Abstract
The impact of global diabetes prevention efforts has been modest despite the promise of landmark diabetes prevention trials nearly twenty years ago. While national and regional initiatives show potential, challenges remain to adapt large-scale strategies in the real-world that fits individuals and their communities. Additionally, the sedentary lifestyle changes during the COVID-19 pandemic and guidelines that now call for earlier screening (e.g., US Preventative Task Force) will increase the pool of eligible adults worldwide. Thus, a more adaptable, person-centered approach that expands the current toolkit is urgently needed to innovate and revitalize our approach to diabetes prevention.
This review identifies key priorities to optimize the population-level delivery of diabetes prevention based on a consensus-based evaluation of the current evidence among experts in global translational programs; key priorities identified include (1) participant eligibility, (2) intervention intensity, (3) delivery components, (4) behavioral economics, (5) technology, and (6) the role of pharmacotherapy. We offer a conceptual framework for a broader, person-centered approach to better address an individual’s risk, readiness, barriers, and digital competency.
Background:
Two decades have passed since landmark diabetes prevention trials demonstrated the efficacy of intensive lifestyle interventions (ILI) and prompted global translation initiatives,1–4 including national and regional translational diabetes prevention efforts that are underway across four continents.5–9 Translational programs have tried to balance fidelity to the evidence generated by clinical trials (e.g., minimum weight loss goals) with adaptations for scaling in real-world settings (e.g., delivery by lay professionals). The potential for impact is promising. For example, the Finnish translational program found 29–37% decreased incidence of type 2 diabetes (T2D) at seven year follow-up among adults who participated in ILI and lost significant weight during the first year.10 In addition, ILI targeting high risk adults appears to be cost-effective, which supports efforts for wider dissemination.11
National efforts in the US and UK have experienced encouraging weight loss outcomes in the real-world (i.e., 2.76–4.2%9), but also highlight challenges in program reach and retention.6,9,12,13 The US National Diabetes Prevention campaign’s success to reach nearly half a million participants in the past decade is only a fraction of the 88 million at-risk.12 An individual’s lack of risk awareness, poor linkage between clinicians and community-based prevention programs and shortage of widespread program sites have contributed to low population reach. In both the US and UK, program participation and retention are lower among racial/ethnic minority, low-income, and younger participants,12,14 highlighting the need to address structural factors that may contribute to program inequities. Moreover, modest reimbursement schemes in the US have struggled to support sustainable program supply and broad access for areas in highest need.14 With an aim to optimize impact on population health, there is growing interest in strategies to bolster the reach and effectiveness of translational programs and improve outcomes for high-risk populations.12,14
The COVID-19 pandemic has further highlighted the importance of diabetes prevention, as the ‘slow-moving’ diabetes pandemic may worsen15 with the increase in sedentary lifestyle and weight gain that appears more common as a result of the COVID-19 pandemic.16 Economic downturns caused by the COVID-19 pandemic are further likely to accentuate social, financial and environmental risk factors (e.g., food insecurity) that may contribute to metabolic risk factors.17 As such, it is more critical than ever to develop global diabetes prevention strategies to better serve eligible adults. Evidence-based, adapted tools that address individual risk, readiness, and environments are critically needed (Figure 1). Existing, high-impact delivery models, such as the International AIDS Society’s Differentiated Service Delivery,18 offer a conceptual framework for broader, person-centered approaches (Figure 2). To meet this important goal, we identified six priority areas through consensus among a panel of interdisciplinary and international experts in diabetes prevention: (1) participant eligibility, (2) intervention intensity, (3) delivery components, (4) behavioral economics, (5) technology, and (6) the role of pharmacotherapy.
Figure 1: Key Components of Diabetes Prevention Service Delivery.
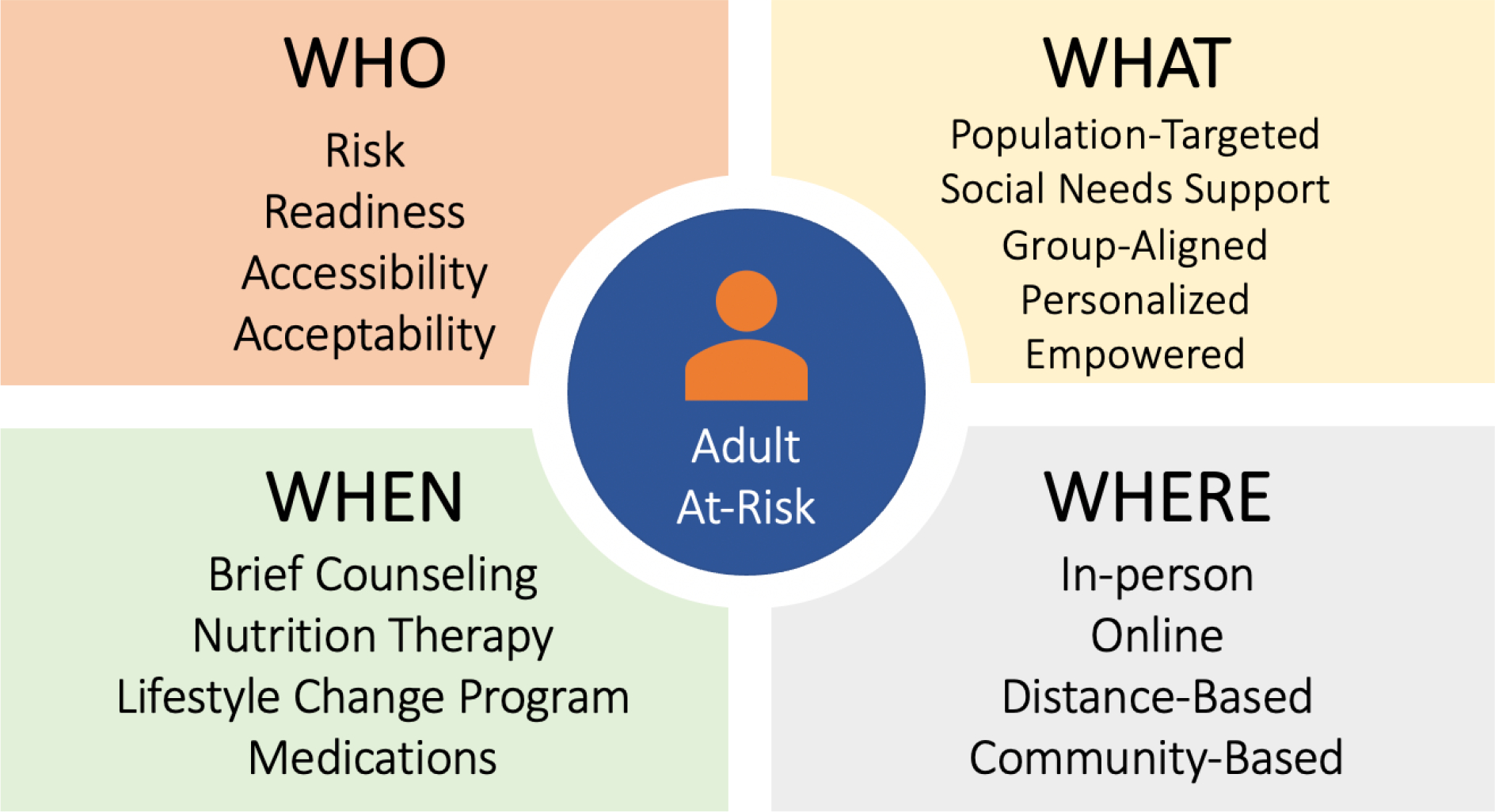
Four key components to optimize the delivery of diabetes preventative services. 1) The ‘who’ revisits risk and readiness for program eligibility; 2) the ‘what’ considers how the program content can be curtailed to the individual; 3) the ‘when’ compliments existing lifestyle change programming with alternate, less intense programming and medications; 4) the ‘where’ addresses how to leverage technology. Addressing these four key areas will better adapt current programming to individuals’ risks, preferences, comorbidities, and settings and broaden the impact of current diabetes prevention efforts.
Figure 2: Essential Elements for Person-Centered Diabetes Prevention Programming.
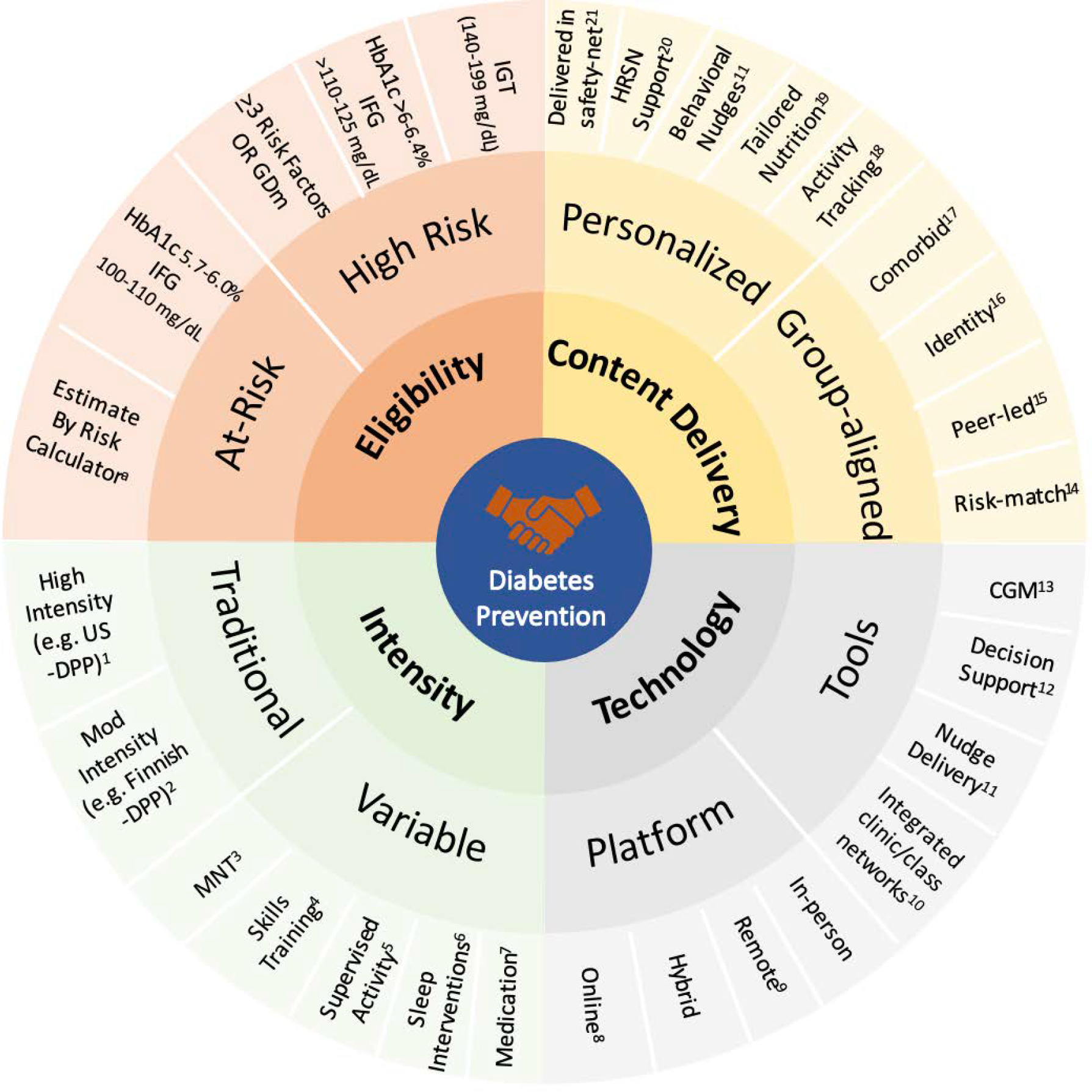
IFG: Impaired Fasting Glucose
IGT: Impaired Glucose Tolerance
GDM: Gestational Diabetes Mellitus
DPP: Diabetes Prevention Program
MNT: Medical Nutrition Therapy
CGM: Continuous Glucose Monitoring
HRSN: Health-related Social Needs
a Risk assessment tools include Centers for Disease Control/American Diabetes Association in the US; AUSDRISK in Australia, FINDRISC in Finland and IDRS in India
A conceptual model of the essential elements to optimize diabetes prevention services with an evidence-based, person-centered approach. This model is based on the conceptual framework used by the International AIDS Society’s HIV Differentiated Care,22 with illustrative examples as references. Responding to the call for national programs to adapt to individual factors, 23,24 this model offers a guide for programs to better match to an individual’s risk, readiness and setting based on key elements of service delivery (eligibility, content delivery, intensity, and technology). For example, a highly motivated 53-year-old woman living with obesity, PTSD and a HbA1c of 6.3% who works shiftwork as a paramedic would be high-risk and ideally matched to an online or remote-based traditional lifestyle change program. She declines given time-constraints (‘When’) and preference (‘Who’) and requests options that more closely fit her schedule (‘Where’) and her background (‘What’). She may be receptive to nutritional counseling and life skills training (‘variable intensity’) with self-directed telephone and app-based approaches (technology) with other female veterans living with PTSD (‘group-aligned’ content delivery).
Methods:
We organized an international team of diabetes prevention experts who have led and/or evaluated translational initiatives to summarize key gaps and opportunities in population-level program delivery based on the current evidence through online correspondence from July 2021 to February 2022. Three authors created an initial outline (IG, MB and TM) which was then reviewed and revised by all authors. The outline was then expanded into a first draft which was reviewed and revised until consensus was achieved by all authors. Studies were identified through review of reference lists, PubMed search terms (e.g., ‘real-world lifestyle program’), PubMed’s related articles feature, and the authors’ archives. Responding to the call for national programs to address individual factors,19 we developed a conceptual model based on the conceptual framework used by the International AIDS Society’s HIV Differentiated Care model,18 with illustrative examples as references.
Priority I: Participant Eligibility – Has the Pendulum Swung Too Far?
In contrast to prospective, randomized trials, translational studies must relax their eligibility criteria to incorporate readily available clinical measures and assessment tools. In Table 1, case examples of the US and Finnish diabetes prevention trials are presented along with the adjusted eligibility criteria for their translational programs. As such, real-world ILI participants are increasingly distinct from those enrolled in clinical trials who were largely defined by impaired glucose tolerance (IGT) with or without impaired fasting glucose (IFG).3 There are opposing views of how these risk phenotypes influence responses to lifestyle modification. A systematic review of lifestyle change trials suggests that phenotype may be less of a factor as lifestyle interventions have a clinically meaningful reduction in metabolic risk markers among adults in the absence of IGT.20 Alternatively, some trials have shown that adults with isolated IFG (iIFG; IFG in the absence of IGT) may have a distinctive phenotype driven by hepatic insulin resistance that is less responsive to ILI compared to adults with isolated IGT (iIGT; IGT in the absence of IFG) or IGT+IFG.21,22 While significant variation by region, ethnicity and diagnostic criteria exists,23 iIFG is among the most commonplace phenotype globally (per American Diabetes Association criteria). Further research is needed to determine effective programming among this substantial subgroup.21 Wagner et al. identified additional distinct pathophysiologic subtypes of prediabetes at higher risk for developing T2D,24 that may have varied treatment response with ILI among these subgroups. Recently, there is increased attention to revisit recommendations for older adults with prediabetes to receive ILI, after an observational study found that many older adults (age >70) with prediabetes had naturally returned to normoglycemia without intervention (13% with baseline HbA1c 5.7–6.4%; 44% with baseline FG 100–125 mg/dL).25 Additionally, there is a need to address prevention needs among high-risk, yet lean adults. While the Da Qing and Indian Diabetes Prevention Program (DPP) trials showed favorable results among lean adults, the remaining landmark trials excluded this group; further studies are therefore needed to identify prevention strategies in this population to complement current programming given overweight/obesity is not the only risk factor for T2D.26
Table 1:
Case Example Comparison of Two Landmark National Diabetes Prevention Trials
| Original Diabetes prevention Study | Study Eligibility | Study Intensity & Duration | Translational Outcomes | National Program | Program Eligibility | Program Intensity & Duration |
|---|---|---|---|---|---|---|
| US Diabetes Prevention Program1 | Age ≥ 25 years BMI ≥24 kg/m2 (Asian ≥22 kg/m2 ) FPG: 95–125 mg/dL OGTT: 140–199 mg/dL |
• ≥16 1:1 sessions 0–6 months followed by ≥6 sessions/7–12 mo (≥22 hours/1 year) • Voluntary supervised activity ≥2 times per week |
• Average one year weight loss among participants in YMCA DPP was 5.6%3 • In a pooled meta-analysis of programs based on US-DPP, mean weight loss was −3.77 kg, mean HbA1c reduction of −0.21% with an average follow-up of 9.3 months4 |
CDC NDPP (US)6 | Age ≥ 18 years + BMI ≥25 kg/m2 (Asian ≥23 kg/m2 ) + HbA1c 5.7–6.4% (39–46 mmol/mol) OR FPG: 100–125 mg/dL OR OGTT: 140–199 mg/dL OR past GDM OR CDC/ADA Prediabetes Risk Testa >5 |
• ≥16 sessions in months 1–6; ≥6 sessions in months 7–12 (≥22 hours) • Medicare (MDPP) two year program extends monthly sessions • >780 program providers CDC-certifiedb |
| Finnish Diabetes Prevention Study2 | Age 40–64 years BMI ≥25 kg/m2 Mean value of two OGTT: 140–199 mg/dL |
• 7 1:1 sessions at weeks 0, 1, 5, 12, 16, 24 and 36 then every 3 months for remaining 2 years (≥ 16 sessions/3 years) • Voluntary supervised activity offered |
>17% of participants in Finnish national translation project achieved ≥5% weight loss5 | NHS DPP (UK)7 | Age ≥ 18 years Hba1c: 6.0–6.4% (42–46 mmol/mol) FPG: 110–125 mg/dL (5.5–6.9 mmol/L) |
≥13 sessions over ≥ 9 months (≥16 hours) • four program providers NHS-certified |
| FIN-D2D (Finland)8 | Age ≥ 18 years FINDRISCc ≥ 15 |
• 4–8 sessions every 1–2 weeks + 1 follow-up session | ||||
| Life! (Australia)9 | Age ≥ 50 years AUSDRISKc ≥12 (Age ≥ 18 years if Aboriginal and Torres Strait Islander descent and/or history of GDM, CVD) |
• 5 sessions every 2 weeks + 1 follow-up session at 8 months • provider network of nonprofit, public sector, and private agencies |
FPG: Fasting Plasma Glucose
OGTT: Oral Glucose Tolerance Test
BMI: Body Mass Index
GDM: Gestational Diabetes Mellitus
CVD: Cardiovascular Disease
Organizations must meet minimum 35% of cohort entering with qualifying blood test or history of gestational diabetes. Medicare DPP requires serum-based testing. CDC/ADA Prediabetes Risk Test is a risk tool comprimised of scored questions to estimate probaiblity of developing T2D.
Fully recognized by the CDC Diabetes Prevention Recognition Program
FINDRISC (8 questions) and AUSDRISK (10 questions) are risk tools comprised of scored questions, with the total test score providing a measure of the probability of developing T2D.
To optimize large-scale preventive lifestyle programming, we need to identify adults more precisely at the greatest risk of incident T2D who may benefit most from ILI, especially as the pool of eligible adults expands with guidelines offering lower age threshold for screening for dysglycemia. The use of risk calculators, that incorporate clinical data and relevant demographic characteristics, may increase precision in estimating incident diabetes risk and the relative benefits of ILI.27 Simple, non-laboratory diabetes risk scores have been developed which have proven useful to identify people at high-risk and suitable for preventive interventions.28 There are ongoing studies implementing risk calculators within electronic health records to inform shared decision making between patients and providers at the point of care.29 We offer a scheme of risk tiers to consider for ILI eligibility to better match higher risk subgroups to more intensive program elements (Figure 2; ‘Eligibility’, High Risk). Multi-tiered approaches (invasive testing following existing screening tools) are also being examined. A one-hour oral glucose tolerance test (OGTT)30 is a potential tool to better target individuals at high-risk for progression to T2D whose β-cell function is still relatively preserved when earlier ILI may have greater benefits.,31 Further work is needed to determine how to best manage high-risk individuals, including the practicality of implementing a confirmatory test such as the one-hour OGTT in primary care. At a systems-level, adopting a tiered-risk stratification approach, similar to atherosclerotic cardiovascular disease risk for dyslipidemia, could help align efforts to prioritize the highest-risk with the goal of efficient resource allocation.32 Screening high-risk adults using non-laboratory diabetes risk scores may be the most practical,28 and cost-effective method33 and persist in low-resource settings.34 Targeted screening strategies to detect which adults identified as high risk by diabetes risk scores have phenotypes that are likely to respond to lifestyle intervention should be reconsidered. For example, the detection of IGT using the one-hour OGTT is cost-saving when compared to two-hour OGTT.35 Further work is needed to examine if novel biomarkers and precision medicine approaches together with utilizing electronic records are practical and effective.
Priority II: Intervention Intensity and Duration – Could Less Mean More?
Translational programs have used varying intensity of ILI, such as six two-hour sessions over three years to twenty two one-hour sessions over one year.36 These studies have demonstrated a dose-response relationship between number of sessions attended, weight loss, and reductions in incident diabetes.37 Alternate measures of intensity, including frequency and duration of contact, program length and fidelity to evidence-based material are also associated with increased weight loss.38 Maintenance strategies after the initial intervention phase also appear important to sustain program effectiveness.4 Finding the optimal intensity and duration are important considerations in real-world settings, as adherence to longer lifestyle programs is more challenging and require more resources.
Offering a menu of prevention tools, including less intensive lifestyle intervention (e.g., medical nutrition therapy), may help balance demands for efficiency, effectiveness, and accommodating individual preferences and provider decision-making. Early evaluation of the UK national program (≥16 hours) resulted in comparable weight loss trends and length of participation as the US national program (≥22 hours; see Table 1 comparing intensities).6,9,12 These findings suggest that a less-intensive approach in a population-based strategy is comparable to more structured and lengthier programs and may have higher uptake at lower costs. To enhance overall impact, alternatives to higher intensity ILI should be incorporated into national and regional programs to offer evidence-based intervention to lower tiered risk groups and/or high-risk adults unwilling or unable to participate in more intensive programs (Figure 2; Intensity, Variable). Additionally, we suggest further examination of intermediate outcomes, such as number and variety of goals identified, as potential measures of participation linked to risk reduction that may serve as program performance metrics. A novel pilot program based on a practical, unstructured, customized approach has been initiated among US veterans, although long-term outcomes from larger studies are awaited.39
Most diabetes prevention strategies to date, aside from ILI, involve medical nutrition training (MNT; in-depth, individualized diet-based treatment by a nutritionist over 2–3 hours per year).40 Despite broad support,41 there is poor access and linkage to MNT in most countries. Other approaches, such as life skills training and coaching (e.g., certified health coaches supporting patient-centered lifestyle goals integrated in a clinic setting over 6–12 months),42,43 may broaden reach. Further cost-effectiveness analyses are needed to examine whether these efforts justify the additional resources needed to train and support such a workforce. It is also important to emphasize that all aspects related to cost-effectiveness drastically vary among countries and population groups. The European IMAGE diabetes prevention toolkit and training curriculum offers structured guidance to support practical, stand-alone efforts around healthy nutrition, sleep hygiene, physical activity, and stress and smoking reduction.44
Priority III: Intervention Content and Delivery – Is There a Need to Further Evolve?
National diabetes prevention campaigns have emphasized fidelity to the core components of ILI as tested in clinical trials.13,45 Since 2011, the Centers for Disease Control and Prevention (CDC) has established standards for delivering the translational US program, including an approved curriculum (publicly available at no cost) and defined national performance metrics. As our understanding of dietary recommendations has evolved over the last two decades, there is growing evidence to support low and very-low carbohydrate diets, as opposed to low-fat, calorie-restricted diets that were tested as part of ILI in the US.46 A recent multinational expert panel recommended healthy eating patterns that appear to reduce incident T2D (Mediterranean47 and Nordic-style48), and that curricula should include individual-tailoring to accommodate diverse needs and cultural and societal factors.49 Dietary strategies, such as modified fasting (e.g., early time restricted feeding with 6-hour feeding) independent of weight loss, also have shown promise with improving insulin sensitivity among adults with prediabetes.50 Further evaluation of updated evidence-based nutritional programs, such as a very low carbohydrate diet with higher fat content51 or the Mediterranean diet41 may improve acceptability (palatability) and thereby retention in ILIs. A recent evaluation showed that healthier and more sustainable dietary patterns are generally lower in cost than current western diets, and can be cost-competitive in low-income settings if a health- and environmentally-sensitive development policy are in place.52 The Finnish DPS showed that diet quality did not significantly change costs and that increased daily fiber lowered food costs.53
Tailoring lifestyle programs for delivery to special populations may also better support T2D risk reduction. We offer illustrative examples of how tailored delivery to groups based on shared comorbidities, demographic characteristics, and/or specific risk factors, may improve program outcomes (Figure 2; Content Delivery, Group-aligned). Mental health conditions, like depression and post-traumatic stress disorder, can interfere with participation in group programming and progress toward intervention goals. Building upon evidence from tailored interventions for T2D and obesity with comorbid depression, ILI coupled with additional resources, such as skill-building exercises from cognitive behavioral therapy, may better address comorbid-specific barriers and improve behavioral change.54–56 Examining programming that further promotes psychosocial factors associated with increased engagement, such as motivation (via motivational interviewing, tailored messaging), activation and social support may improve intervention success. Programs with content tailored for special populations, such as women veterans in the US,57 may promote greater cohesion and synergy. Peer-led58 and peer-coaching59 models offer attractive approaches that may improve uptake and acceptability in highest-risk, hardest to reach subgroups, but the data is conflicting.60 A community-based, peer-support ILI in a low-resource setting in Kerala, India resulted in a nonsignificant reduction in incident T2D after two years at modest cost per participant ($22 USD).61 Additionally, high-risk households may be a target to reinforce long-term behavioral change. Spouses of adults participating in an ILI led to significant reduced weight loss and improved health-related behaviors,62 suggesting potential ‘spillover’ benefit at a household level. Lastly, lifestyle programs may have spillover benefits in sleep, the 3rd pillar of lifestyle in addition to activity and nutrition, that may further reduce diabetes risk.63 Future efforts should consider incorporating sleep hygiene as part of strategies to prevent T2D.
Priority IV: Change Readiness – Can Programming Better Address an Individual’s Competing Needs?
Behavioral change strategies are a key component of ILI to prevent T2D. Various theoretical approaches underpinned the landmark prevention trials (e.g., Social Cognitive Theory, the Transtheoretical Model, and the Theory of Planned Behavior);64 however, translational efforts may have led to compromising on grounded theory to focus more on practical aspects of scaling up ILI delivery.65 Real-world adaptations that promote elements of behavior change theory (e.g., goal setting, individual tailoring, problem solving and increasing self-efficacy) as the primary targets of the program (rather than activity and weight-loss benchmarks) show promise. A recent US pilot study tested an adapted ILI that focused on individually-tailored goals, plus flexibility to adjust goals over time as needed, leading to four-times greater HbA1c reduction and odds of having normoglycemia at follow-up compared to the standard ILI approach with pre-set weight loss and activity goals.66 We thereby support efforts to personalize content delivery to promote engagement (Figure 2; Content Delivery, Personalized). Furthermore, translational efforts should invest to identify strategies to link individuals who are highly motivated, a known predictor of program success, to ILI. Approaches may include building effective, targeted marketing campaigns,67 incorporating change readiness into referral processes and offering a ‘presession’ class to better match enrolled and engaged participants.68 Patient preferences to program characteristics (e.g., delivery platform, communication frequency, group activities and time requirements) may be another strong predictor of engagement. Studies are currently underway to examine how patient preference may predict engagement and how programs can better adapt to address these factors.69,70 For less motivated adults, concepts from behavioral economics, which employs elements of economics and psychology to better understand behavior in the real-world, may optimize engagement by meeting adults “where they are” in their decision-making process. For example, cognitive biases to prioritize short-term over long-term payoffs, (e.g., seeking high-calorie foods despite long-term metabolic risk) may be key unaddressed targets to promote behavioral change.71 Soler et al. also offers a compelling roadmap to improve translational outcomes through small actions that “nudge” individuals to enroll and follow through with their action plans.72
Modest real-world ILI impact raises uncertainty whether strategies that more directly address an individual’s competing priorities leads to improved program uptake. Unaddressed social determinants of health (SDOH) likely fuel competing priorities and the racial/ethnic and income disparities in program outcomes. There is growing evidence of the relationship between SDOH and its impact on behavioral outcomes and thereby diabetes prevention efforts, including social support (e.g., child care), urban planning that supports walkability and recreation to increase physical activity, and food and housing security.73 Qualitative studies suggest strategies to support childcare, transportation needs, and class schedule flexibility may improve referrals and/or enrollment.74 Screening and delivering prevention programs in community-based settings co-aligned to support social needs (e.g. food pantry sites) offers a promising approach that may both concurrently reduce barriers and streamline services (Figure 2;Content Delivery, Personalized, Delivered in safety-net, Health-relate social needs support).75 While outside the scope of this review, further work to identify how SDOH impact ILI engagement and how national campaigns can best support, rather than exacerbate, efforts to combat these factors may be critical if lifestyle intervention translational potential is to be fully realized by everyone.
Priority V: Role of Technology –How to Harness Opportunities with Oversight?
There are increasing global efforts to scale digital tools in chronic disease prevention, as exemplified by the recent WHO Global Strategy on Digital Health.76 Technology-assisted diabetes prevention delivery has shown promise. A randomized trial in southeast India among men with IGT demonstrated that utilizing texts to deliver curriculum content and support resulted in a 36% relative risk reduction in incident T2D over two years compared to usual care77 and a sustained effect over five years,78 yet a similar model was less effective in the US.79 Online (delivered by computer, laptop, tablet, or smart phone) and distance-learning (using video and/or phone to connect to remote teaching/group classes; Figure 2; Technology, Platforms) platforms have rapidly expanded in the past few years, driven by increasing insurance coverage in the US and digital pilot programs in the UK, the need to expand access beyond the reach of in-person programming, and to ensure safety and convenience (e.g., on-demand) during the COVID-19 pandemic. While online programming offers the potential of greater reach at lower cost, its effectiveness in terms of engagement, retention, and weight loss outcomes compared to in-person programming is less clear.13,80 Additionally, traditionally marginalized groups (e.g., racial and ethnic minority and low-income populations) have fewer incremental gains with technology-assisted strategies.81 Nonetheless, early evidence suggests that distance-learning delivery may be especially promising, with a report showing that over half of remote participants complete ≥9 months of the lifestyle programs, versus 20% of online-only participants.13
For online delivery, a challenge is lacking uniform measures to assure sufficient engagement in an asynchronously delivered intervention. For example, an online participant may connect for as little as five minutes or five hours weekly but both scenarios may “count” equally toward adherence standards, which can make it difficult to assure fidelity and compare effectiveness across modalities of program delivery. More work is needed to identify technology-supported engagement benchmarks (e.g., standardized components of weekly interactions and assignments that define engagement on an app-based platform) that link to clinically meaningful outcomes. Policy makers could incorporate these standardized metrics into program certification to ensure program fidelity. The aforementioned behavioral economic tools, such as nudges, delivered via text, online messaging or phone, and may improve program retention and reinforce behavioral change.72 The increasing use of integrated electronic medical records offers the opportunity to build bidirectional referral networks to better match at-risk adults to community-based programs and empower clinicians to support their patients’ goals. The rapid adjustments forced by the COVID-19 pandemic offer a ripe opportunity for researchers and policymakers alike to assess how technology-supported programs may help or hinder delivery to guide future policy based on evidence rather than assumptions and industry incentives.
Priority VI: Role of Pharmacotherapy– Is it Time to Offer Metformin to Adults at Highest Risk?
Although numerous clinical trials show the efficacy of medications to reduce incident diabetes, pharmacotherapy for diabetes prevention remains controversial and relatively few patients are prescribed medication.82 The recent US Preventative Task Force Review found that metformin was associated with less incident T2D (pooled RRs 0.73; 95% CI, 0.64–0.83) when compared to placebo or control group.83 Alpha glucosidase inhibitors have been tested for diabetes prevention with the assumption that reducing post-prandial glucose excursions in persons with IGT may help preserve insulin secretion or action, thus preventing incident T2D.82 The success of GLP1-agonists for sustained weight loss84 offer promise as a powerful, yet costly, pharmacologic option in diabetes prevention for high-risk adults with obesity. However, the use of glucose-lowering drugs raises concerns regarding medicalization of prediabetes, potential side effects, sustainability, costs, and lack of FDA indications for diabetes prevention.4,85 Acarbose has been approved for the indication of IGT in several countries, although it is not marketed for this indication. Although the Indian DPP showed that the addition of metformin to ILI did not provide additive benefits, metformin has been shown to produce cost-savings while further supported by positive long-term safety and reasonable tolerability data.86 Metformin did provide significant levels of risk reduction compared to placebo in the US DPP trial,3 albeit with heterogenous treatment effects, such that participants in the highest risk quartiles benefited from metformin while those in the lowest risk quartile did not.27 Therefore, US clinical care guidelines on the use of metformin for diabetes prevention focuses on adults at highest risk, including those <60 years, more obese (BMI >35 kg/m2), and women with a history of gestational diabetes (GDM).87 The recently revised US Preventive Services Task Force recommendations now also highlight metformin as an effective prevention option.83
A stepwise, risk-stratified approach to pharmacotherapy may mitigate concerns regarding medicalization of prediabetes while addressing diabetes prevention more effectively in real-world settings. For example, in the D-CLIP study,22 adults with iIGT, iIFG, or IFG+IGT were randomized to a culturally-tailored ILI with the stepwise addition of metformin for those at highest risk of incident T2D after ≥4 months of follow-up (i.e., IFG+IGT or IFG+ HbA1c ≥5.7%).22 Similar approaches can be feasibly delivered in many health systems using basic provider-level education and electronic health record enabled tools to support patient identification and follow-up. Early evidence suggests the stepwise addition of a GLP1-agonist to ILI as a highly effective, yet costly, alternative to metformin to reduce incident diabetes among high-risk adults with obesity, but further long-term data on efficacy and safety are needed.88 Novel anti-obesity medications, such as the dual GIP-GLP1-agonist tirzepatide,89 may offer yet more powerful tools on the horizon. Pharmacotherapy appears to have untapped potential as an adjunctive treatment to ILI (e.g., for the highest risk participants, maintenance booster following ILI and/or non-responders) or stand-alone treatment offered to individuals who decline, lack access, or prematurely discontinue ILI (Figure 2; Intensity, Variable, Medication). Despite other medications, such as alpha glucosidase inhibitors and thiazolidinedione, demonstrating short-term efficacy to prevent diabetes, their use is limited by risk of adverse drug effect, lack of sustained long-term benefits and availability on the market.4
Conclusions
Population level reach and engagement in lifestyle interventions cannot exist in a vacuum. The COVID-19 pandemic has further strengthened our collective resolve to also address structural barriers and SDOH. Behavioral strategies, such as ILI delivery, are largely influenced by upstream societal-level policies, such as agriculture, food manufacturing, pricing and accessibility, adaptation to urban, sedentary living and the built environment. Further examination of the attributable risk of these upstream factors to the growing diabetes burden is needed, coupled with population-level interventions, such as smoking ban laws, regulation of advertisements, taxes on sugar-sweetened beverages, promoting green spaces, and healthy food subsidies. Additionally, supporting large-scale lifestyle intervention efforts coupled with the recommended population-level policies may lead to broader healthy lifestyle changes and subsequent reduction in all-cause mortality that ILI independently have failed to demonstrate to-date. Furthermore, efforts to better identify and address risk in children and adolescents is needed. Rigorous evaluation through modelling and natural experiments will help inform multilevel national primary prevention programs to effectively target individuals across the spectrum of risk, engagement, and capacity.90
By leveraging lessons learned over the last two decades, we can innovate and revitalize our approach to diabetes prevention when it is most needed. More precise risk assessment can better match individuals to the intervention that they will draw the greatest benefit(s) from. Less intensive, evidence-based options will offer a menu of actionable, pragmatic tools for at-risk adults who are pre-contemplative and/or unable to participate in intensive programming. Several national care guidelines also endorse metformin as an effective preventive intervention, specifically for higher risk adults as a stepwise addition to ILI. Customizing program content to an individual’s habits, identity, and comorbidities will improve program engagement. Addressing competing social needs and supporting an individual in their decision-making process will further enhance engagement. The promise of technology may bolster these efforts but will require close oversight. Building upon Figure 2 as a conceptional model of the essential elements for person-centered delivery, program developers can draw upon these to support current national strategies that reflect specific resources, risk burden and preferences in their settings. Integrating these tools into existing delivery models is critical to acknowledge the ever-increasing competing demands and time constraints in healthcare settings today. Further work is needed to assess the effectiveness of these individual components in the real-world, how best to incorporate them in existing systems, what are the incremental costs, and if synergistic effects exist. Real-world impact on the prevention of type 2 diabetes can only be fully realized with programming that better acknowledges the current evidence of an individual’s risk, readiness, barriers, and digital competency. The need to deliver effective and population-level diabetes prevention services is timelier than ever.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Competing interests
The authors declare that they have no conflict of interests to report.
References
- 1.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes care. 1997;20(4):537–544. [DOI] [PubMed] [Google Scholar]
- 2.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haw JS, Galaviz KI, Straus AN, et al. Long-term Sustainability of Diabetes Prevention Approaches: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Intern Med. 2017;177(12):1808–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albright AL, Gregg EW. Preventing type 2 diabetes in communities across the U.S.: the National Diabetes Prevention Program. Am J Prev Med. 2013;44(4 Suppl 4):S346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valabhji J, Barron E, Bradley D, et al. Early Outcomes From the English National Health Service Diabetes Prevention Programme. Diabetes Care. 2020;43(1):152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramachandran A, Snehalatha C, Mary S, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49(2):289–297. [DOI] [PubMed] [Google Scholar]
- 8.Saaristo T, Peltonen M, Keinanen-Kiukaanniemi S, et al. National type 2 diabetes prevention programme in Finland: FIN-D2D. Int J Circumpolar Health. 2007;66(2):101–112. [DOI] [PubMed] [Google Scholar]
- 9.Ely EK, Gruss SM, Luman ET, et al. A National Effort to Prevent Type 2 Diabetes: Participant-Level Evaluation of CDC’s National Diabetes Prevention Program. Diabetes care. 2017;40(10):1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rintamaki R, Rautio N, Peltonen M, et al. Long-term outcomes of lifestyle intervention to prevent type 2 diabetes in people at high risk in primary health care. Prim Care Diabetes. 2021;15(3):444–450. [DOI] [PubMed] [Google Scholar]
- 11.Alouki K, Delisle H, Bermudez-Tamayo C, Johri M. Lifestyle Interventions to Prevent Type 2 Diabetes: A Systematic Review of Economic Evaluation Studies. J Diabetes Res. 2016;2016:2159890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannon MJ, Masalovich S, Ng BP, et al. Retention Among Participants in the National Diabetes Prevention Program Lifestyle Change Program, 2012–2017. Diabetes care. 2020;43(9):2042–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruss SM, Nhim K, Gregg E, Bell M, Luman E, Albright A. Public Health Approaches to Type 2 Diabetes Prevention: the US National Diabetes Prevention Program and Beyond. Curr Diab Rep. 2019;19(9):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritchie ND, Baucom KJW, Sauder KA. Current Perspectives on the Impact of the National Diabetes Prevention Program: Building on Successes and Overcoming Challenges. Diabetes Metab Syndr Obes. 2020;13:2949–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerr D, Glantz N. Diabetes, like COVID-19, is a wicked problem. Lancet Diabetes Endocrinol. 2020;8(11):873–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin AL, Vittinghoff E, Olgin JE, Pletcher MJ, Marcus GM. Body Weight Changes During Pandemic-Related Shelter-in-Place in a Longitudinal Cohort Study. JAMA Netw Open. 2021;4(3):e212536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright L, Stallings-Smith S, Arikawa AY. Associations between food insecurity and prediabetes in a representative sample of U.S. Adults (NHANES 2005–2014). Diabetes Res Clin Pract. 2019;148:130–136. [DOI] [PubMed] [Google Scholar]
- 18.International AIDS Society. About The HIV Differentiated Service Delivery. Differentiated Service Delivery 2021; https://differentiatedservicedelivery.org/about. Accessed March 2021. [Google Scholar]
- 19.Shahraz S, Pittas AG, Kent DM. Prediabetes Risk in Adult Americans According to a Risk Test. JAMA Intern Med. 2016;176(12):1861–1863. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Devlin HM, Smith B, et al. Effect of lifestyle interventions on cardiovascular risk factors among adults without impaired glucose tolerance or diabetes: A systematic review and meta-analysis. PLoS One. 2017;12(5):e0176436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell MD, Sathish T, Zimmet PZ, et al. Benefit of lifestyle-based T2DM prevention is influenced by prediabetes phenotype. Nat Rev Endocrinol. 2020;16(7):395–400. [DOI] [PubMed] [Google Scholar]
- 22.Weber MB, Ranjani H, Staimez LR, et al. The Stepwise Approach to Diabetes Prevention: Results From the D-CLIP Randomized Controlled Trial. Diabetes Care. 2016;39(10):1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yip WCY, Sequeira IR, Plank LD, Poppitt SD. Prevalence of Pre-Diabetes across Ethnicities: A Review of Impaired Fasting Glucose (IFG) and Impaired Glucose Tolerance (IGT) for Classification of Dysglycaemia. Nutrients. 2017;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner R, Heni M, Tabak AG, et al. Pathophysiology-based subphenotyping of individuals at elevated risk for type 2 diabetes. Nat Med. 2021;27(1):49–57. [DOI] [PubMed] [Google Scholar]
- 25.Rooney MR, Rawlings AM, Pankow JS, et al. Risk of Progression to Diabetes Among Older Adults With Prediabetes. JAMA Intern Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gujral UP, Narayan KMV. Diabetes in Normal-Weight Individuals: High Susceptibility in Nonwhite Populations. Diabetes Care. 2019;42(12):2164–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sussman JB, Kent DM, Nelson JP, Hayward RA. Improving diabetes prevention with benefit based tailored treatment: risk based reanalysis of Diabetes Prevention Program. BMJ. 2015;350:h454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz PE, Li J, Lindstrom J, Tuomilehto J. Tools for predicting the risk of type 2 diabetes in daily practice. Horm Metab Res. 2009;41(2):86–97. [DOI] [PubMed] [Google Scholar]
- 29.Duru OK. Using Personalized Risk/Benefit Profiles in SDM for Diabetes Prevention. In: Patient-Centered Outcomes Research Institute; 2019. [Google Scholar]
- 30.Bergman M, Manco M, Sesti G, et al. Petition to replace current OGTT criteria for diagnosing prediabetes with the 1-hour post-load plasma glucose>/=155mg/dl (8.6mmol/L). Diabetes Res Clin Pract. 2018;146:18–33. [DOI] [PubMed] [Google Scholar]
- 31.Jagannathan R, Buysschaert M, Medina JL, et al. The 1-h post-load plasma glucose as a novel biomarker for diagnosing dysglycemia. Acta Diabetol. 2018;55(6):519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ackermann RT. From Programs to Policy and Back Again: The Push and Pull of Realizing Type 2 Diabetes Prevention on a National Scale. Diabetes Care. 2017;40(10):1298–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X, Siegel KR, Ng BP, et al. Cost-effectiveness of Diabetes Prevention Interventions Targeting High-risk Individuals and Whole Populations: A Systematic Review. Diabetes Care. 2020;43(7):1593–1616. [DOI] [PubMed] [Google Scholar]
- 34.Sathish T, Shaw JE, Oldenburg B, Mahal A. Comment on Zhou et al. Cost-effectiveness of Diabetes Prevention Interventions Targeting High-risk Individuals and Whole Populations: A Systematic Review. Diabetes Care 2020;43:1593–1616. Diabetes Care. 2020;43(12):e204–e205. [DOI] [PubMed] [Google Scholar]
- 35.Andellini M, Manco M, Esposito MT, Tozzi AE, Bergman M, Ritrovato M. A simulation model estimates lifetime health and economic outcomes of screening prediabetes using the 1-h plasma glucose. Acta Diabetol. 2022. [DOI] [PubMed] [Google Scholar]
- 36.Cefalu WT, Buse JB, Tuomilehto J, et al. Update and Next Steps for Real-World Translation of Interventions for Type 2 Diabetes Prevention: Reflections From a Diabetes Care Editors’ Expert Forum. Diabetes Care. 2016;39(7):1186–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galaviz KI, Weber MB, Straus A, Haw JS, Narayan KMV, Ali MK. Global Diabetes Prevention Interventions: A Systematic Review and Network Meta-analysis of the Real-World Impact on Incidence, Weight, and Glucose. Diabetes Care. 2018;41(7):1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aziz Z, Absetz P, Oldroyd J, Pronk NP, Oldenburg B. A systematic review of real-world diabetes prevention programs: learnings from the last 15 years. Implement Sci. 2015;10:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorcely B, Bergman M, Tenner C, Katz K, Jagannathan R, Pirraglia E. Manhattan Veterans Affairs Medical Center Diabetes Prevention Clinic. Clin Diabetes. 2020;38(3):291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raynor HA, Davidson PG, Burns H, et al. Medical Nutrition Therapy and Weight Loss Questions for the Evidence Analysis Library Prevention of Type 2 Diabetes Project: Systematic Reviews. J Acad Nutr Diet. 2017;117(10):1578–1611. [DOI] [PubMed] [Google Scholar]
- 41.Evert AB, Dennison M, Gardner CD, et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care. 2019;42(5):731–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malecki HL, Gollie JM, Scholten J. Physical Activity, Exercise, Whole Health, and Integrative Health Coaching. Phys Med Rehabil Clin N Am. 2020;31(4):649–663. [DOI] [PubMed] [Google Scholar]
- 43.Dexter AS, Pope JF, Erickson D, Fontenot C, Ollendike E, Walker E. Cooking Education Improves Cooking Confidence and Dietary Habits in Veterans. Diabetes Educ. 2019;45(4):442–449. [DOI] [PubMed] [Google Scholar]
- 44.Lindstrom J, Neumann A, Sheppard KE, et al. Take action to prevent diabetes--the IMAGE toolkit for the prevention of type 2 diabetes in Europe. Horm Metab Res. 2010;42 Suppl 1:S37–55. [DOI] [PubMed] [Google Scholar]
- 45.National Health Service. NHS Diabetes Prevention Program. 2021; https://www.england.nhs.uk/wp-content/uploads/2016/08/dpp-faq.pdf. [Google Scholar]
- 46.Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2015;31(1):1–13. [DOI] [PubMed] [Google Scholar]
- 47.Martin-Pelaez S, Fito M, Castaner O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms. A Review. Nutrients. 2020;12(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tertsunen HM, Hantunen S, Tuomainen TP, Virtanen JK. Adherence to a healthy Nordic diet and risk of type 2 diabetes among men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Nutr. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forouhi NG, Misra A, Mohan V, Taylor R, Yancy W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ. 2018;361:k2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018;27(6):1212–1221 e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hafez Griauzde D, Saslow L, Patterson K, et al. Mixed methods pilot study of a low-carbohydrate diabetes prevention programme among adults with pre-diabetes in the USA. BMJ Open. 2020;10(1):e033397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Springmann M, Clark MA, Rayner M, Scarborough P, Webb P. The global and regional costs of healthy and sustainable dietary patterns: a modelling study. Lancet Planet Health. 2021;5(11):e797–e807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ottelin AM, Lindstrom J, Peltonen M, et al. Costs of a self-selected, health-promoting diet among the participants of the Finnish Diabetes Prevention Study. Diabetes Care. 2007;30(5):1275–1277. [DOI] [PubMed] [Google Scholar]
- 54.Daumit GL, Dickerson FB, Wang NY, et al. A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med. 2013;368(17):1594–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma J, Rosas LG, Lv N, et al. Effect of Integrated Behavioral Weight Loss Treatment and Problem-Solving Therapy on Body Mass Index and Depressive Symptoms Among Patients With Obesity and Depression: The RAINBOW Randomized Clinical Trial. JAMA. 2019;321(9):869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cummings DM, Lutes LD, Littlewood K, et al. Randomized Trial of a Tailored Cognitive Behavioral Intervention in Type 2 Diabetes With Comorbid Depressive and/or Regimen-Related Distress Symptoms: 12-Month Outcomes From COMRADE. Diabetes Care. 2019;42(5):841–848. [DOI] [PubMed] [Google Scholar]
- 57.Dyer KE, Moreau Jl PhD MPH, Finley E PhD MPH, et al. Tailoring an evidence-based lifestyle intervention to meet the needs of women Veterans with prediabetes. Women Health. 2020;60(7):748–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mayer VL, Vangeepuram N, Fei K, et al. Outcomes of a Weight Loss Intervention to Prevent Diabetes Among Low-Income Residents of East Harlem, New York. Health Educ Behav. 2019;46(6):1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heisler M, Kullgren J, Richardson C, et al. Study protocol: Using peer support to aid in prevention and treatment in prediabetes (UPSTART). Contemp Clin Trials. 2020;95:106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sampson M, Clark A, Bachmann M, et al. Effects of the Norfolk diabetes prevention lifestyle intervention (NDPS) on glycaemic control in screen-detected type 2 diabetes: a randomised controlled trial. BMC Med. 2021;19(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thankappan KR, Sathish T, Tapp RJ, et al. A peer-support lifestyle intervention for preventing type 2 diabetes in India: A cluster-randomized controlled trial of the Kerala Diabetes Prevention Program. PLoS Med. 2018;15(6):e1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmittdiel JA, Cunningham SA, Adams SR, Nielsen J, Ali MK. Influence of a New Diabetes Diagnosis on the Health Behaviors of the Patient’s Partner. Ann Fam Med. 2018;16(4):290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuomilehto H, Peltonen M, Partinen M, et al. Sleep duration, lifestyle intervention, and incidence of type 2 diabetes in impaired glucose tolerance: The Finnish Diabetes Prevention Study. Diabetes Care. 2009;32(11):1965–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baker MK, Simpson K, Lloyd B, Bauman AE, Singh MA. Behavioral strategies in diabetes prevention programs: a systematic review of randomized controlled trials. Diabetes Res Clin Pract. 2011;91(1):1–12. [DOI] [PubMed] [Google Scholar]
- 65.Hawkes RE, Miles LM, French DP. The theoretical basis of a nationally implemented type 2 diabetes prevention programme: how is the programme expected to produce changes in behaviour? Int J Behav Nutr Phys Act. 2021;18(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ritchie ND, Sauder KA, Kaufmann PG, Perreault L. Patient-Centered Goal-Setting in the National Diabetes Prevention Program: A Pilot Study. Diabetes Care. 2021;44(11):2464–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams A, Bowen SA, Murphy M, Costa K, Echavarria C, Knight M. Enhancing the Adoption of Evidence-Based Health Marketing and Promotion Strategies in Local Communities: Building a Communication Dissemination and Support System for the National Diabetes Prevention Program. Health Promot Pract. 2021:15248399211013817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ritchie ND, Kaufmann PG, Gritz RM, Sauder KA, Holtrop JS. Presessions to the National Diabetes Prevention Program May be a Promising Strategy to Improve Attendance and Weight Loss Outcomes. Am J Health Promot. 2019;33(2):289–292. [DOI] [PubMed] [Google Scholar]
- 69.Sommer J, Dyczmons J, Grobosch S, et al. Preferences of people with type 2 diabetes for telemedical lifestyle programmes in Germany: protocol of a discrete choice experiment. BMJ Open. 2020;10(9):e036995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dintsios CM, Chernyak N, Grehl B, Icks A. Quantified patient preferences for lifestyle intervention programs for diabetes prevention-a protocol for a systematic review. Syst Rev. 2018;7(1):214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kullgren JT, Hafez D, Fedewa A, Heisler M. A Scoping Review of Behavioral Economic Interventions for Prevention and Treatment of Type 2 Diabetes Mellitus. Curr Diab Rep. 2017;17(9):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soler RE, Proia K, Jackson MC, et al. Nudging to Change: Using Behavioral Economics Theory to Move People and Their Health Care Partners Toward Effective Type 2 Diabetes Prevention. Diabetes Spectr. 2018;31(4):310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harrison CR, Phimphasone-Brady P, DiOrio B, et al. Barriers and Facilitators of National Diabetes Prevention Program Engagement Among Women of Childbearing Age: A Qualitative Study. Diabetes Educ. 2020;46(3):279–288. [DOI] [PubMed] [Google Scholar]
- 75.Cheyne K, Smith M, Felter EM, et al. Food Bank-Based Diabetes Prevention Intervention to Address Food Security, Dietary Intake, and Physical Activity in a Food-Insecure Cohort at High Risk for Diabetes. Prev Chronic Dis. 2020;17:E04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mariano B. Towards a global strategy on digital health. Bull World Health Organ. 2020;98(4):231–231A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramachandran A, Snehalatha C, Ram J, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2013;1(3):191–198. [DOI] [PubMed] [Google Scholar]
- 78.Nanditha A, Snehalatha C, Raghavan A, et al. The post-trial analysis of the Indian SMS diabetes prevention study shows persistent beneficial effects of lifestyle intervention. Diabetes Res Clin Pract. 2018;142:213–221. [DOI] [PubMed] [Google Scholar]
- 79.Fischer HH, Durfee MJ, Raghunath SG, Ritchie ND. Short Message Service Text Message Support for Weight Loss in Patients With Prediabetes: Pragmatic Trial. JMIR Diabetes. 2019;4(2):e12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Golovaty I, Wadhwa S, Fisher L, et al. Reach, engagement and effectiveness of in-person and online lifestyle change programs to prevent diabetes. BMC Public Health. 2021;21(1):1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Samuels-Kalow M, Jaffe T, Zachrison K. Digital disparities: designing telemedicine systems with a health equity aim. Emerg Med J. 2021. [DOI] [PubMed] [Google Scholar]
- 82.Knowler WC, Crandall JP. Pharmacologic Randomized Clinical Trials in Prevention of Type 2 Diabetes. Curr Diab Rep. 2019;19(12):154. [DOI] [PubMed] [Google Scholar]
- 83.Jonas DE, Crotty K, Yun JDY, et al. In: Screening for Prediabetes and Type 2 Diabetes Mellitus: An Evidence Review for the U.S. Preventive Services Task Force. Rockville (MD)2021. [PubMed] [Google Scholar]
- 84.Wilding JPH, Batterham RL, Calanna S, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021;384(11):989. [DOI] [PubMed] [Google Scholar]
- 85.Piller C. The war on ‘prediabetes’ could be a boon for pharma—but is it good medicine? In. Science 2019. [Google Scholar]
- 86.Moin T, Schmittdiel JA, Flory JH, et al. Review of Metformin Use for Type 2 Diabetes Prevention. Am J Prev Med. 2018;55(4):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.American Diabetes A. 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S34–S39. [DOI] [PubMed] [Google Scholar]
- 88.Perreault L, Davies M, Frias JP, et al. Changes in Glucose Metabolism and Glycemic Status With Once-Weekly Subcutaneous Semaglutide 2.4 mg Among Participants With Prediabetes in the STEP Program. Diabetes Care. 2022;45(10):2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jastreboff AM, Aronne LJ, Stefanski A. Tirzepatide Once Weekly for the Treatment of Obesity. Reply. N Engl J Med. 2022;387(15):1434–1435. [DOI] [PubMed] [Google Scholar]
- 90.Chan JCN, Lim LL, Wareham NJ, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet. 2021;396(10267):2019–2082. [DOI] [PubMed] [Google Scholar]
Figure 2 References
- 91.Chae JS, Kang R, Kwak JH, et al. Supervised exercise program, BMI, and risk of type 2 diabetes in subjects with normal or impaired fasting glucose. Diabetes Care. 2012;35(8):1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moin T, Schmittdiel JA, Flory JH, et al. Review of Metformin Use for Type 2 Diabetes Prevention. Am J Prev Med. 2018;55(4):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kirley K, Sachdev N Digital Health-Supported Lifestyle Change Programs to Prevent Type 2 Diabetes. Diabetes Spectr. 2018;31(4):303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vadheim LM, Patch K, Brokaw SM, et al. Telehealth delivery of the diabetes prevention program to rural communities. Transl Behav Med. 2017;7(2):286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rehm CD, Marquez ME, Spurrell-Huss E, Hollingsworth N, Parsons AS. Lessons from Launching the Diabetes Prevention Program in a Large Integrated Health Care Delivery System: A Case Study. Popul Health Manag. 2017;20(4):262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O’Brien MJ. Prevent Diabetes Mellitus (PreDM) Clinical Decision Support Intervention in Community Health Centers. In. Northwestern University: National Institute of Health; 2018. [Google Scholar]
- 97.Yost O, DeJonckheere M, Stonebraker S, et al. Continuous Glucose Monitoring With Low-Carbohydrate Diet Coaching in Adults With Prediabetes: Mixed Methods Pilot Study. JMIR Diabetes. 2020;5(4):e21551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gomez ML, Hieronymus LB, Ashford KB, Barnett JM, Renn TA. Linking Postpartum and Parenting Women With a National Diabetes Prevention Program: Recruitment Efforts, Challenges, and Recommendations. Diabetes Spectr. 2018;31(4):324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoerster KD, Tanksley L, Simpson T, et al. Development of a Tailored Behavioral Weight Loss Program for Veterans With PTSD (MOVE!+UP): A Mixed-Methods Uncontrolled Iterative Pilot Study. Am J Health Promot. 2020;34(6):587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Patel ML, Wakayama LN, Bennett GG. Self-Monitoring via Digital Health in Weight Loss Interventions: A Systematic Review Among Adults with Overweight or Obesity. Obesity (Silver Spring). 2021;29(3):478–499. [DOI] [PubMed] [Google Scholar]
- 101.Htet TD, Godneva A, Liu Z, et al. Rationale and design of a randomised controlled trial testing the effect of personalised diet in individuals with pre-diabetes or type 2 diabetes mellitus treated with metformin. BMJ Open. 2020;10(10):e037859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olchanski N, van Klaveren D, Cohen JT, Wong JB, Ruthazer R, Kent DM. Targeting of the diabetes prevention program leads to substantial benefits when capacity is constrained. Acta Diabetol. 2021;58(6):707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]


