Abstract
Objectives: Urine output is used to evaluate fluid status and is an important marker for acute kidney injury (AKI). Our primary aim was to validate a new automatic urine output monitoring device by comparison to the current practice – the standard urometer. Methods: We conducted a prospective observational study in three ICUs. Urine flow measurements by Serenno Medical Automatic urine output measuring device (Serenno Medical, Yokneam, Israel) were compared to standard urometer readings taken automatically at 5-minutes intervals by a camera, and to hourly urometer readings by the nurses, both over 1 to 7 days. Our primary outcome was the difference between urine flow assessed by the Serenno device and reference camera-derived measurements (Camera). Our secondary outcome was the difference between urine flow assessed by the Serenno device and hourly nursing assessments (Nurse), and detection of oliguria. Results: Thirty-seven patients completed the study, with 1,306 h of recording and a median of 25 measurement hours per patient. Bland and Altman analysis comparing the study device to camera measurements demonstrated good agreement, with a bias of -0.4 ml/h and 95% confidence intervals ranging from − 28 to 27ml/h. Concordance was 92%. The correlation between Camera and hourly nursing assessment of urine output was distinctly worse with a bias of 7.2 ml and limits of agreement extending from − 75 to + 107 ml. Severe oliguria (urine output < 0.3 ml/kg/h) lasting 2 h or more was common and observed in 8 (21%) of patients. Among the severe oliguric events lasting more than 3 consecutive hours, 6 (41%) were not detected or documented by the nursing staff. There were no device-related complications. Conclusion: The Serenno Medical Automatic urine output measuring device required minimal supervision, little ICU nursing staff attention, and is sufficiently accurate and precise. In addition to providing continuous assessments of urine output, it was considerably more accurate than hourly nursing assessments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10877-023-00991-w.
Keywords: Acute kidney Injury, Urine output, Oliguria, Anuria, Continuous monitoring, Intensive care
Introduction
Acute kidney injury (AKI) is common in critically ill patients and associated with prolonged hospitalization, morbidity, mortality, and increased cost of care [1–3]. Most consensus definitions consider both urine output and serum creatinine concentration for diagnosis of AKI [2, 4, 5] because together they better characterize kidney function [6]. Oliguria, defined as urine output < 0.5 ml/kg/h, is an even stronger predictor of AKI than serum creatinine [6].
Most vital characteristics are precisely measured and accurately electronically recorded in intensive care units (ICUs). Urine output differs in being mostly recorded manually and intermittently which can lead to recording errors and delayed diagnosis of AKI. Consistent with this theory, intensive monitoring of urine output is associated with improved patient outcomes [7]. Several systems for continuously monitoring urine output are sensitive and accurate [8].
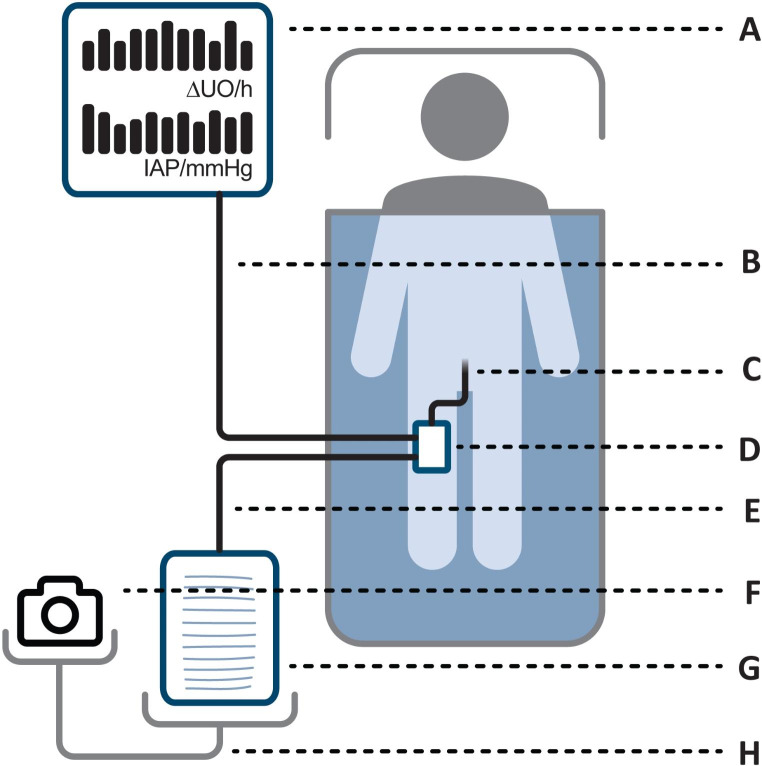
A novel device for continuously monitoring and electronically recording urine output has recently been developed by Serenno Medical (Yokneam, Israel). The study device is intended to continuously measure urine flow and rate over long periods in patients who have a urinary bladder (Foley) catheter. The study device includes a control box (sensing system) located near a patient bed, and a disposable unit connected between the existing catheter and the urine collection bag. The disposable unit is connected to the control box with triple-lumen tubing, and a fourth tube directs urine from the catheter to a collection bag (Fig. 1).
Fig. 1.
System layout. The disposable unit (D) is operated by the controller (A) and connected with an air tube (B) and also connected in-line (E) with the standard urine output monitoring equipment, the catheter (C) and urometer (G). The urometer and camera (F) were stand-mounted (H) for monitoring by both by the nurses and the camera. The urometer tube (E) is positioned in a way that reduces dependent loops
Our goal was to test the Serenno study device in critical care patients with indwelling bladder catheters. Primarily, we compared the study device with urine output recorded by a camera observing the urometer at 5-minute intervals over 1-7-day periods. Secondarily, urine output from the study device and camera values were compared with urine output as assessed by nurses, nominally at 1-hour intervals. And finally, we determined whether the study device detects oliguria more often and earlier than nurses.
Materials and methods
Patients were enrolled at two cardiothoracic and one general critical care units in the Rabin and Sheba Medical Centers. The study protocol was approved by the local ethics committees at each site (0860-18-RMC, 5839-19-SMC) and the study was performed in accordance with Good Clinical Practice and applicable regulatory requirements. The study was observational and experimental results were not available to clinicians. The study therefore did not influence clinical care. Written informed consent was obtained from each patient or an authorized relative before inclusion.
We considered adult critical care patients who already had an indwelling urinary catheter connected to a urometer that was expected to remain in place for at least 12 h. Testing the device in the ICU was chosen to ensure the closest patient supervision and care – better nurse attendance allowing more data from nurses’ measurements, as well as provide a wide range of clinical and operational challenges for the study device. Since the only function of the study device is volume measurement, the clinical history of the patient was not a factor on enrollment and patients who gave consent were included in the study.
As a precaution, we excluded patients who had known urologic pathology including nephrolithiasis, polycystic kidney disease, congenital abnormalities of the kidneys and/or urinary tract, history of obstructive uropathy, and severe chronic renal disease. We also excluded pregnant women and patients with cognitive impairment that precluded informed consent.
The Serenno system (study device)
The Serenno Medical Automatic urine output measuring device consists of an electronic controller operating a pneumatic pressure-sensing disposable unit that moves urine from an input port connected to the bladder catheter to the output port which is connected to the bag/urometer.
The system operates in repeated strokes, with each completed stroke passing exactly 1 ml of fluid (allowing UO calculation). Since the volume of each stroke is known and constant, and the monitoring process is counting and timing the strokes, both the current urine production rate, as well as cumulative urine volume over any period can be calculated.
The system is programmed to only complete the 1-ml stroke when incoming fluid volume from the patient completely fills the 1-ml chamber (observed by the controller as air pressure in the control channel). This will happen under any condition, including different patient orientations, urine properties, sediments, elevation, etc., as long as the fluid volume in the bladder is larger than 1 ml.
If this condition is not met, the system pauses and will not complete the stroke. As more urine is produced and accumulates in the bladder, its volume would eventually be sufficient, and the stroke would resolve within seconds or minutes. The more urine produced - the less the system pauses.
Since the system measures pressures at both input and output ports of the pump, it can detect blocked catheters, full urine collection bags, kinked tubes and connection leaks, the control box can be positioned at eye level (not on the floor). The controller is fail-safe and reverts to a free-flow pass-through mode when dysfunction is detected, even without power.
Measurements
We recorded baseline patient demographic and morphometric details, along with admitting diagnosis and acute physiology and chronic health evaluation (APACHE) 2 scores. Patients were evaluated for up to 7 days so long as they remained in intensive care and required a Foley catheter for clinical reasons.
Study personnel were periodically present to change the controllers’ batteries and adjust tubing to remove kinks and dependent loops that would affect the nurses’ and camera reading (but do not affect the study device). Study personnel also reported to the medical staff when the urometer bag was full. Per clinical routine, nurses were to assess urine output at hourly intervals. We assumed that nurses made assessments when they emptied the urometer and recorded those times.
A camera was positioned to capture images of the standard urometer at 5-minute intervals. Images were later analyzed by 2 independent observers who were blinded to both Serenno device and nursing assessments. When observer assessment differed by more than 5 ml, a third observer adjudicated. Observer assessments of volume in the images never differed more than 10 ml. Adjudicated visually recorded volumes from the urometer bag were considered reference values.
The standard tube connecting the urine bag to the study device has a volume of 50ml when full. Despite investigator efforts, some urine accumulated in the tube when it was not perfectly sloped and then released into the urometer when the nurse emptied it on the next hourly reading, urometer readings may theoretically lag true urine output by up to 50 ml. Since the study device takes a UO reading at the catheter port (before the tube), Serenno reading can be up to 50 ml higher than the urometer (measured by both the camera and nurse) when urine accumulates in the tube. Conversely, Serenno readings can be lower when urine accumulated in the tubing is emptied into the urometer long after being recorded by the Serenno system.
Urine output of less than 0.5 ml/kg/hr for at least 20 min defined an oliguria event, but we also considered urine output < 0.3 ml/kg/hr for at least 20 min as severe oliguria event. Both event types were assessed over sequential 5-minute intervals. We also categorized events by groups as lasting < 1 h, 1-2 h, or 2-4 hs and by those observed and not observed by the nursing staff (according to EMR data). Nursing assessments of oliguria were based on urine flow rates over each nominal hourly assessment.
As a measure of device reliability, we recorded the number and nature of mechanical and electronic malfunctions reported by study staff. We also examined device electronic logs to identify other potential problems and queried nurses who cared for patients enrolled in the study. The urometer and camera were required for study purposes but would not be used during routine care using the study device.
Statistical analysis
Our primary target was a priori defined in the Statistical Analysis Plan as non-Inferiority between the mean absolute delta of Device-Camera readings and the mean absolute delta of Nurse-Camera readings, meaning that the absolute delta of Device-Camera readings should be smaller than the mean absolute delta of Nurse-Camera readings. Specifically, we compared Serenno Medical Automatic urine output assessments (Serenno) with adjudicated camera images-derived urometer volumes (Camera). Urine volumes from the study device were compared with camera assessments at 5-minute intervals.
We initially used Pearson correlation and linear regression analysis to confirm a generally linear relationship. Thereafter, we estimated bias, precision, and limits of agreement with a repeated-measures Bland-Altman analysis. From that analysis, we determined the modified percentage error (PE) which was defined as the standard deviation of the bias divided by mean urine output.
Post-hoc analyses included various calculations to help better understand differences between the device and the nurses’ urine output assessments. For example, we compared reference camera-derived urine output (Camera) to volume assessments obtained by nurses (Nurse) at roughly 1-hour intervals. Our analytic approach was similar to that used for the primary comparison between Serenno measurements and the camera urometer images.
We also compared the number of oliguric (urine output < 0.5 ml/kg/h) and severe oliguric (urine output < 0.3 ml/kg/h) events detected by nurses and by the study device with chi square statistics. Elapsed time between identification of oliguric events by the study device and by nurses were also compared descriptively. We also evaluated urine output reliability by comparing camera-derived reading time and volume vs. nurse-recorded values.
Normally distributed data are presented as means and standard deviations (SD). Non-parametric data are presented as medians and interquartile ranges (IQR). The statistical analysis involved the 2-tailed unpaired Student’s t-test for continuous parameters. Categorical variables were compared with Chi square tests. P-values < 0.05 were considered statistically significant. Statistical analyses were performed with SPSS software 26.
Results
Patients were enrolled between May and October 2019. We a priori planned to enroll at least 30 patients in the study, with up to 90 patients being allowed to better support secondary outcomes. After enrollment of 48 patients, of whom 37 completed measurements, the study ended late 2019 because Covid-19 concerns precluded further research at both study centers. Eleven patients were excluded from the final analysis, mainly because they didn’t reach the minimal monitoring time required or withdrew consent. The Serenno device required minimal nursing supervision and there were no device-related complications.
Thirty-seven patients therefore completed the study, with a total of 1,306 h of recording with a median of 25 measurement hours per patient (range 14–169 h). Patients averaged 58 ± 16 years and 73% were men. The average height was 170 (153–185) cm, and the average weight was 84 (52–135) kg. The body mass index average (± SD) was 29 ± 5 kg/m2. There were 16 trauma patients who had an average APACHE II score of 21 ± 8; the remaining 21 patients were recovering from cardiothoracic surgery.
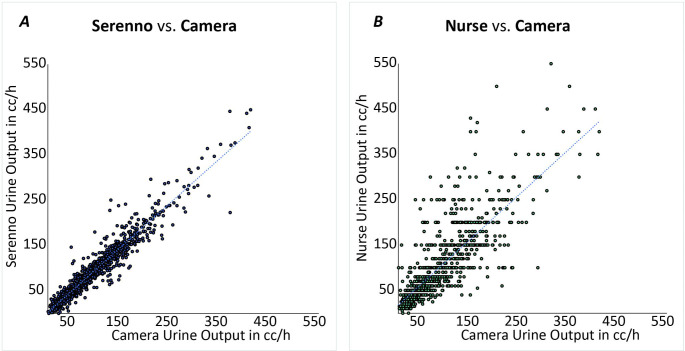
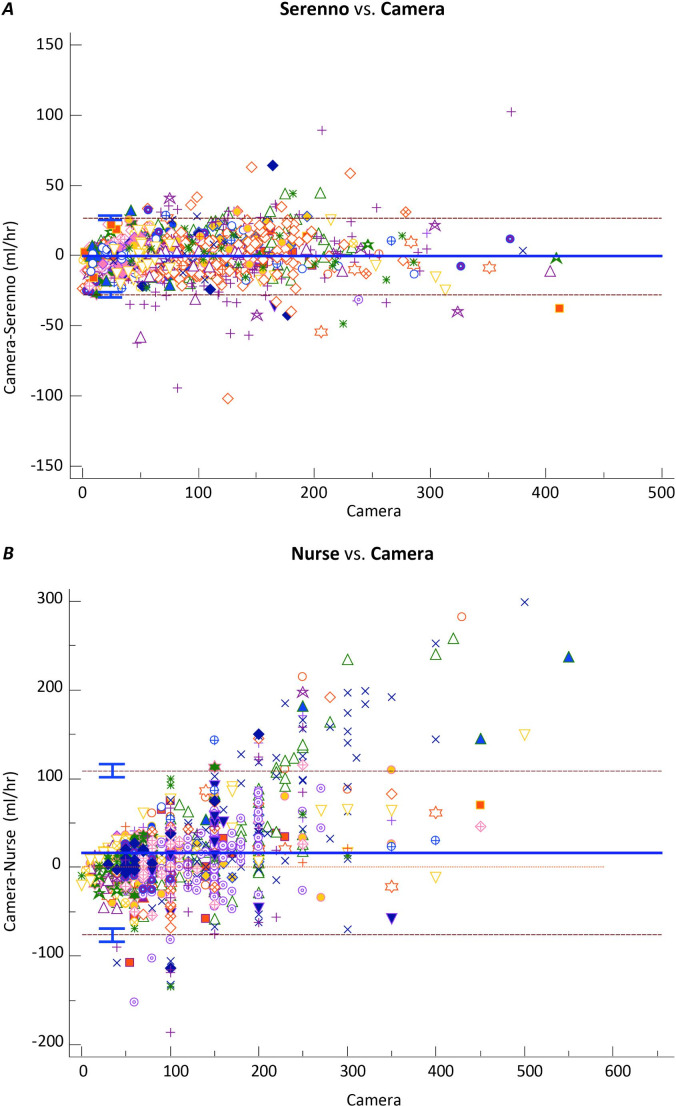
Our statistical analysis plan was based on non-Inferiority between the mean absolute delta of Device-Camera readings and the mean absolute delta of Nurse-Camera readings. This target was clearly reached as is demonstrated in Figs. 2 and 3.
Fig. 2.
Regression plots comparing hourly urine output readings of Serenno and Nurse with reference Camera readings
Fig. 3.
Repeat measurement Bland and Altman analysis comparing Serenno (top chart) and Nurse (Bottom chart) with reference camera urine-output readings. Each symbol represents a different patient. Dashed lines are 95% confidence intervals
There was a strong correlation between Camera and Serenno urine output assessments: Serenno = 1.0 X Camera, R2 = 0.92. Bias was − 0.4 ml, and the limits of agreement extended from − 28 to + 27 ml (Figs. 2a and 3a). The correlation between Camera and Nurse urine output assessments was distinctly worse: Nurse = 1.2 X Camera, R2 = 0.65. Bias was 7.2 ml, with limits of agreement extending from − 107 to + 122 ml (Figs. 2b and 3b).
The Serenno device performed more than 120,000 automated urine output rate determinations with an error standard deviation of 18 ml/hour. In contrast, nurses performed fewer than 1,000 UO rate assessments with an error standard deviation of 46 ml/hour. Nursing urine output measurements were to be performed hourly per routine in participating critical care units. In fact, assessments were performed within 10 min of the hour in only 34% of the monitored hours, and 33% of the assessments were completely missing (urometer was not emptied for that hour).
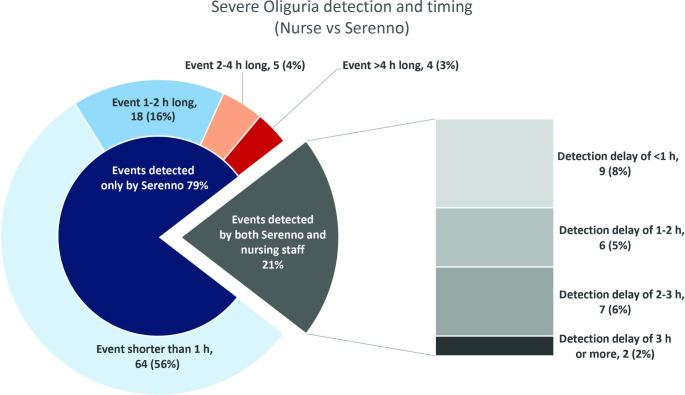
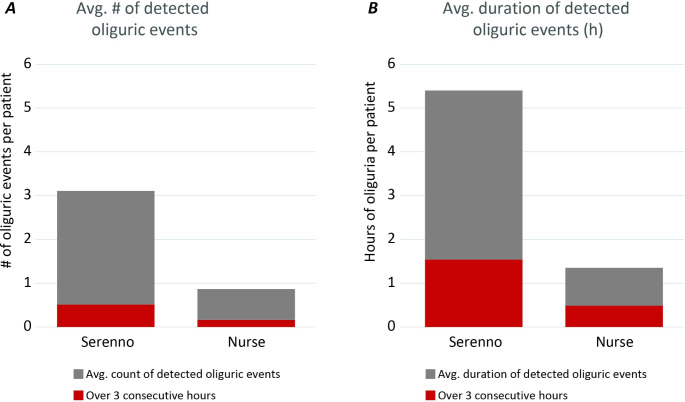
Within the study duration, severe oliguria (urine output < 0.3 ml/kg/h) lasting 2 h or more was common and observed in 8 (21%) of the patients. Among the severe oliguric events lasting more than 3 consecutive hours, 6 (41%) were not detected or documented by the nursing staff. Similarly, 18 (53%) for events lasting 1 to 3 h and 69 (79%) of events lasting an hour or less (but at least 20 min) were missed by the nurse’s measurements (Fig. 4). Events lasting 20 or 40 min are, by definition, unlikely to be detected by the nursing observations, as these observations occur generally on an hourly basis. Even 1-hour long events may go unnoticed when these don’t align with the hourly visits and average out with higher rates of urine flow before or after the event.
Fig. 4.
Comparison of undetected and detected severe oliguric events (< 0.3 ml/Kg/h for > 20 min), by length of event and timing of detection. Numbers are actual number of oliguric events of a total of 115 events
When oliguric events were detected by both nurses and the Serenno device, they were recognized earlier by the Serenno device. Specifically, severe oliguric events of < 0.3 ml/kg/h were detected 1.0 (95% CI 0.4–1.6) hour earlier, while oliguric events of < 0.5 ml/kg/h were detected 1.7 (95% CI 1.0-2.3) hours earlier (Fig. 5).
Fig. 5.
Comparison of detection sensitivity of oliguric and severe oliguric events and their duration. Gray indicates oliguric events, and red indicates severe oliguric events. Oliguric events were observed in 34 patients. There were a total of 115 oliguric events detected by camera and 32 detected by nurses. The total duration of oliguric events detected by the camera was 199 h versus only 37 h detected by nurses
For a more detailed view of the device performance see Supplemental Figs. 1 and 2) showing a typical week-long recording, as well as per patient and cumulative urine output errors.
Discussion
Our major finding is that the Serenno device continuously measured urine flow with error standard deviation of 18 ml/hour and required little staff attention. Measurement bias was close to zero, and the limits of agreement were reasonably low at about ± 28 ml. These results compare favorably against the nursing staff manual measurements as well as other automatic urine output measuring devices such as the Clarity RMS (RenalSense, Jerusalem, Israel) with a reported error standard deviation of 34 ml/hour [8] or the NephroLog, (Fize Research Ltd) with a reported error standard deviation of ≈ 35 ml/hour [9]. While the Serenno LOA is apparently insufficiently accurate to declare oliguria based on a single hour’s reading, it remains an early alarm of suspected oliguric event in progress which can be either confirmed or rejected in subsequent hours.
Automated urine output assessments were far more reliable than nursing assessments which had range of limits of agreement exceeding 200 ml. Furthermore, 32% of the nursing urine output assessments were not performed near the designated times, and 33% were completely missed, likely because the nurses were occupied with higher-priority tasks.
Acute kidney injury is common in critical care cardiac surgery patients, with about 20% developing Stage 1 injury and > 60% developing Stage 2–3 injury in some studies [10]. Among patients who develop Stage 2–3 renal injury, the injury will prove permanent in about 12% of patients [10]. Even in perioperative patients, who are not usually especially sick, a quarter or more of acute kidney injury of any stage appears to be permanent [11]. As might thus be expected, renal injury is a strong predictor of mortality in critically ill patients [12].
The main criteria used for the diagnosis of acute kidney injury (AKI) are reduced urine output (UO) and increased serum creatinine [1–3, 13]. Both markers suffer from significant limitations [4] that can delay diagnosis of functional impairment in kidney function for hours to days compared to more sensitive biomarkers of renal injury [2, 14].
Increased serum creatinine occurs relatively late in the course of AKI, often manifesting 24–48 h after the insult. Furthermore, creatinine is usually measured only once daily, even in critically ill patients. These limitations make creatinine a poor tool for the early detection of AKI [15–17]. Using only serum creatinine for the detection of AKI misses more than a third of cases that would have been diagnosed when also considering oliguria [18]. Consequently, urine output is an important component of AKI identification. For example, including oliguria defined by urine output of less than 0.5 mL/kg.hr or severe oliguria defined by less than 0.3 mL/kg.hr in the definition, leads to earlier and more frequent detection of AKI in critically ill patients [7, 12, 19, 20].
Urine output in most ICU’s is still recorded manually and intermittently. The practice leads to missing, delayed, or inaccurate assessments and adds to the workload on the stuff. Intensive monitoring of urine output leads to earlier diagnosis of AKI and better outcomes and is an early predictor of mortality in critically ill patients [12, 21]. In the last decade, various devices capable of monitoring urine output continuously have been developed [8, 22–24]. In a recent study, for example, an automated urine output device performed considerably better than manual methods. The automated device recognized oliguric event earlier, including many that were completely missed by the manual recordings [8].
Our study demonstrates that the Serenno device identifies oliguria and severe oliguria events earlier than nurses and recognized many oliguric events that were completely missed by the nursing stuff. While it remains unclear what degree and duration of oliguria is meaningful with respect to AKI diagnosis and long-term prognosis, the ability to recognize oliguric events earlier might well facilitate implementation of preventive measures such as the maintenance of adequate hemodynamics, optimization of fluid resuscitation, and reduced use of nephrotoxic agents - each of which might limit the severity of AKI and its complications.
An important limitation of our study is that it was stopped shortly after meeting minimum enrollment. Although three critical care units participated, all patients were admitted for trauma or after cardiothoracic surgery. Our population thus poorly represents the range of critical care patients even in Israel, much less in other countries and settings. But that said, measurement of urine output is fairly technical, and it seems unlikely that measurement accuracy depends much on patient factors. We also lack long-term follow-up and thus are unable to evaluate the long-term consequences of oliguric periods, and what duration might be clinically meaningful. Furthermore, a much larger study would be required to evaluate consequences of oliguria. And finally, our study was purely observational. We therefore cannot comment on whether early intervention for oliguria might reduce the risk of acute kidney injury, much less prevent more serious outcomes.
In summary, in this first-in-human validation study, the Serenno continuous urine output measurement device automatically and accurately assessed urine output in critical care patients. It identified even brief periods of oliguria which might allow interventions to reduce the risk of acute kidney injury. Much additional will be required to determine what severity and duration of oliguria is clinically meaningful, much less whether it is possible to intervene and prevent acute and chronic kidney injury.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Data acquisition: GF, ER, DG, YK, RD, TH, MLNGM, PS. Data interpretation and manuscript preparation: DIS. Statistical analysis: Noam Hadas.
Funding
Funded by Serenno Medical (Yokneam, Israel).
Data Availability
No additional data will be available.
Declarations
Competing interests
DIS and MM have equity interests in Serenno Medical. TH was employed at Serenno Medical during patient enrollment.
Ethics statement
The study protocol was approved by the local ethics committees at each site (0860-18-RMC, 5839-19-SMC) and the study was performed in accordance with Good Clinical Practice and applicable regulatory requirements. The study was observational and experimental results were not available to clinicians. The study therefore did not influence clinical care. Written informed consent was obtained from each patient or an authorized relative before inclusion.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chertow GM, Lazarus JM, Paganini EP, Allgren RL, Lafayette RA, Sayegh MH. Predictors of mortality and the provision of dialysis in patients with acute tubular necrosis. The Auriculin Anaritide Acute Renal failure Study Group. J Am Soc Nephrol. 1998;9(4):692–8. doi: 10.1681/ASN.V94692. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. & Acute Dialysis Quality Initiative, w. (2004). Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care, 8(4), R204-212. 10.1186/cc2872 [DOI] [PMC free article] [PubMed]
- 3.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17(4):1135–42. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 4.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Injury AK. Acute kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, Levey AS, MacLeod AM, Mehta RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M, Uchino S. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Supplements. 2012;2(1):1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 6.Bianchi NA, Stavart LL, Altarelli M, Kelevina T, Faouzi M, Schneider AG. Association of Oliguria with Acute kidney Injury diagnosis, Severity Assessment, and Mortality among patients with critical illness. JAMA Netw open. 2021;4(11):e2133094. doi: 10.1001/jamanetworkopen.2021.33094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin K, Murugan R, Sileanu FE, Foldes E, Priyanka P, Clermont G, Kellum J. Intensive monitoring of urine output is Associated with increased detection of Acute kidney Injury and Improved Outcomes. Chest Nov. 2017;152(5):972–9. doi: 10.1016/j.chest.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 8.11 Minor J, Smith A, Deutsch F, et al. Automated versus manual urine output monitoring in the intensive care unit. Sci Rep. 2021;11:17429. doi: 10.1016/j.chest.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rott D, Hay I, Klempfner R. Urine output measurement by a Novel Electronic Urinometer is much more Accurate than by Conventional Urinometer. J Integr Cardiol Open Access Published: 26 June. 2020 doi: 10.31487/j.JICOA.2020.03.04. [DOI] [Google Scholar]
- 10.Priyanka P, Zarbock A, Izawa J, Gleason TG, Renfurm RW, Kellum JA. The impact of acute kidney injury by serum creatinine or urine output criteria on major adverse kidney events in cardiac surgery patients. J Thorac Cardiovasc Surg. 2020;162(1):143–51. doi: 10.1016/j.jtcvs.2019.11.137. [DOI] [PubMed] [Google Scholar]
- 11.Turan A, Cohen B, Adegboye J, Makarova N, Liu L, Mascha EJ, Qiu Y, Irefin S, Wakefield BJ, Ruetzler K, Sessler DI. Mild Acute kidney Injury after Noncardiac surgery is Associated with Long-term renal dysfunction: a retrospective cohort study. Anesthesiology. 2020;132:1053–61. doi: 10.1097/ALN.0000000000003109. [DOI] [PubMed] [Google Scholar]
- 12.Macedo E, Malhotra R, Bouchard J, Wynn SK, Mehta RL. Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int. 2021;30(7):760–7. doi: 10.1038/ki.2011.150. [DOI] [PubMed] [Google Scholar]
- 13.Ronco C, Bellomo R, Kellum JA, Acute Kidney Injury Lancet. 2019;394:1949–64. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 14.Ronco C. Kidney attack: overdiagnosis of acute kidney injury or comprehensive definition of acute kidney syndromes? Blood Purif. 2013;36(2):65–8. doi: 10.1159/000354768. [DOI] [PubMed] [Google Scholar]
- 15.Chawla LS, Goldstein SL, Kellum JA, Ronco C. Renal angina: concept and development of pretest probability assessment in acute kidney injury. Crit Care Feb. 2015;27(1):93. doi: 10.1186/s13054-015-0779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ronco C, McCullough PA, Chawla LS. Kidney attack versus heart attack: evolution of classification and diagnostic criteria. Lancet Sep. 2013;14(9896):939–40. doi: 10.1016/S0140-6736(13)61932-7. [DOI] [PubMed] [Google Scholar]
- 17.Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015 Sep;26(9):2231–8. 10.1016/S0140-6736(13)61932-7. [DOI] [PMC free article] [PubMed]
- 18.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. (2005) Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005 Nov;16(11):3365-70. 10.1681/ASN.2004090740. [DOI] [PubMed]
- 19.Basu RK, Wang Y, Wong HR, Chawla LS, Wheeler DS, Goldstein SL. Incorporation of biomarkers with the renal angina index for prediction of severe AKI in critically ill children. Clin J Am Soc Nephrol Apr. 2014;9(4):654–62. doi: 10.2215/CJN.09720913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menon S, Goldstein SL, Mottes T, Fei L, Kaddourah A, Terrell T, Arnold P, Bennett MR, Basu RK. Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: a prospective cohort study. Nephrol Dial Transplant. 2016;31(4):586–94. doi: 10.1093/ndt/gfv457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorval JF, Dixon SR, Zelman RB, Davidson CJ, Rudko R, Resnic FS. Feasibility study of the RenalGuard™ balanced hydration system: a novel strategy for the prevention of contrast-induced nephropathy in high-risk patients. Int J Cardiol Jun. 2013;20(2):482–6. doi: 10.1016/j.ijcard.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 22.Chang AJ, Nomura Y, Barodka VM, Hori D, Magruder JT, Katz NM, Berkowitz DE, Hogue CW. (2017) Validation of a Real-Time Minute-to-Minute Urine Output Monitor and the Feasibility of Its Clinical Use for Patients Undergoing Cardiac Surgery. Anesth Analg. Dec;12583 – 1886. 10.1213/ANE.0000000000002217 [DOI] [PMC free article] [PubMed]
- 23.Panagiotou A, Garzotto F, Gramaticopolo S, Piccinni P, Trentin C, Cruz DN, Ronco C. Continuous real-time urine output monitoring for early detection of acute kidney injury. Contrib Nephrol. 2011;171:194–200. doi: 10.1159/000327323. [DOI] [PubMed] [Google Scholar]
- 24.Brotfain E, Klein Y, Toledano R, Koyfman L, Frank D, Shamir MY, Klein M. Urine flow rate monitoring in hypovolemic multiple trauma patients. World J Emerg Surg Aug. 2017;18:41. doi: 10.1186/s13017-017-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No additional data will be available.