Abstract
Purpose
Despite increasing evidence for the potential association between atopic dermatitis (AD) and cardiovascular diseases (CVDs), results have still remained controversial. Therefore, this study investigated the association between AD and subsequent CVDs in adults newly diagnosed with AD.
Methods
Datasets from the National Health Insurance Service-National Sample Cohort in South Korea from 2002 to 2015 were analyzed. The primary outcome was new-onset CVD, which included angina pectoris, myocardial infarction, stroke, or any revascularization procedure. The crude and adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated in the AD group compared with the matched control group using the Cox proportional hazards regression models.
Results
A total of 40,512 individuals with AD were matched with 40,512 control subjects without AD. The overall incidence of CVDs was 2,235 (5.5%) and 1,640 (4.1%) in the AD and matched control groups, respectively. In the adjusted model, AD was associated with an increased risk of CVDs (HR, 1.42; 95% CI, 1.33–1.52), angina pectoris (adjusted HR, 1.49; 95% CI, 1.36–1.63), myocardial infarction (adjusted HR, 1.40; 95% CI, 1.15–1.70), ischemic stroke (adjusted HR, 1.34; 95% CI, 1.20–1.49), and hemorrhagic stroke (adjusted HR, 1.26; 95% CI, 1.05–1.52). Most of the subgroup and sensitivity analysis results were consistent with those of the main analysis.
Conclusions
The current study found that adult patients newly diagnosed with AD were at significantly increased risk for subsequent CVDs, suggesting the need to consider early prevention strategies for CVDs targeting patients with AD.
Keywords: Atopic dermatitis, eczema, cardiovascular disease, myocardial ischemia, stroke
INTRODUCTION
Atopic dermatitis (AD) is a common chronic inflammatory skin disease. Despite being traditionally considered a childhood disease, AD affects around 5%–10% of adults, with its global prevalence showing an increasing trend.1,2,3,4 The close relationship between AD and other atopic comorbidities, such as asthma and allergic rhinitis, is well known as the “atopic march”. 5,6 Recently, however, an increasing number of studies have suggested an association between AD and other nonallergic conditions, such as cardiometabolic diseases beyond the atopic comorbidities.7,8
Globally, the major cause of death is cardiovascular diseases (CVDs), accounting for approximately 30%.9 Moreover, evidence has shown that CVDs are associated with various inflammatory conditions.10 Therefore, the chronic systemic inflammatory component of AD has been suggested to possibly trigger atherosclerosis and CVDs. However, studies conducted to date on the association between AD and CVD outcomes have reported inconsistent results. One previous meta-analysis reported no association between AD and most cardiovascular outcomes, including myocardial infarction (MI), hypertension, or stroke.11 In contrast, a recent systematic review and meta-analysis reported that AD was associated with an increased risk for MI, stroke, angina, and heart failure in a pooled analysis of cohort studies and that increasing AD severity was associated with an increased risk of cardiovascular outcomes.12 Notably, almost all studies conducted to date had one or both limitations with respect to the diagnosis of AD or common cardiovascular risk factors, such as smoking and obesity. Several meta-analyses have reported that smoking and obesity, which are well-known factors that increase CVD risk, are associated with AD.13,14 However, many studies had not considered smoking and obesity in their adjusted analysis.15,16,17,18,19,20,21,22,23,24,25 Moreover, a number of studies had defined AD patients based on self-reported questionnaires, which may possibly cause selection bias.26,27,28,29
Understanding the link between AD and CVD may help lessen the associated burden of these comorbidities in patients with AD. Thus, this study aimed to evaluate the risk of cardiovascular outcomes in patients newly diagnosed with AD by a physician using well-established national cohort data from Korea, accounting for lifestyle risk factors, and investigate whether the risk of CVDs varied according to AD severity.
MATERIALS AND METHODS
Data source
This retrospective propensity score matching (PSM) cohort study used data from National Health Insurance Service-National Sample Cohort (NHIS-NSC) 2.0 database in South Korea from 2002 to 2015. The NHIS is the compulsory single-payer national health care coverage system in South Korea, and the NHIS-NSC is a large-scale, population-based cohort data consisting of an approximately 2.2% representative sample of the general Korean population. Sampling consisted of a systematic stratified random sample with proportional allocation within each stratum. The database contains longitudinal health-related information regarding socio-demographics, disease diagnoses (International Classification of Disease, Tenth Revision [ICD-10]), therapeutic procedures, drug prescriptions (brand name, generic name, prescription date, days of supply, and dose and route of administration), type of medical utilization (outpatient, inpatient, or emergency department), and annual or biennial national health screening examinations that include assessment of cardiovascular risk factors (health questionnaire surveys, physical examinations, and laboratory test).30 A detailed description of these data has been reported elsewhere.30
This study was approved by the Institutional Review Board of Korea University (KUIRB-2020-0043-01) and the Korea NHIS National Health Information Data Request Review Committee (NHIS-2020-2-155). All methods were performed in accordance with the approved guidelines and regulations. Informed consent was waived, as this was a retrospective study of de-identified administrative data.
Study population
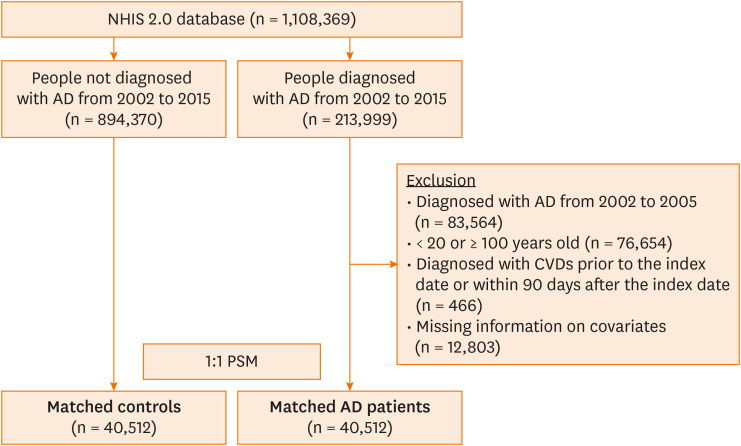
Patients with AD were defined as those having received at least one diagnostic code (ICD-10 code L20) and AD-related treatment on the same day to reduce a probability of misclassification. AD-related treatments included prescriptions such as topical or oral corticosteroids, immunosuppressants (methotrexate, cyclosporine, mycophenolate mofetil, or azathioprine), topical calcineurin inhibitors (tacrolimus or pimecrolimus), or antihistamines. The date of the initial AD diagnosis for each patient was defined as the index date. The exclusion criteria were patients aged < 20 or ≥ 100 years, diagnosed with CVD prior to the index date or within 90 days after the index date, or had incomplete information on covariates. Additionally, patients diagnosed with AD between 2002 and 2005 were excluded, and as a result of this 4-year washout period, we could include new cases of AD. The study population selection process is detailed in Fig. 1.
Fig. 1. Flowchart showing the sample selection. This is a stratified random sampled cohort study with approximately a million subjects. First, the sample was divided into the AD group (n = 213,999) who met the AD criteria defined in this study between 2002 and 2015, and the control group (n = 894,370). Then, in the AD group, if a patient had a previous AD history between 2002 and 2005 (n = 83,564), was < 20 or ≥ 100 years old (n = 76,654), diagnosed with CVD prior to the index date or within 90 days after the index date (n = 466), or had incomplete information on covariates (n = 12,803), then the patient was excluded from the study population. After that, selected patients with AD were matched 1:1 with controls who had never been diagnosed with AD from 2002 to 2015 based on the covariates at the index date of patients with AD using PSM.
AD, atopic dermatitis; CVD, cardiovascular disease; PSM, propensity score matching; NHIS, National Health Insurance Service.
Selected AD patients were matched 1:1 with controls who had never been diagnosed with AD from 2002 to 2015 based on the covariates at the AD patient’s index date using PSM. PSM was used to reduce potential confounders and balance the baseline covariates of the 2 groups.31 Propensity scores were derived from the predicted probability of subjects with versus without AD using a logistic regression model that adjusts for the following confounders: sex, age, household income, region of residence, and comorbidities (hypertension, diabetes, and hyperlipidemia). To reduce immortal time bias, the individual index date was set to the same date as AD diagnosis in patients with AD and matched controls.32 Patients with severe AD were defined as those who had received oral corticosteroids or immunosuppressants. Other AD cases were classified as non-severe.33 For statistical analysis, patients with severe AD were matched 1:1 with patients with non-severe AD based on the covariates of the first date of severe AD using PSM.
Study outcomes and follow-up period
The primary composite endpoint was CVDs defined using diagnostic codes of angina pectoris (I20), MI (I21–I24), ischemic stroke (I60), hemorrhagic stroke (I61–I63), or any procedure involving coronary artery bypass grafts or percutaneous coronary intervention. The secondary outcomes were ischemic heart disease (I20–I24), angina pectoris (I20), MI (I21–I24), stroke (I60–I63), ischemic stroke (I60), and hemorrhagic stroke (I61–I63). To assess the risk of subsequent CVD after a diagnosis of AD, patients with AD were followed up starting 90 days after the index date. Follow-up for matched controls also began on the start date of their matched patient with AD. Follow-up was terminated on the date of the outcome of interest, upon death, or at the end of the study (December 31, 2015), whichever occurred first.
Covariates
To control for potential confounding factors in the analyses, covariates were identified on the basis of previous literature, expert opinion, and the availability of covariates within the data. The covariates used in this study included sex, age, household income, region of residence, body mass index (BMI) and smoking status in the baseline year, and the Charlson comorbidity index (CCI), comorbidities (hypertension, diabetes, and hyperlipidemia), and co-medications for 1 year prior to the index date.
The age groups were classified using 10-year intervals. A total of 7 age groups were included for those aged ≥ 20 years. The household income groups, which were initially categorized according to 11 classes (class 0, lowest income; class 10, highest income) in the NHIS database, were recategorized into 4 classes (low, class 0–2; medium–low, class 3–5; medium–high, class 6–8; and high, 9–10). In this study, the region of residence was recategorized into urban (Seoul, Busan, Daegu, Incheon, Gwangju, Daejeon, and Ulsan) and rural (Gyeonggi, Gangwon, Chungbuk, Chungnam, Jeonbuk, Jeonnam, Gyeongbuk, Gyeongnam, and Jeju) areas. BMI was categorized into < 18, ≥ 18 to < 25, and ≥ 25 kg/m2. Smoking status was categorized as never smoking, former smoking, and current smoking. The patients’ CCI scores were estimated from their disease records using previously validated algorithms.34 Comorbidity was defined as hospitalization for ≥ 2 days or outpatient treatment for ≥ 3 days with the corresponding diagnosis code, as well as a drug prescription for treatment within 1 year before the index date. Co-medications included antihypertensives (angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, β-adrenergic antagonists, calcium channel blockers, or thiazide diuretics), antidiabetics (insulin, glucagon-like peptide 1 receptor agonist, and other oral hypoglycemic agents), lipid-lowering drugs (statin, fibrate, niacin, omega-3 fatty acids, and ezetimibe), antiplatelets, anticoagulants (warfarin and other anticoagulants), and antidepressants, which have been associated with CVDs.
Statistical analysis
Baseline characteristics of the AD and normal control groups were reported as frequencies and percentages and compared using the χ2 test. The Cox proportional hazards model was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for CVD in the AD group compared with the matched control group and in the severe AD group compared with the non-severe AD group. Crude and adjusted models were used. The cumulative incidence of CVDs was estimated using the Kaplan–Meier method and log-rank tests. Subgroup analyses were performed according to sex, age, and a combination of both (sex × age). For sensitivity analysis, the definitions of AD and the start date of follow-up for CVD outcomes were changed such that 1) the definition of AD from at least one diagnosis code and AD-related treatments on the same day was changed to 2 or more on separate days and 2) the start date of follow-up for outcomes was changed to 1 year after the index date. All analyses were conducted using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA), with a 2-sided P value of < 0.05 indicating statistical significance.
RESULTS
Characteristics of the study population
After PSM, 40,512 patients newly diagnosed with AD and 40,512 matched controls were included in this study. The baseline characteristics of the study population are summarized in Table 1. The mean follow-up period was 5.1 ± 2.9 years and 4.7 ± 2.9 years in the AD and control groups, respectively. During the follow-up period, a total of 3,875 CVDs occurred, 2,235 (5.5%) and 1,640 (4.1%) in the AD and control groups, respectively. Given that matching was performed, no significant differences in sex, age, household income, region of residence, and comorbidities were observed between the 2 groups. No significant intergroup differences were observed except for unadjusted variables in PSM such as CCI scores, smoking status, and some kinds of co-medication.
Table 1. Characteristics of the study population after propensity score matching.
| Variables | Matched controls (n = 40,512) | Patients with AD (n = 40,512) | P value | |
|---|---|---|---|---|
| Sex | 0.7584 | |||
| Male | 16,478 (40.67) | 16,435 (40.57) | ||
| Female | 24,034 (59.33) | 24,077 (59.43) | ||
| Age (yr) | 0.9971 | |||
| 20–29 | 5,280 (13.03) | 5,274 (13.02) | ||
| 30–39 | 8,381 (20.69) | 8,411 (20.76) | ||
| 40–49 | 8,825 (21.78) | 8,826 (21.79) | ||
| 50–59 | 8,426 (20.80) | 8,394 (20.72) | ||
| 60–69 | 5,410 (13.35) | 5,385 (13.29) | ||
| 70–79 | 3,305 (8.16) | 3,311 (8.17) | ||
| ≥ 80 | 885 (2.18) | 911 (2.25) | ||
| Household income | 0.9403 | |||
| Low (0–2) | 4,113 (10.15) | 4,167 (10.29) | ||
| Medium–low (3–5) | 9,299 (22.95) | 9,288 (22.93) | ||
| Medium–high (6–8) | 11,675 (28.82) | 11,666 (28.80) | ||
| High (9–10) | 15,425 (38.08) | 15,391 (37.99) | ||
| Region of residence | 0.7948 | |||
| Urban | 19,521 (48.19) | 19,558 (48.28) | ||
| Rural | 20,991 (51.81) | 20,954 (51.72) | ||
| Comorbidity | ||||
| Hypertension | 6,225 (15.37) | 6,216 (15.34) | 0.9301 | |
| Diabetes | 2,233 (5.51) | 2,259 (5.58) | 0.6898 | |
| Hyperlipidemia | 2,227 (5.50) | 2,220 (5.48) | 0.9140 | |
| CCI | < 0.0001 | |||
| 0 | 34,206 (84.43) | 32,417 (80.02) | ||
| 1 | 4,418 (10.91) | 5,645 (13.93) | ||
| 2 | 1,357 (3.35) | 1,696 (4.19) | ||
| ≥ 3 | 531 (1.31) | 754 (1.86) | ||
| Smoking status | < 0.0001 | |||
| Current | 8,256 (20.38) | 7,826 (19.32) | ||
| Former | 4,384 (10.82) | 4,808 (11.87) | ||
| Never | 27,872 (68.80) | 27,878 (68.81) | ||
| BMI (kg/m2) | 0.9056 | |||
| < 18 | 1,263 (3.12) | 1,283 (3.17) | ||
| 18–24.9 | 26,897 (66.39) | 26,908 (66.42) | ||
| ≥ 25 | 12,352 (30.49) | 12,321 (30.41) | ||
| Co-medications | ||||
| ACEIs | 485 (1.20) | 510 (1.26) | 0.4252 | |
| ARBs | 3,665 (9.05) | 3,879 (9.57) | 0.0097 | |
| BBs | 1,619 (4.00) | 1,845 (4.55) | < 0.0001 | |
| CCBs | 4,078 (10.07) | 4,215 (10.40) | 0.1123 | |
| Diuretics | 2,947 (7.27) | 2,980 (7.36) | 0.6561 | |
| Insulin | 25 (0.06) | 29 (0.07) | 0.5861 | |
| Hypoglycemic drugs other than insulin | 2,230 (5.50) | 2,309 (5.70) | 0.2275 | |
| Statins | 2,970 (7.33) | 3,454 (8.53) | < 0.0001 | |
| Lipid-lowering drugs other than statin | 558 (1.38) | 702 (1.73) | < 0.0001 | |
| Antiplatelets | 2,381 (5.88) | 2,993 (7.39) | < 0.0001 | |
| Anticoagulants | 58 (0.14) | 116 (0.29) | < 0.0001 | |
| Antidepressants | 657 (1.62) | 1,055 (2.60) | < 0.0001 | |
Variables are presented as numbers (%), and the P value was derived from the χ2 test.
AD, atopic dermatitis; CCI, Charlson comorbidity index; BMI, body mass index; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta blocker; CCB, calcium channel blocker.
Risk of CVD development in patients diagnosed with AD
Compared to matched controls, patients diagnosed with AD had a 42% higher risk of CVD after fully adjusting for covariates (adjusted HR, 1.42; 95% CI, 1.33–1.52) (Table 2). Analyses according to sub-diseases showed that patients newly diagnosed with AD had a significantly increased risk of angina pectoris (adjusted HR, 1.49; 95% CI, 1.36–1.63), MI (adjusted HR, 1.40; 95% CI, 1.15–1.70), ischemic stroke (adjusted HR, 1.34; 95% CI, 1.20–1.49), and hemorrhagic stroke (adjusted HR, 1.26; 95% CI, 1.05–1.52) compared to matched controls.
Table 2. Comparison of the risk for CVDs between patients with AD and matched controls.
| Variables | No. | Event (%) | Crude HR (95% CI) | P value | Adjusted HR (95% CI)* | P value | ||
|---|---|---|---|---|---|---|---|---|
| CVDs | ||||||||
| AD | 40,512 | 2,235 (5.52) | 1.46 (1.37–1.55) | < 0.0001 | 1.42 (1.33–1.52) | < 0.0001 | ||
| Matched controls | 40,512 | 1,640 (4.05) | Reference | Reference | ||||
| Ischemic heart disease | ||||||||
| AD | 40,512 | 1,310 (3.23) | 1.51 (1.39–1.65) | < 0.0001 | 1.47 (1.35–1.60) | < 0.0001 | ||
| Non-AD | 40,512 | 920 (2.27) | Reference | Reference | ||||
| Angina pectoris | ||||||||
| AD | 40,512 | 1,171 (2.89) | 1.54 (1.41–1.68) | < 0.0001 | 1.49 (1.36–1.63) | < 0.0001 | ||
| Matched controls | 40,512 | 810 (2.00) | Reference | Reference | ||||
| MI | ||||||||
| AD | 40,512 | 243 (0.60) | 1.45 (1.20–1.76) | 0.0002 | 1.40 (1.15–1.70) | 0.0009 | ||
| Matched controls | 40,512 | 178 (0.44) | Reference | Reference | ||||
| Stroke | ||||||||
| AD | 40,512 | 880 (2.17) | 1.37 (1.24–1.51) | < 0.0001 | 1.30 (1.18–1.44) | < 0.0001 | ||
| Matched controls | 40,512 | 682 (1.68) | Reference | Reference | ||||
| Ischemic stroke | ||||||||
| AD | 40,512 | 758 (1.87) | 1.41 (1.27–1.57) | < 0.0001 | 1.34 (1.20–1.49) | < 0.0001 | ||
| Matched controls | 40,512 | 570 (1.41) | Reference | Reference | ||||
| Hemorrhagic stroke | ||||||||
| AD | 40,512 | 262 (0.65) | 1.30 (1.09–1.56) | 0.0043 | 1.26 (1.05–1.52) | 0.0118 | ||
| Matched controls | 40,512 | 215 (0.53) | Reference | Reference | ||||
CVD, cardiovascular disease; HR, hazard ratio; CI, confidence interval; AD, atopic dermatitis; MI, myocardial infarction.
*Adjusted for sex, age, household income, region of residence, body mass index, smoking status, comorbidities (hypertension, diabetes, and hyperlipidemia), Charlson comorbidity index, and co-medications.
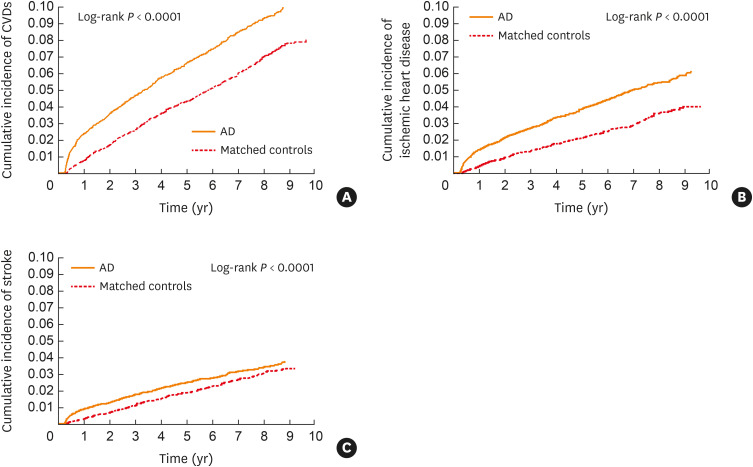
The cumulative incidence of study outcomes over time in adults with AD and matched controls demonstrated that the risk of CVDs, ischemic heart disease, and stroke were significantly higher in patients with AD compared to matched controls (log-rank P < 0.0001; Fig. 2).
Fig. 2. Cumulative incidence of CVD outcomes in patients with AD (solid line) and matched controls (dashed line) over time. The risk of (A) CVDs, (B) ischemic heart disease, and (C) stroke was significantly higher in patients with AD than in matched controls (log-rank P < 0.0001).
CVD, cardiovascular disease; AD, atopic dermatitis.
Risk of CVD development in severe AD
AD severity was significantly associated with an increased risk of CVDs (Table 3). Compared to patients with non-severe AD, those with severe AD had a 62% higher risk of CVDs after fully adjusting for covariates (adjusted HR, 1.62; 95% CI, 1.48–1.77). Analyses according to sub-diseases showed that patients with severe AD had a significantly increased risk for angina pectoris (adjusted HR, 1.70; 95% CI, 1.50–1.93), MI (adjusted HR, 1.63; 95% CI, 1.23–2.15), ischemic stroke (adjusted HR, 1.43; 95% CI, 1.22–1.67), and hemorrhagic stroke (adjusted HR, 1.47; 95% CI, 1.15–1.89) compared with those with non-severe AD.
Table 3. Comparison of the risk for CVDs between patients with severe and non-severe AD.
| Variables | No. | Event (%) | Crude HR (95% CI) | P value | Adjusted HR (95% CI)* | P value | ||
|---|---|---|---|---|---|---|---|---|
| CVDs | ||||||||
| Severe AD | 19,621 | 1,285 (6.55) | 1.67 (1.53–1.82) | < 0.0001 | 1.62 (1.48–1.77) | < 0.0001 | ||
| Non-severe AD | 20,082 | 824 (4.10) | Reference | Reference | ||||
| Ischemic heart disease | ||||||||
| Severe AD | 20,164 | 742 (3.68) | 1.76 (1.57–1.98) | < 0.0001 | 1.70 (1.51–1.92) | < 0.0001 | ||
| Non-severe AD | 20,455 | 451 (2.20) | Reference | Reference | ||||
| Angina pectoris | ||||||||
| Severe AD | 20,230 | 676 (3.34) | 1.77 (1.56–2.00) | < 0.0001 | 1.70 (1.50–1.93) | < 0.0001 | ||
| Non-severe AD | 20,498 | 408 (1.99) | Reference | Reference | ||||
| MI | ||||||||
| Severe AD | 20,772 | 134 (0.65) | 1.74 (1.32–2.29) | < 0.0001 | 1.63 (1.23–2.15) | 0.0007 | ||
| Non-severe AD | 20,824 | 82 (0.39) | Reference | Reference | ||||
| Stroke | ||||||||
| Severe AD | 20,418 | 488 (2.39) | 1.56 (1.36–1.80) | < 0.0001 | 1.45 (1.26–1.67) | < 0.0001 | ||
| Non-severe AD | 20,575 | 331 (1.61) | Reference | Reference | ||||
| Ischemic stroke | ||||||||
| Severe AD | 20,497 | 409 (2.00) | 1.56 (1.34–1.82) | < 0.0001 | 1.43 (1.22–1.67) | < 0.0001 | ||
| Non-severe AD | 20,629 | 277 (1.34) | Reference | Reference | ||||
| Hemorrhagic stroke | ||||||||
| Severe AD | 20,755 | 151 (0.73) | 1.52 (1.19–1.95) | 0.0009 | 1.47 (1.15–1.89) | 0.0025 | ||
| Non-severe AD | 20,800 | 106 (0.51) | Reference | Reference | ||||
CVD, cardiovascular disease; AD, atopic dermatitis; HR, hazard ratio; CI, confidence interval; MI, myocardial infarction.
*Adjusted for sex, age, household income, region of residence, body mass index, smoking status, comorbidities (hypertension, diabetes, and hyperlipidemia), Charlson comorbidity index, and co-medications.
Subgroup analysis
Analysis according to sex showed that among patients diagnosed with AD, male and female had an adjusted HR for CVD risk of 1.42 (95% CI, 1.30–1.56) and 1.43 (95% CI, 1.30–1.56), respectively (Supplementary Table S1). Among males, the risk of any outcome was significantly higher in patients diagnosed with AD than in matched controls. The adjusted HR for MI was the highest at 1.54 (95% CI, 1.21–1.98), followed by a hemorrhagic stroke at 1.48 (95% CI, 1.10–1.99), angina pectoris at 1.42 (95% CI, 1.25–1.61), and ischemic stroke at 1.37 (95% CI, 1.17–1.60). In a subgroup analysis among females, the adjusted HRs for angina pectoris and ischemic stroke risk were 1.57 (95% CI, 1.38–1.79) and 1.32 (95% CI, 1.13–1.55), respectively. Among females, the risk for MI and hemorrhagic stroke was higher in patients diagnosed with AD than in matched controls, although the difference was not significant.
In a subgroup analysis according to age group, patients diagnosed with AD exhibited a significantly higher risk of CVD than matched controls in the age groups of 50 years and older: age group 50–59 (adjusted HR, 1.57; 95% CI, 1.37–1.79), age group 60–69 (adjusted HR, 1.42; 95% CI, 1.26–1.59), age group 70–79 (adjusted HR, 1.42; 95% CI, 1.25–1.62), and ≥ 80 years of age group (adjusted HR, 1.94; 95% CI, 1.49–2.53) (Supplementary Table S2). Even after accounting for sex and age, newly diagnosed AD was significantly associated with a higher risk for subsequent CVD in both females and males over the age of 50 (Supplementary Table S3).
Sensitivity analyses
Most of the sensitivity analysis results using the different definitions of AD or follow-up start dates for CVD outcomes were consistent with the results of the main analysis (Supplementary Tables S4 and S5). The adjusted HR of all the outcomes, except for hemorrhagic stroke, was consistently higher in patients diagnosed with AD than in matched controls, regardless of the definition used. When using the different definitions of AD, the adjusted HR of hemorrhagic stroke in patients diagnosed with AD was 1.32 (95% CI, 1.00–1.73), albeit not statistically significant.
DISCUSSION
This nationwide study using well-established cohort data from Korea showed that AD was associated with a significant increase in the risk for cardiovascular outcomes and that this increased risk was also associated with AD severity. After fully adjusting for covariates, our findings showed that adult patients diagnosed with AD were at a 42% increased risk of CVDs, 40%–49% increased risk of ischemic heart disease, and 26%–34% increased risk of stroke compared to matched controls. Moreover, these positive associations between AD and cardiovascular outcomes remained regardless of sex and age (≥ 50 years).
AD is a prevalent inflammatory skin disease that involves both abnormal skin barrier function and distorted immune response, with tendencies for exaggerated type 2 helper T (Th2) response.35 Despite growing evidence suggesting that the immunological abnormalities of AD can extend beyond the skin or can affect inflammatory process in other organs, patient-based studies on the association between AD and CVD have shown mixed results. These conflicting results open the possibility that previous studies had misclassified AD or that other potential confounding factors affected the association. A number of previous studies using self-reported definitions of AD may have incorrectly included other types of eczema, such as contact dermatitis. Regarding other confounders, some shared risk factors for AD and CVD, such as smoking, may be considered. This is supported by results from a Danish study, which suggested that the statistical significance of an increased risk of stroke and cardiovascular death in AD patients was lost after adjusting for smoking, socioeconomic status, comorbidity, and use of medication.16 As such, the results of our study, which defined AD using a combination of physician diagnosis and AD-related medication history and included smoking status and BMI as covariates in the adjusted model, provide sufficient support for the hypothesis that AD increases the risk of CVDs.
While the underlying mechanisms between AD and CVDs are not yet fully understood, some recent proteomic studies reported upregulated markers correlated with atherosclerosis and cardiovascular risk (e.g., CX3CL1/fractalkine, CCL8, M-CSF, and VEGF-A) in the skin and blood of AD patients.36,37,38 Furthermore, a recent study that evaluated vascular inflammation with positron emission tomography-computed tomography (PET-CT) in young AD patients with no known CVDs found a significant association between vascular inflammation confirmed via PET-CT and Th2-related products (e.g., CCL17, CCL22) in the skin and blood of patients with AD.39 The same study also found that circulating levels of microparticles derived from platelets and endothelial cells, predictive markers of atherosclerosis and coronary artery disease, were significantly higher in patients with severe AD and tended to correlate with vascular inflammation assessed using PET-CT.39 Taken together, these studies suggest that chronic inflammation may play a key role in increasing cardiovascular risk in AD. Besides this, there have been several hypotheses on the association between AD and cardiovascular risk. Such hypotheses include altered plasma fibrin clot properties, increased platelet activation, and oxidative stress leading to the formation of atherogenic plaques in AD.40,41,42,43
In this study, the adjusted HR for CVD risk in patients diagnosed with AD was significant in both males and females. However, the adjusted HR for MI and hemorrhagic stroke was not statistically significant among females, contrary to that observed among males. This finding is in agreement with results from the previous meta-analysis that reported a significantly elevated risk of stroke and MI in males but not in females.44 Several factors related to male sex, such as unhealthy behaviors (e.g., drinking and smoking) or sex hormones, may have contributed to the difference in the results between males and females.
Our findings of a significantly higher CVD risk among elderly AD patients are supported by a recent study evaluating inflammatory marker levels in peripheral blood of elderly patients with AD. Notably, the aforementioned study found that elderly AD patients had significantly higher levels of inflammatory markers compared to both other patient age groups and respective controls and that these markers (e.g., TNFSF14/LIGHT, IL16, CCL4, and CCL7) were most significantly upregulated in the oldest age group.37 These suggest that systemic inflammation increases significantly with age and that the presence of AD may independently aggravate inflammation of the vessels in an already vulnerable elderly population.37 Although AD is mostly considered a childhood disease, there has recently been a new definition for a new elderly AD phenotype (> 60 years old).45 In line with this, the above findings suggest that strategies to prevent the occurrence of CVD in elderly AD patients may be necessary.
Additionally, this study showed that the risk of CVDs varied according to AD severity and that this result is consistent with those presented in previous studies. A cohort study classifying AD severity according to the prescribed medication revealed that patients with severe AD demonstrated significantly higher risks of stroke, MI, angina, and cardiovascular death compared to those with milder disease.46 Another 2019 meta-analysis also reported a significantly increased risk of MI, angina, and cardiovascular death with increased AD severity.12 These findings may be partly explained by dose–response effects and an underlying alternative pathogenesis of non-severe AD compared with severe AD. Therefore, reducing the risk of CVDs among patients with severe AD should be prioritized.
The results of this study are valuable due to the study design with several strengths. First, this study used the NHIS-NSC database of South Korea, which is representative of the entire population and healthcare utilization information in all settings across Korea. Second, to the best of our knowledge, this study is one of the few longitudinal studies that not only defined AD while considering both the physician’s diagnosis and the use of medications to treat AD but also adjusted for lifestyle risk factors, such as smoking and BMI. This patient definition method is supported by studies that have evaluated the specificity or sensitivity of methods for defining AD using diagnostic codes. A study in the UK reported a positive predictive value of 86% when using one AD code and 2 AD-related treatment codes.47 In another study conducted in Korea, no difference was found in the specificity between groups defined only by the 2 diagnostic codes or by combining the diagnostic code and AD laboratory code.48 Additionally, the current study performed sensitivity analyses using various definitions of AD to overcome the possibility of AD misclassification, and all the results were consistent with the main findings. Nevertheless, caution should be exercised when interpreting the results because we cannot completely exclude the possibility of misclassification owing to the inherent limitations of the claims data. Third, this study investigated the risk of CVDs by targeting only adult patients diagnosed with AD. Compared to childhood AD, adult AD has been reported to be associated with distinctly different risk factors, distribution and morphology of lesions, associated signs, comorbidities, and immune mechanisms such as interleukin (IL)-31 and IL-33.49 Owing to the fact that a limited number of studies have focused on adult patients diagnosed with AD, we believe that results of this study can contribute to improving the understanding of adult AD. Lastly, we performed PSM and several sensitivity analyses to reduce the effects of residual confounding and bias.
Despite these strengths, several limitations of the current study need to be acknowledged. First, this study defined adult patients newly diagnosed with AD during the study period by excluding cases diagnosed with AD between 2002 and 2005 from the study group (4-year washout period). However, due to the inherent limitations of the claim database, the possibility that recurrent or persistent AD was enrolled in this study cannot be excluded. Secondly, other allergic diseases that could confound the effects of AD were not considered when selecting subjects. Thus, patients with other allergic diseases may have been included in the case and control groups and contributed to the increased risk of AD. This may have resulted in misclassification of patients with AD in this study. However, the effects of non-differential misclassification would have resulted in a bias toward the null.50 Thirdly, the inherent limitations of the claims database may affect the research results. Despite robust multivariate adjustment, there may be possible residual confounders that cannot be identified by claim data, such as diet or genetic differences. Also, subjects who did not receive a national health screening were excluded from the study population, which may have caused a selection bias. However, the participation rates of Korea’s national health screening program remain very high at around 75%, which lowers the possibility of selection bias.51 Moreover, we also attempted to compensate for these limitations through PSM, robust multivariable adjustment, and multiple sensitivity analyses. Fourthly, we defined patients with severe AD using systemic treatment use, which may have introduced a misclassification bias. Unfortunately, however, there is still no fully validated and agreed upon algorithm for defining AD severity in cohort studies; therefore, almost all studies assessing the risk of cardiovascular outcomes according to AD severity had also defined AD severity based on the use of systemic treatments.15,16,20,21,46 Finally, novel AD treatments such as biologics or small-molecule drugs were not considered in this study. Most of these drugs began to receive approval after 2015 and were not covered by the National Health Insurance in Korea, so it was not possible to accurately consider them.
In conclusion, this nationwide cohort study demonstrated that adult patients newly diagnosed with AD experienced a clinically significant increase in the risk of subsequent CVDs, despite adjusting for potential risk factors for cardiovascular outcomes. These results help us understand the risk of cardiovascular comorbidities in AD patients while also highlighting the need for increased cardiovascular screening efforts to reduce CVDs in adult patients with AD.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2019R1A6A1A03031807). There was no involvement of the funder in the study design, collection, analysis, interpretation of data, writing of the report, or decision to submit the manuscript for publication. There are no conflict of interests to declare.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Subgroup analysis of CVDs risk based on sex
Subgroup analysis of CVDs risk based on age
Subgroup analysis of CVDs risk based on sex and age
Sensitivity analysis according to the different definitions of AD*
Sensitivity analysis according to the different start dates of follow-up*
References
- 1.Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132–1138. doi: 10.1016/j.jaci.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 2.Rönmark EP, Ekerljung L, Lötvall J, Wennergren G, Rönmark E, Torén K, et al. Eczema among adults: prevalence, risk factors and relation to airway diseases. Results from a large-scale population survey in Sweden. Br J Dermatol. 2012;166:1301–1308. doi: 10.1111/j.1365-2133.2012.10904.x. [DOI] [PubMed] [Google Scholar]
- 3.Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947–54.e15. doi: 10.1016/j.jaci.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73:1284–1293. doi: 10.1111/all.13401. [DOI] [PubMed] [Google Scholar]
- 5.Simpson EL, Eichenfield LF, Ellis CN, Mancini AJ, Paller AS. Current issues in atopic comorbidities and preventing the atopic march. Semin Cutan Med Surg. 2012;31:S6–S9. doi: 10.1016/j.sder.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Schneider L, Hanifin J, Boguniewicz M, Eichenfield LF, Spergel JM, Dakovic R, et al. Study of the atopic march: development of atopic comorbidities. Pediatr Dermatol. 2016;33:388–398. doi: 10.1111/pde.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paller A, Jaworski JC, Simpson EL, Boguniewicz M, Russell JJ, Block JK, et al. Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19:821–838. doi: 10.1007/s40257-018-0383-4. [DOI] [PubMed] [Google Scholar]
- 8.Andersen YM, Egeberg A, Skov L, Thyssen JP. Comorbidities of atopic dermatitis: beyond rhinitis and asthma. Curr Dermatol Rep. 2017;6:35–41. doi: 10.1007/s13671-017-0168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO) Cardiovascular diseases (CVDs) fact sheet [Internet] Geneva: WHO; 2022. [cited 2022 Sep 11]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) [Google Scholar]
- 10.Narayan KM, Ali MK, Koplan JP. Global noncommunicable diseases--where worlds meet. N Engl J Med. 2010;363:1196–1198. doi: 10.1056/NEJMp1002024. [DOI] [PubMed] [Google Scholar]
- 11.Thyssen JP, Halling-Overgaard AS, Andersen YM, Gislason G, Skov L, Egeberg A. The association with cardiovascular disease and type 2 diabetes in adults with atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol. 2018;178:1272–1279. doi: 10.1111/bjd.16215. [DOI] [PubMed] [Google Scholar]
- 12.Ascott A, Mulick A, Yu AM, Prieto-Merino D, Schmidt M, Abuabara K, et al. Atopic eczema and major cardiovascular outcomes: a systematic review and meta-analysis of population-based studies. J Allergy Clin Immunol. 2019;143:1821–1829. doi: 10.1016/j.jaci.2018.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantor R, Kim A, Thyssen JP, Silverberg JI. Association of atopic dermatitis with smoking: a systematic review and meta-analysis. J Am Acad Dermatol. 2016;75:1119–1125.e1. doi: 10.1016/j.jaad.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang A, Silverberg JI. Association of atopic dermatitis with being overweight and obese: a systematic review and metaanalysis. J Am Acad Dermatol. 2015;72:606–16.e4. doi: 10.1016/j.jaad.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Andersen YM, Egeberg A, Gislason GH, Hansen PR, Skov L, Thyssen JP. Risk of myocardial infarction, ischemic stroke, and cardiovascular death in patients with atopic dermatitis. J Allergy Clin Immunol. 2016;138:310–312.e3. doi: 10.1016/j.jaci.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Egeberg A, Andersen YM, Gislason GH, Skov L, Thyssen JP. Prevalence of comorbidity and associated risk factors in adults with atopic dermatitis. Allergy. 2017;72:783–791. doi: 10.1111/all.13085. [DOI] [PubMed] [Google Scholar]
- 17.Kwa MC, Silverberg JI. Association between inflammatory skin disease and cardiovascular and cerebrovascular co-morbidities in US adults: analysis of nationwide inpatient sample data. Am J Clin Dermatol. 2017;18:813–823. doi: 10.1007/s40257-017-0293-x. [DOI] [PubMed] [Google Scholar]
- 18.Marshall VD, Moustafa F, Hawkins SD, Balkrishnan R, Feldman SR. Cardiovascular disease outcomes associated with three major inflammatory dermatologic diseases: a propensity-matched case control study. Dermatol Ther (Heidelb) 2016;6:649–658. doi: 10.1007/s13555-016-0144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radtke MA, Schäfer I, Glaeske G, Jacobi A, Augustin M. Prevalence and comorbidities in adults with psoriasis compared to atopic eczema. J Eur Acad Dermatol Venereol. 2017;31:151–157. doi: 10.1111/jdv.13813. [DOI] [PubMed] [Google Scholar]
- 20.Riis JL, Vestergaard C, Hjuler KF, Iversen L, Jakobsen L, Deleuran MS, et al. Hospital-diagnosed atopic dermatitis and long-term risk of myocardial infarction: a population-based follow-up study. BMJ Open. 2016;6:e011870. doi: 10.1136/bmjopen-2016-011870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Standl M, Tesch F, Baurecht H, Rodríguez E, Müller-Nurasyid M, Gieger C, et al. Association of atopic dermatitis with cardiovascular risk factors and diseases. J Invest Dermatol. 2017;137:1074–1081. doi: 10.1016/j.jid.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Su VY, Chen TJ, Yeh CM, Chou KT, Hung MH, Chu SY, et al. Atopic dermatitis and risk of ischemic stroke: a nationwide population-based study. Ann Med. 2014;46:84–89. doi: 10.3109/07853890.2013.870018. [DOI] [PubMed] [Google Scholar]
- 23.Tsai KS, Yen CS, Wu PY, Chiang JH, Shen JL, Yang CH, et al. Traditional Chinese medicine decreases the stroke risk of systemic corticosteroid treatment in dermatitis: a nationwide population-based study. Evid Based Complement Alternat Med. 2015;2015:543517. doi: 10.1155/2015/543517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung HJ, Lee DH, Park MY, Ahn J. Cardiovascular comorbidities of atopic dermatitis: using National Health Insurance data in Korea. Allergy Asthma Clin Immunol. 2021;17:94. doi: 10.1186/s13223-021-00590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivert LU, Johansson EK, Dal H, Lindelöf B, Wahlgren CF, Bradley M. Association between atopic dermatitis and cardiovascular disease: a nationwide register-based case-control study from Sweden. Acta Derm Venereol. 2019;99:865–870. doi: 10.2340/00015555-3235. [DOI] [PubMed] [Google Scholar]
- 26.Drucker AM, Li WQ, Cho E, Li T, Sun Q, Camargo CA, Jr, et al. Atopic dermatitis is not independently associated with nonfatal myocardial infarction or stroke among US women. Allergy. 2016;71:1496–1500. doi: 10.1111/all.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drucker AM, Qureshi AA, Dummer TJ, Parker L, Li WQ. Atopic dermatitis and risk of hypertension, type 2 diabetes, myocardial infarction and stroke in a cross-sectional analysis from the Canadian Partnership for Tomorrow Project. Br J Dermatol. 2017;177:1043–1051. doi: 10.1111/bjd.15727. [DOI] [PubMed] [Google Scholar]
- 28.Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy. 2015;70:1300–1308. doi: 10.1111/all.12685. [DOI] [PubMed] [Google Scholar]
- 29.Treudler R, Zeynalova S, Walther F, Engel C, Simon JC. Atopic dermatitis is associated with autoimmune but not with cardiovascular comorbidities in a random sample of the general population in Leipzig, Germany. J Eur Acad Dermatol Venereol. 2018;32:e44–e46. doi: 10.1111/jdv.14495. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46:e15. doi: 10.1093/ije/dyv319. [DOI] [PubMed] [Google Scholar]
- 31.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo A, Lee SW, Koh HY, Kim MA, Han MY, Yon DK. Incidence of cancer after asthma development: 2 independent population-based cohort studies. J Allergy Clin Immunol. 2021;147:135–143. doi: 10.1016/j.jaci.2020.04.041. [DOI] [PubMed] [Google Scholar]
- 33.Kim JE, Kim HJ, Lew BL, Lee KH, Hong SP, Jang YH, et al. Consensus guidelines for the treatment of atopic dermatitis in Korea (Part II): systemic treatment. Ann Dermatol. 2015;27:578–592. doi: 10.5021/ad.2015.27.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 35.Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139:S65–S76. doi: 10.1016/j.jaci.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pavel AB, Zhou L, Diaz A, Ungar B, Dan J, He H, et al. The proteomic skin profile of moderate-to-severe atopic dermatitis patients shows an inflammatory signature. J Am Acad Dermatol. 2020;82:690–699. doi: 10.1016/j.jaad.2019.10.039. [DOI] [PubMed] [Google Scholar]
- 37.He H, Li R, Choi S, Zhou L, Pavel A, Estrada YD, et al. Increased cardiovascular and atherosclerosis markers in blood of older patients with atopic dermatitis. Ann Allergy Asthma Immunol. 2020;124:70–78. doi: 10.1016/j.anai.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Brunner PM, Suárez-Fariñas M, He H, Malik K, Wen HC, Gonzalez J, et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep. 2017;7:8707. doi: 10.1038/s41598-017-09207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villani AP, Pavel AB, Wu J, Fernandes M, Maari C, Saint-Cyr Proulx E, et al. Vascular inflammation in moderate-to-severe atopic dermatitis is associated with enhanced Th2 response. Allergy. 2021;76:3107–3121. doi: 10.1111/all.14859. [DOI] [PubMed] [Google Scholar]
- 40.Nastałek M, Wojas-Pelc A, Undas A. Plasma fibrin clot properties in atopic dermatitis: links between thrombosis and atopy. J Thromb Thrombolysis. 2010;30:121–126. doi: 10.1007/s11239-010-0478-0. [DOI] [PubMed] [Google Scholar]
- 41.Tamagawa-Mineoka R, Katoh N, Ueda E, Masuda K, Kishimoto S. Elevated platelet activation in patients with atopic dermatitis and psoriasis: increased plasma levels of beta-thromboglobulin and platelet factor 4. Allergol Int. 2008;57:391–396. doi: 10.2332/allergolint.O-08-537. [DOI] [PubMed] [Google Scholar]
- 42.Ji H, Li XK. Oxidative stress in atopic dermatitis. Oxid Med Cell Longev. 2016;2016:2721469. doi: 10.1155/2016/2721469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khosravi M, Poursaleh A, Ghasempour G, Farhad S, Najafi M. The effects of oxidative stress on the development of atherosclerosis. Biol Chem. 2019;400:711–732. doi: 10.1515/hsz-2018-0397. [DOI] [PubMed] [Google Scholar]
- 44.Yuan M, Cao WF, Xie XF, Zhou HY, Wu XM. Relationship of atopic dermatitis with stroke and myocardial infarction: a meta-analysis. Medicine (Baltimore) 2018;97:e13512. doi: 10.1097/MD.0000000000013512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanei R, Hasegawa Y. Atopic dermatitis in older adults: a viewpoint from geriatric dermatology. Geriatr Gerontol Int. 2016;16(Suppl 1):75–86. doi: 10.1111/ggi.12771. [DOI] [PubMed] [Google Scholar]
- 46.Silverwood RJ, Forbes HJ, Abuabara K, Ascott A, Schmidt M, Schmidt SA, et al. Severe and predominantly active atopic eczema in adulthood and long term risk of cardiovascular disease: population based cohort study. BMJ. 2018;361:k1786. doi: 10.1136/bmj.k1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abuabara K, Magyari AM, Hoffstad O, Jabbar-Lopez ZK, Smeeth L, Williams HC, et al. Development and validation of an algorithm to accurately identify atopic eczema patients in primary care electronic health records from the UK. J Invest Dermatol. 2017;137:1655–1662. doi: 10.1016/j.jid.2017.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jung H, Lee J, Jang D, Park M, Ahn J. Criteria to identify patients with atopic dermatitis in the National Health Insurance data in Korea. JAAD Int. 2020;1:124–125. doi: 10.1016/j.jdin.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vakharia PP, Silverberg JI. Adult-onset atopic dermatitis: characteristics and management. Am J Clin Dermatol. 2019;20:771–779. doi: 10.1007/s40257-019-00453-7. [DOI] [PubMed] [Google Scholar]
- 50.Gullen WH, Bearman JE, Johnson EA. Effects of misclassification in epidemiologic studies. Public Health Rep. 1968;83:914–918. [PMC free article] [PubMed] [Google Scholar]
- 51.National Health Insurance Service (NHIS) National health screening statistical yearbook. Seoul: NHIS; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subgroup analysis of CVDs risk based on sex
Subgroup analysis of CVDs risk based on age
Subgroup analysis of CVDs risk based on sex and age
Sensitivity analysis according to the different definitions of AD*
Sensitivity analysis according to the different start dates of follow-up*