Abstract
The development of donor-specific antibodies after lung transplantation is associated with downstream acute cellular rejection (ACR), antibody-mediated rejection (AMR), chronic lung allograft dysfunction (CLAD) or death. It is unknown whether preemptive (early) treatment of de novo donor-specific antibodies (dnDSA), in the absence of clinical signs and symptoms of allograft dysfunction, reduces the risk of subsequent chronic lung allograft dysfunction or death. We performed a multicenter, retrospective cohort study to determine if early treatment of dnDSA in lung transplant patients reduces the risk of the composite endpoint of CLAD or death. In the cohort of 445 patients, 145 patients developed dnDSA post-transplant. 30 patients received early targeted treatment for dnDSA in the absence of clinical signs and symptoms of antibody-mediated rejection. Early treatment of dnDSA was associated with a decreased risk of CLAD or death (HR 0.36; 95% confidence interval (CI), 00.17 – 0.76, p < 0.01). Deferring treatment until the development of clinical antibody-mediated rejection was associated with an increased risk of CLAD or death (HR 3.00; 95% CI, 1.46—6.18, p < 0.01). This study suggests that early, preemptive treatment of donor specific antibodies in lung transplant patients may reduce the subsequent risk of CLAD or death.
Introduction
The development of donor-specific human leukocyte antigen antibodies (donor specific antibodies, DSA) after lung transplantation is a risk factor for the development of antibody mediated rejection (AMR), acute cellular rejection (ACR), chronic lung allograft dysfunction (CLAD) and death1–9. In particular, the development of de novo DSA (dnDSA) has also been associated with an increased risk of CLAD and death10–12. The incidence of dnDSA after lung transplantation based on prior studies ranges from 13–61% depending on the screening protocol and sensitivity of the antibody screening assay10–12. Although the development of dnDSA is common and has potentially severe clinical consequences, it is unknown whether preemptive treatment of DSA in the absence of clinical or histological signs of AMR improves subsequent clinical outcomes.
Given the uncertainty surrounding the management of lung transplant patients who develop dnDSA, practice patterns across lung transplant centers vary widely. Some providers opt to treat patients who develop dnDSA early, prior to the clinical manifestations of AMR, with the goal of preventing the downstream development of allograft dysfunction. Others elect to defer treatment until development of clinical AMR or allograft dysfunction. There remains a lack of quality evidence to help guide these decision-making processes. In this multicenter retrospective cohort study that includes transplant centers with different DSA treatment practices, we aimed to determine if initiating preemptive treatment for dnDSA in the absence of clinical AMR, in comparison to no preemptive treatment, decreased the risk of CLAD and death in lung transplant patients. We hypothesized that initiating preemptive treatment for dnDSA would decrease the risk of AMR and the subsequent development of CLAD or death.
Methods
Study Design and Patient Population
We performed a multicenter, retrospective analysis of three lung transplant cohorts that included patients who were at least 18 years of age and awaiting lung transplantation. The first cohort consisted of 81 patients enrolled in the Genome Transplant Dynamics (GTD) (NCT01985412) study between 12/1/2010 – 12/31/2012, a single-center prospective cohort study at Stanford University Hospital, with follow up recorded until May 1, 2019. The second cohort consisted of 223 patients enrolled in the Genomic Research Alliance for Transplantation (GRAfT) (NCT0243070) study between June 1, 2015 and October 1, 2019, an ongoing multicenter prospective cohort study that began recruitment in 2015 at three centers (Johns Hopkins Hospital, University of Maryland Medical Center, and Inova Fairfax Hospital). The third cohort consisted of lung transplant recipients at Johns Hopkins Hospital between May 1, 2010 and September 1, 2015 who survived the index hospitalization, with follow up recorded until November 1, 2019 (n=141). The primary goal of all three studies was to study acute rejection and its relationship to CLAD or death. An additional aim of the GTD and GRAfT studies was to test the performance of donor-derived cell free DNA as a biomarker for allograft injury. Subjects were monitored prospectively post-transplant with collection of clinical data including pulmonary function testing, DSA, allograft histopathology as well as treatment dates and regimens for ACR and AMR. Patients with a positive prospective crossmatch with their donor, no development of DSA post-transplant, missing or incomplete DSA data, and missing or incomplete data on allograft function were excluded. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement guidelines for reporting observational studies were followed. This study was approved by the Institutional Review Board at each center.
DSA Testing and Measurement
The participating centers performed surveillance DSA testing prior to transplantation and on post-transplant day 7, 14 and month 1, 3, 6, 9, 12, 18 and 24 (coincident with scheduled surveillance bronchoscopy). Patients underwent additional DSA testing for clinical signs or symptoms of allograft dysfunction. DSA was detected at each center by single antigen bead testing using the LABScreen® Single Antigen Bead assay (One Lambda, Canoga Park, CA) and designated as either positive or negative. A positive test was defined as a mean fluorescence intensity (MFI) ≥ 1000 on one occasion or an MFI between 500–1000 on two serial occasions. DSA was categorized as preformed (present prior to transplantation) or de novo – not detected prior to transplantation.
Clinical Variables
The primary outcome was a composite outcome of CLAD or death. We included re-transplantation in this outcome. CLAD was defined according to International Society for Heart and Lung Transplantation (ISHLT) criteria as ≥ 20% decline of the forced expiratory volume in 1 second (FEV1) from baseline at least 3 months post-transplant on 2 separate measurements made ≥ 3 weeks apart13.
DSA treatment categories, AMR and other study endpoints were adjudicated by multidisciplinary adjudication committees. The committee encompassed two transplant pulmonologists, one internist, one transplant immunogeneticist, one transplant pharmacist and two pathologists. AMR was defined according to ISHLT guidelines for possible, probable and definite clinical AMR14, using previously described adjudication protocols15. The definition of clinical AMR was defined as the presence of allograft dysfunction (decline in FEV1 ≥ 10%) and positive DSA plus one of the following: positive histopathology, positive c4d staining or absence of an alternative diagnosis. All patients were DSA positive given the primary goal of this study. In the presence of dnDSA but without signs of allograft dysfunction or clinical AMR, patients were classified as “early treatment” if they received preemptive antibody directed therapy based on the positive dnDSA only, and “no early treatment” if they did not receive antibody directed therapy. The “no early treatment” group consisted of patients who never developed subsequent signs and symptoms of AMR requiring antibody directed therapies at a later point in time as well as patients who eventually developed AMR and required treatment with antibody directed therapies. Antibody directed therapy was defined as the use of intravenous immune globulin (IVIG), rituximab, plasma exchange or a proteasome inhibitor +/− methylprednisolone either alone or in combination. Initial treatment was usually followed by 3–6 months of IVIG therapy. When clinical AMR was present, treatment proceeded in accordance to the individual institutions’ protocol and generally consisted of a combination of methylprednisolone, PLEX and rituximab followed by 3–6 months of IVIG therapy. Outlines of institutional protocols are provided in the Supplementary Index eMethods.
Statistical Analysis
Continuous variables were described using mean (SD) or median (IQR), and categorical variables were summarized using counts (%). Non-parametric tests were used when indicated. Univariate analyses were conducted to compare primary and secondary endpoints using Chi-square or Fisher’s exact test for categorical variables and t-tests for continuous variables. Kaplan-Meier curves were constructed depicting time from transplant to CLAD or death and compared using the log-rank test. Multivariable Cox proportional-hazards models were used to model time to CLAD or death adjusting for confounding variables. Multivariable logistic regression models were used to investigate the association of DSA treatment with subsequent diagnosis of AMR. Co-variates were prespecified based on the potential for influencing the outcome or evidence from prior studies demonstrating an increased risk of CLAD and included transplant center (in order to account for treatment practices, including induction therapy), DQ-Specific DSA, and time to DSA positivity16. Analyses were performed using SAS version 9.4. P-values were 2-sided with significance indicated by a value ≤ 0.05.
Results
During the study period, 512 patients were transplanted at participating centers and 445 of these patients were enrolled. Of these, 96 patients were excluded due to missing or incomplete data for DSA, determination of CLAD, death or treatment type, 28 patients were excluded with pre-formed DSA and 176 patients were excluded that did not develop DSA over the course of the study period leaving 145 dnDSA positive patients included in the final analysis (Figure 1). The mean length of follow-up was 23.8 months. The average age (SD) of the cohort was 50.7 (15.6) years, 110 (76%) had a bilateral transplant procedure, 118 (81%) patients were white and 76 (52%) were female (Table 1). Eight percent of patients who developed dnDSA exclusively developed Class I antibodies, 76% only Class II antibodies and 12% both class I and class II antibodies. Specificity for HLA-DQ antibody (alone and in combination with other antibodies) was present in 79% of patients (Table 2). Thirty (21%) patients received early treatment for dnDSA while 115 (79%) patients did not. The median time to dnDSA positivity was 11 days in the early treatment group and 75 days in the no early treatment group. The most common early treatment regimen consisted of IVIG alone (33%), followed by IVIG + Rituximab (27%) and plasmapheresis + IVIG + Rituximab (13%); with the remainder of the treatment regimens presented in Supplementary Index Table 2.
Figure 1:
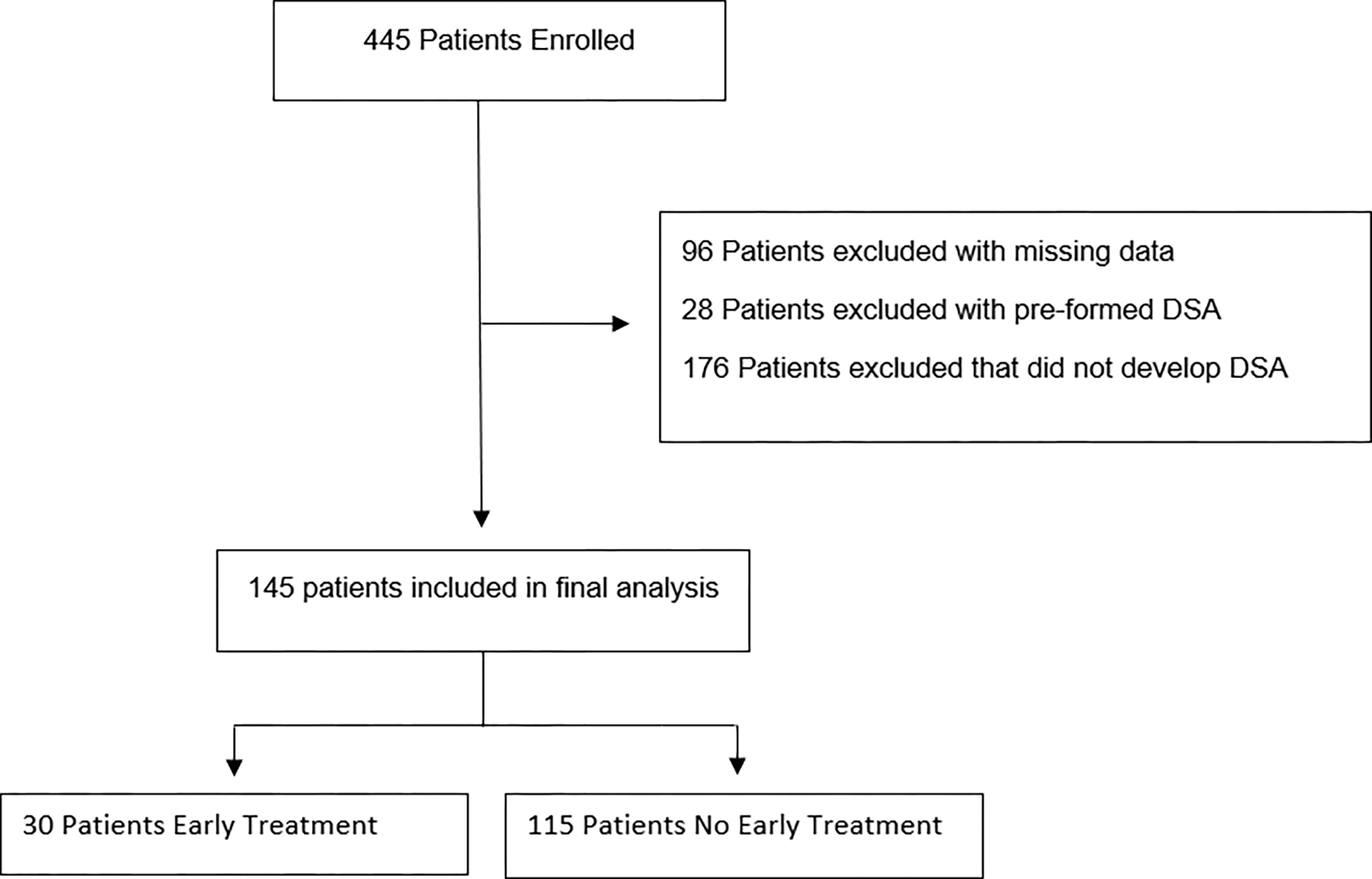
Flowchart of study design. Patients with missing DSA, CLAD or Death data were excluded (n=96). Patients with pre-formed DSA and those that did not develop DSA were then excluded, leaving 145 patients in our final analysis – 30 that received early treatment and 115 that did not receive early treatment. CLAD: Chronic Lung Allograft Dysfunction; DSA: Donor Specific Antibody.
Table 1:
Patient Demographics
| Total (n=145) | Early Treatment (n=30) | No Early Treatment (n=115) | p-value | |
|---|---|---|---|---|
|
| ||||
| Recipient Age – Years (SD) | 50.7 (15.6) | 51.0 (11.1) | 51.4 (15.9) | 0.95 |
|
| ||||
| Donor Age – Years (SD) | 36.8 (14.1) | 37.2 (16.4) | 36.4 (13.6) | 0.54 |
|
| ||||
| Female Recipient (%) | 76 (52%) | 19 (63%) | 57 (50%) | 0.22 |
|
| ||||
| Bilateral Transplant (%) | 110 (76%) | 26 (87%) | 84 (73%) | 0.62 |
|
| ||||
| Diagnosis | 0.08 | |||
| COPD | 33 (23%) | 3 (10%) | 30 (26%) | |
| Cystic Fibrosis | 34 (23%) | 6 (20%) | 28 (24%) | |
| Interstitial Lung Disease | 60 (41%) | 17 (57%) | 43 (37%) | |
| Pulmonary Arterial Hypertension | 3 (2%) | 2 (7%) | 1 (1%) | |
| Sarcoidosis | 9 (6%) | 2 (7%) | 7 (6%) | |
| Other | 3 (2%) | 0 (0%) | 3 (3%) | |
| Re-transplantation | 3 (2%) | 0 (0%) | 3 (3%) | |
|
| ||||
| Race | 0.33 | |||
| White | 117 (81%) | 24 (80%) | 93 (81%) | |
| Black | 21 (14%) | 6 (20%) | 15 (13%) | |
| Asian | 4 (3%) | 0 (0%) | 4 (4%) | |
| Other | 3 (2%) | 0 (0%) | 2 (2%) | |
|
| ||||
| Total HLA Mismatches | 0.63 | |||
| 0 | 1 (1%) | 0 (0%) | 1 (1%) | |
| 1 | 0 (0%) | 0 (0%) | 0 (0%) | |
| 2 | 5 (3%) | 0 (0%) | 5 (4%) | |
| 3 | 13 (9%) | 3 (10%) | 10 (9%) | |
| 4 | 33 (23%) | 6 (20%) | 27 (23%) | |
| 5 | 47(32%) | 13 (43%) | 34 (30%) | |
| 6 | 46 (32%) | 8 (27%) | 38 (33%) | |
| HLA-A-Mismatch | 0.18 | |||
| 0 | 15 (10%) | 2 (7%) | 13 (11%) | |
| 1 | 45 (31%) | 6 (20%) | 39 (34%) | |
| 2 | 85 (59%) | 22 (73%) | 63 (55%) | |
| HLA-B-Mismatch | 0.25 | |||
| 0 | 5 (3%) | 0 (0%) | 5 (4%) | |
| 1 | 35 (24%) | 10 (33%) | 25 (22%) | |
| 2 | 105 (72%) | 20 (67%) | 85 (74%) | |
| HLA-DR-Mismatch | 0.02 | |||
| 0 | 9 (6%) | 0 (0%) | 9 (8%) | |
| 1 | 40 (28%) | 14 (47%) | 26 (23%) | |
| 2 | 96 (66%) | 16 (53%) | 80 (70%) | |
Table 2:
dnDSA Characteristics of Study Population
| Characteristics | dnDSA+ (n=145) | Early Treatment (n=30) | No Early Treatment (n=115) |
|---|---|---|---|
|
| |||
| Median days to dnDSA | 38 | 11 | 75 |
|
| |||
| HLA Class I | 12 (8%) | 3 (10%) | 9 (8%) |
|
| |||
| HLA Class II | 110 (76%) | 16 (53%) | 94 (81%) |
| DQ | 89 (61%) | 13 (43%) | 76 (66%) |
| DP/DR | 12 (8%) | 3 (10%) | 9 (8%) |
| DQ + DP/DR | 9 (8%) | 0 | 9 (8%) |
|
| |||
| HLA Class I and II | 17 (12%) | 6 (20%) | 11 (10%) |
|
| |||
| Other Combinations | 6 (4%) | 5 (17%) | 1 (1%) |
Early DSA Treatment vs. No Early DSA Treatment
In a multivariable Cox regression analysis adjusting for differences in center, presence of DQ antibodies and time to dnDSA positivity; early treatment of dnDSA was associated with a decreased the risk of developing CLAD or death in comparison to not initiating early treatment (HR 0.36; 95% confidence interval (CI), 0.17 – 0.76, p < 0.01) (Figure 2a). Similarly, early treatment of dnDSA was associated with a decreased the risk of developing subsequent clinical AMR (OR 0.26; 95% CI, 0.11 – 0.64, p <0.01). Baseline demographics did not differ between the two groups (Table 1). There were no differences in the rates of subsequent ACR between early treatment vs no early treatment group (p = 0.52). There were no differences in the rates of infection between the groups (p = 0.99). The mean peak FEV1 values did not differ between the early treatment and no early treatment group (2.524 L vs 2.513 L; p = 0.95). There was no difference in the rates of PGD 3 between the groups (OR 0.55; 95% CI: 0.20 – 1.41; p = 0.27).
Figure 2:
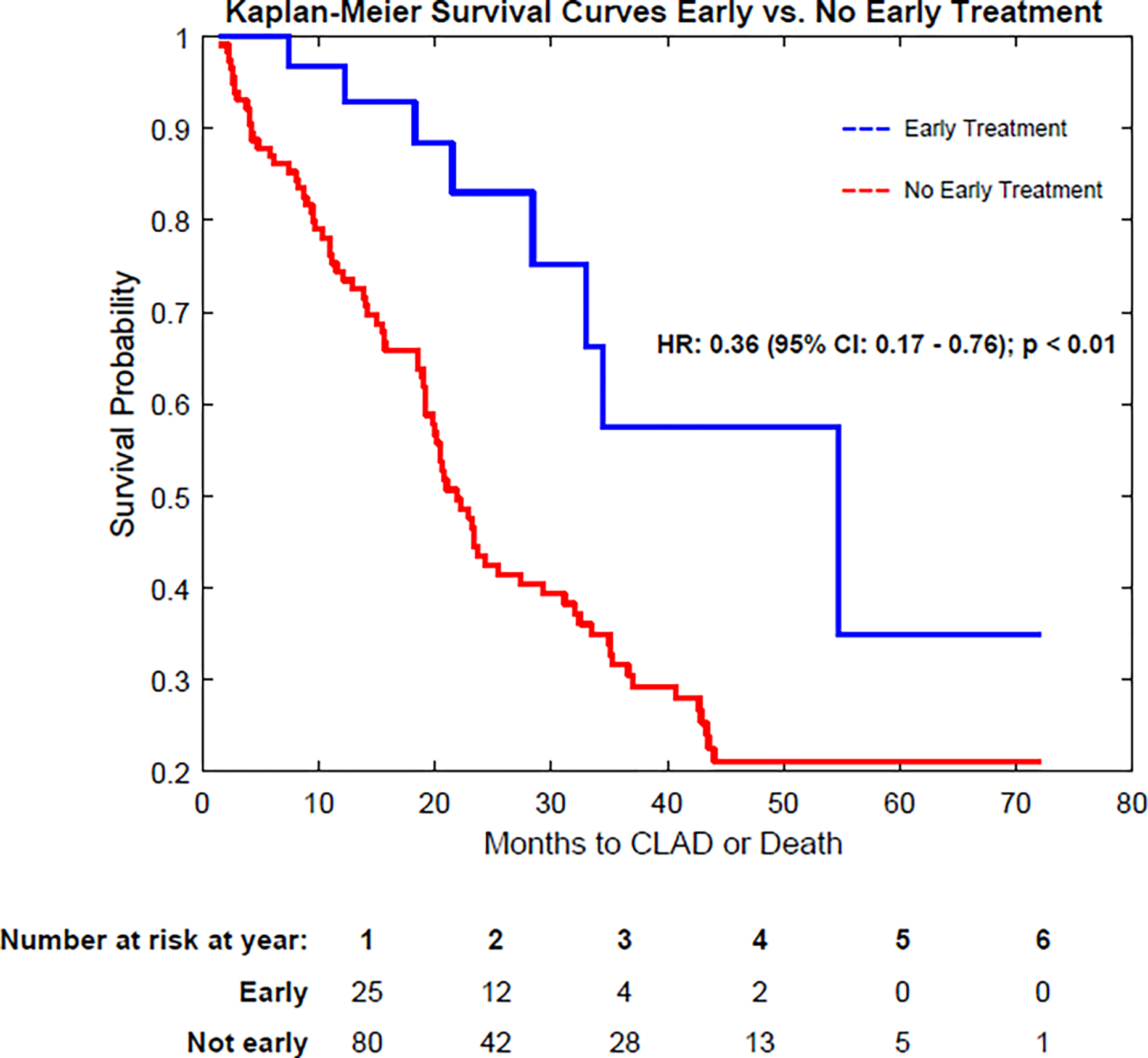
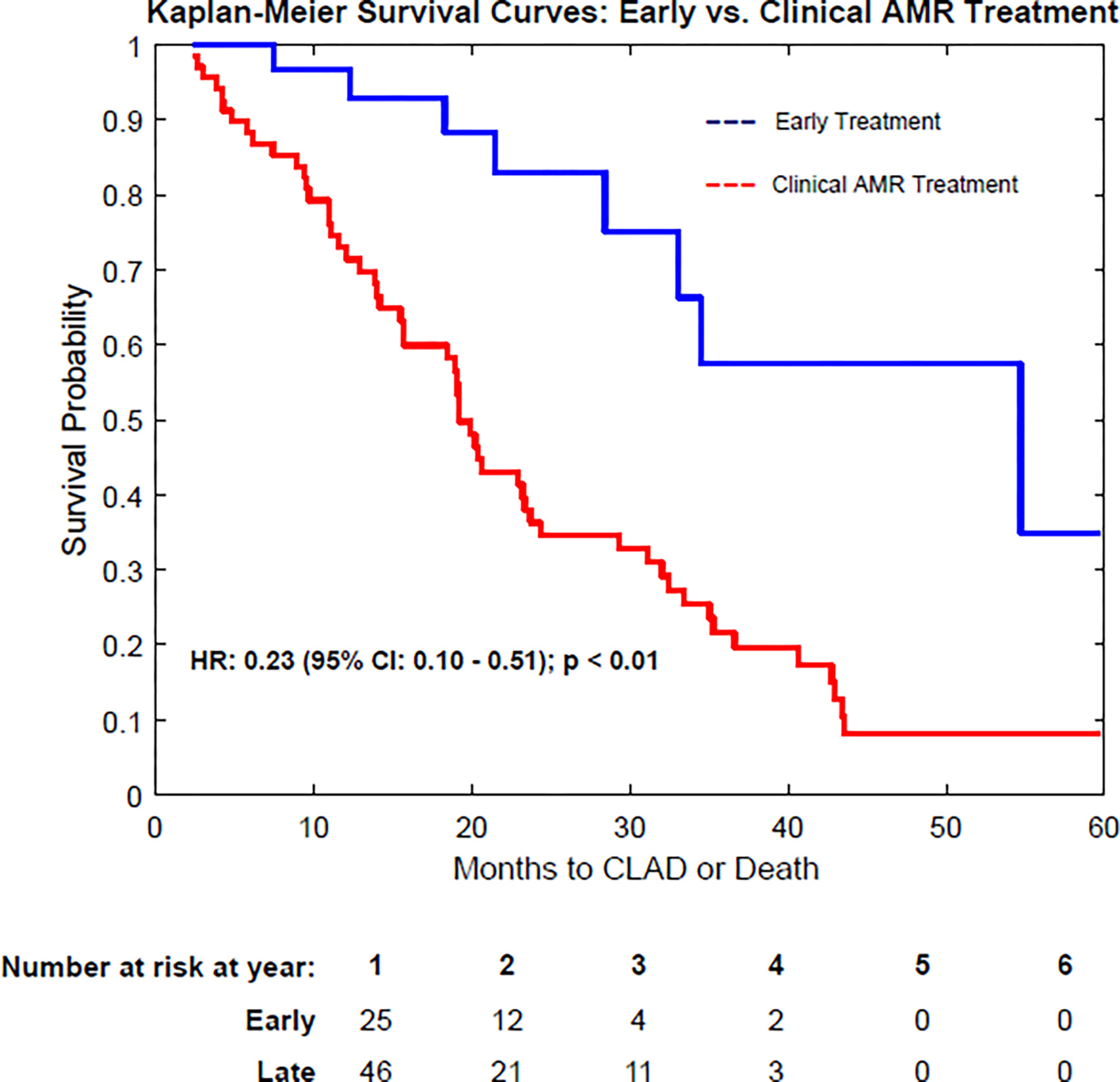
Kaplan-Meier survival curves demonstrating freedom from CLAD or Death in patients receiving early, preemptive treatment of DSA. a) Comparison of early treatment vs. no early treatment. b) Comparison of early treatment vs. Clinical AMR treatment. Note that the “no early treatment” group includes patients that were never treated for dnDSA and patients receiving treatment only in the setting of clinical AMR. CLAD: Chronic Lung Allograft Dysfunction; DSA: Donor Specific Antibody; AMR: Antibody-Mediated Rejection; dnDSA: de novo DSA
Given the differences in time to DSA development between the 2 groups, we performed a sub group analysis comparing the early treatment group vs. the no early treatment group in the subset of patients that developed first dnDSA < 2 months post-transplant. The median time to DSA development was 11 days (IQR 2.3 – 29.8) in the early treatment group (n = 28) and 18 days (13.0 – 32) in the no early treatment group (n = 52). There were also no significant differences in peak lung function, rates of PGD, infection or hospitalization between the two groups. Results from our multivariable Cox Regression model remained similar in that early treatment of dnDSA was associated with a decreased the risk of developing CLAD or death in comparison to not initiating early treatment (HR 0.33; 95% confidence interval (CI), 0.14 – 0.76, p < 0.01). Incorporating the cohort of DSA negative patients into the analysis, the DSA negative group had a lower risk of CLAD or death compared to the early treatment group (HR: 0.46 (95% CI: 0.18 – 1.14); p = 0.09) and the no early treatment group (HR: 0.65 (95% CI: 0.32 – 1.29); p = 0.21), although these did not reach statistical significance (eFigure 1a and 1b).
Within the early treatment arm, the presence of DQ antibodies was independently associated with an increased risk of CLAD or death (HR 5.0; 95% CI, 1.01—24.94, p= 0.049) compared to those in the early treatment arm without DQ antibodies, however, clearance of dnDSA was not associated with the reduced risk of CLAD or death or AMR (p = 0.28) (Table 3). Patients receiving early treatment for dnDSA were more likely to have specificity for HLA-DQ antibodies than those that did not receive early treatment (p <0.01). The decision to provide early treatment for dnDSA varied by center (p < 0.01). Patients that did not receive early dnDSA treatment were also less likely to have received induction therapy (p < 0.01).
Table 3:
Multivariable Cox Regression Analysis for Time to CLAD or Death
| Model | Hazard Ratio (95% CI) | p-value |
|---|---|---|
| Early Treatment vs No Early Treatment of dnDSA (n= 145) | 0.36 (0.17 – 0.76) | <0.01 |
| Early Treatment vs Late Treatment of dnDSA (n=98) | 0.23 (0.10 – 0.51) | < 0.01 |
| Presence of HLA-DQ vs no HLA DQ in Early treatment arm (n=30) | 5.0 (1.01–24.94) | 0.049 |
| Clearance of dnDSA vs No Clearance of dnDSA in Early Treatment arm (n=30) | 3.19 (.38–26.42) | 0.28 |
Early DSA Treatment vs. Clinical AMR Treatment
In a multivariable Cox regression analysis, delaying treatment of dnDSA until the time of clinical manifestations of AMR was associated with a significantly increased risk of CLAD or death (HR 3.00; 95% CI, 1.46—6.18, p < 0.01) (Figure 2b). Median time to CLAD or death was 22.9 months in the early treatment group vs 18.7 months in the late treatment group. There were no differences in rates of ACR or infection between the groups.
Discussion
This multicenter, retrospective cohort study suggests that preemptive treatment of dnDSA prior to the clinical manifestations of AMR in lung transplant patients is associated with a decreased risk of AMR, CLAD and death. Furthermore, delaying treatment of subclinical dnDSA until the development of overt clinical AMR (with signs or symptoms of allograft dysfunction) may increase the risk of CLAD or death. These findings imply that preemptive treatment of dnDSA may be beneficial in lung transplant patients.
Several studies have shown that development of both pre-formed and de-novo anti-HLA antibodies increase the risk of CLAD and death, suggesting that early antibody-directed treatment of DSA could improve outcomes. Prior observational studies have evaluated the impact of treatment of pre-formed and de-novo DSA on clinical outcomes in lung transplant patients. A single-center prospective, cohort study performed by Tinckam et al. consisting of 340 lung transplant recipients demonstrated that patients with preformed DSA treated with an antibody directed desensitization protocol prior to transplantation achieved similar 1-year outcomes as unsensitized patients17. Ius et al. performed a single-center retrospective cohort study evaluating the effects of pre-emptive treatment of early DSA with IVIG on clinical outcomes18. They demonstrated similar 4-year graft survival in patients receiving pre-emptive treatment for DSA in comparison to patients without DSA. Similarly, Hachem et al. performed a prospective cohort study demonstrating that patients who developed DSA and received antibody-directed therapy had similar rates of CLAD and acute rejection as those who did not develop DSA19. These studies were limited by the lack of a control group that developed DSA but were not treated preemptively. Our study supports and further extends the observations of the aforementioned studies by including a control group of patients who developed DSA but did not receive preemptive antibody-directed therapy.
It is notable that the median time to DSA detection was significantly earlier in the early treatment group compared to the no early treatment group (11 days vs. 75 days). The significance of this finding remains unclear but may suggest a difference in post-transplant monitoring between the two groups. Although participating centers in the study performed routine DSA testing on a similar schedule, there may have been a difference between actual surveillance DSA testing between the groups, reflecting differences in post-transplant follow up that may have impacted other surveillance strategies, and therefore, long term outcomes. Despite incorporating time-to-DSA positivity in our multivariable model, residual confounding as a result of this difference may still be present. However, the impact of early dnDSA development on outcomes also remains undefined and several studies have demonstrated an association of early dnDSA development and poor long-term outcomes (including DSA that develops during the index hospitalization and at 1 month post-transplant)7, 12. Further, the results of our study remained consistent in the subgroup analysis comparing Early Treatment vs. No Early Treatment in the subgroup of patients that first developed dnDSA at similar time periods after transplant (< 2 months – Median 11 days vs 18 days in Early Treatment vs No Early Treatment respectively).
The mechanism by which preemptive treatment of DSA may improve clinical outcomes remains unclear. A prior single-center observational cohort study demonstrated that the development of overt clinical AMR may result in severe allograft dysfunction with high mortality rates and progression to CLAD20. Given that the development of DSA may increase the risk of developing AMR, preventing the progression to clinical AMR, by preemptively treating subclinical dnDSA prior to the manifestations of allograft dysfunction, may be vital to improving downstream outcomes. It is notable that clearance of DSA in the early treatment group was not associated with improved clinical outcomes in our study. This contrasts with previous findings suggesting that clearance of DSA with preemptive treatment was associated with decreased risk of BOS and improved survival19. Future research is required to further investigate the immunobiological mechanisms by which preemptive anti-body directed therapy may improve clinical outcomes, even in the absence of DSA clearance.
While our study provides novel insight into the clinical implications of early treatment of dnDSA in lung transplantation, it is subject to several important limitations. This was a retrospective analysis consisting of pooled data from 3 separate cohorts subject to the inherent risk of selection bias and the presence of missing data. The sample size was small (n=145), particularly in the early treatment group (n=30), which may obscure the true effect sizes of our outcomes. Although all patients were enrolled in the post-LAS era, the study period spanned over 12 years, raising the potential for era bias in favoring outcomes towards more recent cohorts who received the majority of early treatment. Adequate DSA, CLAD or death data was missing in approximately 20% of our cohort and thus excluded, leaving the potential for significant selection bias (although we considered this data missing at random). This was not a randomized controlled trial, as such, residual confounding may exist despite multivariable analysis. The decision to treat was based on provider discretion and may have been influenced by pragmatic factors such as insurance coverage and logistic feasibility, providing an additional element of selection bias. In addition, antibody-directed treatment regimens varied widely both within and between centers resulting in an additional element of confounding, even despite adjusting for center variation in the multivariable analysis. Thus, we cannot rule out with certainty that center-specific practices concerning preemptive treatment of dnDSA are simply a proxy for other beneficial practices that may drive improved outcomes such as more aggressive induction therapy or maintenance immunosuppression. Furthermore, the variation in treatment protocols makes it difficult to ascertain precisely which treatment protocols, as well as specific elements of the treatment protocols, were responsible for driving improved outcomes. Several patients were treated with IVIG alone, and although the therapeutic efficacy of IVIG alone as a means of antibody directed therapy remains unclear, similar regimens have been employed in other high-volume transplant centers7, 12, 19. IVIG monotherapy has also demonstrated similar or superior efficacy to other treatment regimens in the setting of other autoimmune diseases such as Guillan Barre Syndrome and chronic inflammatory demyelinating polyneuropathy21–23.
Center and provider variability in the management of immunosuppression, including induction therapy, also exists. While each center in our study utilized a similar definition of dnDSA positivity, this definition is not standardized and may differ by center, limiting the generalizability of our results. We did not include data on specific MFI strength or C1q assay positivity. The decision to institute antibody directed therapy for preemptive treatment of dnDSA should consider the inherent risks of infection, cost of treatment and medication/procedural side effects. Given these limitations, our results should be viewed as hypothesis generating and underscore the need for further investigation, including a randomized controlled trial evaluating the clinical consequences of preemptive treatment of dnDSA in lung transplant patients.
In conclusion, among lung transplant patients who developed dnDSA, preemptive treatment of dnDSA was associated with a risk of AMR and CLAD or death. Further investigation is required in order to determine the appropriate timing and optimal treatment regimen in the treatment of dnDSA in lung transplant patients.
Supplementary Material
Acknowledgements
We would like to thank Kelly Byrnes for her help in constructing the figures and tables for this manuscript.
Abbreviations
- ACR
Acute Cellular Rejection
- ALAD
Acute Lung Allograft Dysfunction
- AMR
Antibody Mediated Rejection
- AR
Acute Rejection
- CLAD
Chronic Lung Allograft Dysfunction
- dnDSA
de novo Donor Specific Antibody
- DSA
Donor Specific Antibody
- FEV1
Forced Expiratory Volume in One Second
- HLA
Human Leukocyte Antigen
- IVIG
Intravenous Immune Globulin
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmer SM, Davis RD, Hadjiliadis D, et al. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation. Sep 27 2002;74(6):799–804. doi: 10.1097/00007890-200209270-00011 [DOI] [PubMed] [Google Scholar]
- 2.Snyder LD, Wang Z, Chen DF, et al. Implications for human leukocyte antigen antibodies after lung transplantation: a 10-year experience in 441 patients. Chest. Jul 2013;144(1):226–233. doi: 10.1378/chest.12-0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girnita AL, McCurry KR, Iacono AT, et al. HLA-specific antibodies are associated with high-grade and persistent-recurrent lung allograft acute rejection. J Heart Lung Transplant. Oct 2004;23(10):1135–41. doi: 10.1016/j.healun.2003.08.030 [DOI] [PubMed] [Google Scholar]
- 4.Girnita AL, Duquesnoy R, Yousem SA, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. Jan 2005;5(1):131–8. doi: 10.1111/j.1600-6143.2004.00650.x [DOI] [PubMed] [Google Scholar]
- 5.Hachem RR, Kamoun M, Budev MM, et al. Human leukocyte antigens antibodies after lung transplantation: Primary results of the HALT study. Am J Transplant. Sep 2018;18(9):2285–2294. doi: 10.1111/ajt.14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrell M R, Pilewski JM, Gries CJ, et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. The Journal of Heart and Lung Transplantation. 2014;33(12):1288–1294. doi: 10.1016/j.healun.2014.07.018 [DOI] [PubMed] [Google Scholar]
- 7.Ius F, Sommer W, Tudorache I, et al. Early donor-specific antibodies in lung transplantation: risk factors and impact on survival. J Heart Lung Transplant. Dec 2014;33(12):1255–63. doi: 10.1016/j.healun.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 8.Verleden SE, Vanaudenaerde BM, Emonds M-P, et al. Donor-specific and -nonspecific HLA antibodies and outcome post lung transplantation. European Respiratory Journal. 2017;50(5):1701248. doi: 10.1183/13993003.01248-2017 [DOI] [PubMed] [Google Scholar]
- 9.Sundaresan S, Mohanakumar T, Smith MA, et al. HLA-A locus mismatches and development of antibodies to HLA after lung transplantation correlate with the development of bronchiolitis obliterans syndrome. Transplantation. Mar 15 1998;65(5):648–53. doi: 10.1097/00007890-199803150-00008 [DOI] [PubMed] [Google Scholar]
- 10.Morrell MR, Pilewski JM, Gries CJ, et al. De novo donor-specific HLA antibodies are associated with early and high-grade bronchiolitis obliterans syndrome and death after lung transplantation. J Heart Lung Transplant. Dec 2014;33(12):1288–94. doi: 10.1016/j.healun.2014.07.018 [DOI] [PubMed] [Google Scholar]
- 11.Tikkanen JM, Singer LG, Kim SJ, et al. De Novo DQ Donor-Specific Antibodies Are Associated with Chronic Lung Allograft Dysfunction after Lung Transplantation. Am J Respir Crit Care Med. Sep 1 2016;194(5):596–606. doi: 10.1164/rccm.201509-1857OC [DOI] [PubMed] [Google Scholar]
- 12.Le Pavec J, Suberbielle C, Lamrani L, et al. De-novo donor-specific anti-HLA antibodies 30 days after lung transplantation are associated with a worse outcome. J Heart Lung Transplant. Sep 2016;35(9):1067–77. doi: 10.1016/j.healun.2016.05.020 [DOI] [PubMed] [Google Scholar]
- 13.Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant. May 2019;38(5):493–503. doi: 10.1016/j.healun.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 14.Levine DJ, Glanville AR, Aboyoun C, et al. Antibody-mediated rejection of the lung: A consensus report of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. Apr 2016;35(4):397–406. doi: 10.1016/j.healun.2016.01.1223 [DOI] [PubMed] [Google Scholar]
- 15.Agbor-Enoh S, Jackson AM, Tunc I, et al. Late manifestation of alloantibody-associated injury and clinical pulmonary antibody-mediated rejection: Evidence from cell-free DNA analysis. J Heart Lung Transplant. Jul 2018;37(7):925–932. doi: 10.1016/j.healun.2018.01.1305 [DOI] [PubMed] [Google Scholar]
- 16.Iasella CJ, Ensor CR, Marrari M, et al. Donor-specific antibody characteristics, including persistence and complement-binding capacity, increase risk for chronic lung allograft dysfunction. J Heart Lung Transplant. Dec 2020;39(12):1417–1425. doi: 10.1016/j.healun.2020.09.003 [DOI] [PubMed] [Google Scholar]
- 17.Tinckam KJ, Keshavjee S, Chaparro C, et al. Survival in sensitized lung transplant recipients with perioperative desensitization. Am J Transplant. Feb 2015;15(2):417–26. doi: 10.1111/ajt.13076 [DOI] [PubMed] [Google Scholar]
- 18.Ius F, Verboom M, Sommer W, et al. Preemptive treatment of early donor-specific antibodies with IgA- and IgM-enriched intravenous human immunoglobulins in lung transplantation. Am J Transplant. Sep 2018;18(9):2295–2304. doi: 10.1111/ajt.14912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hachem RR, Yusen RD, Meyers BF, et al. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant. Sep 2010;29(9):973–80. doi: 10.1016/j.healun.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witt CA, Gaut JP, Yusen RD, et al. Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant. Oct 2013;32(10):1034–40. doi: 10.1016/j.healun.2013.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Meché FG, Schmitz PI. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain-Barré syndrome. Dutch Guillain-Barré Study Group. N Engl J Med. Apr 23 1992;326(17):1123–9. doi: 10.1056/nejm199204233261705 [DOI] [PubMed] [Google Scholar]
- 22.Eftimov F, Winer JB, Vermeulen M, de Haan R, van Schaik IN. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database Syst Rev. Dec 30 2013;(12):Cd001797. doi: 10.1002/14651858.CD001797.pub3 [DOI] [PubMed] [Google Scholar]
- 23.Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome. The Lancet. 1997/January/25/1997;349(9047):225–230. doi: 10.1016/S0140-6736(96)09095-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


