Abstract
During the coronavirus disease 2019 (COVID-19) pandemic, frontline healthcare workers (HCWs) suffered more distress from the possibility of contracting the virus, quarantine, social stigma, and prejudice against their families. Many studies have investigated the impact of the pandemic on HCWs; however, studies or guidelines presenting strategies to overcome these challenges are lacking. As part of a 2020 research project supported by the Ministry of Health and Welfare, titled “Health impact assessment of healthcare workers undertaking coronavirus disease 2019 treatment and management in Korea: Identifying problems and researching effective solutions” (HC20C0003), we created guidelines to respond to serious problems posed by infection control. and burnout among HCWs during COVID-19 response measures throughout the extended pandemic period. We formulated the guidelines by means of a systematic review and collated them with the latest literature. The guidelines will highlight the gravity and impact of infection control and burnout among HCWs responding to COVID-19 and include potential prevention strategies, and they can be used as a reference in the event of another emerging infectious disease outbreak in the future.
Keywords: SARS-CoV-2, Healthcare personnel, Mental health, Infection control
INTRODUCTION
1. Background
In Korea, the first case of coronavirus disease 2019 (COVID-19) was confirmed on January 20, 2020, and as of January 20, 2023, there were approximately 30 million confirmed cases and 33,134 deaths. Despite the high vaccination rate, the number of newly diagnosed COVID-19 cases continues to increase, and critical illness and mortality rates remain high because of the decline in vaccine effectiveness over time and mitigated nationwide containment measures [1].
In the early stages of the pandemic, healthcare workers (HCWs) had to respond to infections with a shortage of personal protective equipment (PPE) and limited manpower [2,3,4]. Furthermore, the psychological and physical difficulties faced by HCWs continue to intensify as a result of prolonged pandemic situations worldwide. Frontline HCWs suffer more distress due to the possibility of contracting the virus, quarantine, social stigma, and prejudice against their families [5]. Consequently, many studies have investigated the impact of the COVID-19 pandemic on HCWs.
However, studies or guidelines presenting strategies to overcome these challenges are lacking. Standardized, evidence-based guidelines protect HCWs, who are frontline personnel in the battle against COVID-19, and are essential sustainable response measures. In this context, we developed guidelines and strategies to overcome these challenges by reviewing the literature on the impact of COVID-19 on HCWs.
2. Formulating key questions and reaching a consensus
We systematically searched the international literature on the impact of COVID-19 on HCWs in PubMed, the Cochrane Library, and Embase as well as KMBase and the Research Information Sharing Service (RISS) for Korean literature (Supplementary Material 1). The key questions for the guidelines were finally selected through several meetings of the Guidelines Development Committee, which comprised two psychiatrists and six specialists in infectious diseases. Six information specialists performed systematic literature searches. We employed a highly sensitive search strategy involving a combination of controlled vocabulary (MeSH terms in PubMed and the Cochrane Library and Emtree terms in Embase) and free-text terms for each key question. We reviewed the identified references and selected 100 references for the guidelines (Supplementary Material 1).
3. Strength of recommendations and level of evidence
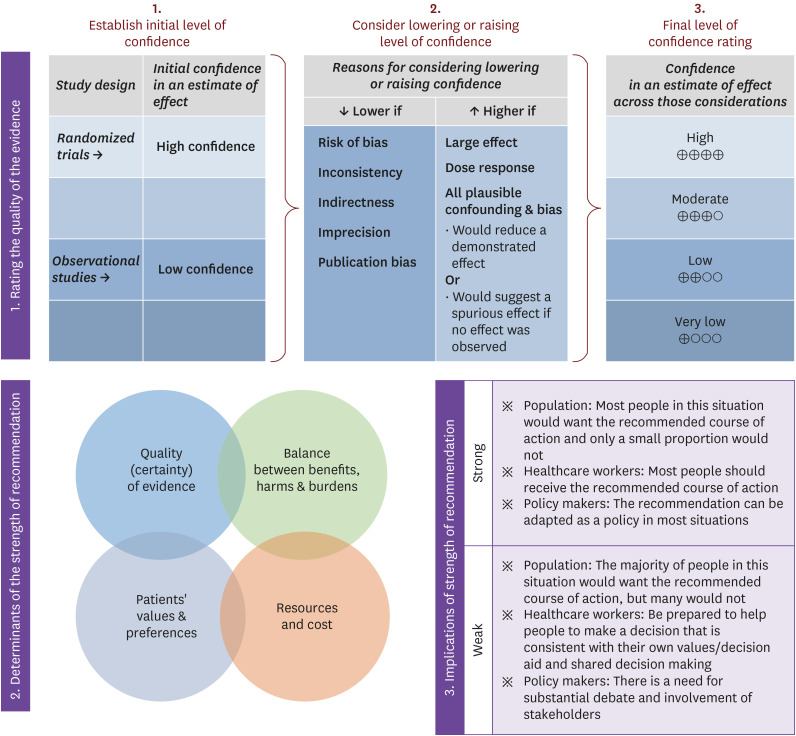
We categorized the level of evidence and the strength of the recommendations using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system as follows: high, moderate, low, and very low for the level of evidence and strong and weak for the strength of each recommendation (Table 1, Fig. 1) [6].
Table 1. Strength and quality of recommendations (GRADE system).
| Evaluation of the quality of evidence | Strength of recommendations | ||||||
|---|---|---|---|---|---|---|---|
| Study design | Initial grading of the quality of evidence | Consider lowering the grade if | Consider raising the grade if | Quality of evidence | |||
| Randomized trials | High | Bias risk | Effect size | High: 4 points | Strong: Believed that benefits are clearly larger or smaller than the harms | ||
| Serious: −1 | Large: +1 | Moderate: 3 points | Weak: All non-strong recommendations | ||||
| Highly serious: −2 | Very large: +2 | Low: 2 points | |||||
| Inconsistency | Positive relationship | Very low: 1 point | |||||
| Serious: −1 | Yes: +1 | ||||||
| Highly serious: −2 | |||||||
| Observational studies | Low | Indirectness | Confounding variables | ||||
| Serious: −1 | Raising the certainty of effect estimation: +1 | ||||||
| Highly serious: −2 | |||||||
| Imprecision | |||||||
| Serious: −1 | |||||||
| Highly serious: −2 | |||||||
| Publication bias | |||||||
| Strongly suspicious: −1 | |||||||
GRADE, grading of recommendations assessment, development and evaluation.
Figure 1. Approach and implication to the rating the quality of evidence and strength of recommendations using the GRADE approach [6].
GRADE, grading of recommendations assessment, development and evaluation.
4. External expert evaluations
The draft guidelines developed through internal meetings among the panel members were reviewed by an external expert panel for secondary opinions. The secondary opinions were addressed in an additional internal meeting among the panel members, and the guidelines were accordingly revised and supplemented. Opinions from a group of other experts were also collected, and the guidelines were finalized on the basis of these discussions. The Korean Society of Infectious Diseases (KSID), the Korean Society for Antimicrobial Therapy (KSAT), and the Korean Society for Healthcare-Associated Infection Control and Prevention endorsed these guidelines.
5. Terminology and abbreviations
Relevant academic terminologies were expressed in Korean using the sixth edition of the Korean Medical Terminology (published by the Korea Medical Association, amended in March 2020). If a Korean term was not clear, the corresponding English term was provided in parentheses. Terms that could not be written in Korean, such as names of pathogens, proper nouns, names of drugs, and units, were written in English.
RECOMMENDATIONS
1. Summary of key questions
1) How does COVID-19 psychologically impact HCWs, and how can this be prevented?
2) How does COVID-19 physically impact HCWs (excluding COVID-19 morbidity), and how can this be prevented?
3) What are the effects of COVID-19-related morbidity among HCWs, and what can be done to prevent them?
4) How does COVID-19 affect social stigma for HCWs, and what can be done to prevent this?
5) How does quarantine due to COVID-19 affect HCWs, and what can be done to prevent it?
6) How does COVID-19 affect the families of HCWs, and what can be done to prevent this?
2. Statements by each key question
Key question 1: How does COVID-19 psychologically affect HCWs, and how can this be prevented?
1. COVID-19 intensifies post-traumatic stress disorder (PTSD), depression, anxiety, and burnout in HCWs. To prevent the origin of these ailments, the workload of HCWs must be carefully monitored and an adequate level of staffing must be maintained (level of evidence: moderate, strength of recommendation: strong).
2. HCWs responding to COVID-19 should be periodically assessed for mental health and required assistance. Psychological counseling and psychiatric consultations should be provided as required (level of evidence: moderate, strength of recommendation: strong).
3. In the event of a novel infectious disease outbreak, HCWs should be provided with psychological support via delivery of information and education about the infectious disease and infection control (level of evidence: low, strength of recommendation: strong).
A meta-analysis of 86 studies reported that during an epidemic, frontline HCWs develop concerns about the potential spread of the virus to their families (60.4%), stress (56.8%), health concerns (46.0%), sleep disorders (39.9%), and burnout (31.8%) [7]. A cross-sectional study in one healthcare facility early after the COVID-19 outbreak in Korea (April 2020) showed that the incidence rates of depression and anxiety were 33.0% and 12.5%, respectively [8]. In particular, the rates of depression and anxiety were higher among nurses, probably because of the fear of contracting COVID-19 and social stigma. Infectious diseases specialists, who played a pivotal role in the response to COVID-19, also suffered from burnout (90.4%), anxiety (20.0%), and stress (4.3%), and they cited pinpointed a shortage of HCWs (64.0%) as the greatest difficulty, followed by the lack of infection control personnel (44.0%) [9].
Mental toll and burnout among HCWs deteriorate the quality of care and treatment outcomes, and this lowers patient satisfaction levels. Furthermore, hiring and training new healthcare staff because of attrition due to burnout incurs additional costs [10]. Some HCWs even feel guilty for taking standard breaks amid a staff shortage and are often quarantined in hospitals or hotels, even during breaks [5]. It is important to implement a flexible work schedule and ensure uninterrupted sleep by securing an adequate pool of healthcare staff [11].
In addition, HCWs responding to COVID-19 should be periodically assessed for mental health and receive the necessary psychological counseling and psychiatric care. A study showed that a psychological support program for HCWs significantly reduced their psychological distress, enhanced their quality of life, and reduced absenteeism. Moreover, their mental health and quality of life improved proportionally with the number of psychiatric counseling sessions [12]. Another study developed a hotline for prompt psychiatric counseling, which appeared to benefit HCWs providing direct COVID-19 care as well as other staff members [13]. Such hotlines can be considered an early intervention strategy for HCWs. To ensure effective and feasible interventions, eHealth and mobile phone apps can also be used [14].
In the event of an outbreak of a novel infectious disease such as COVID-19, HCWs must receive psychological support by receiving information about the disease and infection control protocol [15,16,17]. Clear information about infectious diseases, preventive measures, management feedback, and support are reportedly viable psychological coping strategies [16,17,18]. The provision of a digital learning package may also be helpful. Such a package developed in the United Kingdom (UK) includes evidence-based guidelines, information about communication and reducing social stigma, colleague and family support, psychological emergency response, self-care strategies (e.g., rest, sleep, rotating shifts, fatigue, healthy lifestyles), and emotional regulation (e.g., prevention of moral injury, coping, guilt, sorrow, fear, anxiety, depression, burnout, and psychological trauma) [19].
Key question 2: How does COVID-19 physically impact HCWs (excluding COVID-19-related morbidity), and how can this be prevented?
1. Policies that limit the duration of consecutive work hours while wearing protective coveralls (<1 - 4 hours) and relevant protocols must be implemented to prevent physical side effects from excessive use of protective coveralls (level of evidence: moderate, strength of recommendation: strong).
2. HCWs who use PPE must be instructed to take scheduled breaks on a frequent basis (e.g., every 1 - 2 hours) in order to prevent fatigue and adverse effects (level of evidence: moderate, strength of recommendation: strong).
3. Physical problems among HCWs that occur during work must be continuously monitored, and reporting protocols, plans, and response measures must be developed (level of evidence: moderate, strength of recommendation: strong).
The most common adverse effects of PPE use are skin-related problems, with an incidence of 97% among HCWs who wear a face mask or other protective gear [20]. Hand hygiene, the use of PPE such as face masks or gloves, and respirator fit tests can lead to adverse outcomes such as itching, dryness, dermatitis, hives, discoloration, facial scarring, acne, exacerbation of pre-existing skin conditions, pompholyx, desquamation, cheilitis, and superficial fungal infection [20,21,22,23]. If an HCW develops allergic responses to protective equipment (e.g., specific hand sanitizer or gloves), an alternative product should be provided. Furthermore, individuals with a damaged skin barrier or contact dermatitis frequently use topical skin protectants. Mechanical injury to the skin as a result of wearing protective equipment should be treated by application of powder or skin protectants, and equipment of an appropriate size should be provided. Mechanical injuries inflicted by goggles or face masks can be alleviated using silicone foam or hydrocolloid dressings that reduce pressure. These methods can reduce skin injuries without increasing the risk of additional infection [24]. With regard to superficial fungal diseases or eczema, the duration of protective gear use should also be reduced. Acne is also linked to mental and physical stress; thus, appropriate measures should be considered [23]. Implementation of virtual occupational skin health clinics to provide skin-related consultations for healthcare staff required to wear PPE may also be a good option [25].
Approximately 35% to 90% HCWs suffer from headaches, which may be attributable to superficial nerve compression, poor ventilation, and noise from powered air-purifying respirators (PAPR) [26,27]. Wearing an N-95 mask continuously for ≥4 h is associated with headache, so workers required to wear an N-95 mask must take a break with the mask removed after 4 h of use [28]. Moreover, one study reported that the heart rate, oxygen saturation, and perfusion indices were significantly altered 4 h after use of a protective coverall, compared with those before coverall wear [26]. Most HCWs who developed an adverse event related to the use of PPE wore their protective gear for >6 h in a day [29]. In addition, 45% to 61% workers perspire excessively while wearing protective coveralls [30]. During the Ebola epidemic in West Africa, the standard recommendation was not to exceed 40 min in a protective coverall in order to prevent heat stroke, dehydration, cognitive impairment, and postural instability in Africa’s high-temperature environment [31]. Even though infection care wards in Korea are temperature-controlled, protective coverall use can still cause heat-related problems. Therefore, work hours should be adjusted according to the seasonal influence and work environment (e.g., indoors and outdoors). The World Health Organization (WHO) recommends that hospitals limit the maximum working hours to 1 h in special wards that require the use of protective coveralls in order to prevent heat stress [32]. In addition, ≥8 h of coverall wear in a day can increase the risk of accidents by increasing fatigue and diminishing concentration [33]. Thus, the ideal work hours to prevent potentially adverse outcomes in environments that require PPE are 6 to 8 h per day, and ongoing work while wearing a protective coverall should ideally be limited to 1 h, with a maximum of 4 h.
Furthermore, protective coverall use can trigger physiological problems such as breathing-related chest pounding, increased heart rate, fatigue, dizziness, nausea, and bronchospasm. Cases of vocal disorders due to loud conversations, asthma, and vascular edema as a result of a respirator fit test have also been noted [21]. Moreover, female HCWs are reported to be more frequency affected by burnout due to gender inequality, bias, and family-related work stress [34].
Shorter shifts and more frequent breaks are needed to prevent adverse outcomes related to protective coverall use [35]. The United States Centers for Disease Control and Prevention (CDC) state that frequent short breaks (e.g., every 1 - 2 h) during a shift are more effective in preventing fatigue than are long breaks at longer intervals during a disaster [33]. Scheduled breaks, adequate hydration, and adequate food intake are crucial for preventing headaches, dizziness, impaired concentration, and cognitive impairment. In addition, each facility should devise and enforce specific policies and protocols to curtail physical and physiological issues and protect HCWs from overuse of PPE. These include guaranteed breaks, periodic inspections by frontline managers, and implementation of a system that allows workers to report problems without fear of suffering a disadvantage [30].
Key question 3: What are the effects of COVID-19-related morbidity among HCWs, and what can be done to prevent them?
1. HCWs contract COVID-19 via not only direct contact with patients but also unexpected exposure to a colleague or community members, and they must be instructed to adhere to appropriate physical distancing policies within the facility and strictly adhere to basic containment strategies for COVID-19 (level of evidence: moderate, strength of recommendation: strong).
2. HCWs responding to COVID-19 must practice meticulous contact precautions and wear proper PPE to avoid contracting COVID-19 (level of evidence: moderate; strength of recommendation: strong).
3. Considering the rate of COVID-19 spread in the community, periodic screening tests should be considered for HCWs responding to COVID-19 (level of evidence: low, strength of recommendation: weak).
4. Diagnostic tests for COVID-19 must be performed immediately upon onset of COVID-19 symptoms (level of evidence: moderate, strength of recommendation: strong).
5. HCWs must be vaccinated against COVID-19 to avoid infection (level of evidence: high, strength of recommendation, strong).
The COVID-19 infection rate among HCWs varies widely across countries, and although a simple comparison among nations is hindered by varying diagnostic methods, 551,801 cases of infection (1.34% of all infections in the US) and 1,753 deaths (0.26% of all deaths in the US) among HCWs were reported in the US as of September 14, 2021 [36]. In Italy, 142,722 HCWs (3.09% of all cases) reported COVID-19 infection as of September 13, 2021 [37]. In Korea, 565 HCWs (0.38% of all cases) were infected with COVID-19 by the end of June 2021 [38]. However, these statistics do not distinguish between community- and hospital-acquired infections. According to a recent meta-analysis of 97 studies on COVID-19 infection among HCWs that were published since the outbreak of COVID-19 in 2020, the COVID-19 infection rate was the highest among nurses (48.0%), followed by physicians (25.0%) [39]. The results were consistent with those of a Korean study, where nurses and physicians accounted for 73.5% (n = 415) and 20.0% (n = 113) of 565 HCWs diagnosed with COVID-19, respectively, at the end of June 2021 [38].
COVID-19 infections among HCWs can have multiple effects [40]. First, they have a direct impact on health. In a Chinese study in early 2020, 3.8% of 44,672 COVID-19 patients (n = 1,716) were HCWs, 14.8% of whom developed a severe or critical illness. Five HCWs died of infection [41]. As shown here, the infection rate among HCWs was high in the early days of the COVID-19 pandemic because of high susceptibility [42]. In particular, frontline HCWs who provide COVID-19 care are vulnerable to infection amid a short supply of PPE. Second, infected HCWs can spread the virus to susceptible individuals. It is difficult to pinpoint the cause of a COVID-19 outbreak within a hospital, specifically for patients or HCWs. However, HCWs are generally young; this means that the rate of asymptomatic or mild cases of COVID-19 among HCWs is high, and it is easier for them to spread the virus to vulnerable patients [43]. A study that analyzed massive COVID-19 outbreaks in three hospitals in the Netherlands using genetic information identified HCWs who had contracted the virus in the community as the origin of the outbreak [44]. Third, an increased infection rate among HCWs leads to shortages in healthcare staffing. Several countries had serious staffing issues in early 2020 because of COVID-19 outbreaks among frontline HCWs. Because HCWs need to undergo education and training, immediate replacement of lost staff is difficult. For this reason, infection prevention among HCWs is more important than anything else during a pandemic [42]. Fourth, infected HCWs can spread the virus to their families, colleagues, and communities [43]. Because HCWs are a bridge between healthcare facilities and the community, COVID-19 infection may directly cause an outbreak within a healthcare facility [45]. Thus, prevention of COVID-19 infection among HCWs is crucial for the prevention of infection among patients and fellow HCWs.
Studies on the route of COVID-19 infection among HCWs have reported that HCWs more commonly acquire infection from the community and spread it within the healthcare facility, rather than acquiring it from a COVID-19 patient [44,46]. Mass infections also occur among HCWs in contact with colleagues who do not wear proper PPE [47,48]. The most dreaded scenario, which involves infection via contact with a COVID-19 patient, generally occurs when HCWs come in contact with these patients without proper PPE before the patients are confirmed to have COVID-19 [47,48]. Thus, HCWs must be instructed to adhere to basic infection control and prevention measures at work and in their daily lives. At work, HCWs should follow the social distancing guidelines distributed by the Korea Disease Control and Prevention Agency (KDCA) as well as their hospital guidelines. Most hospital guidelines lay emphasis on body temperature measurement, use of a face mask at work at all times except while using the bathroom and during meals, social distancing (separate work sites, distancing tables, telework, video conference calls), and prompt testing and close contact tracing for HCWs who develop fever or suspected COVID-19 symptoms.
Risk factors for COVID-19 among HCWs include the following: prolonged exposure in a high-risk environment, inappropriate hand hygiene, and inappropriate PPE use in a Chinese study [49]. Inappropriate PPE use was also a risk factor for COVID-19 infection among HCWs in an Indian study [50]. In another study, none of the 420 HCWs who provided direct COVID-19 care in four hospitals in Wuhan, China in early 2020 developed COVID-19 infection because they properly used PPE and strictly adhered to infection control guidelines [51]. In a study on the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) antibody positivity rate among HCWs providing COVID-19 care, conducted by a research team at Vanderbilt University Medical Center in the US, the positivity rate was significantly lower among those who properly used PPE in all situations [52]. In a previous meta-analysis, the COVID-19 infection rate was 4.7% among HCWs who did not wear proper PPE when they came in contact with a COVID-19 patient in the hospital without knowing their infection status; however, the infection rate was 0% when they used PPE properly [39]. As shown here, multiple studies have shed light on the significance of contact precautions, including hand hygiene and the use of appropriate PPE, for the prevention of COVID-19 among HCWs. To guide the proper use of PPE within a healthcare facility, individuals and organizations should refer to the COVID-19 response guidelines for healthcare facilities distributed by the KDCA [53].
Approximately 40% of SARS-CoV-2 transmissions occur in the asymptomatic or presymptomatic stage [54]. Therefore, the need for pre-emptive testing of asymptomatic HCWs has been highlighted. However, because facility-wide pre-emptive testing for asymptomatic HCWs cannot be continuously enforced for extended periods, there is only one report of early detection of infection in asymptomatic HCWs through pre-emptive testing during a particular period [55,56], with no studies examining the long-term effects of pre-emptive testing on COVID-19 detection in asymptomatic HCWs. A few studies have shown that repeated pre-emptive testing of asymptomatic HCWs over a short period halted the spread of COVID-19 [57,58]. While pre-emptive testing of HCWs is an effective strategy to prevent COVID-19 spread within a facility, a variety of factors must be considered before implementing this approach (e.g., community spread, COVID-19 diagnostic testing capacity, early test participation upon onset of symptoms, contact tracing capacity, and vaccination status) [59]. If so, how should pre-emptive testing be performed for HCWs? In the UK, one study indicated that twice-a-week screening if there is active spread within the community and once-a-week screening if the incidence of infection is low within the community can effectively halt the spread of the disease in a high-risk healthcare facility [60]. Another UK study recommended diagnostic tests for symptomatic staff if the incidence of infection is low within the community and pre-emptive screening for asymptomatic staff if there is active spread within the community [58]. Currently, early diagnostic testing upon the onset of symptoms is recommended in Korea because of the lower incidence rate of COVID-19 compared with that in other countries and the capability of performing meticulous contact tracing and active diagnostic testing. However, periodic pre-emptive testing of asymptomatic personnel can be considered in healthcare facilities depending on the incidence of infection among personnel.
Among symptomatic HCWs who tested positive for COVID-19, fever, loss of smell, and muscle pain increased the likelihood of a positive test result [39]. In a study conducted by a research team at Yale University Medical Center, the positive test rate was 0.24% in SARS-CoV-2 screening of asymptomatic healthcare staff, and approximately 16% workers who tested positive had extremely mild symptoms. Likewise, the positive test rate was 3% in pre-emptive testing of approximately 1,000 asymptomatic HCWs in the UK, and 40% of these workers had symptoms [57]. In other words, mild symptoms are easily neglected in several cases; therefore, HCWs should undergo diagnostic testing even if they only have mild symptoms in order to prevent COVID-19 infection and spread [56]. In Korea, mass infections in healthcare facilities were facilitated by multipatient rooms and the high frequency of paid or family caregivers residing in multipatient rooms; there were many cases in which the infection was transmitted from an HCW to a patient, the patient’s family, or the paid caregiver, who then passed it back to HCWs. Thus, a mandatory screening test should be considered for all inpatients and their families or paid caregivers [61].
HCW vaccination is crucial because it can prevent COVID-19 infection in HCWs and patients. Therefore, HCWs are included in the priority group for vaccination as suggested by the WHO Strategic Advisory Group of Experts on Immunization (SAGE) and COVID-19 vaccination campaigns in many countries [62]. The SARS-CoV-2 prevention rates for the primary vaccines used in Korea, namely BNT162b2 (Pfizer, New York, NY, USA), mRNA-1273 (Moderna, Norwood, MA, USA), and ChAdOx1nCoV-19 (AstraZeneca, Cambridge, UK) are 95.0%, 94.1%, and 62.1%, respectively [63,64,65]. This preventive efficacy also applies to HCWs, and real-world studies on HCWs in France, the US, and the UK showed results similar to previously identified vaccine efficacies [66,67,68]. COVID-19 vaccination was globally initiated in early 2021. While vaccination rates vary by country, the infection rate is dramatically increasing, even in countries with a high vaccination rate. This can be attributed to the delta variant of the SARS-CoV-2 virus, but recent studies have also reported that the immunogenicity of the vaccines eventually declines after vaccination. A study that analyzed the effectiveness of the BNT162b2 vaccine (Pfizer, USA) in 4.90 million individuals registered in a large healthcare system in the US reported that the infection prevention rate was 90% in the first month after two doses of vaccination; however, it decreased to 47% after 5 months. However, COVID-19-related hospitalization rates did not increase [69]. Therefore, some countries, including Korea, have approved booster shots for HCWs.
Vaccine hesitancy refers to the refusal or postponement of vaccination [70,71]. Because COVID-19 vaccines have been introduced in many countries under emergency approval, many HCWs as well as the general population exhibit vaccine hesitancy because of safety concerns and doubts about vaccine effectiveness. Vaccine hesitancy among HCWs is associated with the level of vaccine hesitancy among the public [72]. In particular, healthcare providers’ recommendations play a decisive role in the decision to get vaccinated among patients with a pre-existing condition [73]. Reducing vaccine hesitancy among HCWs is critical for increasing the vaccination rate in the general population; as such, some countries have made COVID-19 vaccination mandatory among HCWs [74]. However, given the concerns and anxiety regarding the hasty development of the COVID-19 vaccines, mandatory vaccination is not desirable. Instead, vaccination should be encouraged by transparent disclosure of the adverse reactions and effectiveness and repeated education of HCWs about the safety and effectiveness of the vaccines based on scientific evidence in order to change their perceptions [72].
Key question 4: How does COVID-19 affect social stigma for HCWs, and what can be done to prevent this?
1. An infectious disease pandemic, such as the COVID-19 pandemic, triggers stigma and discrimination against HCWs (level of evidence: moderate).
2. Stigmatization of HCWs intensifies their fatigue and burnout, thereby impairing their work competence (level of evidence: moderate).
3. To minimize stigmatization of HCWs, accurate and prompt delivery of COVID-19-related information or guidelines is essential (level of evidence: low, strength of recommendation: strong).
4. To minimize the impact of COVID-19-related stigma, HCWs must be provided with psychological support or counseling (level of evidence: low, strength of recommendation: strong).
Stigma refers to the attitude or belief that drives an individual to refuse, avoid, or fear individuals or groups linked to a specific trait (e.g., race, sex, and disease), event, or person. Stigma acts through a series of social processes involving categorization and discrimination against others, stopping them from seizing life opportunities or oppressing them. In healthcare, stigma limits individuals from seeking timely care because of personal or collective beliefs about a particular disease.
The stigma associated with an infectious disease is aggravated by fear of the disease, and HCWs, particularly frontline HCWs, are more likely to become stigmatized [75]. The risk of stigma associated with an infectious disease may be elevated if the novel infectious disease is life-threatening with no known treatment or cure [76].
Studies report that frontline HCWs are considered the potential cause of the infectious disease by their community and experience subsequent stigma, even though they provide healthcare services to patients who are exposed to the virus and risk their lives. This can manifest in refusal to receive services, home-related problems, verbal violence, gossip, and social defamation; moreover, the families of affected HCWs are vulnerable to “secondary” stigma [77].
A study that evaluated 10,511 HCWs involved in SARS care reported that a substantial proportion of participants experienced social stigma (49.0%) and were alienated by their families (31.0%), and 31% believed their families were ostracized because of their occupation [78].
In an online study conducted on 3,551 non-HCWs in the US and Canada during a rapid spike in COVID-19 cases, a considerable share of participants stated that HCWs should be prohibited from visiting public places, have their freedom restricted, be socially isolated from the community, and be separated from their families [79]. In an online study regarding COVID-19-related stigma that involved 7,411 individuals (including 837 HCWs) in 173 countries, the odds for COVID-19-related bullying or stigma were significantly higher among HCWs even after adjusting for occupational, personal, geographic, and sociocultural confounders (adjusted odds ratio:1.5, 95% confidence interval: 1.2 - 2.0) [62]. Many cases of violence, bullying, or stigmatization against HCWs, patients, and health-based facilities linked to the COVID-19 pandemic have been reported worldwide, and HCWs were the victims in 67% of recorded violence and bullying cases [80].
The stigma associated with COVID-19 has several adverse effects on HCWs. A study on the Middle East respiratory syndrome (MERS) epidemic reported that stigma and discrimination associated with an infectious disease influenced the self-efficacy of HCWs and increased their psychological distress and physical symptoms [81]. The stigma associated with COVID-19 also increases fatigue and burnout and reduces work satisfaction, thereby causing substantial impairments in the work competence of HCWs [82,83]. In particular, individuals who experienced discrimination and stigma were at greater risk for mental disorders, including anxiety, depression, PTSD, and suicidal impulses [76].
People tend to stigmatize HCWs because they perceive COVID-19 as a highly hazardous illness, fear exposure to an object potentially contaminated by the causative virus, and fear the socioeconomic impact of COVID-19 [79]. COVID-19-associated stigmatization of HCWs may be part of the inclination to overestimate the threat to health and exaggerate perceptions. Media coverage can provoke fear and anxiety among individuals [84]. Selective media reporting of COVID-19 arouses fear of infection, impairs logical thinking, and can lead to social stigmatization or discrimination against vulnerable sectors of society [82]. In particular, the risk of fake news increases amid massive volumes of news coverage, which consequently escalates anxiety or uncertainty, thereby having an adverse impact such as stigma. Prompt delivery of accurate information and clear guidelines may be important factors in avoiding fear or stigma. Therefore, legal measures that impose legal liability for reporting inaccurate information must be implemented [82]. To reduce the stigmatization of HCWs, reliable and credible organizations must promote clear messages that increase COVID-19 awareness without provoking fear [62,75,82]. Furthermore, stigma associated with COVID-19 has several adverse psychological and physical effects. Thus, psychological support or counseling to alleviate the impact of stigma should be provided for HCWs [82].
Key question 5: How does quarantine due to COVID-19 affect HCWs, and what can be done to prevent this?
1. When placing HCWs under quarantine because of COVID-19 exposure, adequate information and evidence about the quarantine process must be provided by government agencies and experts (level of evidence: moderate, strength of recommendation: strong).
2. The psychological state of HCWs placed under quarantine following COVID-19 exposure must be carefully monitored, and colleagues should provide support (level of evidence: moderate, strength of recommendation: strong).
3. HCWs exposed to COVID-19 should be placed in quarantine in a place outside the healthcare facility such that they are not subject to work-related pressure (level of evidence: moderate, strength of recommendation: strong).
HCWs can better understand the reasons for quarantine following COVID-19 exposure than can non-HCWs. However, failure to provide adequate information about quarantine or precautions may have an adverse impact. Although HCWs have a positive attitude toward quarantine to prevent the spread of the virus following COVID-19 exposure, the quarantine can provoke acute stress responses, PTSD symptoms, depressive symptoms, alcohol abuse/dependency symptoms, and alcohol addiction/dependence [85,86]. In addition, HCWs fear infection or spread of the infection to others, have concerns about physical restrictions and colleagues, experience discomfort while remaining under surveillance during the quarantine period, and fear financial loss and stigma [87]. Therefore, adequate evidence and relevant information should be provided to quarantined healthcare providers.
When placing HCWs exposed to COVID-19 under quarantine, their psychological state must be carefully monitored, and an intervention should be provided if needed. Quarantined HCWs suffer from tremendous psychological distress and psychopathology, the effects of which may be retained in the long term, even after 3 years [86]. When HCWs are quarantined, the consequent shortage of staff increases the workload for the rest of the team. Accordingly, it is important to understand how HCWs perceive their co-workers in quarantine. Separation from the workplace or colleagues can also trigger a sense of alienation. As such, emotional support from colleagues is critical. Organizations or managers should provide psychological support to quarantined HCWs and ensure that they are aware of the support received from their colleagues [88].
HCWs should be quarantined outside the healthcare facility, where they are not subject to work-related pressure. According to a 2015 study on the MERS epidemic, HCWs quarantined for an extended period at a hospital displayed depressive symptoms and acute stress disorder for longer durations than did their counterparts quarantined at home [89]. Furthermore, quarantined HCWs may be exposed to additional workload stress compared with non-HCWs [87]. Therefore, emotional interventions should be considered for HCWs who need to be quarantined after COVID-19 exposure. Instead of quarantining them at work, a different location would be more beneficial if the risk of spreading the virus to their families can be minimized [90].
Key question 6: How does COVID-19 affect the families of HCWs, and what can be done to prevent this?
1. HCWs responding to COVID-19 are worried about their families and potentially development of a COVID-19 infection. If possible, they should be allowed to maintain contact and sufficiently communicate with their families and the outside world (level of evidence: moderate, strength of recommendation: strong).
2. Social and public agencies must strive to ensure that HCWs and their families do not suffer from any disadvantages due to social prejudice (level of evidence: very low, strength of recommendation: strong).
3. Appropriate social and mental support needs to be provided for HCWs and their families (level of evidence: high, strength of recommendation, strong).
There is insufficient research on the families of HCWs responding to COVID-19. While some available data show that the presence of and support from one’s family are important sources of mental support, reports have suggested that living with family further elevates anxiety [91,92].
During a nationwide epidemic, the general population intentionally avoided HCWs and their families in public places such as work or school and strove to prohibit them from visiting public places, even work or school, out of fear of potential spread of the virus. Such behaviors and attitudes aggravate the suffering of HCWs because they add mental stress associated with the disadvantages they and their families could face in society, all while being tired and exhausted after caring for their patients [78,79].
HCWs responding to COVID-19 who live with their families are subject to escalated anxiety provoked by their concerns about contracting the virus themselves or spreading it to their families [93]. In particular, children at home or those with a large family scored higher on the anxiety scale. HCWs with children had a higher anxiety score regarding the possibility of spreading the virus to their children than did their counterparts without children, and they intentionally avoided contact with their children, which weakened their family bonds. In a study conducted among nurses in China, having children and stress at work intensified anxiety [94]. A study involving physicians in Pakistan reported that approximately 80% participants were scared of infecting their families, when 60% of them did not even work in hospitals that provided COVID-19 care [95]. Physicians in hospitals that provide COVID-19 care are expected to be more fearful of infecting their families.
If a patient under the care of an HCW dies, the HCW’s fatigue, anxiety, and negative emotions about treatment failure may influence his or her family as well; thus, the incidence and severity of infection among patients under one’s care may be important factors that affect the mental health of HCWs and their families [96].
On the other hand, another study that analyzed the psychology of HCWs responding to COVID-19 found that having two or more children was a protective factor against mental problems [97]. Adequate mental support for the families of HCWs is crucial, and children need more active support. One viable strategy would be to help HCWs actively communicate with their families and friends using communication technologies if face-to-face contact is not possible.
Shanafelt and his research team at the Stanford University School of Medicine published a study on understanding the anxiety of HCWs and identifying measures for anxiety relief during the COVID-19 pandemic. The key triggers of anxiety among HCWs were pertinent to their families, such as being exposed to COVID-19 at work and bringing the virus home, being uncertain whether their organization would provide the necessary support and management should they or their family members develop the infection, using childcare services during extended work hours and school closures, and ensuring support for themselves and their families in response to increased essential needs (food, water, accommodation, and transport) during increased work hours. The research team stressed that governments/organizations must provide active medical and social support to HCWs and their families [98]. Factors such as the need to stay separated because of work and inadequate support for family members were also identified as risk factors for burnout among HCWs [99,100].
To prevent factors that trigger anxiety among HCWs and their families, appropriate social and emotional support is required at the national, regional, and individual levels; to this end, additional epidemiological surveys and other relevant studies are needed to investigate the psychosocial impact on both groups.
CONCLUSION
The present study highlights the effect of COVID-19 on HCWs and their families and suggests intervention methods to prevent COVID-19 transmission and psychological and physical distress. Measures to deal with stigma and quarantine of medical workers exposed to COVID-19 are also discussed. We expect that these guidelines will protect HCWs from infection and burnout and help them continue their response to COVID-19. Furthermore, they will serve as a reference in the event of another emerging infectious disease outbreak in the future.
1. Limitations and contents to be added in subsequent updates
Most studies forming the basis for the recommendations for these guidelines were conducted abroad, and in the absence of sufficient results related to the corresponding domestic situation, precautions should be exercised when preparing and applying the findings of international studies. To overcome this limitation, it is necessary to accumulate data on the impact of domestic COVID-19 response sites and domestic HCWs. In addition, as the epidemic situation of COVID-19 continues to evolve, it may be difficult to immediately apply the content suggested in these guidelines because of changes in government or institutional guidelines. After the COVID-19 outbreak ends, it will become necessary to accumulate domestic data on the long-term effects on HCWs.
2. Conflicts of interest
The development of the guidelines was funded by the MOHW grant for the Patient-Centered Clinical Research Coordinating Center (PACEN) and research to produce evidence for medical technologies to combat infectious diseases. However, the MOHW was not involved in the actual creation of the clinical practice guidelines. The panel that elaborated the guidelines declares no conflicts of interest with government agencies, pharmaceutical companies, hospital organizations, or stakeholders.
3. Plan for revising the guidelines
By reflecting on the results of recent studies conducted in both Korea and other countries, the guidelines will be periodically revised to keep them relevant to the situation in Korea.
4. Note
The Korean version of this manuscript will be secondarily published in "The Korean Journal of Healthcare-associated Infection Control and Prevention" and permissions from both Journals were obtained prior to submission.
Footnotes
Funding: This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC20C0003).
Conflict of Interest: No conflict of interest.
- Conceptualization: KTK.
- Data curation: SYP, HSC, KTK.
- Formal analysis: SYP, HSC, KTK.
- Funding acquisition: KTK.
- Investigation: SYP, HSC, KTK, KMS, STH, SL.
- Methodology: SYP, KTK, USC, SHL.
- Project administration: KTK, SHL.
- Resources: SYP, HSC, KTK, KMS, STH, SL.
- Software: SYP, HSC, KTK, KMS, STH, SL.
- Supervision: KTK.
- Visualization: SYP, KTK.
- Writing – original draft: SYP, HSC, KTK, KMS, STH, and SL.
- Writing – review & editing: SYP, HSC, KTK, KMS, STH, SL, USC, SHL.
SUPPLEMENTARY MATERIAL
Systematic literature search process
References
- 1.Korea Centers for Disease Control and Prevention (KCDC) Coronavirus (COVID-19), Republic of Korea. [Accessed 5 March 2022]. Available at: https://ncov.mohw.go.kr.
- 2.Kim B, Park SY, Jung DS, Jung SI, Oh WS, Kim SW, Peck KR, Chang HH Korean Society of Infectious Diseases. What should we prepare for the next coronavirus disease 2019 outbreak? A survey on the opinions of infectious diseases specialists in South Korea. Korean J Intern Med. 2020;35:1270–1278. doi: 10.3904/kjim.2020.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang Y, Park SY, Kim B, Lee E, Lee S, Son HJ, Park JW, Yu SN, Kim T, Jeon MH, Choo EJ, Kim TH. Infectious diseases physician workforce in Korea. J Korean Med Sci. 2020;35:e428. doi: 10.3346/jkms.2020.35.e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon HH, Kim HI, Kwon KT, Hwang S, Kim SW, Kim Y, Kim HA, Hyun M, Hong HL, Kim MJ, Hur J, Hong KS. Healthcare workforce response to the coronavirus disease outbreak in Daegu, Korea: a multi-center, cross-sectional survey. Infect Chemother. 2022;54:298–307. doi: 10.3947/ic.2022.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billings J, Ching BCF, Gkofa V, Greene T, Bloomfield M. Experiences of frontline healthcare workers and their views about support during COVID-19 and previous pandemics: a systematic review and qualitative meta-synthesis. BMC Health Serv Res. 2021;21:923. doi: 10.1186/s12913-021-06917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyatt GH, Oxman AD, Kunz R, Jaeschke R, Helfand M, Liberati A, Vist GE, Schünemann HJ GRADE Working Group. Incorporating considerations of resources use into grading recommendations. BMJ. 2008;336:1170–1173. doi: 10.1136/bmj.39504.506319.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busch IM, Moretti F, Mazzi M, Wu AW, Rimondini M. What we have learned from two decades of epidemics and pandemics: a systematic review and meta-analysis of the psychological burden of frontline healthcare workers. Psychother Psychosom. 2021;90:178–190. doi: 10.1159/000513733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park C, Hwang JM, Jo S, Bae SJ, Sakong J. COVID-19 Outbreak and Its Association with Healthcare Workers’ Emotional Stress: a Cross-Sectional Study. J Korean Med Sci. 2020;35:e372. doi: 10.3346/jkms.2020.35.e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SY, Kim B, Jung DS, Jung SI, Oh WS, Kim SW, Peck KR, Chang HH Korean Society of Infectious Diseases. Psychological distress among infectious disease physicians during the response to the COVID-19 outbreak in the Republic of Korea. BMC Public Health. 2020;20:1811. doi: 10.1186/s12889-020-09886-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel RS, Bachu R, Adikey A, Malik M, Shah M. Factors related to physician burnout and its consequences: a review. Behav Sci (Basel) 2018;8:98. doi: 10.3390/bs8110098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theorell T. COVID-19 and working conditions in health care. Psychother Psychosom. 2020;89:193–194. doi: 10.1159/000507765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalmasso G, Di Prinzio RR, Gilardi F, De Falco F, Vinci MR, Camisa V, Santoro A, Casasanta D, Raponi M, Giorgi G, Magnavita N, Zaffina S. Effectiveness of psychological support to healthcare workers by the occupational health service: a pilot experience. Healthcare (Basel) 2021;9:732. doi: 10.3390/healthcare9060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geoffroy PA, Le Goanvic V, Sabbagh O, Richoux C, Weinstein A, Dufayet G, Lejoyeux M. Psychological support system for hospital workers during the Covid-19 outbreak: rapid design and implementation of the Covid-Psy hotline. Front Psychiatry. 2020;11:511. doi: 10.3389/fpsyt.2020.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stratton E, Lampit A, Choi I, Calvo RA, Harvey SB, Glozier N. Effectiveness of eHealth interventions for reducing mental health conditions in employees: a systematic review and meta-analysis. PLoS One. 2017;12:e0189904. doi: 10.1371/journal.pone.0189904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kisely S, Warren N, McMahon L, Dalais C, Henry I, Siskind D. Occurrence, prevention, and management of the psychological effects of emerging virus outbreaks on healthcare workers: rapid review and meta-analysis. BMJ. 2020;369:m1642. doi: 10.1136/bmj.m1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SH, Noh JW, Kim Y, Ryoo HW, Park KH, Park SY, Lee S, Cheong HS, Heo ST, Hong KJ, Kim KB, Kwon KT. Expert consensus on measures to promote physical and psychological health among COVID-19-related healthcare workers in Korea using Delphi technique. Infect Chemother. 2022;54:247–257. doi: 10.3947/ic.2021.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Min HS, Moon S, Jang Y, Cho I, Jeon J, Sung HK. The use of personal protective equipment among frontline nurses in a nationally designated COVID-19 hospital during the pandemic. Infect Chemother. 2021;53:705–717. doi: 10.3947/ic.2021.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan AO, Huak CY. Psychological impact of the 2003 severe acute respiratory syndrome outbreak on health care workers in a medium size regional general hospital in Singapore. Occup Med (Lond) 2004;54:190–196. doi: 10.1093/occmed/kqh027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blake H, Bermingham F, Johnson G, Tabner A. Mitigating the psychological impact of COVID-19 on healthcare workers: a digital learning package. Int J Environ Res Public Health. 2020;17:2997. doi: 10.3390/ijerph17092997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan J, Song Z, Miao X, Li H, Li Y, Dong L, Yang J, An X, Zhang Y, Yang L, Zhou N, Yang L, Li J, Cao J, Wang J, Tao J. Skin damage among health care workers managing coronavirus disease-2019. J Am Acad Dermatol. 2020;82:1215–1216. doi: 10.1016/j.jaad.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youakim S. Adverse reactions associated with respirator fit testing of healthcare workers in British Columbia, Canada: a review of compensation claim cases. Arch Environ Occup Health. 2007;62:197–200. doi: 10.3200/AEOH.62.4.197-200. [DOI] [PubMed] [Google Scholar]
- 22.Hu K, Fan J, Li X, Gou X, Li X, Zhou X. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore) 2020;99:e20603. doi: 10.1097/MD.0000000000020603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long H, Zhao H, Chen A, Yao Z, Cheng B, Lu Q. Protecting medical staff from skin injury/disease caused by personal protective equipment during epidemic period of COVID-19: experience from China. J Eur Acad Dermatol Venereol. 2020;34:919–921. doi: 10.1111/jdv.16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacis M, Azor-Ocampo A, Burnett E, Tanasapphaisal C, Coleman B. Prophylactic dressings for maintaining skin integrity of healthcare workers when using N95 respirators while preventing contamination due to the novel coronavirus: a quality improvement project. J Wound Ostomy Continence Nurs. 2020;47:551–557. doi: 10.1097/WON.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chowdhury MM, Bevan N, Ryan K. Covid-19: virtual occupational skin health clinics for healthcare workers. BMJ. 2020;369:m2281. doi: 10.1136/bmj.m2281. [DOI] [PubMed] [Google Scholar]
- 26.Choudhury A, Singh M, Khurana DK, Mustafi SM, Ganapathy U, Kumar A, Sharma S. Physiological effects of N95 FFP and PPE in healthcare workers in COVID intensive care unit: a prospective cohort study. Indian J Crit Care Med. 2020;24:1169–1173. doi: 10.5005/jp-journals-10071-23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jose S, Cyriac MC, Dhandapani M. Health problems and skin damages caused by personal protective equipment: experience of frontline nurses caring for critical COVID-19 patients in intensive care units. Indian J Crit Care Med. 2021;25:134–139. doi: 10.5005/jp-journals-10071-23713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim EC, Seet RC, Lee KH, Wilder-Smith EP, Chuah BY, Ong BK. Headaches and the N95 face-mask amongst healthcare providers. Acta Neurol Scand. 2006;113:199–202. doi: 10.1111/j.1600-0404.2005.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Battista RA, Ferraro M, Piccioni LO, Malzanni GE, Bussi M. Personal protective equipment (PPE) in COVID 19 pandemic: related symptoms and adverse reactions in healthcare workers and general population. J Occup Environ Med. 2021;63:e80–e85. doi: 10.1097/JOM.0000000000002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams J, Hornbeck A, Pollard J, Snyder J. The physiological burden of prolonged PPE use on healthcare workers during long shifts. CDC NIOSH science blog; 2020. Jun 10, [Google Scholar]
- 31.Sprecher AG, Caluwaerts A, Draper M, Feldmann H, Frey CP, Funk RH, Kobinger G, Le Duc JW, Spiropoulou C, Williams WJ. Personal protective equipment for filovirus epidemics: a call for better evidence. J Infect Dis. 2015;212(Suppl 2):S98–100. doi: 10.1093/infdis/jiv153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization (WHO) Occupational safety and health in public health emergencies: a manual for protecting health workers and responders. [Accessed 5 March 2022]. Available at: https://www.who.int/publications/i/item/9789241514347.
- 33.The National Response Team (NRT) Guidance for managing worker fatigue during disaster operations: technical assistance document. [Accessed 5 March 2022]. Available at: https://www.cdc.gov/niosh/topics/oilspillresponse/pdfs/NRT-Fatigue-for-Emergency-Workers.pdf.
- 34.Jones Y, Durand V, Morton K, Ottolini M, Shaughnessy E, Spector ND, O’Toole J ADVANCE PHM Steering Committee. Collateral damage: how COVID-19 is adversely impacting women Physicians. J Hosp Med. 2020;15:507–509. doi: 10.12788/jhm.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Çağlar A, Kaçer İ, Hacımustafaoğlu M, Öztürk B, Öztürk K. Symptoms associated With personal protective equipment among frontline health care professionals during the COVID-19 pandemic. Disaster Med Public Health Prep. 2022;16:987–990. doi: 10.1017/dmp.2020.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC) COVID data tracker. Atlanta, GA: US Department of health and human services; [Accessed 5 March 2022]. Available at: https://covid.cdc.gov/covid-data-tracker/#health-care-personnel. [Google Scholar]
- 37.Epidemiology for public health (EpiCentro) COVID-19 integrated surveillance data in Italy. [Accessed 5 March 2022]. Available at: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-dashboard.
- 38.Medical Tribone. Number of nurses confirmed COVID-19, followed by doctors, dentists, and oriental doctors. [Accessed 5 March 2022]. Available at: http://www.medical-tribune.co.kr/news/articleView.html?idxno=101211.
- 39.Gómez-Ochoa SA, Franco OH, Rojas LZ, Raguindin PF, Roa-Díaz ZM, Wyssmann BM, Guevara SLR, Echeverría LE, Glisic M, Muka T. COVID-19 in health-care workers: a living systematic review and meta-analysis of prevalence, risk factors, clinical characteristics, and outcomes. Am J Epidemiol. 2021;190:161–175. doi: 10.1093/aje/kwaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suárez-García I, Martínez de Aramayona López MJ, Sáez Vicente A, Lobo Abascal P. SARS-CoV-2 infection among healthcare workers in a hospital in Madrid, Spain. J Hosp Infect. 2020;106:357–363. doi: 10.1016/j.jhin.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 42.The Lancet. COVID-19: protecting health-care workers. Lancet. 2020;395:922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbas M, Robalo Nunes T, Martischang R, Zingg W, Iten A, Pittet D, Harbarth S. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrob Resist Infect Control. 2021;10:7. doi: 10.1186/s13756-020-00875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sikkema RS, Pas SD, Nieuwenhuijse DF, O’Toole Á, Verweij J, van der Linden A, Chestakova I, Schapendonk C, Pronk M, Lexmond P, Bestebroer T, Overmars RJ, van Nieuwkoop S, van den Bijllaardt W, Bentvelsen RG, van Rijen MML, Buiting AGM, van Oudheusden AJG, Diederen BM, Bergmans AMC, van der Eijk A, Molenkamp R, Rambaut A, Timen A, Kluytmans JAJW, Oude Munnink BB, Kluytmans van den Bergh MFQ, Koopmans MPG. COVID-19 in health-care workers in three hospitals in the south of the Netherlands: a cross-sectional study. Lancet Infect Dis. 2020;20:1273–1280. doi: 10.1016/S1473-3099(20)30527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Labetoulle R, Detoc M, Gagnaire J, Berthelot P, Pelissier C, Fontana L, Botelho-Nevers E, Gagneux-Brunon A. COVID-19 in health-care workers: lessons from SARS and MERS epidemics and perspectives for chemoprophylaxis and vaccines. Expert Rev Vaccines. 2020;19:937–947. doi: 10.1080/14760584.2020.1843432. [DOI] [PubMed] [Google Scholar]
- 46.Dimcheff DE, Schildhouse RJ, Hausman MS, Jr, Vincent BM, Markovitz E, Chensue SW, Deng J, McLeod M, Hagan D, Russell J, Bradley SF. Seroprevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection among Veterans Affairs healthcare system employees suggests higher risk of infection when exposed to SARS-CoV-2 outside the work environment. Infect Control Hosp Epidemiol. 2021;42:392–398. doi: 10.1017/ice.2020.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider S, Piening B, Nouri-Pasovsky PA, Krüger AC, Gastmeier P, Aghdassi SJS. SARS-Coronavirus-2 cases in healthcare workers may not regularly originate from patient care: lessons from a university hospital on the underestimated risk of healthcare worker to healthcare worker transmission. Antimicrob Resist Infect Control. 2020;9:192. doi: 10.1186/s13756-020-00848-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, Tong X, Wang J, Huang W, Yin S, Huang R, Yang H, Chen Y, Huang A, Liu Y, Chen Y, Yuan L, Yan X, Shen H, Wu C. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect. 2020;81:420–426. doi: 10.1016/j.jinf.2020.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ran L, Chen X, Wang Y, Wu W, Zhang L, Tan X. Risk factors of healthcare workers with coronavirus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020;71:2218–2221. doi: 10.1093/cid/ciaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chatterjee P, Anand T, Singh KJ, Rasaily R, Singh R, Das S, Singh H, Praharaj I, Gangakhedkar RR, Bhargava B, Panda S. Healthcare workers & SARS-CoV-2 infection in India: a case-control investigation in the time of COVID-19. Indian J Med Res. 2020;151:459–467. doi: 10.4103/ijmr.IJMR_2234_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu M, Cheng SZ, Xu KW, Yang Y, Zhu QT, Zhang H, Yang DY, Cheng SY, Xiao H, Wang JW, Yao HR, Cong YT, Zhou YQ, Peng S, Kuang M, Hou FF, Cheng KK, Xiao HP. Use of personal protective equipment against coronavirus disease 2019 by healthcare professionals in Wuhan, China: cross sectional study. BMJ. 2020;369:m2195. doi: 10.1136/bmj.m2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stubblefield WB, Talbot HK, Feldstein LR, Tenforde MW, Ur Rasheed MA, Mills L, Lester SN, Freeman B, Thornburg NJ, Jones ID, Ward MJ, Lindsell CJ, Baughman A, Halasa N, Grijalva CG, Rice TW, Patel MM, Self WH. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for patients with COVID-19-Nashville, Tennessee. Clin Infect Dis. 2021;72:1645–1648. doi: 10.1093/cid/ciaa936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korea Centers for Disease Control and Prevention (KCDC) Response Guideline of healthcare facilities for COVID-19. [Accessed 5 March 2022]. Available at: http://ncov.mohw.go.kr/duBoardList.do.
- 54.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, Lau YC, Wong JY, Guan Y, Tan X, Mo X, Chen Y, Liao B, Chen W, Hu F, Zhang Q, Zhong M, Wu Y, Zhao L, Zhang F, Cowling BJ, Li F, Leung GM. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 55.Hogan CA, Gombar S, Wang H, Röltgen K, Shi RZ, Holubar M, Chang SI, Lee GM, Boyd SD, Zehnder J, Pinsky BA. Large-scale testing of asymptomatic healthcare personnel for severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2021;27:250–254. doi: 10.3201/eid2701.203892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roberts SC, Peaper DR, Thorne CD, Sussman LS, Murray TS, Choi SJ, Pettker CM, Russi MB, Martinello RA. Mass severe acute respiratory coronavirus 2 (SARS-CoV-2) testing of asymptomatic healthcare personnel. Infect Control Hosp Epidemiol. 2021;42:625–626. doi: 10.1017/ice.2021.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivett L, Sridhar S, Sparkes D, Routledge M, Jones NK, Forrest S, Young J, Pereira-Dias J, Hamilton WL, Ferris M, Torok ME, Meredith L, Curran MD, Fuller S, Chaudhry A, Shaw A, Samworth RJ, Bradley JR, Dougan G, Smith KG, Lehner PJ, Matheson NJ, Wright G, Goodfellow IG, Baker S, Weekes MP CITIID-NIHR COVID-19 BioResource Collaboration. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. eLife. 2020;9:e58728. doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395:1418–1420. doi: 10.1016/S0140-6736(20)30917-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grassly NC, Pons-Salort M, Parker EP, White PJ, Ferguson NM Imperial College COVID-19 Response Team. Comparison of molecular testing strategies for COVID-19 control: a mathematical modelling study. Lancet Infect Dis. 2020;20:1381–1389. doi: 10.1016/S1473-3099(20)30630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chin ET, Huynh BQ, Chapman LAC, Murrill M, Basu S, Lo NC. Frequency of routine testing for coronavirus disease 2019 (COVID-19) in high-risk healthcare environments to reduce outbreaks. Clin Infect Dis. 2021;73:e3127–e3129. doi: 10.1093/cid/ciaa1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee U, Kim SE, Lee SY, Wi HN, Choi O, Park JW, Kim D, Kim YJ, Shin HY, Kim M, Kim EJ, Kang SJ, Jung SI, Park KH. Source analysis and effective control of a COVID-19 outbreak in a university teaching hospital during a period of increasing community prevalence of COVID-19. J Korean Med Sci. 2021;36:e179. doi: 10.3346/jkms.2021.36.e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dye TD, Alcantara L, Siddiqi S, Barbosu M, Sharma S, Panko T, Pressman E. Risk of COVID-19-related bullying, harassment and stigma among healthcare workers: an analytical cross-sectional global study. BMJ Open. 2020;10:e046620. doi: 10.1136/bmjopen-2020-046620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC C4591001 clinical trial group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T COVE study group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Voysey M, Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJ, Emary KR, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SH, Izu A, Jackson S, Jenkin D, Joe CC, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AV, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O’Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DP, Vekemans J, Villafana TL, Watson ME, Williams CJ, Douglas AD, Hill AV, Lambe T, Gilbert SC, Pollard AJ Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paris C, Perrin S, Hamonic S, Bourget B, Roué C, Brassard O, Tadié E, Gicquel V, Bénézit F, Thibault V, Garlantézec R, Tattevin P. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in healthcare workers: an observational study using surveillance data. Clin Microbiol Infect. 2021;27:1699.e5–1699.e8. doi: 10.1016/j.cmi.2021.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes A, Lutrick K, Kuntz JL, Dunnigan K, Odean MJ, Hegmann KT, Stefanski E, Edwards LJ, Schaefer-Solle N, Grant L, Ellingson K, Groom HC, Zunie T, Thiese MS, Ivacic L, Wesley MG, Lamberte JM, Sun X, Smith ME, Phillips AL, Groover KD, Yoo YM, Gerald J, Brown RT, Herring MK, Joseph G, Beitel S, Morrill TC, Mak J, Rivers P, Harris KM, Hunt DR, Arvay ML, Kutty P, Fry AM, Gaglani M. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - Eight U.S. Locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hall VJ, Foulkes S, Saei A, Andrews N, Oguti B, Charlett A, Wellington E, Stowe J, Gillson N, Atti A, Islam J, Karagiannis I, Munro K, Khawam J, Chand MA, Brown CS, Ramsay M, Lopez-Bernal J, Hopkins S SIREN Study Group. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, Frankland TB, Ogun OA, Zamparo JM, Gray S, Valluri SR, Pan K, Angulo FJ, Jodar L, McLaughlin JM. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacDonald NE SAGE working group on vaccine hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine. 2015;33:4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 71.Duong MC, Nguyen HT, Duong BT. Who influences the public intention to get a COVID-19 vaccine and what are the public references and concerns? a population survey in Vietnam. Infect Chemother. 2021;53:753–766. doi: 10.3947/ic.2021.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li M, Luo Y, Watson R, Zheng Y, Ren J, Tang J, Chen Y. Healthcare workers’ (HCWs) attitudes and related factors towards COVID-19 vaccination: a rapid systematic review. Postgrad Med J. 2021 doi: 10.1136/postgradmedj-2021-140195. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 73.Chun JY, Kim SI, Park EY, Park SY, Koh SJ, Cha Y, Yoo HJ, Joung JY, Yoon HM, Eom BW, Park CM, Han JY, Kim M, Lee DW, Kim JW, Keam B, Lee M, Kim TM, Choi YJ, Chang YJ, Lim MC. Cancer patients’ willingness to take COVID-19 vaccination: a nationwide multicenter survey in Korea. Cancers (Basel) 2021;13:3883. doi: 10.3390/cancers13153883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shekhar R, Sheikh AB, Upadhyay S, Singh M, Kottewar S, Mir H, Barrett E, Pal S. COVID-19 vaccine acceptance among health care workers in the United States. Vaccines (Basel) 2021;9:119. doi: 10.3390/vaccines9020119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bagcchi S. Stigma during the COVID-19 pandemic. Lancet Infect Dis. 2020;20:782. doi: 10.1016/S1473-3099(20)30498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brewis A, Wutich A, Mahdavi P. Stigma, pandemics, and human biology: looking back, looking forward. Am J Hum Biol. 2020;32:e23480. doi: 10.1002/ajhb.23480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ransing R, Ramalho R, de Filippis R, Ojeahere MI, Karaliuniene R, Orsolini L, Pinto da Costa M, Ullah I, Grandinetti P, Gashi Bytyçi D, Grigo O, Mhamunkar A, El Hayek S, Essam L, Larnaout A, Shalbafan M, Nofal M, Soler-Vidal J, Pereira-Sanchez V, Adiukwu F. Infectious disease outbreak related stigma and discrimination during the COVID-19 pandemic: Drivers, facilitators, manifestations, and outcomes across the world. Brain Behav Immun. 2020;89:555–558. doi: 10.1016/j.bbi.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koh D, Lim MK, Chia SE, Ko SM, Qian F, Ng V, Tan BH, Wong KS, Chew WM, Tang HK, Ng W, Muttakin Z, Emmanuel S, Fong NP, Koh G, Kwa CT, Tan KB, Fones C. Risk perception and impact of Severe Acute Respiratory Syndrome (SARS) on work and personal lives of healthcare workers in Singapore: what can we learn? Med Care. 2005;43:676–682. doi: 10.1097/01.mlr.0000167181.36730.cc. [DOI] [PubMed] [Google Scholar]
- 79.Taylor S, Landry CA, Rachor GS, Paluszek MM, Asmundson GJG. Fear and avoidance of healthcare workers: an important, under-recognized form of stigmatization during the COVID-19 pandemic. J Anxiety Disord. 2020;75:102289. doi: 10.1016/j.janxdis.2020.102289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Devi S. COVID-19 exacerbates violence against health workers. Lancet. 2020;396:658. doi: 10.1016/S0140-6736(20)31858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Almutairi AF, Adlan AA, Balkhy HH, Abbas OA, Clark AM. “It feels like I’m the dirtiest person in the world.”: Exploring the experiences of healthcare providers who survived MERS-CoV in Saudi Arabia. J Infect Public Health. 2018;11:187–191. doi: 10.1016/j.jiph.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muhidin S, Vizheh M, Moghadam ZB. Anticipating COVID-19-related stigma in survivors and health-care workers: Lessons from previous infectious diseases outbreaks - an integrative literature review. Psychiatry Clin Neurosci. 2020;74:617–618. doi: 10.1111/pcn.13140. [DOI] [PubMed] [Google Scholar]
- 83.Ramaci T, Barattucci M, Ledda C, Rapisarda V. Social Stigma during COVID-19 and its impact on HCWs outcomes. Sustainability. 2020;12:3834. [Google Scholar]
- 84.Chaiuk TA, Dunaievska OV. Fear culture in media: an examination on coronavirus discourse. J Hist Cult Art Res. 2020;9:184–194. [Google Scholar]
- 85.Wu P, Liu X, Fang Y, Fan B, Fuller CJ, Guan Z, Yao Z, Kong J, Lu J, Litvak IJ. Alcohol abuse/dependence symptoms among hospital employees exposed to a SARS outbreak. Alcohol Alcohol. 2008;43:706–712. doi: 10.1093/alcalc/agn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu X, Kakade M, Fuller CJ, Fan B, Fang Y, Kong J, Guan Z, Wu P. Depression after exposure to stressful events: lessons learned from the severe acute respiratory syndrome epidemic. Compr Psychiatry. 2012;53:15–23. doi: 10.1016/j.comppsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gómez-Durán EL, Martin-Fumadó C, Forero CG. Psychological impact of quarantine on healthcare workers. Occup Environ Med. 2020;77:666–674. doi: 10.1136/oemed-2020-106587. [DOI] [PubMed] [Google Scholar]
- 88.Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin GJ. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sim MR. The COVID-19 pandemic: major risks to healthcare and other workers on the front line. Occup Environ Med. 2020;77:281–282. doi: 10.1136/oemed-2020-106567. [DOI] [PubMed] [Google Scholar]
- 90.Seong SJ, Kim HJ, Yim KM, Park JW, Son KH, Jeon YJ, Hwang JY. Differences between the psychiatric symptoms of healthcare workers quarantined at home and in the hospital after contact with a patient with Middle East respiratory syndrome. Front Psychiatry. 2021;12:659202. doi: 10.3389/fpsyt.2021.659202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun N, Wei L, Shi S, Jiao D, Song R, Ma L, Wang H, Wang C, Wang Z, You Y, Liu S, Wang H. A qualitative study on the psychological experience of caregivers of COVID-19 patients. Am J Infect Control. 2020;48:592–598. doi: 10.1016/j.ajic.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee SH, Juang YY, Su YJ, Lee HL, Lin YH, Chao CC. Facing SARS: psychological impacts on SARS team nurses and psychiatric services in a Taiwan general hospital. Gen Hosp Psychiatry. 2005;27:352–358. doi: 10.1016/j.genhosppsych.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alzaid EH, Alsaad SS, Alshakhis N, Albagshi D, Albesher R, Aloqaili M. Prevalence of COVID-19-related anxiety among healthcare workers: a cross-sectional study. J Family Med Prim Care. 2020;9:4904–4910. doi: 10.4103/jfmpc.jfmpc_674_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu W, Wang H, Lin Y, Li L. Psychological status of medical workforce during the COVID-19 pandemic: a cross-sectional study. Psychiatry Res. 2020;288:112936. doi: 10.1016/j.psychres.2020.112936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Urooj U, Ansari A, Siraj A, Khan S, Tariq H. Expectations, fears and perceptions of doctors during Covid-19 pandemic. Pak J Med Sci. 2020;36(COVID19-S4):S37–S42. doi: 10.12669/pjms.36.COVID19-S4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Şahan E, Tangılntız A. State and trait anxiety among medical staff during the first month of COVID-19 pandemic: a sample from Turkey. Int J Psychiatry Med. 2022;57:338–356. doi: 10.1177/00912174211042698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu J, Liu X, Xiao Y, Fang X, Cheng Y, Zhang J. Effect of EAP psychological intervention on improving the mental health of medical workers under the novel coronavirus epidemic in China. Front Public Health. 2021;9:649157. doi: 10.3389/fpubh.2021.649157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shanafelt T, Ripp J, Trockel M. Understanding and addressing sources of anxiety among health care professionals during the COVID-19 pandemic. JAMA. 2020;323:2133–2134. doi: 10.1001/jama.2020.5893. [DOI] [PubMed] [Google Scholar]
- 99.Kim JS, Choi JS. Factors influencing emergency nurses’ burnout during an outbreak of Middle East respiratory syndrome coronavirus in Korea. Asian Nurs Res (Korean Soc Nurs Sci) 2016;10:295–299. doi: 10.1016/j.anr.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seo YE, Kim HC, Yoo SY, Lee KU, Lee HW, Lee SH. Factors associated with burnout among healthcare workers during an outbreak of MERS. Psychiatry Investig. 2020;17:674–680. doi: 10.30773/pi.2020.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Systematic literature search process