Abstract
Background
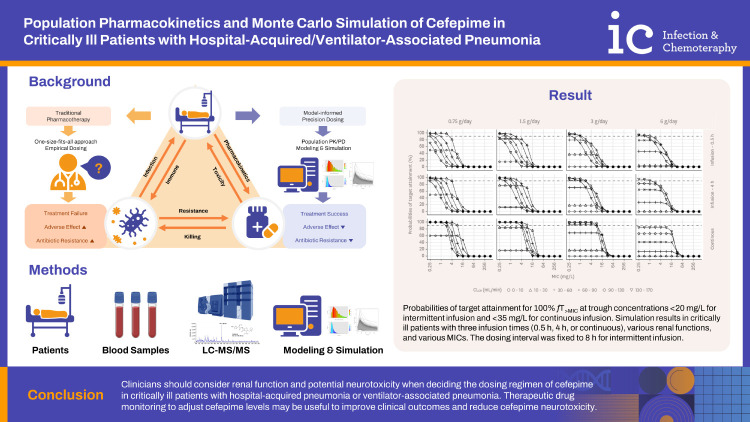
This study aimed to investigate the population pharmacokinetics (PK) profile and determine the optimal dosage regimen of cefepime in critically ill adult patients with hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP).
Materials and Methods
Population-PK models for cefepime were developed using a nonlinear mixed-effect modeling approach. The percentage of time within 24 h in which the free concentration exceeded the minimum inhibitory concentration (MIC) at a steady state (50%fT>MIC, 100%fT>MIC, and 100%fT>4×MIC) for various combinations of dosage regimens and renal function was explored using Monte Carlo simulation.
Results
Twenty-one patients were prospectively enrolled in this study. Cefepime PK was best described using a two-compartment model in which creatinine clearance (CLCR) through Cockcroft-Gault (CG) was a significant covariate for the total clearance of cefepime. The simulation results to determine the optimal cefepime dosing regimen for 50%fT>MIC as treatment target with Cmin <20 mg/L as safety target showed that a dosage regimen of 2 g through intravenous (IV) infusion every 12 h administered over 4 h was optimal at an MIC of 4 mg/L, rather than the currently recommended dosage regimen of 2 g administered through IV infusion every 8 h, in patients with normal renal function (CLCR = 90 - 130 mL/min). For a treatment target of 100%fT>MIC with Cmin <35 mg/L as a safety target, a dosage regimen of 0.75 g administered through continuous infusion over 24 h would be sufficient at an MIC equal to or less than 8 mg/L in patients with renal dysfunction (CLCR = 10 - 30 mL/min).
Conclusion
Our results suggest that clinicians should consider renal function and potential neurotoxicity when deciding the dosing regimen of cefepime in critically ill patients with HAP or VAP. Therapeutic drug monitoring (TDM) to adjust cefepime trough levels may be useful to improve clinical outcomes and reduce cefepime neurotoxicity.
Keywords: Cefepime, Population pharmacokinetics, Hospital-acquired pneumonia, Ventilator-associated pneumonia, Monte Carlo simulation
Graphical Abstract
INTRODUCTION
Cefepime, a fourth-generation cephalosporin, also known as extended-spectrum cephalosporin, is active against most Gram-negative bacilli, including Pseudomonas aeruginosa, and maintains its activity against Gram-positive cocci [1]. Cefepime is less susceptible to inactivation by AmpC β-lactamases. Therefore, cefepime is thought to be an alternative to carbapenem for the treatment of infections caused by AmpC β-lactamase-producing Enterobacteriaceae [2]. Furthermore, cefepime is considered to exhibit high epithelial lining fluid penetration [3]. Therefore, empirical treatment of hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) is recommended [4].
However, there have been concerns regarding the neurological toxicity of cefepime, especially in patients with reduced renal clearance [5], and this is thought to be related to the high plasma concentration of cefepime [6]. Although critically ill patients with organ dysfunction may develop decreased renal clearance, predicting cefepime neurotoxicity is challenging because cefepime therapeutic drug monitoring (TDM) is not routinely performed in all hospitals.
Although previous studies have suggested optimal antibiotic usage based on pharmacokinetics (PK) and pharmacodynamics (PD) for the treatment of HAP and VAP, especially in critically ill patients [3], this is not always feasible in the clinical setting. The PK variability of β-lactams in critically ill patients has been described in previous studies [7,8,9]. When patients are in sepsis, they become hyperdynamic, which leads to increased renal blood flow and glomerular infiltration, resulting in increased clearance of β-lactams [9]. Suboptimal dosing is thought to be associated with reduced efficacy [9] and emergence of antibiotic resistance [10].
Therefore, an optimal dosage regimen based on PK/PD data is needed to reduce the potential toxicity and maximize the clinical benefit of cefepime. This study aimed to investigate the population PK profile and determine the optimal dosage regimen of cefepime in critically ill adult patients with HAP or VAP, especially for the prevention of neurotoxicity.
MATERIALS AND METHODS
1. Study design
Eligible patients participated in the initiation of cefepime administration. The enrolled patients were administered 2 g cefepime (Boryung, Seoul, Korea) for 30 min every 8, 12, or 24 h through intravenous (IV) infusion. Seven blood samples were collected during the first dose period. The planned sampling times for model development were as follows: (1) immediately before dosing and 35, 45, 60, 120, 240, and 480 min after beginning the infusion for the 8-h interval administration; (2) immediately before dosing and 35, 45, 60, 240, 480, and 720 min after beginning the infusion for the 12-h interval administration; and (3) immediately before dosing and 35, 45, 60, 360, 720, and 1,440 min after beginning the infusion for the 24-h interval administration.
2. Ethics statement
This clinical PK study was approved by the Institutional Review Board of Hallym University Sacred Heart Hospital (IRB No. 2019-05-033). A written informed consent form was signed by the subject or the subject's legally authorized representative prior to enrollment. This study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice. For an unconscious patient, a legal representative of each patient signed a written informed consent form prior to participation.
3. Patients
This prospective study was performed at an 840-bed university-affiliated tertiary referral hospital between September 2019 and November 2019 (Hallym University Sacred Heart Hospital, Anyang, Korea). Clinical indications for cefepime include nosocomial pneumonia and the empirical management of septic shock from an unknown source. Patients with a history of β-lactam allergy were excluded.
4. Cefepime assay
Cefepime plasma concentrations were analyzed using a high-performance liquid chromatography (HPLC)-tandem mass spectrometry (MS) assay. The HPLC system consisted of an LC-20A system (Shimadzu, Kyoto, Japan) and Gemini C18 column (Phenomenex, Torrance, CA, USA). MS detection was performed using a quadrupole mass spectrometer (API4000 QTRAP system; SCIEX, Framingham, MA, USA). To prepare the calibration curves, a 100 mm3 aliquot of plasma sample was pipetted into a centrifuge tube. Next, 100 mm3 of acetonitrile containing an internal standard (20 mg/L [Sigma-Aldrich, St. Louis MO, USA]) was added to the tube and vortexed for 1 min. After centrifugation at 12,000 rpm at 4°C for 2 min, the supernatant was transferred to the vial of an autoinjector and diluted 20 times with 20 mM ammonium acetate. An aliquot of 10 mm3 was injected into the LC-MS/MS system. Similar to the calibration curve preparation process, ceftazidime, an internal standard, was added to 100 mm3 of plasma sample, pre-treated with acetonitrile, and then analyzed using LC-MS/MS. From the obtained chromatogram, the ratio of the peak area of cefepime to that of the internal standard was calculated, and the concentration of cefepime in plasma was calculated using the calibration curve equation (weighted by 1/x2) prepared for each batch.
5. Population PK analysis
Population PK analysis was conducted using nonlinear mixed-effects modeling software (version 7.5, NONMEM®, ICON Clinical Research LLC, North Wales, PA, USA). The first-order conditional estimation with the interaction (FOCEI) method was used to estimate the measured and unexplained random effect parameters. The FOCEI allows interaction between the unexplained inter-individual variability (IIV) of PK parameters and the unexplained residual unexplained variability (RUV) of the measured concentrations. RUV was caused by measurement error, assay error, intra-individual variability, and model misspecification. One-, two-, and three-compartment PK model building was performed using ADVAN1 TRANS2, ADVAN3 TRANS4, and ADVAN11 TRANS 4, respectively, from the NONMEM model library. All PK processes, except zero-order infusion, were assumed to follow first-order kinetics. The PK parameter was defined as i = [INSERT FIGURE 001] exp (ηi), where is the typical value of the PK parameter, i is an individual PK parameter, and ηi is a random variable associated with IIV, which was assumed to have a normal distribution with a mean of 0 and a variance of ω2. Additive, proportional, or combined additive and proportional error models were tested for RUV, which was assumed to have a normal distribution with a mean of 0 and a variance of σ2.
The models were evaluated and selected based on NONMEM objective function values (OFVs), precision of parameter estimates (relative standard errors), shrinkage of IIV, and diagnostic goodness-of-fit plots. In a log-likelihood ratio test, a decrease in the OFV (ΔOFV) between two nested models, with one degree of freedom greater than 3.84 or two degrees of freedom greater than 5.99, were considered statistically significant at P <0.05 for model improvement. Diagnostic plots included the following: conditional weighted residuals (CWRES) vs. time, CWRES vs. population predictions (PRED), observation vs. PRED, and observation vs. individual predictions [11]. Perl-speaks-NONMEM software (version 5.3.0, PSN, [https://uupharmacometrics.github.io/PsN], Uppsala University, Uppsala, Svealand, Sweden) was used to search for covariates, evaluate a model with a visual predictive check, and conduct nonparametric bootstrap. Stepwise forward selection and backward elimination processes were performed to search for significant covariates for PK parameters. Statistical significance was set at P <0.01 (ΔOFV <-6.635 for one degree of freedom) for selection and P <0.001 (ΔOFV > 10.83 for one degree of freedom) for elimination. A significant covariate should have statistical and clinical relevance. The tested covariates for PK parameters were sex, age, weight, height, body surface area (BSA), serum protein level, serum albumin level, serum creatinine level, and renal function. The renal function was calculated by applying Cockcroft-Gault (CG) formulations, Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), modified CKD-EPI, Modification of Diet in Renal Disease (MDRD), or modified MDRD. The modified CKD-EPI and the modified MDRD estimates were adjusted using individual BSA values, where BSA was calculated by applying the Du Bois formula [12]. Visual predictive check with prediction and variability-correction (VPCPVC) [13] was performed using PSN software by comparing the final PK model with the observed concentrations with 80% prediction intervals from 1,000 virtual datasets. The median and 95% confidence intervals for the PK parameter estimates of bootstrap samples (n = 2,000) were generated to examine the potential predictive variation in estimates of the final PK model. R software (version 4.1.2, [www.rproject.org], Vienna, Austria) was used to post-process the modeling results and for visualization.
6. PD target attainment
Three Monte Carlo simulations were performed. The first simulation was conducted to explore the appropriateness of the recommended dosage regimen for empirical treatment (creatinine clearance [CLCR] >60 mL/min, 2 g every 8 h through extended 4 h IV infusion; for a CLCR of 30 - 60 mL/min, 2 g every 12 h through IV infusion over 4 h; for a CLCR of 11 - 29 mL/min, 2 g every 24 h through IV infusion over 4 h; and for a CLCR <11 mL/min, 1 g every 24 h through IV infusion over 4 h) of nosocomial pneumonia. A total of 2,000 individual PK parameters were generated for virtual patients, assuming a log-normal distribution for each parameter, with the typical parameter values and the IIV of the final PK model. The selected covariate, creatinine clearance calculated using the CG formulations, was generated using a uniform distribution within the range of 0 - 130 mL/min. Virtual patients were assigned to 13 renal function groups (0 - 130 mL/min in increments of 10 mL/min). Then, 2,000 minimum inhibitory concentrations (MICs) from 0.25 - 128 mg/L were generated and randomly assigned to virtual patients. The MIC distribution of cefepime against Escherichia coli, Klebsiella pneumonia, and P. aeruginosa, collected globally by the European Committee on Antimicrobial Susceptibility Testing (available at: https://mic.eucast.org/Eucast2/accessed on 2022-AUG-22), was used to generate MICs. Two thousand concentration-time (in minutes) profiles at steady state were generated using the 2,000 PK parameters and tested for three treatment targets of the PK/PD index. The antimicrobial activity of cefepime is related to the cumulative percentage of a 24 h period during which the unbound drug concentration exceeds the MIC for a pathogen under steady-state conditions (fT>MIC; f, fraction unbound). The f was fixed at 81% [14]. The tested treatment targets were 50%fT>MIC, 100%fT>MIC, and 100%fT>4xMIC. A dosage strategy was considered adequate if the probability of target attainment (PTA) is greater than or equal to 90%. For each treatment target, three steady-state minimum concentration (Cmin) levels of less than 7.5 mg/L [15], 20 mg/L, and 35 mg/L [6] were also evaluated as safety targets.
The second and the third simulations were conducted to determine the optimal dosage regimens for 50%fT>MIC, 100%fT>MIC, and 100%fT>4XMIC as treatment targets, with Cmin <20 mg/L as a safety target. A total of 1,000 individual PK parameters were generated for virtual patients, assuming a log-normal distribution for each parameter, whereas the covariate was generated by applying a uniform distribution within the range of 0 - 170 mL/min. Patients were divided into six renal function groups (0 <CLCR ≤10, 11 ≤CLCR ≤29, 30 <CLCR ≤60, 60 <CLCR ≤90, 90 <CLCR ≤130, and 130 <CLCR ≤170 mL/min). For the second simulation to evaluate the treatment target of 50%fT>MIC, the steady-state concentration-time profiles of the 1,000 virtual patients were tested for various combinations of the four doses (0.25, 0.5, 1, and 2 g), three dosing intervals (8, 12, and 24 h), three infusion times (0.5, 2, and 4 h), and various MICs (0.25, 0.5, 1, 2, 4, 8, 16, 32, 64, and 128 mg). For the third simulation, to evaluate the treatment target of 100%fT>MIC and 100%fT>4XMIC, the PK profiles were tested for various combinations of the four doses (0.25, 0.5, 1, and 2 g), a dosing interval of 8 h, three infusion times (30 min, extended 4 h, and continuous infusion), and various MICs (0.25, 0.5, 1, 2, 4, 8, 16, 32, 64, and 128 mg/L).
RESULTS
1. Patient characteristics
During the study period, 21 patients (12 men, 9 women) were enrolled. The demographic and clinical characteristics of the patients are shown in Table 1. The median serum creatinine levels (interquartile range [IQR]) were 0.80 mg/dL (0.53 - 1.12 mg/dL) and the median CLCR values (IQR) by the CG equation were 77.2 mL/min (45.3 - 125 mL/min). Creatinine levels in only two patients were >2 mg/dL (3.44 mg/dL and 2.71 mg/dL).
Table 1. Patients characteristics.
| Parameters | Mean (SD) or No. | Median (IQR) |
|---|---|---|
| Sex | Male 12 / Female 9 | |
| Age (yr) | 67.10 (10.47) | 67 (62 - 76) |
| Height (cm) | 164.05 (8.64) | 165 (155 - 172) |
| Weight (kg) | 59.68 (10.53) | 60 (54.4 - 65) |
| Body surface area (m2) | 1.64 (0.17) | 1.60 (1.56 - 1.75) |
| SOFA | 5.05 (3.04) | 4 (4 - 5) |
| APACHE II | 17.33 (4.63) | 17 (14 - 22) |
| Protein (g/dL) | 5.42 (0.67) | 5.5 (4.8 - 5.7) |
| Albumin (g/dL) | 2.87 (0.43) | 2.8 (2.5 - 3.3) |
| BUN (mg/dL) | 22.48 (9.60) | 22.1 (15.2 - 29.5) |
| Scr (mg/dL) | 1.07 (0.80) | 0.80 (0.53 - 1.12) |
| CLCR, Cockcroft-Gault (mL/min) | 79.02 (47.67) | 77.2 (45.3 - 125) |
| GFR, MDRD (mL/min/1.73m2) | 97.12 (59.22) | 91.3 (48.9 - 155) |
| GFR, CKD-EPI (mL/min/1.73m2) | 89.18 (24.68) | 94.9 (69.4 - 109) |
| GFR, modified MDRD (mL/min)a | 93.00 (57.53) | 92 (49.5 - 127) |
| GFR, modified CKD-EPI (mL/min)a | 85.31 (26.42) | 84.3 (70.2 - 102) |
aThe modified MDRD and CKD-EPI equations adjusted to individual BSA are GFR (mL/min) = GFR (MDRD or CKD-EPI) × (BSA/1.73m2).
SD, standard deviation; IQR, interquartile range; SOFA, sequential organ failure assessment; BUN, serum blood urea nitrogen level; Scr; serum creatinine level; CLCR, creatinine clearance; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; BSA, body surface area.
2. Cefepime assay
The lower limit of quantitation was 0.5 mg/L. In the daily analysis results, concentration precision and accuracy of the calibration standard were 1.37 - 0.65% and 96.95 - 104.45%, respectively, at concentrations of 0.05, 0.2, 0.5, 2, 5, 20, 50, and 200 mg/L. The coefficient of determination indicating the linearity of the calibration curve over a range of 0.05 - 200 mg/L was greater than 0.99 for all three batches per day. In intraday analysis of quality control samples, the precision was 6.91% at 0.5 mg/L, 1.99% at 5 mg/L, and 6.33% at 50 mg/L. The accuracy was 97.33% at 0.5 mg/L, 102.47 at 5 mg/L, and 111.60% at 50 mg/L. In inter-day analysis, the precision was 1.61% at 0.5 mg/L, 5.92% at 5 mg/L, and 3.47% at 50 mg/L. The accuracy was 98.18% at 0.5 mg/L, 100.45 at 5 mg/L, and 103.44% at 50 mg/L.
3. Population PK analysis
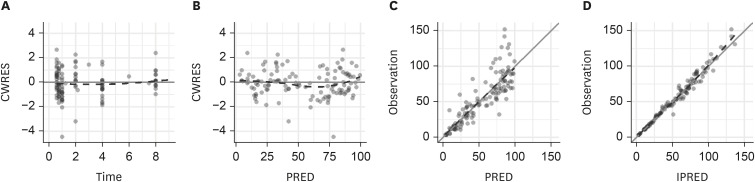
In total, 144 plasma samples were used for the population PK model of cefepime. The concentration-time profile of cefepime was best described using a two-compartment model. The NONMEM OFVs for the one-, two-, and three-compartment structural models without covariates were 307.124, 162.452, and 147.837, respectively. Although the OFV of the three-compartment model was the lowest, it failed to converge. The structural PK parameters for the two-compartment model were the total clearance (CL), central volume of distribution (V1), peripheral volume of distribution (V2), and intercompartmental clearance (Q) between V1 and V2. Inter-individual variability (IIV) was estimated for CL, V1, Q, and V2 (Table 2). In the final PK model (OFV 140.143), CLCR was estimated using the CG equation as a statistically significant covariate of CL. The IIV for CL was reduced from 59.0% to 33.7% after the covariate was included. The diagnostic goodness-of-fit plots for the final PK model are depicted in Figure1. The CWRES are randomly distributed around the zero line, indicating no systemic deviation in the structural (a) or RUV models (b). The observations are also evenly distributed about the line of identity, indicating that there is no evidence of misspecification in the structural, IIV, or RUV models (c, d). Supplementary Figure 1 shows the VPCPVC plots for cefepime. The observed 10th, 50th, and 90th percentiles fell within the 95% confidence intervals of the simulated 10th, 50th, and 90th percentiles, respectively, indicating that the final PK model appropriately explains the observed cefepime concentrations and has a good predictive performance.
Table 2. Population PK parameter estimates for cefepime.
| Parameter | Estimates | RSE (%) [Shrinkage (%)] | Bootstrap median (95% confidence intervals) | ||
|---|---|---|---|---|---|
| Structural model | |||||
| CL = θ1 × (CG/77.21)θ2 | |||||
| θ1 (L/h) | 6.60 | 7.91 | 6.63 (5.55 – 7.64) | ||
| θ2 | 0.656 | 10.7 | 0.650 (0.438 – 0.796) | ||
| V1 (L) | 13.3 | 9.79 | 13.4 (10.8 – 16.5) | ||
| Q (L/h) | 16.5 | 18.7 | 16.2 (9.59 – 23.7) | ||
| V2 (L) | 13.0 | 10.6 | 12.8 (9.87 – 16.3) | ||
| Interindividual variability | |||||
| CL (%) | 33.7 | 25.2 [0.000] | 32.0 (17.0 – 46.8) | ||
| V1 (%) | 34.1 | 24.9 [11.2] | 32.9 (7.52 – 49.5) | ||
| Q (%) | 50.8 | 18.4 [19.6] | 46.5 (0.000 – 65.8) | ||
| V2 (%) | 40.1 | 21.2 [8.84] | 38.6 (16.7 – 54.3) | ||
| Residual variability | |||||
| Proportional error (%) | 7.62 | 9.43 | 7.61 (6.10 – 9.23) | ||
PK, pharmacokinetics; RSE, relative standard error; CL, total clearance; V1, central volume of distribution; Q, intercompartmental clearance between V1 and V2; V2, peripheral volume of distribution.
Figure 1.
Goodness of fit plots for cefepime: (A) conditional weighted residuals (CWRES) versus time, (B) CWRES versus population predicted concentration (PRED), (C) observed concentration versus PRED, and (D) observed concentration versus individual predicted concentration (IPRED). The dashed lines represent smooth curves.
4. PD target attainment
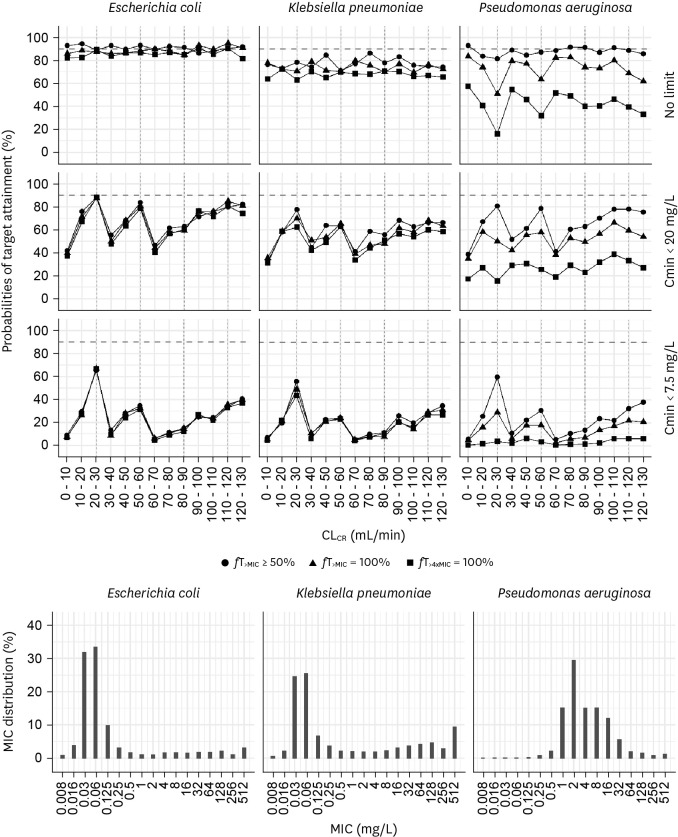
Figure 2 (Supplementary Table 1) and Supplementary Figure 2 (Supplementary Table 2) show the PTA of empirical therapy using the current dosage regimen with extended 4 h infusion and standard 30 min infusion, respectively. For extended infusion, the recommended dosage regimen achieved 90% PTA at 50%fT>MIC when patients were infected with E. coli, whereas this could not attain 90% PTA at 100%fT>4xMIC. When patients were infected with P. aeruginosa, which was distributed where the MIC was higher than that of E. coli, the current regimen did not achieve 90% PTA at 50%fT>MIC (Fig. 2). The probability of achieving the target dropped significantly when a safety target was added to ensure that Cmin did not exceed 7.5, 20, or 35 mg/L.
Figure 2.
Probabilities of target attainment of empirical therapy using the current dosing regimen with a 4-hour extended infusion for patients with CLCR of 0 - 130 mL/min when there is no target trough concentration (Cmin) and when the target Cmin is <20 mg/L or <7.5 mg/L. Bars indicate the MIC distribution for Escherichia coli (left), Klebsiella pneumoniae (middle), and Pseudomonas aeruginosa (right).
MIC, minimum inhibitory concentration.
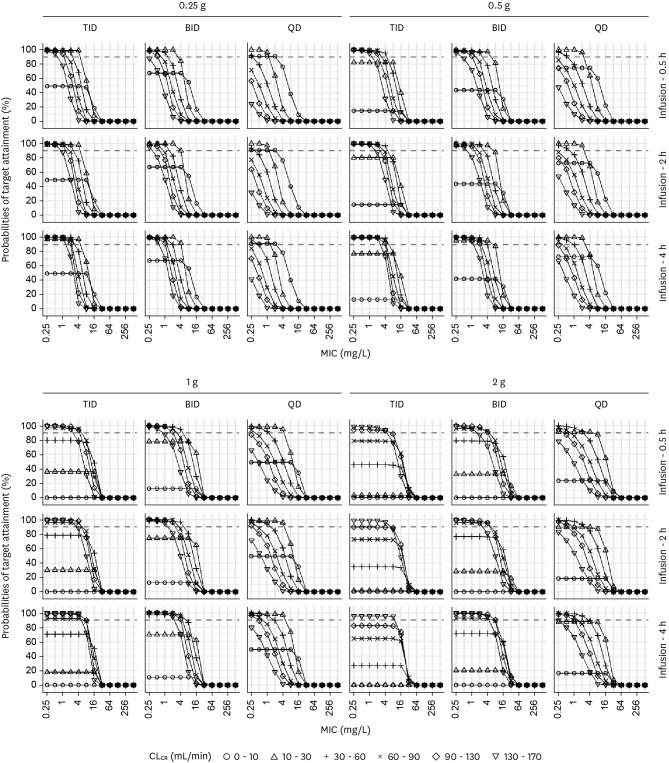
When the safety target of Cmin <20 mg/L was added, the PTAs for the target of 50%fT>MIC with various combinations of renal function, four doses, three dosing intervals, three infusion times, and various MICs are illustrated in Figure 3 (Supplementary Table 3). For patients with a CLCR of 10 - 30 mL/min, a dosage regimen of 0.25 g every 8 h through IV infusion over 0.5 h was optimal when the MIC was 4 mg/L. For patients with a CLCR of 90 - 130 mL/min, a dosage regimen of 2 g every 12 h through IV infusion over 4 h was optimal when the MIC was 4 mg/L, whereas a dosage regimen of 2 g every 8 h through IV infusion over 4 h was not optimal.
Figure 3.
Probabilities of target attainment for 50% fT>MIC at trough concentrations <20 mg/L. Simulation results in critically ill patients with three doses (0.25, 0.5, 1, or 2 g) and three dosing intervals (8, 12, or 24 h), three infusion times (0.5, 2, or 4 h), various renal functions, and various MICs.
MIC, minimum inhibitory concentration; TID, three times a day; BID, twice a day; QD, once a day.
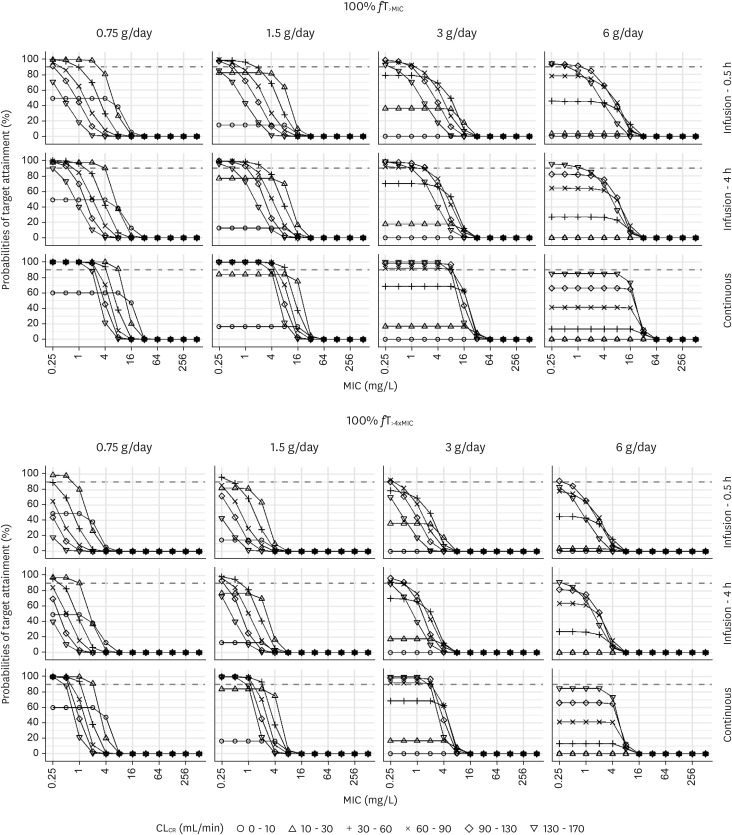
Figure 4 (Supplementary Table 4) shows the PTAs for the target of 100%fT>MIC and 100%fT>4xMIC when the safety targets of Cmin <20 mg/L for intermittent infusion and Cmin <35 mg/L for continuous infusion were added. For patients with an CLCR of 130 - 170 mL/min, a dosage regimen of 3 g/day through continuous IV infusion was optimal when the target was 100%fT>4xMIC and the MIC was 1 mg/L. For patients with a CLCR of 90 - 130 mL/min, a continuous infusion of 3 g/day was optimal rather than a dosage regimen of 1 g every 8 h through IV infusion over 4 h when the target was 100%fT>MIC and the MIC was 8 mg/L.
Figure 4.
Probabilities of target attainment for 100% fT>MIC or 100% fT>4xMIC at trough concentrations <20 mg/L for intermittent infusion and <35 mg/L for continuous infusion. Simulation results in critically ill patients with three infusion times (0.5 h, 4 h, or continuous), various renal functions, and various MICs. The dosing interval was fixed to 8 h for intermittent infusion.
MIC, minimum inhibitory concentration.
DISCUSSION
This study presents the PK properties of cefepime in critically ill adult patients. The PK of cefepime was best described using a two-compartment model in the dense sampling scheme, in which CLCR by CG was a significant covariate of total clearance for cefepime, which was consistent with a previous study reported by Álvarez et al [16]. Our results suggest that current dosing regimens may be inappropriate when the treatment target is 100%fT>MIC and 100%fT>4xMIC rather than 50%fT>MIC. Notably, if not only efficacy but also safety were considered as the treatment goals, these goals could not be achieved with the current one-size-fits-all dosing, and TDM should be considered to achieve efficacy and safety targets simultaneously.
Three PK/PD indices of 50%fT>MIC, 100%fT>MIC, and 100%fT>4xMIC were investigated for efficacy targets. The target of 40 - 50% fT>MIC is appropriate to achieve significant bactericidal activity for amoxicillin and amoxicillin-clavulanate against penicillin susceptible, intermediate resistant, and resistant Streptococcus pneumoniae in two in vivo animal models, and to lead to good efficacy with various β-lactams in patients infected with penicillin intermediate resistant and resistant S. pneumoniae [17,18,19,20]. A more aggressive target of 100%fT>MIC or 100%fT>4xMIC for β-lactams have been proposed in recent studies to achieve improved microbiological and clinical outcomes in critically ill patients [21,22,23,24,25,26]. We also explored three PK/PD indices of Cmin <7.5 mg/L, Cmin <20 mg/L, and <35 mg/L for safety targets, with the three PK/PD indices for efficacy. Boschung-Pasquier et al. suggested targeting a trough concentration of <7.5 mg/L to avoid the risk of cefepime-induced neurotoxicity [15]. Huwyler et al. reported that patients with trough concentrations >20 mg/L and >40 mg/L had a five-fold and a nine-fold higher risk of neurologic events, respectively, and proposed to avoid steady-state Cmin <20 mg/L and Cmin <35 mg/L in patients receiving intermittent and continuous infusion, respectively [6].
Traditionally, prolonged or continuous infusion of β-lactam agents has been advocated in previous studies [27,28,29]. Bauer et al. reported that extended infusion of cefepime 2 g every 8 h over 4 h reduced mortality in patients with P. aeruginosa infection compared to the 30 min infusion [29]. Huang et al. compared continuous versus intermittent infusion of cefepime in neurosurgical patients and reported that continuous infusion significantly enhanced the antibacterial effect and reduced treatment duration [27]. In our study, in patients with a CLCR of 90 - 130 mL/min, a continuous infusion of 3 g/day was more beneficial than a dosage regimen of 1 g every 8 h through IV infusion over 4 h when the target was 100%fT>MIC and the MIC was ≤8 mg/L in case of safety target of Cmin <35 mg/L for continuous infusion and that of Cmin <20 mg for intermittent infusion were considered. Previous studies have shown that cefepime neurotoxicity is associated with high plasma concentrations, especially in patients with renal dysfunction [5,6,15]. In our study, we identified that a dosage regimen of 2 g every 12 h IV infusion over 4 h was optimal at a MIC of 4 mg/L, rather than the currently recommended dosage regimen of 2 g every 8 h through IV infusion, in patients with normal renal function (CLCR = 90 - 130 mL/min) through the simulation of the optimal cefepime dosing regimen for 50%fT>MIC as treatment target with Cmin <20 mg/L as safety target (Fig. 3, Supplementary Table 3). Furthermore, in patients with renal dysfunction (CLCR = 10 - 30 mL/min), a dosage regimen of 0.75 g over 24 h continuous infusion was sufficient at a MIC ≤8 mg/L for a treatment target of 100% fT>MIC with Cmin <35 mg/L as safety target (Fig. 4Supplementary Table 4). Our findings are consistent with a recent study by Cheng et al [30]. Although patient characteristics differed as they evaluated patients receiving extracorporeal membrane oxygenation (ECMO), reduced cefepime clearance in patients receiving ECMO was reported, and the current dosing of cefepime may lead to cefepime toxicity when a safety target of Cmin <20 mg/L is considered. Therefore, TDM of cefepime may be beneficial to avoid neurotoxicity and optimize its effect, which can be reinforced by the current guidelines on antimicrobial stewardship programs in Korea [31].
Previous studies have reported PD target attainment for cefepime in critically ill patients [32,33,34]. Although it was reported that the current dosing regimen succeeded in achieving PD target attainment for bactericidal effects based on 50% or 65% fT>MIC, we evaluated PD target attainment with more diverse parameters, including 50%fT>MIC, 100%fT>MIC, and 100%fT>4xMIC, and added a safety target of Cmin <20 mg/L or <35 mg/L to prevent neurotoxicity. A recent study by Álvarez et al. evaluated PK/PD parameters based on 60%fT>MIC and 100%fT>MIC. [16], and it was reported that intermittent dosing was suboptimal, and continuous infusion could maximize exposure of cefepime. However, they included patients with hematologic malignancies and febrile neutropenia after chemotherapy and did not include safety targets. Therefore, these findings may be attributed to the differences in the results compared with our study.
Our study had some limitations. First, patients from a single center were included, and we did not perform external validation of our suggested dosage regimen. Further study including patients in multicenter studies are required to confirm our findings. Second, we did not evaluate the results of the PK/PD analysis based on clinical outcomes. Despite these limitations, our findings suggest that a more delicate dosage regimen based on renal function is needed to reduce neurotoxicity.
In conclusion, our results suggest that clinicians should consider renal function and potential neurotoxicity when deciding the dosing regimen of cefepime in critically ill patients with HAP or VAP. TDM to adjust cefepime levels may be useful to improve clinical outcomes and reduce cefepime neurotoxicity.
Footnotes
Funding: This study was supported by a grant from Korean Society for Antimicrobial Therapy, 2019.
Conflict of Interest: No conflict of interest.
Author Contributions: Conceptualization: HS, YKK, DHL. Data curation: HS, YKK, DHL. Formal analysis: HS, YKK, DHL. Funding acquisition: YKK. Methodology: HS, YKK, SP, HK, DHL. Software: HS, YKK, DHL. Supervision: YKK, SP, DHL. Writing – original draft: HS, YKK, DHL. Writing – review & editing: HS, YKK, SP, HK, DHL.
SUPPLEMENTARY MATERIALS
Probabilities of target attainment of empirical therapy using the current dosing regimen with a 4-hour extended infusion for patients with CLCR of 0 - 130 mL/min when there is no target trough concentration (Cmin) and when the target Cmin is <20 mg/L or <7.5 mg/L. This table corresponds to Figure 2
Probabilities of target attainment of empirical therapy using the current dosing regimen with a 30-minute infusion for patients with CLCR of 0 - 130 mL/min when there is no target trough concentration (Cmin) and when the target Cmin is <20 mg/L or <7.5 mg/L.
Probabilities of target attainment for 50% fT>MIC at trough concentrations <20 mg/L. Simulation results in critically ill patients with four doses (0.25, 0.5, 1, or 2 g) and three dosing intervals (8, 12, or 24 h), three infusion times (0.5, 2, or 4 h), various renal functions, and various MICs. This table corresponds to Figure 3.
Probabilities of target attainment for 100% fT>MIC or 100% fT>4xMIC at trough concentrations <20 mg/L for intermittent infusion and <35 mg/L for continuous infusion. Simulation results in critically ill patients with three infusion times (0.5 h, 4 h, or continuous), various renal functions, and various MICs. The dosing interval was fixed to 8 h for intermittent infusion.
Visual predictive check from simulated concentrations of 1,000 virtual datasets for cefepime (upper, 0 - 24 h; lower, 0 - 5 h). Closed circles, observed concentrations; solid lines, 10th, 50th and 90th percentiles of observations; dashed lines, 10th, 50th and 90th percentiles of simulated concentrations; and shaded areas, 95% confidence intervals for the 10th, 50th, and 90th percentiles of simulated concentrations.
Probabilities of target attainment of empirical therapy using the current dosing regimen with a 30-minute infusion for patients with CLCR of 0 - 130 mL/min when there is no target trough concentration (Cmin) and when the target Cmin is <20 mg/L or <7.5 mg/L. Bars indicate the MIC distribution for Escherichia coli (left), Klebsiella pneumoniae (middle), and Pseudomonas aeruginosa (right).
References
- 1.Lepak AJ, Andes DR. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Disease. 9th ed. Bennett JE, Dolin R, Blaser MJ, editors. Philadelphia: Elsevier; 2020. Cephalosporins. [Google Scholar]
- 2.D’Angelo RG, Johnson JK, Bork JT, Heil EL. Treatment options for extended-spectrum beta-lactamase (ESBL) and AmpC-producing bacteria. Expert Opin Pharmacother. 2016;17:953–967. doi: 10.1517/14656566.2016.1154538. [DOI] [PubMed] [Google Scholar]
- 3.Sulaiman H, Abdul-Aziz MH, Roberts JA. Pharmacokinetic/pharmacodynamics-optimized antimicrobial therapy in patients with hospital-acquired pneumonia/ventilator-associated pneumonia. Semin Respir Crit Care Med. 2017;38:271–286. doi: 10.1055/s-0037-1602716. [DOI] [PubMed] [Google Scholar]
- 4.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM, Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamoth F, Buclin T, Pascual A, Vora S, Bolay S, Decosterd LA, Calandra T, Marchetti O. High cefepime plasma concentrations and neurological toxicity in febrile neutropenic patients with mild impairment of renal function. Antimicrob Agents Chemother. 2010;54:4360–4367. doi: 10.1128/AAC.01595-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huwyler T, Lenggenhager L, Abbas M, Ing Lorenzini K, Hughes S, Huttner B, Karmime A, Uçkay I, von Dach E, Lescuyer P, Harbarth S, Huttner A. Cefepime plasma concentrations and clinical toxicity: a retrospective cohort study. Clin Microbiol Infect. 2017;23:454–459. doi: 10.1016/j.cmi.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Gómez CM, Cordingly JJ, Palazzo MG. Altered pharmacokinetics of ceftazidime in critically ill patients. Antimicrob Agents Chemother. 1999;43:1798–1802. doi: 10.1128/aac.43.7.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med. 2009;37:840–851. doi: 10.1097/CCM.0b013e3181961bff. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JA, Ulldemolins M, Roberts MS, McWhinney B, Ungerer J, Paterson DL, Lipman J. Therapeutic drug monitoring of beta-lactams in critically ill patients: proof of concept. Int J Antimicrob Agents. 2010;36:332–339. doi: 10.1016/j.ijantimicag.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Roberts JA, Kruger P, Paterson DL, Lipman J. Antibiotic resistance--what’s dosing got to do with it? Crit Care Med. 2008;36:2433–2440. doi: 10.1097/CCM.0b013e318180fe62. [DOI] [PubMed] [Google Scholar]
- 11.Hooker AC, Staatz CE, Karlsson MO. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res. 2007;24:2187–2197. doi: 10.1007/s11095-007-9361-x. [DOI] [PubMed] [Google Scholar]
- 12.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303–311. discussion 312-3. [PubMed] [Google Scholar]
- 13.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamoto MP, Nakahiro RK, Chin A, Bedikian A. Cefepime clinical pharmacokinetics. Clin Pharmacokinet. 1993;25:88–102. doi: 10.2165/00003088-199325020-00002. [DOI] [PubMed] [Google Scholar]
- 15.Boschung-Pasquier L, Atkinson A, Kastner LK, Banholzer S, Haschke M, Buetti N, Furrer DI, Hauser C, Jent P, Que YA, Furrer H, Babouee Flury B. Cefepime neurotoxicity: thresholds and risk factors. A retrospective cohort study. Clin Microbiol Infect. 2020;26:333–339. doi: 10.1016/j.cmi.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 16.Álvarez JC, Cuervo SI, Silva E, Díaz JA, Jiménez LL, Parra DS, Gómez JC, Sánchez R, Cortés JA. Pharmacokinetics and pharmacodynamics of cefepime in adults with hematological malignancies and febrile neutropenia after chemotherapy. Antibiotics (Basel) 2021;10:504. doi: 10.3390/antibiotics10050504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andes D, Craig WA. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob Agents Chemother. 1998;42:2375–2379. doi: 10.1128/aac.42.9.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodnutt G, Berry V. Two pharmacodynamic models for assessing the efficacy of amoxicillin-clavulanate against experimental respiratory tract infections caused by strains of Streptococcus pneumoniae . Antimicrob Agents Chemother. 1999;43:29–34. doi: 10.1128/aac.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heffelfinger JD, Dowell SF, Jorgensen JH, Klugman KP, Mabry LR, Musher DM, Plouffe JF, Rakowsky A, Schuchat A, Whitney CG. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch Intern Med. 2000;160:1399–1408. doi: 10.1001/archinte.160.10.1399. [DOI] [PubMed] [Google Scholar]
- 20.Craig WA. Does the dose matter? Clin Infect Dis. 2001;33(Suppl 3):S233–7. doi: 10.1086/321854. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Du X, Kuti JL, Nicolau DP. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother. 2007;51:1725–1730. doi: 10.1128/AAC.00294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31:345–351. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, DALI Study DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Aziz MH, Lipman J, Mouton JW, Hope WW, Roberts JA. Applying pharmacokinetic/pharmacodynamic principles in critically ill patients: optimizing efficacy and reducing resistance development. Semin Respir Crit Care Med. 2015;36:136–153. doi: 10.1055/s-0034-1398490. [DOI] [PubMed] [Google Scholar]
- 25.Guilhaumou R, Benaboud S, Bennis Y, Dahyot-Fizelier C, Dailly E, Gandia P, Goutelle S, Lefeuvre S, Mongardon N, Roger C, Scala-Bertola J, Lemaitre F, Garnier M. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation-SFAR) Crit Care. 2019;23:104. doi: 10.1186/s13054-019-2378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scharf C, Liebchen U, Paal M, Taubert M, Vogeser M, Irlbeck M, Zoller M, Schroeder I. The higher the better? Defining the optimal beta-lactam target for critically ill patients to reach infection resolution and improve outcome. J Intensive Care. 2020;8:86. doi: 10.1186/s40560-020-00504-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang H, Huang S, Zhu P, Xi X. Continuous versus intermittent infusion of cefepime in neurosurgical patients with post-operative intracranial infections. Int J Antimicrob Agents. 2014;43:68–72. doi: 10.1016/j.ijantimicag.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Sprauten PF, Beringer PM, Louie SG, Synold TW, Gill MA. Stability and antibacterial activity of cefepime during continuous infusion. Antimicrob Agents Chemother. 2003;47:1991–1994. doi: 10.1128/AAC.47.6.1991-1994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bauer KA, West JE, O’Brien JM, Goff DA. Extended-infusion cefepime reduces mortality in patients with Pseudomonas aeruginosa infections. Antimicrob Agents Chemother. 2013;57:2907–2912. doi: 10.1128/AAC.02365-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng V, Abdul-Aziz MH, Burrows F, Buscher H, Corley A, Diehl A, Jakob SM, Levkovich BJ, Pellegrino V, Que YA, Reynolds C, Rudham S, Wallis SC, Welch SA, Zacharias D, Roberts JA, Shekar K, Fraser JF. Population pharmacokinetics of cefepime in critically ill patients receiving extracorporeal membrane oxygenation (an ASAP ECMO study) Int J Antimicrob Agents. 2021;58:106466. doi: 10.1016/j.ijantimicag.2021.106466. [DOI] [PubMed] [Google Scholar]
- 31.Yoon YK, Kwon KT, Jeong SJ, Moon C, Kim B, Kiem S, Kim HS, Heo E, Kim SW Korean Society for Antimicrobial Therapy; Korean Society of Infectious Diseases; Korean Society of Health-System Pharmacist. Guidelines on implementing antimicrobial stewardship programs in Korea. Infect Chemother. 2021;53:617–659. doi: 10.3947/ic.2021.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heil EL, Nicolau DP, Farkas A, Roberts JA, Thom KA. Pharmacodynamic Target Attainment for Cefepime, Meropenem, and Piperacillin-Tazobactam Using a Pharmacokinetic/Pharmacodynamic-Based Dosing Calculator in Critically Ill Patients. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.01008-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roos JF, Bulitta J, Lipman J, Kirkpatrick CM. Pharmacokinetic-pharmacodynamic rationale for cefepime dosing regimens in intensive care units. J Antimicrob Chemother. 2006;58:987–993. doi: 10.1093/jac/dkl349. [DOI] [PubMed] [Google Scholar]
- 34.Ambrose PG, Bhavnani SM, Jones RN. Pharmacokinetics-pharmacodynamics of cefepime and piperacillin-tazobactam against Escherichia coli and Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases: report from the ARREST program. Antimicrob Agents Chemother. 2003;47:1643–1646. doi: 10.1128/AAC.47.5.1643-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Probabilities of target attainment of empirical therapy using the current dosing regimen with a 4-hour extended infusion for patients with CLCR of 0 - 130 mL/min when there is no target trough concentration (Cmin) and when the target Cmin is <20 mg/L or <7.5 mg/L. This table corresponds to Figure 2
Probabilities of target attainment of empirical therapy using the current dosing regimen with a 30-minute infusion for patients with CLCR of 0 - 130 mL/min when there is no target trough concentration (Cmin) and when the target Cmin is <20 mg/L or <7.5 mg/L.
Probabilities of target attainment for 50% fT>MIC at trough concentrations <20 mg/L. Simulation results in critically ill patients with four doses (0.25, 0.5, 1, or 2 g) and three dosing intervals (8, 12, or 24 h), three infusion times (0.5, 2, or 4 h), various renal functions, and various MICs. This table corresponds to Figure 3.
Probabilities of target attainment for 100% fT>MIC or 100% fT>4xMIC at trough concentrations <20 mg/L for intermittent infusion and <35 mg/L for continuous infusion. Simulation results in critically ill patients with three infusion times (0.5 h, 4 h, or continuous), various renal functions, and various MICs. The dosing interval was fixed to 8 h for intermittent infusion.
Visual predictive check from simulated concentrations of 1,000 virtual datasets for cefepime (upper, 0 - 24 h; lower, 0 - 5 h). Closed circles, observed concentrations; solid lines, 10th, 50th and 90th percentiles of observations; dashed lines, 10th, 50th and 90th percentiles of simulated concentrations; and shaded areas, 95% confidence intervals for the 10th, 50th, and 90th percentiles of simulated concentrations.
Probabilities of target attainment of empirical therapy using the current dosing regimen with a 30-minute infusion for patients with CLCR of 0 - 130 mL/min when there is no target trough concentration (Cmin) and when the target Cmin is <20 mg/L or <7.5 mg/L. Bars indicate the MIC distribution for Escherichia coli (left), Klebsiella pneumoniae (middle), and Pseudomonas aeruginosa (right).