Abstract
Protein supplements are extensively used for muscle building, weight loss, recovery from exercise, improving endurance & cardio-performance. Major challenge with protein supplement is undigested protein and impaired gut health which results in nausea, dehydration, diarrhea, constipation, indigestion, stomach pain, and decreased appetite. Several studies have linked plant protein with reduced metabolic syndrome incidence. Probiotics can improve gut health as well. The objective of the study is to assess the efficacy and safety of protein supplement in promoting health and wellbeing in healthy adults. The present trial is a double blind, multi-center, randomized, placebo controlled, clinical trial involving 60 healthy individuals. The treatment duration was of 90 days. The subjects were randomized to receive either protein supplement treatment or placebo control. Protein supplement significantly improved quality-of-life score by 85.76%, VO2 max by 42.92%, distance covered in 6 minutes, 100% individuals with at least 25% reduction in low energy events as compared to the control group. Protein supplement treatment reduced body weight (1.94 kg), waist circumference (2.46 cm), body mass index, waist circumference, hip circumference and body fat. Remarkable and significant improvement in digestive and sleep quality score, percent skeletal muscle was observed among protein supplement treated group. There were no clinically significant changes in hematological, biochemical and vital parameters; indicating safety of protein supplement. Present study concluded that protein supplement is safe and efficacious in weight management, improving high energy events, aerobic capacity, quality of life, digestive behavior score and sleep quality. This study ensures consumers about safety and effectiveness of protein supplement.
Keywords: Protein, Probiotic, Endurance, Oxygen Consumption
INTRODUCTION
Protein and amino acid supplements are extensively marketed as muscle building and improving physical endurance in large consumer base like from athletes, active lifestyle individuals to individuals with sedentary lifestyle [1,2,3]. In another way, low carbohydrate, high protein diet has been used routinely as weight management regimen. Adults appears to be use protein supplement widely for health and fitness purposes like weight loss, recovery from exercise, improving cardio-performance. In case of elderly individuals, largely the protein supplementation is intended at preserving the muscle mass, recovery and maintaining well-being. Protein supplements in growing age are evident to provide the favorable body conditions for growth, development and immunity [2]. There are various applications of a good protein source in keeping health at every age. The effectiveness of protein supplementation varies based on confounders like individual activity level, diet and metabolic state [4,5].
There are some challenges with high-protein diets that more undigested protein-derived constituents end up in the large intestine leading to bacterial amino acid metabolism in the colon. The colonic fermentation of dietary amino acids may result in detrimental effects to colonic health. From the previous research it is also evident that the protein powder supplement consumers suffered from gastrointestinal symptoms, such as nausea, dehydration, diarrhea, constipation, indigestion, stomach pain, and decreased appetite [5,6]. On the contrary, plant proteins are found in plant foods, and positive health benefits of plant foods are linked to dietary fiber, vitamins, minerals, and phytochemicals coming along with the plant derived protein. Consumption of plant-based proteins are also correlated with reduced incidence of metabolic syndrome [7]. It is well known fact that the gut microbiota is an important factor for both energy harvest, storage and production through the protein metabolism [8]. In order to improve the efficacy of protein supplementation and gut health, probiotics either in protein supplement or together consumed is a proper strategy. Probiotics can encourage host digestive protease and peptidase activity, and release exo-enzymes involved in the protein digestion. Probiotics can improve the absorption of small peptides and amino acids by improving the absorption ability of the epithelium and enhancing transport [1]. The aim of the present study is to establish evidence of the efficacy and safety of protein supplement along with probiotics, amino acid, vitamins, minerals and natural ingredient rich fortification in promoting health and wellbeing in healthy adults.
Rationale of the study
The purpose of the research is evaluating safety and efficacy of protein supplement for improving health and wellbeing in healthy individuals. There are many research articles already proposing the health benefits of the key ingredients (plant-based proteins, milk solids, micronutrients like vitamins, minerals, probiotics etc.) individually. It is hypothesized that, protein supplement may provide health benefits to improve quality of life, energy, endurance and digestive behavior compared to placebo (Milk solids).
Objectives of the study
The objectives of the study were to evaluate the efficacy, safety and tolerability of test product-nutritional protein supplement in the improving health and wellbeing of healthy individuals.
METHODS
Study design
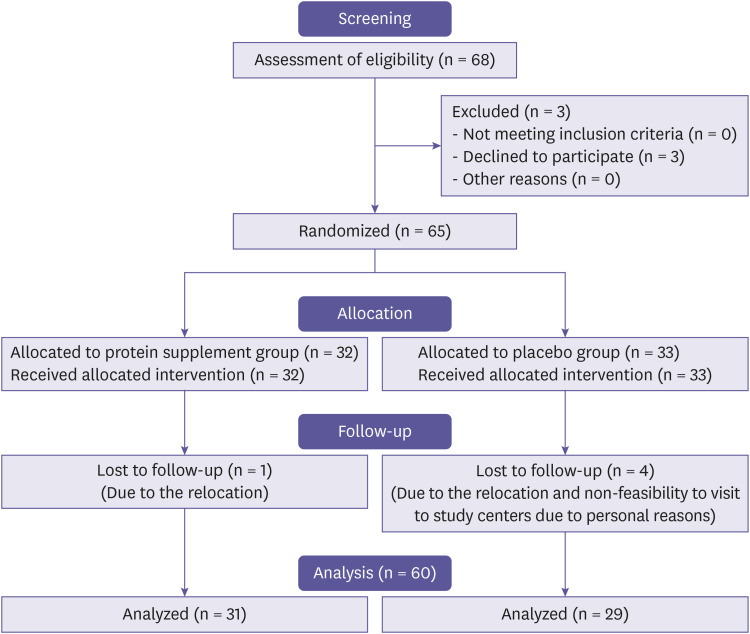
We conducted a double blind, multi-center (2 study sites), randomized, placebo controlled, clinical trial involving healthy individuals recruited from the outpatient department of both the study centers i.e. Lokmanya Medical Research Centre & Hospital, Pune, India and Health Nexus, Pune, India. The study was approved by Institutional Ethics Committee (IEC) Lokmanya Medical Research Centre, Pune, India and Royal Pune Independent Ethics Committee, Pune, India. The trial was registered on CTRI website (CTRI/2022/01/039219). The trial involved parallel design, two groups i.e. protein supplement (Treatment) and Placebo (control). 1:1 allocation of subjects was followed. The consolidated standards of reporting trials (CONSORT) flow of the entire study are depicted in Fig. 1.
Figure 1. CONSORT diagram for the study.
Inclusion criteria
Adults of age group 25–45 years old visiting outpatient department of study site were considered for the study. Those willing to give written informed consent and, able to comply with protocol requirements as per investigator judgement were selected. The criteria of selection of subjects with body mass index (BMI) in the range of 20-26 kg/m2 with good physical condition and sound mental status, willingness to abstain from use of vitamin or mineral supplements, nutritional supplements and/or medical foods if applicable.
Exclusion criteria
The subjects allergic to ingredients in product/ milk with uncontrolled and complicated diabetes (HbA1C not more than [NMT] 5.7% and blood pressure NMT 140/90 mmHg) were excluded from the study. Subjects with past history of addiction abuse, rehabilitation and more than 2 events of alcohol consumption per week were not considered in the study. Subjects with current medical history of any major illness such as cancer, heart disease, chronic obstructive pulmonary disease, asthma etc. as well as subjects having any past history of any acute or chronic illness that may affect the patient’s participation in the study were not considered for participation. Subjects with acute illness or history of major or minor surgery in the past one year, using prescription medications and/or nonprescription medications for weight loss, with deranged liver function test (LFT) and kidney function test (KFT) as per investigator discretion were not included in the study. Female subjects who are currently pregnant and/or breast feeding were excluded. Volunteers not willing to participate in study were excluded.
Study groups
We intended to complete 60 subjects at the end of the study. We screened 68 participants on above mentioned research sites based on the inclusion-exclusion criteria, of which three withdrawn their consent before randomization and were considered screen failure. Total of 65 subjects entered the randomization. Participants were randomized using software i.e. SPSS Version 10.0 (SPSS, Chicago, IL, USA) generated randomization sheet to receive either protein supplement– treatment group and placebo – control group. Fig. 1 presents the flow of events for the subjects considered in the analysis for this manuscript. We received randomization schedule from qualified statistician, investigator enrolled the participants to respective study groups.
Sample size
We intended to enroll 60 subjects at the end of the study. We have considered comparison of Mean Changes in total General Health Questionnaire-28 (GHQ-28) score between treatment and placebo in 90 days (superiority study). 60 patients are required to have a 90% chance of detecting, as significant at the 5% level, an increase in the primary outcome (reduction in GHQ score) measure from 5% in the control group to 35% in the experimental group. Based on this assumption from clinical experience, a qualified statistician evaluated the sample size of total 60 (30 cases in each arm) completed cases needed to assess the study objective at 90% power and alpha 0.05. However, in the present study, the total evaluable subjects were 60 (31 in protein supplement– treatment group and 29 in placebo control group).
Intervention and dosage
Subjects from both the groups will be provided with container each containing 600 g of protein supplement or placebo. Subject will be advised to consume given supplement in a dose of 20 g in 200 ml milk once a day for 90 days.
Proposed intervention is a protein supplement. It is a proprietary product of Netsurf Communication (P) Ltd, Pune, India. The product is composed of plant-based proteins, milk solids, micronutrients like vitamins, minerals, probiotics like Bifidobacterium, Lactobacillus together with some specialized natural ingredients like Colostrum, Omega 3 DHA, Aloe Vera and Zeaxanthin etc.
Outcome measures
As protein supplement is composed of plant-based proteins, micronutrients, probiotics with some specialized natural ingredients is thought to be provide health benefits to improve quality of life, energy, endurance and digestive behavior in healthy individuals. The primary outcomes were changes in total GHQ-28 score, serum total protein, and albumin to globulin ratio, VO2 max by 6-minute walk test, BMI, fat %, skeletal muscle %, through impedance studies for body composition along with waist and hip circumferences.
The secondary outcomes were change in % responders (subjects showing reduction of 25% of low energy scores in 30 days’ time) on daily energy audit questionnaire using patient diary. Energy audit questionnaire allows subject to record his/her experience, activity. As this trial is a general representation of consumers of protein supplement, the subject reported outcome on maintaining energy level could be an important parameter of determining quality of life. This questionnaire was filled by subject every day in four different time ranges i.e. morning, afternoon, evening and night time. Interpretation of energy was based on total number of events from the category of high, moderate and low energy events. To determine changes in total digestive behavior score all patients flled out a questionnaire which included questions about the severity of GI symptoms during the last 4 weeks, rated 0–6, where 0 meant “no complaints” and 6 represented the worst imaginable severity of that symptom. Severity score for all symptoms was analyzed as total mean score. Total sleep quality score was evaluated using questionnaire- disturbed sleep cycle index. Patients were asked to respond to questions and appropriate score was given (0-Did not apply to me at all; 1-Applied to me to some degree, or some of the time; 2-Applied to me to a considerable degree or a good part of time; 3-Applied to me very much or most of the time). Summing up the score, sleep quality was assessed as mild (0-6), moderate (6-12), and severe (12-18). Compliance of intervention was assessed. Safety was assessed by incidence of AEs and SAEs either self-reported by subject or assessed by Investigator. There were no changes in the outcome assessment and amendment to protocol.
Methodology
After receiving Ethics committee’s approval for study protocol, clinical study was registered on CTRI website. subjects between 25 to 45 years of age attending outpatient/consultation department of study site(s) will be screened for eligibility criteria.
On screening visit, a written informed consent was obtained from subject confirming participation in the study. Subject’s demographic details were noted. Subjects underwent clinical examination, complete blood count, LFT, KFT, HbA1c assessment. Subject’s medical, surgical and treatment history was noted. VO2 max by 6-minute walk test and anthropometric analysis was performed and documented. Subject’s current medication (s) if any were noted in the case record from (CRF). Vitals were recorded. The subjects were considered for further evaluation as per the inclusion and exclusion criteria.
During screening visit and the entire study duration subjects were advised to refrain from any protein supplement, antioxidant agents, vitamins, anti-inflammatory drugs, hormones, Nutraceutical, Ayurvedic, Siddha, Unani, herbal /homeopathic medicines for maintenance of health. A screening window of 2-3 days was kept, in case if there is requirement of any tests reports for confirming participation.
Subjects meeting all the inclusion criteria were randomized to the respective study groups as per the computer-generated randomization list. Subject were asked for occurrence of any adverse event during screening period. Subjects from the treatment group and placebo group received respective medication for a total of 90 days. At randomization visit and at every follow up visit (except last follow up visit), subjects were provided with container each containing 600 g of protein supplement or placebo. Subjects were advised to consume given supplement in a dose of 20 g in 200 mL milk once a day for 90 days. Subjects from placebo group received identical investigational product like protein supplement. The packaging, concealment method and flavor of the placebo were identical to that of the counterpart.
The record of concomitant medication other than prohibited ones, was kept in the CRF. Subject were advised to continue the diet and exercise regimen (which they are already following) during the entire study period of 90 days.
Investigational product compliance was assessed by the investigator on each and every follow up visit. If subject continuously missed dosing for > 6 consecutive days or total missed dose > 6 during the one assessment duration (one month), subject was treated as drop out of the study. Subject was allowed to come for follow up either 1-2 days prior or after the scheduled follow up visit, provided subject should continue the given treatment.
Subject were called at respective study sites for follow up visits after each 30 days for 90 days. On baseline and every follow up visit, subjects underwent clinical examination. Subject were assessed with some questionnaires related to health and wellbeing. On last follow up visit (end of treatment visit) subject underwent clinical examination, complete blood count, LFT, KFT, HbA1c assessment. VO2 max by 6-minute walk test and anthropometric assessment. Subject were assessed with some questionnaires related to health and wellbeing.
After completion of 3 months of study treatment, subjects were asked to stop investigational product and take advice of investigator for further treatment. Palatability with dosage compliance was assessed from baseline to end of the study. Subjects were closely monitored for any adverse event starting from baseline visit till the end of the study visit.
Data analysis
Patients without any major protocol violation, consumed at least one dose of intervention, and those who did not take any prohibited medications during the study period were considered for analysis. Continuous variables, such as age and other demographical characteristics, were summarized by using summary statistics, i.e. frequency, and mean, and standard deviation. Categorical values like gender and clinical examination were summarized using frequencies and percentages.
Analysis of primary efficacy parameters
GHQ-28 score was analyzed by Student’s t-test, Mann Whitney U test. Changes in VO2 max by 6-minute walk test was represented using percentage and distance in meters. Other efficacy parameters like serum total protein, albumin to globulin ratio, anthropometric parameters were analyzed by Student’s- t test.
Secondary efficacy parameters
Changes in % responders (subjects showing reduction of 25% of low energy scores in 30 days’ time) on daily energy questionnaire was analyzed using Student’s- t test. Changes in mean total digestive behavior score and total sleep quality score was analyzed by Student’s- t test, Wilcoxon signed ranked test within groups and Mann Whitney U test between groups. The adverse events were represented as number of events and analyzed by Fisher exact test.
RESULTS
Total of 68 subjects were screened, of which three withdrawn their consent before randomization and were considered screen failure. Total of 65 subjects entered the randomization and were randomized in two groups (32 in treatment group and 33 in placebo group). One subject from protein supplement group and four from placebo control group were dropped out. Thus, we evaluated 31 subjects from protein supplement group and 29 from placebo control group (total of 60) in this manuscript.
Demographic characteristics
Both groups were comparable in terms of the mean age and gender distribution. The male to female ratio in both test and control groups was comparable (Table 1).
Table 1. Demographic details of study subjects.
| Parameters | Treatment | Placebo | ||
|---|---|---|---|---|
| Group/Gender* | Male (n = 20) | Female (n = 11) | Male (n = 22) | Female (n = 7) |
| Age (yr)† | 32.15 ± 7.11 | 31.36 ± 6.59 | 33.95 ± 6.18 | 29.43 ± 6.16 |
| Total age (yr) | 32 ± 6.9 | 33 ± 6.4 | ||
*Data analyzed by χ2 test. Not significant. p > 0.05.
†Data analyzed by student t test.
Primary study outcomes
Change in total GHQ-28 score
All the patients were made to answer for the set of questions in the validated version of GHQ-28. The GHQ-28 provides information on extent of psychosomatic symptoms which can provide a fair understanding of overall quality of life. The four domains were considered like somatic symptoms, anxiety & insomnia, social dysfunction, and depressive symptoms. Self-scoring was done ranging from 0-1-2-3 (lower to higher disability respectively). The mean score of all the questions and 4 sub-sections along with total GHQ score were calculated and assessed. There is significantly more improvement in general health and wellbeing of subjects in all four domains from treated group compared to placebo. Reduced score from baseline indicated better quality of life. There was 85.76% improvement in total quality-of-life score in treatment group compared to 28.47%in placebo group (Table 2)
Table 2. Changes in GHQ scores between groups.
| Duration | Test | Placebo | p-value | |
|---|---|---|---|---|
| Somatic symptoms score* (mean ± SD) | ||||
| Baseline | 13.80 ± 1.06 | 14.0 ± 0.90 | 0.4179 | |
| Day 90 | 1.90 ± 1.40 | 9.72 ± 1.47 | < 0.001 | |
| % difference | 86.23 | 30.57 | ||
| Anxiety & insomnia score† (mean ± SD) | ||||
| Baseline | 13.40 ± 1.63 | 13.20 ± 1.28 | 0.5186 | |
| Day 90 | 2.51 ± 1.36 | 10.70 ± 10.70 | < 0.001 | |
| % difference | 81.27 | 18.94 | ||
| Social dysfunction score* (mean ± SD) | ||||
| Baseline | 13.29 ± 1.41 | 13.40 ± 0.98 | 0.6312 | |
| Day 90 | 2.40 ± 1.50 | 10.93 ± 0.75 | < 0.001 | |
| % difference | 81.94 | 18.43 | ||
| Depressive symptoms score† (mean ± SD) | ||||
| Baseline | 13.71 ± 1.41 | 13.10 ± 1.88 | 0.1618 | |
| Day 90 | 0.90 ± 0.74 | 6.90 ± 0.31 | < 0.001 | |
| % difference | 93.43 | 47.33 | ||
| Total GHQ-28 score† (mean ± SD) | ||||
| Baseline | 54.16 ± 2.86 | 53.17 ± 2.13 | 0.1373 | |
| Day 90 | 7.71 ± 3.93 | 38.03 ± 2.03 | < 0.001 | |
| % difference | 85.76 | 28.47 | ||
GHQ-28, General Health Questionnaire-28.
*By Mann Whitney U test between groups. Significant at p < 0.05.
†Data analyzed by Student’s t-test.
Changes in mean serum total protein, and albumin to globulin ratio
There were no statistically (at p < 0.05) as well as clinically significant changes in albumin, globulin, A/G ratio in both treatment and placebo groups (Table 3).
Table 3. Changes in liver function parameters between groups.
| Parameters | Treatment group | Placebo group | ||
|---|---|---|---|---|
| Baseline | Day 90 | Baseline | Day 90 | |
| Bilirubin total | 0.97 ± 0.33 | 0.88 ± 0.17 | 1.05 ± 0.41 | 0.92 ± 0.18 |
| Bilirubin direct | 0.34 ± 0.25 | 0.28 ± 0.10 | 0.37 ± 0.23 | 0.28 ± 0.11 |
| Bilirubin indirect | 0.63 ± 0.19 | 0.60 ± 0.12 | 0.68 ± 0.25 | 0.65 ± 0.12 |
| SGOT | 40.97 ± 23.47 | 33.68 ± 12.15 | 41.52 ± 28.00 | 32.03 ± 8.60 |
| SGPT | 33.87 ± 20.59 | 32.52 ± 17.82 | 34.49 ± 23.57 | 32.66 ± 12.18 |
| Alkaline phosphatase | 81.81 ± 22.93 | 84.42 ± 25.64 | 77.83 ± 17.72 | 84.59 ± 21.57 |
| Total proteins | 7.53 ± 3.11 | 7.11 ± 0.54 | 7.29 ± 0.80 | 7.09 ± 0.51 |
| Serum albumin (A) | 4.24 ± 0.44 | 4.06 ± 0.41 | 4.13 ± 0.39 | 4.03 ± 0.41 |
| Serum globulin (G) | 3.36 ± 1.67 | 3.04 ± 0.26 | 3.16 ± 0.51 | 3.08 ± 0.26 |
| Serum A/G ratio | 1.35 ± 0.61 | 1.31 ± 0.17 | 1.31 ± 0.18 | 1.28 ± 0.15 |
Data analyzed by Student’s t-test. Not significant at p < 0.05.
Changes in mean calculated VO2 max by 6-minute walk test
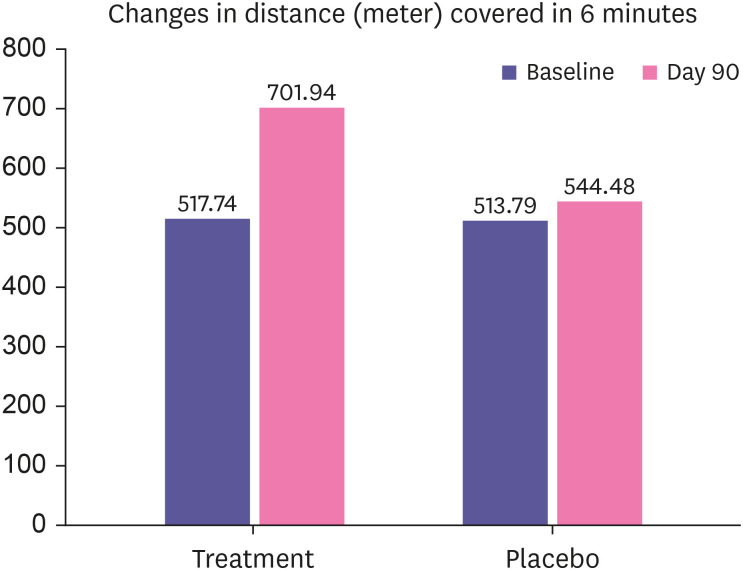
The aerobic capacity of the subjects was assessed by 6-minute walk test and VO2 max by calculation was used as a tool to depict the improvement in the total oxygen consumption capacity of individual. It was evident from the study that the baseline aerobic capacity was comparable between groups, there was significant improvement in distance covered in 6 minutes by subjects in treatment group than placebo (Fig. 2). Consequently, there was significant improvement in VO2 max of treatment group (42.92%) than placebo (6.77%) depicted in Table 4.
Figure 2. Changes in distance covered in 6-minute walk test between groups.
Table 4. Changes in Vo2 max between groups.
| Duration | Mean ± SD (mL/kg/min) | ||
|---|---|---|---|
| Treatment | Placebo | p-value | |
| Baseline | 26.91 ± 4.33 | 26.56 ± 3.90 | 0.7454 |
| Day 90 | 38.46 ± 4.38 | 28.36 ± 3.75 | |
| Mean diff (Baseline – Day 90) | −11.55 ± 2.02 | −1.80 ± 1.90 | < 0.001 |
| % Improvement | 42.92 | 6.77 | |
| p-value | 0.00002 | 0.00002 | |
Data analyzed by Student’s t-test. Non-significant. p > 0.05.
Changes in anthropometric parameters
There was comparable body weight and BMI profile of subjects from both groups at baseline. In treatment group, body weight was reduced with mean difference of 1.94 kg (p < 0.001), BMI was significantly reduced by 2.86% (p < 0.001), waist circumference by 3.05% (2.46 cm reduction) (p < 0.001), hip circumference by 0.53% (p < 0.001) from baseline to end of the study while there were no statistically significant changes in placebo-treated group. Significant reduction in percent body fat by 2.46% (p < 0.001) in test group while, 0.85% increase in placebo group was observed by end of the study. 1.57% (p < 0.01) of percent skeletal muscle mass was increased in protein supplement treated group and there was no significant change in placebo treated group (Table 5).
Table 5. Changes in anthropometric parameters between groups.
| Duration | Test | Placebo | p-value | |
|---|---|---|---|---|
| Body weight (kg) (mean ± SD) | ||||
| Baseline | 64.89 ± 8.64 | 66.12 ± 8.29 | 0.5760 | |
| Day 90 | 62.96 ± 8.64 | 66.31 ± 8.17 | < 0.001 | |
| % difference | 2.97 | 0.28 | ||
| p-value | < 0.001 | 0.3656 | ||
| BMI (kg/m2) (mean ± SD) | ||||
| Baseline | 23.01 ± 0.02 | 23.18 ± 2.17 | 0.7601 | |
| Day 90 | 22.35 ± 2.31 | 23.24 ± 2.16 | < 0.001 | |
| % difference | 2.86 | 0.25 | ||
| p-value | < 0.001 | 0.4108 | ||
| Waist circumference (cm) (mean ± SD) | ||||
| Baseline | 80.43 ± 10.75 | 82.09 ± 9.39 | 0.5292 | |
| Day 90 | 77.97 ± 10.80 | 81.14 ± 9.29 | 0.00006 | |
| % difference | 3.05 | 1.15 | ||
| p-value | < 0.001 | 0.0107 | ||
| Hip circumference (cm) (mean ± SD) | ||||
| Baseline | 86.42 ± 9.98 | 89.98 ± 7.51 | 0.1259 | |
| Day 90 | 85.96 ± 9.96 | 89.59 ± 7.05 | 0.8484 | |
| % difference | 0.53 | 0.43 | ||
| p-value | < 0.001 | 0.3060 | ||
| Body fat (%) (mean ± SD) | ||||
| Baseline | 20.38 ± 3.89 | 19.86 ± 4.53 | 0.6398 | |
| Day 90 | 19.88 ± 3.52 | 20.03 ± 3.54 | 0.0421 | |
| % difference | 2.45 | 0.85 | ||
| p-value | < 0.001 | 0.5476 | ||
| Skeletal muscle (%) (mean ± SD) | ||||
| Baseline | 43.13 ± 5.45 | 42.70 ± 5.60 | 0.7630 | |
| Day 90 | 43.81 ± 5.66 | 42.43 ± 5.50 | 0.0076 | |
| % difference | 1.57 | 0.63 | ||
| p-value | 0.0062 | 0.2846 | ||
Analyzed using Student’s t-test. Significant at p < 0.05.
Secondary study outcomes
Changes in energy events (patient diary) score between groups
The energy audit was performed by calculating frequencies of low, moderate and high energy events reported by subjects in their diaries for each 30 days till 90 days. It was evident from data that there was significant improvement in high energy events in treatment group compared to placebo (p < 0.001). There were significantly more events in moderate to high levels in treatment group at day 30, and the same trend followed till day 90 (Table 6).
Table 6. Changes in patient diary score between groups.
| Duration | Test | Placebo | p-value | |
|---|---|---|---|---|
| No. of high energy events (mean ± SD) | ||||
| Day 30 | 0.29 ± 0.94 | 0.00 ± 0.00 | 0.1010 | |
| Day 60 | 12.16 ± 1.42 | 0.00 ± 0.00 | < 0.001 | |
| Day 90 | 55.58 ± 3.91 | 17.28 ± 2.31 | < 0.001 | |
| No. of moderate energy events (mean ± SD) | ||||
| Day 30 | 111.06 ± 9.01 | 69.21 ± 14.62 | < 0.001 | |
| Day 60 | 102.65 ± 6.19 | 71.21 ± 4.80 | < 0.001 | |
| Day 90 | 57.00 ± 4.31 | 45.00 ± 4.73 | < 0.001 | |
| No. of low energy events (Mean ± SD) | ||||
| Day 30 | 8.65 ± 9.06 | 50.79 ± 14.62 | < 0.001 | |
| Day 60 | 5.19 ± 5.86 | 48.79 ± 4.80 | < 0.001 | |
| Day 90 | 7.42 ± 1.61 | 57.72 ± 4.22 | < 0.001 | |
Data analyzed by Student’s t-test. Significant at p < 0.05.
To understand the response to improvement in energy levels we considered energy audit score comparison between day 30 and day 60 between groups. There were 100% responders i.e., individuals with at least 25% reduction in low energy events in treatment group whereas there were only four (13.33%) responders in placebo group.
Assessment of symptom scores between groups
Digestive behavior score in treatment group was remarkably improved by 78.69%, 90.19% 100% at day 30, day 60 and day 90 from the baseline of the study (p < 0.001) in treatment group while there was not significant change among placebo group. The sleep quality score in treatment group was increased gradually and significantly (p < 0.001) by 31.54%, 32.69% and 33.98% at day 30, day 60, day 90 respectively from baseline. No significant change was observed in placebo group (Table 7).
Table 7. Changes in symptom scores between groups.
| Duration | Test (% difference) | Placebo (% difference) | p-value | |
|---|---|---|---|---|
| Digestive Behavior Score* (mean ± SD) | ||||
| Baseline | 0.61 ± 0.99 | 0.48 ± 0.91 | 0.7039 | |
| Day 30 | 0.13 ± 0.43 (78.69) | 0.52 ± 0.78 (8.33) | ||
| Day 60 | 0.06 ± 0.25 (90.19) | 0.48 ± 0.83 (0) | ||
| Day 90 | 0.00 ± 0.00 (100) | 0.41 ± 0.63 (14.58) | ||
| Mean diff (Baseline – Day 30) | 0.48 ± 1.00 | −0.03 ± 0.82 | 0.0854 | |
| Mean diff (Baseline – Day 60) | 0.55 ± 0.96 | 0.00 ± 0.60 | 0.0643 | |
| Mean diff (Baseline – Day 90) | 0.61 ± 0.99 | 0.07 ± 0.88 | 0.0818 | |
| p-value | 0.00007 | 0.9673 | ||
| Sleep Quality Score† (mean ± SD) | ||||
| Baseline | 7.77 ± 1.67 | 7.31 ± 1.00 | 0.2007 | |
| Day 30 | 5.32 ± 1.28 (31.54) | 6.76 ± 0.95 (7.52) | ||
| Day 60 | 5.23 ± 0.72 (32.69) | 7.10 ± 0.67 (2.87) | ||
| Day 90 | 5.13 ± 0.72 (33.98) | 7.07 ± 0.88 (3.28) | ||
| Mean diff (Baseline – Day 30) | 2.45 ± 1.75 | 0.55 ± 0.83 | < 0.001 | |
| Mean diff (Baseline – Day 60) | 2.55 ± 1.61 | 0.21 ± 1.24 | < 0.001 | |
| Mean diff (Baseline – Day 90) | 2.65 ± 1.85 | 0.24 ± 1.46 | < 0.001 | |
| p-value | < 0.001 | 0.1318 | ||
*Data analyzed by Wilcoxan signed ranked test within groups and Mann Whitney U test between groups. Significant at p < 0.05.
†Data analyzed by Student’s t-test.
Safety outcomes
There are no clinically as well as statistically (at p < 0.05) significant changes in hematological, biochemical, and vital parameters in both groups post treatment (Tables 3, 8, 9, 10, and 11).
Table 8. Changes in hematological parameters between groups.
| Laboratory investigations | Treatment | Placebo | ||
|---|---|---|---|---|
| Baseline | Day 90 | Baseline | Day 90 | |
| Total leukocyte count | 8,145.16 ± 2,212.67 | 7,603.23 ± 1,762.48 | 8,493.10 ± 2,289.09 | 9,041.38 ± 3,392.69 |
| Neutrophil % | 58.87 ± 5.89 | 64.45 ± 8.19 | 59.31 ± 8.69 | 64.79 ± 8.91 |
| Lymphocyte % | 34.10 ± 5.56 | 29.32 ± 7.85 | 32.45 ± 7.42 | 28.79 ± 7.94 |
| Monocyte % | 3.42 ± 1.20 | 3.39 ± 1.58 | 3.83 ± 1.47 | 3.31 ± 1.61 |
| Eosinophil % | 3.61 ± 1.23 | 4.06 ± 1.26 | 4.41 ± 1.88 | 4.10 ± 1.57 |
| Total RBC | 4.63 ± 0.62 | 4.57 ± 0.48 | 4.90 ± 0.54 | 4.64 ± 0.56 |
| Hemoglobin | 12.90 ± 2.00 | 12.96 ± 1.77 | 13.78 ± 1.74 | 13.68 ± 1.56 |
| Platelet count | 228,451.61 ± 60,378.16 | 264,225.81 ± 85,040.27 | 208,379.31 ± 70,893.99 | 245,206.90 ± 69,788.14 |
Data analyzed by Student’s t-test. Not significant at p < 0.05.
Table 9. Changes in kidney function test parameters between groups.
| Laboratory Investigation | Treatment | Placebo | ||
|---|---|---|---|---|
| Baseline | Day 90 | Baseline | Day 90 | |
| Serum calcium | 9.79 ± 3.10 | 9.85 ± 0.73 | 9.85 ± 2.71 | 9.78 ± 0.80 |
| Serum uric acid | 4.76 ± 1.67 | 4.50 ± 1.25 | 5.05 ± 1.43 | 4.58 ± 1.14 |
| Blood urea nitrogen | 12.42 ± 3.05 | 14.05 ± 3.90 | 12.13 ± 4.26 | 13.68 ± 3.35 |
| Serum creatinine | 0.96 ± 0.18 | 0.98 ± 0.17 | 0.99 ± 0.25 | 1.03 ± 0.16 |
| BUN/creatinine ratio | 13.10 ± 3.07 | 14.19 ± 4.53 | 12.39 ± 4.51 | 13.08 ± 2.94 |
Data analyzed by Student’s t-test. Not significant at p < 0.05.
Table 10. Changes in HbA1c parameters between groups.
| Laboratory investigation | Treatment | Placebo | ||
|---|---|---|---|---|
| Baseline | Day 90 | Baseline | Day 90 | |
| HbA1c | 5.53 ± 0.51 | 5.56 ± 0.44 | 5.70 ± 0.43 | 5.62 ± 0.47 |
Data analyzed by Student’s t-test. Not significant at p < 0.05.
Table 11. Changes in vitals between groups.
| Laboratory investigation | Treatment | Placebo | ||
|---|---|---|---|---|
| Baseline | Day 90 | Baseline | Day 90 | |
| Pulse | 72.52 ± 1.06 | 72.23 ± 1.36 | 72.24 ± 1.30 | 71.62 ± 1.95 |
| Temperature | 97.72 ± 0.77 | 97.63 ± 0.80 | 97.71 ± 0.75 | 97.68 ± 0.77 |
| Systolic BP | 121.52 ± 3.53 | 120.90 ± 2.49 | 121.38 ± 3.23 | 121.28 ± 3.26 |
| Diastolic BP | 81.23 ± 1.75 | 80.74 ± 2.00 | 80.93 ± 2.07 | 81.14 ± 1.64 |
| Respiratory rate | 16.16 ± 1.00 | 16.52 ± 1.03 | 16.55 ± 1.02 | 16.24 ± 0.95 |
Data analyzed by Student’s t-test. Not significant at p < 0.05.
Adverse events
There were total 12 adverse events in the study duration, seven events in treatment group and five in placebo group. The difference is not statistically significant. The adverse events were headache, cough and cold, muscle pain, body ache, menstrual pain etc. The adverse events were not related to the investigational product and were mild in nature.
There were 3 adverse events like bloating and hyperacidity in treatment group compared to 7 adverse events related to gastro intestinal tract in placebo group. These adverse events were possibly related to investigational product but were mild in nature and required no change in dosage and got relieved in 1–2 days without rescue medication.
Compliance
There was high i.e. 92% and 90% compliance observed in protein supplement and placebo group, respectively. There were total 5 drop outs from the study population, 1 from treatment group and 4 from the placebo group respectively. Reasons for drop-out from the study were relocation and non-feasibility to visit to study centers due to personal reasons and not the compliance.
DISCUSSION
In the present study, we compared the impact of protein supplement with micronutrient fortification versus placebo in promoting health and wellbeing in healthy adults. Baseline status of the participants in both groups were comparable. All the subjects were representing sedentary or slightly active lifestyle. This avoids the bias and promised data integrity and robustness. After treatment with protein supplement, subject’s general health and wellbeing was significantly improved. 85.56% improvement in quality of life was observed. No change was observed in albumin, globulin and A/G ratio by the end of the study in both the groups. Aerobic capacity was increased in individuals in protein supplement treated group which was calculated by 6-minute walk test (VO2 max). Significant reduction in BMI, waist circumference, % body fat and increased % skeletal muscle mass in treatment group was observed as compared to placebo group. There was remarkable improvement in number of high energy events in the protein supplement group participants compared to placebo group. There was improvement in digestive behavior and sleep quality in protein supplement group than placebo group. There were no clinically significant changes in hematological, biochemical and vital parameters were observed in both groups indicating safety of investigational product.
Gastrointestinal discomfort especially due to milk based marketed products, weight gain, unhealthy spike in blood sugar, dehydration, diarrhea, nausea is some of the common side effects associated with use of protein supplement. A systematic review of experimental and randomized studies analysis revealed that chronic, and excessive use of protein supplementation may cause adverse events related to kidney and liver function, impaired bone metabolism, and increased low density lipoprotein (LDL) [9,10]. All proteins are not easily digested and have varied absorption due to either their source or processing conditions. In recent years there is great interest amongst researchers to study various benefits of plant-based proteins for improving human health. Plant protein are linked with benefits for health and physical function. Plant proteins in the diet can help to supply adequate high-quality protein and also reduces the potential for adverse impact [11,12]. There is increased consumer interest in protein consumption, as well as increased interest in plant-based diet patterns. In the present research, protein supplement possess proteins obtained from rice, pea and soya, together with milk and whey proteins.
Probiotic microorganisms mainly used in human nutrition are species of Lactobacillus and Bifidobacterium. The beneficial effects of probiotics can be used to restore the natural microbiota, counter attack pathogenic intestinal microbiota activity and in digestive processes, the management of food allergies, candidiasis, and tooth decay. Probiotics increase the efficiency of the immune system, absorption of vitamins and minerals, and also stimulate the production of amino acids [13]. As it is evident from the results, probiotics used in the formulation that is Bifido longum and Lactobacillus acidophilus could have potentially reduced gastrointestinal problems evident by negligible side effects of the investigational protein supplement.
Recommendation for daily intake of protein is 0.83 g protein/kg/day in healthy adults regardless of sex [14]. Protein intake is vital to encourage healthy weight loss, treat protein deficiency, help build muscle, aid muscle recovery, perform integral functions like immunological response and to promote general wellbeing of individuals. In athletes and sportspersons protein is needed to enhance endurance and promote muscle growth [1,15,16].
The previous research suggests that, adequate dietary protein supplementation is important for overall health and improving body composition. When protein and energy intakes unable to meet individual nutritional demand, body stores are catabolized to provide energy, leading to the depletion of muscle mass with resulting symptoms such as fatigue or tiredness. Poor nutritional status is also associated with depleted energy levels and reduced physical performance leading to fatigue [17].
As per present study, protein supplement treated group reported better quality of life, improvement in general health and wellbeing, improved energy levels throughout the day. The effectiveness of the protein supplement can be attributed to the synergistic effect of combination of multiple plant proteins, milk protein, probiotics and various micronutrients within their standardized specifications.
Reduced muscle mass, strength, and cardiorespiratory fitness, resulting in an impaired ability to perform daily activities could be the signs of biological aging. Improved maximal oxygen uptake (VO2 max) is a hallmark of physical endurance [18]. In this present study, we have studied effects of protein supplement treatment on VO2 max without any resistance or physical training protocol. There is significant improvement in VO2 max by 42.92% in protein supplement treated group compared to their baselines. It can be assuring parameter for overall improvement in health and activity levels.
Treatment with protein supplement led to reduction in BMI, waist circumference and % body fat which makes it potential candidate for weight management which is very crucial parameter for metabolic health.
Protein supplement can be considered as safe presenting no clinical, vital, hematological and biochemical signs of any abnormal changes including very few, minor in nature adverse events which are not related to study intervention. Product is well tolerable and safe for long term (90 days) use.
The strength of the study is it provides a real time evidence of combination of plant and animal-source based protein and its effectiveness in the healthy population. Study participants were individuals without comorbidities and major health concerns. There is significant improvement in energy levels, general health wellbeing, digestion of formulation, sleep quality, aerobic capacity, quality of life and anthropometric parameters. Present study is more generalizable, relatable to consumers. The fewer sample size could be thought as the limitation of the study. Further research can be carried out with protein supplement using different age groups, gender, groups with varied activity levels and additional comorbidities.
It can be concluded from the present study that protein supplement is safe and efficacious in promoting general health and wellbeing in adults. It can help in weight management with some more dietary modifications, as it is observed to reduce BMI, hip circumference, waist circumference, % body fat. It ensures improvement high energy events, aerobic capacity. Treatment with protein supplement ascertained the fact that it can provide a wholesome improvement in quality of life, digestive behavior score and also improves sleep quality. There was improvement in percent skeletal muscle measured by impedance study.
Negligible gastrointestinal side effects of protein supplement are more assimilated and provided the sustainable results in promoting health and wellbeing. The present research ensures consumers of this product to get the effectiveness to remain energetic, healthy and manage weight.
ACKNOWLEDGMENTS
The authors would like to acknowledge the research team and the back-office team involved in the research work. We would like to acknowledge the support provided by back office, Netsurf Communication (P) Ltd.
Footnotes
Funding: Supported by Netsurf Communication (P) Ltd., Pune, India for material, laboratory testing and other expenses of the trial.
Conflict of Interest: - Authors: Dr. Pranit Ambulkar, Mr. Prashant Hande, Mr. Bhagwat Tambe are employee of the Netsurf Communication (P) Ltd.
- Reviewers: Nothing to declare
- Editors: Nothing to declare
Reviewer: This article was reviewed by peer experts who are not TCP editors.
- Conceptualization: Ambulkar PY.
- Data curation: Ganu G.
- Formal analysis: Ganu G.
- Funding acquisition: Ambulkar PY.
- Investigation: Vaidya VG, Naik N, Agarwal R.
- Methodology: Ganu G.
- Resources: Hande P, Tambe B.
- Supervision: Vaidya VG, Naik N, Agarwal R.
- Validation: Ambulkar PY.
- Visualization: Ambulkar PY.
- Writing - original draft: Ganu G.
- Writing - review & editing: Ambulkar PY.
References
- 1.Kårlund A, Gómez-Gallego C, Turpeinen AM, Palo-Oja OM, El-Nezami H, Kolehmainen M. Protein supplements and their relation with nutrition, microbiota composition and health: is more protein always better for sportspeople? Nutrients. 2019;11:829. doi: 10.3390/nu11040829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianco A, Mammina C, Paoli A, Bellafiore M, Battaglia G, Caramazza G, et al. Protein supplementation in strength and conditioning adepts: knowledge, dietary behavior and practice in Palermo, Italy. J Int Soc Sports Nutr. 2011;8:25. doi: 10.1186/1550-2783-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gannon NP, Schnuck JK, Vaughan RA. BCAA metabolism and insulin sensitivity—Dysregulated by metabolic status? Mol Nutr Food Res. 2018;62:e1700756. doi: 10.1002/mnfr.201700756. [DOI] [PubMed] [Google Scholar]
- 4.Bradley D. Study on food intended for sportspeople: final report. Brussels: European Commission, Directorate General for Health and Food Safety; 2015. [Google Scholar]
- 5.Alhakbany MA, Alzamil HA, Alnazzawi E, Alhenaki G, Alzahrani R, Almughaiseeb A, et al. Knowledge, attitudes, and use of protein supplements among saudi adults: gender differences. Healthcare (Basel) 2022;10:394. doi: 10.3390/healthcare10020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahnen RT, Jonnalagadda SS, Slavin JL. Role of plant protein in nutrition, wellness, and health. Nutr Rev. 2019;77:735–747. doi: 10.1093/nutrit/nuz028. [DOI] [PubMed] [Google Scholar]
- 7.Fardet A. In: Vegetarian and plant-based diets in health and disease prevention. Mariotti F, editor. Cambridge (MA): Academic Press; 2017. New concepts and paradigms for the protective effects of plant-based food components in relation to food complexity; pp. 293–312. [Google Scholar]
- 8.Moreno-Pérez D, Bressa C, Bailén M, Hamed-Bousdar S, Naclerio F, Carmona M, et al. Effect of a protein supplement on the gut microbiota of endurance athletes: a randomized, controlled, double-blind pilot study. Nutrients. 2018;10:337. doi: 10.3390/nu10030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvard Health. The hidden dangers of protein powders [Internet] [Accessed August 15, 2022]. https://www.health.harvard.edu/staying-healthy/the-hidden-dangers-of-protein-powders .
- 10.Vasconcelos QD, Bachur TP, Aragão GF. Whey protein supplementation and its potentially adverse effects on health: a systematic review. Appl Physiol Nutr Metab. 2021;46:27–33. doi: 10.1139/apnm-2020-0370. [DOI] [PubMed] [Google Scholar]
- 11.Hertzler SR, Lieblein-Boff JC, Weiler M, Allgeier C. Plant proteins: assessing their nutritional quality and effects on health and physical function. Nutrients. 2020;12:3704. doi: 10.3390/nu12123704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dallas DC, Sanctuary MR, Qu Y, Khajavi SH, Van Zandt AE, Dyandra M, et al. Personalizing protein nourishment. Crit Rev Food Sci Nutr. 2017;57:3313–3331. doi: 10.1080/10408398.2015.1117412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.María Remes Troche J, Coss Adame E, Ángel Valdovinos Díaz M, Gómez Escudero O, Eugenia Icaza Chávez M, Antonio Chávez-Barrera J, et al. Lactobacillus acidophilus LB: a useful pharmabiotic for the treatment of digestive disorders. Therap Adv Gastroenterol. 2020;13:1756284820971201. doi: 10.1177/1756284820971201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smeuninx B, Greig CA, Breen L. Amount, source and pattern of dietary protein intake across the adult lifespan: a cross-sectional study. Front Nutr. 2020;7:25. doi: 10.3389/fnut.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medical News Today. Health benefits of protein powder [Internet] [Accessed September 18, 2018]. https://www.medicalnewstoday.com/articles/323093 .
- 16.Fitbit. Do you really need protein powder? [Internet] [Accessed October 10, 2017]. https://blog.fitbit.com/protein-powder/nutrition .
- 17.Azzolino D, Arosio B, Marzetti E, Calvani R, Cesari M. Nutritional status as a mediator of fatigue and its underlying mechanisms in older people. Nutrients. 2020;12:444. doi: 10.3390/nu12020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alghannam AF, Templeman I, Thomas JE, Jedrzejewski D, Griffiths S, Lemon J, et al. Effect of carbohydrate-protein supplementation on endurance training adaptations. Eur J Appl Physiol. 2020;120:2273–2287. doi: 10.1007/s00421-020-04450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]