Abstract
Cerebral vasospasm (CV) or delayed cerebral ischemia (DCI) constitutes the main reason for the unfavorable outcomes of patients with aneurysmal subarachnoid hemorrhage (aSAH). The present retrospective cohort study, through an evaluation with computed tomography (CT) perfusion (CTP), aimed to examine the utility of an intravenous or oral administration of sildenafil in preventing DCI that develops due to vasospasm in these patients. A retrospective cohort study was conducted, which included 34 patients in a tertiary care hospital. Of these patients, 18 were males (52.9%), and the median age was 54.4 years. Of these patients, 18 (52.9%) had undergone surgery, and 16 (47.1%) had an endovascular procedure. CTP was performed on the 3rd to the 6th day. The clinical outcome was documented at 30 days using a CT scan and a complete neurological evaluation, including the Glasgow Coma Scale assessment. There was a statistically significant difference in the number of patients who developed an ischemic event at 1 month between those who did not receive sildenafil compared to those who received sildenafil (P<0.05). In addition, the multivariate analysis revealed that cerebral blood flow was an independent factor for detecting an ischemic event in 1 month (P=0.001). On the whole, the findings of the present study indicate that the intravenous or oral administration of sildenafil may be beneficial for the prevention of DCI.
Keywords: subarachnoid hemorrhage, computed tomography perfusion, sildenafil, cerebral vasospasm
Introduction
Cerebral vasospasm (CV) or delayed cerebral ischemia (DCI) occurs in 30-40% of patients with aneurysmal subarachnoid hemorrhage (aSAH) (1). However, despite the use of various therapeutic procedures, 16-65% of these patients develop ischemia (1-4).
The identification of CV (clinically or with angiography) in the early stages of SAH can be complex and challenging (2,3). Although 40-70% of patients exhibit substantial arterial narrowing (on a Doppler ultrasound or in angiography), only 20-30% of these patients present with DCI (2,3). Intra-arterial digital subtraction angiography (DSA) is effective as a screening device for patients whose symptoms and clinical findings are consistent with focal cerebral ischemia (CI) (5). However, when analyzing the global vasculature with DSA, the detailed delineation of the calf vessels may not be accomplished when several stenoses are present and constitute an invasive method. Computed tomography (CT) can illustrate the extent to which tissue is irreversibly damaged (the ischemic core) (6-8), and it indicates that reperfusion therapy may not be necessary or may even be harmful when the ischemic core is large or the perfusion is damaged to a great extent (9,10). By contrast, CT perfusion (CTP) can identify damages not recognized by other methods and may be beneficial for assessing CI related to SAH (11). CTP can be used in daily practice or it can be used as a separate diagnostic tool without the need for magnetic resonance imaging data to predict the outcomes of patients with SAH (12,13). Various devices are employed to enhance cerebral blood flow (CBF) in patients with SAH who develop CV or DCI. For example, intra-aortic balloon counterpulsation, hyperdynamic therapy, intra-arterial and intrathecal drug infusion, as well as novel experimental techniques, such as endovascular treatment and various drugs or their combinations, may be helpful if treatment commences early. However, in the case that a CV does not appear or treatment begins too late, CI may occur even with optimal management (14). Phosphodiesterase-V (PDE-V) is a regulatory enzyme of the endothelial nitric oxide synthase (eNOS)/nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) pathway (15,16). The inhibition of this pathway mainly through sildenafil (a PDE-V inhibitor) in patients with vasospasm due to spontaneous SAH has been found to improve flow velocity on a transcranial Doppler (17). At the same time, animal and patient studies have revealed reduced blood levels of endothelin-1 (18-21). In addition, studies on patients with cardiovascular diseases have proven that sildenafil is safe to administer (22-24). However, to date, to the best of our knowledge, no extensive data are available to confirm the positive effects of sildenafil in patients with vasospasm due to SAH from a ruptured aneurysm.
In this respect, the present retrospective cohort study was performed evaluate the utility of sildenafil following its intravenous or oral administration in preventing DCI that develops due to vasospasm in patients with aSAH.
Patients and methods
Study design and patient population
The present retrospective cohort study analyzed patients diagnosed with spontaneous SAH/aSAH (34 of the 42 patients were diagnosed with spontaneous SAH/aSAH). The Institutional Review Board (IRB) of Nicosia General Hospital, Cyprus approved the study (IRB no. EEBK EΠ 2021.02.158). The study was in line with the Declaration of Helsinki in 1995 (as revised in Edinburgh 2000). Written informed was obtained from all patients for publication of their data.
Inclusion criteria
The inclusion criteria were the following: An age ≥21 years, surgical or endovascular repair of the ruptured aneurysm, a Modified Fisher grade of I to IV hemorrhage on initial CT imaging, and evidence of CI on CTP. The patients were divided into two groups, namely group A, which included patients treated with only intravenous nimodipine, and group B, which included those who received intravenous nimodipine together with sildenafil using a dose escalation scheme (high dose of 20 mg or bioequivalent to a 40 mg oral dose). All individuals received nimodipine at 60 mg every 4 h with close monitoring (including patients' heartbeat, breathing rate, temperature, oxygen saturation and blood pressure) for 21 days. These groups were identified based on age, sex, CT findings, Glasgow Coma Scale (GCS) during intubation and neurosurgical intervention as factors influencing patient outcomes.
Exclusion criteria
Patients were excluded if they exhibited factors predisposed to CI, apart from vasospasm (i.e., those with a history of atrial fibrillation or other arrhythmias, diabetes mellitus, hyperlipidemia and hypotension) or if they exhibited factors predisposed to bleeding (anticoagulant agents and severe liver impairment) and those who were taking any other vasodilators agents for a long period of time, SAH secondary to traumatic or mycotic aneurysm and preictal sildenafil therapy (Fig. 1). All participants had a follow-up for 30 days or until the day of discharge from the hospital. Patient outcomes were evaluated at 30 days using a CT scan, and a complete neurological examination and a GCS assessment were performed. The clinical outcome was categorized according to the presence of neurological or radiological evidence as normal, adverse or mortality.
Figure 1.
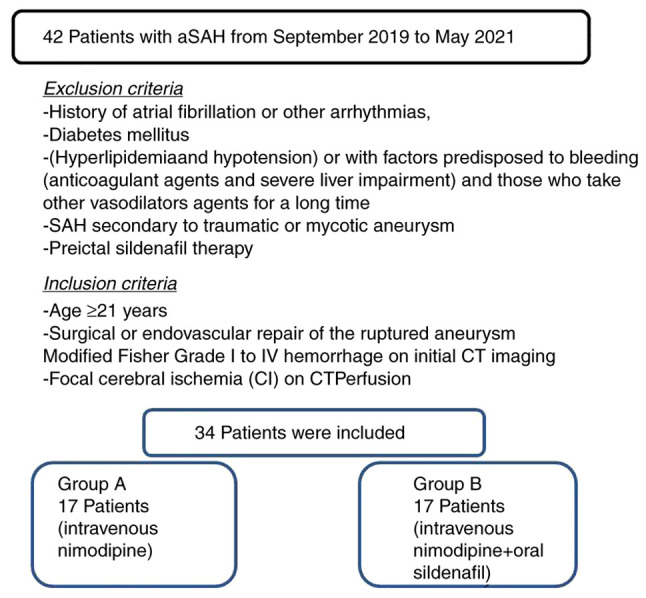
Flow chart of the study design and patient inclusion.
Radiological CV or CI assessment
During the 3rd to the 6th day following the SAH, CTP was performed in all the participants to identify a quantifiable index of CI after CV, given that angiographically detectable cerebral artery constriction is most commonly present 3-10 days after the onset of the condition. The cerebral blood volume (CBV) and CBF values were documented and assessed after receiving two contiguous 10-mm slices placed at the anatomical point of the basal ganglia with similar angulation as for native CT. A bolus of 50 ml non-ionic contrast medium (Imeron 400, Bracco Imaging Deutschland GmbH) accompanied by 30 ml saline was then infused using a power injector at a flow rate of 4 ml/sec. Subsequently, 40 captures were obtained at each slice level at a rate of two images per second (120 kV, 110 mAs, 512x512 matrix). CTP color maps were qualitatively assessed using a visual grading scale, and CTP parameters were established utilizing software platforms (Perfusion CT, Siemens). A positive visual measurement was recorded for side-to-side apparent bilateral abnormalities, suggesting a decline in CBF, CBV and mean transit time (MTT), which were related to the central volume principle: CBF=CBV/MTT (2,25). CBV was determined in milliliters of blood per 100 g of the brain and was established as the volume of blood flow for a certain amount of brain tissue (2,26). MTT was determined as the average time required for blood to move through a particular brain volume and was calculated in seconds (2).
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS 11; SPSS, Inc.). The normality of the distribution of variables was assessed using the Shapiro-Wilk test. Categorical variables were compared between groups using Fisher's exact test and continuous data were compared with the Mann-Whitney U test. Receiver operating characteristic (ROC) analysis was presented to reveal the implementation of TCD or CTP indicators in recognition of unfavorable outcomes. A P-value <0.05 was considered to indicate a statistically significant difference.
Results
In total, 34 of the 42 patients diagnosed with spontaneous SAH/aSAH were enrolled in the present study. A total of 17 patients were included in group A and 17 patients were included in group B. Of the 34 patients included, 18 were males (52.9%), and the median age was 54.4 years. Of these patients, 18 (52.9%) had undergone surgery, and 16 (47.1%) had an endovascular procedure. The baseline characteristics of the study participants and CTP data are presented in Table I. All patients had a regular follow-up for 1 month.
Table I.
Baseline characteristics of the patients included in the present study.
| Parameters | All patients, n=34 (100%) | Group A, n=17 (50%) | Group B, n=17 (50%) | P-value |
|---|---|---|---|---|
| Age, years | 54.4±18 | 56.0±16 | 52.8±20 | 0.679 |
| Sex (male), n (%) | 18 (52.9) | 10 (29.4) | 8 (23.5) | 0.170 |
| GCS at admission | 13.1±2 | 12.6±2 | 13.7±1 | 0.201 |
| Procedure, n (%) | ||||
| Surgical | 18 (52.9) | 10 (29.4) | 8 (23.5) | 0.492 |
| Endovascular | 16 (47.1) | 7 (20.5) | 9 (26.4) | |
| MAP | ||||
| % Reduction from baseline | 7.4±7 | 6.7±7 | 8.2±8 | 0.742 |
| Duration, min | 7.7±11 | 6.0±10 | 9.4±13 | 0.440 |
| Hunt and Hess grade, n (%) | ||||
| I | 8 (23.5) | 3 (8.8) | 5 (14.7) | 0.419 |
| II | 15 (44.1) | 4 (11.7) | 11 (32.3) | 0.016 |
| III | 7 (20.5) | 6 (17.6) | 1 (2.9) | 0.034 |
| IV | 3 (8.8) | 3 (8.8) | 0 (0) | 0.070 |
| V | 1 (2.9) | 1 (2.9) | 0 (0) | 0.310 |
| Modified Fisher grade, n (%) | ||||
| I | 8 (23.5) | 3 (8.8) | 5 (14.7) | 0.419 |
| II | 16 (47.0) | 5 (14.7) | 11 (32.3) | 0.039 |
| III | 7 (20.5) | 6 (17.6) | 1 (2.9) | 0.034 |
| IV | 3 (8.8) | 3 (8.8) | 0 (0) | 0.070 |
| Aneurysm location, n (%) | ||||
| ACoA | 6 (17.6) | 3 (8.8) | 3 (8.8) | NS |
| MCA | 12 (35.2) | 4 (11.7) | 8 (23.5) | 0.151 |
| PICA | 2 (5.8) | 1 (2.9) | 1 (2.9) | NS |
| Pcom | 5 (14.7) | 3 (8.8) | 2 (5.8) | 0.628 |
| ICA | 9 (26.4) | 6 (17.6) | 3 (8.8) | 0.244 |
| CT perfusion (white matter) parameters | ||||
| CBFmean ± SD (mlblood/100 gtissue) | 19.4±11 | 24.0±7 | 14.7±12 | 0.011 |
| CBVmean ± SD (mlblood/100 gtissue) | 1.4±1 | 1.6±0. | 1.2±1 | 0.042 |
| MMTmean ± SD (sec) | 4.3±1.5 | 4.6±1 | 3.9±1 | 0.055 |
GCS, Glasgow coma scale; MAP, mean arterial pressure; ACoA, anterior communicate artery; MCA, middle cerebral artery; PICA, posterior inferior cerebral artery; Pcom, posterior communicating artery; ICA, internal carotid artery; CT, computed tomography; MMTmean, mean value of mean transit time; CBVmean, mean value of cerebral blood volume; CBFmean, mean value of cerebral blood flow; NS, not significant.
Univariate analysis revealed that there was a statistically significant difference in the mean values of CBF, CBV and MTT between the participants who developed adverse ischemic events and between those who did not develop adverse ischemic events (P<0.05, Table II). Overall, there was a statistically significant difference in the number of patients who developed an ischemic event at 1 month in group B compared with those of group A (P<0.05, Table II).
Table II.
Univariate analysis (outcome, ischemic event at 1 month).
| Parameter | Patients with ischemic event, n=14 (41.1%) | Patients without ischemic event, n=20 (58.9%) | P-value |
|---|---|---|---|
| Groups, n (%) | |||
| Group A | 14 (41.1) | 3 (8.8) | 0.001 |
| Group B | 0 (0) | 17(50) | 0.001 |
| Age, years | 58.1±15 | 51.8±20 | 0.336 |
| Sex (male), n(%) | 10 (29.4) | 8 (23.5) | 0.161 |
| GCS at admission | 12.1±2 | 13.9±1 | 0.011 |
| Procedure, n (%) | |||
| Surgical | 10 (29.4) | 8 (23.5) | 0.071 |
| Endovascular | 4 (11.7) | 12 (35.2) | |
| MAP | |||
| % Reduction from baseline | 4.7±3 | 9.4±8 | 0.388 |
| Duration, min | 3.1±4 | 11.0±14 | 0.181 |
| Hunt and Hess grade, n (%) | |||
| I | 1 (2.9) | 7 (20.5) | 0.059 |
| II | 3 (8.8) | 12 (35.2) | 0.026 |
| III | 6 (17.6) | 1 (2.9) | 0.007 |
| IV | 3 (8.8) | 0 (0) | 0.030 |
| V | 1 (2.9) | 0 (0) | 0.225 |
| Modified Fisher grade, n (%) | |||
| I | 1 (2.9) | 7 (20.5) | 0.059 |
| II | 4 (11.7) | 12 (35.2) | 0.071 |
| III | 6 (17.6) | 1 (2.9) | 0.007 |
| IV | 3 (8.8) | 0 (0) | 0.030 |
| Aneurysm location | |||
| ACoA | 3 (8.8) | 3 (8.8) | 0.628 |
| MCA | 2 (5.8) | 10 (29.4) | 0.032 |
| PICA | 1 (2.9) | 1 (2.9) | 0.794 |
| Pcom | 2 (5.8) | 3 (8.8) | 0.954 |
| ICA | 6 (17.6) | 3 (8.8) | 0.070 |
| CT perfusion (white matter) parameters | |||
| CBFmean ± SD (mlblood/100 gtissue) | 26.6±4 | 14.3±11 | 0.001 |
| CBVmean ± SD (mlblood/100 gtissue) | 1.8±0. | 1.1±1 | 0.005 |
| MMTmean± SD (sec) | 4.9±1 | 3.8±1 | 0.001 |
GCS, Glasgow coma scale; MAP, mean arterial pressure; ACoA, anterior communicate artery; MCA, middle cerebral artery; PICA, posterior inferior cerebral artery; Pcom, posterior communicating artery; ICA, internal carotid artery; CT, computed tomography; MMTmean, mean value of mean transit time; CBVmean, mean value of cerebral blood volume; CBFmean, mean value of cerebral blood flow.
Multivariate analysis (Table III) revealed that CBF was an independent factor in detecting an ischemic event at 1 month (P=0.004). ROC analysis demonstrated that, among the CTP variables, a CBF value <18.35 ml/100 g/min with 92.9% sensitivity and 80% specificity and an MTT value >3.7 sec with 85.7% sensitivity and 80% specificity exhibited the optimal performance to diagnose adverse ischemic events at 1 month, as evaluated by an area under the curve standard error [AUC (SE)] of [0.83 (0.07)] and [0.82 (0.07)] (P=0.001, respectively; Table IV and Fig. 2, Fig. 3 and Fig. 4).
Table III.
Multivariate analysis (outcome, ischemic event at 1 month).
| 95% CI for Exp(B) | ||||
|---|---|---|---|---|
| Parameter | P-value | Exp(B) | Lower | Upper |
| CBFmean ± SD (mlblood/100 gtissue) | 0.004 | 1.395 | 1.109 | 1.754 |
| CBVmean ± SD (mlblood/100 gtissue) | 0.028 | 0.079 | 0.008 | 0.758 |
| MMTmean ± SD (sec) | 0.125 | 2.479 | 0.778 | 7.900 |
MMTmean, mean value of mean transit time; CBVmean, mean value of cerebral blood volume; CBFmean, mean value of cerebral blood flow; SD, standard deviation; CI, confidence interval.
Table IV.
ROC analysis.
| 95% CI | |||||
|---|---|---|---|---|---|
| Parameters | Area | Std Error | Lower | Upper | P-value |
| CBFmean ± SD (mlblood/100 gtissue) | 0.836 | 0.077 | 0.684 | 0.988 | 0.001 |
| CBVmean ±S D (mlblood/100 gtissue) | 0.782 | 0.089 | 0.608 | 0.957 | 0.006 |
| MMTmean ± SD (sec) | 0.829 | 0.079 | 0.673 | 0.984 | 0.001 |
CBF, cerebral blood flow; CBV, cerebral blood volume; MTT, mean transit time; PI, pulsatility index; PSV, peak systolic velocity; SD, standard deviation; CI, confidence interval; Std Error, standard error.
Figure 2.
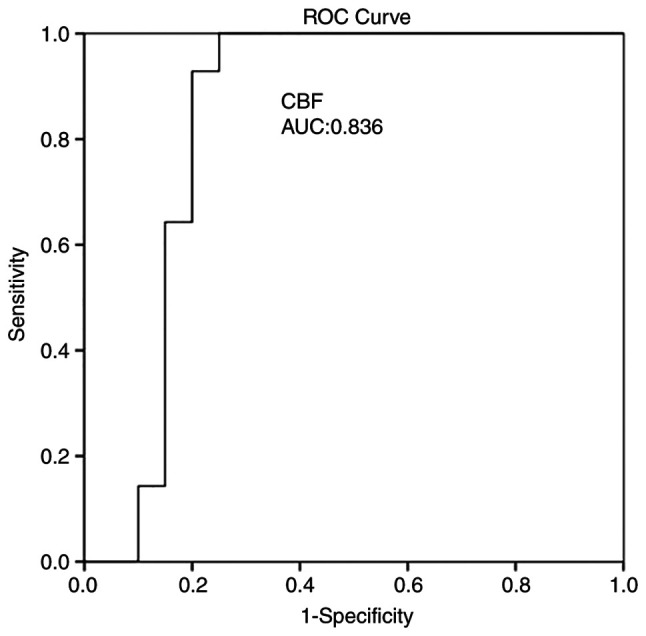
ROC curve for CBF predicting an ischemic event at 1 month. AUC, 0.836. AUC, area under the curve; CBF, cerebral blood flow; ROC, receiver operative characteristic.
Figure 3.
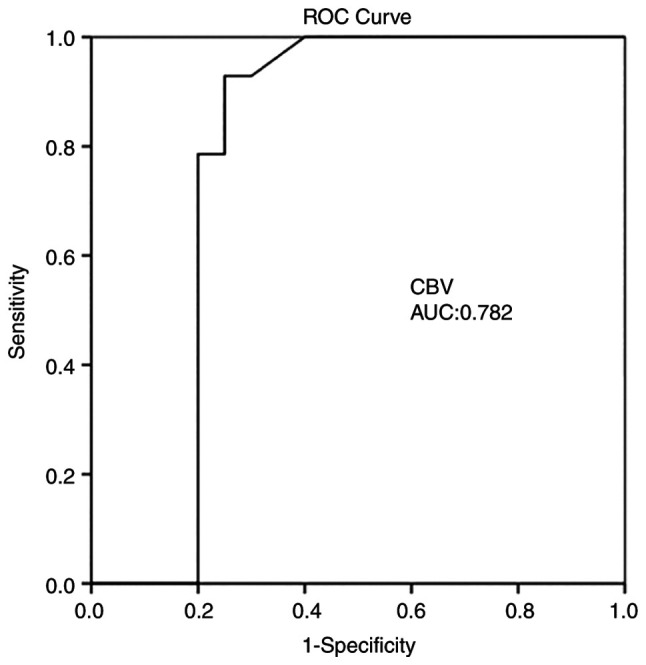
ROC curve for CBV predicting an ischemic event at 1 month. AUC, 0.782. AUC, area under the curve; CBV, cerebral blood volume; ROC, receiver operative characteristic.
Figure 4.
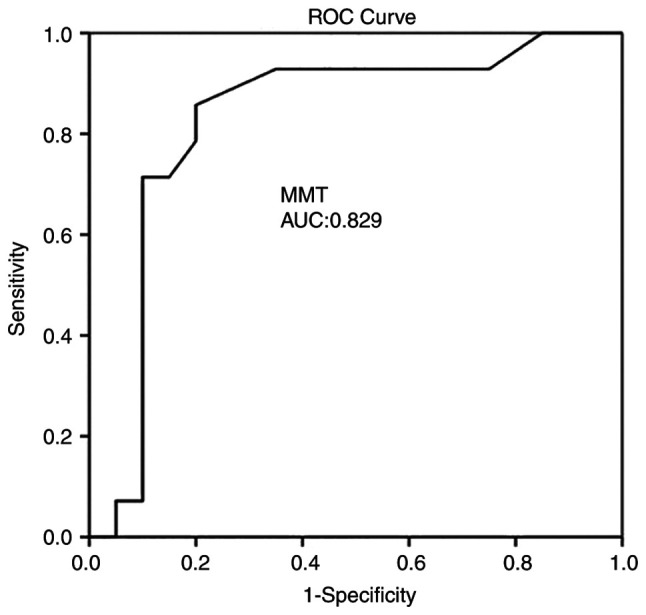
ROC curve for MTT predicting an ischemic event at 1 month. AUC, 0.829. AUC, area under the curve; MTT, mean transit time; ROC, receiver operative characteristic.
Discussion
The results of the present study suggest that using sildenafil, intravenously or by oral administration may be helpful for the prevention of DCI that develops due to vasospasm in patients with SAH following an aneurysm rupture. In addition, CTP, obtainable in everyday practice, provides valuable details regarding SAH-associated CV. Certainly, CTP, mainly CBF and MTT, can identify patients who have developed delayed unfavorable ischemic incidents. Thus, sildenafil constitutes a very promising therapy for the management of SAH. DSA is the gold standard for identifying CV and different cerebrovascular entities (2,27,28). However, the fact that the procedure is invasive and the radiation exposure require careful patient selection and preclude widespread use (29). Therefore, CTP is currently the most commonly used and studied imaging technique (2,28). A range of cut-off values associated with DCI have been mentioned, counting an MTT value >5.0-6.4 sec or a local CBF value <25-40 ml/100 g/min (2,30). In the present study, a CBF value <18.35 ml/100 g/min with 92.9% sensitivity and 80% specificity, and an MTT value >3.7 sec with 85.7% sensitivity and 80% specificity exhibited the optimal performance in diagnosing adverse ischemic events at 1 month, as evaluated using an AUC (SE). The present study examined two groups of patients, depending on sildenafil therapy uptake. At a 1-month follow-up, patients in group B (treated with a combination of nimodipine and sildenafil) exhibited clinical outcomes without ischemic damage in CTP. By contrast, the patients in group A (treated only with nimodipine) exhibited unfavorable clinical outcomes with ischemic damage in CTP.
Vasospasm following aSAH is a multifactored mechanism and the standard of choice is the mainstay for preventing calcium channel blocker nimodipine (31-34). Furthermore, previous studies have indicated that not only angiographic vasospasm, but also a disrupted microcirculation due to microvascular constriction, micro-thrombosis, impaired autoregulation, cortical spreading ischemia and blood-brain barrier disruption may contribute to the development of DCI (35,36). However, therapeutic strategies for vasospasm, refractory to endovascular or microsurgical intervention are still under investigation. Although it has been demonstrated that microsurgical treatment may be helpful for vasospasms (37), others have justified the effects of endovascular intervention as rescue therapy for severe vasospasm on the clinical, as well as the radiological outcomes of patients with aSAH (38). For this reason, the present study was designed using, as a control group, those patients that were treated with the mainstay of prevention, namely the calcium channel blocker, nimodipine, to avoid bias. In addition, it was better to include both endovascular and microsurgical management as the results may would be more clear and more related to the reality.
According to certain studies, sildenafil can cause unacceptable hypotension in the patient population with aSAH, and may thus be associated with unfavorable clinical outcomes (38-40). However, the present study demonstrated that the blood pressure profile was acceptable at a dose of 20 mg without evidence of a prolonged adverse effect or any statistically significant difference between groups (Tables I and II). In addition, while there was a 7.4% mean reduction in mean arterial pressure from baseline levels, this was transient, lasting an average of 6.0 min until it returned to baseline levels. The literature has well documented that the upregulation of the eNOS/NO/cGMP pathway, a component of the development of SAH-induced CV, can improve the clinical outcomes and DCI (41-46). Thus, researchers have administered PDEV inhibitors, such as sildenafil to inhibit SAH-induced DCI. Theoretically, the mechanism through which sildenafil, as a PDE-V inhibitor, utilizes its positive vascular results has been in its capacity to increase intracellular cGMP levels in vascular smooth muscle cells and regulate vascular tone (47). In addition, when CBF to a region reduces to ischemic levels, sildenafil, similar to other orally active PDE5 inhibitors, such as vardenafil, has exhibited the capability to decrease the extent of the infarct and promote recovery by supporting the vasculature in hypoxic areas (48,49). The findings of the present study demonstrate that sildenafil may ‘reverse’ CV and the development of CI.
However, the present study has certain limitations. First, it was a small, single-center study. Therefore, firm conclusions regarding the role of sildenafil in the management of SAH cannot be reached. In addition, as vasospasm following aSAH is a multifactorial mechanism, its role as a rescue therapy for vasospasm, refractory to endovascular or microsurgical intervention is not yet clear. Thus the present study did not distinguish the causes of DCI as it would be impossible, and for this reason, the present study was designed, using as a control group, those patients that were treated with the mainstay of prevention, namely with the calcium channel blocker, nimodipine, to avoid bias. In addition, in the present study, there were a few patients with Hunt and Hess grade IV or V; thus, the development of CV may not have been severe. However, this may be a basis for a future, more extensive clinical studies.
In conclusion, the present study demonstrates that the use of intravenous or oral sildenafil may be helpful for the prevention of DCI that develops due to vasospasm in patients with aSAH. In addition, CTP, mainly CBF and MTT, obtainable in everyday practice, provides valuable details regarding SAH-associated CV. This sequence, along with the evidence of preclinical data in SAH studies and the favorable effects of sildenafil in the treatment of other entities related to vascular endothelium dysfunction, provides a strong justification for more extensive prospective clinical investigations into its efficacy for the prevention of SAH-related DCI.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
GF, AAF and VEG conceptualized the study. KF, VEG, AAF, PP, IT, NT, DAS, VT, NM and KT made a substantial contribution to the analysis and interpretation of the data, and wrote and prepared the draft of the manuscript. EL was the radiologist who examined the patients and analyzed the radiological data. VEG and GF analyzed the data and provided critical revisions. GF and KF confirm the authenticity of all the data. All authors contributed to manuscript revision and have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The Institutional Review Board (IRB) of Nicosia General Hospital, Cyprus approved the study (IRB no. EEBK EΠ 2021.02.158). The study was in line with the Declaration of Helsinki in 1995 (as revised in Edinburgh 2000). Written informed was obtained from all included patients for the publication of their data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cossu G, Messerer M, Oddo M, Daniel RT. To look beyond vasospasm in aneurysmal subarachnoid haemorrhage. Biomed Res Int. 2014;2014(628597) doi: 10.1155/2014/628597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fotakopoulos G, Makris D, Kotlia P, Kapsalaki E, Papanikolaou J, Georgiadis I, Zakynthinos E, Fountas K. The value of computed tomography perfusion & transcranial Doppler in early diagnosis of cerebral vasospasm in aneurysmal & traumatic subarachnoid hemorrhage. Future Sci OA. 2018;4(FSO313) doi: 10.4155/fsoa-2018-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko NU, Rajendran P, Kim H, Rutkowski M, Pawlikowska L, Kwok PY, Higashida RT, Lawton MT, Smith WS, Zaroff JG, Young WL. Endothelial nitric oxide synthase polymorphism (-786T->C) and increased risk of angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2008;39:1103–1018. doi: 10.1161/STROKEAHA.107.496596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumont AS, Dumont RJ, Chow MM, Lin CL, Calisaneller T, Ley KF, Kassell NF, Lee KS. Cerebral vasospasm after subarachnoid hemorrhage: Putative role of inflammation. Neurosurgery. 2003;53:123–135. doi: 10.1227/01.neu.0000068863.37133.9e. [DOI] [PubMed] [Google Scholar]
- 5.Foley WD, Smith DF, Milde MW, Lawson TL, Towne JB, Bandyk DF. Intravenous DSA examination of patients with suspected cerebral ischemia. Radiology. 1984;151:651–659. doi: 10.1148/radiology.151.3.6371889. [DOI] [PubMed] [Google Scholar]
- 6.Muir KW, Baird-Gunning J, Walker L, Baird T, McCormick M, Coutts SB. Can the ischemic penumbra be identified on noncontrast CT of acute stroke? Stroke. 2007;38:2485–2490. doi: 10.1161/STROKEAHA.107.484592. [DOI] [PubMed] [Google Scholar]
- 7.Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, Coutts SB, Demchuk AM, Goyal M, Aviv RI, et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771–177. doi: 10.1161/01.STR.0000227243.96808.53. [DOI] [PubMed] [Google Scholar]
- 8.Wintermark M, Warach SJ. Acute stroke imaging research roadmap II and international survey of acute stroke imaging capabilities: We need your help! AJNR Am J Neuroradiol. 2013;34(1671) doi: 10.3174/ajnr.A3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell BC, Christensen S, Butcher KS, Gordon I, Parsons MW, Desmond PM, Barber PA, Levi CR, Bladin CF, De Silva DA, et al. Regional very low cerebral blood volume predicts hemorrhagic transformation better than diffusion-weighted imaging volume and thresholded apparent diffusion coefficient in acute ischemic stroke. Stroke. 2010;41:82–88. doi: 10.1161/STROKEAHA.109.562116. [DOI] [PubMed] [Google Scholar]
- 10.Yassi N, Parsons MW, Christensen S, Sharma G, Bivard A, Donnan GA, Levi CR, Desmond PM, Davis SM, Campbell BC. Prediction of poststroke hemorrhagic transformation using computed tomography perfusion. Stroke. 2013;44:3039–3043. doi: 10.1161/STROKEAHA.113.002396. [DOI] [PubMed] [Google Scholar]
- 11.Provenzale JM, Shah K, Patel U, McCrory DC. Systematic review of CT and MR perfusion imaging for assessment of acute cerebrovascular disease. AJNR Am J Neuroradiol. 2008;29:1476–1482. doi: 10.3174/ajnr.A1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derex L, Nighoghossian N, Hermier M, Adeleine P, Berthezène Y, Philippeau F, Honnorat J, Froment JC, Trouillas P. Influence of pretreatment MRI parameters on clinical outcome, recanalization and infarct size in 49 stroke patients treated by intravenous tissue plasminogen activator. J Neurol Sci. 2004;225:3–9. doi: 10.1016/j.jns.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Parsons MW, Barber PA, Chalk J, Darby DG, Rose S, Desmond PM, Gerraty RP, Tress BM, Wright PM, Donnan GA, Davis SM. Diffusion- and perfusionweighted MRI response to thrombolysis in stroke. Ann Neurol. 2002;51:28–37. doi: 10.1002/ana.10067. [DOI] [PubMed] [Google Scholar]
- 14.Francoeur CL, Mayer SA. Management of delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care. 2016;20(277) doi: 10.1186/s13054-016-1447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- 16.Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil: From angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov. 2006;5:689–702. doi: 10.1038/nrd2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee KK, Singh SK, Khosla VK, Mohindra S, Salunke P. Safety and efficacy of sildenafil citrate in reversal of cerebral vasospasm: A feasibility study. Surg Neurol Int. 2012;3(3) doi: 10.4103/2152-7806.92164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem. 2005;280:12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 19.García-Cardoso J, Vela R, Mahillo E, Mateos-Cáceres PJ, Modrego J, Macaya C, López-Farré AJ. Increased cyclic guanosine monophosphate production and endothelial nitric oxide synthase level in mononuclear cells from sildenafil citrate-treated patients with erectile dysfunction. Int J Impot Res. 2010;22:68–76. doi: 10.1038/ijir.2009.51. [DOI] [PubMed] [Google Scholar]
- 20.Gebska MA, Stevenson BK, Hemnes AR, Bivalacqua TJ, Haile A, Hesketh GG, Murray CI, Zaiman AL, Halushka MK, Krongkaew N, et al. Phosphodiesterase-5A (PDE5A) is localized to the endothelial caveolae and modulates NOS3 activity. Cardiovasc Res. 2011;90:353–363. doi: 10.1093/cvr/cvq410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konstantinopoulos A, Giannitsas K, Athanasopoulos A, Spathas D, Perimenis P. The impact of daily sildenafil on levels of soluble molecular markers of endothelial function in plasma in patients with erectile dysfunction. Expert Opin Pharmacother. 2009;10:155–1560. doi: 10.1517/14656560802678211. [DOI] [PubMed] [Google Scholar]
- 22.Abbott D, Comby P, Charuel C, Graepel P, Hanton G, Leblanc B, Lodola A, Longeart L, Paulus G, Peters C, Stadler J. Preclinical safety profile of sildenafil. Int J Impot Res. 2004;16:498–504. doi: 10.1038/sj.ijir.3901232. [DOI] [PubMed] [Google Scholar]
- 23.Conti CR, Pepine CJ, Sweeney M. Efficacy and safety of sildenafil citrate in the treatment of erectile dysfunction in patients with ischemic heart disease. Am J Cardiol. 1999;83:29C–34C. doi: 10.1016/s0002-9149(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 24.Fox KM, Thadani U, Ma PT, Nash SD, Keating Z, Czorniak MA, Gillies H, Keltai M. Sildenafil citrate does not reduce exercise tolerance in men with erectile dysfunction and chronic stable angina. Eur Heart J. 2003;24:2206–2212. doi: 10.1016/j.ehj.2003.09.021. CAESAR I (Clinical American and European Studies of Angina and Revascularization) investigators. [DOI] [PubMed] [Google Scholar]
- 25.Meier P, Zierler KL. On the theory of the indicator-dilution method for measurement of blood flow and volume. J Appl Physiol. 1954;6:731–744. doi: 10.1152/jappl.1954.6.12.731. [DOI] [PubMed] [Google Scholar]
- 26.Konstas AA, Goldmakher GV, Lee TY, Lev MH. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 1: Theoretic basis. AJNR Am J Neuroradiol. 2009;30:662–668. doi: 10.3174/ajnr.A1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao GE, Li Q, Jiang XJ, Liu J, Li JL, Zhang LL, Li LL, Zhang J, Xie P. Vasospasm after subarachnoid hemorrhage: A 3D rotational angiography study. Acta Neurochir Suppl. 2011;110:221–225. doi: 10.1007/978-3-7091-0356-2_40. [DOI] [PubMed] [Google Scholar]
- 28.Cremers CH, van der Schaaf IC, Wensink E, Greving JP, Rinkel GJ, Velthuis BK, Vergouwen MD. CT perfusion and delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J Cereb Blood Flow Metab. 2014;34:200–207. doi: 10.1038/jcbfm.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunze E, Pham M, Raslan F, Stetter C, Lee JY, Solymosi L, Ernestus RI, Vince GH, Westermaier T. Value of perfusion CT, transcranial doppler sonography, and neurological examination to detect delayed vasospasm after aneurysmal subarachnoid hemorrhage. Radiol Res Pract. 2012;2012(231206) doi: 10.1155/2012/231206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanelli PC, Ugorec I, Johnson CE, Tan J, Segal AZ, Fink M, Heier LA, Tsiouris AJ, Comunale JP, John M, et al. Using quantitative CT perfusion for evaluation of delayed cerebral ischemia following aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2011;32:2047–2053. doi: 10.3174/ajnr.A2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levati A, Solaini C, Boselli L. Prevention and treatment of vasospasm. J Neurosurg Sci Mar. 1998;42 (1 Suppl 1):S27–S31. [PubMed] [Google Scholar]
- 32.Hansen D, Hannemann L, Specht M, Schaffartzik W. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Therapeutic value of treatment with calcium antagonists, hypervolemic hemodilution and induced arterial hypertension. Anaesthesist. 1995;44:219–229. doi: 10.1007/s001010050148. (In German) [DOI] [PubMed] [Google Scholar]
- 33.Grotenhuis JA, Bettag W. Prevention of symptomatic vasospasm after SAH by constant venous infusion of nimodipine. Neurol Res. 1986;8:243–249. doi: 10.1080/01616412.1986.11739762. [DOI] [PubMed] [Google Scholar]
- 34.Hänggi D, Beseoglu K, Turowski B, Steiger HJ. Feasibility and safety of intrathecal nimodipine on posthaemorrhagic cerebral vasospasm refractory to medical and endovascular therapy. Clin Neurol Neurosurg. 2008;110:784–790. doi: 10.1016/j.clineuro.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Geraghty JR, Testai FD. Delayed cerebral ischemia after subarachnoid hemorrhage: Beyond vasospasm and towards a multifactorial pathophysiology. Curr Atheroscler Rep. 2017;19(50) doi: 10.1007/s11883-017-0690-x. [DOI] [PubMed] [Google Scholar]
- .Maruhashi T, Higashi Y. An overview of pharmacotherapy for cerebral vasospasm and delayed cerebral ischemia after subarachnoid hemorrhage. Expert Opin Pharmacother. 2021;22:1601–1614. doi: 10.1080/14656566.2021.1912013. [DOI] [PubMed] [Google Scholar]
- 36.Ba Y, Zhang C, Huang J, Hua X, Cui T, Zhao S, Gao G. Microsurgical clipping vs. arterial embolization in the treatment of ruptured anterior circulation aneurysms. Am J Transl Res. 2021;13:8040–8048. [PMC free article] [PubMed] [Google Scholar]
- 37.Mielke D, Döring K, Behme D, Psychogios MN, Rohde V, Malinova V. The impact of endovascular rescue therapy on the clinical and radiological outcome after aneurysmal subarachnoid hemorrhage: A safe and effective treatment option for hemodynamically relevant vasospasm? Front Neurol. 2022;13(838456) doi: 10.3389/fneur.2022.838456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atalay B, Caner H, Cekinmez M, Ozen O, Celasun B, Altinors N. Systemic administration of phosphodiesterase V inhibitor, sildenafil citrate, for attenuation of cerebral vasospasm after experimental subarachnoid hemorrhage. Neurosurgery. 2006;59:1102–1107. doi: 10.1227/01.NEU.0000245605.22817.44. [DOI] [PubMed] [Google Scholar]
- 39.Adiga A, Edriss H, Nugent K. Intracranial aneurysm and sildenafil. Proc (Bayl Univ Med Cent) 2016;29:178–1780. doi: 10.1080/08998280.2016.11929407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gokce C, Gulsen S, Yilmaz C, Guven G, Caner H, Altinors N. The effect of the sildenafil citrate on cerebral vasospasm and apoptosis following experimental subarachnoid hemorrhage in rats. J Neurosurg Sci. 2010;54:29–37. [PubMed] [Google Scholar]
- 41.Edwards DH, Byrne JV, Griffith TM. The effect of chronic subarachnoid hemorrhage on basal endothelium-derived relaxing factor activity in intrathecal cerebral arteries. J Neurosurg. 1992;76:830–837. doi: 10.3171/jns.1992.76.5.0830. [DOI] [PubMed] [Google Scholar]
- 42.Inoha S, Inamura T, Ikezaki K, Nakamizo A, Amano T, Fukui M. Type V phosphodiesterase expression in cerebral arteries with vasospasm after subarachnoid hemorrhage in a canine model. Neurol Res. 2002;24:607–612. doi: 10.1179/016164102101200447. [DOI] [PubMed] [Google Scholar]
- 43.Kasuya H, Weir BK, Nakane M, Pollock JS, Johns L, Marton LS, Stefansson K. Nitric oxide synthase and guanylate cyclase levels in canine basilar artery after subarachnoid hemorrhage. J Neurosurg. 1995;82:250–255. doi: 10.3171/jns.1995.82.2.0250. [DOI] [PubMed] [Google Scholar]
- 44.Kim P, Schini VB, Sundt TM Jr, Vanhoutte PM. Reduced production of cGMP underlies the loss of endothelium-dependent relaxations in the canine basilar artery after subarachnoid hemorrhage. Circ Res. 1992;70:248–256. doi: 10.1161/01.res.70.2.248. [DOI] [PubMed] [Google Scholar]
- 45.Thomas JE, Rosenwasser RH. Reversal of severe cerebral vasospasm in three patients after aneurysmal subarachnoid hemorrhage: Initial observations regarding the use of intraventricular sodium nitroprusside in humans. Neurosurgery. 1999;44:48–58. doi: 10.1097/00006123-199901000-00026. [DOI] [PubMed] [Google Scholar]
- 46.Vellimana AK, Milner E, Azad TD, Harries MD, Zhou ML, Gidday JM, Han BH, Zipfel GJ. Endothelial nitric oxide synthase mediates endogenous protection against subarachnoid hemorrhage-induced cerebral vasospasm. Stroke. 2011;42:776–782. doi: 10.1161/STROKEAHA.110.607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han BH, Vellimana AK, Zhou ML, Milner E, Zipfel GJ. Phosphodiesterase 5 inhibition attenuates cerebral vasospasm and improves functional recovery after experimental subarachnoid hemorrhage. Neurosurgery. 2012;70:178–187. doi: 10.1227/NEU.0b013e31822ec2b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahara M, Sata M, Morita T, Nakajima T, Hirata Y, Nagai R. A phosphodiesterase-5 inhibitor vardenafil enhances angiogenesis through a protein kinase G-dependent hypoxia-inducible factor-1/vascular endothelial growth factor pathway. Arterioscler Thromb Vasc Biol. 2010;30:1315–1324. doi: 10.1161/ATVBAHA.109.201327. [DOI] [PubMed] [Google Scholar]
- 49.Zhang L, Zhang RL, Wang Y, Zhang C, Zhang ZG, Meng H, Chopp M. Functional recovery in aged and young rats after embolic stroke: Treatment with a phosphodiesterase type 5 inhibitor. Stroke. 2005;36:847–852. doi: 10.1161/01.STR.0000158923.19956.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


