Abstract
Purpose of review
Given the world-wide problem of obesity, this review considers what types of dietary changes can be utilized to minimize the adverse effects of an obesogenic diet on the intestinal microbiota.
Recent findings
In rodents fed high-fat diets containing lard or Western blend fats to induce obesity, switching to high-fat diets formulated to contain higher amounts of fiber or fiber-containing foods, plant extracts, omega-3 fatty acids or whole grains has beneficial effects on body weight, metabolic alterations, and the intestinal microbiota. Several studies show that the intestinal microbiota has a role in mediating the beneficial health effects of these dietary factors. Many aspects of the microbiota observed in animals when healthful dietary components were added to the feed have also been observed in humans who follow healthful dietary patterns.
Summary
The data shows that specific foods and macronutrients can normalize the obesity-associated microbiota and improve metabolic health. These findings support the design of dietary interventions that would allow individuals to focus on diet quality independently of weight loss to mitigate the adverse sequelae of obesity.
Keywords: Colon, high-fat diet, nutrition, microbiota, obesity
Introduction to the Problem of Dysbiosis Induced by Obesity and High Fat Diets
The Western Diet and Obesity
The transition to a Western style of diet is now affecting a large part of the world as developing nations become more affluent and take on Western lifestyles [1–3]. The Western diet is characterized by higher oil, meat and sugar intakes, and by lower fiber and micronutrient intakes versus that found in most traditional diets, for example the diets that were traditionally consumed in Asia and Greece [4]. Adoption of the Western diet is facilitated by many factors including food availability and shifting lifestyles. Prepared foods, restaurant foods and processed ingredients, are now readily available at affordable costs. Shifting work patterns involving more time spent away from the home also leave little time for preparing traditional meals. Unfortunately, this Western diet and lifestyle is conducive to an energy intakes that exceed energy expenditures, resulting in inappropriate weight gain and obesity.
The global increases in obesity are worrisome. It is now well recognized that Western diets are associated with an increased prevalence of obesity and the obesity-associated chronic diseases that result, namely diabetes, cardiovascular diseases and many cancers [5]. In the U.S., Healthy Eating Index scores in data from the National Health and Nutrition Survey vary from an average of 65 to 51 (of 100 possible points) in groups defined by infrequent versus frequent consumption of fast foods from restaurants, respectively [6]. The U.S. Dietary Guidelines Committee reported that in the U.S., dietary intakes of fruits, vegetables, and whole grains need to be increased, and that intakes of added sugars, refined grains, sodium, and saturated fat need to be decreased [7]. In U.S. older adults, poor diet quality affected more than 60% of a national sample [8]. In more recent years, there is evidence that dietary intakes in Europe and U.S. have improved slightly but still require many changes to meet recommendations [9–12]. Unfortunately, obesity is still a major problem and the most recent data show that only 17.7% of the U.S. population is in the normal weight range [13, 14]. In developing nations, obesity rates continue to rise along with chronic disease risks [15].
Much research has focused on defining the relationships between dietary composition and obesity. In animals, high fat diets are widely utilized to model diet-induced obesity in humans. This typically includes using diets high in saturated fats such as lard or Western blend fats at 45–60% of the dietary energy content. A fat intake of about 45% of energy from calories was reached in the U.S. in the 1980’s [17, 18]. This is much higher than the Acceptable Macronutrient Distribution Range (AMDR) for total dietary fat that is 25–35% of calorie intake with no more than 10% of calorie intake from saturated fat [16]. Animal models of obesity using high saturated fat diets have been widely used in defining the role of the microbiota in diet-induced obesity. This role of the microbiota is now understood to be multi-faceted with roles both in the etiology of the obesity and the adverse effects of obesity.
Obesity-Associated Microbiota
The intestinal microbiota changes that result from “Western”, high saturated fat diets has been reviewed extensively and models a fat intake of at least 45% of energy from calories that was reached in the U.S. in the 1980’s [17, 18]. The high-fat diet induced “dysbiosis” in the intestinal microbiota is broadly recognized to include a higher community abundance ratio of Firmicutes/Bacteroidetes ratio that is driven by a number of changes in individual bacterial species, as well as changes in other bacteria that are less consistent across studies and may vary by model used [17]. Many of these bacteria have a role in regulating the integrity of the intestinal barrier which in turn governs systemic exposures to pro-inflammatory stimuli from the intestinal lumen (Figure 1). This includes an increased abundance of Gram-negative, lipopolysaccharide (LPS)-generating proinflammatory Proteobacteria and a reduction in a reduction in protective Bifidobacterium species [17].
Figure 1.
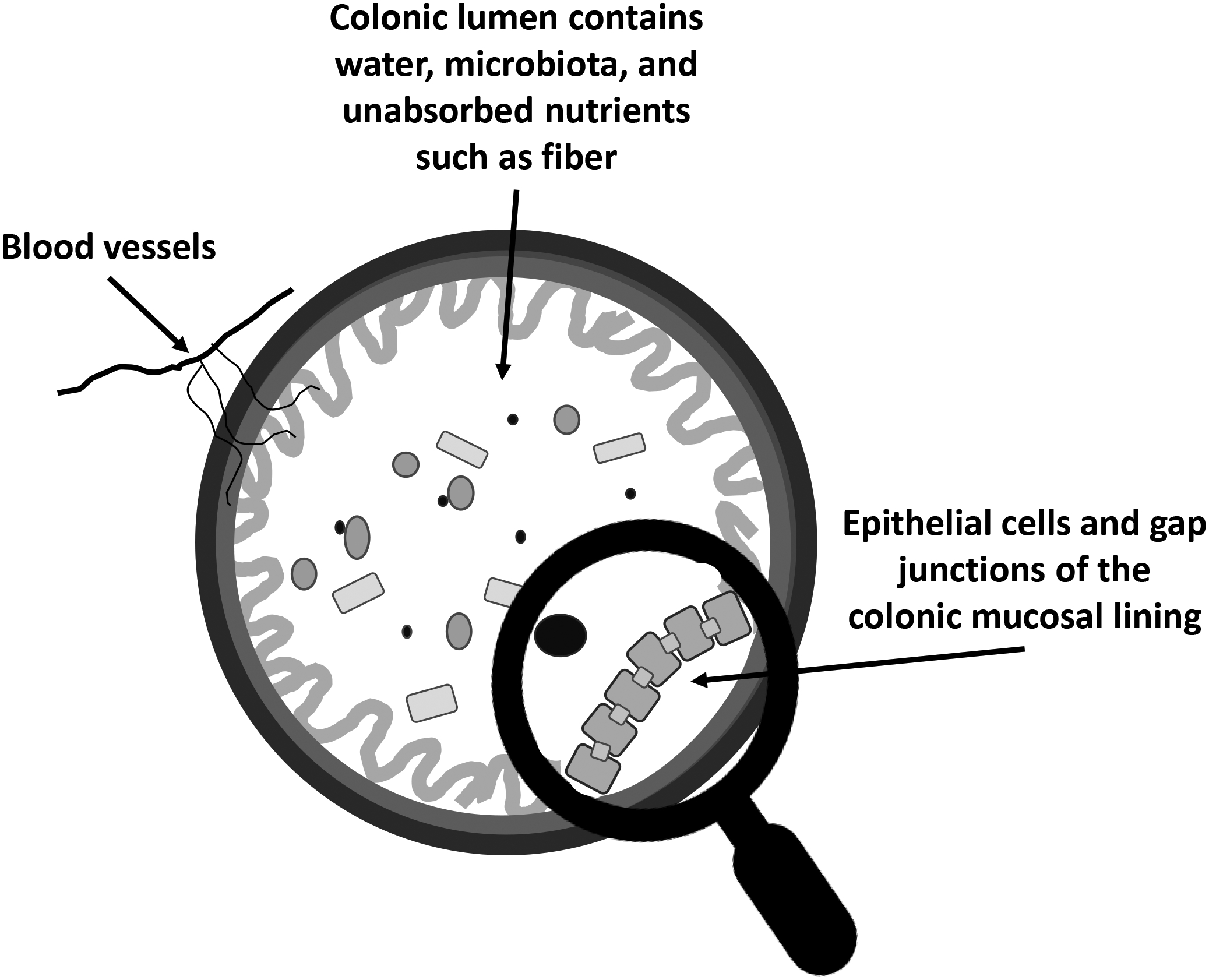
Cross-cut of the colon in a simplistic view. Most nutrients are absorbed in the small intestine, but some nutrients, such as fiber, reach the colon. The microbiota participates in digestion of nutrients, and in the case of fiber, short chain fatty acids that have many beneficial functions are produced. The blood vessels carry nutrients to the colon and also transport compounds absorbed by the colon for systemic distribution. A strong epithelial lining, like that shown under the magnifying glass, resists systemic exposures to pathogenic molecules such as lipopolysaccharides that are known to stimulate immune cell activation. Poor quality diets and obesity are associated with a leaky barrier, contributing to a chronic, low-level pro-inflammatory state. Improvements in diet quality, however, can mitigate some of the effects of obesogenic diets, and microbial metabolism plays an important role in mediating the beneficial effects of healthful dietary components.
Other investigations have also found increases in pathogenic intestinal bacteria during high-fat feeding in rodent models that is done by formulating the diet to include a high content of lard. For example, increases in pathogenic bacteria Alistipes sp. Marseille-P5997 and Alistipes sp. 5CPEGH6 and depletion of the probiotic Parabacteroides distasonis was noted in mice fed a high-fat diet to induce obesity. One of the metabolites produced was lysophosphatidic acid that was implicated in promoting colorectal cell proliferation and impairing the barrier function of gap junctions in the epithelium [19]. Western diets high in fat and low in fiber also alter bile acids in the intestinal lumen, including an increase in free bile acids such as deoxycholic acid that is implicated in gastrointestinal carcinogenesis [20, 21]. Conversely, healthier diets with higher fiber intake promote beneficial bacteria. These beneficial bacteria digest fiber to form short-chain fatty acids that help maintain the integrity of the intestinal barrier via several pathways, including promoting tighter gap junctions in the colonic epithelium [17].
Food Extracts
A recent review has already evaluated the effects of plant extracts (such as polyphenols, alkaloids, terpenoids) on reducing obesity and on normalizing the obesity-associated microbiota [22]. That review of the effects of plant extracts (such as polyphenols, alkaloids, terpenoids) on obesity shows that many of the compounds found in plant extracts normalize the obesity-associated microbiota. For example, Kaempferol, a flavonoid found in kale, beans, tea, spinach, and broccoli, protected against obesity and decreased hyperlipidemia partly through maintaining microbial diversity [22].
Supplementing diets with food extracts is often attractive in that adherence may be easier to achieve than a change in food selection, and it is true that there may be a role of extracts and supplements in delivering specific compounds useful for treatment of diseases. However, for optimal health long-term, choosing foods that provide a variety of nutrients may be most useful for maintaining health in persons without high-risk, medical conditions. Here, recent literature reported on the effects of dietary changes on the intestinal microbiota in obese animal models and in humans is discussed.
Goals of the Present Review.
Given the important role of the microbiota in maintaining the intestinal barrier and hence exposure to pro-inflammatory stimuli, one can begin to understand the important role of the intestinal microbiota in governing the chronic, pro-inflammatory state associated with increased risks of all the major chronic diseases faced today: cancer, diabetes and cardiovascular diseases. Unfortunately, Western style diets and obesity are now becoming prevalent world-wide, and weight loss via calorie restriction, pharmacological agents or bariatric surgery is difficult to maintain in humans, and weight regain is common [23–25]. It is now important to consider what types of dietary changes can be utilized to minimize the adverse effects of an obesogenic diet on the intestinal microbiota. In this review, the focus is on research that has been reported recently on how foods and macronutrients can normalize the obesity-associated microbiota.
Search Methodology
The references cited in this review were mainly obtained by a PubMed search that included the time frame of April 1, 2021- September 20, 2022. The terms used in each of five searches included “High fat”, “diet”, and “microbiota”. Additional terms for individual searches were “nutrients”, “fatty acids”, “calorie restriction”, “omega-3” or “fatty acids”. The references retrieved in PubMed were evaluated individually for suitability for inclusion in this review.
Dietary Patterns and Diet Components Associated with a Beneficial Intestinal Microbiota
Dietary Patterns
Many studies have evaluated how dietary patterns affect the gut bacteria. These data are helpful to summarize here before delving into how diet can attenuate adverse changes instituted by a high-fat, obesogenic diet. Briefly, bacteria in the phylum Firmicutes are the most abundant in the adult human intestine, followed by Bacteroidetes and Actinobacteria. Although there is variation between studies, the evidence is reasonably strong that the ratio of Firmicutes to Bacteroidetes is shifted with low fiber, high-fat diets. A higher Firmicutes to Bacteroidetes ratio that is associated with obesity contributes to maintenance of the obese state via increased energy harvest from foods, and this was confirmed with fecal transplantation experiments [26].
A high-fiber, plant-based diet conversely is generally associated with lower Firmicutes and higher abundance of taxa from the Bacteroidetes phylum, such as Prevotella species and the Bacteroides genus which are thought to be beneficial bacteria for host health [27]. These changes in gut microbiota result from the increased availability of fiber and resistant starch in the colon to support expansion of specific bacteria capable of fiber fermentation, and short-chain fatty acid production is the result [27]. Short chain fatty acids are a source of energy for colonocytes, act to strengthen the intestinal barrier, regulate immune responses, and have other beneficial effects on many metabolic processes including insulin secretion [28–30]. Firmicutes are known for their energy harvesting capabilities and tend to show increases in obese individuals
Other Aspects of Diet
There are two different aspects of diet that need to be considered for improving the adverse effects of a high-fat or Western style of diet on the microbiota: calorie restriction and dietary composition, and this is discussed in the next section. Most of the studies have been done in animal models. In humans, comparing populations with differing eating patterns can be done, but it is difficult if not impossible to exclude the contribution of other lifestyle and genetic factors on shaping the microbiota. In general, isolating the effects of diet on the intestinal microbiota is much easier to accomplish in animals, but dietary manipulation studies also have been done in humans.
In rodents fed high fat diets to induce obesity, a number of studies have shown that natural products found in plant-based foods prevent weight gain and that this could be mediated at least in part by normalization of the gut microbiota. Both black and green tea contain beneficial polyphenols. In mice fed a high fat diet, black tea supplementation of the diet dramatically decreased weight gain to that observed in animals fed a low-fat chow diet [31]. In mice colonized with gut bacteria from mice supplemented with black tea, the weight gain with a high-fat diet was markedly less [31]. Resveratrol is another example of a beneficial compound, and it is found in red grapes. Wistar rats fed a high fat, lard-based diet exhibited an increase in bacterial diversity when red grape juice was added to the diet for 60 days, and this was accompanied by a decrease in systolic blood pressure, serum interleukin 6 (IL6) and oxidative stress markers [32]. In other recent work, resveratrol supplementation of mice fed high fat diets did not reverse the dominant bacteria, such as Firmicutes and Bacteroidetes, but diversity of gut bacteria was increased based on the Shannon index and there were many changes at the class and genus levels [33]. Biomarkers of systemic inflammation improved, and the effects of resveratrol were similar when transplanting fecal microbiota from resveratrol fed animals as when giving resveratrol orally providing evidence that the changes were mediated by the microbiota [33].
Impact of Diet versus Exercise
Finally, diet as a whole is important to consider. In whole food diets, many dietary constituents can work together to shape the microbiota, which then does not necessitate using high quantities of any one component. This again is more easily studied in animals versus in human populations since people who consume better quality diets may also partake in other health behaviors such as higher levels of physical activity. For example, increased physical activity alleviates the high-fat diet induced dysbiosis and increases in intestinal permeability [34, 35]. Another study showed that exercise altered gut microbial composition to increase formation of short chain fatty acids, fiber metabolites that are known to strengthen gut barrier function [36].
Despite the possible role of multiple lifestyle determinants of the intestinal microbiota, one can hypothesize that diet has a stronger impact on the intestinal microbiota than other lifestyle behaviors. In one study that evaluated high fat diets and exercise in mice using a 2X2 study design, the effects of diet on the intestinal microbiota were found to be much more profound than that of exercise [37]. The two groups of mice fed the high fat diet had lower Bacteroidetes to Firmicutes ratios and gut microbial richness than the mice fed the chow diet, regardless of whether or not the mice were exercised on a treadmill [37]. However, mice in the two high-fat diet groups had higher diversity (Shannon) and evenness. There were many changes in taxa identified, including higher Proteobacteria with high-fat feeding. The increase in diversity was suggested to be consistent with an induction of bacteria that metabolize carbohydrates, perhaps as a compensatory mechanism [37].
Effects of Calorie Restriction in Dietary-Induced Obesity on the Gut Microbiota
One very important aspect of the whole diet is the amount of food that is consumed. Consuming an excess of calories beyond energy needs stimulates lipogenesis and expansion of adipose stores. Energy restriction instituted after high fat feeding to induce obesity has multiple beneficial effects on the gut microbiota. In rodents, 40% calorie restriction is typically used but the diets are formulated in such a way that micronutrient intakes are kept constant. The result is healthier rodents that live longer and are healthier [38].
Calorie restriction can also have long-term benefits that may extend beyond the restricted period. When rodents are fed high-fat diets, it is well known there is an overall increase in body weight and body fat versus that of animals fed a low-fat chow diet. However, there is inter-animal heterogeneity in weight gain, with about a third of the animals consumed less total diet and avoided the relatively large increase in weight gain. These animals are referred to as “obesity-resistant” [39].
Interestingly, calorie restriction of a high-fat diet has recently been shown to shape an obesity-resistant gut microbiota in mice [40]. This study evaluated lasting effects of calorie restriction by first feeding mice with either a low-fat diet, a low-fat diet with 30% calorie restriction, or a high fat diet. After four weeks, the mice fed the restricted diet were then placed on either a low-fat diet or a high-fat diet for an additional 8 weeks while the animals fed the low-fat or high-fat diets ad lib continued with their original diets [40]. The animals fed the high-fat diet throughout the experiment exhibited varying degrees of weight gain, as expected, and were classified as either obesity-prone or obesity-resistant. In comparing the animals fed the ad lib high-fat diet continuously with those fed the restricted diet first followed by an ad lib high-fat diet, those with exposure to the calorie restricted diet first were more similar to the obesity-resistant than the obesity-prone animals fed the high fat diet ad lib throughout in terms of body weight and microbiota composition at the end of the experiment. This delay in body weight gain due to the initial calorie restricted period appeared to be mediated by an increase in Clostridiales which has a biological role in energy metabolism [40].
In another study, a 50% restricted high-fat diet had beneficial effects on community richness in mice, but benefits were maximized when the restricted diet contained a mixture of yogurt, fruits and vegetables [41]. The supplemented, restricted diet improved intestinal barrier function, increased hepatic Akkermansia and multiple biomarkers of health status [41]. The role of the microbiota in the observed health effects was confirmed by fecal transplantation experiments [41].
Effects of Dietary Fiber on the Gut Microbiota
Perhaps the best well-studied dietary component with regard to its’ effects on the microbiota is dietary fiber. The majority of the gut microbiota that ferments fiber resides in the colon, and the small intestine has a more limited capacity to metabolize fiber. There are many forms of fiber, and the major classes are fiber soluble in water, including pectin and inulin, and fiber insoluble in water, including cellulose which is less readily fermented by the gut bacteria. Although very little cellulose can be fermented in the gut, it does play a critical role in maintaining colonic health [42].
Types of Fiber
A number of investigations have evaluated the ability of fiber to alleviate the dysbiosis induced by high-fat diets. One study compared insoluble with soluble fiber extracted from kiwifruit, administered via an intra-gastric route, on gut microbiota in rats [43]. Both types of fiber decreased food intake and improved body composition along with metabolic improvements. Although both types of fiber were beneficial, they had very different effects on abundance of specific microbial populations. The soluble fiber treatment resulted in larger improvements in both formation of short-chain fatty acids and markers of metabolic health [43].
It should be noted that newer studies are starting to evaluate synthetic fiber that is designed to optimize the beneficial properties of fiber such as viscosity, capacity to hold water and fermentability in the gut, and one such product was shown to have beneficial effects on the gut bacteria and result in reduced body weight [44]. Other examples of alternative fiber supplementation include flour made from high fiber foods. This includes yacon flour which decreased body fat gain and inflammatory stress in mice fed a high lard diet [45]. Another example is green banana flour that contains high amounts of resistant starch that reaches the colon to reduce the abundance of Firmicutes and increase the abundance of Bacteroidetes [46]. The green banana flour diet also exert beneficial effects on reducing obesity-associated systemic inflammation in mice fed high fat diets [46].
Several studies have been investigating inulin, a soluble fiber, as a potential dietary supplement to alleviate the sequelae of high fat diets and obesity. A combination of egg or whey protein with either cellulose (insoluble fiber) or inulin (soluble fiber) was evaluated for improving weight gain and obesity-associated metabolic alterations in in obese rats fed high fat diets and exercised on a treadmill [47]. This experiment then evaluated the additional beneficial effects dietary fiber could have in exercising rats. The results showed that both inulin-containing diets decreased food intake and weight gain and improved glycemic control more so than cellulose-containing diets, and this was similar to previous work in non-exercised rats [47]. Diet type explained 46% of the total variation in cecal microbiota composition, and plasma butyric acid, a short-chain fatty acid, was the plasma metabolite that was most strongly associated with the predicted functional pathways identified from metagenomic sequencing [47].
Another recent study also evaluated the combination of inulin supplementation with exercise, but these researchers specifically investigate whether exercise can improve the metabolic response to inulin in obese humans and obese rats since exercise can alter transit time and therefore substrate availability in the colon [48]. The results showed greater gut fermentation of inulin (resulting in fewer GI symptoms in humans) and improved glucose homeostasis when exercise is combined with inulin in both species, but there were different effects on the microbiota changes in rats and humans [48]. For example, in humans, but not mice, the exercise/inulin combination stimulated growth of Bifidobacterium and augmented weight loss [48].
Pulses
Many other studies focused on intake of beans, lentils and dry peas as good sources of fiber. Pulses are the seed of the legume plant and contain soluble and insoluble fibers in addition to protein and starch as the major macronutrient components. The published studies overwhelmingly show that beans can alleviate high-fat diet induced dyslipidemia and that this is either correlated with or mediated by normalization of the gut microbiota.
Commonly consumed pulses namely lentils, chickpeas, common beans, and dry peas added to mouse diets to replace 35% of the protein all increased α-diversity of the gut microbiota, increased the abundance of Bacteroidetes and decreased the abundance of Proteobacteria and Firmicutes. Verrucomicrobiota increased with all the pulses except dry peas [49]. The authors noted the beneficial effects on the microbiota were consistent with the anti-obesogenic effects of adding pulses to the diets. Interestingly, the supplemented diets also increased synthesis of vitamins and organic cofactors, such as thiamin, heme, pyridoxal 5′-phosphate, flavin, folic acid (via 6-hydroxymethyl-dihydropterin diphosphate), nicotinamide adenine dinucleotide (NAD), phospho-pantothenate, and coenzyme A indicating multi-faceted benefits [49].
Another study using supplementation of a high-fat diet with 15% Adzuki beans also significantly reduced the ratio of Firmicutes/Bacteroidetes (F/B), increased the abundance of beneficial bacteria such as Bifidobacterium, and decreased the abundance of several taxa induced by a high-fat diet towards the normal state, as defined by mice fed a low-fat control diet [50].
The effects of pulses, however, cannot be ascribed to fiber alone. The fermentation broth from kidney beans, which is rich in polyphenols and flavones, also was shown to increase gut microbial diversity indices and to decrease the abundance of Firmicutes in obese rats fed a high fat diet while improving the dyslipidemia that was observed [51]. This makes it difficult to disentangle the effects of fiber per se from other components of whole beans.
Whole Grains
Finally, there are studies that evaluated the effects of whole grains for improving the high-fat diet induced dysbiosis. These have included coarse, partly milled cereals to maximize fiber delivery to the colon by minimizing digestion and absorption in the small intestine. The recent studies showing beneficial effects on microbiota changes in rodents fed high-fat diets included a study on partly milled barley that showed enrichment of beneficial bacteria, such as Lactobacillus, Bifidobacterium, and Ileibacterium, and reduction in several HFD-dependent taxa [52], and a study on coarse ground oats and tartary buckwheat that showed increased abundance of colonic Lactobacillus and Romboutsia as well as increased short chain fatty acid production [53]. Both studies also showed improvements in biomarkers of obesity-associated metabolic disturbances in the host animal.
Effects of Dietary Fat Type on the Gut Microbiota
In evaluating the effects of high fat diets, it is important to recognize the importance of different fat types. Lard, a saturated animal fat, is typically utilized to induce obesity in animal models. Hence, the adverse effects of high fat diets might be mediated by saturated fatty acids and not by the amount of fat per se. High saturated fat diets are known to be associated with obesity and increased risks of cardiovascular diseases. A number of studies have evaluated the relative impact of different types of fatty acids.
In a study that compared the effects of high-fat diets made with 11 different types of oils and lard on the fecal microbiota of mice, the high fat diets all increased Firmicutes abundance and reduced Bacteroidetes abundance with the lard diet having the largest change and the canola oil diet resulting in minimal changes in these phyla [53]. Of note, the high-fat canola oil diet increased α-diversity the most and the lard diet resulted in the largest changes in bacterial taxa versus the low-fat, chow diet control [53]. This is consistent with results in humans that the ratios of Firmicutes to Bacteroidetes abundance are increased in obesity [17].
In humans, there appear to be clear benefits of limiting saturated fat intakes. In a randomized clinical trial with healthy participants, replacing saturated fats with polyunsaturated fats resulted in beneficial health effects such as reducing total blood cholesterol, and there were increases in the abundance of Lachnospiraceae and several species of Bifidobacterium in fecal samples. These bacteria are both associated with beneficial health effects such as lower serum cholesterol levels [54]. Furthermore, the reduction in total cholesterol after the change in dietary fat quality correlated positively with changes in the gut microbiota family Lachnospiraceae. This was attributed to metabolism of dietary and biliary cholesterol in the intestine to coprostanol which is then excreted, essentially clearing cholesterol from the blood [54]. In an observational study, healthy individuals with relatively higher saturated fatty acid intakes had a higher abundance of the Fusobacterium genus, and one species within that genus has been associated with colon cancer risk, and individuals with higher trans fatty acid intakes had lower α-diversity. In evaluating all the major fatty acid types, the relative abundance of Sutterella, Tyzzerella, and Fusobacterium differed significantly by fatty acid intake [55].
In contrast to saturated fats, omega-3 polyunsaturated fatty acids have been noted to have many beneficial effects. This is especially true of long chain omega-3 fatty acids such as eicosapentaenoic acid (EPA). EPA supplementation of obese mice prevents many metabolic complications of a high fat diet. In a study using obese female mice, which are less often studied, EPA ethyl ester supplementation improved metabolic parameters (insulin, glucose) and increased the abundance of enteric Akkermansia mucinophilia, an organism with putative beneficial effects on the colon [56]. In another study, although fish oil supplementation resulted in lower body weight and lower inflammatory status in both male and female mice fed a high fat diet, the fish oil diet did not correct the low Bacteroidetes-to-Firmicutes ratio elicited by a high fat diet in female mice [57]. An oil high in the short-chain omega-3 fatty acid, α-linolenic acid that is metabolically elongated to form EPA, also was shown to improve high-fat diet induced dysbiosis as defined by alterations in the abundance of Firmicutes and Bacteroidetes [58].
In humans, high-fat, Western diets with low omega-3 fatty acid content are worrisome. Higher omega-6 fatty acid intakes increase inflammatory stress and decrease beneficial taxa such as Bifidobacterium while higher omega-3 fatty acid intakes increase Lactobacillus abundance [17]. Moreover, a high-fat diet driven microbiota is low in bacteria that promote a healthy intestinal barrier, such as Akkermansia muciniphila and several species within Bifidobacterium, Bacteroidetes, Lactobacillus and Clostridiales, and higher in microbes that contribute to disruption of the gut barrier integrity, such as the Oscillibacter and Desulfovibri species [17]. The weakened barrier facilitates absorption of bacterial products such as lipopolysaccharide that activate the immune system. This effect does not appear to be related to diet type. High saturated fat diets have been noted to cause similar pro-inflammatory effects as high polyunsaturated fat diets [17].
Conclusion
Obesity is on the rise world-wide, and it now becomes important to develop strategies that can mitigate the adverse health effects of obesity. Unfortunately, weight loss via any means is difficult to maintain in humans, and weight regain is common. This review therefore focuses on identifying dietary factors outside of calorie restriction that could be used to mitigate the adverse health effects of obesity. Animal models of human obesity have been useful in this regard. Obesity in rodents is typically induced with high-fat diets using lard or Western blend fats high in saturated fat. The animal data on dietary manipulations to ameliorate the adverse effects of obesity is encouraging. In rodents fed an obesogenic, high-fat diet, switching to high-fat diets formulated to contain higher amounts of fiber or fiber-containing foods, plant extracts, omega-3 fatty acids or whole grains has beneficial effects on body weight, metabolic alterations, and the intestinal microbiota. The role of the intestinal microbiota in mediating the beneficial health effects of these dietary factors in the face of an obesogenic diet was supported using bacterial transplantation experiments in animal models. Many aspects of the microbiota observed in animals when healthful dietary components were added to the feed have also been observed in humans who follow healthful dietary patterns. These data then support the design of dietary interventions that would allow individuals to focus on diet quality independently of weight loss to mitigate the adverse sequelae of obesity.
Key Points.
Improved dietary composition can mitigate the adverse health effects of obesity.
High-fat diets formulated to contain higher amounts of fiber or fiber-containing foods, plant extracts, omega-3 fatty acids or whole grains have beneficial effects on body weight, metabolic alterations, and the intestinal microbiota.
Many features of the microbiota observed in animals fed diets with healthful dietary components have also been observed in humans who follow healthful dietary patterns.
These data then support the design of dietary interventions that would allow individuals to focus on diet quality independently of weight loss to mitigate the adverse sequelae of obesity.
Financial support and sponsorship:
This work was supported by NCI grant RO1 CA1255743 and UG1 CA242632
Footnotes
Conflicts of interest: None.
References
Bulleted references: One bullet (*) for special interest and two bullets (**) for outstanding interest publications.
- 1.Moriguchi Watanabe L, Bernardes Pereira Delfino H, Augusta de Souza Pinhel M, et al. Food and Nutrition public policies in brazil: from malnutrition to obesity. Nutrients. 2022;14(12):2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casari S, Di Paola M, Banci E, et al. Changing Dietary Habits: The impact of urbanization and rising socio-economic status in families from Burkina Faso in Sub-Saharan Africa. Nutrients. 2022;14(9):1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueroa C, Echeverria G, Villarreal G, et al. Introducing plant-based mediterranean diet as a lifestyle medicine approach in latin america: Opportunities Within the Chilean Context. Front Nutr. 2021;8:680452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cyr-Scully A, Howard AG, Sanzone E, et al. Characterizing the urban diet: development of an urbanized diet index. Nutr J. 2022;21(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopp WH How western diet and lifestyle drive the Pandemic of obesity and civilization diseases. Diabetes Metab Syndr Obes. 2019;12:2221–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoy MK, Murayi T, Moshfegh AJ. Diet Quality of frequent fast-food consumers on a non-fast food intake day is similar to a day with fast food, What We Eat in America, NHANES 2013–2016. J Acad Nutr Diet. 2022;122(7):1317–25. [DOI] [PubMed] [Google Scholar]
- 7.Agricultural Research Service. Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services. Washington, D.C.: U.S. Department of Agriculture; 2020. [Google Scholar]
- 8.Long T, Zhang K, Chen Y, et al. Trends in diet quality among older US adults from 2001 to 2018. JAMA Netw Open. 2022;5(3):e221880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dokova KG, Pancheva RZ, Usheva NV, et al. Nutrition Transition in Europe: east-west dimensions in the last 30 years-A narrative review. Front Nutr. 2022;9:919112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiFrancesco L, Fulgoni VL 3rd, Gaine PC, et al. Trends in added sugars intake and sources among U.S. adults using the National Health and Nutrition Examination Survey (NHANES) 2001–2018. Front Nutr. 2022;9:897952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juul F, Parekh N, Martinez-Steele E, et al. Ultra-processed food consumption among US adults from 2001 to 2018. The American Journal of Clinical Nutrition. 2022;115(1):211–21. [DOI] [PubMed] [Google Scholar]
- 12.Vinyard M, Zimmer M, Herrick KA, et al. Healthy Eating Index-2015 scores vary by types of food outlets in the United States. Nutrients. 2021;13(8):2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Overweight & Obesity Statistics 2021. [Available from: https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity#:~:text=Fast%20Facts&text=Nearly%201%20in%203%20adults%20(30.7%25)%20are%20overweight.&text=More%20than%202%20in%205%20adults%20(42.4%25)%20have%20obesity.&text=About%201%20in%2011%20adults%20(9.2%25)%20have%20severe%20obesity.
- 14.Stierman B, Afful J, Carroll MD, et al. National Health and Nutrition Examination Survey 2017–March 2020 prepandemic data files—development of files and prevalence estimates for selected health outcomes. In: Surveys DoHaNE, editor. Hyattsville, MD: National Center for Health Statistics; 2021. [Google Scholar]
- 15.World Health Organization. Fact Sheets on Obesity and Overweight, Diabetes and Cardiovascular Diseases: World Health Organization,; 2022. [Available from: https://www.who.int/news-room/fact-sheets. [Google Scholar]
- 16.U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. 8th Edition. 2015. [Google Scholar]
- 17.Malesza IJ, Malesza M, Walkowiak J, et al. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells. 2021;10(11):3164. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This review summarizes the effects of hihg-fat diet on the gut microbiota.
- 18.National Research Council. Diet and Health: Implications for Reducing Chronic Disease Risk. Washington, DC: National Academies Press; 1989. [PubMed] [Google Scholar]
- 19.Yang J, Wei H, Zhou Y, et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology. 2022;162(1):135–49 e2. [DOI] [PubMed] [Google Scholar]; *This review highlights the role of natural products on mitigating obesity.
- 20.Jin D, Huang K, Xu M, et al. Deoxycholic acid induces gastric intestinal metaplasia by activating STAT3 signaling and disturbing gastric bile acids metabolism and microbiota. Gut microbes. 2022;14(1):2120744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao Y, Li X, Xu B, et al. Cholecystectomy promotes colon carcinogenesis by activating the Wnt signaling pathway by increasing the deoxycholic acid level. Cell Commun Signal. 2022;20(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang HN, Xiang JZ, Qi Z, et al. Plant extracts in prevention of obesity. Crit Rev Food Sci Nutr. 2022;62(8):2221–34. [DOI] [PubMed] [Google Scholar]
- 23.Flore G, Preti A, Carta MG, et al. Weight maintenance after dietary weight loss: systematic review and meta-analysis on the effectiveness of behavioural intensive intervention. Nutrients. 2022;14(6):1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ard J, Fitch A, Fruh S, et al. Weight Loss and Maintenance Related to the Mechanism of Action of Glucagon-Like Peptide 1 Receptor Agonists. Adv Ther. 2021;38(6):2821–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Athanasiadis DI, Martin A, Kapsampelis P, et al. Factors associated with weight regain post-bariatric surgery: a systematic review. Surg Endosc. 2021;35(8):4069–84. [DOI] [PubMed] [Google Scholar]
- 26.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. [DOI] [PubMed] [Google Scholar]
- 27.Beam A, Clinger E, Hao L. Effect of diet and dietary components on the composition of the gut microbiota. Nutrients. 2021;13(8):2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiweck C, Edwin Thanarajah S, Aichholzer M, et al. Regulation of CD4(+) and CD8(+) T Cell Biology by Short-Chain Fatty Acids and Its Relevance for Autoimmune Pathology. Int J Mol Sci. 2022;23(15):8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosli NSA, Abd Gani S, Khayat ME, et al. Short-chain fatty acids: possible regulators of insulin secretion. Mol Cell Biochem. 2022;in press August 2022. [DOI] [PubMed] [Google Scholar]
- 30.Pohl K, Moodley P, Dhanda A. The effect of increasing intestinal short-chain fatty acid concentration on gut permeability and liver injury in the context of liver disease: A systematic review. J Gastroenterol Hepatol. 2022;37(8):1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Hu G, Wang A, et al. Black Tea Reduces Diet-Induced Obesity in Mice via Modulation of Gut Microbiota and Gene Expression in Host Tissues. Nutrients. 2022;14(8):1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Fonseca Cardoso LM, de Souza Monnerat JA, de Medeiros Silva IWS, et al. Beverages Rich in resveratrol and physical activity attenuate metabolic changes induced by high-fat diet. J Am Coll Nutr. 2021;40(6):485–95. [DOI] [PubMed] [Google Scholar]
- 33.Wang P, Ma Y, Wang D, et al. Protective effects of dietary resveratrol against chronic low-grade inflammation mediated through the gut microbiota in high-fat diet mice. Nutrients. 2022;14(10):1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu C, Liu S, Niu Y, et al. Exercise protects intestinal epithelial barrier from high fat diet- induced permeabilization through SESN2/AMPKalpha1/HIF-1alpha signaling. J Nutr Biochem. 2022;107:109059. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Zhang Q, Xia J, et al. Moderate Treadmill Exercise Modulates Gut Microbiota and Improves Intestinal Barrier in High-Fat-Diet-Induced Obese Mice via the AMPK/CDX2 Signaling Pathway. Diabetes Metab Syndr Obes. 2022;15:209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang WC, Tung CL, Yang YSH, et al. Endurance exercise ameliorates Western diet-induced atherosclerosis through modulation of microbiota and its metabolites. Sci Rep. 2022;12(1):3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yun EJ, Imdad S, Jang J, et al. Diet is a stronger covariate than exercise in determining gut microbial richness and diversity. Nutrients. 2022;14(12):2507. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study demonstrates a microbiome-dependent mechanism by which the microbiome synergizes with host dietary intakes to regulate body weight.
- 38.Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32(3):159–221. [DOI] [PubMed] [Google Scholar]
- 39.Madsen AN, Hansen G, Paulsen SJ, et al. Long-term characterization of the diet-induced obese and diet-resistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J Endocrinol. 2010;206(3):287–96. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Lin Y, Li T, et al. Calorie restriction on normal body weight mice prevents body weight regain on a follow-up high-fat diet by shaping an obesity-resistant-like gut microbiota profile. Food Funct. 2022;13(14):7684–96. [DOI] [PubMed] [Google Scholar]
- 41.Kong CY, Li ZM, Chen HL, et al. An energy-restricted diet including yogurt, fruit, and vegetables alleviates high-fat diet-induced metabolic syndrome in mice by modulating the gut microbiota. The Journal of nutrition. 2022;in press Aug 2022. [DOI] [PubMed] [Google Scholar]
- 42.Baky MH, Salah M, Ezzelarab N, et al. Insoluble dietary fibers: structure, metabolism, interactions with human microbiome, and role in gut homeostasis. Crit Rev Food Sci Nutr. 2022:1–15. [DOI] [PubMed] [Google Scholar]
- 43.Wang K, Wang Y, Chen S, et al. Insoluble and soluble dietary fibers from kiwifruit (actinidia deliciosa) modify gut microbiota to alleviate high-fat diet and streptozotocin-induced TYPE 2 diabetes in rats. Nutrients. 2022;14(16):3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M, Liu J, Li C, et al. Functional fiber reduces mice obesity by regulating intestinal microbiota. Nutrients. 2022;14(13):2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Abreu Ribeiro Pereira J, de Fatima Piccolo Barcelos M, Valerio Villas Boas E, et al. Combined effects of yacon flour and probiotic yogurt on the metabolic parameters and inflammatory and insulin signaling proteins in high-fat-diet-induced obese mice. J Sci Food Agric. 2022;in press Jun 2022. [DOI] [PubMed] [Google Scholar]
- 46.Rosado CP, Rosa VHC, Martins BC, et al. Green banana flour supplementation improves obesity-associated systemic inflammation and regulates gut microbiota profile in mice fed high-fat diets. Appl Physiol Nutr Metab. 2021;46(12):1469–75. [DOI] [PubMed] [Google Scholar]
- 47.Avirineni BS, Singh A, Zapata RC, et al. Diets Containing egg or whey protein and inulin fiber improve energy balance and modulate gut microbiota in exercising obese rats. Mol Nutr Food Res. 2022;66(7):e2100653. [DOI] [PubMed] [Google Scholar]; * This study evaluates the impact of dietary factors over and above the possible beneficial effects of exercise.
- 48.Rodriguez J, Neyrinck AM, Van Kerckhoven M, et al. Physical activity enhances the improvement of body mass index and metabolism by inulin: a multicenter randomized placebo-controlled trial performed in obese individuals. BMC Med. 2022;20(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lutsiv T, Weir TL, McGinley JN, et al. Compositional Changes of the high-fat diet-induced gut microbiota upon consumption of common pulses. Nutrients. 2021;13(11):3992. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study demonstrates microbiota-associated benefits of pulse consumption in the context of high-fat diet.
- 50.Zhao Q, Hou D, Fu Y, et al. Adzuki Bean Alleviates Obesity and Insulin Resistance Induced by a High-Fat Diet and Modulates Gut Microbiota in Mice. Nutrients. 2021;13(9):3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pang W, Wang D, Zuo Z, et al. Kidney Bean fermented broth alleviates hyperlipidemic by regulating serum metabolites and gut microbiota composition. Nutrients. 2022;14(15):3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li S, Wang M, Li C, et al. Beneficial effects of partly milled highland barley on the prevention of high-fat diet-induced glycometabolic disorder and the modulation of gut microbiota in mice. Nutrients. 2022;14(4):762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.An J, Wang Q, Yi S, et al. The source of the fat significantly affects the results of high-fat diet intervention. Sci Rep. 2022;12(1):4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Telle-Hansen VH, Gaundal L, Bastani N, et al. Replacing saturated fatty acids with polyunsaturated fatty acids increases the abundance of Lachnospiraceae and is associated with reduced total cholesterol levels-a randomized controlled trial in healthy individuals. Lipids Health Dis. 2022;21(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This clinical trial shows how microbiome changes might contribute to the mechanisms behind the beneficial effects of polyunsaturated fats.
- 55.Xu AA, Kennedy LK, Hoffman K, et al. Dietary Fatty acid intake and the colonic gut microbiota in humans. Nutrients. 2022;14(13):2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pal A, Sun S, Armstrong M, et al. Beneficial effects of eicosapentaenoic acid on the metabolic profile of obese female mice entails upregulation of HEPEs and increased abundance of enteric Akkermansia muciniphila. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867(1):159059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abbas MM, Soto P, Ramalingam L, et al. Sex differences in fish oil and olanzapine effects on gut microbiota in diet-induced obese mice. Nutrients. 2022;14(2):349. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study finds sex-specific effects of diet on gut microbiota.
- 58.Kangwan N, Pratchayasakul W, Kongkaew A, et al. Perilla Seed oil alleviates gut dysbiosis, intestinal inflammation and metabolic disturbance in obese-insulin-resistant rats. Nutrients. 2021;13(9):3141. [DOI] [PMC free article] [PubMed] [Google Scholar]


