Abstract
Background:
Transesophageal echocardiograms (TEEs) performed during transcatheter structural cardiac interventions may have higher complications than those performed in the non-operative setting or even those performed during cardiac surgery. However, there are limited data on complications associated with TEE during these procedures. We evaluated the prevalence of major complications among these patients in the United States (US).
Methods:
A retrospective cohort study was conducted using an electronic health record database (TriNetX Research Network) from large academic medical centers across the US for patients undergoing TEE during transcatheter structural interventions from January 2012 to January 2022. Using the American Society of Echocardiography endorsed ICD-10 codes, patients undergoing TEE during a transcatheter structural cardiac intervention, including transaortic, mitral or tricuspid valve repair, left atrial appendage occlusion, atrial septal defect closure, patent foramen ovale closure, and paravalvular leak repair were identified. The primary outcome was major complications within 72 hours of the procedure (composite of bleeding, esophageal and upper respiratory tract injury). The secondary aim was the frequency of major complications, death, or cardiac arrest within 72 hours of patients who completed intraoperative TEE during surgical valve replacement.
Results:
Among 12,043 adult patients (mean age: 74 years old, 42% females) undergoing TEE for transcatheter structural cardiac interventions, 429 (3.6%) patients had a major complication. Complication frequency was higher in patients on anticoagulation or antiplatelet therapy compared with those not on therapy (3.9% vs. 0.5%, RR: 8.09, p < 0.001). Compared with those aged <65 years, patients aged ≥ 65 years had a higher frequency of major complications (3.9% vs. 2.2%, RR: 1.75, p < 0.001). Complication frequency was similar among males and females (3.5% vs 3.7%, RR: 0.96, p = 0.67). Among 28,848 patients who completed surgical valve replacement with TEE guidance, 728 (2.5%) suffered a major complication.
Conclusions:
This study found that more than 3% of patients undergoing TEE during transcatheter structural cardiac interventions have a major complication which is more common among those on anticoagulant or antiplatelet therapy or who were elderly. With a shift of poor surgical candidates to less invasive percutaneous procedures, the future of TEE-guided procedures relies on comprehensive risk discussion and updating practices beyond conventional methods to minimize risk for TEE-related complications.
Introduction
Transesophageal echocardiograms (TEE) allow for higher-resolution images of posterior cardiac structures compared with transthoracic echocardiograms (TTE), and their diagnostic capabilities have expanded to improved visualization of valvular pathology, workup of recurrent strokes, and evaluation for thromboembolic risk prior to cardioversion in atrial fibrillation.1–3 More recently, the role has evolved beyond simple image acquisition to real-time intraoperative visualization utilized to guide percutaneous structural interventions such as transcatheter aortic valve replacement (TAVR), transcatheter mitral valve repair (MVR), transcatheter tricuspid valve repair (TVR), left atrial appendage occlusion (LAAO), atrial septal defect (ASD) repair, ventricular septal defect (VSD) repair, patent foramen ovale (PFO) repair, and paravalvular leak repair (PVLR).3–6 TEE has been considered a generally safe procedure with the frequency of major complications ranging from 0.2–1.4% in both operative and non-operative situations; however, it does carry an inherent risk as an invasive imaging technique.7,8 Historically, complications during TEE have been rare injuries to the gastrointestinal tract because of direct mechanical trauma.9,10 With the growing role in structural cases, complications have been more frequently reported secondary to constant probe manipulation needed during procedures.11,12
There is limited contemporary literature detailing the frequency of complications associated with TEE in transcatheter structural cardiac interventions. The potentially increased risk for TEE-related complications is concerning as we continue to see a dynamic shift to completing percutaneous procedures on these previously poor surgical candidates.13 Over 38,000 LAAOs were completed from January 2016 to December 2018, and over 11,000 transcatheter MVRs were completed in 2019 in the United States.14,15 While smaller studies outside of the United States have reviewed this, the frequency of TEE-related complications for transcatheter structural cardiac interventions in the United States has not been previously described.11,12 Additionally, studies have not previously contrasted the frequency of TEE-related complications from intraoperative TEE in cardiac surgery.
The current study aimed to evaluate the short-term complications for United States adult patients undergoing TEE for transcatheter interventions that include TAVR, MVR, TVR, LAAO, PVLR, ASD, VSD, and PFO closure. It also aimed to contrast the frequency of complications seen during cardiac surgery with intraoperative TEE guidance as well.
Methods
Data Source
We used the TriNetX (Cambridge, MA) Research Network database for this study.16–22 This federated health research network database uses a combination of natural language processing and standardized clinical data entries to integrate electronic health records from multiple institutions into a cloud-based aggregate of nearly 59 million patients. The health entities contributing to the database were composed of approximately 75% academic medical centers and 25% community practices. The data are de-identified at the patient and organization levels to ensure patient privacy and Health Insurance Portability and Accountability Act (HIPAA) compliance. This dataset includes lab values, medications, and procedures for analysis.23 The dataset undergoes a rigorous quality assessment to ensure adequate data representation and remove incomplete records.22 The TriNetX software monitors the temporal trend of data volume to ensure data validity. Additionally, The TriNetX database allows for the comparison of data across several databases to ensure that referential integrity is maintained.16–20,24 The TriNetX database uses the International Statistical Classification of Diseases and Related Health Problems Clinical Modifications 10th Edition (ICD-10-CM) to identify patients’ diagnoses. The database uses Current Procedural Terminology (CPT) codes to record procedures. Finally, the dataset uses the standard Logical Observation Identifiers Names and Codes (LOINC) to capture lab values.16–20, 22 Group-level data are available to researchers at participating healthcare organizations at www.trinetx.com. The ethical oversight for this study was provided by the University of Alabama at Birmingham (UAB) Institutional Review Board.
Patient Population
Our analysis included patients undergoing TEE for transcatheter interventions (TAVR, MVR, TVR, LAAO, PVLR, ASD, VSD, and PFO closure) and traditional valve surgery [MVR, TVR, PVR (pulmonary valve repair), and SAVR (surgical aortic valve replacement)] between January 2012 and January 2022 at medical centers in the United States. The TriNetX Research Network includes large academic medical centers including UAB.24 Using the American Society of Echocardiography approved ICD-10 and CPT codes (93355) (Table 1), an aggregated patient population of patients undergoing a TEE-guided transcatheter intracardiac or great vessel structural intervention was identified for this analysis.25,26 As a secondary analysis, a separate aggregated cohort of patients who completed an intraoperative TEE on the same day of cardiac surgery was identified. First a group of patients who completed an intraoperative TEE using approved American Society of Echocardiography CPT codes (93312–5; 93317–8; 93320–1; 93325) was identified. Afterward, we matched the date of the TEE to the date of the cardiac surgery by using CPT codes corresponding to aortic valve (33400–1; 33403; 33405–6; 33410–7), mitral valve (33420; 33422; 33425–7; 33430), pulmonic valve (33463–4), and tricuspid valve (33475) surgical procedures.27 The TEE was considered intraoperative if the date of the TEE claim was performed on the same calendar day as the cardiothoracic surgical procedure.
Table 1:
Periprocedural Transesophageal Echocardiography Related Outcomes in the Study Cohort
| Clinical outcome | ICD-10-CM code | % ( n ) |
|---|---|---|
| Death | 0.58 (70) | |
| Cardiac arrest | I46 | 1.74 (210) |
| Major complications as defined below: | 3.6 (429) * | |
| Respiratory system: | ||
| Intraoperative hemorrhage and hematoma of a respiratory system organ or structure complicating a procedure | J95.6 | 0.3 (41) |
| Postprocedural hemorrhage of a respiratory system organ or structure following a procedure | J95.83 | 0.4 (51) |
| Gastrointestinal system: | ||
| Other specified disease of the esophagus | K22.8 | 0.1 (13) |
| Postprocedural hemorrhage of a digestive system organ or structure following a procedure | K91.84 | 0.4 (50) |
| Gastrointestinal hemorrhage, unspecified | K92.2 | 2.8 (338) |
| Hemorrhage from throat | R04.1 | 0.03 (4) |
| Hemorrhage from other sites in respiratory passages | R04.89 | 0.1 (13) |
| Unspecified injury of esophagus (thoracic part) | S27.819 | 0 (0) |
| Unspecified open wound of pharynx and cervical esophagus | S11.20 | 0 (0) |
| Perforation of esophagus | K22.3 | 0.02 (2) |
Due to individual patients with multiple complications from TEE, the sum of the major complications is not equal to the number of major complications seen in our patient population.
The TriNetX Database was queried for applicable patients within the past decade as HIPAA-covered entities had largely transitioned to ICD-10 coding by January 2012.28 Additionally, devices utilized in transcatheter structural cardiac interventions became approved by the Food and Drug Administration (FDA) during 2012 as well, with TAVR for symptomatic aortic stenosis becoming approved in late 2011.29 The patient population who completed a TEE-guided percutaneous intervention was further stratified based on age (ages ≤64 years old or ≥ 65 years old), sex (male or female), and if they were taking either anticoagulation or an antiplatelet within a week of their procedure. Temporal trends of major complications were identified by comparing the frequency of major complications for the first five years of the decade versus the second half (January 1st, 2012 to January 1st, 2022).
Measures and Outcomes:
After developing our patient population for analysis, we identified their baseline characteristics, including past medical history, medications, and laboratory values on the day of the procedure. We defined a major complication as suffering from one of the following: 1) intraoperative hemorrhage and hematoma of a respiratory system organ or structure complicating a procedure [ICD-10: J95.6], 2) postprocedural hemorrhage of a respiratory system organ or structure following a procedure [J95.83], 3) postprocedural hemorrhage of a digestive system organ or structure following a procedure [K91.84], 4) gastrointestinal hemorrhage, unspecified [K92.2], 5) Other specified diseases of the esophagus [K22.8], 6) hemorrhage from throat [R04.1], 7) hemorrhage from other sites in respiratory passages [R04.89], 8) unspecified injury of esophagus (thoracic part) [S27.819], 9) unspecified open wound of the pharynx and cervical esophagus [S11.20], and 10) perforation of the esophagus [K22.3]. The day of the procedure was defined as the index event. The primary study outcome was any major complication within 72 hours of the procedure. We also identified the frequency of deaths and cardiac arrest within 72 hours of the procedure as well. A secondary analysis assessed during this study was comparing the frequency of major complications (as previously defined) from intraoperative TEE during surgical valvular procedures. An additional secondary analysis involved assessing temporal trends regarding the frequency of major complications. Each procedure was counted as one separate event so patients who completed multiple procedures between the first half of the decade and the second half were included multiple times in the denominator of total cases. We also evaluated the frequency of major complications between all geographic regions. The individual codes used in the study for the definition of a major complication are listed in Table 1.
Statistical Analysis:
This is a retrospective cross-sectional analysis of patients who completed a TEE-guided transcatheter structural cardiac intervention. The baseline characteristics for the patient population were reported as a mean ±standard deviation for continuous data and as numbers and percentages for categorical data. The study outcomes were reported as comparisons between age, sex, and patients on anticoagulation or antiplatelets. Additional comparisons were reported between the patient population who completed a TEE-guided percutaneous intervention versus a TEE-guided surgical intervention. Characteristics of continuous data were compared using an independent-sample t-test. Categorical variables, such as age or ethnicity, were compared using a Pearson chi-square test. All primary and secondary outcomes were reported in both the overall patient population and within each subset. Logistic regression was performed to assess the odds of TEE-related complications for the sub-group stratification by sex, age (<65 vs. ≥65 years), and medication (antiplatelet and anticoagulant use). The TriNetX Analytic program utilizes a combination of JAVA, R, and Python for statistical analyses.16–20,22–24 The study outcomes are reported as estimates (odds ratios and risk ratios) with 95% confidence intervals. For all analyses, a 2-sided type I error of 0.05 was considered statistically significant.30
Results
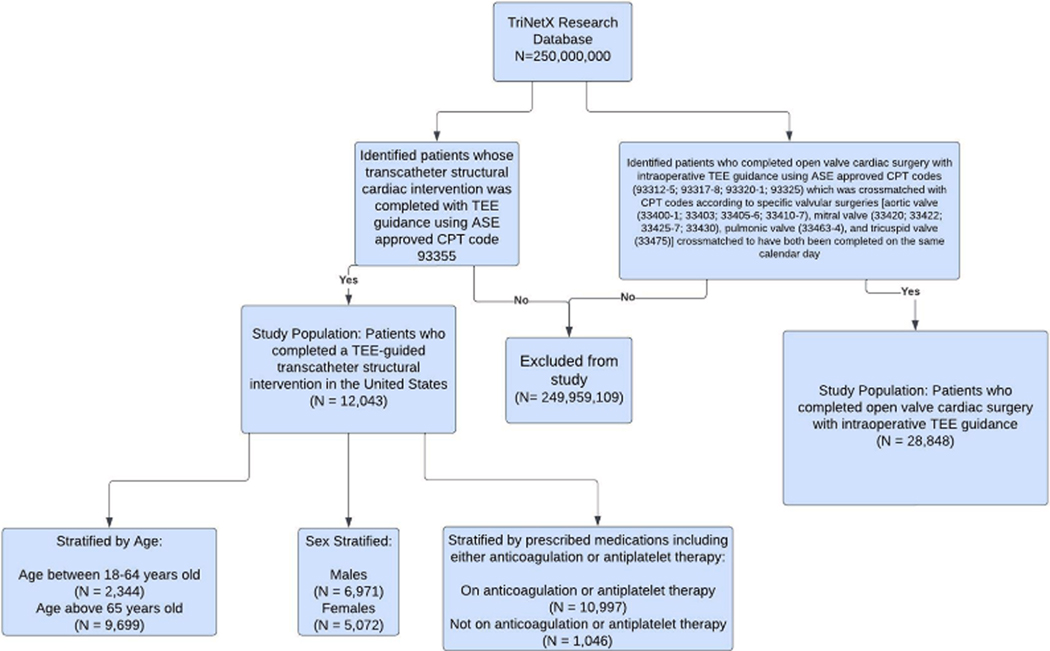
We identified 12,043 adult patients who underwent a TEE-guided structural intervention (TAVR, MVR, TVR, LAAO, PVLR, ASD, or PFO closure) between January 2012 and January 2022. Figure 1 describes the derivation of the study population and the separate cohort of patients who completed TEE-guided valvular cardiac surgeries. Overall, the geographic distribution of the study population, determined by the location of the health care organization headquarters, was composed of 43.9% ( n = 5,284) of patients from the South, 39.5% ( n = 4,756) from the Northeast, 8.3% ( n = 995) from the Midwest, and 2.8% ( n = 336) from the West; 5.6% ( n = 672) of patients were unable to be identified as being from a specific geographic region. The baseline clinical demographics of the study population are summarized in Table 2. The study population had a mean age of 74 ± 14 years, 57.8% ( n = 6,971) were male, and 83.6% ( n = 10,062) were White. At baseline, there was a relatively high prevalence of comorbidities such as hypertension (73.7%), ischemic heart disease (65%), heart failure (53.4%), mitral valve disease (48%), aortic valve disease (47%), and tricuspid valve disease (25%). The baseline lab parameters for the study population are described in Table 3.
Figure 1: Derivation of the Study Population.
The TriNetX database was queried for applicable patients by using the American Society of Echocardiography-approved ICD-10 and CPT codes. After developing a study population from the database, they were further sub-stratified by age, sex, and prescribed medications. A separate cohort of patients who completed cardiac valve surgery with TEE guidance was also identified.
Table 2:
Baseline Characteristics of the Study Cohort
| Clinical characteristic | Value |
|---|---|
| Age at index, years | 70.2 ± 14.5 |
| Gender: | |
| Male | 6,971 (57.8) |
| Female | 5,072 (42.2) |
| Ethnicity: | |
| Not Hispanic or Latino | 9,960 (82.7) |
| Unknown | 1,585 (13.2) |
| Hispanic/Latino | 498 (4.1) |
| Race: | |
| White | 10,062 (83.6) |
| Black or African American | 1,100 (9.1) |
| Unknown | 742 (6.2) |
| Asian | 110 (0.9) |
| American Indian or Alaska Native | 22 (0) |
| Native Hawaiian or Other Pacific Islander | 10 (0) |
| Diagnosis: | |
| Essential (primary) hypertension | 8,870 (73.7) |
| Coronary artery disease | 7,472 (62.0) |
| Heart failure | 6,436 (53.4) |
| Cerebrovascular diseases | 4,767 (39.6) |
| Neoplasms | 4,412 (36.6) |
| Medications: | |
| Anticoagulants | 9,239 (76.7) |
| Beta-blockers | 8,603 (71.4) |
| Antiplatelets | 8,337 (69.2) |
| Angiotensin-converting enzyme inhibitors/angiotensin II inhibitor | 8,327 (69.1) |
| Antilipemic drugs | 8,069 (67.0) |
| Vitals: | |
| Heart rate | 75 ± 15.7 |
| BMI | 29.4 ± 6.7 |
| Systolic blood pressure | 129 ± 20.6 |
| Diastolic blood pressure | 71.7 ± 12.1 |
Table 3:
Laboratory Measures in the Study Cohort
| Characteristics | Mean +/− SD | Reference |
|---|---|---|
| Hematologic Parameters | ||
| Leukocytes (Cells × 103/μL) | 7.54 ± 3.35 | 4.3 to 10.8 (Cells × 103/μL) |
| Hemoglobin (g/dL) | 12.3 ± 2.12 | 12 to 17 g/dL |
| Platelets (Cells × 103/μL) | 212 ± 78.9 | 150 to 400 (Cells × 103/μL) |
| Liver Function | ||
| Alanine aminotransferase (IU/L) | 28.5 ± 89.8 | 10 to 40 IU/L |
| Albumin (g/dL) | 3.91 ± 0.91 | 3.5 to 5.5 g/dL |
| Alkaline phosphatase (IU/L) | 90.2 ± 49.9 | 30 to 120 IU/L |
| Aspartate aminotransferase (IU/L) | 35.8 ± 259 | 10 to 40 IU/L |
| Bilirubin total (mg/dL) | 0.77 ± 0.867 | 0.3 to 1.0 mg/dL |
| Protein (g/dL) | 6.86 ±0.76 | 5.5 to 9.0 g/dL |
| Renal Function | ||
| Creatinine (mg/dL) | 1.42 ± 2.78 | 0.8 to 1.2 mg/dL |
| Blood urea nitrogen (mg/dL) | 24.5 ± 14.7 | 6 to 20 mg/dL |
| Electrolytes | ||
| Calcium (mg/dL) | 9.26 ± 0.592 | 8.5 to 10.2 mg/dL |
| Potassium (mEq/L) | 4.25 ± 0.471 | 3.7 to 5.2 mEq/L |
| Sodium (mEq/L) | 139 ± 3.33 | 136 to 144 mEq/L |
| Bicarbonate (mEq/L) | 26.6 ± 3.5 | 23 to 29 mEq/L |
| Coagulation Function | ||
| Activated Partial Thromboplastin | 36.7 ± 16.8 | 30 to 50 seconds |
| Time (s) | ||
| Prothrombin time (s) | 15.2 ± 5.77 | 12 to 13 seconds |
| INR | 1.36 ± 1.26 | 0.8 to 1.2 |
| Endocrine | ||
| A1c (%) | 6.29 ±1.51 | 4 to 5.6% |
Laboratory values are presented as an average mean with one standard deviation.
In the overall cohort, 3.6% (n = 429) suffered a major complication as defined above within 72 hours of their procedure. Table 1 shows the number of events stratified by each major individual complication. Additionally, 0.6% (70) of patients died, and 1.7% (210) suffered a cardiac arrest within 72 hours after their TEE-guided transcatheter structural cardiac intervention. The most frequent major complication reported following the procedure was gastrointestinal hemorrhage, with 338 (79%) documented as having suffered from this. Within this group, 6.5% (22) suffered a clinically significant bleed requiring transfusion within 24 hours of their procedure; the rest of the group suffered a minor bleed not requiring transfusion. Within our cohort of 429 patients who suffered a major complication; 0.9% (3) died and 6.1% (26) suffered a cardiac arrest within 72 hours of their procedure. Compared to the cohort who did not suffer a major complication, only 0.6% (67) died and 1.6% (184) suffered a cardiac arrest within 72 hours of their procedure. Thus, patients who suffered a TEE complication had a statistically significant increased risk of cardiac arrest (RR: 3.98 [95% CI, 2.57 – 5.70], p < 0.001) but not death (RR: 1.26 [95% CI, 0.40 – 3.98], p = 0.37).
In the sex-stratified comparison of the study population, 6,971 males and 5,072 females were identified. The frequency of major complications was 3.5% (244/6,971) in males and 3.7% (185/5,072) in females (RR: 0.96 [95% CI, 0.80 –1.16], p = 0.67). The risk of suffering cardiac arrest within 72 hours of their intervention was not statistically significant (RR: 0.90 [95% CI, 0.69 – 1.18], p = 0.43) between males (1.7%, 116/6,971) and females (1.9%, 94/5,072). The risk of suffering death within 72 hours of their intervention was lower between males (0.5%, 33/6,971) and females (0.8%, 37/5,072) although not statistically significant (RR: 0.65 [95% CI, 0.41 – 1.04], p = 0.07).
In the age-stratified analysis, there was a higher risk for major complications seen in patients ≥ 65 years old. Our study population identified 9,699 patients ≥ 65 years old and 2,344 patients between the ages of 18–64 years. In the older age group, the frequency of major complications was 3.9% (377/9,699) compared with the middle-aged and younger patients group, wherein the frequency was 2.2% (52/2,344) (RR: 1.75 [95% CI, 1.32 – 2.33], p < 0.001). The risk of suffering death within 72 hours of their intervention was higher in the older age group (0.7%, 65/9,669) versus the younger age group (0.2%, 5/2,344) and the relative risk was statistically significant (RR: 3.14 [95% CI, 1.27 – 7.80], p = 0.01). The risk of suffering cardiac arrest within 72 hours of their intervention was not statistically significantly different between the younger (2.0%, 47/2,344) and the older group (1.7%, 163/9,699) (RR: 0.84 [95% CI, 0.60 – 1.60], p = 0.28).
There were 10,997 (91.3%) patients completing a TEE-guided transcatheter structural intervention who were on anticoagulation or an antiplatelet within seven days of their procedure. In patients on anticoagulation or antiplatelet therapy, there was a higher frequency of major complications (3.9%) compared with those not on anticoagulation or antiplatelets (0.5% (RR: 8.09 [95%, CI: 3.36 – 19.5], p < 0.001). The risk of suffering cardiac arrest within 72 hours of their intervention was not statistically significant (RR: 1.44 [95% CI, 0.83 – 2.52], p = 0.20) between patients on anticoagulation or antiplatelet therapy (1.8%, 197/10,997) and patients who were not (1.2%, 13/1,046). The risk of suffering death within 72 hours of their intervention was similar (RR: 1.24 [95% CI, 0.50 – 3.07], p = 0.13).
There were 28,848 patients identified who completed cardiac surgeries with intraoperative TEE guidance. In this group, the frequency of suffering a major complication was 2.5% (728). Notably, the relative risk of suffering a major complication related to TEE-guided percutaneous interventions was higher compared to intraoperative TEE guidance during cardiac surgery (RR: 1.41 [95% CI, 1.26–1.59], p < 0.001). In contrast, the relative risk of suffering death within 72 hours was lower in the group who completed a TEE-guided percutaneous intervention versus the group who completed cardiac surgeries (RR: 0.76 [95% CI, 0.58 – 0.997], p = 0.05). The relative risk of suffering from cardiac arrest in the TEE-guided percutaneous intervention group was similar when compared to the cardiac surgery group (RR: 1.0 [95% CI, 0.85 –1.17], p=0.99; Figure 2).
Figure 2: Comparing Frequency of Major Complications, Cardiac Arrest, and Death within 72 hours of Intervention between TEE used during transcatheter interventions and intraoperative TEE during cardiac valve surgeries.
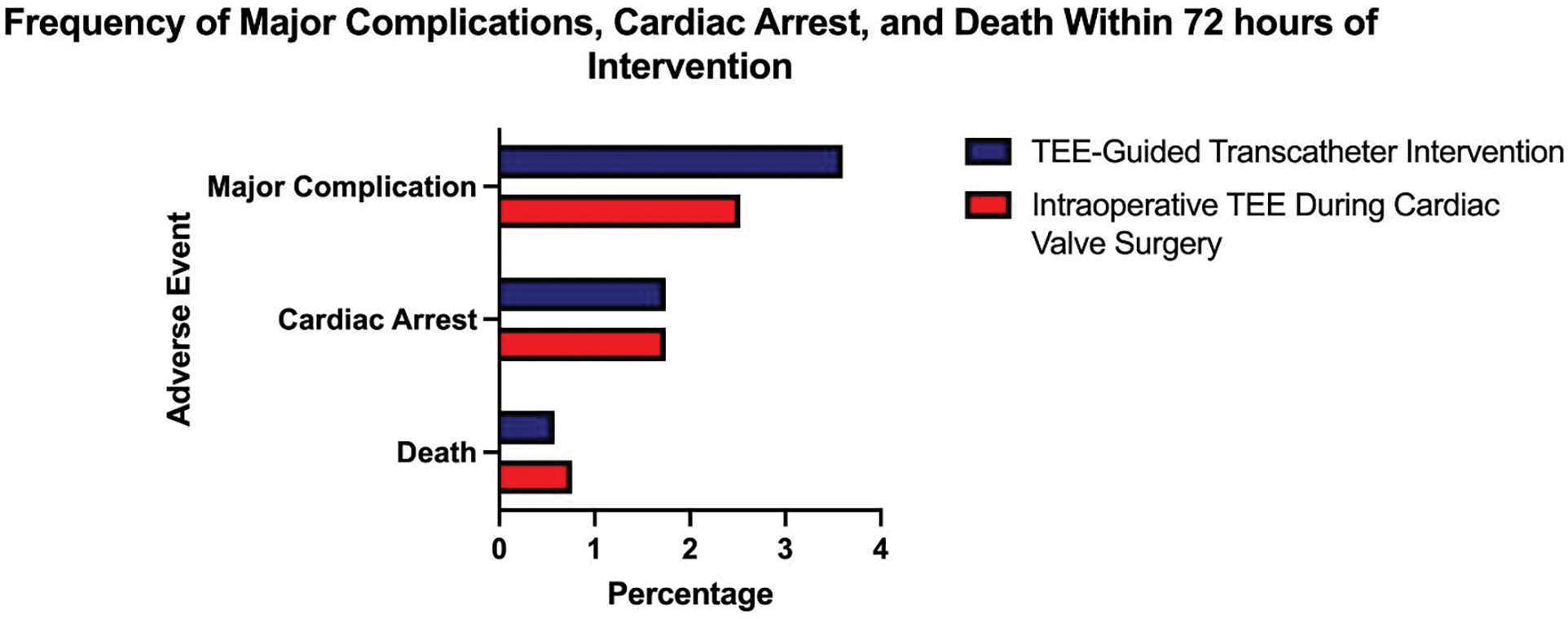
The frequency of major complications, cardiac arrests, and death within 72 hours of a TEE-guided transcatheter intervention versus intraoperative TEE during cardiac valve surgery.
When reviewing temporal trends of major complications over the past ten years, there was a similar frequency of major complications from January 1st, 2012 to December 31st, 2016 (74/2,157) compared to January 1st, 2017 to January 1st, 2022 (355/9,936) (RR: 0.96 [0.75 – 1.23], p = 0.37). The frequency of cardiac arrests from 2012–2017 was higher at 2.3% (50/2,157); whereas in 2017–2022 it was at 1.6% (160/9,936) (RR: 1.44 [95% CI, 1.05 – 1.97], p = 0.01). The frequency of deaths from 2012–2017 was 0.7% (14/2,157) versus in 2017–2022 it was 0.6% (56/9,936) (RR: 1.15 [95% CI, 0.64 – 2.07], p = 0.59) (Figure 3). Over the study period of a decade, 47 patients had multiple procedures completed.
Figure 3: Temporal Trends of Major Complications, Cardiac Arrest, and Death Within 72 Hours of TEE-Guided Transcatheter Intervention.
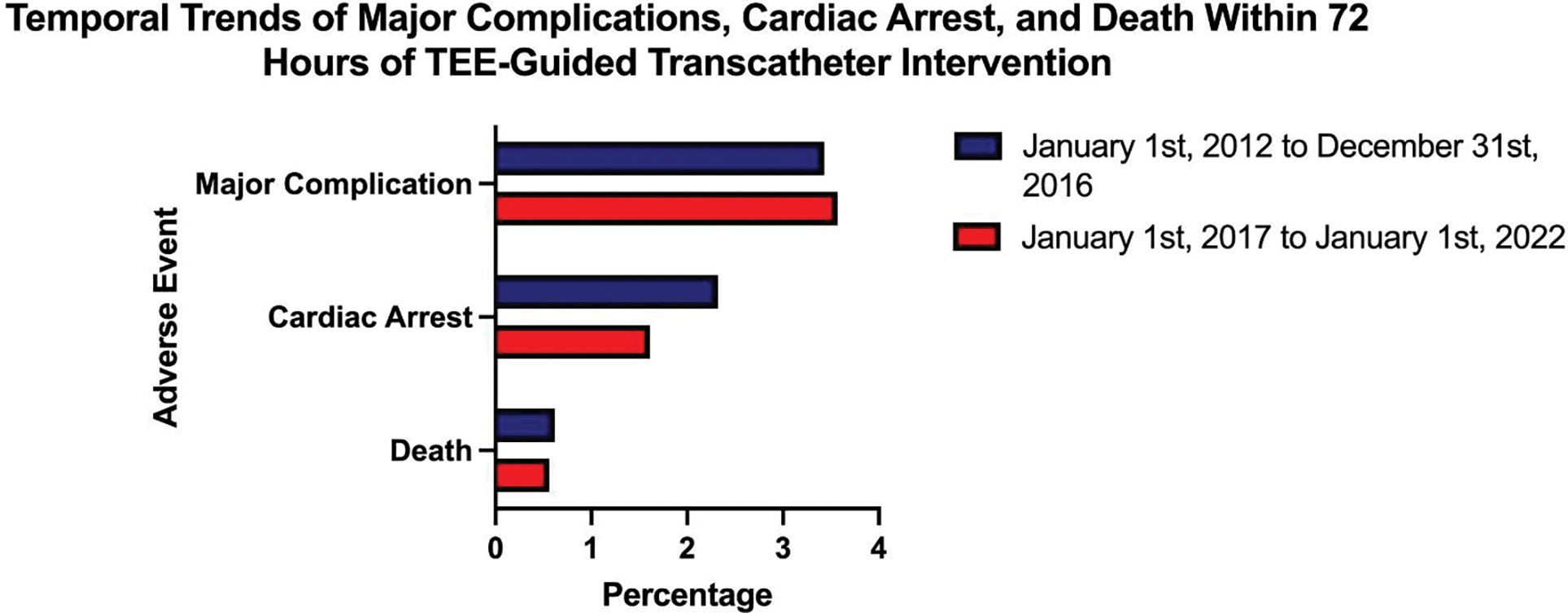
The frequency of major complications within 72 hours of a TEE-guided structural procedure between January 1st, 2012 to December 31st, 2016, and January 1st, 2017 to January 1st, 2022 is presented as a percentage.
When stratified by intervention type, 6,978 patients (57.9%) out of the entire cohort of 12,043 patients were able to be stratified by the type of intervention they completed. For patients who completed a TAVR procedure, the relative frequency of a major complication was 1.8% (32/1,761). In patients who completed an LAAO procedure, the frequency of a major complication was higher at 8.6% (262/3,042). The most reported type of complication suffered was gastrointestinal hemorrhage at 95.4% (250/262). Patients who completed a transcatheter MVR also had a high frequency of major complications at 2.5% (42/1,711). The most frequent complication reported was again gastrointestinal hemorrhage at 81% (34/42). Patients undergoing transcatheter pulmonary valve repair, transcatheter tricuspid valve repair, and VSD repair also had higher rates of major complications but the sample size from which it was completed was much smaller. The full breakdown regarding the frequency of major complications, cardiac arrest, and death within 72 hours by intervention type is shown in Table 4.
Table 4:
Periprocedural Transesophageal Echocardiography Related Outcomes Stratified by Intervention Type
| Procedure | Total Number of Patients | Death - Percentage (Number of Patients) | Cardiac Arrest - Percentage (Number of Patients) | Major Complications - Percentage (Number of Patients) |
|---|---|---|---|---|
| Left Atrial Appendage Occlusion | 3,042 | 0.13% (4) | 1.12% (34) | 8.61% (262) |
| Transcatheter Aortic Valve Replacement | 1,761 | 0.85% (15) | 1.76% (31) | 1.82% (32) |
| Transcatheter Mitral Valve Repair | 1,711 | 0.53% (9) | 2.34% (40) | 2.46% (42) |
| Paravalvular Leak Replacement | 168 | 0% (0) | 0.60% (1) | 1.79% (3) |
| Atrial Septal Defect Repair | 154 | 0.65% (1) | 0.65% (1) | 2.60% (4) |
| Patent Foramen Ovale Repair | 40 | 0% (0) | 0% (0) | 0% (0) |
| Ventricular Septal Defect Repair | 37 | 8.1% (3) | 10.8% (4) | 2.70% (1) |
| Transcatheter Pulmonary Valve Replacement | 36 | 0% (0) | 0% (0) | 5.56% (2) |
| Transcatheter Tricuspid Valve Repair | 29 | 0% (0) | 3.45% (1) | 6.90% (2) |
The frequency of major complications, death, and cardiac arrest within 72 hours of a TEE-guided structural procedure stratified by intervention type.
When reviewing geographic trends of major complications by region, the frequency of suffering a major complication within 72 hours of the procedure was highest in the Midwest at 6.4% compared to other regions. The Northeast had the lowest frequency of major complications at 2.5%. The frequency of major complications in the South was 3.7% and in the West was 3.0%.
Discussion
This study is the first to analyze the outcomes and complications associated with TEE guidance in transcatheter structural cardiac interventions in the United States. It is also the first to contrast the frequency of TEE-related complications between percutaneous interventions and cardiac valve surgery. In this study cohort, 3.6% suffered a major complication. The frequency of major complications in patients undergoing transcatheter structural cardiac interventions was higher among older individuals (≥65 years old) and those previously taking anticoagulants or antiplatelets. This study reaffirmed the increased risk for TEE-related complications in complex structural interventions versus TEE alone for image acquisition seen previously in smaller studies. Additionally, it is interesting to note that TEE used during transcatheter interventions was found to have an overall higher relative risk for major complications compared to TEE for intraoperative interventions. This is likely related to the role of TEE to help guide procedures for percutaneous interventions, whereas during surgical intervention TEE plays less of an active role. The higher relative risk of death is a likely sequela of the inherent risk with open heart surgery compared to minimally invasive procedures. The trends in the frequency of major complications stratified by geographic region are interesting given the significantly higher risk of a major complication in the Midwest compared to other regions. Despite the results, it is difficult to draw any conclusions due to the nature of the database and the number of participating institutions in each region. These results represent an area of future study to compare the frequency of complications from TEE-guided percutaneous interventions amongst different regions and institutions. The temporal trends in the frequency of major complications are also important to note given the massive switch to transcatheter structural heart interventions over the last several years. This lack of improvement is likely related to TEE operation remaining largely unchanged over the past decade compared to an improvement in transcatheter approaches. While TEE guidance during a procedure is classically thought to be relatively benign, there are known risks and the frequency of complications has persisted.
A retrospective review of 1,249 transcatheter structural cardiac cases with TEE guidance at Quebec Heart Institute (1,037 undergoing TAVR and 214 other procedures) noted that the complication frequency for those undergoing transcatheter MVR, PVLC, and LAAO was 2.8%. In those undergoing TAVR, the frequency of major complications was significantly lower at 0.6%, with both procedure type and time determined to be independent risk factors for TEE-related complications.12 Given the higher frequency of TEE-related complications in non-TAVR structural interventions, the same group completed a prospective study of TEE-related injuries with pre and post-procedural esophagogastroduodenoscopies (EGDs) to identify the percentage of injuries seen. A staggering 86% (43/50) of patients’ post-procedural EGD were found to have a new injury, and 40% (20/50) of patients developed a complex lesion such as an intramural hematoma or mucosal laceration of the esophagus.11 All TEEs were performed by a highly experienced and trained Interventional Echocardiographer with more than one year of experience. Despite this, over 80% of patients suffered a complication following TEE. In the Freitas-Ferraz study, they completed a prospective study with every patient completing an EGD after their TEE-guided procedure to identify both major and minor complications following their procedure.11 In our retrospective cross-sectional study, we identified complications as reported by individual operators who reported complications following their procedure with ICD-10 coding. Thus, the frequency of TEE-related complications may be underreported in the patient population included in this study. In a retrospective study of patients completing adult cardiac surgery, Purza et al identified 1,074 patients, of which 73 (6.8%) suffered an esophageal or gastric injury. Advanced age, low body mass index (BMI), and prolonged cardiopulmonary bypass time were noted to be independent risk factors associated with TEE-related complications.8 Regardless, TEEs are frequently cited as having a strong safety profile with complications described as exceedingly rare during routine TEEs.11,12 Long probe manipulation time during transcatheter structural cardiac interventions, performed in older individuals with multiple comorbidities, contributed to the relatively higher frequency of TEE-related complications.8,11,12 This was highlighted in our study by the relatively increased risk for a major complication during LAAO and mitral valve repair. Transcatheter mitral valve repairs have a higher dependence on TEE guidance and are usually longer in duration compared with other transcatheter-based interventions, thereby, increasing their risk for TEE-related complications.13,14 LAAO occlusion is typically much shorter in duration; however, the patient population typically receiving this implant has a known history of either major bleeding, non-major bleeding, or a higher risk to bleed.6,12,15 A small subset of patients who completed VSD repair was found to have suffered a higher frequency of complications. We suspect this is because these procedures were performed in patients who suffered from acute myocardial infarction complicated by cardiogenic shock; thus, representing a sicker patient population than typically encountered for the other procedures. The poor clinical profile in these patients may explain the higher frequency of complications compared with other procedures. Unfortunately, in our database, we were unable to assess the clinical profile of patients before these procedures were completed. Further investigations are needed to assess the granular phenotypic characteristics and prognostic implications of TEE-related complications among patients undergoing complex transcatheter structural cardiac interventions.
This study offers some key insights into the risk of TEE-related complications during transcatheter structural cardiac procedures. Previous studies evaluating the risk of TEE-related injuries during procedures only involved a single-center design, whereas this study assesses the data across multiple large academic medical centers in the United States. Additionally, our study population mirrors the patient population undergoing transcatheter structural interventions. These patients have high surgical mortality given their age, comorbidities, and medications including anticoagulation.31 Percutaneous interventions offer a less invasive approach to repairing and replacing valvular issues than conventional surgery. Studies show that these transcatheter interventions have effective clinical outcomes in this high-risk elderly population with an improved safety profile.32–34 Accordingly, multiple randomized controlled trials have established the use of TEE for intraprocedural guidance. In a study evaluating mortality after transcatheter aortic valve implantation, they found the use of intraoperative TEE was associated with a lower risk of mortality (hazard ratio: 0.57).35 As the role of less invasive percutaneous techniques continues to expand, the need for concurrent use of TEE is equally important to provide real-time visualization of both cardiac structures and catheter location. Thus, it is important to develop an understanding of common TEE-related complications and ways to mitigate the risk of complications.
As noted in our investigation and by others, there are multiple non-modifiable risk factors for TEE-related complications.8,11,12,31 Patient-related factors that increase their risk for negative outcomes include their age, past medical history, and prescribed medications. In our cohort, over 91% of patients were either on anticoagulants or platelet-aggregation inhibitors increasing their risk for intramural hematomas and hemorrhages during TEE. Another factor is the complexity of the procedure. For example, a mitral valve repair is a prolonged procedure that requires frequent manipulation of the probe to obtain the optimal views needed for procedural guidance.36 The necessities involved in completing these procedures increase the risk for adverse outcomes from direct mechanical trauma, high contact pressure at the surface of the mucosa, and thermal injury caused by the heat of the TEE probe.7,8
Multiple modifiable risk factors can be addressed during the procedure including sedation techniques. When patients are under general anesthesia, they are unable to swallow the probe and the operators must blindly insert the probe with potential forced manipulation leading to esophageal injury secondary to direct mechanical trauma. In contrast, patients under conscious sedation can swallow to help facilitate insertion and minimize risk.11 In our study, 79% of the major complications were secondary to gastrointestinal hemorrhage. Avoiding unnecessary manipulation of the probe or keeping it locked for long periods can also help to minimize harm as a longer duration of active TEE use has been noted to be an independent risk factor for complications.11,12,31 After the procedure, patients should be closely followed to assess for evidence of any complications. Minor complications may not be clinically significant, but any symptomatic burden a patient experiences after TEE can be relieved through conservative management with either viscous lidocaine (i.e., a gastrointestinal cocktail) or proton-pump inhibitors.
Another technique that can be incorporated into transcatheter structural interventions is intracardiac echocardiography (ICE), which allows for high-resolution visualization of intracardiac anatomy, to guide catheter manipulation.37 While it has been widely utilized in the electrophysiology lab, it has more recently gained traction in some transcatheter structural cases such as ASD and PFO closures.38–40 Additionally, a recent study evaluating ICE in transcatheter left atrial appendage closure was associated with a decreased risk for GI complications which was the most common complication TEE-guided structural interventions face. However, there was a higher risk of developing vascular complications and while there was no difference in the length of hospital stay, the associated costs of using ICE were significantly higher.41 Developing smaller probes for more-frail patients should also be strongly considered. Purza et al. showed in their large trial that patients with a lower weight had a greater risk for major complications.8 In pediatric patients, TEE probe selection is based on the weight of the patient and the size of the probe.42,43 Creating a standardized approach to assign probe sizes to specific BMI categories could help further decrease the risk for complications, although it would be important to ensure image quality is not sacrificed as a result. This study has notable implications from the public health perspective. In the setting of complex cardiovascular disease, a comprehensive heart team approach is needed to optimize education about risks, benefits, and alternative options in a patient-centric approach.44 The field of structural cardiology continues to evolve and offers interventions to reduce symptomatic burden in patients where surgery was not a viable option. As the volume of percutaneous interventions increases, we must remain cognizant of the risks of TEE-related complications in TEE-guided procedures.45 Discussion with patients about the risks of upper gastrointestinal hemorrhage, an esophageal injury requiring surgical intervention, and even death allow for clearly defined expectations, especially in non-TAVR procedures that carry a higher risk of adverse events. Further efforts are needed to study mechanisms to reduce the risk of TEE complications and potential alternative approaches that can be utilized. This study highlights the importance of implementing new practices in clinical care as they can help reduce the risk for both major and minor complications.
There are several limitations to our study. First, we collected patient outcomes based on the ICD-10 coding schema. While this structured approach allowed for maximal data extraction, there is an inherent risk for incomplete data results secondary to coding errors and incomplete reporting.46,47 However, the coding protocol is largely standardized across healthcare systems which helps counteract this issue.48 Secondly, there is selection bias in our patient population as we are limited to institutions that integrate their electronic health record (EHR) into the TriNetX Research database.23 Another limitation of using the TriNetX research database is that most healthcare organizations included in our study are from the Southeast. There are fewer healthcare organizations from the west coast included in the database. This limited our ability to achieve a more balanced geographic distribution of the population for our analysis. The third limitation stems from using group-level data as a proxy for individual-level data. By using TriNetX, we are unable to complete a standardized pre- and post-procedure evaluation for major complications that may have resulted after a TEE-guided structural intervention.49 Fourthly, due to the limitations of the database, we were unable to assess if procedure duration was a contributing factor to complications. Furthermore, we could not evaluate if complications led to an increased length of hospital stay. Additionally, the database we used lacked the indication for procedures performed which limits our ability to understand the role of the patient’s clinical profile in complications. Finally, it should be noted that our findings reflect practices in large academic medical centers and may not apply to other types of practices given the nature of our database. Regardless, this largest-to-date nationwide study demonstrates that there is an increased risk of major complications from TEE being used concurrently to guide structural heart cases. Likewise, it is important to develop a standardized process with a heart team approach to evaluate the likelihood of potential risks a patient may develop undergoing a transcatheter structural cardiac intervention.
In conclusion, this study found the prevalence of complications from TEE used to guide structural heart interventions was 3.6% which represents a higher level of risk than classically thought. With a shift of poor surgical candidates completing less invasive procedures, the future of TEE-guided procedures relies on an updated, comprehensive risk discussion. While the benefits of TEE-guided procedures are innumerable, there are serious risks that require attention. Further studies are needed to see if additional modalities, such as 3-D TEE or ICE during structural cardiac interventions, help decrease the risk of TEE-related complications compared to conventional methods.50
Highlights:
The risk of suffering a TEE-related complication was 3.6% over the past decade
Gastrointestinal hemorrhage is the most frequently reported complication
Intraoperative TEE during cardiac valve surgery had a lower risk of complications
Sources of Funding:
Dr. Pankaj Arora is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health (NIH) awards R01HL160982, R01HL163852, R01HL163081, and K23HL146887, and by the Doris Duke Charitable Foundation COVID-19 Fund to Retain Clinician Scientists (Grant #2021255); UAB COVID-19 CARES Retention Program (CARES at UAB).
Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR003096. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: None of the other authors had any conflicts of interest or financial disclosures to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.).Khandheria BK, Seward JB, Tajik AJ. Transesophageal echocardiography. Mayo Clin Proc. 1994;69(9):856–863. doi: 10.1016/s0025-6196(12)61788-1 [DOI] [PubMed] [Google Scholar]
- 2.).Bailey SR, Weiner RB, Alagona P Jr, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol 2011; 57:1126. [DOI] [PubMed] [Google Scholar]
- 3.).Peterson GE, Brickner ME, Reimold SC. Transesophageal echocardiography: clinical indications and applications. Circulation. 2003;107(19):2398–2402. doi: 10.1161/01.CIR.0000071540.97144.89 [DOI] [PubMed] [Google Scholar]
- 4.).Perk G, Lang RM, Garcia-Fernandez MA, et al. “Use of real time three-dimensional transesophageal echocardiography in intracardiac catheter based interventions”. J Am Soc Echocardiogr 2009;22:865–882. [DOI] [PubMed] [Google Scholar]
- 5.).Ender J, Sgouropoulou S. Value of transesophageal echocardiography (TEE) guidance in minimally invasive mitral valve surgery. Ann Cardiothorac Surg. 2013;2(6):796–802. doi: 10.3978/j.issn.2225-319X.2013.10.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.).Zhang H, Tang Z, Han Z, et al. Role of real time-three dimensional transesophageal echocardiography in left atrial appendage closure with LACBES® devices. Exp Ther Med. 2019;17(2):1456–1462. doi: 10.3892/etm.2018.7086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.).Hilberath JN, Oakes DA, Shernan SK, et al. Safety of transesophageal echocardiography. J Am Soc Echocardiogr. 2010;23(11):1115–1221. doi: 10.1016/j.echo.2010.08.013 [DOI] [PubMed] [Google Scholar]
- 8.).Purza R, Ghosh S, Walker C, et al. Transesophageal Echocardiography Complications in Adult Cardiac Surgery: A Retrospective Cohort Study. Ann Thorac Surg. 2017;103(3):795–802. doi: 10.1016/j.athoracsur.2016.06.073 [DOI] [PubMed] [Google Scholar]
- 9.).Vignon P, Guéret P, Chabernaud JM, et al. Echecs et complications de l’échocardiographie transoesophagienne. A propos de 1,500 examens consécutifs [Failure and complications of transesophageal echocardiography. Apropos of 1500 consecutive cases]. Arch Mal Coeur Vaiss. 1993;86(6):849–855. [PubMed] [Google Scholar]
- 10.).Chee TS, Quek SS, Ding ZP, et al. Clinical utility, safety, acceptability and complications of transoesophageal echocardiography (TEE) in 901 patients. Singapore Med J. 1995;36(5):479–483. [PubMed] [Google Scholar]
- 11.).Freitas-Ferraz AB, Bernier M, Vaillancourt R, et al. Safety of Transesophageal Echocardiography to Guide Structural Cardiac Interventions. J Am Coll Cardiol. 2020;75(25):3164–3173. doi: 10.1016/j.jacc.2020.04.069 [DOI] [PubMed] [Google Scholar]
- 12.).Freitas-Ferraz AB, Rodés-Cabau J, Junquera Vega L, et al. Transesophageal echocardiography complications associated with interventional cardiology procedures. Am Heart J. 2020;221:19–28. doi: 10.1016/j.ahj.2019.11.018 [DOI] [PubMed] [Google Scholar]
- 13.).Rosengart TK, Feldman T, Borger MA, et al. Percutaneous and minimally invasive valve procedures: a scientific statement from the American Heart Association Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology, Functional Genomics and Translational Biology Interdisciplinary Working Group, and Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2008;117(13):1750–1767. doi: 10.1161/CIRCULATIONAHA.107.188525 [DOI] [PubMed] [Google Scholar]
- 14.).Mack M, Carroll JD, Thourani V, et al. Transcatheter Mitral Valve Therapy in the United States: A Report from the STS/ACC TVT Registry. Ann Thorac Surg. 2022;113(1):337–365. doi: 10.1016/j.athoracsur.2021.07.030 [DOI] [PubMed] [Google Scholar]
- 15.).Freeman JV, Varosy P, Price MJ, et al. The NCDR Left Atrial Appendage Occlusion Registry. J Am Coll Cardiol. 2020;75(13):1503–1518. doi: 10.1016/j.jacc.2019.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.).Parcha V, Booker KS, Kalra R, et al. A retrospective cohort study of 12,306 pediatric COVID-19 patients in the United States. Sci Rep. 2021;11(1):10231. Published 2021 May 13. doi: 10.1038/s41598-021-89553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.).Parcha V, Kalra R, Glenn AM, et al. Coronary artery bypass graft surgery outcomes in the United States: Impact of the coronavirus disease 2019 (COVID-19) pandemic. JTCVS Open. 2021;6:132–143. doi: 10.1016/j.xjon.2021.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.).Harrison SL, Fazio-Eynullayeva E, Lane DA, et al. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. 17, e1003321. 10.1371/journal.pmed.1003321 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.).Singh S, Khan A, Chowdhry M, et al. Risk of severe coronavirus disease 2019 in patients with infammatory bowel disease in the United States: A multicenter research network study. Gastroenterology 10.1053/j.gastro.2020.06.003 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.).Alkhouli M, Nanjundappa A, Annie F, et al. Sex diferences in case fatality rate of COVID-19: Insights from a multinational registry. Mayo Clin. Proc. 95, 1613–1620. 10.1016/j.mayocp.2020.05.014 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.).Harrison PJ, Luciano S, Colbourne L. Rates of delirium associated with calcium channel blockers compared to diuretics, renin-angiotensin system agents and beta-blockers: An electronic health records network study. J. Psychopharmacol. 34, 848–855. 10.1177/0269881120936501 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.).Taquet M, Luciano S, Geddes JR, et al. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry 10.1016/S2215-0366(20)30462-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.).Pfaff ER, Girvin AT, Gabriel DL, et al. Synergies between centralized and federated approaches to data quality: a report from the national COVID cohort collaborative. J Am Med Inform Assoc. 2022;29(4):609–618. doi: 10.1093/jamia/ocab217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.).TriNetX. TriNetX Health Data Network [Internet] Cambridge, MA: TriNetX, Inc; Available from: http://www.trinetx.com. [Google Scholar]
- 25.).Centers for Disease Control and Prevention. NCHS International Classifcation of Diseases, Tenth Revision, Clinical Modifcation (ICD-10-CM). https://www.cdc.gov/nchs/icd/icd10cm.htm.
- 26.).Nicoara A, Skubas N, Ad N, et al. Guidelines for the Use of Transesophageal Echocardiography to Assist with Surgical Decision-Making in the Operating Room: A Surgery-Based Approach: From the American Society of Echocardiography in Collaboration with the Society of Cardiovascular Anesthesiologists and the Society of Thoracic Surgeons [published correction appears in J Am Soc Echocardiogr. 2020 Nov;33(11):1426]. J Am Soc Echocardiogr. 2020;33(6):692–734. doi: 10.1016/j.echo.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 27.).MacKay EJ, Groeneveld PW, Fleisher LA, et al. Practice Pattern Variation in the Use of Transesophageal Echocardiography for Open Valve Cardiac Surgery. J Cardiothorac Vasc Anesth. 2019;33(1):118–133. doi: 10.1053/j.jvca.2018.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.).HIPAA Journal. HIPAA History. HIPAA Journal. Published 2015. https://www.hipaajournal.com/hipaa-history/ [Google Scholar]
- 29.).Mahmaljy H, Tawney A, Young M. Transcatheter Aortic Valve Replacement. In: StatPearls. Treasure Island (FL): StatPearls Publishing; August 13, 2021. [PubMed] [Google Scholar]
- 30.).Hua-Gen Li M, Hutchinson A, Tacey M, et al. Reliability of comorbidity scores derived from administrative data in the tertiary hospital intensive care setting: a cross-sectional study. BMJ Health Care Inform. 2019;26(1):e000016. doi: 10.1136/bmjhci-2019-000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.).Kumar S, Brown G, Sutherland F, et al. The transesophageal echo probe may contribute to esophageal injury after catheter ablation for paroxysmal atrial fibrillation under general anesthesia: a preliminary observation. J Cardiovasc Electrophysiol. 2015;26(2):119–126. doi: 10.1111/jce.12575 [DOI] [PubMed] [Google Scholar]
- 32.).Kodali SK, Velagapudi P, Hahn RT, et al. Valvular Heart Disease in Patients ≥80 Years of Age. J Am Coll Cardiol. 2018;71(18):2058–2072. doi: 10.1016/j.jacc.2018.03.459 [DOI] [PubMed] [Google Scholar]
- 33.).Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation [published correction appears in N Engl J Med. 2011 Jul 14;365(2):189. Glower, Donald G [corrected to Glower, Donald D]]. N Engl J Med. 2011;364(15):1395–1406. doi: 10.1056/NEJMoa1009355 [DOI] [PubMed] [Google Scholar]
- 34.).Maisano F, Franzen O, Baldus S, et al. Percutaneous mitral valve interventions in the real world: early and 1-year results from the ACCESS-EU, a prospective, multicenter, nonrandomized post-approval study of the MitraClip therapy in Europe. J Am Coll Cardiol. 2013;62(12):1052–1061. doi: 10.1016/j.jacc.2013.02.094 [DOI] [PubMed] [Google Scholar]
- 35.).de Brito FS Jr, Carvalho LA, Sarmento-Leite R, et al. Outcomes and predictors of mortality after transcatheter aortic valve implantation: results of the Brazilian registry. Catheter Cardiovasc Interv. 2015;85(5):E153–E162. doi: 10.1002/ccd.25778 [DOI] [PubMed] [Google Scholar]
- 36.).Shah M, Jorde UP. Percutaneous Mitral Valve Interventions (Repair): Current Indications and Future Perspectives. Front Cardiovasc Med. 2019;6:88. Published 2019 Jul 12. doi: 10.3389/fcvm.2019.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.).Enriquez A, Saenz LC, Rosso R, et al. Use of Intracardiac Echocardiography in Interventional Cardiology: Working With the Anatomy Rather Than Fighting It. Circulation. 2018;137(21):2278–2294. doi: 10.1161/CIRCULATIONAHA.117.031343 [DOI] [PubMed] [Google Scholar]
- 38.).Alqahtani F, Bhirud A, Aljohani S, et al. Intracardiac versus transesophageal echocardiography to guide transcatheter closure of interatrial communications: Nationwide trend and comparative analysis. J Interv Cardiol. 2017;30(3):234–241. doi: 10.1111/joic.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.).Basman C, Parmar YJ, Kronzon I. Intracardiac Echocardiography for Structural Heart and Electrophysiological Interventions. Curr Cardiol Rep. 2017;19(10):102. Published 2017 Sep 6. doi: 10.1007/s11886-017-0902-6 [DOI] [PubMed] [Google Scholar]
- 40.).Saji M, Rossi AM, Ailawadi G, et al. Adjunctive intracardiac echocardiography imaging from the left ventricle to guide percutaneous mitral valve repair with the MitraClip in patients with failed prior surgical rings. Catheter Cardiovasc Interv. 2016;87(2):E75–E82. doi: 10.1002/ccd.25981 [DOI] [PubMed] [Google Scholar]
- 41.).Zahid S, Gowda S, Hashem A, et al. Feasibility and Safety of Intracardiac Echocardiography Use in Transcatheter Left Atrial Appendage Closure Procedures. Journal of the Society for Cardiovascular Angiography & Interventions. Published 2022 Nov 3. Doi: 10.1016/k.jscai.2022.100510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.).Puchalski MD, Lui GK, Miller-Hance WC, et al. Guidelines for Performing a Comprehensive Transesophageal Echocardiographic: Examination in Children and All Patients with Congenital Heart Disease: Recommendations from the American Society of Echocardiography [published correction appears in J Am Soc Echocardiogr. 2019 May;32(5):681] [published correction appears in J Am Soc Echocardiogr. 2019 Oct;32(10):1373–1378]. J Am Soc Echocardiogr. 2019;32(2):173–215. doi: 10.1016/j.echo.2018.08.016 [DOI] [PubMed] [Google Scholar]
- 43.).Helmcke F, Mahan EF 3rd, Cooper JW, et al. Use of the smaller pediatric transesophageal echocardiographic probe in adults. Echocardiography. 1990;7(6):727–737. doi: 10.1111/j.1540-8175.1990.tb00425.x [DOI] [PubMed] [Google Scholar]
- 44.).Holmes DR Jr, Rich JB, Zoghbi WA, et al. The heart team of cardiovascular care. J Am Coll Cardiol. 2013;61(9):903–907. doi: 10.1016/j.jacc.2012.08.1034 [DOI] [PubMed] [Google Scholar]
- 45.).Khouzam RN. Advances in Cardiology, Interventional Cardiology, Electrophysiology, and Structural Heart Disease: A Moving Target. Ann Transl Med. 2018;6(1):1. doi: 10.21037/atm.2017.12.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.).Khera R, Angraal S, Couch T, et al. Adherence to Methodological Standards in Research Using the National Inpatient Sample. JAMA. 2017;318(20):2011–2018. doi: 10.1001/jama.2017.17653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.).Ostrominski JW, Amione-Guerra J, Hernandez B, et al. Coding Variation and Adherence to Methodological Standards in Cardiac Research Using the National Inpatient Sample. Front Cardiovasc Med. 2021;8:713695. Published 2021 Nov 2. doi: 10.3389/fcvm.2021.713695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.).Mainor AJ, Morden NE, Smith J, et al. ICD-10 Coding Will Challenge Researchers: Caution and Collaboration may Reduce Measurement Error and Improve Comparability Over Time. Med Care. 2019;57(7):e42–e46. doi: 10.1097/MLR.0000000000001010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.).Roux AVD. The study of group-level factors in epidemiology: rethinking variables, study designs, and analytical approaches. Epidemiol Rev. 2004;26:104–111. doi: 10.1093/epirev/mxh006 [DOI] [PubMed] [Google Scholar]
- 50.).Faletra FF, Pedrazzini G, Pasotti E, et al. 3D TEE during catheter-based interventions. JACC Cardiovasc Imaging. 2014;7(3):292–308. doi: 10.1016/j.jcmg.2013.10.012 [DOI] [PubMed] [Google Scholar]