Cell mechanosensing is the process that cell senses extracellular mechanical cues through mechanosensors and transduces them to downstream signaling pathways to alter cell mechanics and behaviors, which is involved in embryonic development (Gaetani et al., 2020), tissue regeneration (Fu et al., 2019), inflammatory response (Worbs et al., 2017), and tumor invasion and metastasis (Gargalionis et al., 2018). This ability is crucial for cells to adapt to mechanical stimuli (compressive force, shear stress, substrate rigidity, topology, adhesiveness, etc.) and maintain homeostasis (Chen et al., 2017).
The primary site of force transmission to a cell is the plasma membrane (PM) (Martino et al., 2018). As a dynamic mechanosensor, caveolae are PM-invaginated microdomains with specific lipid and protein composition, which are widely abundant in mechanically challenged tissues (muscles, lungs, vessels, etc.) (Del Pozo et al., 2021). Caveolae can bud to generate endocytic vesicles named caveosome or undergo disassembly to play a role in mechanoprotection, mechanosensation, endocytosis, oncogenesis, and uptake of pathogens (Li et al., 2005; Pelkmans, 2005; Parton and del Pozo, 2013; Del Pozo et al., 2021). As one of the essential structural proteins of caveolae, caveolin-1 (Cav-1) is essential for multiple biological processes such as membrane trafficking, signal transduction, and tumorigenesis (Feng et al., 2013).
The second messenger of mechano- transduction is ensured by cytoskeleton tension (Discher et al., 2005). Cellular cytoskeleton is composed of three components, actin filaments (F-actin), microtubules (MTs), and intermediate filaments (IFs). Each of the components displays a highly organized structure contributing to multifaceted functions, including physically and biochemically connecting to the external environment and generating coordinated forces for cell migration and morphologic changes (Fletcher and Mullins, 2010). Stress fibers are contractile actomyosin bundles composed of F-actin, myosin II, and crosslinking proteins (e.g. α-actinin, filamin A, and fascin) (Martino et al., 2018). The structural and contractile properties of stress fibers underlie many cellular processes, including cell migration, adhesion, and mechanosensing (Kassianidou and Kumar, 2015).
Cell migration is a mechanical phenomenon that cell moves in response to extracellular cues and is driven by intracellular biochemical and biomechanical organization (Mak et al., 2016). The key mechanical machinery of cell migration involves actin filaments, myosin motors, and adhesion complexes. Actin fila-ments and myosin generate contractile forces and drive protrusions. Adhesion complexes connect the cell to the external environment and enable force transmission (Mak et al., 2016). Besides, another cytoskeletal component, vimentin, a well-known IF protein, is also involved in cell mechanosensing and migration (Swoger et al., 2022). Here, we discuss how Cav-1 interacts with F-actin and vimentin IFs, and summarize their roles in cell mechanosensing and migration.
Interactions between Cav-1 and actin cytoskeleton in terms of cell mechanosensing and migration
Caveolae, as a dynamic mechano-sensor, can change their shape and organization to buffer membrane tension induced by mechanical stress (Sinha et al., 2011). Increased membrane tension results in flattened caveolae, which leads to release of specific proteins to further mediate mechanotransduction (Torrino et al., 2018). Decreased membrane tension leads to caveolae forming clusters called rosettes (Golani et al., 2019). Cav-1 can interact with cell membrane to cause membrane curvature and clustering of specific lipids, which is essential for caveolae formation and function (Prakash et al., 2022). Actin filaments, especially stress fibers, are intimately associated with caveolae. It has been proved that uncontrolled actin polymerization leads to reduction of caveolae rosettes and caveolae flattening, which results from the increased membrane tension caused by the excessive forces generated by actin filaments (Kozera et al., 2009; Echarri et al., 2012). In this section, we focus on how Cav-1 interacts with actin cytoskeleton to participate in cell mechanosensing and migration.
Crosstalk between mechanosensory Cav-1 and actin stress fibers in cell mechanosensing
Shear stress can be sensed by cells through mechanical effectors on cell membrane and activate several signaling pathways to regulate actin cytoskeleton remodeling (Qin et al., 2021). Cav-1 has been reported to participate in regulating tumor cell responses to shear stress. Cav-1 silence reduces actin stress fibers traversing the cytoplasm and cell motility, which can be rescued by low shear stress (LSS) treatment (Xiong et al., 2017a). Specifically, LSS exposure downregulates the expression levels of the F-actin depolymerization factor cofilin and phosphorylated myosin light chain (p-MLC) whereas upregulates filamin A expression in Cav-1 knockdown cells (Xiong et al., 2017a). The alterations of these actin-associated proteins promote actin bundling, stress fiber formation, and cell migration. These data indicate that LSS regulates tumor cell functions such as motility and adhesion in a Cav-1-dependent manner, emphasizing the importance of interactions between Cav-1 and actin filaments in tumor cell translocation under shear stress during tumor cell passage in the blood vessels (Xiong et al., 2017a). Besides, caveolae control contractile tension for epithelia to eliminate tumor cells (Teo et al., 2020). Depletion of Cav-1 increases the levels of phosphoinositide-4,5-bisphosphate (PtdlIns(4,5)P2), recruits the F-actin elongation factor FMNL2 to the cortex for F-actin bundling, which then increases steady-state tensile stresses in epithelial monolayers (Teo et al., 2020). These results suggest that Cav-1 can regulate active mechanical tension for epithelial homeostasis by governing lipid signaling to actin cytoskeleton (Teo et al., 2020).
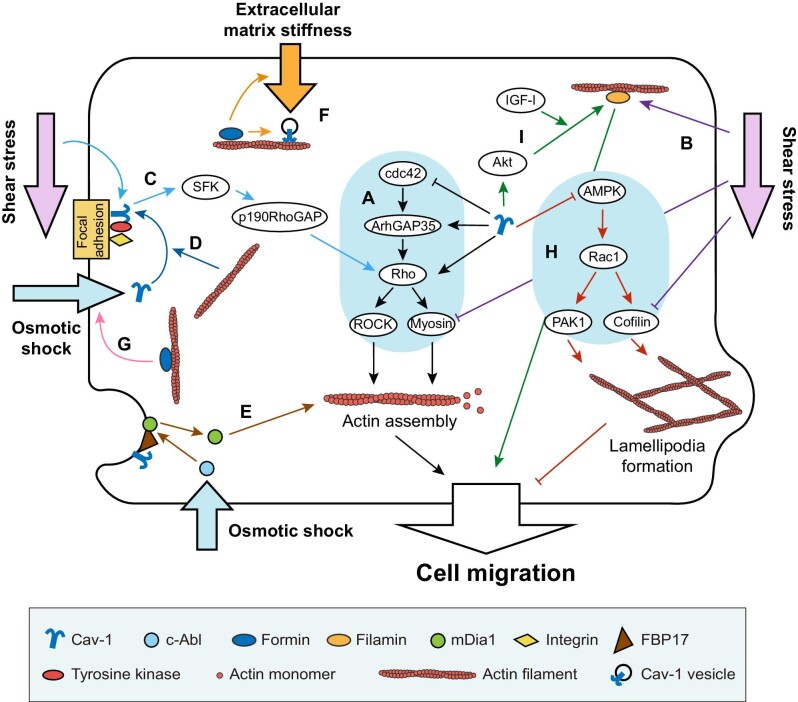
Signaling pathways have been identified involving in shear stress-induced actin remodeling (Figure 1). For example, disruption of cytoskeleton by ROCK inhibitor reduces actin stress fiber formation and cell proliferation, which can be rescued by LSS treatment in control cells but not in Cav-1-depleted cells, suggesting that LSS triggers events by Cav-1-mediated ROCK/p-MLC pathways (Figure 1A and B; Xiong et al., 2017a). In addition, Src-family kinase (SFK) is considered as a key part in Cav-1-mediated mechanosensing and actin reorganization (Grande-García et al., 2007; Yang et al., 2011). Acute shear stress results in integrin-dependent tyrosine phosphorylation of Cav-1 via p38 mitogen-activated protein kinase and SFK (Figure 1C and D; Volonté et al., 2001). This integrin/Cav-1 mechanosignaling complex temporally regulates RhoA activity, which is critical for the actin cytoskeleton reorganization to maintain long-term adaptiveness to shear stress (Yang et al., 2011). However, depletion of p190RhoGAP, a downstream signaling molecule of SFK, or depletion of Cav-1 disturbs temporal regulation of RhoA activity, thereby attenuating actin organization induced by flow (Yang et al., 2011). These findings reveal that p190RhoGAP links β1 integrin–Cav-1 complex to RhoA in a mechanotransduction cascade, which plays a role in endothelial adaptation to flow (Figure 1C; Yang et al., 2011).
Figure 1.
Interactions between actin filaments and Cav-1 and their effects on cell migration and mechanosensing. (A) Black arrows show that Cav-1 regulates the cdc42–ArhGAP35–Rho–myosin signaling pathway to facilitate or inhibit actin filament assembly and cell migration. (B) Purple arrows show that LSS upregulates filamin A and downregulates p-MLC and cofilin to facilitate cell migration. (C) Light blue arrows show that the integrin/Cav-1 complex senses shear stress and regulates downstream SFK–p190RhoGAP–RhoA signaling pathway to alter actin assembly. (D) Dark blue arrows show that actin facilitates Cav-1 localizing to focal adhesion and phosphorylated by tyrosine kinase to sense osmotic shock. (E) Brown arrows show that FBP17 functions as a bridge between caveolae mechanosensing of osmotic shock and the change of mDia1 localization from plasma membrane to cytoplasm to regulate actin assembly. (F) Orange arrows show that formin regulates organization and dynamics of Cav-1 vesicles and affects mechanosensation of extracellular matrix stiffness by Cav-1. (G) Pink arrow shows that formin affects mechanosensation of osmotic shock by interacting with Cav-1. (H) Red arrows show that Cav-1 downregulates the AMPK–Rac1–PAK1/cofilin signaling pathway to inhibit unregulated lamellipodia formation to ensure directional cell migration. (I) Green arrows show that Cav-1 regulates filamin expression and cell migration through Akt in an IGF-I-dependent manner.
Moreover, caveolae can cooperate with the membrane curvature regulator formin-binding protein 17 (FBP17) to contribute to cell mechanoprotection (Figure 1E). By Cav-1 inward trafficking assay, researchers found that FBP17 colocalizes with Cav-1, regulates inward trafficking of Cav-1, and induces the formation of caveolar rosettes (Echarri et al., 2019). Depletion of FBP17 results in Cav-1 moving away from the plasma membrane at a lower rate than control, indicating a defect in the early stages of caveolae redistribution (Echarri et al., 2019). Furthermore, FBP17 depletion and Cav-1 depletion increase the sensitivity of cells to prolonged mechanical stretching treatments like osmotic shock (Echarri et al., 2019). Besides, FBP17 inhibits mDia1-mediated actin polymerization and reduces stress fiber and membrane bending (Figure 1E; Echarri et al., 2019). Combined with the phenomenon that the density of stress fiber positively correlates with plasma membrane tension increase (Burridge and Wittchen, 2013), these results uncover part of the basic mechanisms for cells to sense mechanical cues through caveolae and subsequently regulate actin cytoskeleton to adapt to extracellular mechanical environment (Echarri et al., 2019).
Apart from being the downstream effector of caveolae, actin and actin-associated proteins can inversely regulate Cav-1 in cell mechanosensing. Oligomers of the ATPase EHD2 restrict caveolae to the plasma membrane through association with actin cortex (Stoeber et al., 2012). Besides, scaffold protein IQGAP1 and its downstream mDia1 are recruited to actin cortex to promote caveolin trafficking to the plasma membrane, which allows stable insertion of caveolae into the plasma membrane (Wickström et al., 2010). Complete depolymerization of actin cyto-skeleton by latrunculin A triggers rapid and massive movements of caveolin-positive structures, suggesting that actin cytoskeleton is important for caveolar membrane traffic (Mundy et al., 2002). Our recent work explored the relationship between actin nucleator formins and Cav-1. Depletion of formin proteins FHOD1 and Dia1 results in enlarged size, decreased number, and slower move-ment velocity of Cav-1 vesicles (Shi et al., 2021a). Furthermore, formin proteins are proved to be substantial for the tension-sensing function of Cav-1 when cells grow on softer matrix or are challenged by hypo-osmotic shock (Figure 1F and G; Shi et al., 2021a). These results indicate that Cav-1 and actin cytoskeleton influence each other in the process of cell response to mechanical stress.
Cav-1 regulates cell migration by interacting with actin cytoskeleton
Shear stress-induced cancer cell migration is a key step of tumor metastasis (Qin et al., 2021). Cav-1 is involved in many signaling pathways in cancer cell metabolism (Nwosu et al., 2016). Several studies reported that cancer cell migration is related to the expression level of Cav-1. For instance, Cav-1 expression is increased in hepatic stellate cells upon liver cirrhosis (Yokomori et al., 2002). Overexpression of Cav-1 could increase the fluorescence intensity of actin, reorganize F-actin cytoskeleton, change cell morphology, and promote cell migration and cell proliferation in murine liver (GRX) cells (Ilha et al., 2019). These results indicate that interconnection between Cav-1 expression and actin filaments contributes to hepatic stellate cell activation, an important process of liver fibrosis by producing and altering the extracellular matrix in acute injury of the liver (Ilha et al., 2019). Analogously, increased Cav-1 level can promote the metastasis of rhabdomyosarcoma to the lung (Codenotti et al., 2019). Depletion of Cav-1 compromises lung metastasis of bladder cancer cells (Thomas et al., 2011), indicating that Cav-1 expression positively correlates with cancer cell motility and metastasis. Apart from cancer cells, Cav-1 expression is also associated with microglial morphology and activity. The expression level of Cav-1 is lower in inactivated microglia cells whereas increases in activated microglia cells. Depletion of Cav-1 reduces overall migration of mouse microglia BV2 cells, indicating an important role of Cav-1 in microglia activation and providing a promising novel therapeutic target in central nervous system injury or disease (Niesman et al., 2013).
Stress fibers are contractile actomyosin bundles in non-muscle cells and provide forces for cell migration. The interactions between Cav-1 and stress fibers have been demonstrated to regulate cancer cell migration in several studies. Our recent work found that contractile myosin bundles are essential for the organization and dynamics of cytoplasmic Cav-1. Cav-1 vesicles display actin-associated motility by sliding along actin filaments or coupling to do retrograde flow with stress fibers in human osteosarcoma (U2OS) cells (Shi et al., 2021b). Inhibition of stress fiber reduces the phosphorylation level of Cav-1 on site Tyr14, enlarges size, and decreases motility of Cav-1 vesicles (Shi et al., 2021b). In turn, Cav-1 expression is necessary for stress fiber formation. In metastatic bladder cancer UMUC-3 cells, Cav-1 depletion reduces activation levels of RhoA, RhoC, and the Rho effector ROCK1, which further results in a significant loss of actin stress fibers and compromised cell migration, suggesting that Cav-1 modulates actin reorganization and promotes cancer cell dissemination through regulating Rho activity (Figure 1A; Thomas et al., 2011). Similar phenomenon was also found in U2OS cells. Phospho-deficiency or depletion of Cav-1 compromises the contractile myosin bundles by deactivating RhoA-dependent myosin phosphorylation, results in enhanced lamellipodia formation by activating adenosine monophosphate-activated protein kinase (AMPK) followed by Rac1-dependent p21-activated kinase 1 (PAK1) and cofilin phosphorylation, and further leads to a failure of the establishment of polarized cell morphology and directional cell migration (Figure 1A and H; Shi et al., 2021b). In human metastatic melanoma Me665/1 cells, Cav-1 deficiency results in the imbalance of actin cytoskeleton equilibrium achieved by RhoA and Rac1/cdc42 counteracting activities and subsequently delays the cell spreading (Figure 1A and H; Fecchi et al., 2012). In addition, Cav-1 overexpression promotes the metastatic progression of embryonal rhabdomyosarcoma in an Erk-dependent way and also leads to increased stress fiber and reorganized actin cytoskeleton (Codenotti et al., 2019). These results indicate that Cav-1 and stress fibers regulate each other to maintain proper functions and promote metastasis. The involved signaling pathways of Cav-1 and actin filaments and their effects on cell migration are summarized in Figure 1.
Actin-associated proteins also interact with Cav-1 during cell migration. Cav-1 is connected to stress fibers through the actin filament crosslinker filamin A (Stahlhut and van Deurs, 2000; Muriel et al., 2011). Overexpression of Cav-1 increases the mRNA and protein levels of filamin A, and enhances insulin-like growth factor I (IGF-I)-dependent MCF-7 cell migration through Akt (Figure 1I; Ravid et al., 2008). Flotillins are peripherally membrane-associated proteins, which can interact with F-actin and thus link rafts to cytoskeleton (Langhorst et al., 2007). A study revealed that Cav-1 colocalizes with flotillin and α-actinin, another F-actin-crosslinking protein, both of which are enriched in lipid rafts and play an important role in cell migration and axon ensheathment (Campos et al., 2021). Interactions between Cav-1 and actin-associated proteins and their effects on cell migration are also summarized in Figure 1.
On the other hand, Cav-1 expression can inhibit cell migration and re-epithelialization of non-healing wounds. Cav-1 was found localized to basal keratinocytes and spatiotemporally downregulated during acute wound healing (Jozic et al., 2019; Sawaya et al., 2019). Cav-1 antagonizes the glucocorticoid receptor repressor ArhGAP35, which increases activation of RhoA and diminishes activation of cdc42 (Figure 1A; Jozic et al., 2021). Increase of Cav-1 expression level contributes to impaired actin-cytoskeletal signaling and further leads to aberrant keratinocyte migration, which prevents the wound closure (Jozic et al., 2021). The opposite regulation of Cav-1 on cell migration indicates that the role of Cav-1 appears to vary with cell types, microenvironmental factors, and pathology conditions.
Interactions between Cav-1 and vimentin IFs in terms of cell mechanosensing and migration
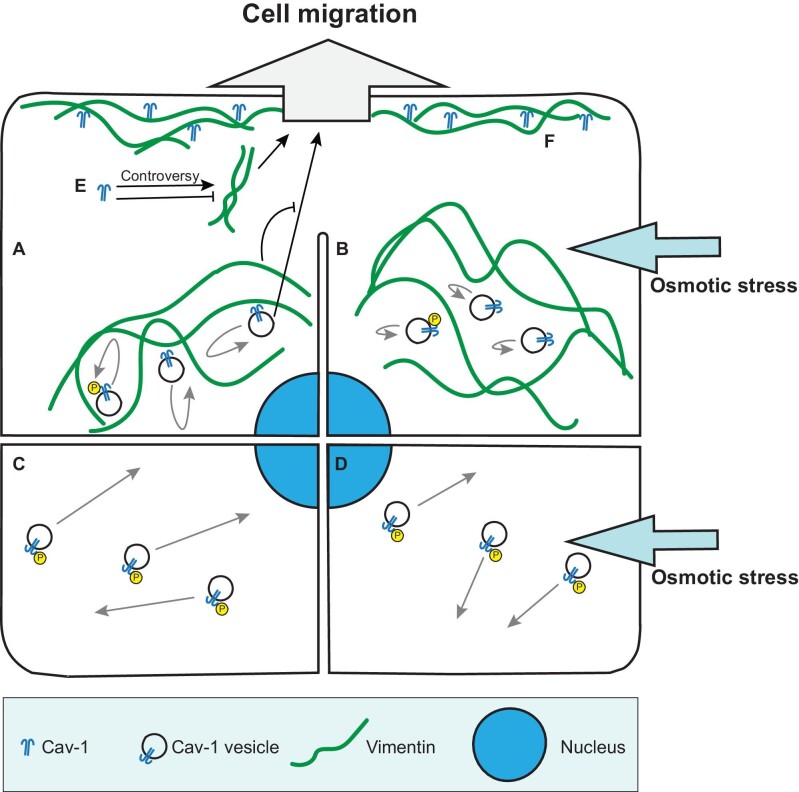
Interactions between Cav-1 and IFs are less studied compared to that between Cav-1 and actin cytoskeleton. Several studies reported interactions or regulations between Cav-1 and IF proteins including keratin (Sotgia et al., 2005; Yamaguchi et al., 2015), nestin (Chen et al., 2021), and vimentin (Kamibeppu et al., 2018; Sun et al., 2020). Among them, only interactions between vimentin IFs and Cav-1 are discussed in the context of mechanosensing and cell migration. Vimentin IFs are important players in the migration process and cell mechanoprotection. Absence of vimentin impairs cellular mechanical stability, migration, and contractile capacity (Eckes et al., 1998; Brown et al., 2001). Recent studies have revealed the role of vimentin in Cav-1-mediated cell migration and mechanosensing, as discussed below (Figure 2).
Figure 2.
Interactions between IFs and Cav-1 and their effects on cell migration and mechanosensing. (A) Vimentin functions as physical barriers to constrain movement of Cav-1 vesicles and inhibits Cav-1-promoted cell migration. (B) Osmotic stress reduces the motility of Cav-1 vesicles. (C) Depletion of vimentin results in increased motility and phosphorylation of Cav-1. (D) Depletion of vimentin partially rescues the reduced motility of Cav-1 vesicles in cells under osmotic stress. (E) Cav-1 regulates vimentin expression level to alter cell migration. Whether Cav-1 upregulates or downregulates vimentin expression remains controversial and may depend on cell types and experimental systems. (F) Cav-1 and vimentin colocalize at the front of cells and interact with each other during three-dimensional cell migration.
Several studies pointed out that Cav-1 affects cell migration through regulating the expression level of vimentin. Cav-1 and vimentin are representative epithelial–mesenchymal transition biomarkers of metastatic tumors (Chanvorachote et al., 2014; Yu et al., 2016; Xiong et al., 2017b). Silence of Cav-1 increases vimentin expression and cell migration rate, which promotes cell metastasis in head and neck squamous cell carcinoma (Sun et al., 2020). However, different phenomenon is observed in prostate cancer PC3 cells, in which Cav-1 knockdown decreases mRNA level of vimentin and downregulates cell motility (Kamibeppu et al., 2018). These data suggest that the expression level of vimentin is positively correlated with cell motility, but the relationship between Cav-1 expression and vimentin may vary with cell types. The underlying mechanisms and involved signaling pathways still require further investigation.
In turn, vimentin can also regulate characteristics of Cav-1. Previous study found that Cav-1 and vimentin colocalize and interact with each other, both polarizing and accumulating at the front of transmigrating bovine aortic endothelial cells (Santilman et al., 2007). Vimentin deficiency leads to patch and diffusion of Cav-1 instead of polarized distribution, suggesting that the interaction between Cav-1 and vimentin is required for Cav-1 anterior polarization in transmigrating cells (Santilman et al., 2007). Besides, vimentin filaments can affect the motility of cytoplasmic Cav-1 vesicles. In our work, vimentin IFs function as a physical barrier to restrain movement of the vesicles during the intracellular trafficking of Cav-1 in U2OS cells (Jiu, 2018). Depletion of vimentin promotes the release of vimentin-associated Cav-1 vesicles, and then increases the motility of Cav-1 vesicles (Jiu, 2018). These results reveal a negative role of vimentin IFs in regulating the trafficking of intracellular Cav-1 vesicles, which is crucial for caveolae rapid assembly and disassembly in response to mechanical stress (Sinha et al., 2011). Moreover, Tyr14 residue of Cav-1, a substrate for SFK, is necessary for binding to vimentin (Santilman et al., 2007). Vimentin depletion significantly increases the level of phosphorylation on Cav-1 Tyr14 site without influencing total protein level of Cav-1, which partially recovers the reduced intracellular motility of Cav-1 vesicles upon hypo-osmotic shock (Jiu, 2018; Shi et al., 2020), suggesting that vimentin is associated with Cav-1-mediated cell mechanoprotection. To sum up, vimentin can interact with Cav-1 and regulate its cellular localization, motility, and phosphorylation to participate in cell migration and cell mechanosensing.
Concluding remarks
In summary, the studies discussed here suggest that mechanical stimulation can induce cytoskeletal reorganization and change cell migration in a Cav-1-dependent manner, indicating that cells use similar mechanisms for mechanoprotection and mechanotransduction. On the one hand, the expression level and phosphorylation at Tyr14 of Cav-1 influence contractile actin bundle organization by interacting with actin-associated proteins or regulating several signaling pathways such as ROCK, Rho, and Src. On the other hand, the complete actin network ensures the dynamic movement of Cav-1 vesicles. Our studies on the interactions between Cav-1 and actin network differentiate themselves from previous reports: (i) we identified actin nucleator formin proteins FHOD1 and mDia1 as indispensable factors to maintain the proper mechanosensing function of Cav-1; (ii) we decoded the underlying molecular mechanisms that Cav-1 expression influences AMPK activation, followed by Rac-1-dependent PAK1/cofilin phosphorylation, and subsequently affects lamellipodia formation and distribution. From our point of view, there are still three major challenges in studying the interactions between Cav-1 and actin network: (i) further detailed studies on the underlying molecular mechanisms of crosstalk among the known signaling pathways; (ii) further investigation on the interactions between Cav-1 and actin-associated proteins; and (iii) in-depth studies on how interactions between Cav-1 and F-actin regulate mechanosensing and cell migration in the context of related diseases, such as cancer metastasis and hypertension-derived diseases. Different cell types and animal models, microenvironmental factors, and patho-logy conditions need to be carefully considered and modulated to gain new insights into potential therapies.
There are few studies focusing on the interactions between Cav-1 and IFs in cell migration and mechanosensing. Cav-1 can influence cell migration by regulating the expression of vimentin protein. In turn, vimentin IFs can influence cell behavior through the regulation of distribution and motility of Cav-1 vesicles. Our studies on the interactions between Cav-1 and vimentin also differentiate themselves from previous reports: (i) we discovered vimentin functioning as a barrier to physically affect the intracellular motility of Cav-1 vesicles; (ii) we explored the effects of vimentin on Cav-1 expression and cell migration; (iii) we revealed the regulation of mechanosensing by vimentin and Cav-1 interactions. The primary unsolved challenge is the in-depth study of molecular mechanisms underlying interactions between IFs (especially IF proteins other than vimentin) and Cav-1, as well as their regulation on cell mechanosensing and migration. Given that vimentin is an important marker of tumors, the interactions between Cav-1 and vimentin may provide a potential perspective in cancer studies.
Contributor Information
Daijiao Tang, Unit of Cell Biology and Imaging Study of Pathogen Host Interaction, The Center for Microbes, Development and Health, CAS Key Laboratory of Molecular Virology and Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai 200031, China; University of Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing 100049, China.
Yue Zhang, Unit of Cell Biology and Imaging Study of Pathogen Host Interaction, The Center for Microbes, Development and Health, CAS Key Laboratory of Molecular Virology and Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai 200031, China; University of Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing 100049, China.
Jie Mei, Unit of Cell Biology and Imaging Study of Pathogen Host Interaction, The Center for Microbes, Development and Health, CAS Key Laboratory of Molecular Virology and Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai 200031, China; University of Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing 100049, China.
Jing Zhao, Unit of Cell Biology and Imaging Study of Pathogen Host Interaction, The Center for Microbes, Development and Health, CAS Key Laboratory of Molecular Virology and Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai 200031, China.
Chenglin Miao, Unit of Cell Biology and Imaging Study of Pathogen Host Interaction, The Center for Microbes, Development and Health, CAS Key Laboratory of Molecular Virology and Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai 200031, China; University of Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing 100049, China.
Yaming Jiu, Unit of Cell Biology and Imaging Study of Pathogen Host Interaction, The Center for Microbes, Development and Health, CAS Key Laboratory of Molecular Virology and Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai 200031, China; University of Chinese Academy of Sciences, Chinese Academy of Sciences, Beijing 100049, China.
[This work was supported by the National Key Research and Development Program of China (2022YFC2303502 and 2021YFC2300204), the National Natural Science Foundation of China (32222022, 92054104, and 31970660), CAS–VPST Silk Road Science Fund (GJHZ2021138), and Shanghai Municipal Science and Technology Major Project (2019SHZDZX02). D.T. made the initial draft and drew the figures. D.T. and Y.Z. made the revised draft. J.M. modified the manuscript and figures. C.M. and J.Z. modified the manuscript. Y.J. conceived the study, made the outline, and wrote the manuscript.]
References
- Brown M.J., Hallam J.A., Colucci-Guyon E.et al. (2001). Rigidity of circulating lymphocytes is primarily conferred by vimentin intermediate filaments. J. Immunol. 166, 6640–6646. [DOI] [PubMed] [Google Scholar]
- Burridge K., Wittchen E.S. (2013). The tension mounts: stress fibers as force-generating mechanotransducers. J. Cell Biol. 200, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos F.S.O., Piña-Rodrigues F.M., Reis A.et al. (2021). Lipid rafts from olfactory ensheathing cells: molecular composition and possible roles. Cell. Mol. Neurobiol. 41, 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanvorachote P., Pongrakhananon V., Chunhacha P. (2014). Prolonged nitric oxide exposure enhances anoikis resistance and migration through epithelial–mesenchymal transition and caveolin-1 upregulation. Biomed. Res. Int. 2014, 941359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Cai J., Wang J.et al. (2021). Targeting Nestin+ hepatic stellate cells ameliorates liver fibrosis by facilitating TβRI degradation. J. Hepatol. 74, 1176–1187. [DOI] [PubMed] [Google Scholar]
- Chen Y., Ju L., Rushdi M.et al. (2017). Receptor-mediated cell mechanosensing. Mol. Biol. Cell 28, 3134–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codenotti S., Faggi F., Ronca R.et al. (2019). Caveolin-1 enhances metastasis formation in a human model of embryonal rhabdomyosarcoma through Erk signaling cooperation. Cancer Lett. 449, 135–144. [DOI] [PubMed] [Google Scholar]
- Del Pozo M.A., Lolo F.N., Echarri A. (2021). Caveolae: mechanosensing and mechanotransduction devices linking membrane trafficking to mechanoadaptation. Curr. Opin. Cell Biol. 68, 113–123. [DOI] [PubMed] [Google Scholar]
- Discher D.E., Janmey P., Wang Y.L. (2005). Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143. [DOI] [PubMed] [Google Scholar]
- Echarri A., Muriel O., Pavón D.M.et al. (2012). Caveolar domain organization and trafficking is regulated by Abl kinases and mDia1. J. Cell Sci. 125(Pt 13), 3097–3113. [DOI] [PubMed] [Google Scholar]
- Echarri A., Pavón D.M., Sánchez S.et al. (2019). An Abl–FBP17 mechanosensing system couples local plasma membrane curvature and stress fiber remodeling during mechanoadaptation. Nat. Commun. 10, 5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckes B., Dogic D., Colucci-Guyon E.et al. (1998). Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J. Cell Sci. 111, 1897–1907. [DOI] [PubMed] [Google Scholar]
- Fecchi K., Travaglione S., Spadaro F.et al. (2012). Human melanoma cells express FGFR/Src/Rho signaling that entails an adhesion-independent caveolin-1 membrane association. Int. J. Cancer 130, 1273–1283. [DOI] [PubMed] [Google Scholar]
- Feng H., Guo W., Han J.et al. (2013). Role of caveolin-1 and caveolae signaling in endotoxemia and sepsis. Life Sci. 93, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher D.A., Mullins R.D. (2010). Cell mechanics and the cytoskeleton. Nature 463, 485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Liu G., Halim A.et al. (2019). Mesenchymal stem cell migration and tissue repair. Cells 8, 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetani R., Zizzi E.A., Deriu M.A.et al. (2020). When stiffness matters: mechano-sensing in heart development and disease. Front. Cell Dev. Biol. 8, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargalionis A.N., Basdra E.K., Papavassiliou A.G. (2018). Tumor mechanosensing and its therapeutic potential. J. Cell. Biochem. 119, 4304–4308. [DOI] [PubMed] [Google Scholar]
- Golani G., Ariotti N., Parton R.G.et al. (2019). Membrane curvature and tension control the formation and collapse of caveolar superstructures. Dev. Cell 48, 523–538.e4. [DOI] [PubMed] [Google Scholar]
- Grande-García A., Echarri A., de Rooij J.et al. (2007). Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J. Cell Biol. 177, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilha M., Moraes K.D.S., Rohden F.et al. (2019). Exogenous expression of caveolin-1 is sufficient for hepatic stellate cell activation. J. Cell. Biochem. 120, 19031–19043. [DOI] [PubMed] [Google Scholar]
- Jiu Y. (2018). Vimentin intermediate filaments function as a physical barrier during intra-cellular trafficking of caveolin-1. Biochem. Biophys. Res. Commun. 507, 161–167. [DOI] [PubMed] [Google Scholar]
- Jozic I., Abujamra B.A., Elliott M.H.et al. (2021). Glucocorticoid-mediated induction of caveolin-1 disrupts cytoskeletal organization, inhibits cell migration and re-epithelialization of non-healing wounds. Commun. Biol. 4, 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozic I., Sawaya A.P., Pastar I.et al. (2019). Pharmacological and genetic inhibition of caveolin-1 promotes epithelialization and wound closure. Mol. Ther. 27, 1992–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamibeppu T., Yamasaki K., Nakahara K.et al. (2018). Caveolin-1 and -2 regulate cell moti-lity in castration-resistant prostate cancer. Res. Rep. Urol. 10, 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassianidou E., Kumar S. (2015). A biomechanical perspective on stress fiber structure and function. Biochim. Biophys. Acta 1853, 3065–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozera L., White E., Calaghan S. (2009). Caveolae act as membrane reserves which limit mechanosensitive I(Cl,swell) channel activation during swelling in the rat ventricular myocyte. PLoS One 4, e8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhorst M.F., Solis G.P., Hannbeck S.et al. (2007). Linking membrane microdomains to the cytoskeleton: regulation of the lateral mobility of reggie-1/flotillin-2 by interaction with actin. FEBS Lett. 581, 4697–4703. [DOI] [PubMed] [Google Scholar]
- Li X.A., Everson W.V., Smart E.J. (2005). Caveolae, lipid rafts, and vascular disease. Trends Cardiovasc. Med. 15, 92–96. [DOI] [PubMed] [Google Scholar]
- Mak M., Spill F., Kamm R.D.et al. (2016). Single-cell migration in complex microenvironments: mechanics and signaling dynamics. J. Biomech. Eng. 138, 021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino F., Perestrelo A.R., Vinarský V.et al. (2018). Cellular mechanotransduction: from tension to function. Front. Physiol. 9, 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy D.I., Machleidt T., Ying Y.S.et al. (2002). Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J. Cell Sci. 115, 4327–4339. [DOI] [PubMed] [Google Scholar]
- Muriel O., Echarri A., Hellriegel C.et al. (2011). Phosphorylated filamin A regulates actin-linked caveolae dynamics. J. Cell Sci. 124, 2763–2776. [DOI] [PubMed] [Google Scholar]
- Niesman I.R., Zemke N., Fridolfsson H.N.et al. (2013). Caveolin isoform switching as a molecular, structural, and metabolic regulator of microglia. Mol. Cell. Neurosci. 56, 283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwosu Z.C., Ebert M.P., Dooley S.et al. (2016). Caveolin-1 in the regulation of cell metabolism: a cancer perspective. Mol. Cancer 15, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R.G., Pozo M.A. (2013). Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 14, 98–112. [DOI] [PubMed] [Google Scholar]
- Pelkmans L. (2005). Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim. Biophys. Acta 1746, 295–304. [DOI] [PubMed] [Google Scholar]
- Prakash S., Malshikare H., Sengupta D. (2022). Molecular mechanisms underlying caveolin-1 mediated membrane curvature. J. Membr. Biol. 255, 225–236. [DOI] [PubMed] [Google Scholar]
- Qin X., Zhang Y., He Y.et al. (2021). Shear stress triggered circular dorsal ruffles formation to facilitate cancer cell migration. Arch. Biochem. Biophys. 709, 108967. [DOI] [PubMed] [Google Scholar]
- Ravid D., Chuderland D., Landsman L.et al. (2008). Filamin A is a novel caveolin-1-dependent target in IGF-I-stimulated cancer cell migration. Exp. Cell Res. 314, 2762–2773. [DOI] [PubMed] [Google Scholar]
- Santilman V., Baran J., Anand-Apte B.et al. (2007). Caveolin-1 polarization in transmigrating endothelial cells requires binding to intermediate filaments. Angiogenesis 10, 297–305. [DOI] [PubMed] [Google Scholar]
- Sawaya A.P., Jozic I., Stone R.C.et al. (2019). Mevastatin promotes healing by targeting caveolin-1 to restore EGFR signaling. JCI Insight 4, e129320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Fan C., Jiu Y. (2020). Unidirectional regulation of vimentin intermediate filaments to caveolin-1. Int. J. Mol. Sci. 21, 7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Tang D., Xing Y.et al. (2021a). Actin nucleator formins regulate the tension-buffering function of caveolin-1. J. Mol. Cell Biol. 13, 876–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Wen Z., Wang Y.et al. (2021b). Feedback-driven mechanisms between phosphorylated caveolin-1 and contractile actin assemblies instruct persistent cell migration. Front. Cell Dev. Biol. 9, 665919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B., Köster D., Ruez R.et al. (2011). Cells respond to mechanical stress by rapid dis-assembly of caveolae. Cell 144, 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotgia F., Williams T.M., Cohen A.W.et al. (2005). Caveolin-1-deficient mice have an increased mammary stem cell population with upregulation of Wnt/β-catenin signaling. Cell Cycle 4, 1808–1816. [DOI] [PubMed] [Google Scholar]
- Stahlhut M., van Deurs B. (2000). Identification of filamin as a novel ligand for caveolin-1: evidence for the organization of caveolin-1-associated membrane domains by the actin cytoskeleton. Mol. Biol. Cell 11, 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeber M., Stoeck I.K., Hänni C.et al. (2012). Oligomers of the ATPase EHD2 confine caveolae to the plasma membrane through association with actin. EMBO J. 31, 2350–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Lu Y., Yu C.et al. (2020). Involvement of the TGF-β1 pathway in caveolin-1-associated regulation of head and neck tumor cell metastasis. Oncol. Lett. 19, 1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoger M., Gupta S., Charrier E.E.et al. (2022). Vimentin intermediate filaments mediate cell morphology on viscoelastic substrates. ACS Appl. Bio. Mater. 5, 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teo J.L., Gomez G.A., Weeratunga S.et al. (2020). Caveolae control contractile tension for epithelia to eliminate tumor cells. Dev. Cell 54, 75–91.e7. [DOI] [PubMed] [Google Scholar]
- Thomas S., Overdevest J.B., Nitz M.D.et al. (2011). Src and caveolin-1 reciprocally regulate metastasis via a common downstream signaling pathway in bladder cancer. Cancer Res. 71, 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrino S., Shen W.W., Blouin C.M.et al. (2018). EHD2 is a mechanotransducer connecting caveolae dynamics with gene transcription. J. Cell Biol. 217, 4092–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonté D., Galbiati F., Pestell R.G.et al. (2001). Cellular stress induces the tyrosine phosphorylation of caveolin-1 (Tyr14) via activation of p38 mitogen-activated protein kinase and c-Src kinase. Evidence for caveolae, the actin cytoskeleton, and focal adhesions as mechanical sensors of osmotic stress. J. Biol. Chem. 276, 8094–8103. [DOI] [PubMed] [Google Scholar]
- Wickström S.A., Lange A., Hess M.W.et al. (2010). Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev. Cell 19, 574–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbs T., Hammerschmidt S.I., Förster R. (2017). Dendritic cell migration in health and disease. Nat. Rev. Immunol. 17, 30–48. [DOI] [PubMed] [Google Scholar]
- Xiong J., Wang D., Wei A.et al. (2017b). Deregulated expression of miR-107 inhibits metastasis of PDAC through inhibition PI3K/Akt signaling via caveolin-1 and PTEN. Exp. Cell Res. 361, 316–323. [DOI] [PubMed] [Google Scholar]
- Xiong N., Li S., Tang K.et al. (2017a). Involvement of caveolin-1 in low shear stress-induced breast cancer cell motility and adhesion: roles of FAK/Src and ROCK/p-MLC pathways. Biochim. Biophys. Acta Mol. Cell Res. 1864, 12–22. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Watanabe Y., Watanabe T.et al. (2015). Decreased expression of caveolin-1 contributes to the pathogenesis of psoriasiform dermatitis in mice. J. Invest. Dermatol. 135, 2764–2774. [DOI] [PubMed] [Google Scholar]
- Yang B., Radel C., Hughes D.et al. (2011). p190 RhoGTPase-activating protein links the β1 integrin/caveolin-1 mechanosignaling complex to RhoA and actin remodeling. Arterioscler. Thromb. Vasc. Biol. 31, 376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomori H., Oda M., Ogi M.et al. (2002). Enhanced expression of endothelial nitric oxide synthase and caveolin-1 in human cirrhosis. Liver 22, 150–158. [DOI] [PubMed] [Google Scholar]
- Yu S., Yan C., Yang X.et al. (2016). Pharmacoproteomic analysis reveals that metapristone (RU486 metabolite) intervenes E-cadherin and vimentin to realize cancer metastasis chemoprevention. Sci. Rep. 6, 22388. [DOI] [PMC free article] [PubMed] [Google Scholar]