Abstract
Rationale
The majority of chronic obstructive pulmonary disease (COPD) patients have chronic bronchitis, for which specific therapies are unavailable. Acquired cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction is observed in chronic bronchitis, but has not been proven in a controlled animal model with airway disease. Furthermore, the potential of CFTR as a therapeutic target has not been tested in vivo, given limitations to rodent models of COPD. Ferrets exhibit cystic fibrosis-related lung pathology when CFTR is absent and COPD with bronchitis following cigarette smoke exposure.
Objectives
To evaluate CFTR dysfunction induced by smoking and test its pharmacological reversal by a novel CFTR potentiator, GLPG2196, in a ferret model of COPD with chronic bronchitis.
Methods
Ferrets were exposed for 6 months to cigarette smoke to induce COPD and chronic bronchitis and then treated with enteral GLPG2196 once daily for 1 month. Electrophysiological measurements of ion transport and CFTR function, assessment of mucociliary function by one-micron optical coherence tomography imaging and particle-tracking microrheology, microcomputed tomography imaging, histopathological analysis and quantification of CFTR protein and mRNA expression were used to evaluate mechanistic and pathophysiological changes.
Measurements and main results
Following cigarette smoke exposure, ferrets exhibited CFTR dysfunction, increased mucus viscosity, delayed mucociliary clearance, airway wall thickening and airway epithelial hypertrophy. In COPD ferrets, GLPG2196 treatment reversed CFTR dysfunction, increased mucus transport by decreasing mucus viscosity, and reduced bronchial wall thickening and airway epithelial hypertrophy.
Conclusions
The pharmacologic reversal of acquired CFTR dysfunction is beneficial against pathological features of chronic bronchitis in a COPD ferret model.
Shareable abstract
These studies provide the first in vivo data evaluating CFTR potentiation in an experimental animal model of chronic bronchitis, an approach being pursued in COPD. Findings support targeting CFTR in COPD with drugs that potentiate CFTR.
Introduction
Chronic obstructive lung disease (COPD) is the fourth leading cause of death in the USA [1] and was estimated to be responsible for nearly $50 billion in healthcare costs in 2020 [2]. Current COPD management fails to alter its natural history or effectively address chronic cough and sputum production associated with the chronic bronchitis reported in up to 70% of COPD patients [3].
Inherited mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene cause cystic fibrosis (CF) [4]. COPD patients with chronic bronchitis, versus those with solely emphysema, the other classically defined COPD phenotype, exhibit pathological features comparable to CF [5–7], including goblet cell hyperplasia, mucin hyperexpression and mucus accumulation [8]. Cigarette smoke inhibits CFTR in vitro [9–14] and cigarette smoking induces acquired CFTR dysfunction even in the absence of congenital mutations [9–12, 15, 16]. Still, the contribution of acquired CFTR dysfunction to COPD pathogenesis remains unknown.
CFTR modulators restore CFTR function and have prominent effects on the treatment of CF lung disease, including improved mucociliary clearance [17], reduced mucus retention [18] and improved bacterial infection [19, 20], all prominent pathogenic features of chronic bronchitis [21]. Ivacaftor, a CFTR potentiator, restores CFTR activity in wild-type cells exposed to cigarette smoke and tissues from patients with COPD [10, 22]. Pilot studies have suggested CFTR potentiators may improve respiratory outcomes in patients with chronic bronchitis [23, 24]. However, these studies have not been definitive, necessitating animal studies to determine the role of acquired CFTR dysfunction and its treatment in COPD.
We previously reported that ferrets chronically exposed to cigarette smoke exhibit COPD and, uniquely as compared with rodents, features of chronic bronchitis, including mucus hyperexpression [25], delayed mucociliary clearance [26] and bronchial wall thickening by microcomputed tomography (μCT) imaging [27]. These features are associated with CFTR dysfunction in humans [28, 29]. Here, we evaluated smoke-exposed ferrets to determine the pharmacological activity of a CFTR potentiator as a therapy for COPD-related chronic bronchitis. We used GLPG2196 because, unlike ivacaftor, it activated wild-type ferret CFTR and it is a derivative of GLPG2451, an effective CFTR potentiator amenable to once-daily oral administration in people with CF [30].
Methods
Full experimental details are available in the supplementary material.
Ferret pharmacokinetics
Ferrets were administered GLPG2196 orally as a single oesophageal gavage at a dose of 5 or 30 mg·kg−1 and then anesthetised before whole blood samples were collected via the tail vein at 1, 3, 6 and 24 h post-administration for analysis.
Cigarette smoke exposure in ferrets, necropsy and histopathology, CFTR protein expression by immunoblot, RNA isolation, cDNA synthesis, reverse transcription PCR and reagents
Oral administration of CFTR modulators in a ferret model of COPD
Following 6 months of cigarette smoke exposure, ferrets were administered GLPG2196 (30 mg·kg−1) orally once daily for 1 month, followed by post-treatment assessments.
Ferret tracheal short-circuit current and nasal potential difference
Ion transport function of CFTR was assessed in primary ferret bronchial epithelial cells and ex vivo ferret trachea using short-circuit current (Isc) analysis and in anaesthetised ferrets using nasal potential difference (NPD), as detailed previously [31].
One-micron-resolution optical coherence tomography imaging
Video-rate micro-optical coherence tomography (μOCT) images of ferret tracheal explants were captured promptly after excision to analyse airway functional microanatomy [26, 32, 33].
Particle-tracking microrheology analysis
Ferret tracheal explants imaged by μOCT were subsequently plugged at each end for mucus accumulation followed by a 2-h treatment with carbachol (10 μM) and phenylephrine (20 μM; Sigma-Aldrich). Mucus was collected, and particle-tracking microrheology (PTM) was used to determine mucus viscosity [26, 34].
μCT imaging
Airway wall metrics of the right and left upper lobe were obtained using non-contrast μCT imaging of anaesthetised ferrets [27].
Statistics
Descriptive statistics are reported as mean±SEM unless indicated otherwise and compared using a t-test or ANOVA, as appropriate. All statistical tests were two-sided and performed at a 0.05 significance level. Scatter plots were used for individual data, unless the number per condition exceeded 50, when box and whisker plots were used to demonstrate variance.
Study approval
All animal protocols were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Results
Chronic cigarette smoke induces acquired CFTR dysfunction in ferrets
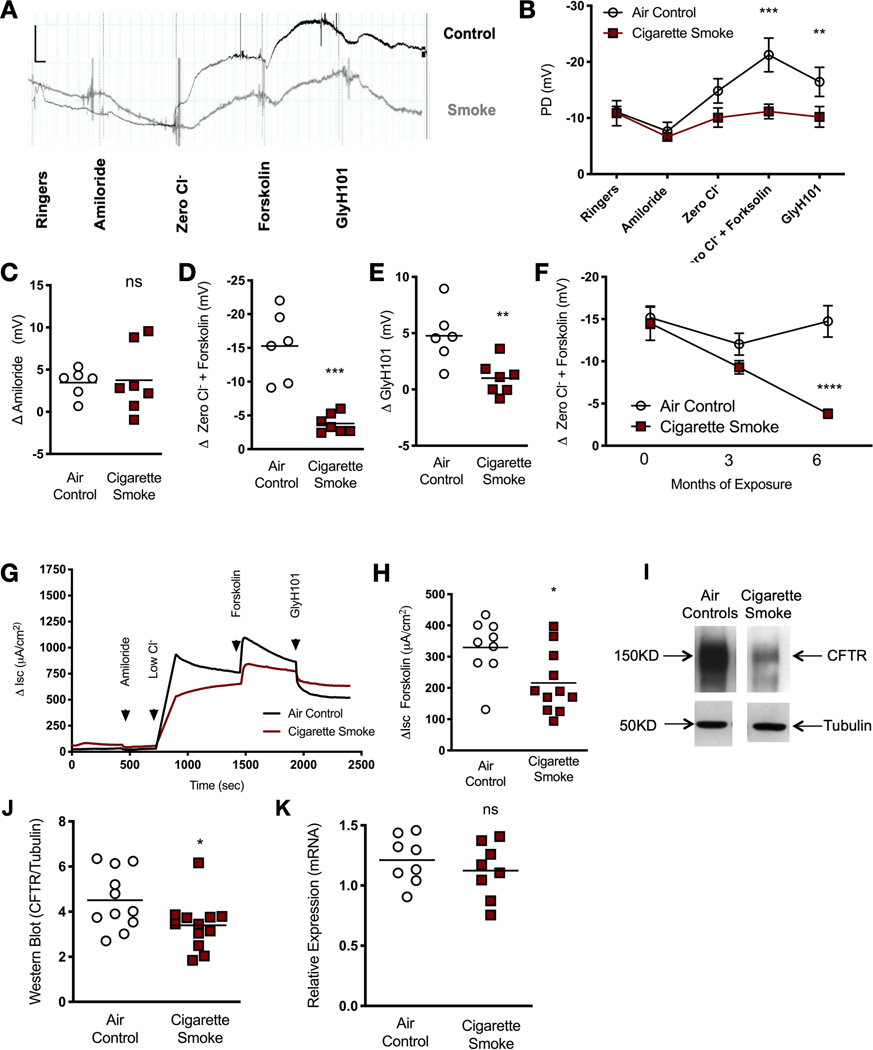
To determine the association of CFTR dysfunction with chronic bronchitis, wild-type ferrets were exposed to cigarette smoke for 6 months in a custom-designed, nose-only exposure system connected to an automated smoke generator, as previously described [31]. NPD, an electrophysiological measurement of ion transport and CFTR function applicable to ferrets [31], was performed at baseline and every 3 months. Representative NPD tracings at 6 months are shown in figure 1a and summary data in figure 1b–f. Smoke-exposed ferrets exhibited diminished CFTR-dependent changes in potential difference (PD) response to chloride-free conditions plus forskolin (figure 1a, b, d), including the response to the CFTR pore-blocking inhibitor GlyH101 (figure 1a, b, e). These changes occurred without an apparent effect on amiloride-sensitive PD (figure 1a–c), a measure of epithelial sodium channel activity, noting that amiloride-sensitive voltage changes in ferrets are known to be small [31]. The magnitude of the CFTR impairment (80.1% decrement ΔCl−-free Ringer’s + forskolin, p=0.0001) in comparison to controls was similar to that observed in cigarette smoke-exposed mice (71% reduction) and human smokers (53% reduction) [10, 12]. The effects of smoke exposure on CFTR-mediated ion transport depended on the duration of exposure, suggesting cumulative decrements over time and suggesting causality (figure 1f). These findings were corroborated by short-circuit current (ΔIsc) analysis (figure 1g, h), which demonstrated a depletion of forskolin-stimulated ΔIsc across the epithelium of tracheal tissue excised from ferrets exposed to smoke (215.8±30.1 μA·cm−2) versus air control (329.3±30.4 μA·cm−2, p=0.031, figure 1h). Functional deficits were accompanied by reduced CFTR protein expression in smoke-exposed ferrets as detected by immunoblot analysis of whole-lung homogenates (27.7% reduction, p=0.039, figure 1i, j). By contrast, CFTR mRNA expression was largely unaffected (figure 1k), consistent with an analysis of bronchial airway brushings from COPD patients [35]. In total, these data demonstrate that cigarette smoke causes an acquired CFTR dysfunction phenotype in ferrets, as also reported in humans with COPD [9–12].
FIGURE 1.
Cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction is caused by cigarette smoke exposure in ferrets. a) Representative nasal potential difference (NPD) tracings of ferrets exposed to whole cigarette smoke or room air via a nose-only exposure system for 6 months. Tracings represent mean potential difference (PD) following perfusion with Ringer’s, amiloride (100 μM), chloride-free forskolin (Zero Cl−) (20 μM), forskolin (10 μM) and GlyH101 (20 μM). b) Summary data of ferret NPD for ferrets in air control and smoke-exposed cohorts. Data are shown as mean±SEM. Significance was determined by two-way ANOVA with post hoc Tukey. c–e) The effect of smoke exposure on CFTR function in individual ferrets is shown by quantifying mean change in amiloride-sensitive (c), forskolin-stimulated (d) and GlyH101-inhibited (e) PD (n=7, air control and n=7, cigarette smoke, unpaired t-test). f) Temporal change in CFTR function measured by chloride-free forskolin. Data are shown as mean±SEM. Significance was determined by two-way ANOVA with post hoc Sidak; individual replicates at 6 months are shown in d. g) Representative short-circuit current (Isc) measurements of excised trachea from ferrets exposed to air or cigarette smoke (n=11) and treated with forskolin in the setting of amiloride. h) Mean change in Isc upon treatment with forskolin in tissues representing both experimental ferret groups (n=9, air control and n=11, cigarette smoke, Mann–Whitney U test). i) Representative images of immunoblot analysis of CFTR protein in whole-lung lysates of ferrets exposed to smoke or room air for 6 months. j) Summary of mean relative expression of CFTR normalised to the housekeeping protein tubulin (n=11, air control and n=12, cigarette smoke, unpaired t-test). k) Relative expression of ferret CFTR mRNA is shown for whole-lung lysates with and without smoke exposure (n=8 per group, Mann–Whitney U test). NS: not significant; *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001.
GLPG2196 stimulates wild-type CFTR in ferrets with and without cigarette smoke exposure
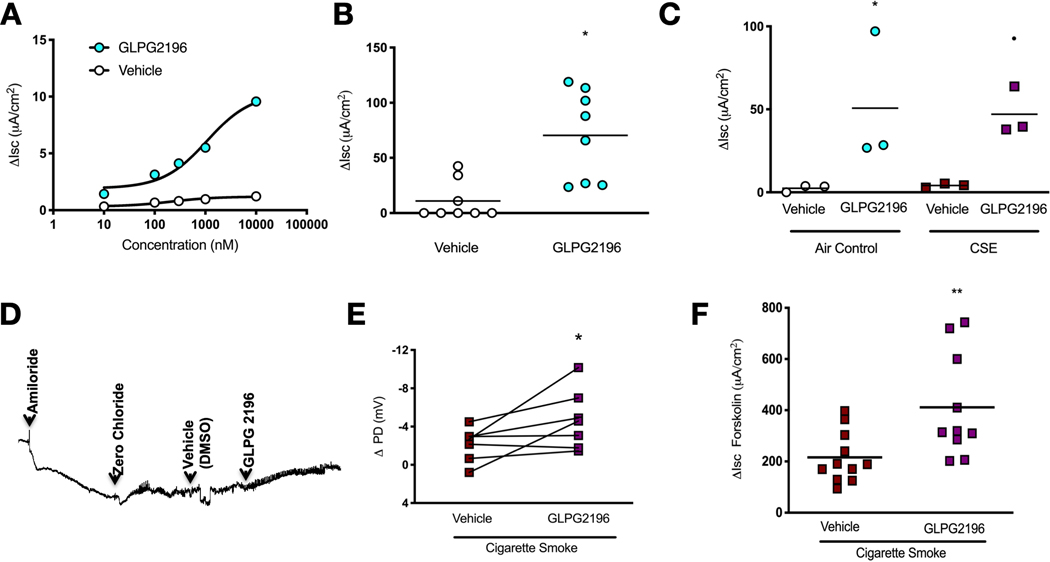
To assess the therapeutic potential and further establish causality, we sought to reverse CFTR dysfunction with a novel CFTR potentiator, GLPG2196. We used GLPG2196 because it is clinically relevant and ivacaftor was ineffective against wild-type ferret CFTR (supplementary figure S1), despite its known activity in ferrets engineered with the G551D-CFTR mutation [36]. As an initial test of GLPG2196, we performed electrophysiological measurements in ferrets to assess the efficacy of the CFTR potentiator GLPG2196 in ameliorating smoke-induced decrements in CFTR function. Using chamber analysis of well-differentiated ferret bronchial epithelial cells revealed that sequential addition of GLPG2196 up to a dose of 10 μM significantly activated CFTR-dependent Isc (figure 2a). In excised trachea from wild-type ferrets, GLPG2196 (10 μM) stimulated Isc (60.3±22.1 μA·cm−2) versus vehicle control (11.0±6.2 μA·cm−2, p=0.0186, figure 2b) in the absence of CFTR impairment due to smoke exposure. A similar increase was also observed in trachea after cigarette smoke extract was applied to induce acquired CFTR dysfunction (GLPG2196, 47.1±8.3 μA·cm−2 versus vehicle, 4.2±0.7 μA·cm−2; p=0.054; figure 2c). We then confirmed the activity of GLPG2196 in ferrets with acquired CFTR dysfunction by cigarette smoke exposure. NPD analysis showed GLPG2196 perfusion to the nasal mucosa stimulated CFTR-dependent PD changes in smoke-exposed ferrets (−4.7±1.2 mV) versus vehicle (−2.2±0.7 mV, p=0.0469, figure 2d, e). Further, acute addition of GLPG2196 after forskolin stimulation to trachea excised from smoke-exposed ferrets resulted in significantly elevated CFTR-dependent ΔIsc (411.1±64.2 μA·cm−2) compared to vehicle (215.8±30.1 μA·cm−2, p=0.006, figure 2f). These findings indicate GLPG2196 is efficacious in activating wild-type ferret CFTR even after antecedent cigarette smoke exposure, setting the stage for in vivo evaluation.
FIGURE 2.
GLPG2196 activates wild-type cystic fibrosis transmembrane conductance regulator (CFTR) in vitro, in vivo and ex vivo. a) Dose-response assessment of GLPG2196 compared to vehicle in well-differentiated primary ferret bronchial epithelial cells using Ussing chamber analysis. The experiment included serial addition of amiloride (100 μM), chloride secretory gradient with amiloride, followed by increasing concentrations of GLPG2196 or vehicle control (n=5 monolayers per group). b) Mean change in CFTR-dependent short-circuit current (Isc) in trachea excised from normal ferrets and treated with GLPG2196 (10 μM) or vehicle (n=8 monolayers, vehicle and n=8 monolayers, GLPG2196, Mann–Whitney U test). c) Mean change in CFTR-dependent Isc in trachea excised from normal ferrets and exposed to room air (air control) or cigarette smoke extract and treated with GLPG2196 (10 μM) or vehicle (n=3 monolayers per group, Kruskal–Wallis one-way ANOVA). d) Representative nasal potential difference (PD) tracing in ferrets exposed to cigarette smoke for 6 months followed by serial addition of amiloride (100 μM), chloride secretory gradient with amiloride, and vehicle control or GLPG2196 (10 μM). e) Summary data showing the effect of smoke exposure on CFTR function in smoke-exposed ferrets by quantifying change in PD. Each line represents a ferret (n=7 ferrets, Wilcoxon rank-sum test). f) Summary data depicting the change in CFTR-dependent Isc in response to forskolin in freshly isolated tracheal explants from ferrets exposed to cigarette smoke for 6 months. Trachea were treated with vehicle or GLPG2196 (10 μM) upon excision (n=11, vehicle and n=10, GLPG2196, Mann–Whitney U test). *: p<0.05; **: p<0.01.
Pharmacokinetics and drug study design
Pharmacokinetic analysis showed that GLPG2196 administered at 5 and 30 mg·kg−1 achieved adequate absorption and peak plasma concentration (Cmax) of ~900 ng·mL−1 and ~4100 ng·mL−1, respectively. Peak pharmacokinetic curves showed sustained plasma concentrations through 24 h, irrespective of ferret sex, allowing once-daily oral administration (supplementary figure S2). Micromolar-range concentrations were thought to be sufficient to exhibit bioactivity given in vitro and ex vivo studies in figure 1; thus, 30 mg·kg−1 was chosen for subsequent studies. Cigarette smoke-exposed ferrets were randomised to receive GLPG2196 or vehicle control for 28 days at 30 mg·kg−1 once daily by oral gavage while continuing daily smoke exposure. An additional air-control ferret group, matched for age and sex, was treated with a vehicle to compare drug efficacy.
GLPG2196 reversed airway remodelling in smoke-exposed ferrets
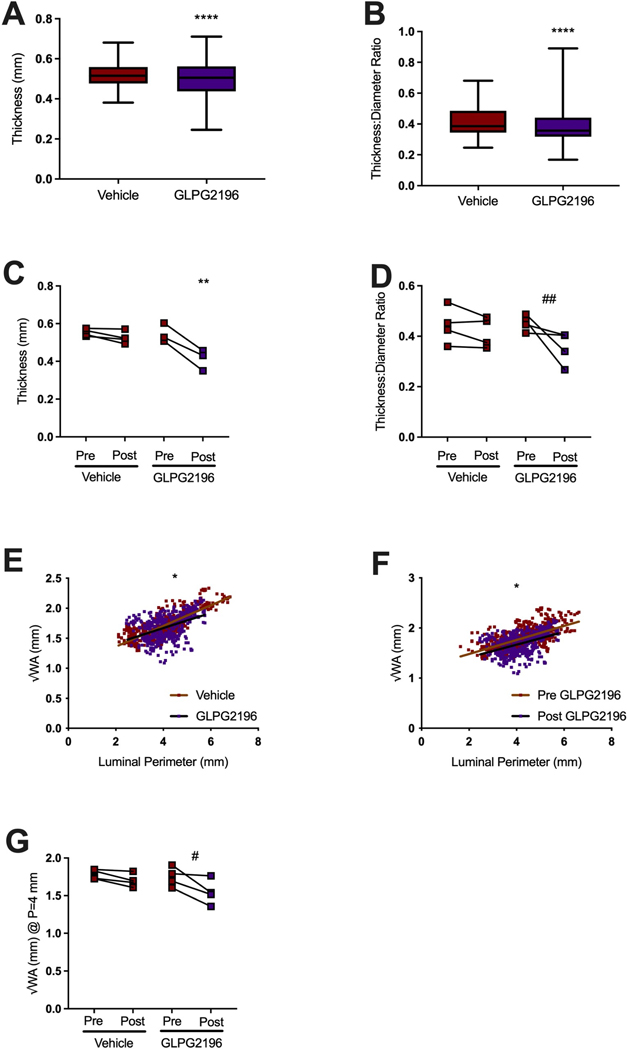
As seen in chronic bronchitis patients [7, 12, 14, 23], smoke-exposed ferrets demonstrate increased bronchial wall thickness (BWT) [27], indicative of mucus stasis, airway wall injury and remodelling [27]. Using a semi-automated high-resolution μCT image analysis [27], each animal was analysed for airway metrics of the fourth through sixth generation airway (presented as mean±SD of all images acquired) [37]. Smoke-exposed GLPG2196-treated ferrets had 4% decreased BWT (0.50±0.09 mm) compared to smoke-exposed vehicle-treated animals (0.52±0.06 mm, p<0.0001, figure 3a). Similarly, the ratio of BWT to luminal diameter (LD) (figure 3b) decreased by 10% in GLPG2196-treated (0.39±0.12) versus vehicle-treated (0.43±0.12, p<0.0001) ferrets. To evaluate this on an individual ferret basis, we analysed changes to ferret airways compared to baseline μCT findings. We found that 1 month of GLPG2916 treatment decreased BWT by 24% (0.41±0.05 mm) versus pre-treatment baseline values (0.55±0.05 mm, p=0.0019, figure 3c); this change was not evident in vehicle-treated ferrets. A trend towards decreased BWT/LD ratio (figure 3d) in GLPG2196-treated ferrets was not statistically significant (p=0.125).
FIGURE 3.
GLPG2196 reverses airway remodelling in smoke-exposed ferrets. Semi-automated measurements were derived from image slices (n~100) for each animal at one airway per apical lobe between the fourth and sixth. a) Semi-automated measurements of airway bronchial wall thickness (BWT) and b) BWT/luminal diameter (LD) ratio of smoke-exposed ferrets administered vehicle or GLPG2196. Comparisons by unpaired t-test. Data are shown as box and whisker plots with Tukey or minimum-to-maximum whiskers, where appropriate. c) Semi-automated measurements of BWT pre- and post-treatment with vehicle or GLPG2196 in smoke-exposed ferrets (n=3, ordinary one-way ANOVA with post hoc Tukey. Each line represents a ferret. d) Semi-automated measurements of BWT/LD ratio pre- and post-treatment with vehicle or GLPG2196 in smoke-exposed ferrets (n=4, Wilcoxon rank-sum test). e, f) Semi-automated scatter plots of the square root of BWT (√WA) versus airway luminal perimeter of a smoke-exposed vehicle-treated and a smoke-exposed GLPG2196-treated ferret (e) and of a smoke-exposed ferret pre- and post-treatment with GLPG2196 (f). Plots include data from n=4 ferrets per group. g) Semi-automated calculated √WA of the theoretical 4 mm perimeter airway (Pi4) for each smoke-exposed ferret pre- and post-treatment with vehicle or GLPG2196 (n=4, paired t-test). #: p=0.0629; ##: p=0.0125; *: p<0.05; **: p<0.01; ****: p<0.0001.
We recently reported an individualised measure of BWT in ferrets based on the standardised theoretical bronchial wall area in a ferret airway with a luminal perimeter of 4 mm (Pi4) as a sensitive indicator of cigarette smoke exposure [27]. This Pi4 measurement corresponds to the Pi10 measurement in humans, a proven biomarker of chronic bronchitis [38–41]. As seen in the scatter plots of the square root of BWT (√WA) versus luminal perimeter (figure 3e), linear regression analyses demonstrated significantly lower wall areas with GLPG2196 across luminal perimeters (p<0.05 for slope). Pre-treatment baseline scans compared to scans obtained after 1 month of GLPG2196 treatment also showed reduced BWT (figure 3f, p<0.05 for y-intercept). Summarised on an individual ferret basis, changes in Pi4 support improved BWT with GLPG2196 treatment (pre-GLPG2196, 1.75±0.13 mm versus post-GLPG2196, 1.54±0.17 mm; p=0.0629; figure 3g). We conclude that GLPG2196 reversed bronchial wall thickening, an indicator of airway remodelling in smoke-exposed ferrets.
GLPG2196 restores mucus transport and improves mucus viscosity
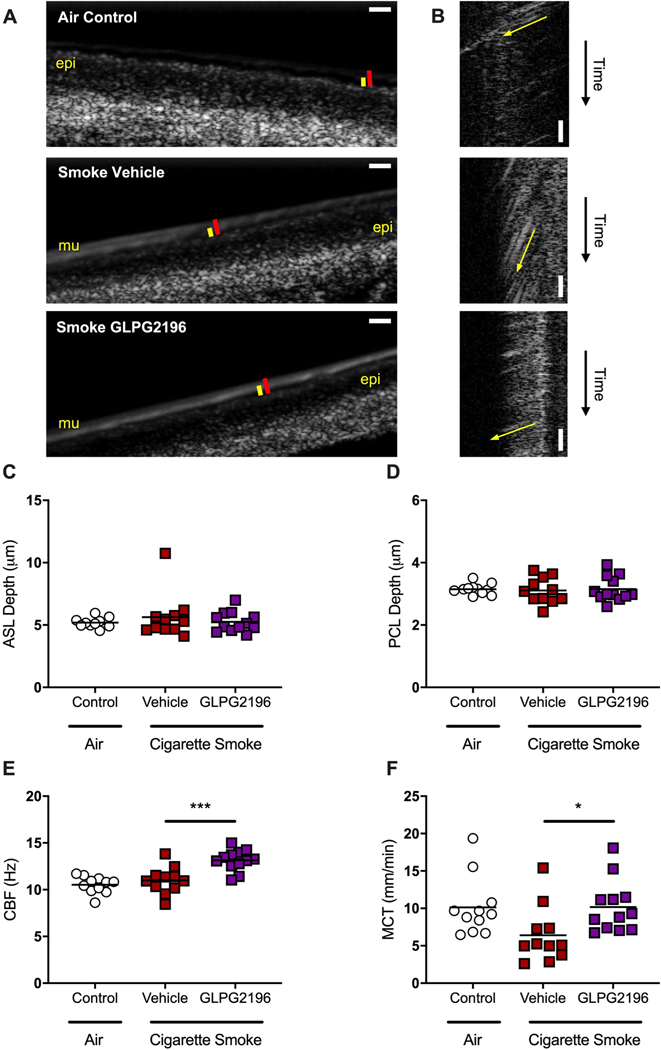
μOCT enables simultaneous evaluation of fundamental facets of CFTR-dependent airway function at the cellular level [32–34]. Prior studies showed decreased mucociliary transport (MCT) and elevated mucus viscosity in ferret tracheal explants after 6 months of smoke exposure [26]. Figure 4a and supplementary videos S1 and S2 depict representative μOCT images of excised ferret trachea after 28 days of treatment with GLPG2196 or vehicle or air controls; figure 4b shows corresponding M-mode images to measure MCT rates. We observed significantly higher MCT rates in the tracheal tissue of smoke-exposed GLPG2196-treated ferrets (10.2±1.0 mm·min−1) versus that of smoke-exposed vehicle-treated animals (6.4±1.1 mm·min−1, p=0.046 Kruskal–Wallis), with rates restored to levels similar to air controls (10.1±1.2 mm·min−1, figure 4f). Improvements in mucus transport with GLPG2196 treatment were accompanied by increased ciliary beat frequency (GLPG2196, 13.1±0.3 Hz versus vehicle, 11.0±0.4 Hz; p=0.0003; figure 4e), but minimal changes in airway surface liquid (figure 4c) and periciliary layer (figure 4d) depths, noting that smoke-exposed animals did not exhibit detectable reductions in airway hydration previously unless subject to multivariate analysis [26].
FIGURE 4.
GLPG2196 increases mucus transport and ciliary beating in smoke-exposed ferret tracheal explants. a, b) Representative micro-optical coherence tomography images of trachea excised from ferrets exposed to room air for 6 months and then receiving enteral administration of vehicle, and from ferrets exposed to whole cigarette smoke via a nose-only exposure system for 6 months and then receiving enteral administration of vehicle or GLPG2196. Epithelial cell monolayer (epi), mucus layer (mu), airway surface liquid (ASL) depth (red bar), periciliary layer (PCL) depth (yellow bar). Scale bars: 10 μm. Representative videos from which these still images were derived are presented in supplementary videos E1 and E2. b) Resliced images in which the slope of the diagonal streak (yellow arrow) indicates the vectorial transport of mucus particles over time, allowing for visualisation of mucociliary transport (MCT) rate on still images. Steeper slopes are indicative of slower MCT rates. c–f) Quantification of mean ASL depth (c), PCL depth (d), ciliary beat frequency (CBF) (e) and MCT rate (f) in tracheal explants from air-exposed vehicle-treated, smoke-exposed vehicle-treated, and smoke-exposed GLPG2196-treated ferrets (n=11–12 trachea per group, ordinary one-way ANOVA for CBF and Kruskal–Wallis one-way ANOVA for MCT). *: p<0.05; ***: p<0.001.
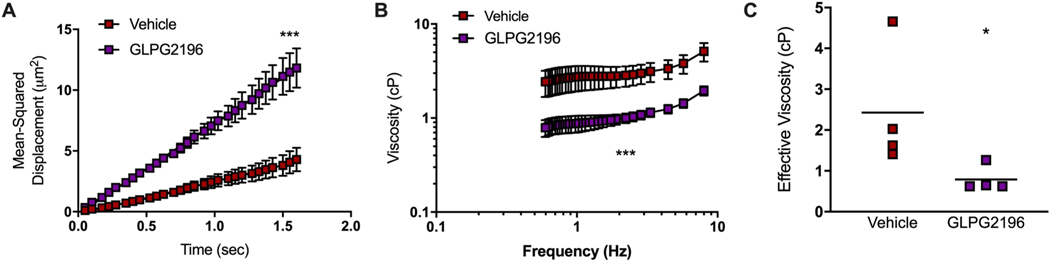
Viscosity analysis by PTM of mucus procured from the same tracheal explants used for studies presented in figure 5 revealed that Brownian motion of particles measured by mean squared displacement was more prominent in the mucus of smoke-exposed GLPG2196-treated versus smoke-exposed vehicle-treated ferrets (figure 5a), and was apparent in viscoelastic curve showing lower viscosities with GLPG2196 across frequencies (figure 5b). Effective viscosity, measured at a near-static frequency of 0.6 Hz, was significantly lower with GLPG2196 (0.789±0.159 cP) versus vehicle (2.429±0.753 cP, p=0.029, figure 5c). Together, these studies demonstrate that GLPG2196 restores mucus transport and reduces mucus viscosity in smoke-exposed animals.
FIGURE 5.
GLPG2196 improves effective viscosity in smoke-exposed ferret mucus. Particle-tracking microrheology was performed using mucus that was extracted from ferret tracheal explants. Ferrets were either exposed to room air via a nose-only exposure system for 6 months and then administered vehicle, or exposed to whole cigarette smoke for 6 months and administered vehicle or GLPG2196. a) Graphical representation of mean squared displacement (MSD) of individual beads in the mucus layer over time. b) Viscoelastic curves over a frequency sweep of 0.46–17.36 Hz with c) quantitation of mean effective viscosity at 0.9 Hz (n=4 samples per group, Mann–Whitney U test). All statistics denote the evaluation of GLPG2196 versus vehicle. *: p<0.05; ***: p<0.001.
GLPG2196 modulates histopathological signs of lung injury in smoke-exposed ferrets
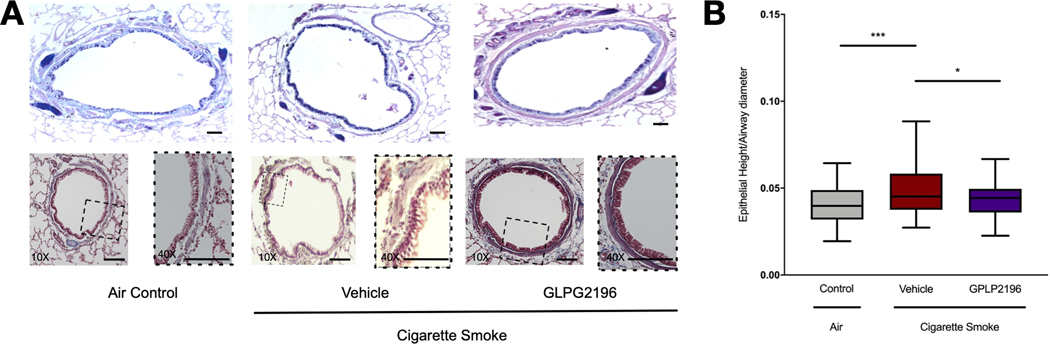
Next, we examined necropsy specimens for histopathological changes associated with chronic cigarette smoke exposure in ferrets [25]. As seen by representative Alcian blue/periodic acid–Schiff (AB-PAS) staining, GLPG2196-treated animals demonstrated less goblet cell hyperplasia than smoke-exposed vehicle-treated controls, although these changes were patchy in distribution (figure 6a). GLPG2196-treated ferrets had a lower epithelial height/LD ratio (0.0438±0.0), a measure of epithelial hypertrophy due to injury, in their large and medium airways than smoke-exposed vehicle-treated ferrets (0.04919±0.0, p=0.033). This ratio was increased in vehicle-treated ferrets compared to air-control ferrets (0.04087±0.0, p=0.0002), a finding documented previously [25].
FIGURE 6.
GLPG2196 modulates epithelial hypertrophy in smoke-exposed ferrets. a) Mucus-expressing goblet cells and submucosa were stained with periodic acid–Schiff (PAS) (magenta) in tracheal sections of ferrets exposed to room air or cigarette smoke for 6 months and administered vehicle or GLPG2196. Lung sections from the same ferrets were stained with Alcian blue/periodic acid–Schiff (AB-PAS) to highlight mucus-expressing cells (deep blue). Scale bars: 100 μm; magnifications on lower panels are of ×10 (left) and ×40 (right). Images are representative of 10–12 sections from n=8 ferrets per condition. b) Quantitative analyses demonstrate that GLPG2196 ameliorates epithelial hyperplasia in smoke-exposed ferrets, as measured by decreased epithelial cell height controlled for the luminal area. Data are shown as box and whisker plots with Tukey whiskers (n=74–89 observations per group, one-way ANOVA with post hoc Tukey). *: p<0.05; ***: p<0.001.
Discussion
Novel therapies for COPD are needed, particularly for individuals affected by chronic bronchitis who exhibit recalcitrant mucus obstruction and are prone to frequent exacerbation. CFTR dysfunction has been observed in patients with COPD, but its role in disease pathogenesis is not yet confirmed. Here, we establish the physiological significance of acquired CFTR dysfunction conferred by cigarette smoking in an animal model of COPD with chronic bronchitis. We demonstrate that augmentation of CFTR function by potentiating wild-type CFTR can accelerate mucus transport and reverse mucus retention. These findings illustrate the bioactivity of a novel therapeutic approach in a large animal model that exhibits chronic bronchitis.
Recent in vivo studies in mice [12, 42] and humans [10–12, 16] demonstrate the presence of acquired CFTR dysfunction due to smoking and that the defect can persist in both the lung and periphery despite smoking cessation [43]. Acquired CFTR dysfunction has been associated with chronic bronchitis severity in several independent cohorts [10–12, 16], underscoring the need for animal studies to confirm causation and evaluate therapeutic approaches. Mice are poorly suited for this because they do not develop significant bronchitis with cigarette smoke exposure [44]. Hence, we developed a smoke-exposed ferret model that manifests features of COPD with chronic bronchitis that more closely resemble human COPD [25]. We subsequently showed that bronchitis in COPD ferrets is associated with delayed mucociliary clearance [26], although the mechanistic basis was not yet determined. Here, we establish that smoke-exposed ferrets develop acquired CFTR dysfunction in a time-dependent fashion. The severity of CFTR dysfunction exhibited was similar to that observed in humans with chronic cigarette smoking in that the degree of dysfunction was ~50% of normal in the nasal airway [10], lung [11] and intestine [12]. The observed imbalance between reduced CFTR-mediated fluid secretion coupled with mucus overexpression induced by cigarette smoke likely explains increased mucus viscosity, delayed mucociliary clearance and the clinical features of chronic bronchitis evident in this model, a concept postulated in prior cell culture studies [10, 13, 14].
To verify if CFTR is a valid therapeutic target in COPD, we evaluated the efficacy of the CFTR potentiator GLPG2196. This agent is closely related to a clinical development candidate for the treatment of CFTR gating mutations and a multidrug corrector-potentiator regimen for people with specific genetic forms of CF. Previous studies have shown that GLPG1837, a compound related to GLPG2196, improved sweat chloride and lung function (i.e. forced expiratory volume in 1 s) in CF [45], and we know from the evaluation of other potentiators in animal models [46] and people with CF that this is likely to address abnormal mucus clearance [17, 47]. In vitro studies confirmed that GLPG2196 activated non-mutant, wild-type CFTR in ferrets, a finding we were unable to document with ivacaftor even though it is known to activate ferret G551D-CFTR [36], perhaps due to small differences in the CFTR amino acid sequence in sufficient proximity to its putative binding site or other aspects that govern allosterically mediated activation [48]. Furthermore, CFTR function could be potentiated by GLPG2196 in ferret cells exposed to cigarette smoke, and the effect was observed with perfusion of GLPG2196 to the nasal surface, or when added to excised trachea from smoke-exposed ferrets. These crucial studies established that the potential of GLPG2196 was related to pharmacological restoration of CFTR function, as we have previously shown with the CFTR potentiator ivacaftor in vitro [10, 22]. It was notable that the response to CFTR agonists, whether GLPG2196 or forskolin, was not completely inhibited by GlyH101 in cell or tissue culture studies, a finding we have seen previously even in the absence of GLPG2196 [31]. We suspect that is due to incomplete potency and efficacy of GlyH101, which functions as a pore blocker, in the acutely administered conditions we used in our studies, but we cannot rule out that GLPG2196 also stimulates alternative chloride channels that contribute to the electrophysiological stimulus in ferrets.
Following pharmacokinetic studies that documented stable pharmacokinetics in ferrets, we established that GLPG2196 reversed two important features of the acquired CFTR dysfunction phenotype upon systemic administration. Using μOCT imaging of excised trachea, a method sensitive to evaluating the mucociliary clearance abnormality conferred by smoking [26], GLPG2196 augmented MCT rate to a level that approached air controls and accelerated ciliary beat frequency. MCT improvement was likely explained by a 2-fold reduction in mucus viscosity. Prior studies have shown the MCT apparatus is extremely sensitive to changes in mucus viscosity [49], likely explaining how these improvements could occur without changes in air surface liquid or periciliary layer depths, especially because these measures of airway hydration are less sensitive in excised ferret trachea [26]. This may be because trachea excision influences the delivery of airway fluids from the distal lung and influences neurogenic secretion, factors that may have negatively impacted the ability to identify effects on airway hydration. This concept could ultimately be tested with in vivo μOCT imaging once available in ferrets.
Augmented mucus clearance would be expected to reduce mucus retention of the lung and later alter airway remodelling. To track this, we recently developed a μCT-based metric of bronchial wall thickening in ferrets that is elevated by smoking in a time-dependent fashion [27], akin to findings in humans that have been associated with CFTR dysfunction [15]. Indeed, BWT decreased by 24% in GLPG2196 ferrets compared to vehicle-treated controls. Likewise, the calculated Pi4 showed a similar reduction (by 12%) with GLPG2196 treatment. Our prior studies in ferrets showed 6 months of smoke exposure increased BWT by 34% and Pi4 by 20%, respectively [27]. Thus we conclude bronchial wall thickening improved substantially with GLGP2196 treatment, although did not return to normal, pre-smoke-exposed baseline. Overall, these findings indicate that activation of CFTR not only improved mucociliary clearance but also reduced mucus retention as detected by changes in μCT morphology.
Histopathological findings with GLPG2196 treatment were relatively limited, acknowledging that the duration of the study was not sufficient to expect substantial changes in airway remodelling. The most apparent finding was that CFTR potentiation reduced epithelial height, a general indicator of epithelial injury previously shown to be elevated in smoke-exposed ferrets [25]. We suspect this is attributable to reduced toxic exposure of the epithelial surface to cigarette smoke constituents through improved mucus clearance. However, secondary effects on subclinical bacterial infection are also possible. There was also reduced mucus expression, in the form of less apparent mucus staining within goblet cells, although the findings were sporadic, limiting quantitative assessments. Given the patchy nature of mucus hypersecretion likely related to deposition patterns of cigarette smoke, longer studies may be needed to examine definitive effects on lung pathology. No ferrets exhibited spontaneous infection in the study; thus, the impact of CFTR potentiation on bacterial infection may need to be evaluated in future studies involving bacterial exacerbation, as recently established in smoke-exposed ferrets [50]. While the beneficial effects of reversing cigarette smoke-induced CFTR dysfunction on airway physiology are clearly demonstrated by these studies, it is also evident that smoking induces many other pathologies that would not be reversed by potentiation of CFTR. We did not attempt to address systemic manifestations of CFTR potentiation, because effects on the gastrointestinal tract are likely to have a much longer time domain, and an equivalent to sweat chloride is not available. These end-points could be of interest in future studies.
In summary, this report establishes in a controlled animal model that cigarette smoking causes CFTR dysfunction and that pharmacological potentiators of CFTR can restore mucus transport and reduce mucus retention. These data also establish how modest dysfunction of a protein associated with a rare, monogenetic disorder may also contribute to a common and complex multifactorial disorder, suggesting a novel treatment opportunity.
Supplementary Material
Acknowledgments
Support statement: These studies were supported by NIH National Heart, Lung, and Blood Institute (NHLBI) Grant R35HL135816 (to S.M.R.) and National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK072482 (to S.M.R). Funding information for this article has been deposited with the Crossref Funder Registry.
Footnotes
Conflict of interest: S.M. Rowe has received consulting fees from Abbvie related to the design and conduct of clinical trials in cystic fibrosis. M. Borgonovi and K. Conrath are employees of Galapagos. All other authors declare no competing interests.
References
- 1.Centers for Disease Control and Prevention. National Center for Health Statistics. Chronic Obstructive Pulmonary Disease (COPD). Includes: Chronic Bronchitis and Emphysema. www.cdc.gov/nchs/fastats/copd.htm [Google Scholar]
- 2.Centers for Disease Control and Prevention. COPD Costs. www.cdc.gov/copd/infographics/copd-costs.html [Google Scholar]
- 3.Kim V, Criner GJ. Chronic bronchitis and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005; 352: 1992–2001. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Garcia MA, Soler-Cataluna JJ, Donat Sanz Y, et al. Factors associated with bronchiectasis in patients with COPD. Chest 2011; 140: 1130–1137. [DOI] [PubMed] [Google Scholar]
- 6.Allinson JP, Hardy R, Donaldson GC, et al. The presence of chronic mucus hypersecretion across adult life in relation to COPD development. Am J Respir Crit Care Med 2015; 192: 943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 2645–2653. [DOI] [PubMed] [Google Scholar]
- 8.Saetta M, Turato G, Baraldo S, et al. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med 2000; 161: 1016–1021. [DOI] [PubMed] [Google Scholar]
- 9.Cantin AM, Hanrahan JW, Bilodeau G, et al. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med 2006; 173: 1139–1144. [DOI] [PubMed] [Google Scholar]
- 10.Sloane PA, Shastry S, Wilhelm A, et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One 2012; 7: e39809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dransfield MT, Wilhelm AM, Flanagan B, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest 2013; 144: 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raju SV, Jackson PL, Courville CA, et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med 2013; 188: 1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreindler JL, Jackson AD, Kemp PA, et al. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol 2005; 288: L894–L902. [DOI] [PubMed] [Google Scholar]
- 14.Clunes LA, Davies CM, Coakley RD, et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J 2012; 26: 533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teerapuncharoen K, Wells JM, Raju SV, et al. Acquired CFTR dysfunction and radiographic bronchiectasis in current and former smokers: a cross-sectional study. Ann Am Thorac Soc 2018; 16: 150–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Courville CA, Tidwell S, Liu B, et al. Acquired defects in CFTR-dependent β-adrenergic sweat secretion in chronic obstructive pulmonary disease. Respir Res 2014; 15: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowe SM, Heltshe SL, Gonska T, et al. Clinical mechanism of the CFTR potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 2014; 190: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoare S, McEvoy S, McCarthy CJ, et al. Ivacaftor imaging response in cystic fibrosis. Am J Respir Crit Care Med 2014; 189: 484. [DOI] [PubMed] [Google Scholar]
- 19.Hisert KB, Heltshe SL, Pope C, et al. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med 2017; 195: 1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heltshe SL, Mayer-Hamblett N, Burns JL, et al. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis 2015; 60: 703–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sethi S, Murphy TF. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin Microbiol Rev 2001; 14: 336–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raju SV, Lin VY, Liu L, et al. The cystic fibrosis transmembrane conductance regulator potentiator ivacaftor augments mucociliary clearance abrogating cystic fibrosis transmembrane conductance regulator inhibition by cigarette smoke. Am J Respir Cell Mol Biol 2017; 56: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomon GM, Hathorne H, Liu B, et al. Pilot evaluation of ivacaftor for chronic bronchitis. Lancet Respir Med 2016; 4: e32–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowe SM, Jones I, Dransfield MT, et al. Efficacy and safety of the CFTR potentiator icenticaftor (QBW251) in COPD: results from a phase 2 randomized trial. Int J Chron Obstruct Pulmon Dis 2020; 15: 2399–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raju SV, Kim H, Byzek SA, et al. A ferret model of COPD-related chronic bronchitis. JCI Insight 2016; 1: e87536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin VY, Kaza N, Birket SE, et al. Excess mucus viscosity and airway dehydration impact COPD airway clearance. Eur Respir J 2020; 55: 1900419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanford D, Kim H, Bodduluri S, et al. Airway remodeling in ferrets with cigarette smoke induced COPD using microCT imaging. Am J Physiol Lung Cell Mol Physiol 2020; 319: L11–L20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teerapuncharoen K, Wells JM, Raju SV, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction and radiographic bronchiectasis in current and former smokers: a cross-sectional study. Ann Am Thorac Soc 2019; 16: 150–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheikh SI, Long FR, McCoy KS, et al. Computed tomography correlates with improvement with ivacaftor in cystic fibrosis patients with G551D mutation. J Cyst Fibros 2015; 14: 84–89. [DOI] [PubMed] [Google Scholar]
- 30.Van der Plas SE, Kelgtermans H, Mammoliti O, et al. Discovery of GLPG2451, a novel once daily potentiator for the treatment of cystic fibrosis. J Med Chem 2021; 64: 343–353. [DOI] [PubMed] [Google Scholar]
- 31.Kaza N, Raju SV, Cadillac JM, et al. Use of ferrets for electrophysiologic monitoring of ion transport. PLoS One 2017; 12: e0186984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L, Shastry S, Byan-Parker S, et al. An autoregulatory mechanism governing mucociliary transport is sensitive to mucus load. Am J Respir Cell Mol Biol 2014; 51: 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Chu KK, Houser GH, et al. Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography. PLoS One 2013; 8: e54473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu KK, Mojahed D, Fernandez CM, et al. Particle-tracking microrheology using micro-optical coherence tomography. Biophys J 2016; 111: 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tilley AE, Staudt MR, Salit J, et al. Cigarette smoking induces changes in airway epithelial expression of genes associated with monogenic lung disorders. Am J Respir Crit Care Med 2016; 193: 215–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun X, Yi Y, Yan Z, et al. In utero and postnatal VX-770 administration rescues multiorgan disease in a ferret model of cystic fibrosis. Sci Transl Med 2019; 11: 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ou C, Li Y, Wei J, et al. Numerical modeling of particle deposition in ferret airways: a comparison with humans. Aerosol Science and Technology 2017; 51: 477–487. [Google Scholar]
- 38.Grydeland TB, Thorsen E, Dirksen A, et al. Quantitative CT measures of emphysema and airway wall thickness are related to DLCO. Respir Med 2011; 105: 343–351. [DOI] [PubMed] [Google Scholar]
- 39.Kim V, Desai P, Newell JD, et al. Airway wall thickness is increased in COPD patients with bronchodilator responsiveness. Respir Res 2014; 15: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mair G, Maclay J, Miller JJ, et al. Airway dimensions in COPD: relationships with clinical variables. Respir Med 2010; 104: 1683–1690. [DOI] [PubMed] [Google Scholar]
- 41.Patel BD, Coxson HO, Pillai SG, et al. Airway wall thickening and emphysema show independent familial aggregation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 178: 500–505. [DOI] [PubMed] [Google Scholar]
- 42.Raju SV, Tate JH, Peacock SK, et al. Impact of heterozygote CFTR mutations in COPD patients with chronic bronchitis. Respir Res 2014; 15: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Courville CA, Raju SV, Liu B, et al. Recovery of acquired cystic fibrosis transmembrane conductance regulator dysfunction after smoking cessation. Am J Respir Crit Care Med 2015; 192: 1521–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groneberg DA, Chung KF. Models of chronic obstructive pulmonary disease. Respir Res 2004; 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies JC, Van de Steen O, van Koningsbruggen-Rietschel S, et al. GLPG1837, a CFTR potentiator, in p.Gly551Asp (G551D)-CF patients: an open-label, single-arm, phase 2a study (SAPHIRA1). J Cyst Fibros 2019; 18: 693–699. [DOI] [PubMed] [Google Scholar]
- 46.Birket SE, Davis JM, Fernandez-Petty CM, et al. Ivacaftor reverses airway mucus abnormalities in a rat model harboring a humanized G551D-CFTR. Am J Respir Crit Care Med 2020; 202: 1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donaldson SH, Laube BL, Corcoran TE, et al. Effect of ivacaftor on mucociliary clearance and clinical outcomes in cystic fibrosis patients with G551D-CFTR. JCI Insight 2018; 3: e122695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu F, Zhang Z, Levit A, et al. Structural identification of a hotspot on CFTR for potentiation. Science 2019; 364: 1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fernandez-Petty CM, Hughes GW, Bowers HL, et al. A glycopolymer improves vascoelasticity and mucociliary transport of abnormal cystic fibrosis mucus. JCI Insight 2019; 4: e125954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hunt BC, Stanford D, Xu X, et al. Haemophilus influenzae persists in biofilm communities in a smoke-exposed ferret model of COPD. ERJ Open Res 2020; 6: 00200–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.