Abstract
Raw bioelectrical impedance measurements are often used as a prognosticator of health status given their association with disease states and malnutrition. While studies consistently show the effect of physical characteristics on bioelectrical impedance, few investigations describe the effect of race, particularly for Black adults, and many bioelectrical impedance standards were produced from primarily White adults almost two decades ago. Therefore, this study sought to evaluate the racial differences in bioelectrical impedance measurements using bioimpedance spectroscopy between non-Hispanic White and non-Hispanic Black adults matched for age, sex, and BMI. We hypothesized that Black adults would have a lower phase angle (PA) from higher resistance (R) and lower reactance (Xc) compared to White adults. One-hundred non-Hispanic White (n=50) and non-Hispanic Black (n=50) males and females (M=34, F=66) matched for sex, age, and BMI completed this cross-sectional study. Participants underwent several anthropometric assessments including height, weight, waist circumference, hip circumference, bioimpedance spectroscopy, and dual-energy X-ray absorptiometry. Bioelectrical impedance measures of R, Xc, PA, and impedance (Z) were all collected at frequencies of 5, 50, and 250kHz and bioelectrical impedance vector analysis (BIVA) was performed using 50kHz data. There were no significant differences for any anthropometric variable between Black and White participants in the total sample or by sex groups. In addition, there were no significant racial differences for any bioelectrical impedance assessment including BIVA. Differences in bioelectrical impedance are likely not a function of race between Black and White adults and concerns regarding its utility should not be based on this characteristic.
Keywords: body composition, race, ethnicity, obesity, bioelectrical impedance
Graphical Abstract
After accounting for all relevant physical attributes known to influence bioelectrical impedance measurements, these assessments, including bioimpedance vector analyses, were not significantly different between non-Hispanic Black and White adults. Concerns regarding its utility for Black adults should not be based on race. The absence of race-dependent bioelectrical values may improve clinical confidence and prognostic value in these techniques and limit racial health disparities for Black adults.
1. INTRODUCTION
Bioelectrical impedance analysis (BIA), a method used to quantify the body’s responses to injected electrical currents, has traditionally been used for body fluid or composition estimation. [1] However, body composition estimation from BIA is often limited by its inherent algorithmic challenges, particularly for those with pathologies associated with abnormal hydration status. [2] Consequently, raw bioelectrical values have gained renewed attention outside of body composition estimation due to their ability to assess body tissue characteristics, such as cellular composition, size, and integrity. [3] Resistance (R) – the opposition to electrical currents – and reactance (Xc) – indicative of the capacitive properties of the cell membrane and tissues – are the two primary raw bioelectrical values, which can subsequently be used to calculate impedance (Z) and phase angle (PA). In fact, PA is now considered to be an important prognosticator of health status [4] given its association with several disease states, [5–8] malnutrition, [9] and mortality. [10]
Despite the utility of bioimpedance assessment in practice, there are several physical characteristics that should be considered prior to interpretation. For example, several studies report that sex, age, and body mass index (BMI) are the primary determinants of bioelectrical impedance values. [3,11–13] While the influence of these general characteristics are consistently reported, few investigations describe the effect of race with the majority of studies being conducted almost exclusively in White participants. [3] As such, the majority of established standards for bioelectrical impedance values are derived from primarily White adults and children [14], and the only studies, to our knowledge, addressing racial differences in adults are conflicting and from almost two decades ago [11,12]; which is, interestingly, when BIA was excluded from US health surveillance protocols. So, while bioimpedance assessments may be useful in practice, generalizing these measures without a complete understanding of potential racial variations may lead to inaccurate assessment and interpretation for minority patients.
Unfortunately, health assessment and diagnostic accuracy continues to be an issue for racial minorities, [15] but are particularly important given these group’s disparately high incidence of disease, underdiagnosis, and medical mistrust. [16] Specifically, Black adults are at the highest risk for developing chronic disease [17] and, relative to White adults, have an increased risk of developing cancer, [18] cardiovascular, [19] liver, [18] and kidney disease; [20] all of which are associated with bioimpedance standards produced mostly from White adults. Furthermore, Black adults possess fat-free mass (FFM) characteristics that are the most distinct from White adults. [21] Given that components of FFM are the primary conductors of the electrical currents distributed using BIA, and because there are inherent differences in FFM characteristics between White and Black adults, it is possible that racial variation in body composition characteristics also results in varying bioelectrical impedance values. [22,23] However, because several anthropometric characteristics differ between White and Black individuals in national datasets and in practice, it is difficult to distinguish the true racial differences in bioelectrical impedance. Although it is statistically possible to identify the independent effects of race on bioelectrical impedance through the addition of influential attributes into linear regression models (the most common technique), excessive overfitting is likely and produces error; where the associations between race and bioelectrical impedance appear within the original sample but fail to replicate in other samples or in clinical practice. [24,25] Therefore, the purpose of this study was to evaluate the racial differences in raw and calculated bioimpedance values, produced from bioimpedance spectroscopy (BIS), between non-Hispanic White and non-Hispanic Black adults matched for age, sex, and BMI. We hypothesized that there would be differences between races; specifically, that PA would be lower in Black adults as a product of higher R and lower Xc compared to White adults. These results will provide a better understanding of racial differences in BIA for improved use in clinical practice.
2. MATERIALS AND METHODS
2.1. Participants
A priori sample size calculations for one-tailed independent sample means using a Cohen’s d effect size of 0.50 and an α = 0.05 revealed that an n = 102 (51/group) would produce 80% power. Thus, total of 112 healthy non-Hispanic White (n = 56) and non-Hispanic Black (n = 56) adults between the ages 18 and 65 years were prospectively recruited for this cross-sectional study. Participants were excluded if they were younger than 18 or older than 65; were missing any limbs or part of a limb; had a substantial amount of metal such as a complete joint replacement; were pregnant; trying to become pregnant; or lactating. No participants reported having any chronic cardiovascular or lung disease other than postural orthostatic tachycardia syndrome (n = 1) and asthma (n = 1) and no instance of diabetes, liver, kidney, or thyroid disease. Black participants who were eligible were matched with a White participant for sex, age, and BMI. Of the 56 Black participants recruited, six (F: 2, M: 4) did not have a BMI that matched within ± 2.5 kg/m2 of their White participant pair and thus, these participants and their matched-pairs were excluded (n = 12). There were no mismatches by sex, all BMIs matched within ± 2.5 kg/m2 for an average difference of 0.32 kg/m2, and all participants matched by age using the clusters defined by the American Aging Association [26] for an average difference of 0.04 years. Only three pairs (all females) had a difference in BMI > 2.0 kg/m2 but remained within the same weight classification (obese). Therefore, a total of 100 sex, age, and BMI matched White (n = 50) and Black (n = 50) adults (F: 66, M: 34) were included in the final analysis. The study took place from November 2021 to September 2022, was approved by the University of Southern Mississippi Institutional Review Board (IRB #21-213), and was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants prior to participation.
2.2. Procedures
Participants arrived at the laboratory after an ≥8 h abstention from food, beverage, supplements and medication, and exercise. Upon arrival, participants reported their hydration status using an 8-point color chart [27] where they rated the color of their urine after voiding with all participants indicating adequate hydration status (< 6 on the chart). Following preliminary questionnaires, participants were asked to remove all accessories and metal so that they were wearing only light athletic clothing, and underwent several anthropometric assessments including height, weight, waist circumference (WC), hip circumference (HC), bioimpedance spectroscopy (BIS), and dual-energy X-ray absorptiometry (DXA).
2.3. Body Composition Assessment
Height was measured using a digital stadiometer (SECA 769, Hamburg, Germany) to the nearest tenth (cm) and weight using a calibrated digital scale (SECA, Hamburg, Germany) to the nearest hundredth (kg). Body composition estimates including body fat percent (BF%), fat mass (FM), and fat-free mass (FFM) were collected using DXA (Lunar iDXA, General Electric, Boston, MA, USA) with version 18 enCORE software and used to describe our sample and verify similar body composition components between groups. Participants were positioned on the DXA according to recommended guidelines and reflection scanning techniques were used for larger participants who were unable to fit within the DXA scanning dimensions. [28,29] WC was collected at the level of the superior iliac crest and HC was collected at the widest lateral portion of the hip using a flexible aluminum tape measure. WC and HC were collected in duplicate and averaged to produce a final estimate (TEM: 0.43 cm). Further reliability metrics from our laboratory can be found elsewhere. [30] Waist-to-hip ratio was calculated as the average WC divided by the average HC.
2.4. Bioimpedance Spectroscopy
Bioelectrical impedance was collected using the BIS hand-to-foot electrode model (SFB7, ImpediMed, Carlsbad, CA, USA). BIS, which has been validated against deuterium dilution, [31] and DXA, [32] were also used to collect estimates of total body water (TBW) and body composition, respectively. All BIS assessments were collected immediately after DXA and thus, all participants were supine for ≥3 min prior to testing. Electrodes were placed on the posterior hand and wrist and the anterior foot and ankle, separated by approximately five centimeters in the positions recommended by the manufacturer. Two continuous assessments with a measurement limit of two were conducted, resulting in four total assessments (two individual assessments that collect two bioelectrical impedance measurements each) that were subsequently averaged to produce a final estimate. Coefficients for sex, body density/proportion, and hydration were the default suggested by the manufacturer as previously reported [33] and visual inspection of Cole plots were conducted after each assessment. Three frequencies (5, 50, 250 kHz) were used for the analysis of R, Xc, and PA. PA was calculated from R and XC using the formula arctan(Xc/R) x 180°/π at each frequency. Measured frequencies provided by the associated Bioimp® software (ImpediMed, Carlsbad, CA, USA) were used. These measured frequencies are not collected at the integer values (i.e., 5, 50, 250 kHz) and are instead collected at 5.0669 kHz, 50.0078 kHz, and 250.5112 kHz. As such, these measured frequencies have been defined as their rounded frequencies (i.e., 5, 50, 250 kHz) throughout the manuscript for clarity. The 50 kHz frequency was selected given its consideration as the standard frequency, and 5 kHz and 250 kHz were selected to provide a representative lower and higher frequency relative to the 50 kHz standard. These frequencies were also selected due to their use across other bioelectrical impedance devices which would allow for more appropriate comparisons. In addition, measures of TBW, extracellular fluid, and intracellular fluid were collected and used to describe our sample.
2.5. Bioelectrical Impedance Vector Analysis
Resistance/height (R/H) and reactance/height (Xc/H) in ohm/m were calculated at 50 kHz and used for bioelectrical impedance vector analysis (BIVA); a graphical analysis that plots Z as a vector of R and Xc after adjusting for height (i.e., R/H and Xc/H). [34] Both confidence and tolerance ellipses were generated from BIVA values as suggested by Piccoli et al. [34,35] using BIVA 2002® software developed by Professor Antonio Piccoli, University of Padova. [36] Confidence ellipses were produced for White and Black participants for the total sample and for males and females separately by plotting the group mean of R/H and Xc/H as an Z vector with its 95% confidence interval. The size of the confidence ellipse was determined by sample size, the vector variance using the group standard deviation, and the shape of the ellipse using calculated Pearson product moment correlations. Tolerance ellipses for males and females were generated using bivariate Z vector intervals from a healthy reference population, where White male and White female references from Piccoli et al. [37] were used to generate tolerance regions of 50%, 75%, and 95% which ultimately produce the R/Xc graph. On the R/Xc graph, R/H and Xc/H for each participant were plotted as an individual Z vector.
2.6. Statistical Analyses
Participant characteristics and descriptive information were assessed using independent t-tests. All BIA measures at 5, 50, and 250 kHz were compared between White and Black participants in the total sample and by sex group using independent t-tests with Cohen’s d effect sizes. Mean vectors produced from BIVA confidence ellipses were assessed using Hotelling’s T2 and visual inspection of the interval overlap and vector length. The R/Xc graphs produced from BIVA tolerance ellipses were visually interpreted as the individual vector position relative to the reference. Vectors outside of the 75% tolerance ellipse indicate an abnormal Z with vector displacements on the major axis (vertical) representing abnormal tissue hydration and vector displacements on the minor axis (horizontal) representing abnormal soft tissue mass. Statistical significance was determined at p < 0.050. Data was analyzed using IBM SPSS version 27, Microsoft Excel version 16, and BIVA 2002®. [36]
3. RESULTS
3.1. Participant characteristics
Participant characteristics and results of the independent t-tests are presented in Table 1. There were no significant differences between White and Black participants in the total sample or by sex for any anthropometric variable including age, BMI, height, weight, WC, HC, waist-to-hip ratio, BF%, FM, FFM, TBW, extracellular fluid, and intracellular fluid (all p > 0.050).
Table 1.
Participant Characteristics
| Total (n = 100) |
White (n = 50) |
Black (n = 50) |
Female (n = 66) |
Male (n = 34) |
pa | pb | pc | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| White | Black | White | Black | |||||||
| Male (n) | 34 | 17 | 17 | |||||||
| Female (n) | 66 | 33 | 33 | |||||||
| Age (y) | 23.8 ± 8.1 | 23.8 ± 7.8 | 23.8 ± 8.5 | 23.7 ± 7.7 | 23.9 ± 9.4 | 23.9 ± 8.0 | 23.7 ± 6.8 | 0.990 | 0.932 | 0.909 |
| Height (cm) | 168.6 ± 8.5 | 169.0 ± 8.1 | 168.1 ± 8.8 | 164.9 ± 5.7 | 163.5 ± 5.8 | 177.1 ± 5.6 | 177.0 ± 6.7 | 0.572 | 0.333 | 0.945 |
| Weight (kg) | 77.8 ± 20.7 | 77.6 ± 19.1 | 78.0 ± 22.4 | 71.4 ± 16.8 | 71.4 ± 21.3 | 89.7 ± 17.7 | 90.9 ± 19.0 | 0.921 | 0.999 | 0.849 |
| BMI (kg/m2) | 27.2 ± 6.4 | 27.1 ± 6.1 | 27.4 ± 6.8 | 26.3 ± 6.1 | 26.5 ± 6.9 | 28.6 ± 5.8 | 29.1 ± 6.4 | 0.783 | 0.862 | 0.814 |
| Waist (cm) | 89.3 ± 16.8 | 88.6 ± 17.1 | 90.0 ± 16.8 | 85.9 ± 16.5 | 87.9 ± 17.9 | 93.9 ± 17.4 | 94.1 ± 13.9 | 0.678 | 0.638 | 0.963 |
| Hip (cm) | 101.5 ± 13.7 | 100.7 ± 14.6 | 102.3 ± 12.8 | 99.8 ± 15.9 | 100.7 ± 12.7 | 102.5 ± 11.8 | 105.4 ± 12.9 | 0.557 | 0.791 | 0.494 |
| Waist-to-hip ratio | 0.88 ± 0.07 | 0.88 ± 0.07 | 0.88 ± 0.07 | 0.86 ± 0.06 | 0.87 ± 0.08 | 0.91 ± 0.07 | 0.89 ± 0.04 | 0.918 | 0.680 | 0.394 |
| Body fat % | 31.3 ± 9.5 | 31.7 ± 9.8 | 31.0 ± 9.2 | 34.7 ± 8.2 | 32.9 ± 9.3 | 25.8 ± 10.2 | 27.3 ± 8.1 | 0.722 | 0.413 | 0.652 |
| Fat mass (kg) | 25.3 ± 12.8 | 25.3 ± 12.3 | 25.3 ± 13.4 | 25.8 ± 11.6 | 25.0 ± 13.7 | 24.4 ± 14.0 | 25.9 ± 13.2 | 0.989 | 0.797 | 0.755 |
| Fat-free mass (kg) | 52.5 ± 12.1 | 52.3 ± 11.9 | 52.8 ± 12.5 | 45.6 ± 7.1 | 46.5 ± 9.04 | 65.3 ± 8.0 | 65.0 ± 8.5 | 0.852 | 0.683 | 0.931 |
| Total body water (L) | 39.3 ± 9.4 | 40.0 ± 9.2 | 38.5 ± 9.7 | 35.3 ± 6.1 | 34.0 ± 7.5 | 49.2 ± 6.9 | 47.2 ± 7.2 | 0.419 | 0.450 | 0.411 |
| Total body water (%) | 50.5 ± 5.8 | 51.4 ± 6.0 | 49.7 ± 5.5 | 49.7 ± 5.5 | 48.7 ± 5.4 | 54.8 ± 5.8 | 51.6 ± 5.2 | 0.122 | 0.432 | 0.097 |
| Extracellular Fluid (L) | 16.0 ± 3.9 | 16.2 ± 3.7 | 15.8 ± 4.0 | 14.2 ± 2.3 | 13.8 ± 3.0 | 20.2 ± 2.6 | 19.6 ± 2.9 | 0.527 | 0.547 | 0.474 |
| Extracellular Fluid (%) | 41.2 ± 3.0 | 41.0 ± 2.6 | 41.4 ± 3.4 | 40.4 ± 1.7 | 40.6 ± 1.8 | 42.1 ± 3.6 | 43.0 ± 5.1 | 0.446 | 0.584 | 0.553 |
| Intracellular Fluid (L) | 23.2 ± 5.7 | 23.8 ± 5.6 | 22.7 ± 5.8 | 21.0 ± 3.9 | 20.2 ± 4.6 | 29.0 ± 4.6 | 27.6 ± 4.8 | 0.375 | 0.439 | 0.389 |
| Intracellular Fluid (%) | 59.2 ± 1.9 | 59.3 ± 1.9 | 59.0 ± 2.0 | 59.6 ± 1.7 | 59.7 ± 1.8 | 58.8 ± 2.0 | 58.3 ± 2.2 | 0.401 | 0.584 | 0.505 |
Data presented for the “male” and “female” rows are expressed as n. All other data are presented as mean ± standard deviation.
Statistical significance is determined by independent t-tests.
= p value for race comparisons in the total sample;
= p value for race comparisons in female participants;
= p value for race comparisons in male participants.
3.2. Bioelectrical Impedance Analysis
Raw BIA measurements between Black and White participants are presented in Table 2 and individual participant values are illustrated in Figure 1. There were no significant differences between Black and White participants in the total sample or by sex groups for Z, PA, R, and Xc at any frequency (including 5 kHz and 250 kHz for PA) or for R/H and Xc/H at 50 kHz.
Table 2.
Bioelectrical Analysis Between Sex, Age, and BMI-matched White and Black Adults
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total (n = 100) | White* (n = 50) | Black (n = 50) | Female (n = 66) | Male (n = 34) | d (95%CI)a | d (95%CI)b | d (95%CI)c | |||
|
| ||||||||||
| White* | Black | White* | Black | |||||||
| 5 kHz | ||||||||||
| Impedance (Ω) | 649.7 ± 105.8 | 636.9 ± 97.8 | 662.6 ± 112.7 | 677.2 ± 87.8 | 699.3 ± 115.3 | 558.7 ± 63.7 | 591.3 ± 64.0 | 0.24 (−0.15, 0.64) | 0.22 (−0.27, 0.70) | 0.51 (−0.18, 1.18) |
| Phase angle (°) | 3.12 ± 0.58 | 3.17 ± 0.59 | 3.07 ± 0.56 | 2.92 ± 0.46 | 2.89 ± 0.41 | 3.67 ± 0.51 | 3.42 ± 0.64 | 0.18 (−0.21, 0.58) | 0.07 (−0.42, 0.55) | 0.43 (−0.26, 1.10) |
| Resistance (Ω) | 650.5 ± 105.1 | 636.0 ± 97.94 | 665.0 ± 110.9 | 676.3 ± 87.8 | 703.6 ± 110.8 | 557.9 ± 64.1 | 590.2 ± 64.1 | 0.28 (−0.12, 0.67) | 0.27 (−0.21, 0.76) | 0.51 (−0.18, 1.18) |
| Reactance (Ω) | 35.0 ± 5.3 | 34.6 ± 5.0 | 35.3 ± 5.6 | 34.0 ± 4.6 | 35.4 ± 5.3 | 35.6 ± 5.7 | 35.1 ± 6.1 | 0.14 (−0.26, 0.53) | 0.27 (−0.22, 0.76) | 0.09 (−0.58, 0.76) |
|
| ||||||||||
| 50 kHz | ||||||||||
| Impedance (Ω) | 562.6 ± 99.1 | 546.8 ± 93.7 | 578.4 ± 102.7 | 586.4 ± 84.1 | 614.1 ± 100.9 | 469.9 ± 57.0 | 509.1 ± 64.8 | 0.32 (−0.07, 0.72) | 0.30 (−0.19, 0.78) | 0.64 (−0.05, 1.33) |
| Phase angle (°) | 6.59 ± 0.94 | 6.60 ± 0.94 | 6.57 ± 0.94 | 6.22 ± 0.74 | 6.23 ± 0.69 | 7.36 ± 0.84 | 7.22 ± 1.03 | 0.04 (−0.35, 0.43) | 0.02 (−0.46, 0.50) | 0.15 (−0.53, 0.82) |
| Resistance (Ω) | 559.3 ± 99.7 | 546.8 ± 95.2 | 571.9 ± 103.5 | 587.9 ± 83.9 | 610.4 ± 100.6 | 466.9 ± 58.2 | 497.2 ± 59.9 | 0.25 (−0.14, 0.65) | 0.24 (−0.24, 0.73) | 0.51 (−0.17, 1.19) |
| Reactance (Ω) | 63.8 ± 9.2 | 62.2 ± 7.8 | 65.4 ± 10.2 | 63.4 ± 7.8 | 66.8 ± 10.8 | 59.9 ± 7.5 | 62.5 ± 8.4 | 0.35 (−0.05, 0.74) | 0.36 (−0.13, 0.85) | 0.33 (−0.35, 1.00) |
| R/H (Ω/m) | 333.9 ± 68.8 | 325.2 ± 64.1 | 342.7 ± 72.7 | 356.9 ± 52.1 | 374.4 ± 67.0 | 263.7 ± 32.4 | 281.0 ± 33.0 | 0.25 (−0.14, 0.65) | 0.29 (−0.20, 0.78) | 0.53 (−0.16, 1.21) |
| Xc/H (Ω/m) | 38.0 ± 6.3 | 36.9 ± 5.3 | 39.1 ± 7.0 | 38.5 ± 5.1 | 41.0 ± 7.1 | 33.8 ± 4.2 | 35.4 ± 5.3 | 0.35 (−0.05, 0.74) | 0.39 (−0.10, 0.88) | 0.34 (−0.34, 1.01) |
|
| ||||||||||
| 250 kHz | ||||||||||
| Impedance (Ω) | 498.7 ± 91.9 | 487.2 ± 88.4 | 510.2 ± 94.8 | 525.8 ± 77.9 | 546.5 ± 90.6 | 412.3 ± 52.6 | 439.5 ± 55.8 | 0.25 (−0.14, 0.64) | 0.25 (−0.24, 0.73) | 0.50 (−0.18, 1.18) |
| Phase angle (°) | 4.38 ± 0.63 | 4.44 ± 0.61 | 4.33 ± 0.66 | 4.32 ± 0.55 | 4.21 ± 0.69 | 4.66 ± 0.67 | 4.57 ± 0.55 | 0.16 (−0.23, 0.55) | 0.18 (−0.31, 0.65) | 0.14 (−0.54, 0.81) |
| Resistance (Ω) | 498.7 ± 93.0 | 486.1 ± 88.2 | 511.4 ± 96.8 | 524.8 ± 77.5 | 549.2 ± 92.0 | 410.9 ± 52.5 | 438.2 ± 55.8 | 0.27 (−0.12, 0.67) | 0.29 (−0.20, 0.77) | 0.50 (−0.18, 1.18) |
| Reactance (Ω) | 37.8 ± 7.4 | 37.4 ± 6.8 | 38.2 ± 8.0 | 39.5 ± 6.4 | 40.0 ± 8.8 | 33.4 ± 5.6 | 34.8 ± 4.6 | 0.12 (−0.28, 0.51) | 0.07 (−0.41, 0.55) | 0.29 (−0.39, 0.96) |
Data are presented as mean ± standard deviation. Statistical significance is determined by independent t-tests and effect sizes are reported as Cohen’s d (95% confidence interval).
= Cohen’s d for race comparisons in the total sample;
= Cohen’s d for race comparisons in female participants;
= Cohen’s d for race comparisons in male participants.
= not significantly different from Black participants at p < 0.050 across all measurements.
R/H: resistance/height in meters; Xc/H: reactance/height in meters.
Figure 1. Raw Bioelectrical Impedance Analysis Between Black and White Adults.
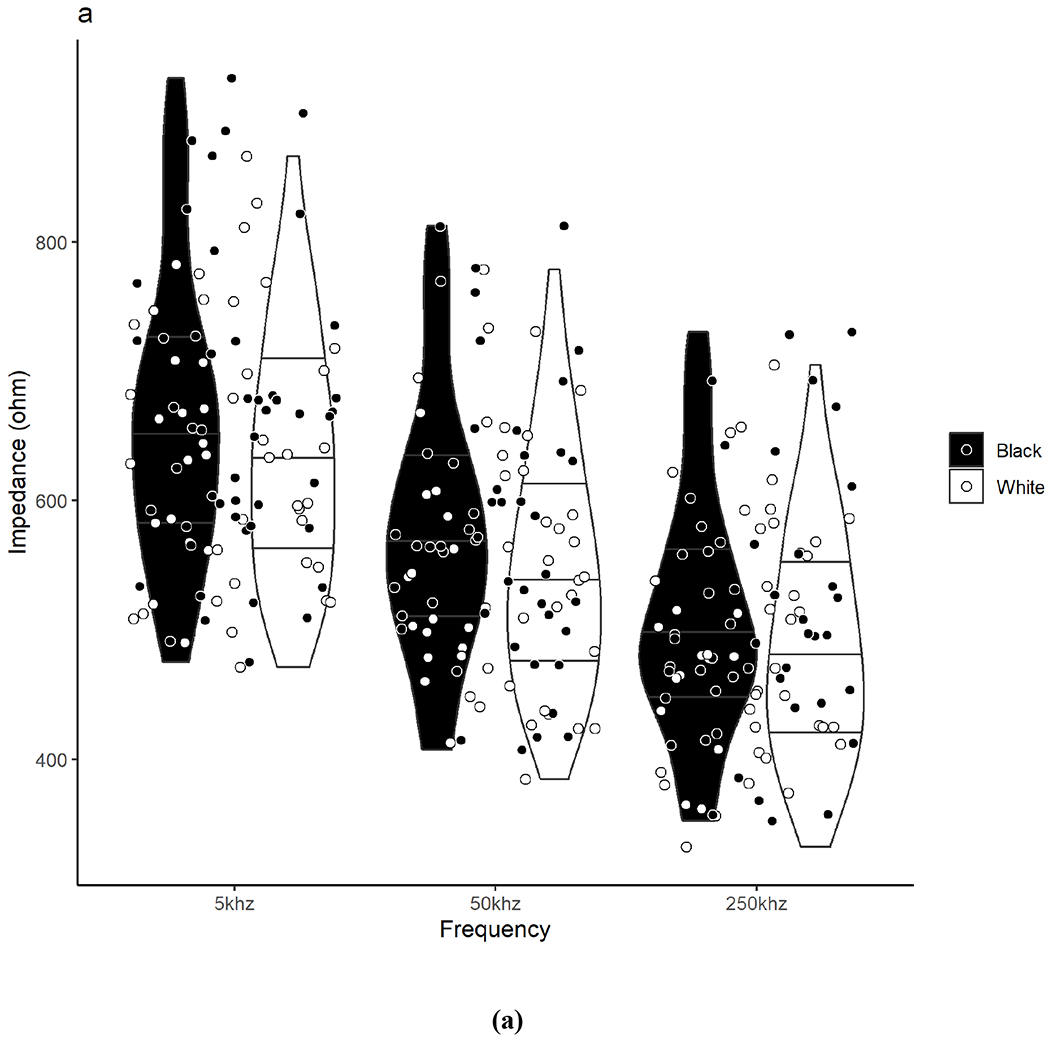
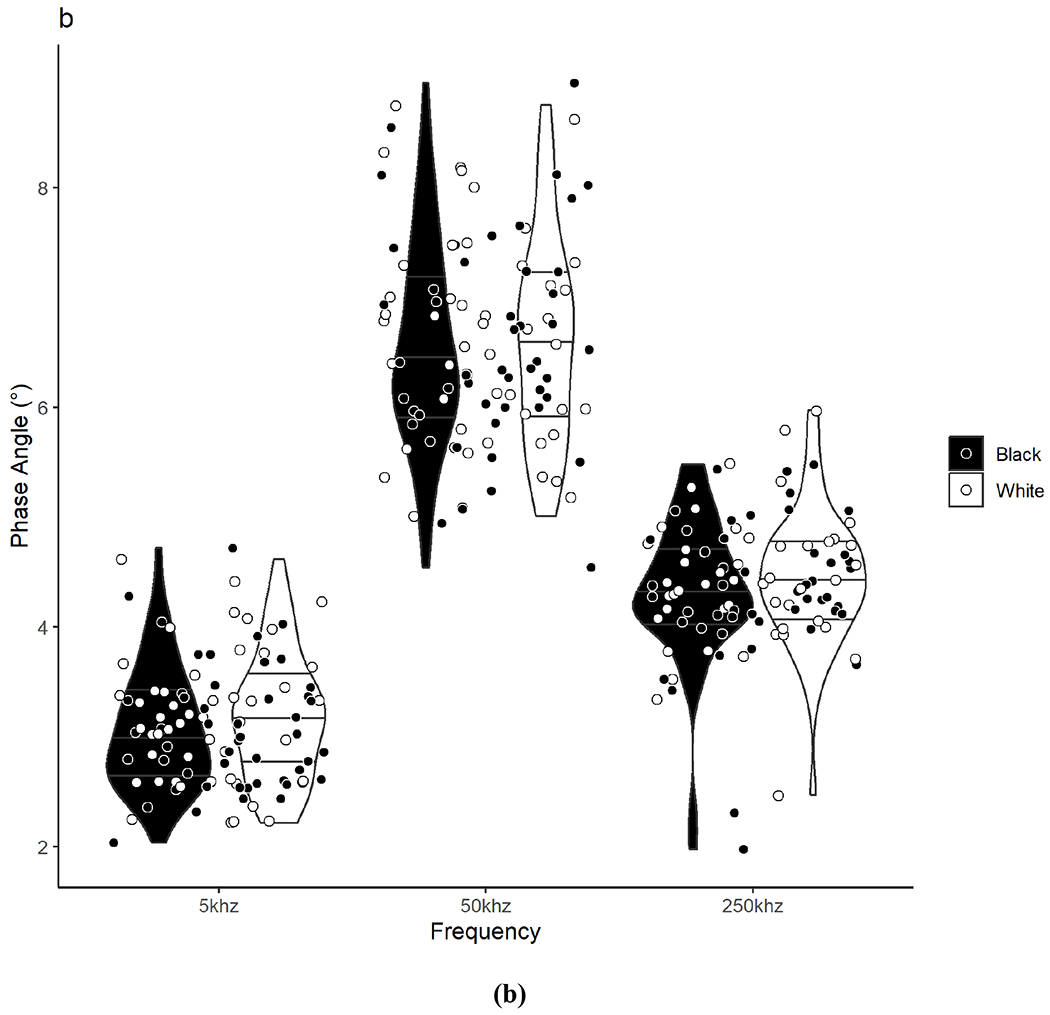
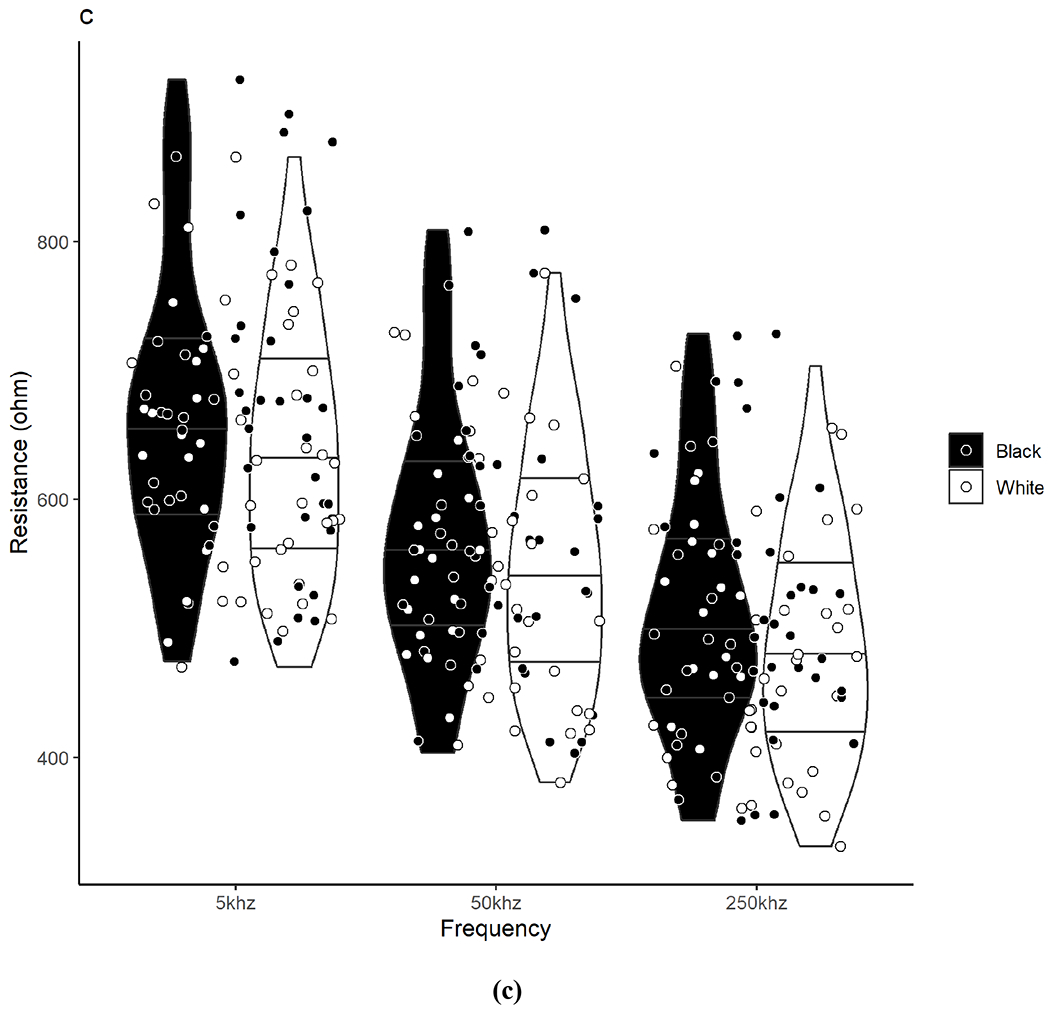
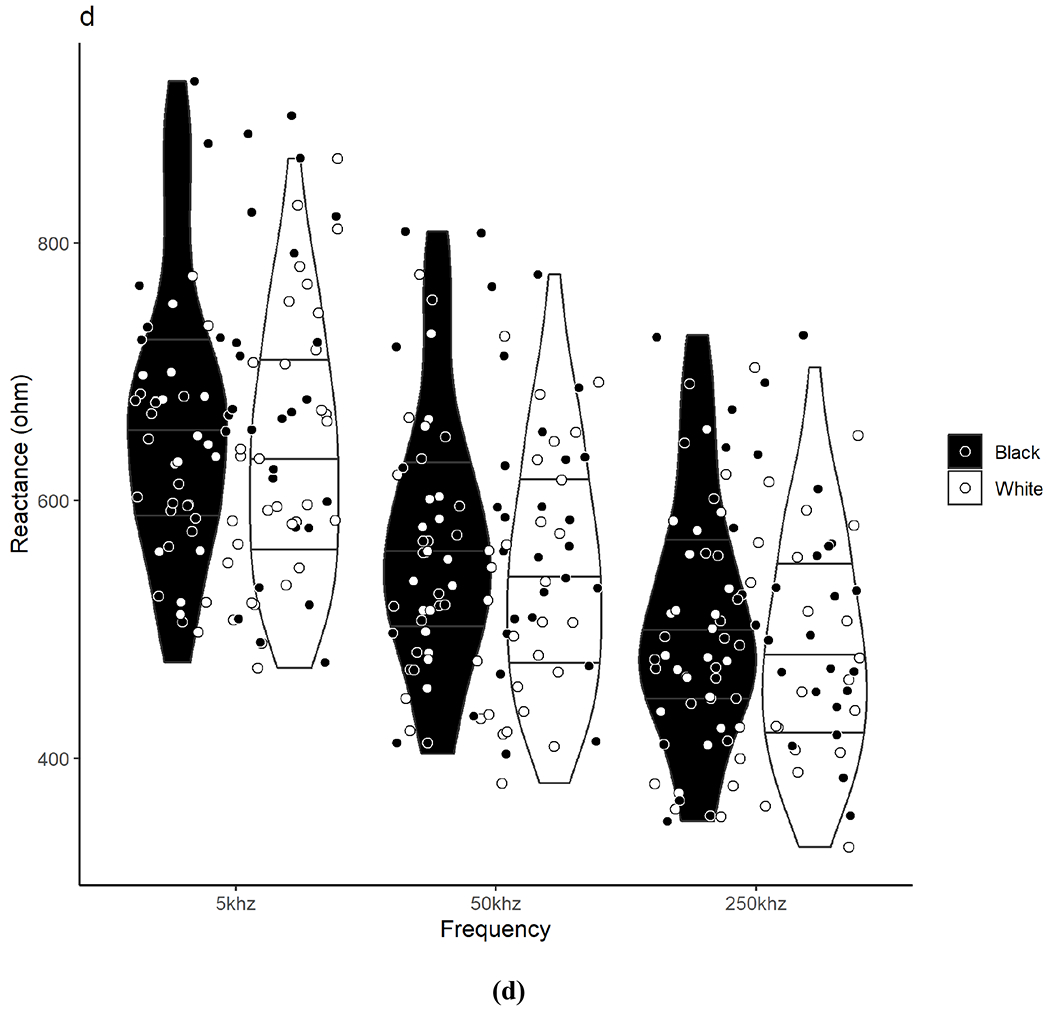
Violin plots show the density/distribution of the group data and individual datapoints for each Black and White participant for assessments of (a) impedance, (b) phase angle, (c) resistance, and (d) reactance measured at 5, 50, and 250 kHz. The y-axis represents the raw bioelectrical impedance values and the x-axis represents the measured frequencies. The bottom line within each violin represents the 25th quartile, the middle line represents the 50th quartile, and the top line represents the 75th quartile. Data were analyzed using independent t-tests. No significant differences were observed between Black and White adults for any measure across frequencies.
3.3. Bioelectrical Impedance Vector Analysis
Pearson correlation coefficients for R/H and Xc/H were conducted for all Black participants (r = 0.79, p < 0.001), all White participants (r = 0.70, p < 0.001), Black and White females (B: r = 0.83, p < 0.001; W: r = 0.63, p < 0.001), and Black and White males (B: r = 0.49, p = 0.046; W: r = 0.58, p = 0.015) and used to produce the corresponding confidence ellipses presented in Figure 2.
Figure 2. Mean Impedance Vectors and Confidence Ellipses between Black and White Adults.
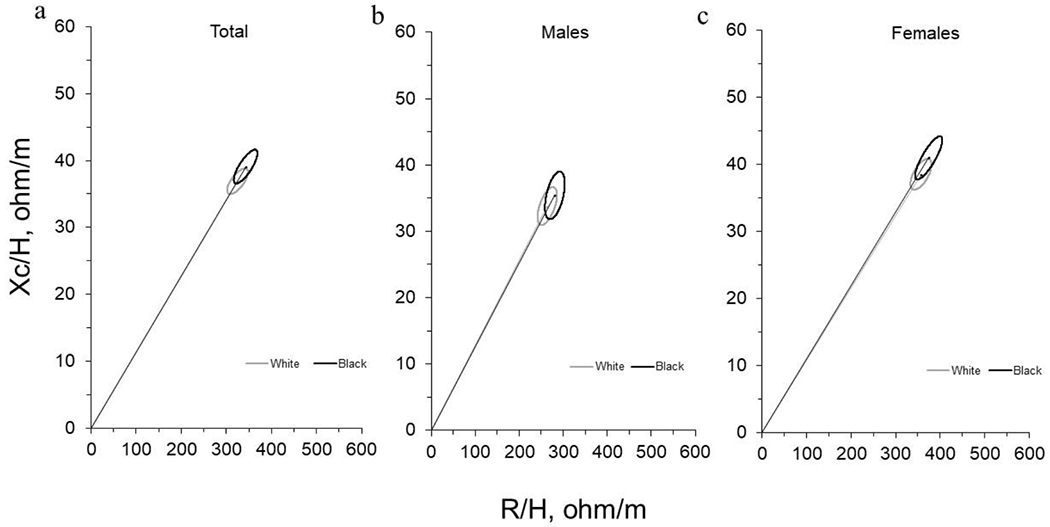
Mean impedance vectors with 95% confidence ellipses for Black adults and White adults in the (a) total sample, (b) for males, and (c) for females are presented. Data were assessed using Hotelling’s T2. No significant differences were observed between Black and White adults for any measure.
BIVA confidence ellipses using the two-sample Hotelling’s T2 tests were not significantly different between White and Black participants for the total sample (T2 = 3.1, F = 1.6, p = 0.216, Mahalanobis D = 0.35), for females (T2 = 2.6, F = 1.3, p = 0.283, Mahalanobis D = 0.43), or for males (T2 = 2.4, F = 1.2, p = 0.323, Mahalanobis D = 0.53). The mean vectors for Black participants were slightly longer than mean vectors for White participants for all groups; however, all 95% confidence ellipses were overlapping indicating no significant differences for the mean vectors by race.
Figure 3 illustrates the individual vectors of each participant by race relative to the 50%, 75%, and 95% tolerance ellipses produced from the previously mentioned White male and female reference populations. Overall, 35.3% of Black males were inside the 50% tolerance ellipse (n = 6) compared to 41.2% of White males (n = 7). In addition, 29.4% of Black males were outside of the 75% tolerance ellipse (n = 5) compared to 23.5% of White males (n = 4). All male participants outside of the 75% tolerance ellipse, other than one White male participant, had individual vectors that were shifted downward on the major axis (R) indicating greater tissue hydration relative to the White male reference population. One of the five Black male participants and two of the four White male participants outside of the 75% tolerance ellipse were shifted to the left on the minor axis (Xc) indicating greater soft tissue mass. All White and Black males were within the 95% tolerance ellipse of the reference population.
Figure 3. Mean Impedance Vectors between Black and White Adults using Tolerance Ellipses Generated from a White Adult Reference.
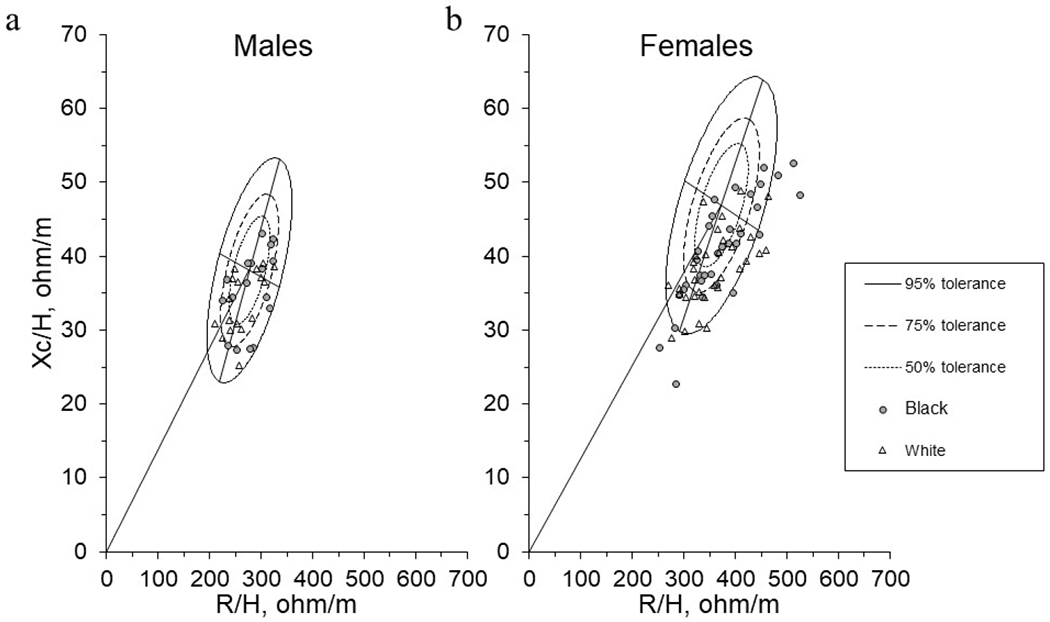
Mean impedance vectors with tolerance ellipses of 50%, 75%, and 95% produced from White (a) male and (b) female adult references. Tolerance ellipses were visually interpreted as the individual vector position relative to the reference. Vectors outside of the 75% tolerance ellipse indicate an abnormal Z with vector displacements on the major axis (vertical) representing abnormal tissue hydration and vector displacements on the minor axis (horizontal) representing abnormal soft tissue mass.
For females, 24.2% of Black females were inside the 50% tolerance ellipse (n = 8) compared to 21.2% of White females (n = 7). Additionally, 48.5% of Black females (n = 16) and 57.6% of White females (n = 19) were outside of the 75% tolerance ellipse; with 24.2% of Black females (n = 8) and 15.2% of White females (n = 5) outside of the 95% tolerance ellipse. Of the female participants outside of the 75% tolerance ellipse, only one White female was shifted upward on the major axis compared to six Black females. Only five Black female participants and four White female participants outside of the 75% tolerance ellipse were shifted to the left on the minor axis.
DISCUSSION
Using a rigorous matched-pairs design, our study showed that after matching for relevant physical attributes, particularly those known to influence bioelectrical impedance (i.e., sex, age, BMI, BF%), [12] these important assessments did not differ between Black and White adults. Moreover, when the only physical attribute that differs is Black or White race, there are only minor differences in how these groups compare to a White adult reference population (primarily tissue hydration differences in females); further supporting little to no racial differences when physical characteristics are similar. Although we failed to reject the null hypothesis for the current study (that there would be no racial differences in bioelectrical impedance assessments), our findings may be viewed as a net positive in the lens of Black adult racial health disparities; where the absence of race-dependent values may improve clinical confidence and prognostic value and alleviate potential hesitancy when using these assessments with Black patients.
The findings from our study contrast with previous studies showing an effect of race on bioelectrical impedance measures. [11] Previous studies that have shown racial and ethnic differences in PA in a multiethnic sample [12] have also noted that these differences were no longer attributable to race or ethnicity after complex regression modelling. As discussed, there are inherent errors due to overfitting during complex regression techniques, where elaborate regression models uniquely fit the data at hand but often do not translate to other datasets or practice. The specificity of the regression models to the peculiarities of the sample in question may be why some studies report racial differences in bioelectrical impedance while others do not. Given that our matched-pairs design allowed us to employ the most parsimonious model to evaluate the effect of race and showed no differences, it is likely that any observed variation between Black and White participants are not due to race. Rather, differences observed in prior research or in practice may be due to racial variation in BMI and other body composition characteristics that are not controlled for in real-world settings. For example, the racial variation in bioelectrical impedance standards referred to today were produced from National Health and Nutrition Examination Survey (NHANES) data up to 2004 [11] or other datasets produced during this time. [12] NHANES data from 2004 also showed that Black adults had the highest obesity prevalence in 2003-2004, at a rate that was 15% higher than White adults during the same period. [38] While our study did not examine this is in other racial groups, other studies report racial differences in bioelectrical impedance at a given BMI in multiethnic samples, noting that these differences are likely a function of differing body composition components between races [39]. Given that our study demonstrated no racial differences for any body composition component, it is unsurprising that our study showed no differences in bioelectrical impedance between White and Black adults. However, this cannot be translated across other racial and ethnic groups and thus, further evaluation of the ethnic differences in bioelectrical impedance is warranted.
Assuming that the body composition characteristics from a nationally representative sample translate to practice, it is likely that racial variation in body composition, and not race itself, explains the observed bioimpedance differences between Black and White adults. Understanding common racial differences in body composition is of particular importance in clinical practice. For example, clinicians may be concerned with the validity of BIA after reports of racial variation and choose not to utilize this prognostic tool for fear of misdiagnosis. As result, when race, rather than anthropometric characteristics, is used as a primary determinant, Black patients may not receive important health information that they would otherwise. In some cases, health assessments that primarily focus on race as a factor have an increased risk for misdiagnosis given that this assumes that the patients genetic history is a product of a single racial group. [40] It should also be noted that reference values may also be device specific [13] and that algorithmic differences may also contribute to different findings across studies.
In conclusion, our study shows that differences in bioelectrical impedance outcomes for Black adults are likely not a function of race, and concerns regarding its utility relative to White adults should not be based on this characteristic. Our data support the broad use of bioelectrical impedance measures in healthy Black adults, and showing that BIA may not need to rely on race as a factor is a step forward in addressing minority health disparities. Future studies should examine these differences in other racial and ethnic groups and continue to evaluate the prognostic value of BIA in practice.
ACKNOWLEDGEMENT
The authors have no acknowledgments to declare.
SOURCES OF SUPPORT
This work was supported by start-up funds for AJG from the University of Southern Mississippi. This work was also supported, in part, by the Mississippi INBRE, funded by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103476.
Declaration of Competing Interest
AJG: none; CB: none; RA: none; GMT has received research support through in-kind equipment donation or loan from manufacturers of body composition assessment methods, including bioimpedance analyzers (RJL Systems and Biospace, Inc.). These entities played no role in the present manuscript.
List of Abbreviations:
- BF%
body fat percent
- BIA
bioelectrical impedance analysis
- BIS
bioimpedance spectroscopy
- BIVA
bioelectrical impedance vector analysis
- BMI
body mass index
- DXA
dual-energy X-ray absorptiometry
- FFM
fat-free mass
- FM
fat mass
- HC
hip circumference
- PA
phase angel
- R
resistance
- R/H
resistance/height
- TBW
total body water
- WC
waist circumference
- Xc
reactance
- Xc/H
reactance/height
- Z
impedance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journa6l pertain.
REFERENCES
- [1].Norman K, Stobäus N, Pirlich M, Bosy-Westphal A. Bioelectrical phase angle and impedance vector analysis – clinical relevance and applicability of impedance parameters. Clin Nutr 2012;31:854–61. doi: 10.1016/j.clnu.2012.05.008. [DOI] [PubMed] [Google Scholar]
- [2].Marra M, Sammarco R, De Lorenzo A, Iellamo F, Siervo M, Pietrobelli A, et al. Assessment of body composition in health and disease using bioelectrical impedance analysis (BIA) and dual energy x-ray absorptiometry (DXA): a critical overview. Contrast Media Mol Imaging 2019;2019:3548284. doi: 10.1155/2019/3548284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mattiello R, Amaral MA, Mundstock E, Ziegelmann PK. Reference values for the phase angle of the electrical bioimpedance: systematic review and meta-analysis involving more than 250,000 subjects. Clin Nutr 2020;39:1411–7. doi: 10.1016/j.clnu.2019.07.004. [DOI] [PubMed] [Google Scholar]
- [4].Gonzalez MC, Barbosa-Silva TG, Bielemann RM, Gallagher D, Heymsfield SB. Phase angle and its determinants in healthy subjects: influence of body composition. The Am J Clin Nutr 2016;103:712–6. doi: 10.3945/ajcn.ll5.116772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gonzalez MC, Barbosa-Silva TG, Heymsfield SB. Bioelectrical impedance analysis in the assessment of sarcopenia. Curr Opin Clin Nutr Metab Care 2018;21:366–74. doi: 10.1097/MCO.0000000000000496. [DOI] [PubMed] [Google Scholar]
- [6].Tanaka S, Ando K, Kobayashi K, Hida T, Ito K, Tsushima M, et al. A low phase angle measured with bioelectrical impedance analysis is associated with osteoporosis and is a risk factor for osteoporosis in community-dwelling people: the Yakumo study. Arch Osteoporos 2018;13:39. doi: 10.1007/sl1657-018-0450-8. [DOI] [PubMed] [Google Scholar]
- [7].Pereira MME, Queiroz M dos SC, de Albuquerque NMC, Rodrigues J, Wiegert EVM, Calixto-Lima L, et al. The prognostic role of phase angle in advanced cancer patients: a systematic review. Nutr Clin Pract 2018;33:813–24. doi: 10.1002/ncp.10100. [DOI] [PubMed] [Google Scholar]
- [8].de Borba EL, Ceolin J, Ziegelmann PK, Bodanese LC, Gonçalves MR, Cañon-Montañez W, et al. Phase angle of bioimpedance at 50 kHz is associated with cardiovascular diseases: systematic review and meta-analysis. Eur J Clin Nutr 2022;76:1366–73. doi: 10.1038/s41430-022-01131-4. [DOI] [PubMed] [Google Scholar]
- [9].Barbosa-Silva MCG, Barros AJD, Post CLA, Waitzberg DL, Heymsfield SB. Can bioelectrical impedance analysis identify malnutrition in preoperative nutrition assessment? Nutrition 2003;19:422–6. doi: 10.1016/S0899-9007(02)00932-2. [DOI] [PubMed] [Google Scholar]
- [10].Garlini LM, Alves FD, Ceretta LB, Perry IS, Souza GC, Clausell NO. Phase angle and mortality: a systematic review. Eur J Clin Nutr 2019;73:495–508. doi: 10.1038/s41430-018-0159-1. [DOI] [PubMed] [Google Scholar]
- [11].Kuchnia AJ, Teigen LM, Cole AJ, Mulasi U, Gonzalez MC, Heymsfield SB, et al. Phase angle and impedance ratio: reference cut-points from the United States National Health And Nutrition Examination Survey 1999-2004 from bioimpedance spectroscopy data. J Parenter Enteral Nutr 2017;41:1310–5. doi: 10.1177/0148607116670378. [DOI] [PubMed] [Google Scholar]
- [12].Barbosa-Silva MCG, Barros AJD, Wang J, Heymsfield SB, Pierson RN. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. Am J Clin Nutr 2005;82:49–52. doi: 10.1093/ajcn.82.1.49. [DOI] [PubMed] [Google Scholar]
- [13].Bosy-Westphal A, Danielzik S, Dörhöfer R-P, Later W, Wiese S, Müller MJ. Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. J Parenter Enteral Nutr 2006;30:309–16. doi: 10.1177/0148607106030004309. [DOI] [PubMed] [Google Scholar]
- [14].Tanabe RF, de Azevedo ZMA, Fonseca VM, Peixoto MVM, dos Anjos LA, Gaspar-Elsas MIC, et al. Distribution of bioelectrical impedance vector values in multi-ethnic infants and pre-school children. Clin Nutr 2012;31:144–8. doi: 10.1016/j.clnu.2011.08.006. [DOI] [PubMed] [Google Scholar]
- [15].Kim EJ, Kim T, Conigliaro J, Liebschutz JM, Paasche-Orlow MK, Hanchate AD. Racial and ethnic disparities in diagnosis of chronic medical conditions in the USA. J Gen Intern Med 2018;33:1116–23. doi: 10.1007/s11606-018-4471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Arnett MJ, Thorpe RJ, Gaskin DJ, Bowie JV, LaVeist TA. Race, medical mistrust, and segregation in primary care as usual source of care: findings from the exploring health disparities in integrated communities study. J Urban Health 2016;93:456–67. doi: 10.1007/s11524-016-0054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Der Ananian C, Winham DM, Thompson SV, Tisue ME. Perceptions of heart-healthy behaviors among African American adults: a mixed methods study. Int J Environ Res Public Health 2018;15:2433. doi: 10.3390/ijerphl5112433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Conway B, Sudenga S, McClain D, Blot W. Diabetes and liver cancer risk: a stronger effect in Whites than Blacks? J Diabetes Complications 2021;35:107816. doi: 10.1016/j.jdiacomp.2020.107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update. Circulation 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- [20].Assari S Racial disparities in chronic kidney diseases in the United States; a pressing public health challenge with social, behavioral and medical causes. J Nephropharmacol 2015;5:4–6. [PMC free article] [PubMed] [Google Scholar]
- [21].Tinsley GM, Smith-Ryan AE, Kim Y, Blue MNM, Nickerson BS, Stratton MT, et al. Fat-free mass characteristics vary based on sex, race, and weight status in US adults. Nutr Res 2020;81:58–70. doi: 10.1016/j.nutres.2020.07.002. [DOI] [PubMed] [Google Scholar]
- [22].Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr 2004;23:1226–43. doi: 10.1016/j.clnu.2004.06.004. [DOI] [PubMed] [Google Scholar]
- [23].Siddiqui NI, Khan SA, Shoeb M, Bose S. Anthropometric predictors of bio-impedance analysis (BIA) phase angle in healthy adults. J Clin Diagn Res 2016;10:CC01–4. doi: 10.7860/JCDR/2016/17229.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Babyak MA What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med 2004;66:411–21. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- [25].Zhang Z Too much covariates in a multivariable model may cause the problem of overfitting. J Thorac Dis 2014;6:E196–7. doi: 10.3978/j.issn.2072-1439.2014.08.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Geifman N, Cohen R, Rubin E. Redefining meaningful age groups in the context of disease. Age 2012;35:2357–66. doi: 10.1007/sll357-013-9510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Graybeal AJ, Moore ML, Cruz MR, Tinsley GM. Body composition assessment in male and female bodybuilders: a 4-compartment model comparison of dual-energy X-ray absorptiometry and impedance-based devices. J Strength Cond Res 2020;34:1676–89. doi: 10.1519/JSC.0000000000002831. [DOI] [PubMed] [Google Scholar]
- [28].Moço AV, Matias CN, Santos DA, Rocha PM, Minderico CS, Cyrino ES, et al. Usefulness of reflection scanning in determining whole-body composition in broadly built individuals using dual-energy X-ray absorptiometry. J Clin Densitom 2019;22:429–36. doi: 10.1016/j.jocd.2018.03.007. [DOI] [PubMed] [Google Scholar]
- [29].Tinsley GM, Moore ML, Graybeal AJ. Precision of dual-energy X-ray absorptiometry reflection scans in muscular athletes. J Clin Densitom 2020;23:647–55. doi: 10.1016/j.jocd.2018.09.005. [DOI] [PubMed] [Google Scholar]
- [30].Graybeal AJ, Brandner C, Tinsley, GM. Evaluation of automated anthropometrics produced by smartphone-based machine learning: A comparison with traditional anthropometric assessments. Br J Nutr 2023;1–29. doi: 10.1016/j.clnu.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moon JR, Smith AE, Tobkin SE, Lockwood CM, Kendall KL, Graef JL, et al. Total body water changes after an exercise intervention tracked using bioimpedance spectroscopy: a deuterium oxide comparison. Clin Nutr 2009;28:516–25. doi: 10.1016/j.clnu.2009.04.025. [DOI] [PubMed] [Google Scholar]
- [32].Esco MR, Fedewa MV, Freeborn TJ, Moon JR, Wingo JE, Cicone Z, et al. Agreement between supine and standing bioimpedance spectroscopy devices and dual-energy X-ray absorptiometry for body composition determination. Clin Physiol Funct Imaging 2019;39:355–61. doi: 10.1111/cpf.12585. [DOI] [PubMed] [Google Scholar]
- [33].Moon JR, Smith AE, Tobkin SE, Lockwood CM, Kendall KL, Graef JL, et al. Total body water changes after an exercise intervention tracked using bioimpedance spectroscopy: a deuterium oxide comparison. Clin Nutr 2009;28:16–25. doi: 10.1186/1743-7075-5-7. [DOI] [PubMed] [Google Scholar]
- [34].Piccoli A, Rossi B, Pillon L, Bucciante G. A new method for monitoring body fluid variation by bioimpedance analysis: the RXc graph. Kidney Int 1994;46:534–9. doi: 10.1038/ki.1994.305. [DOI] [PubMed] [Google Scholar]
- [35].Piccoli A, Nigrelli S, Caberlotto A, Bottazzo S, Rossi B, Pillon L, et al. Bivariate normal values of the bioelectrical impedance vector in adult and elderly populations. Am J Clin Nutr 1995;61:269–70. doi: 10.1093/ajcn/61.2.269. [DOI] [PubMed] [Google Scholar]
- [36].Piccoli A, Pastori G. Department of Medical and Surgical Sciences, University of Padova, Italy: E-mail: apiccoli@unipd.it n.d.:17. [Google Scholar]
- [37].Piccoli A, Pillon L, Dumler F. Impedance vector distribution by sex, race, body mass index, and age in the United States: standard reference intervals as bivariate z scores. Nutrition 2002;18:153–67. doi: 10.1016/S0899-9007(01)00665-7. [DOI] [PubMed] [Google Scholar]
- [38].Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- [39].Siervo M, Davies AA, Jebb SA, Jalil F, Moore SE, Prentice AM. Ethnic differences in the association between body mass index and impedance index (Ht2/Z) in adult women and men using a leg-to-leg bioimpedance method. Eur J of Clin Nutr 2007;61:1337–40. doi: 10.1038/sj.ejcn.1602678. [DOI] [PubMed] [Google Scholar]
- [40].Hsu C, Yang W, Parikh RV, Anderson AH, Chen TK, Cohen DL, et al. Race, genetic ancestry, and estimating kidney function in CKD. N Engl J Med 2021;385:1750–60. doi: 10.1056/NEJMoa2103753. [DOI] [PMC free article] [PubMed] [Google Scholar]


