Abstract
We examine neural correlates of discrete expressions of negative emotionality in infants to determine whether the microstructure of white matter tracts at 1 month of age foreshadows the expression of specific negative emotions later in infancy. Infants (n=103) underwent neuroimaging at 1-month, and mothers reported on infant fear, sadness, and anger at 6, 12, and 18 months using the Infant Behavior Questionnaire-Revised. Levels and developmental change in fear, sadness, and anger were estimated from mother reports. Relations between MRI and infant emotion indicated that 1-month white matter microstructure was differentially associated with level and change in infant fear, but not anger or sadness, in the left stria terminalis (p < .05, corrected), a tract that connects frontal and tempo-parietal regions and has been implicated in emerging psychopathology in adults. More relaxed constraints on significance (p < .10, corrected) revealed that fear was associated with lower white matter microstructure bilaterally in the inferior portion of the stria terminalis and regions within the sagittal stratum. Results suggest the neurobehavioral uniqueness of fear as early as 1 month of age in regions that are associated with potential longer-term outcomes. This work highlights the early neural precursors of fearfulness, adding to literature explaining the psychobiological accounts of affective development.
Keywords: neuroimaging, infant emotion, fear, DTI
Emotion-related motivations can promote behaviors that enhance safety and protection; however, negative emotional expressions are not always adaptive and can lead to later behavioral problems (see Beauchaine & Hinshaw, 2008). Negative emotion is often linked to mood and behavior problems, with early emerging negativity being a key developmental risk factor for poor academic performance (Molfese et al., 2010; Stright et al., 2008) and social behaviors in childhood and adolescence (Sanson et al., 2004). A better understanding of the neural biomarkers underlying discrete negative emotions will allow for the improved identification of individuals at risk for negative emotional behaviors, dysregulation, and later psychopathology, thereby aiding in the development of targeted interventions early in life.
Negative Emotion in Infants
A central tenet in Darwin’s original thesis on human emotion, The Expression of Emotion in Man and Animals (Darwin, 1872), is that emotions serve evolutionary functions that promote survival. Fear serves to protect us in the face of uncertainty or insecurity (Plutchik, 1980); anger motivates us to work toward or protect a goal or overcome an obstacle thwarting a goal (Frijda, 1986); sadness can elicit maternal caregiving when the infant is unable to meet his/her own needs (Buss & Kiel, 2004). As such, emotional expressions are biological imperatives and therefore distinct and similarly expressed across all humans. More recently, proponents of Discrete or Basic Emotion Theories, building on Darwin’s original ideas, characterize the expression of specific emotions (e.g., fear) by a set of relatively fixed and distinct expressed and experienced components that might be evident early in infancy (Ekman, 1999; Ekman & Cordaro, 2011; Goldsmith, 1993; Goldsmith et al., 1987; Izard & Malatesta, 1987). Thus, discrete emotion theories define specific emotions such as fear, anger, sadness, disgust, surprise, interest, and pleasure as having characteristic expressions and elicitors. Yet historically, the infant literature has characterized emotion more broadly, often examining an overall negativity component rather than decomposing negative emotion into individual discrete emotions (Derryberry & Rothbart, 1997; Rothbart & Derryberry, 2002). Such characterization neglects the complex neurobehavioral processes that may underlie different negative affective states (Belsky et al., 1998). Yet, little empirical evidence on the neural bases of emotion exists to differentiate a discrete versus general emotions view of negativity during infancy (Tracy & Randles, 2011). Indeed, only a few studies using similar modalities to those proposed here examine neurobehavioral origins of infant fear (Graham et al., 2016; Thomas et al., 2019), with none delineating among fear, anger, and sadness. Here, we examine infants’ neural architecture and how it relates of expressions of fear, sadness, and anger to better understand the emergence and differentiation of negative emotional expression early in life and provide evidence for discrete or more general negative affect approaches to understanding infant emotionality.
Brain Structure of Negative Emotion
Even though limbic areas of the brain often associated with emotional behavior are early developing (Stiles & Jernigan, 2010), research examining the neural architecture underlying emotional expression in the early postnatal period is limited. Previous research indicates that the brain’s white matter microstructure underlying the connectivity of limbic and prefrontal brain regions, such as the uncinate fasciculus, temporoparietal regions (superior and inferior longitudinal fasciculi, arcuate fasciculus), inferior fronto-occipital fasciculus, and corpus callosum, play a critical role in the expression of negative emotion (Herbet et al., 2018; Lai & Wu, 2014; Lai & Wu, 2016; Pisner et al., 2019; Von Der Heide et al., 2013). However, much of our understanding of neural correlates of negative emotional expression stems from non-human animal and adult human studies (Henderson et al., 2015; Pine, 2003; Rifkin-Graboi et al., 2019).
We know very little about neural precursors of or regions of interest relating to infant sadness and anger expression. Limited adult research identifies subcortical structures, including the thalamus and amygdala, as well as frontoparietal structures, such as the precuneus and posterior cingulate cortex, and white matter tracts connecting these brain regions, such as the uncinate fasciculus, as relevant to the expression of anger (see Alia-Klein et al., 2020 for a review on the neuroscience of anger). In addition, adults with extreme anger and impulsive aggression show decreased white matter microstructure in the superior longitudinal fasciculus, a white matter region connecting the parietal and frontal lobes that important for processing of emotion (Lee et al., 2016). The expression of sadness has been associated with increased neural activity in numerous structures, including the inferior orbitofrontal cortex, anterior insular cortex, middle and posterior temporal cortices, lateral cerebellum, the midbrain, and the putamen and caudate (Lane et al., 1997). In infancy, abnormalities in cerebellar structure, defined using clinical cutoffs as described in Tamm and colleagues (2020), predicted lower fear and sadness in term versus pre-term infants (Tamm et al., 2020). However, abnormalities, as defined here, are nonspecific and do not identify regions in the brain where emotional expressions and development may be most salient.
On the other hand, emerging research identifies neural precursors to the development of infant fear expressions. For instance, amygdala connectivity in neonates relates to fear at six months of age using functional connectivity neuroimaging (Graham et al., 2016). Specifically, higher six-month fear was related to stronger functional connectivity from the left amygdala to several regions connecting the amygdala to temporal and prefrontal lobes: the bilateral anterior insula, right inferior frontal gyrus, left parahippocampal gyrus, right putamen, right temporoparietal junction, and right superior temporal gyrus. The left amygdala also showed greater negative connectivity with the left occipital cortex.
Notably, this work highlights functional connectivity. But the structural connectivity and underlying white matter microstructure, which subserves and facilitates this functional connectivity, is less understood. An examination of white matter microstructure in typically developing neonates and later emotional behaviors suggested that infants who have higher internalizing scores (indexed by higher withdrawal, general and separation anxious behaviors, inhibition and distress to novelty) at 12 months of age showed lower white matter microstructure as neonates, measured using diffusion neuroimaging techniques, than infants lower in internalizing (Rifkin-Graboi et al., 2015). Results were localized to regions salient in emotional experience and response, namely the right insula, inferior frontal, middle occipital, middle temporal, and parahippocampal gyrus. In this relatively small sample (n=25), significant relations did not remain so after correcting for multiple comparisons. Importantly, this work did not examine distinct emotional expressions, and to our knowledge, no study has identified differential neural processes involved in the early emergence of fear, sadness, and anger in infancy. Thus, here, we aim to detect white matter microstructural differences that may presage infants’ later variation in negative emotional expression.
Developmental Changes in Emotion and Brain Development
Increasing research highlights differences between the development of specific negative emotions, including fear, sadness, and anger early in life (Ekman & Cordaro, 2011; Izard, 2011; Levenson, 2011; Panksepp & Watt, 2011). Although fewer studies include the examination of developmental changes in infant sadness, consistent evidence indicates that expressions of fear and anger increase throughout early childhood (Denham et al., 1995). Infants exhibit normative developmental changes in fear in infancy, with marked increases between 6-15 months of life (Braungart-Rieker et al., 2010; Brooker et al., 2013; Goldsmith, 1993; Lewis, 2012; Scarr & Salapatek, 1970). Anger also increases from infancy into toddlerhood with flattening trajectories in the school years (Brooker et al., 2014; Denham, 2006; Gagne & Goldsmith, 2011; Liu et al., 2018; Liu et al., 2021; Saarni et al., 2006).
In one neuroimaging study, stronger neonatal amygdala-insular functional connectivity predicted fear development from six to 24 months of age (Thomas et al., 2019), yet we do not know how structural connectivity might relate to such emotional development. Importantly, white matter microstructure develops exponentially throughout infancy and early childhood (Dean et al., 2017; Knickmeyer et al., 2008; Lebel et al., 2008; Stiles & Jernigan, 2010). Core structures involved in emotion, such as the amygdala and hippocampus, undergo rapid and robust growth during the first years of life (Uematsu et al., 2012) and continue to develop into later childhood and adolescence (Giedd & Rapoport, 2010; Lenroot & Giedd, 2006). Early emergence and development of these brain areas may be tied to the developmental shifts observed in emotional expressions and behaviors as well.
Diffusion Tensor Imaging: a non-invasive technique to assess neurobiology in infants
Neuroimaging techniques, such as magnetic resonance imaging (MRI), are used to characterize the rapid macro- and microstructural changes occurring during infancy. Diffusion MRI is a non-invasive quantitative MRI technique used to characterize the brain’s white matter microstructure (Alexander et al., 2007, 2011; Pierpaoli et al., 1996). Information gleaned using diffusion MRI has enhanced our understanding of white matter development and microstructural changes that occur throughout early development (Dubois et al., 2014). For example, we know that compared to full-term infants, pre-term infants have lower white matter microstructure throughout early childhood (Eaton-Rosen et al., 2015; Kelly et al., 2016) and infants with early trauma or disease also show altered white matter development (Kline-Fath et al., 2018; Taylor et al., 2015; Tortora et al., 2018). However, few studies have examined individual differences in white matter in typically developing neonates (Dean et al., 2017; Dowe et al., 2020; D. Kim et al., 2016; S. J. Lee et al., 2015), and none examine white matter in relation to developmental patterns of emotion.
Until recently, technical and logistical limitations that have made using MRI in neonates difficult (Dean et al., 2014). Advancements in MRI protocols and strategies for imaging infants and young children (Howell et al., 2019; Raschle et al., 2012; Torres et al., 2020) as well as increasing interest in early brain development have allowed researchers to use MRI to understand more about interrelations among early brain and behavioral development (Dean et al., 2017; Dowe et al., 2020; O’muircheartaigh et al., 2014). Here, we examine whether white matter microstructure at one month of age foreshadows the expression of negative emotions in infancy.
Current Study
We examine mother reports of infant fear, sadness, and anger from 6 to 18 months of age in relation to early (1-month) brain architecture. Our goal is to identify whether white matter microstructure early in life foreshadows the emergence of discrete negative emotionality in infants. If we accept that discrete emotional expressions are early emerging, then we might expect to see variation in the neural architecture underlying developing emotional expressions in early infancy as well. In general, we expect that infants will increase in fear and anger across infancy, with no a priori expectation relating to infant sadness; little work has examined developmental shifts in sadness. Further, we propose that expressions of fear, sadness, and anger will exhibit potentially divergent early neural architecture in the brain. We expect to find associations between emotion and white matter microstructure in brain regions connecting limbic and prefrontal brain regions, specifically the uncinate fasciculus, temporoparietal regions, inferior fronto-occipital fasciculus, and corpus callosum to be related to infant emotional expression. As previously noted, these regions are all implicated in expressions of fear, with the uncinate fasciculus additionally being associated with anger. We do not have regional hypotheses relating to sadness, as no previous work has identified neurobiological pathways of infant sadness. All but one previous study has examined function and not structural connectivity, and the singular study found lower white matter microstructure associated with higher fear. Thus, our hypotheses all follow this direction as well with lower white matter predicting higher and increasing fear expressions. Findings from this work will add to a growing literature on the neurobiology of emotional development in childhood.
Method
Participants
Data were collected as part of a longitudinal study investigating how early life experiences affect children’s brain and behavioral development. Mothers (N=149) were recruited during the second trimester of pregnancy (<28 weeks’ gestation) using the following inclusion and exclusion criteria: between 18 and 40 years of age, expecting singleton births, no diagnosis of major psychiatric illnesses (i.e., schizophrenia, bipolar disorder, borderline personality disorder), no pre-existing neurological conditions or major head trauma, no major autoimmune disease or infections during pregnancy. Families were also excluded if there was significant birth trauma or the infant was diagnosed with a neurological disorder at birth. Criteria were identified through maternal questionnaire and hospital birth record data for each infant. In total, 149 typically developing infants (76 female) were enrolled. Forty-eight infants had incomplete or poor diffusion imaging data quality (described below). Thus, the final sample consisted of 103 infants (52 female) that had complete imaging and at least two instances of behavioral data. Of note, there were no differences between infants who were and were not included in analyses based on demographic variables (child sex, age at scan, gestational period, mother age, or mother education) or variables of interest (fear, sadness, and anger across time). Additional demographic information of the full sample is provided in Table 1. The local Institutional Review Board approved all study procedures and parental consent was obtained at each time point from each participating family.
Table 1.
Participant demographic characteristics
| Sample Characteristics (N=103) | Mean | Std. Dev. |
|---|---|---|
| Mother Age at Infant Birth (years) | 32.84 | 3.94 |
| Mother Education at Infant Birth (years) | 16.81 | 4.62 |
| Infant Age at 1-month Scan (days) | 33.03 | 5.99 |
| Gestation (weeks) | 39.52 | 1.38 |
| Birth weight (lbs) | 7.64 | 1.15 |
| Birth Length (in) | 20.20 | 1.18 |
| Head Circumference (in) | 13.67 | .58 |
| APGAR Score (1 Min/5 Min) | 7.87/8.80 | 1.84/.70 |
|
| ||
| Infant Sex | n = 52 female | |
| Family Income at Infant Birth | Median = $80,001 to $100,000 | |
| Mothers’ Marital Status at Infant Birth |
n = 90 married to father; n = 3 single; n = 1 divorced; n = 1 separated; n = 5 missing |
|
|
| ||
| Infant Racial Background | ||
| African American/Black | 2 | |
| Asian | 6 | |
| Caucasian/White | 89 (12 Hispanic) | |
| Native Hawaiian or Other Pacific Islander | 2 | |
| Mixed Race | 1 | |
Procedures
At approximately 1 month (±2 weeks) of age, infants underwent magnetic resonance imaging (MRI) during natural, non-sedated sleep. At infant age 6, 12, and 18 months, mothers completed a series of demographic questionnaires as well as a measure of infant emotional disposition online.
MRI Data Acquisition and Preprocessing.
MRI was performed using a 3 Tesla General Electric MR750 Discovery scanner using a 32 channel receive-only head RF array coil (Nova Medical, Wakefield, MA). Infants were scanned during natural, non-sedated sleep (Dean et al., 2014, 2017, 2018), with sessions scheduled around the infant’s daily nap schedule. Mothers fed and swaddled their infant and rocked the infant to sleep before moving into the darkened MRI scanner suite. Several procedures were performed to enhance neuroimaging data collection from the sleeping infant; first, we optimized imaging protocols to limit peak gradient slew-rates, slowing down the scan and reducing the scanner noise, creating a more soothing environment in which the infant could sleep. In addition, scanner acoustical noise was reduced by inserting a noise-attenuating foam insert inside the bore of the MRI scanner and the infant wore ear plugs and MiniMuff® (Natus Medical Incorporated) ear covers. Finally, we used a MedVac vacuum immobilization unit (CFI Medical Solutions, USA) and foam around the infant’s head to prevent movement. Infants were monitored throughout the scan by a trained research staff member, and mothers remained in the scanner room throughout the scan if they chose to do so.
Diffusion weighted (DW) MRI were acquired with a single shot spin-echo echo-planar imaging pulse sequence and parallel imaging (factor 2) to reduce acquisition time and distortions from magnetic field inhomogeneities. A total of 69 volumes were acquired, sampling diffusion encoding directions with b-values of 350 s/mm2 [9 directions], 800 s/mm2 [18 directions], and 1500 s/mm2 [36 directions]; the remaining 6 volumes were acquired with no diffusion weighting (i.e., b-value = 0 s/mm2). Additional imaging parameters consisted of repetition time [TR] = 8400 ms, echo time [TE] = 94 ms, and bandwidth = 3906 Hz/pixel. Imaging field of view [FOV] was 25.6 cm×25.6 cm with an acquisition matrix of 128×128, providing a 2 mm×2 mm in-plane resolution. Coverage across the whole brain was achieved by acquiring 60 sagittal-oriented contiguous slices with a slice thickness of 2.0 mm. Total acquisition time was approximately 10 minutes.
DW-MRI data underwent standard pre-processing procedures using in-house processing pipelines (Dean et al., 2017). Briefly, images were visually examined for motion artifacts to correct for eddy current-induced distortions and subject movement (Jenkinson et al., 2002), diffusion encoding directions were rotated as necessary (Leemans & Jones, 2009), and non-brain signal removed. A measure of total head motion was calculated from the average displacement of each DW image relative to the first image slice and used as a covariate in subsequent analyses (Yendiki et al., 2014).
Measures
Infant Fear, Sadness, and Anger.
Mothers completed the 191-item Infant Behavior Questionnaire-Revised (IBQ-R; Gartstein & Rothbart, 2003) at 6, 12, and 18 months of age. The IBQ-R asks mothers to respond to questions about their infant’s behavior on a 1 (never) to 7 (always) point Likert scale. The measure consists of 14 scales, though here we only use the Fear/Distress to Novelty (16 items), Sadness (14 items), and Anger/Distress to Limitations (16 items) scales. IBQ-R questions were designed to assess an infant’s style of responding in specific situational contexts. For example, in the Fear scale, mothers report on the frequency with which the infant cries or shows distress when in the presence of a stranger or unfamiliar adult, or when a loud noise occurs. In the Sadness scale, mothers report on the extent to which infants display sadness when the caregiver leaves for a long period of time, how often the infant became sad when the mother was busy, and how tearful the infant gets when tired or at bedtime. In the Anger scale, mothers report on how often the infant had tantrums or got upset when s/he wanted something and how often the infant fusses or cries when being put to bed, when left in their crib, and when being bathed. Reliability for each scale across time points was strong (Table 2). Maternal report on the IBQ-R is widely used to assess infant emotions in infancy and has strong internal consistency in our work and others’ (Braungart-Rieker et al., 2010; Planalp & Braungart-Rieker, 2013).
Table 2.
Intercorrelations and other Descriptive Statistics for Fear, Sadness, and Anger at 6, 12, and 18 months of age
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | IBQ Scale Reliability (alpha) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. 6 Month Fear | 1 | .91 | ||||||||||
| 2. 12 Month Fear | .45*** | 1 | .85 | |||||||||
| 3. 18 Month Fear | .47*** | .55*** | 1 | .88 | ||||||||
| 4. 6 Month Sadness | .18 | .075 | .19 | 1 | .78 | |||||||
| 5. 12 Month Sadness | .08 | .26** | .26* | .38** | 1 | .80 | ||||||
| 6. 18 Month Sadness | .23* | .23** | .31** | .33** | .58** | 1 | .71 | |||||
| 7. 6 Month Anger | .26* | .16 | .22* | .65** | .44** | .32** | 1 | .91 | ||||
| 8. 12 Month Anger | .05 | .23* | .17 | .38** | .57** | .38** | .56** | 1 | .95 | |||
| 9. 18 Month Anger | .11 | .33*** | .22* | .31** | .42** | .47** | .48** | .69** | 1 | .88 | ||
| 10. Gestational Age at scan | .14 | −.01 | −.09 | .15 | −.01 | .15 | .02 | .03 | .02 | 1 | ||
| 11. Scan motion | .01 | .09 | .03 | −.03 | −.03 | −.08 | .02 | −.02 | .01 | −.04 | 1 | |
|
| ||||||||||||
| Mean | 2.10 | 2.87 | 3.14 | 3.46 | 3.50 | 3.32 | 3.42 | 4.04 | 3.95 | 33.08 | 2.27 | |
| S.D. | 0.70 | 0.82 | 0.81 | 0.78 | 0.72 | 0.69 | 0.70 | 0.72 | 0.81 | 5.92 | 1.78 | |
| Range | 1 - 4.69 | 1.07 - 4.67 | 1 – 4.69 | 1.85 - 5.58 | 1.6 – 5.5 | 1.75 – 5 | 1.75 – 5.69 | 2.63 – 6.69 | 2.31 – 5.88 | 18 – 50 | 0.48 – 13.17 | |
|
| ||||||||||||
| Male Mean | 2.08 | 2.84 | 3.14 | 3.46 | 3.36 | 3.28 | 3.49 | 4.06 | 3.92 | 32.88 | 1.93 | |
| Female Mean | 2.11 | 2.90 | 3.15 | 3.46 | 3.62 | 3.35 | 3.36 | 4.02 | 3.97 | 33.26 | 2.57 | |
| t | −0.14 | −0.34 | −0.05 | 0.04 | −1.81 | −0.48 | 0.90 | 0.30 | −0.34 | −0.33 | −1.84 | |
| (df) | 97 | 99 | 93 | 97 | 99 | 93 | 97 | 99 | 93 | 101 | 101 | |
| p-value for difference | 0.886 | 0.732 | 0.962 | 0.967 | 0.073 | 0.634 | 0.369 | 0.763 | 0.736 | 0.746 | 0.069 | |
Note:
p<.0001,
p<.01,
p<.05.
Imaging Parameters.
Microstructural characteristics of white matter were probed using metrics derived from the measurement of water diffusion in the brain using diffusion tensor imaging (DTI) and neurite orientation dispersion and diffusion imaging (NODDI; Zhang et al., 2012). NODDI is a biophysical model proposed to model three types of water diffusion (e.g. restricted vs. free diffusion) within brain tissue, estimates the microstructural complexity with more specificity than DTI (Dean et al., 2016; Zhang et al., 2012) and provides metrics sensitive to underlying neurite density (ND) and orientation dispersion index (ODI).
DTI.
Diffusion tensors were estimated at each voxel using the robust estimation of tensors by an outlier rejection (RESTORE; Chang et al., 2005) algorithm as part of the Diffusion Imaging in Python open source software package (DIPY; (Garyfallidis et al., 2014). Quantitative maps of FA, MD, AD, and RD (Basser & Pierpaoli, 2011) were derived. FA is a normalized standard deviation of the diffusion tensor eigenvalues that describes the degree of anisotropy of the diffusion tensor; FA is a widely used index of white matter microstructure (Alexander et al., 2007). MD corresponds to the average diffusion across all directions and is sensitive to cellularity and the brain’s water content (Alexander et al., 2007). AD is the rate of diffusion along the principal direction of diffusion and is responsive to axonal changes whereas RD describes the rate of diffusion perpendicular to the principal diffusion direction and is sensitive to alterations in myelin structure (Winklewski et al., 2018). FA and AD are inversely related to MD and RD, with higher levels of FA and AD and lower MD and RD typically considered indicative of more mature white matter microstructure (Alexander et al., 2007, 2011).
NODDI.
DW-MRI data were also fit to the three-compartment NODDI tissue model, and we computed measures of ND and ODI (Zhang et al., 2012), which reflect the microstructural complexity of axons and dendrites (‘neurites’) and provide additional information about the brain tissue microstructure beyond DTI parameters.
Following calculation of DTI and NODDI parameter maps, we used DTI-TK (Zhang et al., 2007) to construct a population specific template. Individual FA maps for each infant were nonlinearly registered to the template using diffeomorphic registration algorithms as part of the Advanced Normalization Tools (ANTs) software (Avants et al., 2008, 2011) and resulting transformations were applied to the DTI (FA, MD, AD, RD) and NODDI (ND, ODI) measures to bring these maps in alignment with the template. The study-specific FA template threshold was set at FA>0.15 (determined through visual inspection) to create an overall white matter mask.
Analytic Plan
First, we examined descriptive statistics for fear, sadness, and anger across time and infant sex. Substantive research questions pertain to neural precursors of emotional development across infancy. Thus, rather than examine each score separately or a composite across time that would preclude examining developmental change in emotion, we used estimated Fear, Sadness, and Anger intercept and slope parameters for further analyses. All infants contributed data for at least two time points. We constructed a series of multilevel models using proc mixed in SAS 9.4, which imposed a random intercept and slope, providing individual linear regression lines for fear, sadness, and anger for each infant as well as estimates of overall variation. Each emotion at each time point was grand mean centered so that developmental change was represented as deviations from the mean. The intercept represents the infants’ estimated (hypothetical) level of emotional expression at the initial measurement of 6 months, and the slope represents the average rate of change across the three measured time points.
To examine whether early white matter microstructure foreshadows emerging negativity in infants, we examined voxel-wise associations of 1-month DTI and NODDI parameters with the 6-month levels and developmental changes in fear, sadness, and anger using a joint inference model with Permutation Analysis of Linear Models (PALM) (Winkler et al., 2014). Separate voxel-wise models were performed for each 6-month level and developmental change score, while controlling for 6-month levels and developmental changes of the other 2 emotions. Each model incorporated all 6 DTI and NODDI parameters (FA, MD, AD, RD, ND, ODI) into non-parametric combination analysis (NPC; Winkler, Webster, et al., 2016), creating an overall voxel-wise statistic of the association between white matter microstructure and emotion, while additionally allowing examination of the separate contribution of each individual parameter (Bergsland et al., 2018). Voxel-wise NPC analyses were restricted to the previously described white matter mask and performed with 500 permutations using tail acceleration (Winkler, Ridgway, et al., 2016). For all analyses, infant chronological age (corrected to a 40-week gestation), sex, and head motion were included as covariates. Significance was defined as p < .05, corrected for multiple comparisons by controlling family-wise error (FWE) rate after Threshold-Free Cluster Enhancement (Smith & Nichols, 2009). Of note, all analyses were run with maternal education as a proxy for SES and included sex as an interaction term predicting emotion, but results did not differ by SES or sex of the child and therefore these covariates were removed.
Results
Descriptive Statistics and Individual Variation
Means, standard deviations, and zero-order correlations for infant negative emotion at each age are presented in Table 2, along with scale internal consistency reliability estimates. Within emotions, measures were intercorrelated across ages, with stronger intercorrelations across adjacent ages and higher correlations from 12 to 18 months than from 6 to 12 months. Correlations of fear with anger and sadness were positive but weak and often insignificant. Anger and sadness were more strongly intercorrelated. No significant sex differences emerged for any of the negative emotion scales at any age.
Estimated individual infant fear, sadness, and anger trajectories are depicted in Figure 1, panels 1–3, with means highlighted in each individual panel. Figure 1, panel 4 shows infant emotion mean slopes on the same scaling compared with one another. Intercept and slope estimates indicate that infant fear and anger changed over time, whereas sadness remained relatively stable. These differences encourage the following analyses of earlier brain white matter microstructure.
Figure 1:
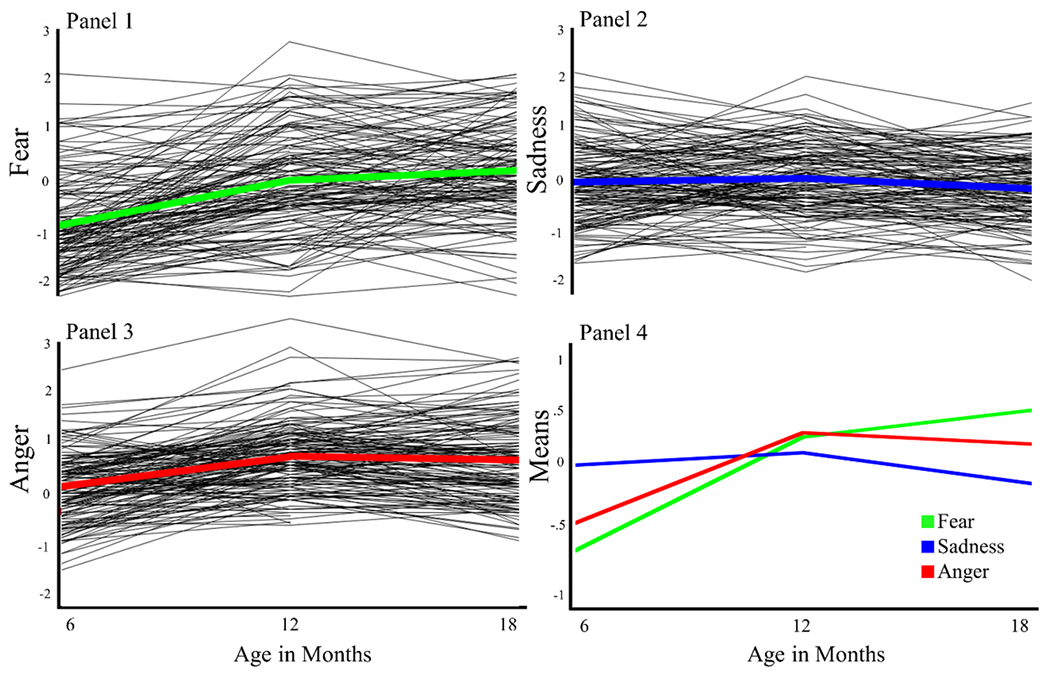
Infant Emotion Trajectories: data are grand mean centered to show deviations from the average in each behavior and all behaviors are on the same scaling. Note: The axis labels for the individual trajectories and the means plots differ.
Infant 1-month White Matter Microstructure Foreshadows Negative Emotion
Six-month levels of sadness and anger were not significantly associated with 1-month white matter microstructure. There were significant regions of interest predicting 6-month level and developmental increases in infant fear, indicated by significant associations with lower 1-month white matter microstructure of the left inferior stria terminalis (p < 0.05, FWE corrected; Figure 2). Specifically, examining the contribution of the individual DTI and NODDI parameters derived from the omnibus NPC indicated that this association was largely driven by 1-month fractional anisotropy (FA, p < .05, FWE corrected). This region was significantly negatively associated with 6-month fear levels (range of t-statistics from cluster: 4.30 – 4.56) and developmental changes in infant fear (range of t-statistics from cluster: 4.37 – 4.65), such that lower FA in the left stria terminalis at 1-month is associated with higher levels and steeper increases in infant fear across the first 18 months of life. Additional associations were observed between infant fear measures (6-month level and developmental change) and white matter regions including the inferior portion of the stria terminalis and regions of the inferior longitudinal fasciculus (ILF), inferior frontal occipital fasciculus (IFOF), uncinate fasciculus (UF), and posterior thalamic radiations. However, these associations failed to maintain significance after multiple comparison correction (Supplementary Figure 1).
Figure 2:
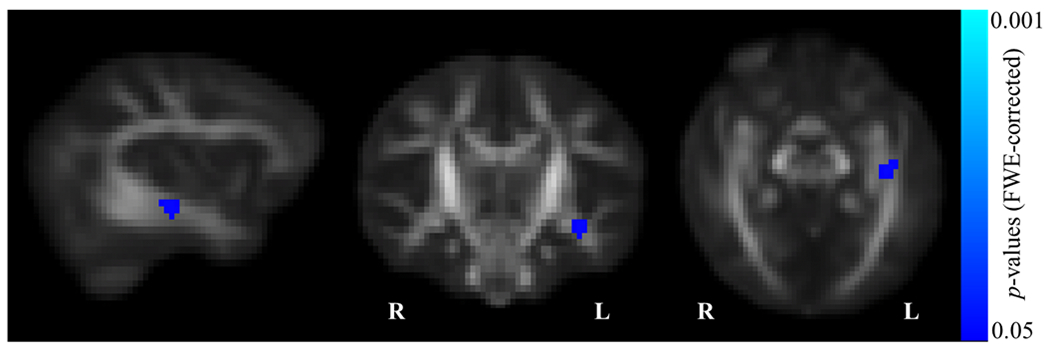
Infant fear significantly associated with white matter microstructure in the stria terminalis (p<.05, corrected).
Discussion
The only neurobehavioral associations elucidated here involved infant fear. Because fear is an adaptive emotional response in the face of novelty and uncertainty, it is also ubiquitous in infancy when encounters with novel stimuli and uncertain contexts are common. Results offer evidence that various white matter tracts involved in emotion show early differentiation, specifically in terms of fear.
The most pervasive finding here was that infants who showed both higher initial levels of fear and an increase in fear at a steeper rate had lower white matter microstructure, indexed by lower fractional anisotropy, in the stria terminalis. The stria terminalis is a white matter track that starts at the bed nucleus and loops around toward the amygdala (Theiss et al., 2017). The stria terminalis follows an early developmental pattern that starts early prenatally and during early brain development, potentially earlier than other white matter tracts (i.e., the uncinate fasciculus). However, it shows a protracted developmental trajectory, peaking later than other regions (Dubois et al., 2014). The stria terminalis is implicated in hypervigilant threat monitoring, including sustained, ambiguous, or distant threat cues (Lebow & Chen, 2016; Somerville et al., 2010). In addition, recent research has suggested that increased resting state connectivity between the bed nucleus of the stria terminalis and the central nucleus of the amygdala is associated with increased physiological reactivity to negative stimuli (Pedersen et al., 2020). Due to its relevance in threat processing, the stria terminalis plays an important role in fearful behaviors and related anxiety disorders (Lebow & Chen, 2016). Extant research in both adult and children have focused on the implications associated with early fear, our work included. Indeed, early fear is a salient risk factor of generalized and social anxiety during childhood and adolescence (Buss, 2011; Copeland et al., 2014; Van Hulle et al., 2017). Yet developmental increases in infant fear, here associated with lower white matter microstructure in the stria terminalis, are also indicative of typical development, during which infants’ learn to navigate their environment in conjunction with their ability to more independently explore their surroundings (Leppänen & Nelson, 2012). Thus, multiple potential interpretations are possible: first, lower white matter microstructure in the stria terminalis may allow us to identify infants predisposed to fearful responses as early as 1 month of age. Or alternatively, infants with lower white matter microstructure in this region may only be beginning to explore their environments successfully, indicated by higher fear (i.e., distress to novelty) and lower microstructure if this white matter pathways has not yet developed with repeated exposures to their environment. How this observation of lower white matter microstructure in the stria terminalis is related to measures of resting state functional connectivity requires additional research. In addition, longitudinal work may be better able to build upon such findings.
Temporoparietal Regions of Emotional Significance
Because of the limited research on the neurobiology of infant emotion and the fact that our sample is relatively small and homogenous with many exclusionary factors, it is possible that regions in addition to the stria terminalis may be of import in the development of infant emotional expression. Here, we briefly note several potential regions that future research may include to better understand the neurobiology of neonatal emotion. Lesion studies suggest that the inferior longitudinal fasciculus (ILF) is important for the integration of visual and emotional information (Fischer et al., 2016), and the inferior frontal occipital fasciculus (IFOF) is believed to play a role in attention and visual processing (Doricchi et al., 2008). In infancy, fear is often thought of as distress or unease when confronted with something novel or unusual. People, and in this case infants as young as 1-month of age, with lower white matter integrity or less developed white matter in the ILF and IFOF may have difficult with contextualizing emotional input (Fischer et al., 2016). Much of the previous work linking white matter microstructure to fear, anger, or mental health problems highlights the uncinate fasciculus’ (UF) import to emotional expression and regulation (Bhatia et al., 2018; Kim et al., 2019; Koch et al., 2017; Phan et al., 2009). The UF follows a fairly protracted developmental course that lasts into adulthood (Olson et al., 2015). Thus, perhaps stronger, robust individual differences in UF white matter microstructure and emotional expression occur later than could be assessed here. In sum, multiple temporoparietal regions could impact infant fear expressions and though we only found relations that withstood multiple comparisons corrections in the stria terminalis, future work should build and expand upon this work to regions implicated in behaviors such as attentional control, memory, threat monitoring, and later anxiety disorders.
Clinical Implications of Negative Emotions
Repeated instances of extreme fear or anger, associated with decreased regulatory abilities, may foretell later internalizing and externalizing disorders, respectively (Kerr & Schneider, 2008; Van Hulle et al., 2017). Consequently, the literature has systemically focused on negativity throughout the lifespan (De Pauw & Mervielde, 2010; Nigg, 2006). We acknowledge that we, too, focused solely on negative emotions and neglect positive emotions and regulatory behaviors, other broad components often conceptualized alongside negativity in the infant emotion literature (Derryberry & Rothbart, 1997; Goldsmith et al., 1987). Indeed, frequent expression of negative affect may inhibit normal exploratory behaviors, hindering an infant’s normative development of emotion regulation strategies (Coffey, 2019; Thomas et al., 2017). Future work should incorporate early developing positive emotions and regulatory behaviors more broadly to better understand the early microstructure underlying emotional development and disorder. Nonetheless, a better understanding of the neural biomarkers underlying discrete negative emotions will allow for the improved identification of individuals at risk for negative emotional behaviors, dysregulation, and later psychopathology, thereby aiding in the development of targeted interventions early in life.
Empirical Evidence to Support Theories of Emotion
We use temporal precedence to infer that early neural architecture predisposes infants to exhibit or express negative emotions, in essence suggesting a biological basis for emerging emotional expression. However, it is also possible that experiencing the emotion itself refines the brain regions of interest. In other words, infants who experience fear earlier than the 1-month time point examined here may have had fearful experiences that already altered their neural architecture. Analyses used the whole brain and did not pre-select specific tracts, allowing us to explore where white matter may be related to emotion in the whole brain agnostically, though we did have hypotheses relating to specific regions.
We offer initial evidence that fear is biologically motivated, but we did not find the same patterns for sadness or anger. The white matter is still rapidly developing and sensitive to the child’s experiences during this time – so perhaps, as children develop these other emotions more – we would expect to observe stronger associations with sadness and anger in white matter. Constructivist theories of emotion propose that emotions result from multiple underlying psychological experiences which can include social and cultural events (Feldman Barrett, 2011). Expressions of sadness and anger convey the infant’s needs and desires to a caregiver, with both being typical responses to goal blockage that serve as tools for effective communication with caregivers (Lewis et al., 2006).
Meta-analyses on the functional neuroanatomy of emotion suggest that prominent areas of emotional activation during anger are rooted in the prefrontal cortex (Murphy et al., 2003; Phan et al., 2009), which is only beginning to develop in infancy. In addition, because sadness may develop as a tool to communicate needs to caregivers (Buss & Kiel, 2004), individual differences in sadness, along with associated neural correlates, likely develop later in infancy and were not observed here. Thus, alternative causal interpretations are plausible.
Limitations and Conclusions
Neurodevelopmental trajectories of infant emotion may be altered in high-risk or stressful family environments, which were not considered here. Previous work from our lab and others indicates that developmental trajectories of fear and anger expression increase in the presence of higher levels of parent and family stressors and lower parenting quality (Braungart-Rieker et al., 2010; Brooker et al., 2013). Our sample was not high risk, perhaps not experiencing extreme levels of stress and parenting; thus, we probably did not capture the most extreme forms of negativity in infancy. Further, this work uses mother reports of infants’ emotional expressions but does not include direct observations of infants’ responses to specific stimuli. Mother reports may be influenced by her own characteristics (Gagne et al., 2015; Stifter et al., 2008), which would presumably attenuate emotion-DTI associations. Nevertheless, the early architecture of emotional neurobiology likely exists in the absence of emotion eliciting stimuli (Davidson & Irwin, 1999) and should be important for the emergence of later emotional expression. In addition, we found that FA was sensitive to variation in fear expression as hypothesized, but other DTI and NODDI metrics were not. Non-significant associations (particularly with ND and RD) suggest that perhaps its not the neurite microstructure that is associated with emotional expression, but rather maybe alternative neurobiological change that is not captured using standard DTI metrics. Studies using more myelin specific techniques (e.g. relaxometry, myelin water fraction imaging, magnetization transfer) may be better able to explore this in the future. Nonetheless, the field of infant behavioral neuroscience is relatively new; thus, our findings linking 1-month FA to 6-, 12-, and 18-month behavioral outcomes, particularly those that were marginal, provides a basis of knowledge that requires replication before we have a thorough understanding of these brain-behavior relations. Our findings may prove useful for future work that can address such questions in samples with greater statistical power, or test generalization with different measurement modalities and different infant ages.
In infancy, negative emotion is not necessarily maladaptive, but can motivate needed caregiver responses. Infant emotional expression is an effective way to communicate with a caregiver when more sophisticated means of communication (e.g., verbalizing) are not yet available. For example, an infant who fusses or cries when she does not have access to a favored toy is essentially asking the caregiver to bring the toy to her. Therefore, we do not assume that alterations in white matter microstructure relating to infant emotion indicate development of later mental health or behavior problems. In addition, it remains to be seen whether alterations in function may be due to the underlying microstructure or a bidirectional feedback loop amongst microstructure and function, which we were not able to examine. Yet, we offer novel evidence that neural substrates of fear exist early in life, and are potentially indicative of the earliest developmental trajectories of emotion.
Supplementary Material
Supplementary Figure 1. Infant fear significantly associated with white matter microstructure in the stria terminalis and sagittal stratum, including the ILF, IFOF, and posterior thalamic radiations (p<.005, uncorrected or p<.10, corrected.
Research Highlights.
Expressions of infant fear and anger, but not sadness, increase from 6 to 18 months of age.
Early neural architecture in the stria terminalis is related to higher initial levels and increasing fear in infancy.
After accounting for fear, anger and sadness do not appear to be associated with differences in early white matter microstructure.
This work identifies early neural precursors of fearfulness as early as 1-month of age.
Acknowledgements:
We thank the families who participated in this research. This work was supported by the National Institutes of Mental Health (P50 MH100031 to HHG, ALA, RJD and R01 MH101504 to HHG). EMP and KD are supported by T32 MH018931, EMP is also supported by K01 MH113710; DCD is supported by T32 HD007489 and R00 MH11059. Infrastructure support was also provided by a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30 HD03352 and U54 HD090256). Data are available upon request from the last author. The authors have no conflicts of interest to report. Data collection was approved by the University of Wisconsin—Madison’s Institutional Review Board.
References
- Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, Tromp DPM, Zakszewski E, & Field AS (2011). Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connectivity, 1(6), 423–446. 10.1089/brain.2011.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, & Field AS (2007). Diffusion tensor imaging of the brain. Neurotherapeutics, 4(3), 316–329. 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia-Klein N, Gan G, Gilam G, Bezek J, Bruno A, Denson TF, Hendler T, Lowe L, Mariotti V, & Muscatello MR (2020). The feeling of anger: From brain networks to linguistic expressions. Neuroscience & Biobehavioral Reviews, 108, 480–497. [DOI] [PubMed] [Google Scholar]
- Basser PJ, & Pierpaoli C (2011). Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. 1996. Journal of Magnetic Resonance (San Diego, Calif.: 1997), 213(2), 560–570. 10.1016/j.jmr.2011.09.022 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, & Hinshaw SP (Eds.). (2008). Child and adolescent psychopathology. John Wiley & Sons. [Google Scholar]
- Belsky J, Hsieh K-H, & Crnic K (1998). Mothering, fathering, and infant negativity as antecedents of boys’ externalizing problems and inhibition at age 3 years: Differential susceptibility to rearing experience? Development and Psychopathology, 10(2), 301–319. [DOI] [PubMed] [Google Scholar]
- Bergsland N, Schweser F, Dwyer MG, Weinstock-Guttman B, Benedict RHB, & Zivadinov R (2018). Thalamic white matter in multiple sclerosis: A combined diffusion-tensor imaging and quantitative susceptibility mapping study. Human Brain Mapping, 39(10), 4007–4017. 10.1002/hbm.24227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia KD, Henderson LA, Hsu E, & Yim M (2018). Reduced integrity of the uncinate fasciculus and cingulum in depression: A stem-by-stem analysis. Journal of Affective Disorders, 235, 220–228. [DOI] [PubMed] [Google Scholar]
- Braungart-Rieker JM, Hill-Soderlund AL, & Karrass J (2010). Fear and anger reactivity trajectories from 4 to 16 months: The roles of temperament, regulation, and maternal sensitivity. Developmental Psychology, 46(4), 791. [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA, Lemery-Chalfant K, Aksan N, Davidson RJ, & Goldsmith HH (2013). The development of stranger fear in infancy and toddlerhood: Normative development, individual differences, antecedents, and outcomes. Developmental Science, n/a-n/a. 10.1111/desc.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA, Lemery-Chalfant K, Aksan N, Davidson RJ, & Goldsmith HH (2014). Profiles of observed infant anger predict preschool behavior problems: Moderation by life stress. Developmental Psychology, 50(10), 2343–2352. 10.1037/a0037693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA (2011). Which fearful toddlers should we worry about? Context, fear regulation, and anxiety risk. Developmental Psychology, 47(3), 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, & Kiel EJ (2004). Comparison of Sadness, Anger, and Fear Facial Expressions When Toddlers Look at Their Mothers. Child Development, 75(6), 1761–1773. 10.1111/j.1467-8624.2004.00815.x [DOI] [PubMed] [Google Scholar]
- Chang L-C, Jones DK, & Pierpaoli C (2005). RESTORE: Robust estimation of tensors by outlier rejection. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 53(5), 1088–1095. [DOI] [PubMed] [Google Scholar]
- Coffey JK (2019). Cascades of infant happiness: Infant positive affect predicts childhood IQ and adult educational attainment. Emotion. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Angold A, Shanahan L, & Costello EJ (2014). Longitudinal patterns of anxiety from childhood to adulthood: The Great Smoky Mountains Study. Journal of the American Academy of Child & Adolescent Psychiatry, 53(1), 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C (1872). The expression of the emotions in man and animals. John Murray. [Google Scholar]
- Davidson RJ, & Irwin W (1999). The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences, 3(1), 11–21. [DOI] [PubMed] [Google Scholar]
- De Pauw SSW, & Mervielde I (2010). Temperament, Personality and Developmental Psychopathology: A Review Based on the Conceptual Dimensions Underlying Childhood Traits. Child Psychiatry and Human Development, 41, 313–329. 10.1007/s10578-009-0171-8 [DOI] [PubMed] [Google Scholar]
- Dean DC, Dirks H, O’Muircheartaigh J, Walker L, Jerskey BA, Lehman K, Han M, Waskiewicz N, & Deoni SC (2014). Pediatric neuroimaging using magnetic resonance imaging during non-sedated sleep. Pediatric Radiology, 44(1), 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, O’muircheartaigh J, Dirks H, Travers BG, Adluru N, Alexander AL, & Deoni SC (2016). Mapping an index of the myelin g-ratio in infants using magnetic resonance imaging. NeuroImage, 132, 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, Planalp EM, Wooten W, Adluru N, Kecskemeti SR, Frye C, Schmidt CK, Schmidt NL, Styner MA, & Goldsmith HH (2017). Mapping white matter microstructure in the one month human brain. Scientific Reports, 7(1), 9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, Planalp EM, Wooten W, Schmidt CK, Kecskemeti SR, Frye C, Schmidt NL, Goldsmith HH, Alexander AL, & Davidson RJ (2018). Investigation of brain structure in the 1-month infant. Brain Structure and Function, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham SA (2006). The emotional basis of learning and development in early childhood education. [Google Scholar]
- Denham SA, Lehman EB, Moser MH, & Reeves SL (1995). Continuity and change in emotional components of infant temperament. Child Study Journal. [Google Scholar]
- Derryberry D, & Rothbart MK (1997). Reactive and effortful processes in the organization of temperament. Development and Psychopathology, 9(4), 633–652. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Thiebaut de Schotten M, Tomaiuolo F, & Bartolomeo P (2008). White matter (dis)connections and gray matter (dys)functions in visual neglect: Gaining insights into the brain networks of spatial awareness. Cortex, 44(8), 983–995. 10.1016/j.cortex.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Dowe KN, Planalp EM, Dean III DC, Alexander AL, Davidson RJ, & Goldsmith HH (2020). Early microstructure of white matter associated with infant attention. Developmental Cognitive Neuroscience, 45, 100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, & Hertz-Pannier L (2014). The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience, 276, 48–71. [DOI] [PubMed] [Google Scholar]
- Eaton-Rosen Z, Melbourne A, Orasanu E, Cardoso MJ, Modat M, Bainbridge A, Kendall GS, Robertson NJ, Marlow N, & Ourselin S (2015). Longitudinal measurement of the developing grey matter in preterm subjects using multi-modal MRI. NeuroImage, 111, 580–589. [DOI] [PubMed] [Google Scholar]
- Ekman P (1999). Basic emotions. In Dalgleish T & Power M (Eds.), Handbook of cognition and emotion (Vol. 98, p. 16). John Wiley & Sons, Ltd. [Google Scholar]
- Ekman P, & Cordaro D (2011). What is meant by calling emotions basic. Emotion Review, 3(4), 364–370. [Google Scholar]
- Feldman Barrett L (2011). Constructing emotion. Psihologijske Teme, 20(3), 359–380. [Google Scholar]
- Fischer DB, Perez DL, Prasad S, Rigolo L, O’Donnell L, Acar D, Meadows M-E, Baslet G, Boes AD, Golby AJ, & Dworetzky BA (2016). Right inferior longitudinal fasciculus lesions disrupt visual-emotional integration. Social Cognitive and Affective Neuroscience, 11(6), 945–951. 10.1093/scan/nsw011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijda NH (1986). The emotions. Cambridge University Press. [Google Scholar]
- Gagne JR, & Hill Goldsmith H (2011). A longitudinal analysis of anger and inhibitory control in twins from 12 to 36 months of age. Developmental Science, 14(1), 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JR, Prater JC, Abramson L, Mankuta D, & Knafo-Noam A (2015). An Israeli study of family expectations of future child temperament. Family Science, 6(1), 356–361. [Google Scholar]
- Gartstein MA, & Rothbart MK (2003). Studying infant temperament via the revised infant behavior questionnaire. Infant Behavior and Development, 26(1), 64–86. [Google Scholar]
- Garyfallidis E, Brett M, Amirbekian B, Rokem A, Van Der Walt S, Descoteaux M, & Nimmo-Smith I (2014). Dipy, a library for the analysis of diffusion MRI data. Frontiers in Neuroinformatics, 8. 10.3389/fninf.2014.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, & Rapoport JL (2010). Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron, 67(5), 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH (1993). Temperament: Variability in developing emotion systems. In Lewis M & Haviland J (Eds.), Handbook of Emotions. Guilford Press; 353–364. [Google Scholar]
- Goldsmith HH, Buss AH, Plomin R, Rothbart MK, Thomas A, Chess S, Hinde RA, & McCall RB (1987). Roundtable: What is temperament? Four approaches. Child Development, 53, 505–529. [PubMed] [Google Scholar]
- Graham AM, Buss C, Rasmussen JM, Rudolph MD, Demeter DV, Gilmore JH, Styner M, Entringer S, Wadhwa PD, & Fair DA (2016). Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Developmental Cognitive Neuroscience, 18, 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Pine DS, & Fox NA (2015). Behavioral inhibition and developmental risk: A dual-processing perspective. Neuropsychopharmacology, 40(1), 207–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbet G, Zemmoura I, & Duffau H (2018). Functional anatomy of the inferior longitudinal fasciculus: From historical reports to current hypotheses. Frontiers in Neuroanatomy, 12, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BR, Styner MA, Gao W, Yap P-T, Wang L, Baluyot K, Yacoub E, Chen G, Potts T, & Salzwedel A (2019). The UNC/UMN Baby Connectome Project (BCP): An overview of the study design and protocol development. NeuroImage, 185, 891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard CE (2011). Forms and Functions of Emotions: Matters of Emotion–Cognition Interactions. Emotion Review, 3(4), 371–378. 10.1177/1754073911410737 [DOI] [Google Scholar]
- Izard CE, & Malatesta CZ (1987). Perspectives on emotional development I: Differential emotions theory of early emotional development. John Wiley & Sons. [Google Scholar]
- Jenkinson M, Bannister PR, Brady M, & Smith S (2002). Improved Optimization for the Robust and Accurate Linear Registration and Motion Correction of Brain Images. NeuroImage, 17, 825–841. 10.1006/nimg.2002.1132 [DOI] [PubMed] [Google Scholar]
- Kelly CE, Thompson DK, Chen J, Leemans A, Adamson CL, Inder TE, Cheong JL, Doyle LW, & Anderson PJ (2016). Axon density and axon orientation dispersion in children born preterm. Human Brain Mapping, 37(9), 3080–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MA, & Schneider BH (2008). Anger expression in children and adolescents: A review of the empirical literature. Clinical Psychology Review, 28(4), 559–577. [DOI] [PubMed] [Google Scholar]
- Kim D, Park H-K, Kim N-S, Hwang S-J, & Lee HJ (2016). Neonatal diffusion tensor brain imaging predicts later motor outcome in preterm neonates with white matter abnormalities. Italian Journal of Pediatrics, 42(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Elliott ML, d’Arbeloff TC, Knodt AR, Radtke SR, Brigidi BD, & Hariri AR (2019). Microstructural integrity of white matter moderates an association between childhood adversity and adult trait anger. Aggressive Behavior, 45(3), 310–318. 10.1002/ab.21820 [DOI] [PubMed] [Google Scholar]
- Kline-Fath BM, Horn PS, Yuan W, Merhar S, Venkatesan C, Thomas CW, & Schapiro MB (2018). Conventional MRI scan and DTI imaging show more severe brain injury in neonates with hypoxic-ischemic encephalopathy and seizures. Early Human Development, 122, 8–14. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, & Gilmore JH (2008). A structural MRI study of human brain development from birth to 2 years. The Journal of Neuroscience, 28(47), 12176–12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SB, Van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, & Olff M (2017). Decreased uncinate fasciculus tract integrity in male and female patients with PTSD: A diffusion tensor imaging study. Journal of Psychiatry & Neuroscience: JPN, 42(5), 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C-H, & Wu Y-T (2014). Alterations in white matter micro-integrity of the superior longitudinal fasciculus and anterior thalamic radiation of young adult patients with depression. Psychological Medicine, 44(13), 2825–2832. 10.1017/S0033291714000440 [DOI] [PubMed] [Google Scholar]
- Lai C-H, & Wu Y-T (2016). The alterations in regional homogeneity of parieto-cingulate and temporo-cerebellum regions of first-episode medication-naive depression patients. Brain Imaging and Behavior, 10(1), 187–194. [DOI] [PubMed] [Google Scholar]
- Lane R, Reiman E, Ahern G, Schwartz G, & Davidson R (1997). Neuroanatomical correlates of happiness, sadness, and disgust. The American Journal of Psychiatry. 10.1176/AJP.154.7.926 [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, & Beaulieu C (2008). Microstructural maturation of the human brain from childhood to adulthood. NeuroImage, 40(3), 1044–1055. 10.1016/j.neuroimage.2007.12.053 [DOI] [PubMed] [Google Scholar]
- Lebow MA, & Chen A (2016). Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21(4), 450–463. 10.1038/mp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Arfanakis K, Evia AM, Fanning J, Keedy S, & Coccaro EF (2016). White matter integrity reductions in intermittent explosive disorder. Neuropsychopharmacology, 41(11), 2697–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Steiner RJ, Luo S, Neale MC, Styner M, Zhu H, & Gilmore JH (2015). Quantitative tract-based white matter heritability in twin neonates. Neuroimage, 111, 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A, & Jones DK (2009). The B-matrix must be rotated when correcting for subject motion in DTI data. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 61(6), 1336–1349. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, & Giedd JN (2006). Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews, 30(6), 718–729. [DOI] [PubMed] [Google Scholar]
- Leppänen JM, & Nelson CA (2012). Early development of fear processing. Current Directions in Psychological Science, 21(3), 200–204. [Google Scholar]
- Levenson RW (2011). Basic Emotion Questions. Emotion Review, 3(4), 379–386. 10.1177/1754073911410743 [DOI] [Google Scholar]
- Lewis M (2012). Children’s emotions and moods: Developmental theory and measurement. Springer Science & Business Media. [Google Scholar]
- Lewis M, Ramsay DS, & Sullivan MW (2006). The relation of ANS and HPA activation to infant anger and sadness response to goal blockage. Developmental Psychobiology, 48(5), 397–405. 10.1002/dev.20151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Moore GA, Beekman C, Pérez-Edgar KE, Leve LD, Shaw DS, Ganiban JM, Natsuaki MN, Reiss D, & Neiderhiser JM (2018). Developmental patterns of anger from infancy to middle childhood predict problem behaviors at age 8. Developmental Psychology, 54(11), 2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Phillips JJ, Ji F, Shi D, & Bell MA (2021). Temperamental Shyness and Anger/Frustration in Childhood: Normative Development, Individual Differences, and the Impacts of Maternal Intrusiveness and Frontal Electroencephalogram Asymmetry. Child Development, 92(6), 2529–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfese VJ, Rudasill KM, Beswick JL, Jacobi-Vessels JL, Ferguson MC, & White JM (2010). Infant Temperament, Maternal Personality, and Parenting Stress as Contributors to Infant Developmental Outcomes. Merrill-Palmer Quarterly, 56(1), 49–79. JSTOR. [Google Scholar]
- Murphy FC, Nimmo-Smith IAN, & Lawrence AD (2003). Functional neuroanatomy of emotions: A meta-analysis. Cognitive, Affective, & Behavioral Neuroscience, 3(3), 207–233. [DOI] [PubMed] [Google Scholar]
- Nigg JT (2006). Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry, 47(3/4), 395–422. 10.1111/j.1469-7610.2006.01612.x [DOI] [PubMed] [Google Scholar]
- Olson IR, Von Der Heide RJ, Alm KH, & Vyas G (2015). Development of the uncinate fasciculus: Implications for theory and developmental disorders. Developmental Cognitive Neuroscience, 14, 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’muircheartaigh J, Dean DC, Ginestet CE, Walker L, Waskiewicz N, Lehman K, Dirks H, Piryatinsky I, & Deoni SC (2014). White matter development and early cognition in babies and toddlers. Human Brain Mapping, 35(9), 4475–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, & Watt D (2011). What is Basic about Basic Emotions? Lasting Lessons from Affective Neuroscience. Emotion Review, 3(4), 387–396. 10.1177/1754073911410741 [DOI] [Google Scholar]
- Pedersen WS, Schaefer SM, Gresham LK, Lee SD, Kelly MP, Mumford JA, Oler JA, & Davidson RJ (2020). Higher resting-state BNST-CeA connectivity is associated with greater corrugator supercilii reactivity to negatively valenced images. NeuroImage, 207, 116428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, & Arfanakis K (2009). Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biological Psychiatry, 66(7), 691–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, & Di Chiro G (1996). Diffusion tensor MR imaging of the human brain. Radiology, 201(3), 637–648. [DOI] [PubMed] [Google Scholar]
- Pine DS (2003). Developmental psychobiology and response to threats: Relevance to trauma in children and adolescents. Biological Psychiatry, 53(9), 796–808. [DOI] [PubMed] [Google Scholar]
- Pisner DA, Shumake J, Beevers CG, & Schnyer DM (2019). The superior longitudinal fasciculus and its functional triple-network mechanisms in brooding. NeuroImage: Clinical, 24, 101935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planalp EM, & Braungart-Rieker JM (2013). Temperamental precursors of infant attachment with mothers and fathers. Infant Behavior and Development, 36(4), 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutchik R (1980). A general psychoevolutionary theory of emotion. Theories of Emotion, 1(3–31), 4. [Google Scholar]
- Raschle N, Zuk J, Ortiz-Mantilla S, Sliva DD, Franceschi A, Grant PE, Benasich AA, & Gaab N (2012). Pediatric neuroimaging in early childhood and infancy: Challenges and practical guidelines. Annals of the New York Academy of Sciences, 1252(1), 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A, Meaney MJ, Chen H, Bai J, Hameed WB, Tint MT, Broekman BFP, Chong Y-S, Gluckman PD, Fortier MV, & Qiu A (2015). Antenatal Maternal Anxiety Predicts Variations in Neural Structures Implicated in Anxiety Disorders in Newborns. Journal of the American Academy of Child & Adolescent Psychiatry, 54(4), 313–321.e2. 10.1016/j.jaac.2015.01.013 [DOI] [PubMed] [Google Scholar]
- Rifkin-Graboi A, Tan HM, Shaun GKY, Sim LW, Sanmugam S, Chong YS, Tan KH, Shek L, Gluckman PD, & Chen H (2019). An initial investigation of neonatal neuroanatomy, caregiving, and levels of disorganized behavior. Proceedings of the National Academy of Sciences, 116(34), 16787–16792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, & Derryberry D (2002). Temperament in children. Psychology at the Turn of the Millennium, 2, 17–35. [Google Scholar]
- Saarni C, Campos JJ, Camras LA, & Witherington D (2006). Emotional development: Action, communication, and understanding.
- Sanson A, Hemphill SA, & Smart D (2004). Connections between Temperament and Social Development: A Review. Social Development, 13(1), 142–170. 10.1046/j.1467-9507.2004.00261.x [DOI] [Google Scholar]
- Scarr S, & Salapatek P (1970). Patterns of fear development during infancy. Merrill-Palmer Quarterly of Behavior and Development, 16(1), 53–90. [Google Scholar]
- Smith SM, & Nichols TE (2009). Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44(1), 83–98. 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, & Kelley WM (2010). Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry, 68(5), 416–424. 10.1016/j.biopsych.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifter CA, Willoughby MT, & Towe-Goodman N (2008). Agree or agree to disagree? Assessing the convergence between parents and observers on infant temperament. Infant and Child Development, 17(4), 407–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles J, & Jernigan TL (2010). The Basics of Brain Development. Neuropsychology Review, 20(4), 327–348. 10.1007/s11065-010-9148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stright AD, Gallagher KC, & Kelley K (2008). Infant Temperament Moderates Relations Between Maternal Parenting in Early Childhood and Children’s Adjustment in First Grade. Child Development, 79(1), 186–200. 10.1111/j.1467-8624.2007.01119.x [DOI] [PubMed] [Google Scholar]
- Tamm L, Patel M, Peugh J, Kline-Fath BM, Parikh NA, & Study CINEP (2020). Early brain abnormalities in infants born very preterm predict under-reactive temperament. Early Human Development, 144, 104985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Jacobson SW, van der Kouwe A, Molteno CD, Chen G, Wintermark P, Alhamud A, Jacobson JL, & Meintjes EM (2015). A DTI-based tractography study of effects on brain structure associated with prenatal alcohol exposure in newborns. Human Brain Mapping, 36(1), 170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss JD, Ridgewell C, McHugo M, Heckers S, & Blackford JU (2017). Manual segmentation of the human bed nucleus of the stria terminalis using 3T MRI. NeuroImage, 146, 288–292. 10.1016/j.neuroimage.2016.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E, Buss C, Rasmussen JM, Entringer S, Ramirez JS, Marr M, Rudolph MD, Gilmore JH, Styner M, & Wadhwa PD (2019). Newborn amygdala connectivity and early emerging fear. Developmental Cognitive Neuroscience, 37, 100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JC, Letourneau N, Campbell TS, Tomfohr-Madsen L, & Giesbrecht GF (2017). Developmental origins of infant emotion regulation: Mediation by temperamental negativity and moderation by maternal sensitivity. Developmental Psychology, 53(4), 611–628. 10.1037/dev0000279 [DOI] [PubMed] [Google Scholar]
- Torres ER, Tumey TA, Dean III DC, Kassahun-Yimer W, Lopez-Lambert ED, & Hitchcock ME (2020). Non-pharmacological strategies to obtain usable magnetic resonance images in non-sedated infants: Systematic review and meta-analysis. International Journal of Nursing Studies, 106, 103551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortora D, Martinetti C, Severino M, Uccella S, Malova M, Parodi A, Brera F, Morana G, Ramenghi LA, & Rossi A (2018). The effects of mild germinal matrix-intraventricular haemorrhage on the developmental white matter microstructure of preterm neonates: A DTI study. European Radiology, 28(3), 1157–1166. [DOI] [PubMed] [Google Scholar]
- Tracy JL, & Randles D (2011). Four models of basic emotions: A review of Ekman and Cordaro, Izard, Levenson, and Panksepp and Watt. Emotion Review, 3(4), 397–405. [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, & Nishijo H (2012). Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PloS One, 7(10), e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hulle C, Moore MN, Lemery-Chalfant K, Goldsmith HH, & Brooker RJ (2017). Infant stranger fear trajectories predict anxious behaviors and diurnal cortisol rhythm during childhood. Development and Psychopathology, 29(03), 1119–1130. 10.1017/S0954579417000311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, & Olson IR (2013). Dissecting the uncinate fasciculus: Disorders, controversies and a hypothesis. Brain, 136(6), 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Douaud G, Nichols TE, & Smith SM (2016). Faster permutation inference in brain imaging. Neuroimage, 141, 502–516. 10.1016/j.neuroimage.2016.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, & Nichols TE (2014). Permutation inference for the general linear model. Neuroimage, 92, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Webster MA, Brooks JC, Tracey I, Smith SM, & Nichols TE (2016). Non-parametric combination and related permutation tests for neuroimaging. Human Brain Mapping, 37(4), 1486–1511. 10.1002/hbm.23115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, & Szarmach A (2018). Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes—What Do We Know? Frontiers in Neurology, 9. 10.3389/fneur.2018.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, & Fischl B (2014). Spurious group differences due to head motion in a diffusion MRI study. Neuroimage, 88, 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, & Alexander DC (2012). NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage, 61(4), 1000–1016. 10.1016/j.neuroimage.2012.03.072 [DOI] [PubMed] [Google Scholar]
- Zhang H, Yushkevich PA, Rueckert D, & Gee JC (2007). Unbiased white matter atlas construction using diffusion tensor images. International Conference on Medical Image Computing and Computer-Assisted Intervention, 211–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Infant fear significantly associated with white matter microstructure in the stria terminalis and sagittal stratum, including the ILF, IFOF, and posterior thalamic radiations (p<.005, uncorrected or p<.10, corrected.


