Abstract
Perylene bisimide (PBI) dyes are known as red, maroon and black pigments, whose colors depend on the close π−π stacking arrangement. However, contrary to the luminescent monomers, deep-red and black PBI pigments are commonly non- or only weakly fluorescent due to (multiple) quenching pathways. Here, we introduce N-alkoxybenzyl substituted PBIs that contain close π stacking arrangement (exhibiting dπ−π ≈ 3.5 Å, and longitudinal and transversal displacements of 3.1 Å and 1.3 Å); however, they afford deep-red emitters with solid-state fluorescence quantum yields (ΦF) of up to 60%. Systematic photophysical and computational studies in solution and in the solid state reveal a sensitive interconversion of the PBI-centred locally excited state and a charge transfer state, which depends on the dihedral angle (θ) between the benzyl and alkoxy groups. This effectively controls the emission process, and enables high ΦF by circumventing the common quenching pathways commonly observed for perylene black analogues.
Subject terms: Excited states, Crystal engineering, Organic molecules in materials science
Perylene bisimide dyes are high-performance pigments that have red, maroon or black colors, but with typically only weak fluorescence in the solid state. Here, the authors report a series of π-stacked PBI derivatives that show highly efficient deep-red fluorescence in the solid state.
Introduction
Luminescence of organic dyes and pigments has fascinated scientists since the mid-19th century but retains enduring appeals till now. Numerous organic color pigments are deeply rooted in the archeological and historic contexts, and experience a renaissance in organic electronics1,2, such as organic light-emitting diodes (OLEDs)3,4, dye lasers5,6, solar concentrator7 and biological imaging8,9. The rapid development of the organic electronics field has successfully explored abundant building block using common pigments such as perylene-3,4:9,10-bis(dicarboximides) (PBIs)10–13.
PBI derivatives, which cover various shades in the solid state from red to black as commercial pigments, have favorable (opto-)electronic properties and high thermal and (photo-)chemical stability2,14–16. More than for most other pigment chromophores, the color of PBI solids depends much on the longitudinal and transverse displacement of neighboring dye molecules17–20. In particularly, pigments with significant longitudinal but small transverse intermolecular slip, as for instance observed for the commercial pigment Perylene Black 32 (PBk32), are able to absorb all the visible light. The broad band absorption, which is responsible for the black appearance, was ascribed to short range charge transfer (CT) interactions21,22, which modulates the classical Kasha molecular exciton model, based on long-range Coulombic interactions, with its distinction in J- and H-aggregates23,24. In any case, other factors may play a role such as shadowing effects in samples of high optical density25, and polarization effects26, which can strongly modulate the absorption band shapes as a function of the angle of observation27. Despite the interesting absorption properties of black PBI pigments, however, these materials usually suffer from luminescence quenching in the solid state. The quenching processes may include competitive pathways like singlet fission28–32, symmetry-breaking charge separation33–35, and formation of excimer traps36, which are all closely related to the specific intermolecular arrangement in these materials. In fact, in a microscopic view, the mixed electronic states of two neighboring identical chromophores directs the exciton fate37.
In general, avoiding strong π-π interaction and isolating the chromophore appeared as a suitable solution for high solid-state fluorescence25,38–42. Unfortunately, however, such chromophore isolation reduces the usefulness of these solid-state materials for those applications where charge carrier transport is required. Therefore, we focused our attention on stacked PBIs and indeed could demonstrate recently that a polymorph of PBk32, i.e. PBI substituted with 4-methoxybenzyl groups at the peripheral nitrogen atoms (mb-PBI), exhibits a close-to magic-angle stacking mode, and shows solid-state fluorescence with over 0.50 eV redshift relative to the monomer owing to the strong π-π interactions43. Most notably, the crystalline film exhibited appreciable near-infrared (NIR) emission with a fluorescence quantum yield ΦF of about 10%. Therefore, the complex mb-PBI structure concept appeared very promising to generate emissive perylene black analogues, compared to earlier attempts with less complex structure modulation.
In the current work, we therefore systematically elucidate a series of PBI derivatives with different length of branched alkoxy chains at the para-position of the N-benzyl rings, also offering better solubility in comparison with mb-PBI. In solution, the compounds are all moderately emissive (ΦF of 20% in toluene) to low emissive (ΦF of 1–2% in dichloromethane (DCM)) due to the presence of a low-lying CT state. In crystalline films, deep-red (DR) emission with solid-state luminescence enhancement44 is observed, instead of quenching by the predicted excitonic relaxation to multi-exciton states, charge-separated states or excimers in such π-stacked arrangement of PBI chromophores45; however, the enhancement depends largely on the length of the alkoxy substituents, as well as morphological factors, so that ΦF in films ranges from 2% to up to 60%. This is considered as a specific asset of the compounds reported here, as bright DR/NIR solid-state emitters are still a major challenge in the field, also beyond PBIs46,47. Systematic photophysical studies in solution and solid state under varying environmental conditions, combined with computational studies fully rationalize the experimental results. Accordingly, we have discovered a long sought-after packing structure for this important class of pigments, now providing highly fluorescence solid state DR emitters.
Results
Synthesis
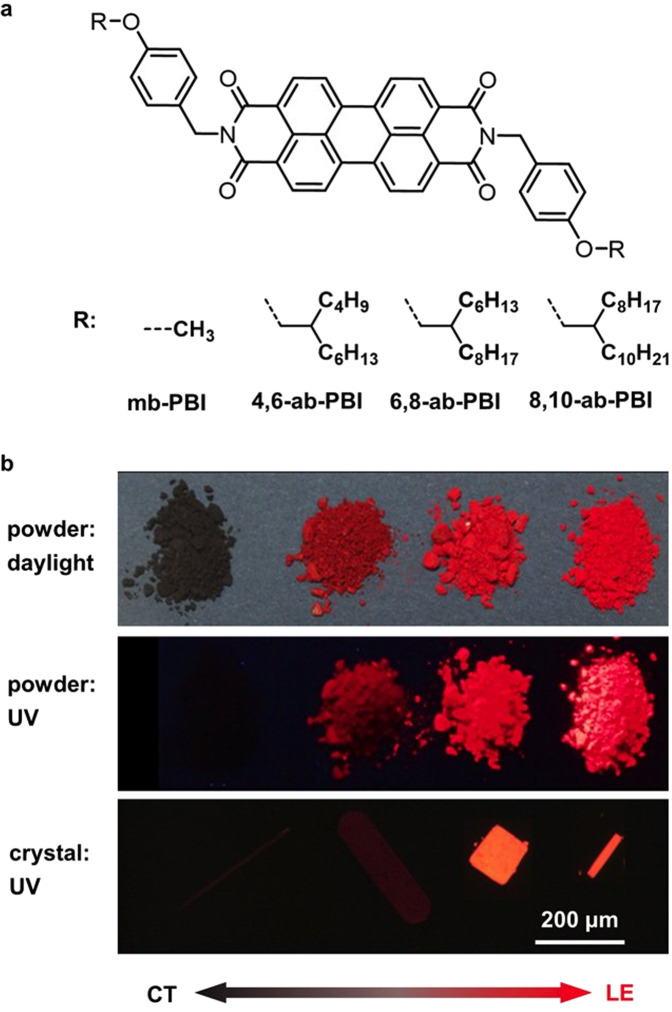
Derived from the parent compound mb-PBI, the methoxyl groups were replaced by branched alkoxy chains with different lengths, giving 4,6-ab-PBI, 6,8-ab-PBI and 8,10-ab-PBI (Fig. 1a). All three alkoxybenzyl substituted PBIs were synthesized by a straightforward method with yields of 50% to 70% according to the previously reported procedures15,48, and were characterized by 1H NMR and 13C NMR (Supplementary Figs. 2–13 in the Supplementary Information; SI). The crude products were purified by column chromatography on silica gel using the mixture of petroleum ether and DCM as eluent. All compounds are well soluble in common organic solvents like toluene, chloroform and chlorobenzene but less soluble in polar solvent such as methanol. The detailed synthetic procedures and characterization data of the PBIs are given in the SI.
Fig. 1. Molecular structure and photographs of powder and crystals.
a Chemical structures of alkoxybenzyl substituted PBIs. b Photographs of the corresponding powders upon UV irradiation (365 nm) and daylight as well as fluorescence images of single crystals with exposure time of 158 milliseconds.
Crystal structures
The molecular structures and stacking patterns of the PBIs were determined by single crystal X-ray diffraction measurements. Bright red single crystals of PBIs were successfully obtained by the liquid-liquid diffusion method, where ethanol (poor solvent) diffused slowly into chloroform solution (good solvent) at room temperature for ~3 days. Plate-like crystals formed at the bottom of the solution (Fig. 1b). Suitable single crystals were selected, and measured in a nitrogen stream at 150 K. The crystal data and structure refinement are summarized in Supplementary Table 1.
All three PBIs showed similar geometries, consisting of a rigid and planar backbone (Fig. 2), and N-substituted benzyl groups pointing to the opposite sides, which resulted in a centrosymmetric chair conformation (Fig. 2c). The chiral carbon atoms of the alkoxy chains in the centrosymmetric positions led to an optical mesomer. A significant difference in the molecular structures is seen in the dihedral angle between the benzyl and alkoxy groups (θ), giving 9° for 4,6-ab-PBI, but 33° and 26° for 6,8-ab-PBI and 8,10-ab-PBI respectively, which indeed influenced the electronic transitions (vide infra).
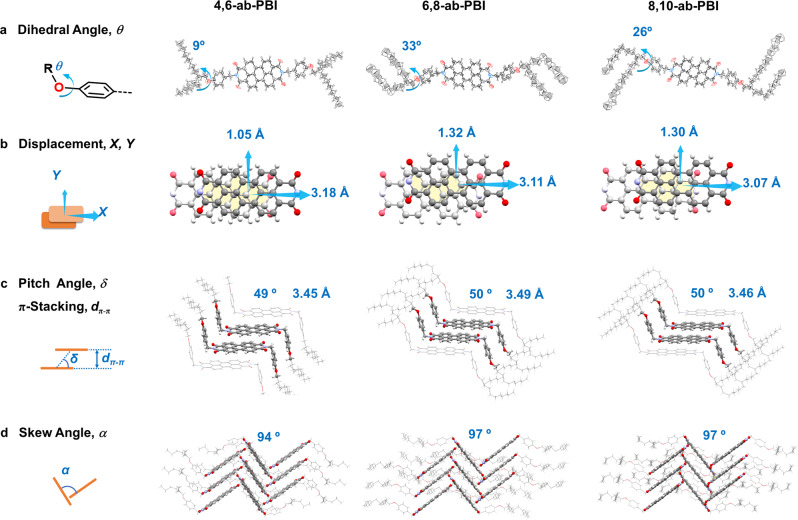
Fig. 2. X-ray crystal structures of the compounds under study.
a ORTEP drawings of the molecular structures (ellipsoid probability 50%), indicating the dihedral angle (θ) between the benzyl and alkoxy groups. b Perspective view along the π-π stacking direction, indicating longitudinal (X) and transverse displacements (Y) between neighboring molecules. c Side view of the PBI π-core, indicating counter pitch angle (δ) for the longitudinal slip and the distance between the π-planes (dπ-π). d View along the column axis, indicating the skew angle (α) between neighboring columns.
All PBIs are aligned in a slipped π-stacked (face-to-face) fashion with a π-π distance of about 3.5 Å and large π-π overlap. In fact, about half of the π-surface overlaps with that of the neighbor molecules (Fig. 2b). The longitudinal (X), transversal (Y) slips are given in Table 1, giving counter pitch angles (δ) of 49–50° for 4,6-ab-PBI, 6,8-ab-PBI and 8,10-ab-PBI, respectively. Neighboring slipped-stacked columns are oriented in a skew angle of 94–97°, giving a zig-zag arrangement following the general classification scheme of ref. 25. Between the stacks, multiple intermolecular C-H···O interactions are found with distances of 2.5–2.8 Å, corresponding indeed to the expected O···H van-der-Waals distance of 2.7 Å, see Supplementary Fig. 14. Along the long molecular (N-N) axis direction, the hydrophobic interactions by branched alkoxy chains are expected to be much weaker compared to the π-π and H-bonding interactions, indicating that this direction was perpendicular to the slow growing faces, which therefore dominates the lamellar morphology. Therefore, the lamellar structure was readily assembled on the spin-coated film with post-annealing, which was verified by the orientation analysis form grazing-incidence wide-angle X-ray scattering (GIWAXS) measurements (Supplementary Fig. 22).
Table 1.
Structural parameters extracted from the crystal structures, as illustrated in Fig. 2
| X /Å | Y /Å | dπ-π /Å | δ / ° | θ/ ° | α/ ° | |
|---|---|---|---|---|---|---|
| 4,6-ab-PBI | 3.18 | 1.05 | 3.45 | 49 | 9 | 94 |
| 6,8-ab-PBI | 3.11 | 1.32 | 3.49 | 50 | 33 | 97 |
| 8,10-ab-PBI | 3.07 | 1.30 | 3.46 | 50 | 26 | 97 |
Optical properties
The optical and photophysical properties of the three PBIs were measured both in dilute solution and in annealed spin-coated thin films with thickness of about 30 nm (Fig. 3). In solution, all three PBIs exhibit well-resolved absorption bands at 528, 490, and 460 nm, attributed to the apparent 0–0, 0–1 and 0–2 vibronic progression of the S0 → S1 transition49; the latter corresponds to an excitation between the highest occupied and lowest unoccupied molecular orbitals (HOMO; LUMO) of the PBI core. Here, 0–0 is the most intense sub-band, as commonly observed in rigid π-core systems with little geometrical reorganization upon electronic excitation. Consequently, small Stokes shifts (ΔEνStokes) between absorption and fluorescence are observed; for instance, in chloroform 9 meV (4,6-ab-PBI and 6,8-ab-PBI) and 13 meV (8,10-ab-PBI). Furthermore, the fluorescence spectra are approximately mirror-symmetrical to the absorption spectra.
Fig. 3. Basic optical properties of 4,6-, 6,8-, 8,10-ab-PBI.
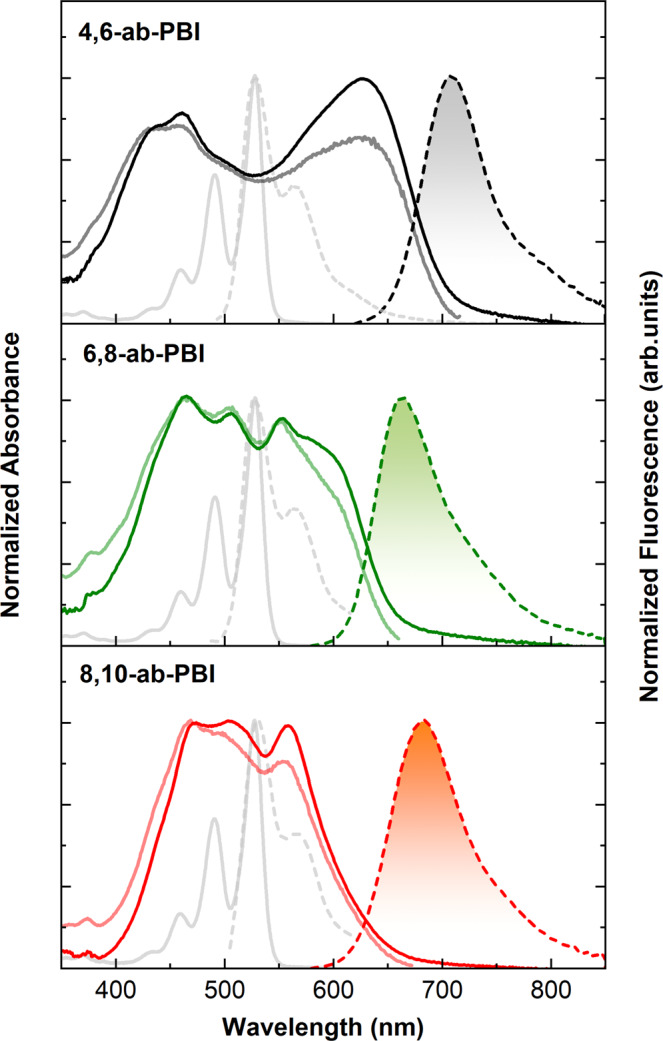
Normalized UV-vis absorption (solid lines), excitation (light solid lines) and fluorescence (dashed lines with filled area) spectra of 4,6-, 6,8-, 8,10-ab-PBI (top to bottom) annealed spin-coated thin films on quartz substrates, respectively (Supplementary Fig. 22). Gray solid and dashed lines show the absorption and fluorescence spectra of the monomers in chloroform solutions (1 × 10−5 M for UV-vis absorption spectra and 1 × 10−7 M for fluorescence spectra).
Nevertheless, the fluorescence properties depend significantly on the environmental conditions. In non-polar solvents, such as toluene, the fluorescence spectra are similarly sharp as in absorption, and ΦF are relatively high; i.e., 7–10% in chlorobenzene and 22–25% in toluene (Supplementary Figs. 15–17, and Supplementary Tables 4–6). In any case, the ΦF are much smaller for the three compounds when compared with common PBIs, whose ΦF are close to unity14. Concomitantly, the fluorescence lifetimes of the three compounds are significantly shortened against the common PBIs, giving about 1.6 ns in toluene. In contrast, in polar, aprotic solvents such as chloroform, DCM or tetrahydrofurane, the fluorescence spectra are significantly broadened vs. absorption, and hypsochromically shifted with respect to non-polar solvents. At the same time, ΦF drops to only 1–2%, and the fluorescence decay in chloroform (Supplementary Fig. 18) is well-fitted by a bi-exponential decay with a short component of ca. 0.3–0.6 ns and a long one of 4–6 ns (Table 2).
Table 2.
Optical and photophysical properties of the PBI derivatives in chloroform solution, and as annealed spin-coated PBI thin films
| Sample | λabsa/nm | λema/nm | τF/ns | ΦF/% | |
|---|---|---|---|---|---|
| 4,6-ab-PBI | Solution | 528 | 530 |
0.3 (40%), 4.1 (60%)b |
1 |
| Film | 628 | 708 | 0.7 | 2 | |
| 6,8-ab-PBI | Solution | 528 | 530 |
0.3 (72%), 4.7 (28%)b |
1 |
| Film | 557/577 | 668 | 1.9 | 27 | |
| 8,10-ab-PBI | Solution | 527 | 530 |
0.2 (52%), 4.3 (48%)b |
2 |
| Film | 559 | 684 | 6.1 | 60 | |
aThe given values are those for the maximum of the largest wavelength absorption and emission band.
bBiexponential fits, obtained by deconvolution; fractional intensities are given in parentheses.
The annealed spin-coated thin films show ‘double-hump’ absorption spectra with blue- and red-shifted components relative to the monomer spectra (Fig. 3). This common feature for black perylene pigments has been ascribed to the presence of low-lying CT states between closely stacked PBIs at longitudinal displacements of ~3 Å that afford two distinct bands due to strong Frenkel/CT-state mixing (Supplementary Table 3)21,22, whose specific shape may further be affected by polarization effects (vide supra)26,27. The position of the high energy band is quite constant for the three compounds, located at ca. 470 nm, while the position of the low energy band largely differs, giving 628, 557/577 and 559 nm for 4,6-ab-PBI, 6,8-ab-PBI, and 8,10-ab-PBI, respectively. This explains the somewhat darker red color of 4,6-ab-PBI under daylight illumination compared to the other compounds (Fig. 1b). We emphasize in this context that mb-PBI has a black appearance under daylight as the low energy band is significantly more red-shifted to ca. 680 nm in the film, so that the absorption onset is found at 800 nm43, and all visible light is absorbed in the powder sample shown in Fig. 1b. Finally, the long extending tails of the absorption spectra in the red-part of the absorption spectra are entirely due to light scattering of the polycrystalline films, as verified by the comparison with the fluorescence excitation spectra in Fig. 314. From this, the absorption edge can be estimated to about 700 nm, 650 nm and 660 nm for 4,6-, 6,8- and 8,10-ab-PBI, respectively.
In contrast to the structured fluorescence spectra in solution, the fluorescence spectra of the films do not exhibit pronounced vibronic features, and reveal rather small widths of about 0.17 eV (FWHM), pointing to coherence effects as typically observed for J-type aggregates14. Due to the differences in the position of the low-energy absorption band, the bathochromic solid-state fluorescence shifts against solution are somewhat different, giving 0.59 eV, 0.48 eV and 0.53 eV for 4,6-ab-PBI, 6,8-ab-PBI, and 8,10-ab-PBI, respectively. Because of the fluorescence emission in the DR, the emission color is very similar to the absorption color as in fact seen in the very similar hues of the powders under UV and daylight illumination (Fig. 1b). Significant differences are however observed for ΦF. As shown in Table 2, 4,6-ab-PBI exhibits a very low fluorescence quantum yield in the film state of only 2% with a relatively short fluorescence lifetime of 0.7 ns. In sharp contrast, 6,8-ab-PBI gives 27% and 1.9 ns, and ΦF for 8,10-ab-PBI reaches 60% and 6.1 ns for 8,10-ab-PBI. This is surprisingly high as PBI derivatives with broad ‘double hump’ absorption features usually do not effectively emit in the solid state28,30,50,51. The pronounced difference between the low-emissive 4,6-ab-PBI and the well-emissive 6,8-ab-PBI and 8,10-ab-PBI is also seen in the powder and crystal samples under UV irradiation (Fig. 1b)25; this excludes morphological reasons for the observed variance, but suggests differences in the intra- and/or intermolecular solid-state architecture.
Discussion
Solution properties
Different from many other common PBIs with ΦF yield close to unity14, the dyes studied here showed only weak emission in dilute solutions of polar, aprotic solvents. In common PBIs, due to the fact that the nitrogen atoms exhibit orbital nodes for both HOMO and LUMO, substituents at the N-position hardly affect the characteristic optical S0 → S1 transition; the latter is described as a locally excited (LE) state within the PBI core. In the current case however, the alkoxybenzyl substituents introduce two low-lying singlet excited CT states, formed between the frontier MOs of the alkoxybenzyl donor (D) and the PBI acceptor (A), see Fig. 4a and Supplementary Fig. 28. According to our time-dependent density functional theory (TD-DFT) calculations on the fully optimized structure, the CT states correspond to S1,2 with vanishing oscillator strength f1,2; the PBI-localized LE state is then found ca. 0.10 eV above (S3; with f3 ≈ 1.0). After photoexcitation from the ground state (GS) to the LE state, photoinduced electron transfer (PeT) takes place, generating the formal CT state; this process is expected to be highly efficient at ambient temperatures due to the spatial proximity of D and A52. Due to the charge-separated nature of the CT state, subsequent back electron transfer recovers GS. Concomitantly, very small ΦF are expected in this electronic situation in agreement with our experimental results in polar solution, and as indeed found in other PBI-based D-A dyads or D-A-D triads with short linkers53,54.
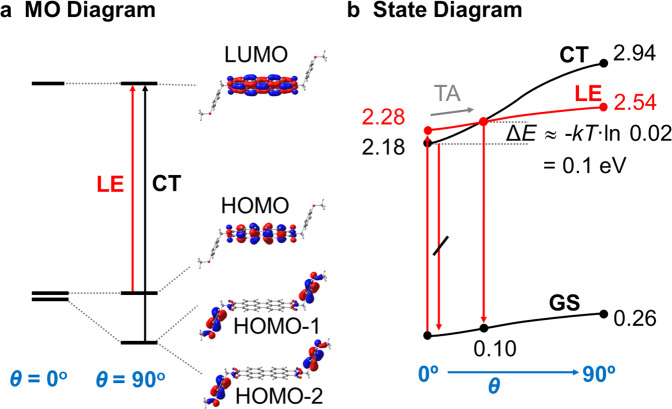
Fig. 4. Deactivation model for the PBI derivatives in solution according to single point TD-DFT calculations.
a Frontier MO energy levels and topologies for torsional angle θ between the alkoxy and benzyl groups of 0° and 90°. b Potential energy surface (PES) of GS, CT, and LE states along θ. The energy levels indicated in the diagram is in eV.
From the simple PeT scenario, mono-exponential fluorescence decay is expected, where the PeT rate shows an exponential dependence on the D-A separation52. Interestingly however, in the current case, bi-exponential kinetics are observed (Table 2 and Supplementary Figs. 18–21). This suggests a somewhat more complex kinetic model, i.e., by repopulation of the LE state, which is thermally accessible from the CT state. In fact, the energy of the CT state depends sensitively on the rotation of the alkoxy group relative to the benzyl group (θ) as the CT state is sufficiently stabilized only close to the co-planar conformation (θ = 0°) due to the destabilization of the donor-localized occupied MOs through the enhanced conjugation in the alkoxy-benzyl moiety; see Fig. 4b. Thus, at larger angles θ, this can lead to a crossover of the CT and LE states (Fig. 4b). Due to the small separation ΔELE-CT, this crossover can be thus induced by thermal fluctuations of θ. At room temperature, the energy barrier of 0.01 eV corresponds to an equilibrium constant KLE/CT = 0.02 which correspond well to the measured ΦF values in Table 2 if we (reasonably) assume a non-emissive CT state and a fully emissive LE state. This 3-state model (i.e., GS, LE, CT) with communicating CT and LE states in fact agrees with the observed bi-exponential kinetics found in solution (Table 2), as such behavior is indeed expected for a consecutive-competitive reaction with a reversible step55.
The energy separation ΔELE-CT depends critically on the solvent polarity; the latter commonly induces significant bathochromic shifts for CT states due to pronounced solvent reorganization in the excited state, which stabilizes the latter56–58. On the other hand, the LE state can experience small bathochromic59, or hypsochromic shifts58, depending on the change of ground and excited state dipole moments. Thus, ΔELE-CT should increase with solvent polarity, and should thus decrease ΦF; this indeed agrees with the experimental observations, which gave ΦF ≈ 1% in DCM, but ΦF ≈ 23% in toluene (Supplementary Figs. 18–21 and Supplementary Tables 4–7).
Upon cooling of the chloroform solution down to 190 K, the fluorescence intensity increases by about a factor of two, and elongates the mean lifetime, i.e., to about 1 ns; this is due to the increase of the (dominant) short lifetime, while the long lifetime changes little, however with a smaller statistical weight (Supplementary Figs. 19–21). We ascribe the increase of the lifetime to the commonly observed thermal activation of the PeT process, as described by the Marcus equation52, to populate the CT state. The temperature dependence is however not very pronounced as the repopulation of the LE state is as well thermally activated. Furthermore, we note that also CT and LE state energies are expected to change somewhat upon cooling.
Solid-state properties
In the solid state, the photophysics of π-conjugated molecules are often strongly modulated against solution by geometrical changes, by intermolecular interactions and by morphological factors, which all impact spectral positions and band shapes, as well as radiative and non-radiative rates25. For the PBIs under study, strong intermolecular interactions are evident from the pronounced bathochromic shift of the rather narrow fluorescence spectra vs. solution, originating from the PBI-centered LE state. Significant interactions are expected from the considerable π-π overlap as discussed further up. In PBI-type materials, depending on the specific region of the excited state potential hypersurface21, π-stacked arrangements with such small transversal slips give usually rise to ultrafast nonradiative exciton deactivation via singlet fission, symmetry-breaking charge separation, or excimer formation45. Thus, the reported high fluorescence quantum yield and long lifetimes within the current PBI series, despite the similar structural arrangement, may surprise at the first glance.
Strong differences for ΦF of the annealed films of the current PBI series are indeed observed, giving only 2% for 4,6-ab-PBI, while 6,8-ab-PBI and 8,10-ab-PBI are highly emissive with 27% and 60%, respectively. Nevertheless, we note that the interpretation of solid-state fluorescence data is generally complicated by morphological issues. In fact, small domain sizes in polycrystalline samples may promote trapping25. Moreover, polymorphism is also frequently observed for π-conjugated organic materials, which may significantly impact ΦF25. Such morphological issues are also evident in the current case; in fact, ΦF of powder samples vary from those in the annealed films, giving 2% for 4,6-ab-PBI, while 17% and 15% are observed for 6,8-ab-PBI and 8,10-ab-PBI. In any case, the very pronounced difference between low-emissive 4,6-ab-PBI and well-emissive 6,8-ab-PBI and 8,10-ab-PBI is evident also here, and furthermore in the single crystal samples under UV illumination (Fig. 1b); in these homogenous 0.1–1 mm crystals, trapping at surfaces or interfaces can be indeed excluded because of low defect concentration, small surface/volume ratio, and large average distance of the exciton to the surface25. In all, this suggests intrinsic, i.e., intra- and/or intermolecular, reasons for the pronounced drop of ΦF in 4,6-ab-PBI vs. 6,8-ab-PBI and 8,10-ab-PBI.
Evidently, as the π-stacking arrangement of the compounds does not significantly differ from each other, the reason for the largely varying ΦF is thus not ascribed to intermolecular factors, but rather to intramolecular ones, i.e., to the difference of the angle θ between the alkoxy group relative to the benzyl group. Indeed, as our solution study clearly showed, this strongly impacts the photophysics. As a matter of fact, in single crystals of 6,8-ab-PBI and 8,10-ab-PBI, θ is rather large with 33° and 26°, respectively (Fig. 2 and Table 1); in sharp contrast, θ is small for 4,6-ab-PBI (9°). This gives a plausible explanation for the observations in the thin films, consistent with our electronic scheme in Fig. 4. In 6,8-ab-PBI and 8,10-ab-PBI θ is large enough to promote the CT state toward high enough energy to prohibit the fluorescence quenching of the LE state. In fact, according to our calculations, the CT state is found 0.05 eV below LE at 26° and 0.02 eV at 33°, respectively; on the other hand, for 4,6-ab-PBI with θ = 9°, CT is calculated to be 0.09 eV below LE, so that here ΦF remains small. Concomitantly, in mb-PBI, where θ = 2°, the fluorescence is even further quenched. In any case, although the main factor for the large differences in ΦF is seen in the variation of θ, morphology is evidently contributing as well in the polycrystalline thin film samples. This is in fact seen in the significant smaller ΦF in the thin film sample of 6,8-ab-PBI compared to 8,10-ab-PBI, despite the somewhat smaller θ in the latter.
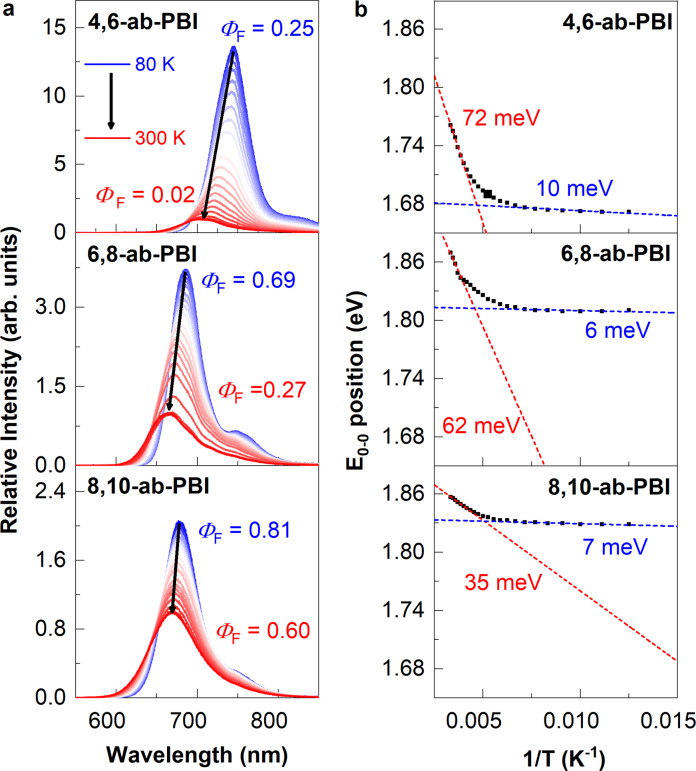
Evidently, as in solution, quenching via PeT in the solid state is a thermally activated process, so that cooling of the films to 80 K significantly enhances ΦF in particular for 4,6-ab-PBI (25%, see Fig. 5); for 6,8-ab-PBI and 8,10-ab-PBI, ΦF of about 70% and 80%, respectively, are observed under this condition. The fluorescence enhancement upon cooling is accompanied by an elongation of the fluorescence lifetimes in the same order of magnitude (Supplementary Figs. 23–25 and Supplementary Table 8), indeed indicating the suppression of the nonradiative quenching pathway upon cooling. The observed bathochromic shift of the LE emission upon cooling (Fig. 5b) is ascribed to the increased effective polarizability60,61; this stabilization of the LE state may additionally contribute to the fluorescence enhancement upon cooling. In fact, there is a systematic trend on the enhancement factor ΦF (80 K)/ΦF (300 K) with the extent of the bathochromic shift, being the largest for 4,6-ab-PBI (Fig. 5b). Finally, it is remarked that the torsional angle is not expected to be constant with temperature. This is evident from ab initio molecular dynamics (AIMD) simulations (Supplementary Figs. 26–27 for details); these evidence negligible intermolecular dynamics, but significant variations of dihedral angles in the solid state. Importantly, no major differences in the dynamics are observed between the three different compounds, as the mean deviation from the respective equilibrium values of θ are rather smaller in 4,6- compared to 6,8- and 8,10-ab-PBI, see (Supplementary Fig. 27). This excludes a dynamic origin for the difference between the low emissive 4,6- vs. the highly emissive 6,8- and 8,10-ab-PBIs, i.e., by a less restricted conformational space in the former. Therefore, the AIMD results further support our scenario on a specific locking of distinct dihedral angles θ for the different compounds, which regulates the access of the emissive LE state.
Fig. 5. Temperature-dependent steady-state fluorescence spectra of PBIs crystalline films.
a Temperature-dependent steady-state fluorescence spectra of PBIs crystalline films (heating from 80 K to 300 K, λex = 480 nm). b Temperature dependence of 0–0 emission peak energy (Supplementary Figs. 23a–25a) and the Gaussian energetic disorder calculated according to the slopes at each extreme along with the temperature-dependent fluorescence spectra.
To summarize, we have reported a series of N-alkoxybenzyl substituted perylene bisimides (4,6-, 6,8-, 8,10-ab-PBI) with different alkoxy substitution pattern, which showed a strong dependency of the fluorescence quantum yields ΦF on the environmental conditions. While in polar, aprotic solvents all compounds were weakly emissive, they became considerably emissive upon cooling, as well as in non-polar solvents. In the solid state, ΦF was low for 4,6-ab-PBI and high for 6,8- and 8,10-ab-PBI. A significant increase of ΦF was observed upon cooling for all compounds. All observations could be consistently explained by a model based on an emissive PBI-core-centered LE state vs. a low-lying non-emissive CT state formed between the PBI acceptor (A) and the alkoxybenzyl donor (D) with a distinct dihedral angle θ between the benzyl and alkoxy groups. The CT state is accessed from the LE state by fast thermally activated photo-induced intramolecular electron transfer (PeT), which can repopulate LE. The equilibrium between LE and CT is subtly influenced by temperature, polarity and the angle θ, which allowed to rationalize the observation in solution under the various conditions. In the solid state, the anomalous high fluorescence efficiency and longer lifetime indicated the suppression of nonradiative exciton deactivation through multi-exciton states, charge-separated states or excimers, which are otherwise commonly observed in PBI chromophores with such pronounced π-stacked arrangement. On the other hand, varying ΦF in the current PBI series is ascribed to intramolecular factors, where the much smaller dihedral angle θ for 4,6-ab-PBI in comparison with 6,8-, 8,10-ab-PBI rationalizes the low ΦF of the former vs. the high ΦF of the latter. The study showed that complex D-A-D PBI triads enable to modulate solution and solid-state fluorescence properties to a large extent. Most importantly, this allows to create highly desired emissive DR solid-state emitters, despite close π-stacking, which commonly leads to quenching in perylene black analogues. Future research will be dedicated to the exploration of how to systematically tune θ and thus ΦF by targeted design of the molecular backbone and the (alkyl) substituents, as well as on the application of such highly emissive solid-state materials in organic electronic devices as for instance in light-emitting field effect transistors and solar concentrators.
Methods
Sample preparation
The synthesis and analysis of single-crystal X-ray diffraction of PBIs are described in the Supplementary Information. All raw materials were purchased from commercial sources and used without further purification, unless otherwise noted. Column chromatography was performed on silica gel (Hai Yang Silica 60, particle size 200–300 nm). NMR spectra were recorded on a Bruker-500 spectrometer at 500 MHz in deuterated solvents at 298 K. Chemical shifts were reported as δ values (ppm) relative to the residual solvent signal.
Films were prepared by spin-coating (2500 rpm, 30 s) from solution (chloroform for 4,6-ab-PBI and 6,8-ab-PBI, chlorobenzene for 8,10-ab-PBI) onto substrates, and were further annealed in air (4,6-ab-PBI and 6,8-ab-PBI at 180 °C for 30 min, 8,10-ab-PBI at 160 °C for 30 min). Single crystals were successfully grown by liquid-liquid diffusion method of ethanol into chloroform solutions.
X-ray diffraction
Suitable single crystals were selected and the X-ray diffraction data were collected in a Rigaku XtaLAB P2000 FR-X setup with a rotating copper anode and a Pilatus 200 K detector.
Grazing-incidence wide-angle X-ray scattering (GIWAXS) measurements were performed in a Xenocs Xeuss 2.0 system, equipped with a MetalJet-D2 Excillum source (λ = 1.34144 Å); the beam size was 0.8 × 0.8 mm2 in high flux mode. The scattered signals were collected by a Pilatus3R 1 M detector, Dectris (Pixel Size: 0.172 × 0.172 mm2). A movable beamstop was deposited at direct beam position to protect the detector from saturation within a long exposure time (1800 s).
UV-vis absorption and fluorescence measurements
The UV-vis spectra of 1 × 10−5 M solutions were carried out with a Shimadzu UV-3600 spectrometer. Steady-State fluorescence excitation and emission spectra of solutions with maximum extinction ~0.1 and of the spin-coated films (ca. 30 nm) were recorded in an Edinburgh FLS980 spectrometer. All spectra were corrected for the characteristics of the lamp source and detection unit, respectively.
Absolute fluorescence quantum yields were obtained in a calibrated integrating sphere (Hamamatsu, C11347-11). Fluorescence images of the single crystals were recorded using a Leica DM4000 microscope with an EL6000 light source and excitation filters.
Temperature-dependent steady-state spectra and time-resolved fluorescence spectra were recorded by homebuilt optical paths. Film and solution samples were placed inside a vacuum nitrogen cryostat (OptistatDN, Oxford). Heating of the film samples was carried out from 80 K to 300 K in vacuum, using a Mercury iTC temperature controller. The samples were excited with a femtosecond laser pulse at 480 nm with a pulse duration of 80 fs and a repetition rate of 80 MHz (Spectra Physics, Mai Tai & Inspire HF 100). Steady-state spectra were obtained by integration with a CCD camera (Princeton Instruments, Pixis 100B). Fluorescence time traces were recorded by time-correlated single photon counting, using a monochromator (Princeton Instruments, Acton 2300i) and a PicoQuant HydraHarp 400 system).
DFT calculations
Geometry optimizations of the molecules were carried out at the DFT level of theory without geometry restriction, as well as with fixed dihedral angles θ between the benzyl and alkoxy groups. Single point TD-DFT calculations were performed to obtain the vertical transition energies in implicit solvent (DCM) through the polarizable continuum model (PCM). In all cases, the B3LYP functional with the standard 6–311 G* basis set with dispersion correction (D3) was applied, as defined in the Gaussian16 program package62. While standard functionals like B3LYP are known to fail for states with partial CT character63, they operate well for states with very pronounced CT character (as in the current case)64,65; a comparison with long-range corrected functionals is given in the SI. It is noted that the computational modelling of solvent effects for CT states is non-trivial as the adiabatic approximation (on which the PCM model is based) does not necessarily holds, as discussed in ref. 58. It is further noted that the excited state ordering in the TD-DFT calculations at θ = 0° differs from the one-electron configurations in DFT due to configuration interaction. In fact, in the DFT-based MO diagram in Fig. 4 the donor-located occupied MO are lower than the acceptor-located ones; however, in the TD-DFT description, this situation reverses.
The geometry of the dimers was optimized by means of a QM/MM (quantum mechanics/molecular mechanics) approach using the ONIOM implementation in Gaussian16. Here, the torsion angles were fixed to those extracted from the experimental X-Ray data at 150 K and 300 K to give a reliable crystal environment. Electronic transitions were performed by single-point TD-DFT calculations.
The dynamics of the PBI derivatives in a realistic environment were carried out by a 4 ps AIMD simulations, using the ORCA 5.0 software66–68 at the B3LYP-D3/6-31 G* level of theory at 150 K and 300 K, where the input files were prepared with the Multiwfn program69,70, based on the initial geometries from the X-Ray data. The initial atomic velocity was set according to Boltzmann-Maxwell distribution. The relative motion was investigated between nearest neighbor molecules, and quantitative statistics on the trajectory was performed.
Supplementary information
Acknowledgements
We sincerely thank the financial supports from National Natural Science Foundation of China (NSFC) (21975076, 52003089, 52103206, 21733005, 51873068) (Z.X. and J.Z.), the Fund of the Key Laboratory of Luminescence from Molecular Aggregates of Guangdong Province (2019B030301003) (Z.X.). The work in Madrid was supported by the Spanish Ministerio de Economía y Competitividad (MINECO-FEDER project CTQ2017-87054), by the Severo Ochoa program for Centers of Excellence in R&D of the MINECO/MICINN (SEV-2016-0686, CEX2020-001039-S) and by the Campus of International Excellence (CEI) UAM + CSIC (J.G.). We also thank the support from the 111 Project (G2022163017L) (F.W. and Z.X.) and Science and Technology Projects in Guangzhou (202102020561, 202102020401) (J.Z.).
Source data
Author contributions
Z.X. conceived the project and suggested the molecular structures. N.T. performed the chemical synthesis, grew single crystals, and carried out most of the characterization and data collection. J.Z. conducted X-ray diffraction measurements and GIWAXS measurement. L.W. and J.G. conducted the DFT calculations and analyzed the calculated results. G.X., X.W., and L.L. contributed to the construction of the laser path of temperature-dependent fluorescence spectra. M.S. confirmed the UV-vis spectra, PL spectra, lifetime and PLQY tests. N.T. and J.Z. wrote the original draft. F.W., J.G., and Z.X. supervised the work in the groups, and guided data interpretation, paper writing and revisions. All the authors discussed the results and commented on the paper.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
All data generated in this study are provided in the paper and Supplementary Information, and the raw data supporting this study are available from the Source Data file. Source data are provided with this paper.
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2211699, 2211700, 1974107, 2211720, 2211721. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jiadong Zhou, Email: zhoujd@scut.edu.cn.
Frank Würthner, Email: wuerthner@uni-wuerzburg.de.
Johannes Gierschner, Email: johannes.gierschner@imdea.org.
Zengqi Xie, Email: msxiez@scut.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-37171-y.
References
- 1.Gsänger M, et al. Organic semiconductors based on dyes and color pigments. Adv. Mater. 2016;28:3615–3645. doi: 10.1002/adma.201505440. [DOI] [PubMed] [Google Scholar]
- 2.Zhan X, et al. Rylene and related diimides for organic electronics. Adv. Mater. 2011;23:268–284. doi: 10.1002/adma.201001402. [DOI] [PubMed] [Google Scholar]
- 3.Kim KH, et al. Phosphorescent dye-based supramolecules for high-efficiency organic light-emitting diodes. Nat. Commun. 2014;5:4769. doi: 10.1038/ncomms5769. [DOI] [PubMed] [Google Scholar]
- 4.Dayneko SV, Rahmati M, Pahlevani M, Welch GC. Solution processed red organic light-emitting-diodes using an N-annulated perylene diimide fluorophore. J. Mater. Chem. C. 2020;8:2314–2319. doi: 10.1039/C9TC05584C. [DOI] [Google Scholar]
- 5.Hales JE, et al. Virus lasers for biological detection. Nat. Commun. 2019;10:3594. doi: 10.1038/s41467-019-11604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gierschner J, Varghese S, Park SY. Organic single crystal lasers: a materials view. Adv. Opt. Mater. 2016;4:348–364. doi: 10.1002/adom.201500531. [DOI] [Google Scholar]
- 7.Huang CS, et al. Energy harvesting textiles: using wearable luminescent solar concentrators to improve the efficiency of fiber solar cells. J. Mater. Chem. A. 2021;9:25974–25981. doi: 10.1039/D1TA04984D. [DOI] [Google Scholar]
- 8.Luo S, et al. A review of NIR dyes in cancer targeting and imaging. Biomaterials. 2011;32:7127–7138. doi: 10.1016/j.biomaterials.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Cosco ED, et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem. 2020;12:1123–1130. doi: 10.1038/s41557-020-00554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H, Bao Z, Oh JH. High-performance phototransistors based on single-crystalline n-channel organic nanowires and photogenerated charge-carrier behaviors. Adv. Funct. Mater. 2013;23:629–639. doi: 10.1002/adfm.201201848. [DOI] [Google Scholar]
- 11.Wen X, et al. Phenol-functionalized perylene bisimides as amine-free electron transporting interlayers for stable nonfullerene organic solar cells. Adv. Funct. Mater. 2022;32:211170. doi: 10.1002/adfm.202111706. [DOI] [Google Scholar]
- 12.Shang X, et al. Supramolecular Nanostructures of Chiral Perylene Diimides with Amplified Chirality for High-Performance Chiroptical Sensing. Adv. Mater. 2017;29:1605828. doi: 10.1002/adma.201605828. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt R, et al. High-performance air-stable n-channel organic thin film transistors based on halogenated perylene bisimide semiconductors. J. Am. Chem. Soc. 2009;131:6215–6228. doi: 10.1021/ja901077a. [DOI] [PubMed] [Google Scholar]
- 14.Würthner F, et al. Perylene bisimide dye assemblies as archetype functional supramolecular materials. Chem. Rev. 2016;116:962–1052. doi: 10.1021/acs.chemrev.5b00188. [DOI] [PubMed] [Google Scholar]
- 15.Nowak-Król A, Würthner F. Progress in the synthesis of perylene bisimide dyes. Org. Chem. Front. 2019;6:1272–1318. doi: 10.1039/C8QO01368C. [DOI] [Google Scholar]
- 16.Sun M, Müllen K, Yin M. Water-soluble perylenediimides: design concepts and biological applications. Chem. Soc. Rev. 2016;45:1513–1528. doi: 10.1039/C5CS00754B. [DOI] [PubMed] [Google Scholar]
- 17.Klebe G, Graser F, Hadicke E, Berndt J. Crystallochromy as a solid-state effect: correlation of molecular conformation, crystal packing and colour in perylene-3,4:9,10-bis(dicarboximide) pigments. Acta Crystallogr. Sect. B: Struct. Sci. 1989;45:69–77. doi: 10.1107/S0108768188010407. [DOI] [Google Scholar]
- 18.Kazmaier PM, Hoffmann R. A theoretical study of crystallochromy. Quantum interference effects in the spectra of perylene pigments. J. Am. Chem. Soc. 1994;116:9684–9691. doi: 10.1021/ja00100a038. [DOI] [Google Scholar]
- 19.Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 14, 1564–1579 (2004). [DOI] [PubMed]
- 20.Hecht M, Würthner F. Supramolecularly engineered J‑aggregates based on perylene bisimide dyes. Acc. Chem. Res. 2021;54:642–653. doi: 10.1021/acs.accounts.0c00590. [DOI] [PubMed] [Google Scholar]
- 21.Hestand NJ, Spano FC. Interference between Coulombic and CT-mediated couplings in molecular aggregates: H- to J-aggregate transformation in perylene-based π-stacks. J. Chem. Phys. 2015;143:244707. doi: 10.1063/1.4938012. [DOI] [PubMed] [Google Scholar]
- 22.Hestand NJ, Spano FC. Expanded theory of H- and J‑molecular aggregates: the effects of vibronic coupling and intermolecular charge transfer. Chem. Rev. 2018;118:7069–7163. doi: 10.1021/acs.chemrev.7b00581. [DOI] [PubMed] [Google Scholar]
- 23.Kasha M, Rawls HR, El-Bayoumi MA. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965;11:371–392. doi: 10.1351/pac196511030371. [DOI] [Google Scholar]
- 24.Ramakrishnan R, Niyas MA, Lijina MP, Hariharan M. Distinct crystalline aromatic structural motifs: Identification, classification, and implications. Acc. Chem. Res. 2019;52:3075–3086. doi: 10.1021/acs.accounts.9b00320. [DOI] [PubMed] [Google Scholar]
- 25.Gierschner J, et al. Luminescence in crystalline organic materials: from molecules to molecular solids. Adv. Opt. Mater. 2021;9:2002251. doi: 10.1002/adom.202002251. [DOI] [Google Scholar]
- 26.Mizuguchi J, Tojo K. Electronic structure of perylene pigments as viewed from the crystal structure and excitonic interactions. J. Phys. Chem. B. 2002;106:767–772. doi: 10.1021/jp012909p. [DOI] [Google Scholar]
- 27.Egelhaaf H-J, Haiber J, Gierschner J, Oelkrug D. Optical constants of highly oriented oligothiophene films and nanoparticles. Opt. Mater. 1999;12:395–401. doi: 10.1016/S0925-3467(99)00045-2. [DOI] [Google Scholar]
- 28.Rachford AA, Goeb S, Castellano FN. Accessing the triplet excited state in perylenediimides. J. Am. Chem. Soc. 2008;130:2766–2767. doi: 10.1021/ja800333y. [DOI] [PubMed] [Google Scholar]
- 29.Hong Y, et al. Steering the multiexciton generation in slip-stacked perylene dye array via exciton coupling. Nat. Commun. 2022;13:4488. doi: 10.1038/s41467-022-31958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eaton SW, et al. Singlet exciton fission in polycrystalline thin films of a slip-stacked perylenediimide. J. Am. Chem. Soc. 2013;135:14701–14712. doi: 10.1021/ja4053174. [DOI] [PubMed] [Google Scholar]
- 31.Sasikumar D, John AT, Sunny J, Hariharan M. Access to the triplet excited states of organic chromophores. Chem. Soc. Rev. 2020;49:6122–6140. doi: 10.1039/D0CS00484G. [DOI] [PubMed] [Google Scholar]
- 32.Kevin MF, Grozema FC. Singlet fission in crystalline organic materials: recent insights and future directions. J. Phys. Chem. Lett. 2019;10:7208–7214. doi: 10.1021/acs.jpclett.9b00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Y, et al. Ultrafast photoinduced symmetry-breaking charge separation and electron sharing in perylene diimide molecular triangles. J. Am. Chem. Soc. 2015;137:13236–13239. doi: 10.1021/jacs.5b08386. [DOI] [PubMed] [Google Scholar]
- 34.Lin C, et al. Accelerating symmetry-breaking charge separation in a perylenediimide trimer through a vibronically coherent dimer intermediate. Nat. Chem. 2022;14:786–793. doi: 10.1038/s41557-022-00927-y. [DOI] [PubMed] [Google Scholar]
- 35.Sebastian E, Sunny J, Hariharan M. Excimer evolution hampers symmetry-broken charge-separated states. Chem. Sci. 2022;13:10824–10835. doi: 10.1039/D2SC04387D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufmann C, et al. Ultrafast exciton delocalization, localization, and excimer formation dynamics in a highly defined perylene bisimide quadruple π-stack. J. Am. Chem. Soc. 2018;140:4253–4258. doi: 10.1021/jacs.7b11571. [DOI] [PubMed] [Google Scholar]
- 37.Young RM, Wasielewski MR. Mixed electronic states in molecular dimers: connecting singlet fission, excimer formation, and symmetry-breaking charge transfer. Acc. Chem. Res. 2020;53:1957–1968. doi: 10.1021/acs.accounts.0c00397. [DOI] [PubMed] [Google Scholar]
- 38.Langhals H, Krotz O, Polborn K, Mayer P. A novel fluorescent dye with strong, anisotropic solid-state fluorescence, small stokes shift, and high photostability. Angew. Chem. Int. Ed. 2005;44:2427–2428. doi: 10.1002/anie.200461610. [DOI] [PubMed] [Google Scholar]
- 39.Sabatini RP, et al. Molecularly isolated perylene diimides enable both strong exciton-photon coupling and high photoluminescence quantum yield. J. Mater. Chem. C. 2019;7:2954–2960. doi: 10.1039/C9TC00093C. [DOI] [Google Scholar]
- 40.Zhang B, et al. Highly fluorescent molecularly insulated perylene diimides: effect of con- centration on photophysical properties. Chem. Mater. 2017;29:8395–8403. doi: 10.1021/acs.chemmater.7b02968. [DOI] [Google Scholar]
- 41.Schmidt D, et al. Protein-like enwrapped perylene bisimide chromophore as a bright micro- crystalline Emitter Material. Angew. Chem. Int. Ed. 2019;58:13385–13389. doi: 10.1002/anie.201907618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stolte M, et al. 1-Mono- and 1,7-disubstituted perylene bisimide dyes with voluminous groups at bay positions: in search for highly effective solid-state fluorescence materials. Chem. Mater. 2020;32:6222–6236. doi: 10.1021/acs.chemmater.0c02115. [DOI] [Google Scholar]
- 43.Zhou J, et al. Magic-angle stacking and strong intermolecular π-π interaction in a perylene bisimide crystal: an approach for efficient near-infrared (NIR) emission and high electron mobility. J. Phys. Chem. Lett. 2018;9:596–600. doi: 10.1021/acs.jpclett.7b03251. [DOI] [PubMed] [Google Scholar]
- 44.Shi J, et al. Solid-state luminescence enhancement in π‑conjugated materials: unraveling the mechanism beyond the framework of AIE/AIEE. J. Phys. Chem. C. 2017;121:23166–23183. doi: 10.1021/acs.jpcc.7b08060. [DOI] [Google Scholar]
- 45.Bialas D, et al. Perspectives in dye chemistry: a rational approach toward functional materials by understanding the aggregate state. J. Am. Chem. Soc. 2021;143:4500–4518. doi: 10.1021/jacs.0c13245. [DOI] [PubMed] [Google Scholar]
- 46.Zampetti A, Minotto A, Cacialli F. Near-infrared (NIR) organic light-emitting diodes (OLEDs): challenges and opportunities. Adv. Funct. Mater. 2019;29:1807623. doi: 10.1002/adfm.201807623. [DOI] [Google Scholar]
- 47.Kim M, Whang DR, Gierschner J, Park SY. A distyrylbenzene based highly efficient deep-red/near-infrared emitting organic solid. J. Mater. Chem. C. 2015;3:231–234. doi: 10.1039/C4TC01763C. [DOI] [Google Scholar]
- 48.Jaschke H, Bastani-Oskoui H, Speckbacher M, Langhals H. Perylene dyes with high resistance to alkali. Eur. J. Org. Chem. 2005;20:4313–4321. [Google Scholar]
- 49.Gierschner J, Mack H-GL, Lüer L, Oelkrug D. Fluorescence and absorption spectra of oligophenylenevinylenes: vibronic coupling, band shapes, and solvatochromism. Chem. Phys. 2002;116:8596–8609. [Google Scholar]
- 50.Yagai S, et al. Transformation from H- to J-aggregated perylene bisimide dyes by complexation with cyanurates. Angew. Chem. Int. Ed. 2008;47:3367–3371. doi: 10.1002/anie.200705385. [DOI] [PubMed] [Google Scholar]
- 51.Gisslén L, Scholz R. Crystallochromy of perylene pigments: Interference between Frenkel excitons and charge-transfer states. Phys. Rev. B. 2009;80:115309. doi: 10.1103/PhysRevB.80.115309. [DOI] [Google Scholar]
- 52.Gilbert M, Albinsson B. Photoinduced charge and energy transfer in molecular wires. Chem. Soc. Rev. 2015;44:845–862. doi: 10.1039/C4CS00221K. [DOI] [PubMed] [Google Scholar]
- 53.Wang YJ, et al. The fluorescence properties and aggregation behavior of tetraphenylethene–perylenebisimide dyads. J. Mater. Chem. C. 2015;3:3559–3568. doi: 10.1039/C4TC02550D. [DOI] [Google Scholar]
- 54.Xu B, et al. Photoinduced electron transfer in asymmetrical perylene diimide: understanding the photophysical processes of light-absorbing nonfullerene acceptors. J. Phys. Chem. C. 2017;121:5498–5502. doi: 10.1021/acs.jpcc.7b00263. [DOI] [Google Scholar]
- 55.Korobov, V. I. & Ochkov, V. F. Inverse chemical kinetics problem. In: chemical kinetics with mathcad and maple. (Springer Vienna, 2011).
- 56.Lippert E. Dipolmoment und Elektronenstruktur von angeregten Molekülen. Z. Naturforsch. A. 1955;10a:541–545. doi: 10.1515/zna-1955-0707. [DOI] [Google Scholar]
- 57.Noboru M, Yozo K, Masao K. Solvent Effects upon Fluorescence Spectra and the Dipolemoments of Excited Molecules. Bull. Chem. Soc. Jpn. 1956;29:465–470. doi: 10.1246/bcsj.29.465. [DOI] [Google Scholar]
- 58.Dhali R, et al. Thermally activated delayed fluorescence: a critical assessment of environmental effects on the singlet-triplet energy gap. J. Chem. Phys. 2021;154:134112. doi: 10.1063/5.0042058. [DOI] [PubMed] [Google Scholar]
- 59.Makitra RG, Reichardt C. Solvents and solvent effects in organic chemistry, Weinheim: Wiley-VCH, 2003, 630. Russ. J. Gen. Chem. 2005;75:664. doi: 10.1007/s11176-005-0294-y. [DOI] [Google Scholar]
- 60.Egelhaaf H-J, Gierschner J, Oelkrug D. Polarizability effects and energy transfer in quinquethiophene doped bithiophene and OPV films. Synth. Met. 2002;127:221–227. doi: 10.1016/S0379-6779(01)00627-0. [DOI] [Google Scholar]
- 61.Gierschner J, et al. Highly emissive H-aggregates or aggregation-induced emission quenching? The photophysics of all-trans para-distyrylbenzene. J. Phys. Chem. Lett. 2013;4:2686–2697. doi: 10.1021/jz400985t. [DOI] [Google Scholar]
- 62.Gaussian 16, Revision A.03. Frisch, M. J. et al. Gaussian, Inc., Wallingford CT, https://gaussian.com/citation/ (2016).
- 63.Dreuw A, Head-Gordon M. Single-reference ab initio methods for the calculation of excited states of large molecules. Chem. Rev. 2005;105:4009–4037. doi: 10.1021/cr0505627. [DOI] [PubMed] [Google Scholar]
- 64.Olivier Y, et al. Computational design of thermally activated delayed fluorescence materials: the challenges ahead. J. Phys. Chem. Lett. 2018;9:6149–6163. doi: 10.1021/acs.jpclett.8b02327. [DOI] [PubMed] [Google Scholar]
- 65.Milian-Medina B, Gierschner J. Computational design of low singlet-triplet gap all-organic molecules for OLED application. Org. Electron. 2012;13:985–991. doi: 10.1016/j.orgel.2012.02.010. [DOI] [Google Scholar]
- 66.Neese F. Software update: the ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018;8:e1327. doi: 10.1002/wcms.1327. [DOI] [Google Scholar]
- 67.Neese F. The ORCA program system. WIREs Comput. Mol. Sci. 2012;2:e1327. doi: 10.1002/wcms.81. [DOI] [Google Scholar]
- 68.Neese F. Software update: the ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022;12:e1606. doi: 10.1002/wcms.1606. [DOI] [Google Scholar]
- 69.Lu T, Chen F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 2012;33:580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- 70.Liu Z, Lu T, Chen Q. Intermolecular interaction characteristics of the all-carboatomic ring, cyclo[18]carbon: Focusing on molecular absorption and attacking. Carbon. 2021;171:514–523. doi: 10.1016/j.carbon.2020.09.048. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in this study are provided in the paper and Supplementary Information, and the raw data supporting this study are available from the Source Data file. Source data are provided with this paper.
The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2211699, 2211700, 1974107, 2211720, 2211721. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.