Abstract
Context
Prader-Willi syndrome (PWS) is a complex rare genetic syndrome. Mortality in patients with PWS is 3% per year. In nearly half of the patients, the cause of death is of cardiopulmonary origin. Prevention, diagnosis and treatment of cardiovascular (CV) disease in PWS adults is complicated by the behavioral phenotype, reduced ability to express physical complaints, high pain threshold and obesity.
Objective
To describe the challenges in prevention, diagnosis and treatment of CV disease in PWS adults, in order to increase awareness and improve medical care.
Methods
Retrospective study of medical records of adults visiting the Dutch PWS reference center.
Results
We describe the challenges encountered during diagnosis and treatment of four PWS adults with heart failure. All had pre-existent peripheral edema. CV risk factors in these patients were obesity (n=4), type 2 diabetes mellitus (n=2), hypertension (n=2), hypogonadism (n=3) and sleep apnea (n=2). Remarkably, all patients were younger than 40 years during their first cardiac decompensation. All patients presented with progressive shortness of breath and/or orthopnea and progressive pitting edema. In 117 controls with PWS without CV problems, 31% had leg edema.
Conclusion
Diagnosing CV problems in PWS adults is challenging. Peripheral edema is common in PWS adults without CV morbidity, which makes edema in general a poor marker for heart failure. However, when edema is of the pitting kind and progressive, this is a strong predictor of cardiac decompensation. We provide practical recommendations for diagnosing and treating CV problems in this vulnerable patient population.
Keywords: Prader-Willi syndrome, cardiovascular system, comorbidity, heart failure, cardiovascular abnormalities
Introduction
Prader-Willi syndrome (PWS) is a complex genetic disorder caused by the loss of function of a cluster of paternally expressed genes on chromosome 15q11.2-q13. It occurs in 1:15.000 – 25.000 live births (1). PWS can result from a paternal deletion (65-75%), a maternal uniparental disomy 15 (mUPD, 20-30%), an imprinting center defect (ICD, 1-3%) or a paternal chromosomal translocation (0.1%) (2, 3). During infancy, patients with PWS often have muscular hypotonia, low muscle mass, feeding difficulties, failure to thrive and delayed development. During childhood, most patients develop an insatiable appetite, often leading to obesity. Patients with PWS have an abnormal body compositon with a high fat mass and low muscle mass (4, 5). Additionally, patients with PWS have hypothalamic dysfunction resulting in pituitary hormone deficiencies, abnormal temperature regulation and inadequate pain registration (6–9).
Mortality in patients with PWS is as high as 3% per year (10, 11). In nearly half of the patients, the cause of death is of cardiopulmonary origin and three-quarters of deaths are unexpected (10, 12). Cardiovascular (CV) abnormalities can occur already early in life (13) and patients with PWS have an increased risk to develop CV disease at a young age (10, 14–16). As previously described (17), this increased CV risk is caused by a complex interplay between somatic and behavioral aspects of the syndrome. Musculoskeletal problems associated with the syndrome (i.e. scoliosis and foot problems), hypotonia and pituitary hormone deficiencies can lead to poor exercise tolerance, which can be further aggravated by behavioral challenges. This exercise intolerance combined with hyperphagia, can lead to an increase in body fat and decrease in lean body mass. This leads to a low basal rest metabolism, which further deteriorates body composition. Increased body fat eventually leads to an increased prevalence of CV risk factors like type 2 diabetes mellitus (DM2), hypertension, hypercholesterolemia and sleep apnea (10, 18–24).
Besides abnormal body composition, appetite regulating hormones like ghrelin and leptin can also affect the CV system. PWS is associated with high ghrelin levels and an elevated acylated ghrelin/unacylated ghrelin (AG/UAG) ratio (25). While high levels of both AG and UAG seem to have protective CV effects (26–28), high ghrelin levels could cause weight gain and glucose intolerance (29, 30). Another key player in appetite regulation, leptin, has been associated with the presence, severity, extent and lesion complexity of coronary atherosclerosis (31). Leptin levels in non-obese PWS males are nearly five times higher than in non-obese control males (32). However, the interplay between leptin levels, obesity and CV pathology in PWS has not been investigated.
Apart from the complex etiology, the diagnostic trajectory of CV disease in adults with PWS is also complex. Physicians usually rely on common symptoms of heart disease, that are reliable indicators of cardiac problems in the general population, such as chest pain, orthopnoea, shortness of breath, decreased exercise tolerance, palpitations, fatigue and peripheral edema (33, 34). However, in PWS, some of these symptoms are less reliable. Chest pain can be easily missed due to the high pain threshold in PWS and many PWS adults lack the verbal skills to express complex physical complaints like orthopnoea and palpitations. Physical signs like decreased exercise tolerance and peripheral edema are already common in adults with PWS without CV disease (17, 22, 23) and are therefore unreliable parameters. This combination of factors can easily lead to diagnostic delay (3, 6, 7). The diagnostic process can be further hindered by obesity, which complicates the interpretation of transthoracic cardiac ultrasound (35) and can lead to false-normal NT-proBNP values (36).
In the current case series, we describe the challenges that occurred during the diagnostic trajectory and treatment of four adults with PWS and CV problems. Moreover, we compare clinical characteristics between patients with and without CV events. Based on this comparison and literature data, we provide clinical recommendations for the diagnosis and treatment of CV disease in adults with PWS.
Methods
This study was approved by the Medical Ethics Committee of the Erasmus University Medical Center, Rotterdam, the Netherlands. In this retrospective study, we reviewed medical files of adults with PWS who underwent our routine systematic health screening at the multidisciplinary outpatient clinic of the Center for Adults with rare genetic syndromes at the Erasmus University Medical Center, Rotterdam, the Netherlands between 2015 and 2022. This systematic screening consists of a structured interview, a complete physical examination, a medical questionnaire, a review of the medical file and biochemical measurements, as described previously (17). Biochemical measurements that were routinely measured were: low density lipoprotein (LDL)-cholesterol, nonfasting glucose, hemoglobin A1c, thyroid-stimulating hormone, free thyroxine, luteinizing hormone, follicle-stimulating hormone, estradiol or testosterone, sex hormone binding globulin, aspartate transaminase, alanine transaminase, alkaline phosphatase, gamma glutamyl transpeptidase, total bilirubin, lactate dehydrogenase, urea, creatinine, estimated glomerular filtration rate, hemoglobin, hematocrit, mean corpuscular volume, leukocytes, thrombocytes and 25-hydroxyvitamin D.
We systematically assessed symptoms of CV disease like orthopnea, dyspnea, nocturia, swollen legs and chest pain. During physical examination the presence of edema was objectified and cardiac auscultation was performed. Additional cardiac diagnostics, like NT-proBNP, chest X-ray, cardiac ultrasound or electrocardiogram (ECG) were not performed routinely, but only if CV problems were suspected.
We describe four adults with PWS who had suffered a CV event in their medical history or who developed CV problems while under treatment at the outpatient clinic of our center. We compare these patients to 117 control patients with PWS without known CV events.
Data analysis
Descriptive statistics for continuous variables are reported as median [interquartile range (IQR)]. For dichotomous variables we display the number and the percentage of people, n (%).
Results
We describe four patients with CV events, all with manifestations of heart failure, of whom detailed clinical information was available.
Patient 1
A 39-year-old male was hospitalized with severe dyspnea and weight gain. Physical examination revealed orthopnea, bronchospasm, severe pitting edema up to his chest and generalized rhonchi. He had severe tachypnea with up to 40 breaths per minute. NT-proBNP was normal (20 pmol/L). Based on the acute clinical presentation, pulmonary congestion was suspected. The ECG showed no abnormalities. Chest X-ray was normal (i.e. no signs of pulmonary congestion), besides a slightly blurred left heart margin. Intravenous diuretics were started to reduce edema and beta-agonist inhalers were started to reduce bronchospasm. Within 48 hours, he had lost three kilograms and his respiration frequency had returned to normal (16 breaths per minute). Afterwards, cardiac ultrasound showed normal systolic left ventricular function and no signs of significant diastolic dysfunction. Right ventricular pressure was increased. Although computed tomography angiography (CTA) of the coronary arteries did not reveal any significant obstructions, the calcium score was 146 (> 90th percentile), which indicated the presence of coronary sclerosis. He was prescribed atorvastatine and reduced his cigarette use from 75 to 21 cigarettes per week.
CV risk factors included morbid obesity (body mass index (BMI) 45 kg/m2), heavy smoking, hypertension and dyslipidemia. Another risk factor was hypogonadism (37), which was untreated as testosterone therapy had caused behavioral challenges. Detailed analysis of additional CV risk factors revealed frequent hypoglycemias (38). The patient used insulin, which he administered himself. It turned out that he injected himself with too much insulin, in order to induce hypoglycemia and receive extra food. Finally, sleep apnea (apnea-hypopnea index (AHI) of 15) was also present. To reduce pulmonary resistance and optimize cardiac function, continuous positive airway pressure (CPAP) was started.
Patient 2
A 37-year-old female was hospitalized for analysis and treatment of generalized edema, with clinical suspicion of heart failure. Nephrogenic causes of edema, like nephrotic syndrome, had been excluded. CV risk factors included a family history with CV disease, DM2, morbid obesity and hyperlipidemia. ECG showed negative T waves in V1-3, indicating right ventricular hypertrophy. Cardiac ultrasound showed tricuspid valve insufficiency with signs of elevated right ventricular pressure and a dilated vena cava inferior, but transthoracic ultrasound quality was poor due to obesity. It was hypothesized that she had pulmonary hypertension caused by morbid obesity (BMI 44 kg/m2) and severe sleep apnea (AHI of 59). CPAP was initiated, which led to recompensation. Three months later, she was hospitalized again. An upper respiratory tract infection had caused an increase of her pre-existent pulmonary hypertension, which caused decompensated right heart failure. After a short stay in the hospital, she could be dismissed, but returned half a year later. Then, she admitted that she had discontinued CPAP. Insufficient surveillance in her residential facility had led to nonadherence to CPAP. She had also gained a lot of weight, due to insufficient external food control. She had access to her own debit card, which she used to buy food.
Patient 3
A 29-year-old female with hypogonadism and central hypothyroidism was hospitalized because of orthopnea, progressive exercise-related shortness of breath, hyponatremia (134 mmol/L) and progressive pitting edema. NT-proBNP was increased (99 pmol/L). Chest X-ray showed an enlarged heart. During her stay at the hospital, she developed severe epileptic seizures which eventually required intubation and transfer to the intensive care unit (ICU). Magnetic resonance imaging (MRI) and electroencephalography (EEG) did not show any abnormalities. A year earlier, she had undergone cardiac evaluation because of peripheral edema. At that time, ECG did not show any signs of ischemia and cardiac ultrasound came back normal. However, retrospectively, interpretation at that time was probably already hindered by her morbid obesity. A new cardiac ultrasound, performed at the ICU, showed a normal systolic left ventricle function (LVEF 55%), but a dilated right ventricle and tricuspid valve insufficiency. This was probably the result of right heart failure due to pulmonary hypertension, resulting from restrictive lung function caused by scoliosis and morbid obesity (BMI 48 kg/m2). The patient was treated with intravenous diuretics and fluid and salt restriction, after which she recompensated. After 3 weeks she was sent home with oxygen therapy.
Patient 4
A 32-year-old male presented for the first time at our outpatient clinic. He had progressive exercise-related shortness of breath, extreme peripheral edema with blisters and weight gain of 20 kilograms in one month. He had morbid obesity (BMI 53 kg/m2). At physical examination, his oxygen saturation was 80%. Initially, he refused physical examination and venipuncture. ECG showed left axis cardiac deviation and a right bundle branch block, but no signs of acute ischemia. Chest X-ray showed an enlarged heart and hilar enlargement. He was admitted to the hospital for treatment of his congestive heart failure, but refused medication, oxygen and other medical interventions. As he refused medical treatment without being able to understand the consequences, he was considered to be a danger to himself. The medical staff tried to arrange the legal documents needed for involuntary commitment. However, his aggressive behavior made it impossible to keep him on the ward and the patient left the hospital. As the stress of forced hospitalization and forced treatment was expected to further aggravate his cardiac problems, it was decided to try to treat him in the residential home where he lived. He was treated with oral diuretics (bumetanide) and a salt and water restriction. Eventually, he took the prescribed medication for two days and agreed to undergo venipuncture, which showed an increased NT-proBNP (230 pmol/L). The physician for intellectual disabilities (ID physician) reported that, after an initial weight loss of 3 kilograms, he had gradually increased in weight again. The patient adhered less and less to his salt and water restriction. His hyperphagia, aggressive behavior and the complex psychosocial situation made it impossible for his physician and caregivers to improve adherence to treatment. Eventually, he ate a large amount of salty food and spent two nights drinking water in his shower. He was readmitted to the hospital due to shortness of breath, where he died shortly after arrival. Autopsy confirmed that his death was the result of congestive heart failure. There was severe peripheral edema, the heart was enlarged and the right ventricle was dilated. Apart from congestion of the abdominal organs and the brain, autopsy revealed ascites and pericardial effusion which supported the clinical diagnosis fluid retention due to congestive heart failure.
Comparison to controls
In Tables 1 , 2 , we compare the four cases with CV events to a control population of 117 adults with PWS without CV events. Compared to the cases, controls had a lower BMI and more often had used growth hormone treatment during childhood. CV risk factors were also frequent in control patients. Peripheral edema was present in 31% of the controls.
Table 1.
Patient characteristics, cardiovascular risk factors and childhood GH status of four patients with PWS with cardiovascular events, compared to 117 PWS adults without cardiovascular events.
| Age (years) a | Genotype | BMI (kg/m2) | Type 2 diabetes mellitus | Hyper-tension | Hyperchol-esterolemia | Sleep apnea | GH during childhood | |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 39 | Deletion | 45 | Yes | Yes | Yes | Yes | No |
| Patient 2 | 37 | Deletion | 44 | Yes | No | Yes | Yes | No |
| Patient 3 | 29 | Deletion | 48 | No | No | No | No | No |
| Patient 4 | 32 | Unknown | 53 | NA | Yes | No | NA | No |
| Control (n=117) | 28 [20 – 38] | Deletion: 61 (52%) mUPD: 42 (36%) ICD: 3 (3%) Unknown: 11 (9%) |
29 [26 – 35] | 13 (11%) | 17 (15%) | 20 (17%) | 17 (15%) | 58 (50%) |
Data presented as yes (present), no (absent) or NA (not available) for individual patients and as n (%) for control PWS adults. For controls the age and BMI are given as median [IQR].
Abbreviations: body mass index (BMI), growth hormone (GH), imprinting center defect (ICD), maternal parental disomy (mUPD). a Age at first event for cases and age at data collection for controls.
Table 2.
Physical complaints and signs of cardiac decompensation of four patients with PWS with cardiovascular events, compared to 117 PWS adults without cardiovascular events.
| Chest pain | Peripheral edema | Orthopnea | Progression of edema | Increased NT-proBNP | Difficulty interpreting cardiac ultrasound | Signs of pulmonary hypertension a | |
|---|---|---|---|---|---|---|---|
| Patient 1 | No | Yes | Yes | Yes | No | Yes | No |
| Patient 2 | Yes | Yes | NA | Yes | NA | Yes | Yes |
| Patient 3 | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Patient 4 | No | Yes | Yes | Yes | Yes | NA | Yes |
| Controlb | 2/91 (2%) | 29/95 (31%) | 1/90 (1%) | NA | NA | NA | NA |
Data presented as yes (present), no (absent), NA (not available) for individual patients and as number of controls with the outcome / total number of controls for whom the outcome was known (%) for the group of control patients.
a Signs of pulmonary hypertension include: right axis deviation on electrocardiogram, signs of increased systolic right ventricle pressure on echocardiogram (in absence of pulmonary valve stenosis): increased tricuspid regurgitation velocity, enlarged vena cava and/or right ventricle dilatation or hypertrophy. b Number of controls differs as a result of missing values.
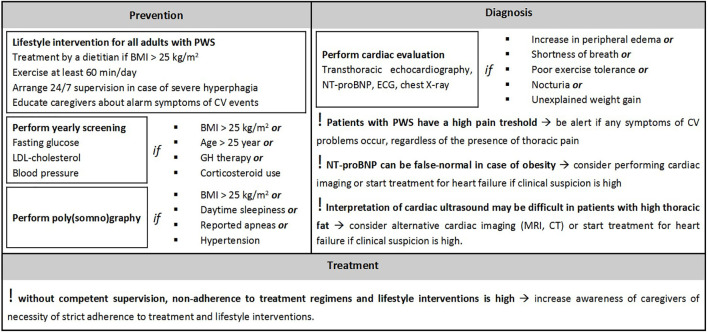
Based on the described cases, the literature and our clinical expertise, we formulated recommendations for prevention, diagnosis, and treatment of CV events in adults with PWS, see Figure 1 .
Figure 1.
Recommendations for prevention, diagnosis and treatment of cardiovascular events in adults with Prader-Willi syndrome. Abbreviations: 24 hours a day, 7 days a week (24/7), body mass index (BMI), computed tomography (CT), cardiovascular (CV), electrocardiogram (ECG), growth hormone (GH), low-density lipoprotein (LDL), minutes (min), magnetic resonance imaging (MRI), Prader-Willi syndrome (PWS). These recommendations only consider factors related to cardiovascular disease. For the full screening protocol for prevention, see Pellikaan et al. (17).
Discussion
We describe the syndrome-specific challenges encountered during the diagnosis and treatment of severe CV problems, i.e. heart failure, of four adults with PWS. Diagnosis was complicated by obesity (BMI between 44 and 53 kg/m2) and pre-existent peripheral edema.
Pulmonary hypertension played a key role in the pathogenesis of CV disease in all patients described. Dilated right ventricle and dilated vena cava inferior, both signs of increased right heart pressure, were present in most patients. Left ventricle function was usually normal.
Factors contributing to CV disease were obesity (n=4), DM2 (n=2), hypertension (n=2), hypogonadism (n=3) and sleep apnea (n=2) (37). Remarkably, all patients had their first cardiac decompensation before the age of 40, the youngest patient being 29 years old. This is in contrast with the low prevalence of heart failure of 0.1-0.5% found in non-PWS adults in this age category (with and without overweight) (39).
CV disease in adults with PWS is caused by a complex interplay of several syndrome-specific characteristics, which eventually leads to obesity (17). Obesity is associated with systemic low-grade inflammation and oxidative stress, hypertension, hypercholesterolemia, insulin resistance and DM2, all associated with increased CV risk (40–43). Furthermore, obesity can lead to obesity-associated hypoventilation syndrome (OAHS) (44), with subsequent pulmonary hypertension and right ventricular failure, if left untreated. In addition to the obesity-related increase in CV risk, patients with PWS have an additional risk due to decreased microvascular function that is associated with the syndrome (45), as endothelium and microvessels may play an important role in the pathogenesis of heart failure (46, 47). Lastly, scoliosis is often present in patients with PWS (23). If severe, scoliosis can cause restrictive pulmonary dysfunction, pulmonary hypertension and eventually CV decompensation (48, 49).
Due to the cumulative effect of the above-mentioned mechanisms, the patients we described developed cardiac decompensation at an exceptionally young age. To prevent the development of CV disease, it is important to identify and treat CV risk factors early in life. Prevention and treatment of obesity may be complicated due to intellectual disability and hyperphagia. Therefore a multidisciplinary approach is needed. A (pediatric) endocrinologist, specialized dietitian, physiotherapist and, if needed, a behavioral expert or psychologist should work together to avoid or treat obesity. Additionally, it is essential to have adequate supervision at home to control food intake. Specialized PWS homes can be beneficial to ensure this supervision. If PWS homes are unavailable, caregivers should receive clear instructions about restricting the patient’s food intake and increasing physicial activity. Additionally, the endocrinologist should screen yearly for CV risk factors like hypertension, DM2, and hypercholesterolemia. For this screening, our previously described algorithm may be helpful (17). These measures might prevent long-term debilitating CV complications as seen in the four patients we described.
If, despite prevention, heart failure develops, the diagnosis of heart failure can be challenging in patients with PWS, especially when obesity is present. Peripheral non-pitting edema (lipedema and lymphedema) is common in PWS adults without CV morbidity (31%), which makes edema, in general, a poor marker for CV deterioration. However, in all four patients who eventually developed cardiac decompensation, edema was of the pitting kind and progressive. Therefore, an increase in peripheral pitting edema should be considered an alarm symptom and should trigger further investigation.
Apart from progressive pitting edema, all cases showed orthopnea and/or progressive shortness of breath. Although not systematically assessed, none of the patients complained of nocturia. When symptoms of heart failure like dyspnea and/or orthopnea are present, additional testing is needed to rule out cardiac problems. It should be emphasized that, in obese subjects, false negative NT-proBNP values can put physicians on the wrong track. Likewise, cardiac ultrasound can be hard to interpret due to poor imaging quality resulting from obesity (35).
Besides diagnostic challenges, it can also be challenging to initiate and maintain adequate cardiac treatment. In general, diuretics and fluid and salt restriction are essential for treatment of congestive heart failure. However, due to hyperphagia and intellectual disability, nonadherence to fluid and salt restriction is common in adults with PWS. Adherence can only be guaranteed by competent supervision, which means that 24/7 supervision is often crucial in heart failure treatment in PWS. Non-adherence to CPAP may also occur. As patients with OAHS benefit from CPAP, this is a useful intervention. However, the CPAP mask can cause anxiety, especially in case of intellectual disability. In that case, stepwise introduction of the mask (starting with a few minutes per day) and gradual increase in usage is crucial to prevent non-compliance. Also, for the acceptance of CPAP, it may be useful to involve a psychologist or behavioral expert.
Strengths and limitations
One of the strengths of the current study is the detailed description of four cases of PWS adults with CV events. To our knowledge, we are the first to describe cases of PWS adults with CV events and to provide practical recommendations based on the similarities between these cases. A limitation of this study is that this study was retrospective and therefore some details were unknown. Moreover, the diagnostic tests were performed in different hospitals, which may have caused some variation in results of imaging and/or biochemical tests.
Conclusion
In conclusion, the diagnostic trajectory and treatment of CV disease in adults with PWS can be extremely challenging. Peripheral edema, a reliable marker of right-sided heart failure in the general population, is frequently present in the general PWS population and is therefore not a good indicator of heart failure. Diagnosis of heart failure is further hindered by the decreased reliability of NT-proBNP levels and increased technical challenges in transthoracic echocardiography in case of obesity. To prevent doctors’ delay, it is important to inform general practitioners, ID physicians, internists and cardiologists about these diagnostic pitfalls. To prevent patient delay, it is important to inform caregivers about early signs of cardiac failure, like exercise-related shortness of breath, progression of peripheral edema and unexplained weight gain. Diagnosis and treatment can be complicated by PWS-specific behavior, non-compliance to salt and water restriction, fear of CPAP and refusal of medication. Therefore, preventive measures, diagnostics and treatment of CV disease should preferably be guided by a multidisciplinary team.
Data availability statement
The datasets presented in this article are not readily available in order to protect the privacy of the patients participating in this study. As Prader–Willi syndrome is a rare syndrome, individual patient data could be traced back to the individual. Requests to access the datasets should be directed to Laura de Graaff, l.degraaff@erasmusmc.nl.
Ethics statement
The studies involving human participants were reviewed and approved by the medical ethical committee of the Erasmus MC. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Conceptualization, KP and LG. Methodology, KP and LG. Formal analysis, KP. Investigation, KP, PW and LG. Resources, LG. Writing—original draft preparation, KP and PW. Writing—review and editing, all authors. Visualization, KP. Supervision, LG and AL. Project administration, KP and LG. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank the patients with PWS, their families and their caregivers who contributed to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1145066/full#supplementary-material
References
- 1. Whittington JE, Holland AJ, Webb T, Butler J, Clarke D, Boer H. Population prevalence and estimated birth incidence and mortality rate for people with prader-willi syndrome in one UK health region. J Med Genet (2001) 38(11):792–8. doi: 10.1136/jmg.38.11.792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheon CK. Genetics of prader-willi syndrome and prader-Will-Like syndrome. Ann Pediatr Endocrinol Metab (2016) 21(3):126–35. doi: 10.6065/apem.2016.21.3.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cassidy SB, Driscoll DJ. Prader-willi syndrome. Eur J Hum Genet (2009) 17(1):3–13. doi: 10.1038/ejhg.2008.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brambilla P, Bosio L, Manzoni P, Pietrobelli A, Beccaria L, Chiumello G. Peculiar body composition in patients with prader-Labhart-Willi syndrome. Am J Clin Nutr (1997) 65(5):1369–74. doi: 10.1093/ajcn/65.5.1369 [DOI] [PubMed] [Google Scholar]
- 5. Bekx MT, Carrel AL, Shriver TC, Li Z, Allen DB. Decreased energy expenditure is caused by abnormal body composition in infants with prader-willi syndrome. J Pediatr (2003) 143(3):372–6. doi: 10.1067/S0022-3476(03)00386-X [DOI] [PubMed] [Google Scholar]
- 6. Angulo MA, Butler MG, Cataletto ME. Prader-willi syndrome: A review of clinical, genetic, and endocrine findings. J Endocrinol Invest (2015) 38(12):1249–63. doi: 10.1007/s40618-015-0312-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M. Speakers contributors at the second expert meeting of the comprehensive care of patients with PWS. recommendations for the diagnosis and management of prader-willi syndrome. J Clin Endocrinol Metab (2008) 93(11):4183–97. [DOI] [PubMed] [Google Scholar]
- 8. Cassidy SB. Prader-willi syndrome. J Med Genet (1997) 34(11):917–23. doi: 10.1136/jmg.34.11.917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY, et al. Prader-willi syndrome: Consensus diagnostic criteria. Pediatrics (1993) 91(2):398–402. doi: 10.1542/peds.91.2.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Proffitt J, Osann K, McManus B, Kimonis VE, Heinemann J, Butler MG, et al. Contributing factors of mortality in prader-willi syndrome. Am J Med Genet A (2019) 179(2):196–205. doi: 10.1002/ajmg.a.60688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hedgeman E, Ulrichsen SP, Carter S, Kreher NC, Malobisky KP, Braun MM, et al. Long-term health outcomes in patients with prader-willi syndrome: A nationwide cohort study in Denmark. Int J Obes (Lond) (2017) 41(10):1531–8. doi: 10.1038/ijo.2017.139 [DOI] [PubMed] [Google Scholar]
- 12. Butler MG, Manzardo AM, Heinemann J, Loker C, Loker J. Causes of death in prader-willi syndrome: Prader-willi syndrome association (USA) 40-year mortality survey. Genet Med (2017) 19(6):635–42. doi: 10.1038/gim.2016.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kobayashi S, Murakami N, Oto Y, Toide H, Kimura N, Hayashi A, et al. Subtle cardiovascular abnormalities in prader-willi syndrome might begin in young adulthood. Intern Med (2021) 60(21):3377–84. doi: 10.2169/internalmedicine.7073-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brás DR, Semedo P, Picąrra BC, Fernandes R. Prader-willi syndrome: A nest for premature coronary artery disease? BMJ Case Rep (2018) 2018. doi: 10.1136/bcr-2017-222828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamb AS, Johnson WM. Premature coronary artery atherosclerosis in a patient with prader-willi syndrome. Am J Med Genet (1987) 28(4):873–80. doi: 10.1002/ajmg.1320280412 [DOI] [PubMed] [Google Scholar]
- 16. Laurance BM, Brito A, Wilkinson J. Prader-willi syndrome after age 15 years. Arch Dis Child (1981) 56(3):181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pellikaan K, Rosenberg AGW, Kattentidt-Mouravieva AA, Kersseboom R, Bos-Roubos AG, Veen-Roelofs JMC, et al. Missed diagnoses and health problems in adults with prader-willi syndrome - recommendations for screening and treatment. J Clin Endocrinol Metab (2020) e4671-e4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laurier V, Lapeyrade A, Copet P, Demeer G, Silvie M, Bieth E, et al. Medical, psychological and social features in a large cohort of adults with prader-willi syndrome: Experience from a dedicated centre in France. J Intellect Disabil Res (2015) 59(5):411–21. [DOI] [PubMed] [Google Scholar]
- 19. Höybye C, Hilding A, Jacobsson H, Thorén M. Metabolic profile and body composition in adults with prader-willi syndrome and severe obesity. J Clin Endocrinol Metab (2002) 87(8):3590–7. [DOI] [PubMed] [Google Scholar]
- 20. van Nieuwpoort IC, Sinnema M, Castelijns JA, Twisk JW, Curfs LM, Drent ML. The GH/IGF-I axis and pituitary function and size in adults with prader-willi syndrome. Horm Res Paediatr (2011) 75(6):403–11. [DOI] [PubMed] [Google Scholar]
- 21. Coupaye M, Tauber M, Cuisset L, Laurier V, Bieth E, Lacorte JM, et al. Effect of genotype and previous GH treatment on adiposity in adults with prader-willi syndrome. J Clin Endocrinol Metab (2016) 101(12):4895–903. doi: 10.1210/jc.2016-2163 [DOI] [PubMed] [Google Scholar]
- 22. Sinnema M, Maaskant MA, van Schrojenstein Lantman-de Valk HM, van Nieuwpoort IC, Drent ML, Curfs LM, et al. Physical health problems in adults with prader-willi syndrome. Am J Med Genet A (2011) 155A(9):2112–24. doi: 10.1002/ajmg.a.34171 [DOI] [PubMed] [Google Scholar]
- 23. Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-willi syndrome. Genet Med (2012) 14(1):10–26. doi: 10.1038/gim.0b013e31822bead0 [DOI] [PubMed] [Google Scholar]
- 24. van Mil EA, Westerterp KR, Gerver WJ, Curfs LM, Schrander-Stumpel CT, Kester AD, et al. Energy expenditure at rest and during sleep in children with prader-willi syndrome is explained by body composition. Am J Clin Nutr (2000) 71(3):752–6. [DOI] [PubMed] [Google Scholar]
- 25. Tauber M, Coupaye M, Diene G, Molinas C, Valette M, Beauloye V. Prader-willi syndrome: A model for understanding the ghrelin system. J Neuroendocrinol (2019) 31(7):e12728. doi: 10.1111/jne.12728 [DOI] [PubMed] [Google Scholar]
- 26. Khatib MN, Simkhada P, Gode D. Cardioprotective effects of ghrelin in heart failure: from gut to heart. Heart Views (2014) 15(3):74–6. doi: 10.4103/1995-705X.144792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yano Y, Nakazato M, Toshinai K, Inokuchi T, Matsuda S, Hidaka T, et al. Circulating des-acyl ghrelin improves cardiovascular risk prediction in older hypertensive patients. Am J Hypertens (2014) 27(5):727–33. doi: 10.1093/ajh/hpt232 [DOI] [PubMed] [Google Scholar]
- 28. Mao Y, Tokudome T, Kishimoto I. Ghrelin as a treatment for cardiovascular diseases. Hypertension (2014) 64(3):450–4. doi: 10.1161/HYPERTENSIONAHA.114.03726 [DOI] [PubMed] [Google Scholar]
- 29. Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, et al. Ghrelin. Mol Metab (2015) 4(6):437–60. doi: 10.1016/j.molmet.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mani BK, Shankar K, Zigman JM. Ghrelin's relationship to blood glucose. Endocrinology (2019) 160(5):1247–61. doi: 10.1210/en.2019-00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Katsiki N, Mikhailidis DP, Banach M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol Sin (2018) 39(7):1176–88. doi: 10.1038/aps.2018.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Butler MG, Moore J, Morawiecki A, Nicolson M. Comparison of leptin protein levels in prader-willi syndrome and control individuals. Am J Med Genet (1998) 75(1):7–12. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Metra M, Teerlink JR. Heart failure. Lancet (2017) 390(10106):1981–95. doi: 10.1016/S0140-6736(17)31071-1 [DOI] [PubMed] [Google Scholar]
- 34. Lu L, Liu M, Sun R, Zheng Y, Zhang P. Myocardial infarction: Symptoms and treatments. Cell Biochem Biophys (2015) 72(3):865–7. doi: 10.1007/s12013-015-0553-4 [DOI] [PubMed] [Google Scholar]
- 35. Siadecki SD, Frasure SE, Lewiss RE, Saul T. High body mass index is strongly correlated with decreased image quality in focused bedside echocardiography. J Emerg Med (2016) 50(2):295–301. doi: 10.1016/j.jemermed.2015.07.023 [DOI] [PubMed] [Google Scholar]
- 36. Krauser DG, Lloyd-Jones DM, Chae CU, Cameron R, Anwaruddin S, Baggish AL, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: A ProBNP investigation of dyspnea in the emergency department (PRIDE) substudy. Am Heart J (2005) 149(4):744–50. doi: 10.1016/j.ahj.2004.07.010 [DOI] [PubMed] [Google Scholar]
- 37. Potenza M, Shimshi M. Male Hypogonadism: The unrecognized cardiovascular risk factor. J Clin Lipidol (2008) 2(2):71–8. doi: 10.1016/j.jacl.2008.01.011 [DOI] [PubMed] [Google Scholar]
- 38. Chopra S, Kewal A. Does hypoglycemia cause cardiovascular events? Indian J Endocrinol Metab (2012) 16(1):102–4. doi: 10.4103/2230-8210.91203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol (2018) 15(4):230–40. doi: 10.1038/nrcardio.2017.154 [DOI] [PubMed] [Google Scholar]
- 40. Reho JJ, Rahmouni K. Oxidative and inflammatory signals in obesity-associated vascular abnormalities. Clin Sci (Lond) (2017) 131(14):1689–700. doi: 10.1042/CS20170219 [DOI] [PubMed] [Google Scholar]
- 41. Ayinapudi K, Singh T, Motwani A, Le Jemtel TH, Oparil S. Obesity and pulmonary hypertension. Curr Hypertens Rep (2018) 20(12):99. doi: 10.1007/s11906-018-0899-2 [DOI] [PubMed] [Google Scholar]
- 42. Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med (2001) 163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008 [DOI] [PubMed] [Google Scholar]
- 43. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J Am Coll Cardiol (2009) 53(21):1925–32. doi: 10.1016/j.jacc.2008.12.068 [DOI] [PubMed] [Google Scholar]
- 44. Masa JF, Pépin JL, Borel JC, Mokhlesi B, Murphy PB, Sánchez-Quiroga M. Obesity hypoventilation syndrome. Eur Respir Rev (2019) 28(151). doi: 10.1183/16000617.0097-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Patel S, Harmer JA, Loughnan G, Skilton MR, Steinbeck K, Celermajer DS. Characteristics of cardiac and vascular structure and function in prader-willi syndrome. Clin Endocrinol (Oxf) (2007) 66(6):771–7. doi: 10.1111/j.1365-2265.2007.02808.x [DOI] [PubMed] [Google Scholar]
- 46. Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J (2018) 39(37):3439–50. doi: 10.1093/eurheartj/ehy531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. D'Amario D, Migliaro S, Borovac JA, Restivo A, Vergallo R, Galli M, et al. Microvascular dysfunction in heart failure with preserved ejection fraction. Front Physiol (2019) 10:1347. doi: 10.3389/fphys.2019.01347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koumbourlis AC. Scoliosis and the respiratory system. Paediatr Respir Rev (2006) 7(2):152–60. doi: 10.1016/j.prrv.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 49. Lin Y, Shen J, Chen L, Yuan W, Cong H, Luo J, et al. Cardiopulmonary function in patients with congenital scoliosis: An observational study. J Bone Joint Surg Am (2019) 101(12):1109–18. doi: 10.2106/JBJS.18.00935 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this article are not readily available in order to protect the privacy of the patients participating in this study. As Prader–Willi syndrome is a rare syndrome, individual patient data could be traced back to the individual. Requests to access the datasets should be directed to Laura de Graaff, l.degraaff@erasmusmc.nl.