Abstract
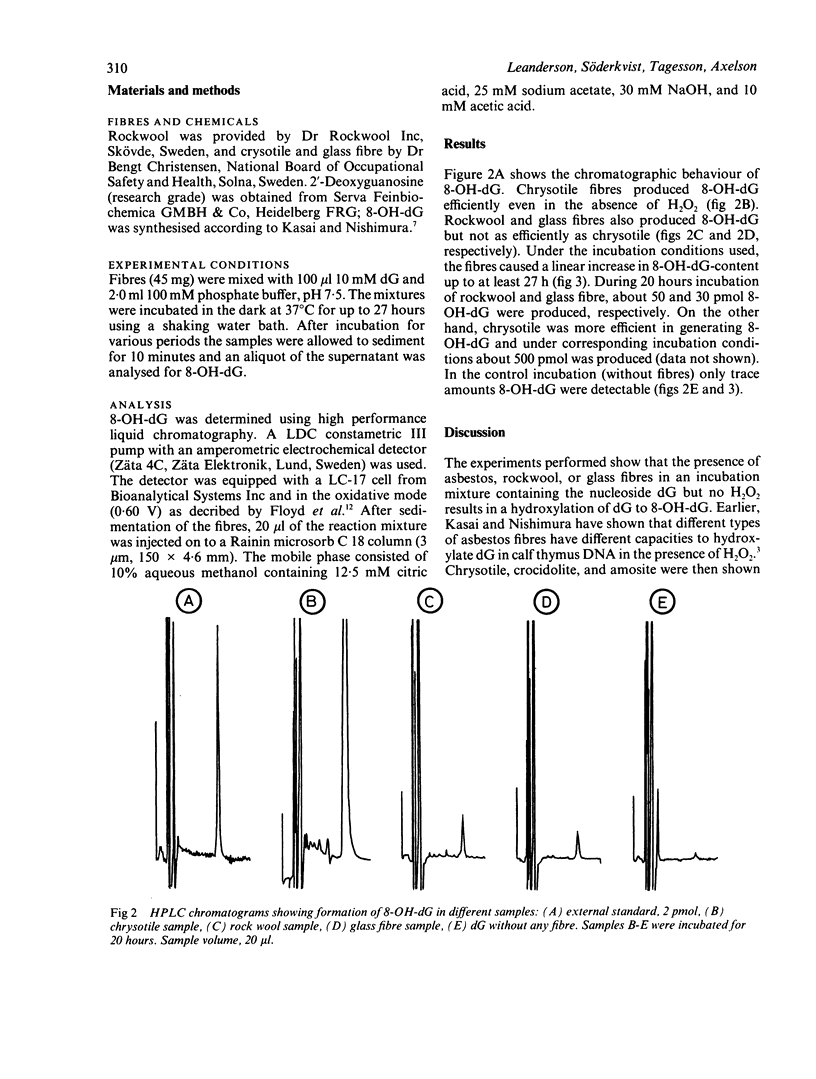
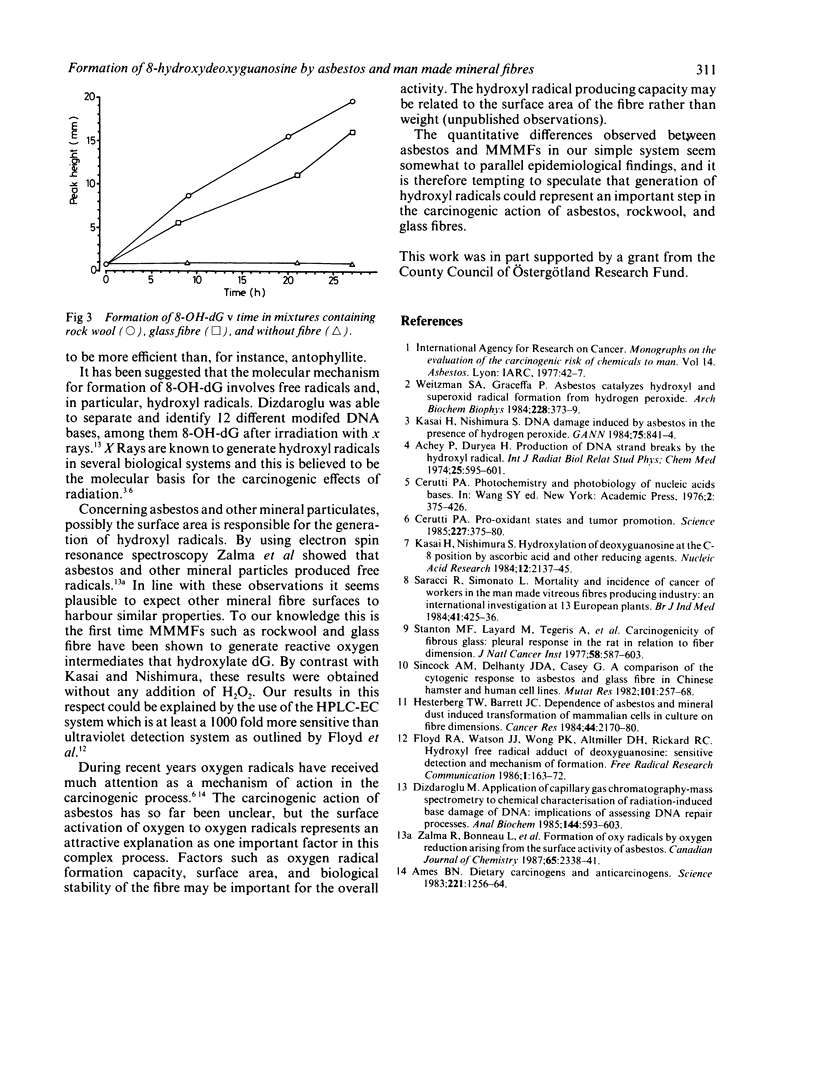
Samples of rockwool and glass fibre were compared with chrysotile fibres for their capacity to hydroxylate 2-deoxyguanosine to 8-hydroxydeoxyguanosine, a reaction that is mediated by formation of hydroxyl radicals. All three fibres produced 8-hydroxydeoxyguanosine in the absence of H2O2. The chrysotile fibres were most potent and produced about ten times more of the modified nucleoside than rockwool and glass fibre. This investigation shows that not only asbestos but also man made mineral fibres are able to modify nucleosides.
Full text
PDF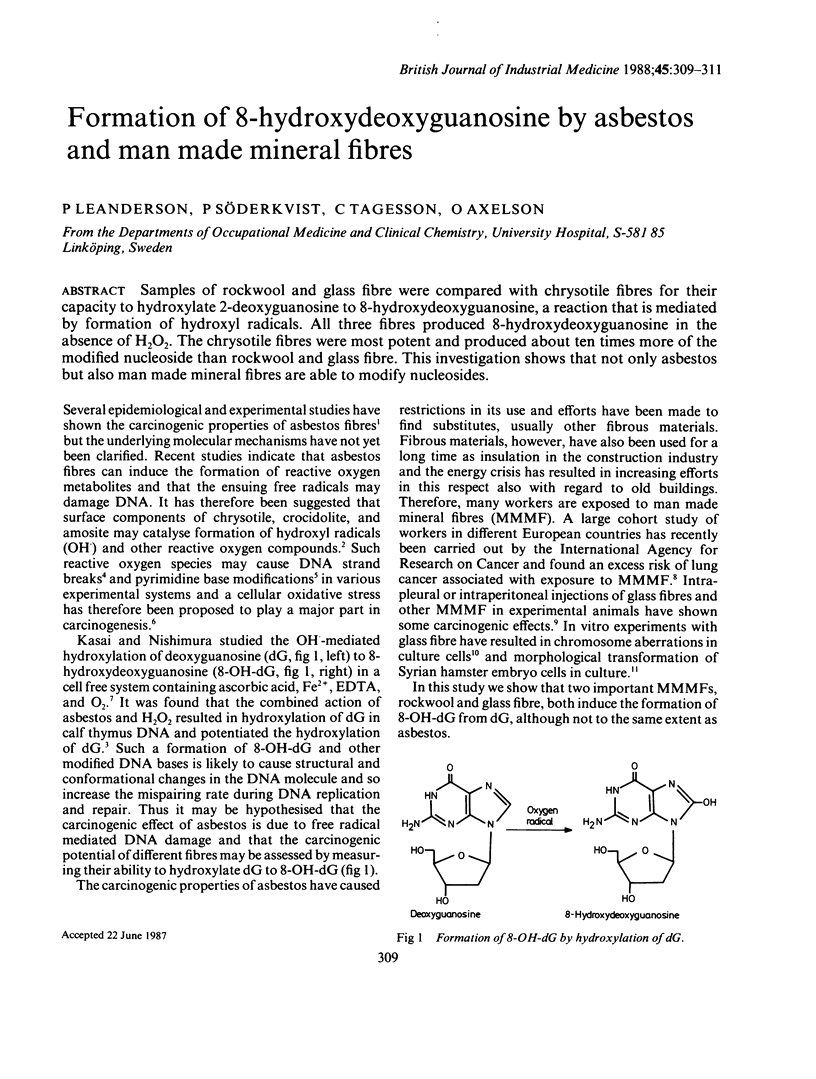
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achey P., Duryea H. Production of DNA strand breaks by the hydroxyl radical. Int J Radiat Biol Relat Stud Phys Chem Med. 1974 Jun;25(6):595–601. doi: 10.1080/09553007414550791. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M. Application of capillary gas chromatography-mass spectrometry to chemical characterization of radiation-induced base damage of DNA: implications for assessing DNA repair processes. Anal Biochem. 1985 Feb 1;144(2):593–603. doi: 10.1016/0003-2697(85)90158-7. [DOI] [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Wong P. K., Altmiller D. H., Rickard R. C. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radic Res Commun. 1986;1(3):163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- Hesterberg T. W., Barrett J. C. Dependence of asbestos- and mineral dust-induced transformation of mammalian cells in culture on fiber dimension. Cancer Res. 1984 May;44(5):2170–2180. [PubMed] [Google Scholar]
- Kasai H., Nishimura S. DNA damage induced by asbestos in the presence of hydrogen peroxide. Gan. 1984 Oct;75(10):841–844. [PubMed] [Google Scholar]
- Kasai H., Nishimura S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984 Feb 24;12(4):2137–2145. doi: 10.1093/nar/12.4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sincock A. M., Delhanty J. D., Casey G. A comparison of the cytogenetic response to asbestos and glass fibre in Chinese hamster and human cell lines. Demonstration of growth inhibition in primary human fibroblasts. Mutat Res. 1982 May;101(3):257–268. doi: 10.1016/0165-1218(82)90157-4. [DOI] [PubMed] [Google Scholar]
- Stanton M. F., Laynard M., Tegeris A., Miller E., May M., Kent E. Carcinogenicity of fibrous glass: pleural response in the rat in relation to fiber dimension. J Natl Cancer Inst. 1977 Mar;58(3):587–603. doi: 10.1093/jnci/58.3.587. [DOI] [PubMed] [Google Scholar]
- Weitzman S. A., Graceffa P. Asbestos catalyzes hydroxyl and superoxide radical generation from hydrogen peroxide. Arch Biochem Biophys. 1984 Jan;228(1):373–376. doi: 10.1016/0003-9861(84)90078-x. [DOI] [PubMed] [Google Scholar]


