Abstract
Purpose of Review:
This review highlights recent findings regarding the prevalence, public health impact, clinical presentation, intervention access, and conceptualization of fetal alcohol spectrum disorders (FASD). Despite ongoing work in prevention and identification of this population, the rates of drinking during pregnancy have increased and significant gaps remain in diagnosis and intervention.
Recent findings:
Prenatal alcohol exposure is the most common preventable cause of developmental disability in the world. Research has focused on improving diagnostic clarity, utilizing technology and neuroimaging to facilitate identification, engaging broader stakeholders (including self-advocates) to inform understanding and needs, and increasing access to effective interventions. There is an emerging focus on developmental trajectories and experiences in young and middle adulthood. Public policy advocacy has also made great strides in recent years.
Summary:
Increases in public awareness, greater concordance of diagnostic schema, leveraged use of novel technology, and the development of targeted interventions within a holistic, strengths-based conceptualization are important considerations for this population.
Keywords: FASD, fetal alcohol spectrum disorders, prenatal alcohol exposure, neurodevelopment
Introduction
Alcohol exposure in utero produces by far the most serious neurobehavioral effects compared to other substances of abuse including heroin and cocaine (1). Fetal Alcohol Syndrome (FAS) was first diagnosed 50 years ago, and the broader effects of alcohol exposure on brain and behavior have been conceptualized under a non-diagnostic umbrella term of Fetal Alcohol Spectrum Disorders (FASD). FASD captures the wide continuum of physical, neurological, cognitive, adaptive functioning, and behavioral that can occur following prenatal alcohol exposure. While there is a large body of evidence that alcohol use during pregnancy can lead to significant negative outcomes, prevention and mitigation efforts have been difficult to implement and the rate of drinking has increased (2, 3).
Over the past decade, the rate of reported drinking during pregnancy as tracked by the CDC has increased by 50%, and binge drinking has doubled. Based on the most recent United States reported data, in 2020 14.3% of pregnant people reported current drinking (at least 1 drink in the past 30 days while pregnant) compared to 9.2% in 2011. Further, 6.1% of pregnant people reported binge drinking (4 or more drinks per occasion at least once in the past 30 days), compared to 2.5% in 2011 (2). Almost half of all pregnancies are unplanned and there has been an increase in women with alcohol use disorders and related problems during the pandemic (4), leading this to be a public health priority.
Understanding specific maternal risk factors (e.g., mental illness, exposure to abuse, and alcohol consumption of partners) may also inform successful prevention efforts (5). There are some programs in place aimed to reduce drinking during pregnancy (e.g., CDC CHOICES, (6)), though additional brief, evidence-based interventions to treat active substance use disorders in pregnant women are needed. While there is standard public messaging that the safest choice would be to abstain from alcohol during pregnancy, women still encounter mixed information. Further, it is difficult to determine why some children are affected more than others. There are a variety of potential variables to consider, including amount of drinking, timing and pattern of drinking, genetics, prematurity or gestational age, polysubstance use, nutritional status, maternal weight, and other pre-existing risk and resilience factors that have been targets of recent investigations (7–10).
In light of the well-documented increase in drinking during pregnancy, it has been difficult to track the prevalence and incidence of FASD. Accurate identification has been difficult in part due to no clear consensus for diagnostic criteria as well as other factors including lack of awareness and stigma. Further, while there are physical manifestations of FAS, the majority of individuals affected by prenatal alcohol exposure do not show clear facial dysmorphology (Figure 1). Despite these challenges, prevalence estimates have been attempted though various methodologies. Recent studies using a random sample and active case ascertainment reported the prevalence rates of FASD to range from 1-7% (11,12).
Figure 1. Facial characteristics that are associated with fetal alcohol exposure.
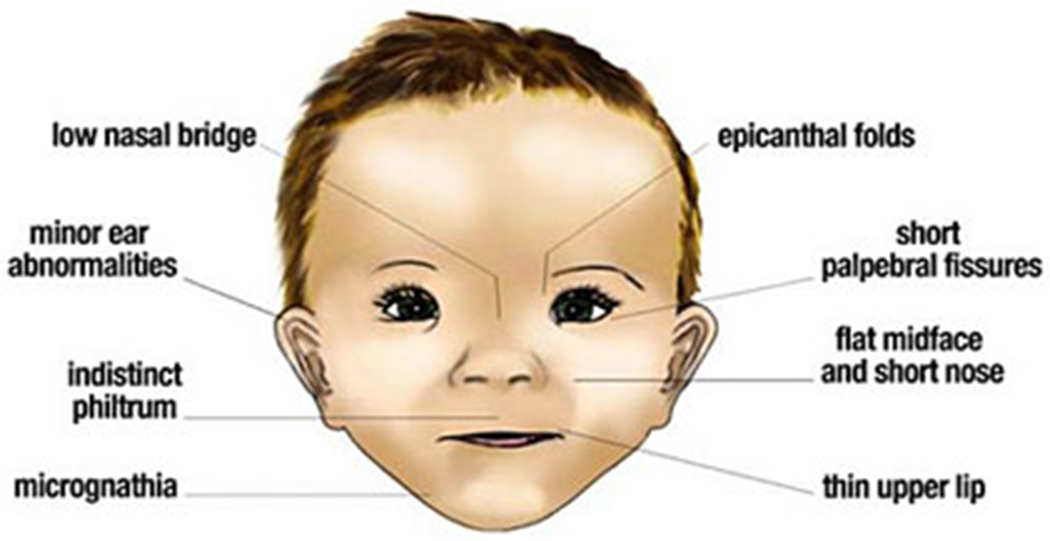
Figure in public domain - https://pubs.niaaa.nih.gov/publications/aa82/aa82.htm
Overall, the effects of alcohol remain missed and are often misdiagnosed; and individuals who are identified have difficulty accessing effective interventions and navigating support systems (13). Increased awareness, standardized diagnostic criteria, and leveraging technological advances can help improve identification. Additionally, use of a strength-based framework and greater involvement of various stakeholders (including self-advocates) can also help in both increasing understanding as well as improving outcomes in a variety of settings and across the lifespan (14,15).
Identification
Efforts are underway to increase access to diagnostic services, improve early intervention, and FASD-informed care across the lifespan (16,17,18). Despite attempts to standardize and formalize diagnostic schema to capture the spectrum of potential effects of prenatal alcohol exposure, there continues to be discordance in research and clinical communities (19). The Diagnostic and Statistical Manual, 5th Edition (DSM-5) lists Neurobehavioral Disorder associated with Prenatal Alcohol Exposure (ND-PAE) as a condition under further review, though also notes alcohol exposure as a model modifier for Specified Neurodevelopmental Disorder (20), see Table 1. The ICD codes used by medical professionals also have different codes that can be used for FAS/FASD, whereas the American Academy of Pediatrics (AAP), has a number of different diagnostic schema for pediatricians to consider (Institute of Medicine guidelines and 4-digit code).
Table 1.
Overview of Criteria for Neurobehavioral Disorder Associated with Prenatal Alcohol Exposure (ND-PAE).
| A. More than minimal exposure to alcohol during gestation |
| B. Impaired neurocognitive functioning (one or more of the following): |
| 1. Impairment in global intellectual performance |
| 2. Impairment in executive functioning |
| 3. Impairment in learning |
| 4. Memory impairment |
| 5. Impairment in visual-spatial reasoning |
| C. Impaired self-regulation (one or more of the following): |
| 1. Impairment in mood or behavioral regulation |
| 2. Attention deficit |
| 3. Impairment in impulse control |
| D. Impairments in adaptive functioning (must include (1) or (2)): |
| 1. Communication deficit |
| 2. Impairment in social communication and interaction |
| 3. Impairment in daily living skills |
| 4. Impairment in motor skills |
| E. The onset of the disorder occurs in childhood |
| F. The disturbance causes clinically significant distress or impairment in social, academic, occupational, or other important areas of functioning. |
| G. The disorder is not better explained by other causes. |
Note: ND-PAE is listed as a condition for further study in the Diagnostic and Statistical Manual of Mental Disorders – Fifth Edition (DSM-5), American Psychiatric Association. (2013). https://doi.org/10.1176/appi.books.9780890425596
Without clear and consistent diagnostic criteria, not only is it hard to identify those in need it is also difficult to educate providers, teachers, and parents about the importance of screening for potential areas of concern related to FASD, especially compared to other neurodevelopmental conditions (e.g., Autism, attentional disorders). Other complicating factors that limit identification include the wide spectrum of cognitive, behavioral and adaptive function, ongoing stigma related to FASD, and high rates of comorbidity and adverse childhood experiences (21), see Table 2.
Table 2.
Considerations underlying missed or misdiagnosis of fetal alcohol spectrum disorders
| ■ High rate of comorbidity with other neurodevelopmental and psychiatric diagnoses |
| ■ Lack of consensus for standard assessment or diagnostic criteria |
| ■ Stigma related to biological parents, adoptive/foster care, and individuals with prenatal alcohol exposure |
| ■ Heterogenous clinical presentation |
| ■ Lack of awareness and minimal training for medical providers and educators |
| ■ Lack of an accurate, sensitive, and specific biomarker or blood test |
| ■ Minimal access to multidisciplinary teams to ensure accurate diagnosis |
| ■ Often inadequate or missing histories of prenatal alcohol exposure for children or adults |
| ■ Other confounding factors such as polysubstance use, poor nutrition, or other maternal risk factors |
Neurobehavioral Profile and Other Considerations
Another barrier to the development and use of a robust and comprehensive diagnostic schema is the ongoing refinement of the neurobehavioral profile associated with prenatal alcohol exposure and raising awareness in the community (22,23). The wide spectrum and heterogeneity of cognitive, behavioral, and adaptive skills can further make it difficult to accurately identify individuals in need. Even within a domain of strength, there are variations in the profile; for example, an individual with strengths in language can show wide variation in regards to receptive versus expressive and functional communication versus social communication (24).
There are a number of attempts to help improve screening and identification, such as the development and validation of a postnatal risk score that helps accurately identify children with prenatal alcohol exposure (25) as well as a clinical decision tree (26). Further, there are promising results found using telemedicine to improve access and accuracy of physical, and cognitive examinations of children with FASD (27,28), which can improve access. Use of initial screenings can indicate that an individual requires a broad multidisciplinary assessment to confirm the diagnosis (29,30). However, even within broad, comprehensive assessments, there are continued debates over the threshold of what would qualify as a weakness (1 or 1.5 SD), how to best address not knowing a prenatal alcohol exposure history, and how to achieve the best sensitivity and specificity in identifying those in need (25,26,31). A recent study described the validation of the FASD-Tree as a screening tool for FASD. This tool incorporates readily accessible parent questionnaires and a physical exam and provides two metrics: a numeric risk score and a dichotomous (yes/no) outcome indicating the screening outcome. The FASD-Tree was effective as a screening tool, with accuracy rates of 75-84% and fair to good discrimination (32).
The lack of education of providers and stigma associated with prenatal alcohol exposure can also hinder those screened or referred for an assessment or services . Unfortunately, FASD has often not been a primary consideration in healthcare settings (pediatricians, primary care, OB-GYN, social workers, psychologists, psychiatrists) and has significantly less funding and attention than other less prevalent neurodevelopmental disorders. There have been small steps of improvement. For example, the American Academy of Pediatrics (AAP) recently released a new, free online course covering ‘Fetal Alcohol Spectrum Disorders: Recognition and Management’ to provide pediatric professionals to increase awareness and empower them to recognize and determine a coordinated referral and treatment approach.
Neuroimaging and other Potential Biomarkers
Over the past few decades, there has been a growing base of research literature describing the structural and functional changes associated with prenatal alcohol exposure to help differentiate those with alcohol exposure from typically developing youth (33) and identify potential clinical correlates that may be provide etiology of deficits or targets for intervention (34). Smaller overall brain size is among the most reported findings, underscoring alcohol’s global teratogenic effect on the brain; however, this is not specific to prenatal alcohol exposure as it also occurs in other disorders. However, there does appear to be some selectivity to alcohol’s effects on the brain. Some regions appear to be more negatively affected by alcohol than others, evidenced by structures that are smaller than one would expect given the total brain size (i.e., disproportionately smaller regions). The corpus callosum, basal ganglia, and cerebellum are all regions that have rather consistently been found to have disproportionately smaller volumes among individuals with prenatal alcohol exposure (35).
Independent examination of brain region morphology, however, may be underutilizing the vast amounts of MRI data available. Studies using multivariate analyses of brain region volumes have discriminated youth with prenatal alcohol exposure from controls, achieving 77% accuracy, 64% sensitivity, and 88% specificity (36). Further, the combination of neuroimaging with psychometric cognitive measures has been shown to be extremely effective in correctly classifying children with prenatal alcohol exposure. Using the Connors 3 Hyperactivity/Impulsivity score, magnetic resonance spectroscopy, and diffusion tensor imaging of supraventricular white matter resulted in highly accurate (92%) discrimination of children with prenatal alcohol exposure and attention-deficit/hyperactivity disorder (ADHD) from children with ADHD but without such exposure, achieving perfect sensitivity (100%) and high specificity (82%) (37). This is particularly exciting given the high difficulty in discriminating between children with and without prenatal alcohol exposure, who may need different clinical treatments or have different trajectories or prognoses. This research suggests that neuroimaging may be beneficial in the diagnostic process.
Additionally, functional MRI studies have mapped the hemodynamic response to a number of tasks among individuals with prenatal alcohol exposure, generally finding altered patterns of brain activation and/or recruitment of a broader range of brain regions when completing tasks (35,36). Further, several studies have demonstrated that disrupted resting-state functional connectivity in children with prenatal alcohol exposure relates to poorer performance on cognitive tests (36,38,39). These imaging studies indicate that we may have more objective methods to identify individuals with prenatal alcohol exposure and they also can help illustrate the potential relation between neurological changes and clinical presentation.
There are also new efforts underway to find biomarkers such as meconium or baby teeth to more objectively assess levels of prenatal alcohol exposure (40,41) as well as fecal microbiota in preclinical trials (42). While still limited, this research can help to combat the difficulty of ascertaining information about the history of prenatal alcohol exposure, which is often missing or incomplete for various reasons, despite the importance in diagnostic clarity.
Conceptualizations of FASD and Novel Research Areas
In the past few years, there has been a new movement focused on strength-based reframing and understanding the potentially modifiable factors that could improve outcomes (43). Some argue that the lack of strength-based research has perpetuated the stress and stigma experienced by those with prenatal alcohol exposure (15). A review of recent strength-based literature finds that individuals with FASD, particularly young adults and adults, have strong self-awareness, are receptive to support, have capacity and often seek human connection, are resilient, and have hope for the future. These strengths and a growth mindset grounded in hope and change in the future can facilitate greater quality of life. Individuals with FASD can have challenges across settings (home, school, medical system, justice system) and there is a great need for strengths-based, comprehensive, FASD-informed care that is coordinated across environments (44,45). An additional hope of a strength-based and informed conceptualization of FASD is to reduce stigma. Stigma and shame actively impede access to diagnosis and treatment from both the provider and the patient perspective. In addition to education requirements for healthcare providers, other areas should be explored that can help birth mothers, adoptive families, and self-advocates better seek treatment.
Understanding of Midlife and Adulthood
FASD is a lifelong condition that can have differential effects in childhood, young adulthood, and middle adulthood as abilities, environment, and expectations change. As FAS was first codified in 1973 by Jones and Smith (46), those that were diagnosed in infancy at that time are now entering midlife. Recent research has attempted to capture the areas of concern individuals are experiencing across the lifespan as well as increase understanding of their developmental trajectories (e.g., developmental delay versus stable weakness) (8,33,47). Based on the emerging findings, prenatal alcohol exposure was associated with greater rates of mental health disorders in middle adulthood (48), including higher rates of depression, anxiety, bipolar disorder, and attention-deficit/hyperactivity disorder, as well as increased suicidality (15). These increased rates were mediated by greater environmental stressors, adverse childhood events, and lower socioeconomic status, which are common for individuals with prenatal alcohol exposure (21). Given these preliminary findings, more research is needed to understand the effects of prenatal alcohol exposure across the lifespan, where much remains unknown.
Recent Policy Advances
In consideration of a continuing and clearly documented need for action, there have been a number of bills introduced to help codify access to services and recognition of FASD. A recent bill passed in California in 2022, (SB1016, (49)) which specifically includes “fetal alcohol spectrum disorder” in the definition of ‘other health impairment’ that is entitled to special education and related services. Additionally, there is a bipartisan bill before the US congress introduced in 2021, the FASD Respect Act (HR 4151), which would support the coordination of research, surveillance, and related activities to diagnose, prevent, and treat FASD including establishing centers for excellence, implementing best practices, providing additional services, and supporting the development of systems of care for those affected by FASD (50). In September 2021, the UK government published a comprehensive health needs assessment for FASD (51).
Intervention Modalities and Targets
The wide continuum of potential outcomes associated with prenatal alcohol exposure leads to a complex clinical presentation that necessitates development of targeted evidence based interventions (52,53). Focus has been on cognitive (particularly executive function, inhibitory control), academic (predominately math), behavioral (mood, secondary diagnoses), legal/justice (overrepresented and prone to confabulation, (54)), adaptive function (daily living skills, social skills, care facilities), and health outcomes (sleep, nutrition, cardiac, medication). In addition to some of the few FASD-specific interventions (e.g., MILE program for math (55) and Children’s Friendship Training for social skills, (56)), there are several novel interventions that are also being piloted, such as music training to increase attention (57). Understanding the underlying etiology of areas of weakness can also assist in targeted, clinical intervention. For example, understanding that behavioral regulation weaknesses are associated with poor adaptive function may point to a specific cognitive area to target for broader positive outcomes (58).
Mobile apps to support intervention have also been developed for both children (work by Dr. Christie Petrenko - Families Moving Forward (FMF) Connect for caregivers of children with FASD, ages 3-12) and adults (My Health Coach). These apps were developed by working closely with individuals with prenatal alcohol exposure using theory-guided planning to increase access to care and ideally ease the barriers to effective intervention. Additionally, there is active development of new resources to help children and young people with FASD understand their diagnosis and become self-advocates through Me and My FASD, which is one of the first peer-to-peer FASD resources (59).
There are still very few interventions specifically for individuals with prenatal alcohol exposure, though there have been more resources that have been made available to schools (60), justice systems, parents, and healthcare settings to help improve outcomes. There is also great opportunity for the repurposing of interventions that can target specific behaviors associated with prenatal alcohol exposure, if they are implemented effectively (e.g., considering the full profile of functioning and environment).
Use of Supplements and Psychopharmacological Intervention
Over the past decade, there has been a focus in both preclinical and clinical research on the use of choline to help mitigate the effects of prenatal alcohol exposure by using supplementation during critical developmental windows to enhance brain plasticity. Clinical findings indicate that young children who receive choline had higher neurocognitive skills, including non-verbal intelligence, visual spatial skills, working memory, verbal memory, and fewer behavioral symptoms (61). Additionally, maternal choline supplementation may be neuroprotective (62). In addition to human studies, preclinical data shows that choline supplementation may be able to mitigate long-term effects of prenatal alcohol exposure (63). Although additional longitudinal follow-up and study is needed, including on short- and long-term risks of any treatment, based on the preliminary findings (64) choline may be a worthwhile intervention for a population that has very few options available. Preclinical data is also investigating the use of iron supplementation to improve fetal outcomes in prenatal alcohol exposure (65).
There is a dearth of research regarding the best course of action regarding psychopharmacological intervention for FASD, though many children, adolescents, and adults are on various medications to manage behavioral and psychiatric symptoms. New research is trying to address this concern by understanding prior utilization of psychotropic medications in children with FASD (66) as well as evaluating the efficacy of medication management (67).
Conclusion
Though we have come a long way in the identification and intervention of FASD, there are clear ongoing documented areas of need. We need better (or better used) screening procedures that coalesce on a single diagnostic schema to assist with earlier identification. Increased access to effective intervention as well as tailored supports are required across the lifespan. Broader, holistic conceptualization of FASD, the use of a strength-based framework, and encouragement of self-advocacy can also help in both increasing understanding as well as improving outcomes and quality of life. This is the time to increase prevention efforts and raise awareness in healthcare settings and broader policy to improve access to services to those affected by prenatal alcohol exposure.
Key Points:
Fetal alcohol spectrum disorders are a significant public health concern
There is a need for increased public awareness and diagnostic clarity to better identify and support those affected by prenatal alcohol exposure
Leveraged use of technology can assist with identification and intervention
Utilizing a holistic and strengths-based approach can facilitate positive outcomes
Consultation across settings and stakeholders is paramount to effective assessment and treatment across the lifespan
Acknowledgements:
Thank you to the families and individuals affected by prenatal alcohol exposure who generously volunteer their expertise, time, energy, and selves to further advance research and understanding.
Financial Support and Sponsorship:
This article was prepared while Eileen Moore was employed at the San Diego State University. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government. Sarah Mattson was supported by NIAAA Grant U01 AA014834. Eileen Moore was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (R01 AA026994) and the National Institute on Minority Health and Health Disparities (U54 MD012397).
Footnotes
Conflicts of Interest: None.
Citations
- 1.Institute of Medicine. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment [Internet]. Washington, DC: The National Academies Press; 1996. Available from: https://nap.nationalacademies.org/catalog/4991/fetal-alcohol-syndrome-diagnosis-epidemiology-prevention-and-treatment [Google Scholar]
- 2.Gosdin Lucas K., Deputy NP, Kim SY, et al. Alcohol Consumption and Binge Drinking During Pregnancy Among Adults Aged 18–49 Years — United States, 2018–2020. MMWR Morb Mortal Wkly Rep [Internet]. 2022. ;71. Available from: https://www.cdc.gov/mmwr/volumes/71/wr/mm7101a2.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobsen B, Lindemann C, Petzina R, Verthein U. The Universal and Primary Prevention of Foetal Alcohol Spectrum Disorders (FASD): A Systematic Review. J Prev. 2022. Jun;43(3):297–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollard MS, Tucker JS, Green HD Jr. Changes in Adult Alcohol Use and Consequences During the COVID-19 Pandemic in the US. JAMA Netw Open. 2020. Sep 29;3(9):e2022942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward N, Correia H, McBride N. Maternal Psycho-Social Risk Factors associated with Maternal Alcohol Consumption and Fetal Alcohol Spectrum Disorder: A Systematic Review. Arch Gynecol Obstet. 2021. Dec;304(6):1399–407. [DOI] [PubMed] [Google Scholar]
- 6.CDC. CHOICES as a Program to Prevent Alcohol-Exposed Pregnancies [Internet]. Centers for Disease Control and Prevention. 2019. Available from: https://www.cdc.gov/ncbddd/fasd/choices-program-prevent-alcohol-exposed-pregnancies.html [Google Scholar]
- 7.Lange S, Probst C, Gmel G, et al. Global Prevalence of Fetal Alcohol Spectrum Disorder among Children and Youth. JAMA Pediatr. 2017. Oct;171(10):948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bandoli G, Kable JA, Coles CD, et al. Trajectories of Prenatal Alcohol Exposure and Behavioral Outcomes: Findings from a Community-Based Sample. Drug Alcohol Depend. 2022. Apr 1;233:109351. * Cluster analysis methodology to determine patterns and trajectories of prenatal alcohol exposure and relation with behavior at first grade.
- 9.Hasken JM, Marais AS, de Vries M, et al. Gestational Age and Birth Growth Parameters as Early Predictors of Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2021. Aug;45(8):1624–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasken JM, Adair LS, Martin SL, et al. The Influence of Maternal Weight and Alcohol Exposure on Infant Physical Characteristics and Neurodevelopmental Outcomes. Curr Res Toxicol. 2022;3:100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.May PA, Chambers CD, Kalberg WO, et al. Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA. 2018. Feb 6;319(5):474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.May PA, Hasken JM, Hooper SR, et al. Estimating the Community Prevalence, Child Traits, and Maternal Risk Factors of Fetal Alcohol Spectrum Disorders (FASD) from a Random Sample of School Children. Drug Alcohol Depend. 2021. Oct 1;227:108918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chasnoff IJ, Wells AM, King L. Misdiagnosis and Missed Diagnoses in Foster and Adopted Children with Prenatal Alcohol Exposure. Pediatrics. 2015. Feb;135(2):264–70. [DOI] [PubMed] [Google Scholar]
- 14. Kautz-Turnbull C, Adams TR, Petrenko CLM. The Strengths and Positive Influences of Children With Fetal Alcohol Spectrum Disorders. Am J Intellect Dev Disabil. 2022. Sep 1;127(5):355–68. * Caregivers reported wide-ranging strengths and positive influences of children with FASD.
- 15. Flannigan K, Wrath A, Ritter C, et al. Balancing the Story of Fetal Alcohol Spectrum Disorder: A Narrative Review of the Literature on Strengths. Alcohol Clin Exp Res. 2021. Dec;45(12):2448–64. ** Review of strengths-based FASD research to inform research, practice, and policy.
- 16.Popova S, Dozet D, Burd L. Fetal Alcohol Spectrum Disorder: Can We Change the Future? Alcohol Clin Exp Res. 2020. Apr;44(4):815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Currie BA, Hoy J, Legge L, et al. Adults with Fetal Alcohol Spectrum Disorder: Factors Associated with Positive Outcomes and Contact with the Criminal Justice System. J Popul Ther Clin Pharmacol J Ther Popul Pharmacol Clin. 2016;23(1):e37–52. [PubMed] [Google Scholar]
- 18.Duko B, Pereira G, Tait RJ, et al. Prenatal Alcohol Exposure and Offspring Subsequent Alcohol Use: A Systematic Review. Drug Alcohol Depend. 2022. Mar 1;232:109324. [DOI] [PubMed] [Google Scholar]
- 19.Hoyme HE, Kalberg WO, Elliott AJ, et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics. 2016. Aug;138(2):e20154256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- 21.Tan GKY, Symons M, Fitzpatrick J, et al. Adverse Childhood Experiences, Associated Stressors and Comorbidities in Children and Youth with Fetal Alcohol Spectrum Disorder across the Justice and Child Protection Settings in Western Australia. BMC Pediatr. 2022. Oct 10;22(1):587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattson SN, Bernes GA, Doyle LR. Fetal Alcohol Spectrum Disorders: A Review of the Neurobehavioral Deficits Associated With Prenatal Alcohol Exposure. Alcohol Clin Exp Res. 2019. Jun;43(6):1046–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson JL, Akkaya-Hocagil T, Ryan LM, et al. Effects of Prenatal Alcohol Exposure on Cognitive and Behavioral Development: Findings from a Hierarchical Meta-analysis of Data from Six Prospective Longitudinal U.S. cohorts. Alcohol Clin Exp Res. 2021. Oct;45(10):2040–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poth LD, Love T, Mattson SN. Profiles of Language and Communication Abilities in Adolescents with Fetal Alcohol Spectrum Disorders. J Int Neuropsychol Soc. 2022. Nov 3;1–10. doi: 10.1017/S1355617722000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernes GA, Courchesne-Krak NS, Hyland MT, et al. Development and validation of a postnatal risk score that identifies children with prenatal alcohol exposure. Alcohol Clin Exp Res. 2022. Jan;46(1):52–65. ** Development of an efficient and accurate risk score to identify and differentiate those with prenatal alcohol exposure from those without.
- 26.Goh PK, Doyle LR, Glass L, et al. A Decision Tree to Identify Children Affected by Prenatal Alcohol Exposure. J Pediatr. 2016. Oct;177:121–127.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Campo M, Beach D, Wells A, Jones KL. Use of Telemedicine for the Physical Examination of Children With Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2021. Feb;45(2):409–17. [DOI] [PubMed] [Google Scholar]
- 28.Whittingham LM, Coons-Harding KD. Connecting People with People: Diagnosing Persons with Fetal Alcohol Spectrum Disorder Using Telehealth. J Autism Dev Disord. 2021. Apr 1;51(4):1067–80. [DOI] [PubMed] [Google Scholar]
- 29.Lim YH, Watkins RE, Jones H, Kippin NR, Finlay-Jones A. Fetal Alcohol Spectrum Disorders Screening Tools: A Systematic Review. Res Dev Disabil. 2022. Mar;122:104168. [DOI] [PubMed] [Google Scholar]
- 30.Grubb M, Golden A, Withers A, et al. Screening Approaches for Identifying Fetal Alcohol Spectrum Disorder in Children, Adolescents, and Adults: A Systematic Review. Alcohol Clin Exp Res. 2021. Aug;45(8):1527–47. [DOI] [PubMed] [Google Scholar]
- 31.Kable JA, Coles CD, Holton JE, et al. Characteristics of the Symptoms of the Proposed ND-PAE Disorder in First Grade Children in a Community Sample. Child Psychiatry Hum Dev. 2022. Aug 30; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattson SN, Jones KL, Chockalingam G, et al. Validation of the FASD-Tree as a Screening Tool for Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore EM, Xia Y. Neurodevelopmental Trajectories Following Prenatal Alcohol Exposure. Front Hum Neurosci. 2021;15:695855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aghamohammadi-Sereshki A, McMorris CA, Ben Gibbard W, et al. Effects of Prenatal Alcohol Exposure on Neurobehavioural Development and Volume of Rostral Cingulate Cortex Subregions. J Psychiatry Neurosci JPN. 2022. Aug;47(4):E272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernes G, Moore EM, Vaurio L, Mattson SN. Fetal Alcohol Spectrum Disorders. In: Pediatric Neuropsychology: Research, Theory, and Practice, 3rd Edition. 3rd Edition. New York: The Guillford Press; 2022. p. 179–205. [Google Scholar]
- 36.Little G, Reynolds J, & Beaulieu C (2018). Altered Functional Connectivity Observed at Rest in Children and Adolescents Prenatally Exposed to Alcohol. Brain Connectivity, 8(8), 503–515. [DOI] [PubMed] [Google Scholar]
- 37.O’Neill J, O’Connor MJ, Kalender G, et al. Combining Neuroimaging and Behavior to Discriminate Children with Attention Deficit-Hyperactivity Disorder with and without Prenatal Alcohol Exposure. Brain Imaging Behav. 2022. Feb;16(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wozniak JR, Mueller BA, Mattson SN, et al. Functional Connectivity Abnormalities and Associated Cognitive Deficits in Fetal Alcohol Spectrum Disorders (FASD). Brain Imaging Behav. 2017. Oct;11(5):1432–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ware AL, Long X, Lebel C. Functional Connectivity of the Attention Networks is Altered and Relates to Neuropsychological Outcomes in Children with Prenatal Alcohol Exposure. Dev Cogn Neurosci. 2021. Apr 1;48:100951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Min MO, Minnes S, Momotaz H, et al. Fatty Acid Ethyl Esters in Meconium and Substance Use in Adolescence. Neurotoxicol Teratol. 2021. Feb;83:106946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montag AC, Chambers CD, Jones KL, et al. Prenatal Alcohol Exposure can be Determined from Baby Teeth: Proof of Concept. Birth Defects Res. 2022. Aug 15;114(14):797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bodnar TS, Lee C, Wong A, et al. Evidence for Long-Lasting Alterations in the Fecal Microbiota Following Prenatal Alcohol Exposure. Alcohol Clin Exp Res. 2022. Apr;46(4):542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skorka K, McBryde C, Copley J, et al. Experiences of Children with Fetal Alcohol Spectrum Disorder and Their Families: A Critical Review. Alcohol Clin Exp Res. 2020. Jun;44(6):1175–88. [DOI] [PubMed] [Google Scholar]
- 44.Boys CJ, Bjorke J, Dole KN, et al. Improving Educational Outcomes in Fetal Alcohol Spectrum Disorder Through Interagency Collaboration. J Pediatr Neuropsychol. 2016. Jun 1;2(1):50–7. [Google Scholar]
- 45.Turchi RM, Smith VC, Committee on Substance Use and Prevention, Council on Children with Disabilities., Ryan SA, Camenga DR, et al. The Role of Integrated Care in a Medical Home for Patients With a Fetal Alcohol Spectrum Disorder. Pediatrics. 2018. Oct 1;142(4):e20182333. [DOI] [PubMed] [Google Scholar]
- 46.Jones KL, Smith DW. Recognition of the Fetal Alcohol Syndrome in Early Infancy. Lancet Lond Engl. 1973. Nov 3;302(7836):999–1001. [DOI] [PubMed] [Google Scholar]
- 47.Lindinger NM, Jacobson JL, Dodge NC, et al. Stability and Change in the Interpretation of Facial Emotions in Fetal Alcohol Spectrum Disorders from Childhood to Adolescence. Alcohol Clin Exp Res. 2022. Jul;46(7):1268–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Coles CD, Grant TM, Kable JA, Stoner SA, Perez A, Collaborative Initiative on Fetal Alcohol Spectrum Disorders. Prenatal alcohol Exposure and Mental Health at Midlife: A Preliminary Report on Two Longitudinal Cohorts. Alcohol Clin Exp Res. 2022. Feb;46(2):232–42. * Prenatal alcohol is associated with greater rates of mental health disorders in middle adulthood, as well as greater environmental stressors.
- 49.Bill Text - SB-1016 Special education: Eligibility: Fetal Alcohol Spectrum Disorder. [Internet]. [cited 2022 Oct 15]. Available from: https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=202120220SB1016
- 50.H.R.4151 - 117th Congress (2021-2022): FASD Respect Act | Congress.gov | Library of Congress; [Internet]. [cited 2022 Oct 15]. Available from: https://www.congress.gov/bill/117th-congress/house-bill/4151?s=1&r=77 [Google Scholar]
- 51.Fetal Alcohol Spectrum Disorder: Health Needs Assessment [Internet]. GOV.UK. [cited 2022 Oct 16]. Available from: https://www.gov.uk/government/publications/fetal-alcohol-spectrum-disorder-health-needs-assessment/fetal-alcohol-spectrum-disorder-health-needs-assessment [Google Scholar]
- 52.Ordenewitz LK, Weinmann T, Schlüter JA, et al. Evidence-based Interventions for Children and Adolescents with Fetal Alcohol Spectrum Disorders - A Systematic Review. Eur J Paediatr Neurol. 2021. Jul;33:50–60. [DOI] [PubMed] [Google Scholar]
- 53.Paley B, O’Connor MJ. Behavioral Interventions for Children and Adolescents with Fetal Alcohol Spectrum Disorders. Alcohol Res Health J Natl Inst Alcohol Abuse Alcohol. 2011;34(1):64–75. [PMC free article] [PubMed] [Google Scholar]
- 54.Brown J, Jonason A, Asp E, et al. Fetal Alcohol Spectrum Disorder and Confabulation in Psycholegal Settings: A Beginner’s Guide for Criminal Justice, Forensic Mental Health, and Legal Interviewers. Behav Sci Law. 2022. Feb;40(1):46–86. [DOI] [PubMed] [Google Scholar]
- 55.Kully-Martens K, Pei J, Kable J, et al. Mathematics Intervention for Children with Fetal Alcohol Spectrum Disorder: A Replication and Extension of the Math Interactive Learning Experience (MILE) program. Res Dev Disabil. 2018. Jul;78:55–65. [DOI] [PubMed] [Google Scholar]
- 56.O’Connor MJ, Laugeson EA, Mogil C, et al. Translation of an Evidence-Based Social Skills Intervention for Children with Prenatal Alcohol Exposure in a Community Mental Health Setting. Alcohol Clin Exp Res. 2012. Jan;36(1):141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gleichmann DC, Pinner JFL, Garcia C, et al. A Pilot Study Examining the Effects of Music Training on Attention in Children with Fetal Alcohol Spectrum Disorders (FASD). Sensors. 2022. Jul 28;22(15):5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gardiner E, Hutchison SM, McLachlan K, et al. Behavior Regulation Skills are Associated with Adaptive Functioning in Children and Adolescents with Prenatal Alcohol Exposure. Appl Neuropsychol Child. 2022. Dec;11(4):691–701. [DOI] [PubMed] [Google Scholar]
- 59.Home - Learn about FASD - Me & My FASD [Internet]. [cited 2022 Nov 23]. Available from: https://fasd.me/
- 60. Lees B, Riches J, Mewton L, et al. Fetal Alcohol Spectrum Disorder Resources for Educators: A Scoping Review. Health Promot J Aust Off J Aust Assoc Health Promot Prof. 2022. Jul;33(3):797–809. * A large review of resources for school age individuals with FASD
- 61.Wozniak JR, Fink BA, Fuglestad AJ, et al. Four-Year Follow-up of a Randomized Controlled Trial of Choline for Neurodevelopment in Fetal Alcohol Spectrum Disorder. J Neurodev Disord. 2020. Mar 12;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warton FL, Molteno CD, Warton CMR, et al. Maternal Choline Supplementation Mitigates Alcohol Exposure Effects on Neonatal Brain Volumes. Alcohol Clin Exp Res. 2021. Sep;45(9):1762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baker JA, Breit KR, Bodnar TS, et al. Choline Supplementation Modifies the Effects of Developmental Alcohol Exposure on Immune Responses in Adult Rats. Nutrients. 2022. Jul 13;14(14):2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuglestad AJ, Miller NC, Fink BA, et al. Neurophysiological Correlates of Memory Change in Children with Fetal Alcohol Spectrum Disorders Treated with Choline. Front Psychol. 2022;13:936019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Helfrich KK, Saini N, Kwan STC, et al. Gestational Iron Supplementation Improves Fetal Outcomes in a Rat Model of Prenatal Alcohol Exposure. Nutrients. 2022. Apr 15;14(8):1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Durr MRR, Petryk S, Mela M, et al. Utilization of Psychotropic Medications in Children with FASD: A Retrospective Review. BMC Pediatr. 2021. Nov 16;21(1):512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ritfeld GJ, Kable JA, Holton JE, Coles CD. Effectiveness of Psychotropic Medications in Children with Prenatal Alcohol and Drug Exposures: A Case Series and Model of Care. Child Psychiatry Hum Dev. 2022. Oct 1; * Review of medication use in FASD through case study methodology as well as the use of continuous performance tests to evaluate response to intervention and guide decision making.


