Abstract
Introduction:
Older adults with breast cancer receiving neo/adjuvant chemotherapy are at high risk for poor outcomes and are underrepresented in clinical trials. The ADVANCE (ADjuVANt Chemotherapy in the Elderly) trial evaluated the feasibility of two neo/adjuvant chemotherapy regimens in parallel-enrolling cohorts of older patients with human epidermal growth factor receptor 2-negative breast cancer: cohort 1—triple-negative; cohort 2—hormone receptor-positive.
Materials and Methods:
Adults age ≥70 years with stage I-III breast cancer warranting neo/adjuvant chemotherapy were enrolled. Cohort 1 received weekly carboplatin (area under the curve 2) and weekly paclitaxel 80 mg/m2 for twelve weeks; cohort 2 received weekly paclitaxel 80 mg/m2 plus every-three-weekly cyclophosphamide 600 mg/m2 over twelve weeks. The primary study endpoint was feasibility, defined as ≥80% of patients receiving ≥80% of intended weeks/doses of therapy. All dose modifications were applied per clinician discretion.
Results:
Forty women (n=20 per cohort) were enrolled from March 25, 2019 through August 3, 2020 from three centers; 45% and 35% of patients in cohorts 1 and 2 were age >75, respectively. Neither cohort achieved targeted thresholds for feasibility. In cohort 1, eight (40.0%) met feasibility (95% confidence interval [CI]=19.1–63.9%), while ten (50.0%) met feasibility in cohort 2 (95% CI=27.2–72.8). Neutropenia was the most common grade 3–4 toxicity (cohort 1—65%, cohort 2—55%). In cohort 1, 80% and 85% required ≥1 dose holds of carboplatin and/or paclitaxel, respectively. In cohort 2, 10% required dose hold(s) for cyclophosphamide and/or 65% for paclitaxel.
Discussion:
In this pragmatic pilot examining chemotherapy regimens in older adults with breast cancer, neither regimen met target goals for feasibility. Developing efficacious and tolerable regimens for older patients with breast cancer who need chemotherapy remains an important goal.
Keywords: Chemotherapy, older adults, breast cancer, carboplatin, paclitaxel, cyclophosphamide
INTRODUCTION
The incidence of breast cancer among older women is rising,1 and one-third of all breast cancer diagnoses occur in those aged ≥70 years.2 Although most older patients with breast cancer present with favorable hormone receptor-positive (HR+) tumor subtypes3 and early stages of disease,4,5 the breast cancer-specific outcomes for older patients are often less favorable than those in younger women, despite significant competing causes of death.4–7 For women aged ≥70 years in particular, survival improvements over time have occurred at a slower pace compared with younger women,8 and older patients experience lower breast cancer-specific survival regardless of breast cancer stage and subtype, including those with both HR+ and triple-negative (TN) disease.9 Many factors lead to worse outcomes in older patients, including comorbid illness, undertreatment, treatment-related toxicity, early discontinuation of hormonal therapy, and variable disease biology.
Approximately 80% of breast cancers in those aged ≥70 years are HR+, while a substantial minority are TN (approximately 10% or ≅7,000 cases/year).3,9 Although adjuvant hormonal therapy is sufficient systemic treatment in the setting of lower-risk HR+ disease, a considerable proportion of older patients with higher-risk HR+ or TN breast cancer may benefit from chemotherapy. However, when toxicity or comorbidity concerns are paramount, older patients are in jeopardy of undertreatment and/or receipt of non-guideline concordant care10–12 because they lack clear alternatives to standard chemotherapy. In the setting of TN disease in particular, older patients do not have non-chemotherapy systemic therapy options and often do not receive treatment.13,14
The most frequently prescribed and often preferred adjuvant chemotherapy regimen in older patients with human epidermal growth factor receptor 2 (HER2)-negative breast cancer is docetaxel-cyclophosphamide (TC).15–17 However, nearly 50% of those aged ≥65 develop grade ≥ 3 adverse events during their chemotherapy course,15,18 with hospitalizations11,16 and functional decline experienced by many.15,18 Despite trials of alternative therapeutic approaches for older patients,19,20 no regimen has demonstrated sufficient efficacy and tolerability to replace standard combination chemotherapy, and most patients eventually complete TC, even when toxicity is observed.15
This pilot study, designed for older adults with breast cancer, prospectively evaluated the feasibility of two potential regimens for neo/adjuvant chemotherapy. The goal was to provide preliminary data to support the design of a larger clinical trial for older patients at higher risk for poor outcomes and who are underrepresented in clinical trials.
MATERIALS AND METHODS
Study Overview (Figure 1)
Figure 1.
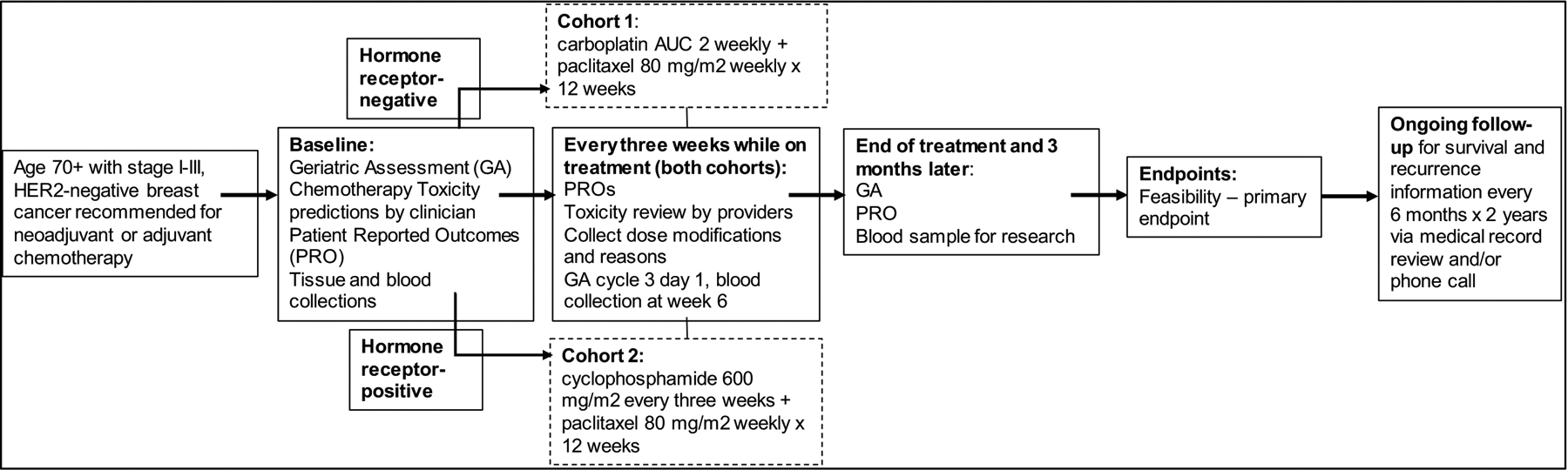
Study Schema
HER-2, human epidermal growth factor receptor 2; AUC, area under the curve
In the ADVANCE (ADjuVANt Chemotherapy in the Elderly) trial, we evaluated the feasibility of administering chemotherapy in two simultaneously-enrolling cohorts of 20 patients for those age ≥70 years who were recommended by their clinicians to receive neoadjuvant or adjuvant chemotherapy for HER2-negative breast cancer. In cohort 1 (TN cohort), planned treatment included weekly carboplatin-paclitaxel for twelve weeks. In cohort 2 (HR+), planned treatment included every-three-week cyclophosphamide plus weekly paclitaxel for twelve weeks, adapted from traditional TC,21 with weekly paclitaxel in place of every-three-week docetaxel.22
Patient Eligibility
Study eligibility included consenting patients aged ≥70 years, with histologically confirmed, non-metastatic, HER2-negative invasive breast cancer.23 This study was approved by the institutional review boards of each participating site prior to enrollment of participants (DFCI protocol #19–031). All patients provided written informed consent and the study was carried out in accordance with the Declaration of Helsinki.
Tumors with immunohistochemistry staining of <10% for estrogen receptor (ER) and progesterone receptor (PR), per local pathology review, were considered TN and these patients were assigned to cohort 1; tumors with ≥10% staining for ER and/or PR were considered HR+ and patients were assigned to cohort 2. Patients were advised to receive neoadjuvant or adjuvant chemotherapy by their treating oncologist. Any additional cancer treatment including breast and nodal surgery, radiation, or hormonal therapy was at the discretion of the treating provider but could not be administered concurrently with study therapy. Patients could receive additional chemotherapy (e.g., anthracycline or post-neoadjuvant capecitabine) if delivered after any study-related chemotherapy. There were no specific requirements for life expectancy, performance status, or laboratory criteria.
Key study exclusions included prior chemotherapy received for the current cancer. Patients initially receiving preoperative hormonal therapy who were then recommended for adjuvant chemotherapy remained eligible, and hormonal therapy was held during study treatment. Prior breast cancer diagnoses were allowed, provided that the treating clinician deemed the current cancer to represent a new primary breast cancer and not recurrent disease. Prior chemotherapy was allowed in the setting of other/prior cancers, with exception of prior carboplatin (cohort 1), cyclophosphamide (cohort 2), or paclitaxel (both cohorts) receipt in the last two years.
Selection of Regimens
Carboplatin-paclitaxel (cohort 1)
In breast cancer, neo/adjuvant carboplatin and paclitaxel have been widely examined in clinical trials when combined sequentially with other agents, such as anthracycline- and alkylating-based therapies in GeparSixto,24 Cancer and Leukemia Group B (CALGB) 40603,25 and GEICAM.2006–003.26 However, there are increasing data supporting the use of platinum and taxanes as primary therapy for those with TN disease, such as a trial administering neoadjuvant carboplatin-docetaxel where pathological complete response rates were high.27 In another study of patients with locally advanced breast cancer of various subtypes, 80% of tumors had some degree of response to taxane plus carboplatin with a relatively low incidence of grade ≥3 toxicity.28 Although no study has directly compared the efficacy and toxicity of every-three-weekly vs. weekly carboplatin in the adjuvant setting, once per week dosing is a well-established method of administration and is ‘titratable’ week-to-week. There are also data using weekly carboplatin-paclitaxel in the setting of metastatic breast and lung cancer, demonstrating activity and tolerability.29–32 Further, while ADVANCE was underway, additional promising efficacy data33 with adjuvant carboplatin-paclitaxel were published, adding further support for its use.
Cyclophosphamide-paclitaxel (cohort 2)
Adjuvant TC is a well-established adjuvant breast cancer regimen21 and is frequently administered to older patients to completion.15,16 To develop a regimen with similar efficacy but perhaps better tolerability, we selected a weekly paclitaxel-based regimen (rather than every-three-week docetaxel) as the therapeutic partner for cyclophosphamide. First, weekly taxane may offer better therapeutic efficacy than every-three-weekly taxane.22,34,35 Second, though paclitaxel and docetaxel have similar efficacy, paclitaxel may have more favorable tolerability.22,36,37 However, it is of note that dosing of docetaxel in these cited studies was higher than that used in modern-era TC, and growth factor was not systematically applied. Third, although formal neo/adjuvant evaluations of our ‘modified TC’ have not been conducted, taxane plus cyclophosphamide are standard agents in multiple regimens, with well-established tolerability and efficacy.38 Further, a similar modified TC regimen was evaluated in the metastatic setting in a small study, and we thus planned a regimen of weekly paclitaxel 80 mg/m2 and every-three-week cyclophosphamide 600 mg/m2.39 Other small studies in metastatic disease have also examined alternative taxane-cyclophosphamide regimens demonstrating tolerability and preliminary efficacy.40–42
Treatment Administration
Cohort 1 participants initiated twelve consecutive weeks of commercially supplied, weekly carboplatin dosed at area under the curve (AUC) 2 and paclitaxel 80 mg/m2. Cohort 2 participants initiated weekly paclitaxel 80 mg/m2 plus standard dose cyclophosphamide 600 mg/m2 every three weeks over twelve consecutive weeks. Cycles in both cohorts were three weeks, with a total of four cycles planned. Treatments were administered intravenously per institutional guidelines without planned breaks, unless clinically indicated. There were no mandated laboratory requirements or growth factor use; this was left to clinician discretion.
During treatment, protocol guidance was provided on when to consider dose modifications/holds for the offending agent for grade ≥3 toxicity (other than alopecia) or a clinically significant grade 1–2 toxicity. However, clinicians could proceed with treatment as they felt appropriate, including any treatment modifications/omissions, with make-up doses allowed to complete up to four full cycles of treatment. In the absence of treatment delays due to adverse event(s) or unacceptable toxicity, treatments continued until completion of protocol-defined therapy, disease progression, intercurrent illness that prevented further treatment, or participant preference.
Study visits occurred on day 1 of each cycle; toxicities were graded using the revised National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. During treatment, we collected information on treatment delays, omissions, and modifications. After therapy completion, additional study visits at three months and one year allowed for collection of information on longer-term toxicity, recurrence, and vital status; medical record review was allowed for up to two years. To inform future correlative analyses, archived tissue was collected at baseline and sequential research blood samples for correlative endpoints were collected for all enrolled patients (data forthcoming).
Survey Instruments
Additional patient assessments included a geriatric assessment (GA)43–46 and frailty (Rockwood Index)47 at baseline, cycle three day one, end-of-treatment, three months post-treatment [+/− one month], and twelve months post-treatment [+/− one month]). We also collected patient-reported outcomes (PROs) using the NCI PRO-CTCAE grading system48 at baseline, day one of each cycle, end-of-treatment, and three months post-treatment [+/− one month].
At baseline, clinicians were surveyed via email about estimations for the likelihood of severe toxicity for that patient, the type of toxicities anticipated, and if having GA information was useful. All patient and clinician surveys are provided in the supplemental file.
Statistical Analysis
The primary study endpoint was feasibility for each cohort, defined as ≥80% of patients receiving ≥80% of intended therapy, in order to align with historical studies which suggest an impact on outcome if <85% relative dose intensity is administered.49 To meet study feasibility, participants in each cohort had to receive ≥ ten weeks of scheduled therapy including up to two dose modification ‘events.’ This was defined as having up to two holds/reductions in total (i.e., one dose change for one agent or two dose changes/delays for one agent). Any patient who received one dose of study treatment was considered evaluable, and any patient who registered but never started therapy was replaced, for a goal of 40 evaluable patients. Feasibility would be achieved if the upper bound of a two-sided 90% confidence interval (CI) using the exact binomial methods covered an 80% response rate, which was consistent with observing 29 patients meeting feasibility requirements among the 40 patients enrolled.
Secondary endpoints include the proportion of patients with NCI CTCAE version 5.0. toxicities, dose modifications, treatment omissions, or early discontinuation of the therapy within each cohort. We also present the toxicity predictions from clinician surveys at baseline and how these aligned with CTCAE-graded toxicities. Finally, we examined whether clinicians found the baseline GA information useful. Given small sample sizes, all secondary outcomes are descriptive and exploratory. Longer-term clinical endpoints and biological correlatives will be reported separately.
RESULTS
Forty women enrolled between March 25, 2019 and August 3, 2020 at three centers (Dana-Farber Cancer Institute, Boston, MA, and its satellite practices in Milford and Weymouth [both in MA]; City of Hope Duarte, CA; and Novant Health Cancer Institute, Kernersville, NC). The median age of study participants was 75 years (range 69–83) in cohort 1 and 74 years (range 69–86) in cohort 2 (one woman in each cohort was allowed to enroll within six months of her 70th birthday).
Table 147 displays participant characteristics by study cohort; 15% were non-White, 60–70% were born in the US. In cohort 1, 35%, 45%, and 15% had stage I, II, III disease, respectively. In cohort 2, 25%, 35%, and 35% of participants had stage I, II, and III disease, respectively; 60% of participants in both cohorts received treatment in the adjuvant (as opposed to neoadjuvant) setting. At baseline, only 20% in cohort 1 and 5% in cohort 2 met criteria for frailty (Table 2).47,50–56 Despite their ages, most patients had a high degree of physical function and independence as self-reported in measures of daily living and social support, with infrequent falls in the last six months.
Table 1.
Participant Characteristics by Cohort
| Characteristic | Cohort 1 (triple-negative; n=20) (n, %) | Cohort 2 (hormone receptor- positive; n=20) (n, %) |
|---|---|---|
| Age (years) | ||
| 69–75 | 11(55) | 13(65) |
| 76–80 | 7(35) | 6(30) |
| ≥81 | 2(10) | 1(5) |
| Median age (range) | 74.5(69–83) | 73.5(69–86) |
| Baseline frailty scorea | ||
| Robust (0–0.2) | 8(40) | 18(90) |
| Pre-frail (>0.2–0.35) | 6(30) | 1(5) |
| Frail (>0.35) | 4(20) | 1(5) |
| Not done | 2 (10) | 0 (0) |
| Female | 20(100) | 20(100) |
| Non-White race | 3(15) | 3(15) |
| Hispanic ethnicity | 2(10) | 1(5) |
| Born in U.Sb | 14(70) | 12(60) |
| Highest educational | ||
| attainment | 5(25) | 3(15) |
| High school graduate or less | 6(30) | 2(10) |
| Some college or tech school | 7(35) | 8(40) |
| College or higher | 2(10) | 7(35) |
| Not answered | ||
| Income | ||
| Less than $20,000 | 1(5) | 1(5) |
| $20,000 to $40,000 | 4(20) | 1(5) |
| $40,001 to $60,000 | 2(10) | 3(15) |
| $60,001 to $80,000 | 1(5) | 5(25) |
| $80,001 or more | 5(25) | 2(10) |
| I prefer not to answer | 4(20) | 1(5) |
| Missing income / did not answer | 3 (15) | 7 (35) |
| Married/with domestic partner | 6(30) | 6(30) |
| Household composition | ||
| Lives alone | 4(20) | 5(25) |
| Lives with someone | 14(70) | 8(40) |
| Not answered | 2(10) | 7(35) |
| With whom do you live? | ||
| Alone | 4(20) | 5(25) |
| Spouse/partner +/− children | 6(30) | 5(25) |
| A child/children | 6(30) | 1(5) |
| A friend | 1(5) | -- |
| Other relative | 1(5) | 2(10) |
| Not answered | 2(10) | 7(35) |
| Location of residence | ||
| Urban | 2(10) | 3(15) |
| Suburban | 14(70) | 6(30) |
| Rural | 2(10) | 4(20) |
| Not answered | 2 (10) | 7(35) |
| Employment | ||
| Employed | 3(15) | 3(15) |
| Retired/not working | 15(75) | 10(50) |
| Not answered | 2(10) | 7(35) |
| Stage of diseasec | ||
| I | 7(35) | 5(25) |
| II | 9(45) | 7(35) |
| III | 3(15) | 7(35) |
| Not classified | 1(5) | 1(5) |
| Treatment setting | ||
| Neoadjuvant | 8(40) | 8(40) |
| Adjuvant | 12(60) | 12(60) |
Rockwood Index47
Countries of birth outside the US included Ireland, Mexico, Pakistan, and Portugal. The first language reported across both cohorts was English for all women except for 1 Portuguese, 1 Urdu, and 1 Spanish.
Stage is presented as clinical stage if a patient received neoadjuvant therapy and pathological if a patient received adjuvant therapy
Table 2.
Geriatric Assessment Findings at Baseline by Cohort
| Baseline Geriatric Assessment Domains | Cohort 1 (triple-negative; n=20) (n, %) | Cohort 2 (hormone receptor- positive; n=20) (n, %) | |||
|---|---|---|---|---|---|
| Description | Mean (SD) | Median (range) | Mean (SD) | Median (range) | |
| Functional status | |||||
| ADLs (Medical Outcomes study [MOS]) subscale50 | Measures limitations in physical function | 68.8(29) | 75.0(5.0–100) | 77.7(21.8) | 85.0(35–100) |
| Instrumental Activities of Daily Living (IADLs) using Older American Resources and Services (OARS) subscale51 | Measures ability to complete activities to maintain independence | 12.8(1.7) | 14.0(9.0–14.0) | 13.8(0.4) | 14.0(13–14) |
| Falls | Number with at least 1 fall in last 6 months (%) | -- | 3(15%) | -- | 3(15%) |
| Karnofsky performance status (by study staff)52 | Global scale for patient function (0–100) | 92.9(6.9) | 90(80–100) | 93.9(8.5) | 100(70–100) |
| Timed up and go53 (seconds) (by study staff) | Time it takes for individual to stand up, walk 10 feet, return to chair, and sit back down (>12 seconds is at risk for falls) | 11.8(4.1) | 11.0(4–22) | 11.4(3.6) | 11.0(6–19.8) |
| Comorbidity OARS physical health scale51 | Assesses the presence of 13 comorbid conditions and effect of illness on daily activities | 3.2(1.7) | 3.0(0–6) | 15(1.3) | 1.0(0–4) |
| Psychological state Mental health inventory54 | Evaluates level of depression and anxiety in the last month | 80.1(16.4) | 82.4(40.0-100) | 78.6(12.5) | 82.2(56.9–97.3) |
| Social activity 54,55 | 62.3(17.2) | 66.7(25.0-83.3) | 57.7(20.3) | 58.3(16.7–83.3) | |
| MOS social support (Overall) | Overall score | 90.4(12.0) | 97.9(68.8-100) | 87.3(15.3) | 91.7(52.1–100) |
| MOS social support (tangible subscale) | Evaluates the self-reported availability of tangible/physical support | 87.9(16.0) | 93.8(56.3-100) | 88.9(16.6) | 100(43.8–100) |
| MOS social support (emotional/informational subscale) | Evaluates the self-reported availability of emotional/informational support | 91.7(12) | 100(68.8–100) | 86.5(18.1) | 90.6(37.5–100) |
| Nutrition | |||||
| Body mass index | Weight (kg) / height (m2) | 28.5(5.3) | 27.9(20.4–41.7) | 30.9(6.1) | 30.5(23.4–47.1) |
| Weight loss | % with ≥5% unintentional weight loss in last 6 months | 2(10%) | 1(5%) | 2(10%) | 1(5%) |
| Cognition (Blessed Orientation Memory Concentration56 (% with <11) | Cognitive assessment, score ≥11 represents impairment | 16(80%) | 3(15%) | 19(95%) | 1(5%) |
ADLs, Activities of Daily Living; IADLs, Instrumental Activities of Daily Living; OARS, Older American Resources and Services
Feasibility (Figure 2 and Supplemental Table S1)
Figure 2.
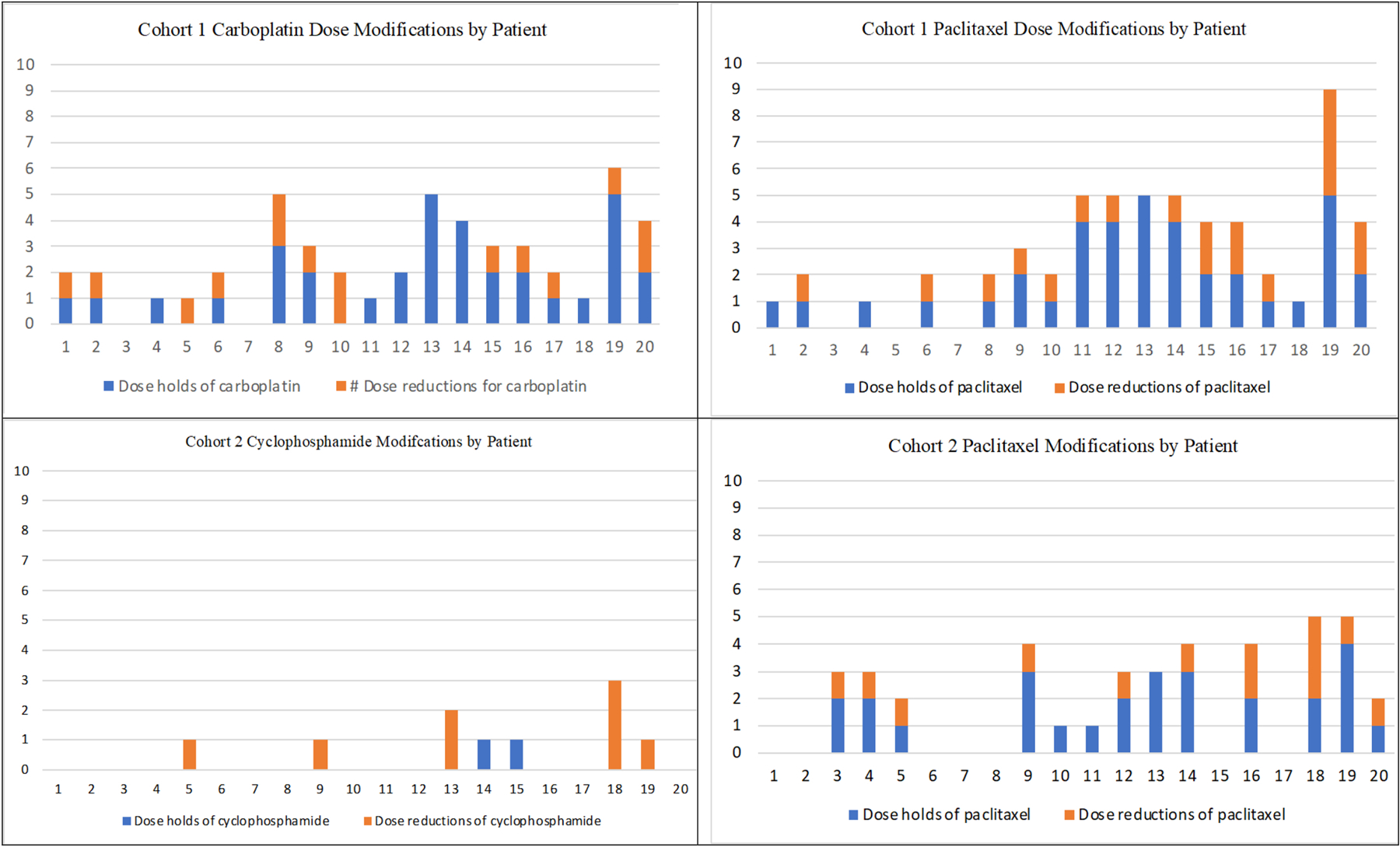
Dose modifications per patient (1–20) in each cohort by agent (A=cohort 1, carboplatin; B=cohort 1, paclitaxel; C=cohort 2, cyclophosphamide; D=cohort 2, paclitaxel). In all graphs, the Y axis represents frequency of dose holds or reductions. The x-axis represents individual patients in each cohort (1–20).
The thresholds for treatment feasibility were not met for participants in cohort 1 or cohort 2. In cohort 1, eight (40.0%) of patients met feasibility endpoints for weekly carboplatin-paclitaxel therapy (95% CI=19.1–63.9%), while ten (50.0%) patients met criteria in cohort 2 (95% CI=27.2–72.8). In cohort 1, sixteen (80%) required ≥1 carboplatin dose hold (range 0–5 dose holds per patient), and seventeen (85%) required ≥1 paclitaxel dose hold (range 0–5 dose holds per patient). This resulted in a total duration of any administered therapy of five to sixteen weeks, with seven (35%) receiving < ten doses of carboplatin and six (30%) patients receiving < ten doses of paclitaxel over that period. In addition, dose reductions were applied in twelve (60%) for carboplatin and thirteen (65%) for paclitaxel. Overall, eleven (55%) met feasibility criteria for carboplatin and ten (50%) for paclitaxel, with eight (40%) patients meeting criteria for both treatments, as above. The reasons for discontinuation of therapy in cohort 1 included therapy complete (n=14, 70%), unacceptable toxicity or clinician discretion due to toxicity (n=4, 20%), and patient withdrawal (n=2, 10%).
In cohort 2, the maximum possible doses of cyclophosphamide were four because it was administered every three weeks. Overall, two (10%) had cyclophosphamide doses held (one hold for each), and dose reductions were applied for five patients (10%; three times in one patient, one to two times in others). For paclitaxel in this cohort, thirteen (65%) had doses held (range 0–4 doses held per patient) and ten (50%) had dose-reductions applied. This resulted in a duration of therapy of one to sixteen weeks, and three (15%) participants did not complete ≥10 weeks of therapy (one participant withdrew after one dose of paclitaxel and cyclophosphamide, and two patients stopped study treatment after four and nine weeks due to toxicity). Overall, sixteen (80%) patients met feasibility for cyclophosphamide, ten (50%) for paclitaxel, and ten for both treatments (50%), as above. The reasons for study treatment discontinuation in cohort 2 included treatment completed (n=16, 80%), patient withdrawal (n=2, 10%), and unacceptable toxicity (n=2, 10%).
Toxicity
Grade 2 and higher toxicities observed and deemed related to any study treatment are shown in Table 3 (cohort 1) and Table 4 (cohort 2). There were no treatment-related deaths or unexpected toxicities observed.
Table 3.
Adverse events related to either chemotherapy in the triple-negative cohort (cohort 1) (n, %)
| Toxicity | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Neutrophil count decreased | 2(10) | 9(45) | 4(20) |
| White blood cell decreased | 2(10) | 3(15) | -- |
| Fatigue | 10(50) | 1(5) | -- |
| Peripheral sensory neuropathy | 7(35) | -- | -- |
| Anemia | 3(15) | 3(15) | -- |
| Alopecia | 5(25) | -- | -- |
| Diarrhea | 2(10) | 2(10) | -- |
| Infusion related reaction | 4(20) | -- | -- |
| Nausea | 4(20) | -- | -- |
| Dysgeusia | 3(15) | -- | -- |
| Hyponatremia | 2(10) | 1(5) | -- |
| Mucositis oral | 3(15) | -- | -- |
| Constipation | 2(10) | -- | -- |
| Creatinine increased | 2(10) | -- | -- |
| Generalized muscle weakness | 1(5) | 1(5) | -- |
| Platelet count decreased | 2(10) | -- | -- |
| Rash acneiform | 1(5) | 1(5) | -- |
| Urinary tract infection | 2(10) | -- | -- |
| Alanine aminotransferase increased | 1(5) | -- | -- |
| Anorexia | 1(5) | -- | -- |
| Arthralgia | 1(5) | -- | -- |
| Aspartate aminotransferase increased | 1(5) | -- | -- |
| Blood bilirubin increased | 1(5) | -- | -- |
| Blurred vision | 1(5) | -- | -- |
| Chills | 1(5) | -- | -- |
| Dehydration | 1(5) | -- | -- |
| Dry mouth | 1(5) | -- | -- |
| Dyspepsia | 1(5) | -- | -- |
| Dyspnea | -- | 1(5) | -- |
| Enterocolitis infectious | -- | 1(5) | -- |
| Fall | 1(5) | -- | -- |
| Febrile neutropenia | -- | 1(5) | -- |
| Gastroesophageal reflux disease | 1(5) | -- | -- |
| Gastrointestinal disorders - Other, specify | 1(5) | -- | -- |
| Insomnia | 1(5) | -- | -- |
| Myalgia | 1(5) | -- | -- |
| Paresthesia | 1(5) | -- | -- |
| Pruritus | 1(5) | -- | -- |
| Rash maculo-papular | 1(5) | -- | -- |
| Skin infection | -- | 1(5) | -- |
| Vaginal dryness | 1(5) | -- | -- |
| Vomiting | 1(5) | -- | -- |
| Weight loss | 1(5) | -- | -- |
Table 4.
Adverse events related to either chemotherapy in the hormone receptor-positive cohort (cohort 2) (n, %)
| Toxicity | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|
| Neutrophil count decreased | -- | 3(15) | 8(40) |
| White blood cell decreased | 2(10) | 4(20) | 2(10) |
| Alopecia | 7(35) | -- | -- |
| Fatigue | 5(25) | 1(5) | -- |
| Anemia | 4(20) | 1(5) | -- |
| Lymphocyte count decreased | 3(15) | 2(10) | -- |
| Diarrhea | 3(15) | -- | -- |
| Febrile neutropenia | -- | 1(5) | 2(10) |
| Peripheral sensory neuropathy | 2(10) | -- | -- |
| Urinary tract infection | 1(5) | 1(5) | -- |
| Anorexia | 1(5) | -- | -- |
| Arthralgia | 1(5) | -- | -- |
| Concentration impairment | 1(5) | -- | -- |
| Constipation | 1(5) | -- | -- |
| Generalized edema | -- | 1(5) | -- |
| Hypoalbuminemia | 1(5) | -- | -- |
| Mucositis oral | 1(5) | -- | -- |
| Nausea | 1(5) | -- | -- |
| Papulopustular rash | 1(5) | -- | -- |
| Pneumonitis | -- | 1(5) | -- |
| Rash maculo-papular | 1(5) | -- | -- |
In cohort 1, the most common grade 2–4 toxicity was neutropenia, with 10%, 45%, and 20% of patients having grade 2, 3, and 4 neutropenia, respectively. One patient had grade 3 febrile neutropenia. No other grade 4 events were observed. Fatigue was also reported as grade 2 in 50% of patients and grade 3 in 5%. Neuropathy was reported as grade 2 only and occurred in 35% of patients.
In cohort 2, neutropenia was also commonly reported and was observed as grade 3 in 15% and grade 4 in 40% of patients. Febrile neutropenia grade 3 and 4 events occurred in 5% and 10% of patients, respectively. Neuropathy occurred as grade 2 only (in 10% of patients), and fatigue was reported as grade 2 in 25% and grade 3 in 5%. One patient (5%) developed grade 3 pneumonitis.
Over the course of treatment, four (20%) and eight (40%) patients in cohorts 1 and 2, respectively, received myeloid growth factor support, administered per clinician discretion. Patients had functional setbacks with treatment in both cohorts; the proportion of patients categorized as frail47 at end of treatment visits was numerically higher in both cohorts (vs. baseline), with 30% (from 20%) of patients in cohort 1 and 15% (from 5%) in cohort 2.
Clinician surveys
At baseline, 30 clinician surveys were submitted that estimated likelihood of grade ≥3 toxicity during chemotherapy for their patient. Responses included the following for likelihood of toxicity: >60% (n=1), 41–60% (n=1), 21–40% (n=8), 10–20% (n=15), or ≤10% (n=5). The most frequently anticipated toxicities (not mutually exclusive; clinicians could select up to three) were neutropenia (n=22), neuropathy (n=17), fatigue (n=17), anemia (n=14), and thrombocytopenia (n=7).
Among the 29 clinicians responding to the surveys on the utility of summary GA, 24 (82.8%) stated they reviewed the summary results provided over email, and sixteen (55.2%) found the information useful; eleven additional clinicians stated they were not sure if the information was helpful and two stated it was not helpful. In just over 10% of surveys, clinicians reported that the information from the GA resulted in “some change(s) in management or referrals they would not have otherwise made, even if something minor.”
DISCUSSION
Older patients with breast cancer need prospective evidence to guide treatment decisions and to improve upon existing chemotherapy-based options, which have a high degree of toxicity and risk for functional decline. In this pilot study evaluating twelve weeks of combination neo/adjuvant carboplatin-paclitaxel or cyclophosphamide-paclitaxel as potential chemotherapeutic options, neither regimen met pre-specified thresholds for feasibility. However, despite a high degree of neutropenia requiring treatment modifications, treatments were otherwise well tolerated, and most (70% cohort 1, 80% cohort 2) eventually completed protocol therapy, in line with other studies administering similar regimens.25,33,57,58 This may be due to the fact that myelosuppression can be an asymptomatic adverse event discovered by laboratory testing alone. Interestingly, on baseline surveys, clinicians in our study underestimated the likelihood of toxicity but aligned well with the types of toxicities observed, further emphasizing the value of including objective measures to predict toxicity in older adults.15 Future analyses from our study will evaluate PROs, their agreement with clinician-reported toxicity, and longitudinal assessments of the GA, which will better inform tolerability and the impact on quality of life from the patient perspective.
Prior studies administering similar regimens also report myelosuppression as the most common adverse event.21,25,33,39,57–60 In our study, the proportion of grade 3–4 neutropenia observed was comparable or lower than observed in some studies (not limited to older patients) with similar agents,33,57,58 but was higher than other studies where mandatory myeloid growth factor and/or different schedules of treatment were utilized.15,25,57 In addition, the frequency of dose reductions was higher in our study than reported previously for similar regimens, though past studies have not typically reported the same degree of detail for dose modifications and dose holds, and there is variability in platinum dosing schedules and taxane agents.25,33,57,59,60 For example, in the carboplatin-paclitaxel arm of Sikov et. al.’s neoadjuvant study25 carboplatin was dosed every three weeks, with 60% of participants receiving 11–12 doses of paclitaxel and 25% receiving 9–10 doses, perhaps a higher dose intensity than seen in our study, though additional information on dose reductions was not reported in detail.
There are several possible reasons why the regimens tested in our study did not prove feasible, some of which are related to study limitations. First, our thresholds for feasibility were strict, limiting the likelihood of reaching our endpoint. Given the lack of standard definitions for feasibility across studies,61,62 our definitions for feasibility were pragmatically selected based on historical data suggesting a threshold of 85% of intended dose intensity to maintain efficacy.49 However, data on optimal chemotherapy dosing for older patients are limited and several studies with carboplatin and taxanes report frequent dose modifications with preservation of benefit.25,33,57–60 Second, because the study design intended to promote a real-world assessment of treatment in academic and community oncology settings, we did not mandate dose reductions or growth factor use (less than 50% of patients received growth factor), and clinicians adjusted treatments according to their comfort, judgment, and practice style. Given the weekly schedule of therapy, patient chemotherapy schedules were busy, and clinicians had regular opportunities to adapt treatment plans and did so readily. As evidence of this, in cohort 2, all but one patient received all four doses of cyclophosphamide, and weekly paclitaxel administration was the limiting agent for feasibility, with five patients receiving < ten doses of paclitaxel. In addition, study enrollment occurred during the peak of the COVID-19 pandemic, and this may have impacted clinician thresholds for treatment modification and cessation.
Other attempts to develop effective, tolerable, and pragmatic alternatives to standard chemotherapy regimens for older adults with HER2-negative breast cancer19,20,63 have also highlighted challenges with tolerability. In future explorations to develop regimens in this context, considerations for up-front dose reductions, mandated use of myeloid growth factor, treatment breaks (e.g., treating three weeks on/one week off), or improved supportive care may promote safety and tolerability while maintaining dose intensity. As an example, the upcoming multi-center DOROTHY trial (Dose Reduction of doceTaxel-based cHemotherapY) in vulnerable older adults with early breast cancer will be evaluating a starting dose reduction of docetaxel (vs. standard dosing) as part of TC, with the goal to maintain dose intensity without sacrificing efficacy. Another future approach would be to further explore single-agent, sequential treatment strategies, known to be as effective as combination regimens but less toxic.38
We recognize additional study limitations. Although the pragmatic nature and multi-center and multi-geographical presence of this trial was a strength (with 15% non-White participants and 30–40% born outside the US), clinician flexibility and the small samples sizes for each cohort limited interpretability. In addition, we did not incorporate immunotherapy into preoperative management for TN disease given the timing of presentation and publication of the KEYNOTE-522 study,64,65 though at least 35% of patients (those with stage I disease) with TN breast cancer on study would not have met the conditions for immunotherapy receipt. Further, given the pilot nature of this study, treatment efficacy data were not included as a study endpoint, though we will explore outcomes at two years of follow-up for all study participants.
In summary, this study directly addressed gaps in knowledge by obtaining pilot, prospective data on the feasibility of two potential neo/adjuvant chemotherapy treatment options for older patients with breast cancer. These patients were anticipated to significantly benefit from neo/adjuvant chemotherapy, with the majority completing protocol therapy; however, this study did not meet feasibility endpoints. There is significant national momentum to increase clinical trial participation for older adults with cancer and advance the evidence base for treatments. We demonstrated proof-of-concept that chemotherapy-based trials for older adults are urgently needed and can enroll to completion through strong multi-center collaborations. Our results underscore the need for deeper insights into how best to treat older patients with breast cancer. Developing efficacious and tolerable regimens remains a priority for this population.
Supplementary Material
Acknowledgements:
We thank all the patients and clinicians who participated in this study. We thank Valerie Hope Goldstein and Kaitlyn Bifolck for their assistance with manuscript preparation and submission (both are full-time employees of Dana-Farber Cancer Institute).
Funding:
This work was supported by an Alliance Cancer Control Program Pilot Award (to RAF), a Susan F. Smith Center for Women’s Cancers Breast & Gynecologic Cancer Innovation Award (to RAF), Bakes for Breast Cancer (to RAF), Susan G. Komen (CCRCR18552788 to RAF), the American Cancer Society (MRSG-14-240-01-CPPB to RAF), the Department of Defense (BCRP W81XWH2010472 to SSM), through support as the Rob and Karen Hale Distinguished Chair in Surgical Oncology (to EAM), the National Cancer Institute (K12CA001727 to MSS), and the National Institute on Aging (R03AG064377 to MSS).
Disclosures:
RAF reports institutional funding from Puma Biotechnology. EAM reports compensated service on scientific advisory boards for AstraZeneca, Exact Sciences, Merck, and Roche/Genentech; uncompensated service on steering committees for Bristol Myers Squibb, Lilly, and Roche/Genentech; and institutional research support from Roche/Genentech (via SU2C grant) and Gilead. MSS reports institutional funding for research from Novartis, Seattle Genetics, Eli Lilly, and Pfizer. ELM reports consulting fees from Lilly, Gilead, AstraZeneca, Novartis. PMG reports receiving consulting fees from Foundation Medicine, Inc. HAP reports institutional funding from Puma Biotechnology. All remaining authors have declared no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifier: NCT03858322
Data Availability Statement:
Data are available from the authors, upon reasonable request.
REFERENCES
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–65. doi: 10.1200/JCO.2008.20.8983 [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Breast Cancer Facts & Figures. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf. Last accessed April 7, 2022. [Google Scholar]
- 3.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055. doi: 10.1093/jnci/dju055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schonberg MA, Marcantonio ER, Li D, Silliman RA, Ngo L, McCarthy EP. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28(12):2038–45. doi: 10.1200/JCO.2009.25.9796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schonberg MA, Marcantonio ER, Ngo L, Li D, Silliman RA, McCarthy EP. Causes of death and relative survival of older women after a breast cancer diagnosis. J Clin Oncol. 2011;29(12):1570–7. doi: 10.1200/JCO.2010.33.0472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120(2):104–10. [DOI] [PubMed] [Google Scholar]
- 7.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–92. doi:joc00907 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Smith BD, Jiang J, McLaughlin SS, Hurria A, Smith GL, Giordano SH, et al. Improvement in breast cancer outcomes over time: are older women missing out? J Clin Oncol. 2011;29(35):4647–53. doi: 10.1200/JCO.2011.35.8408 [DOI] [PubMed] [Google Scholar]
- 9.Freedman RA, Keating NL, Lin NU, Winer EP, Vaz-Luis I, Lii J, et al. Breast cancer-specific survival by age: Worse outcomes for the oldest patients. Cancer. 2018;124(10):2184–91. doi: 10.1002/cncr.31308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griggs JJ, Culakova E, Sorbero ME, Poniewierski MS, Wolff DA, Crawford J, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007;25(18):2522–7. [DOI] [PubMed] [Google Scholar]
- 11.Vaz-Luis I, Keating NL, Lin NU, Lii H, Winer EP, Freedman RA. Duration and toxicity of adjuvant trastuzumab in older patients with early-stage breast cancer: a population-based study. J Clin Oncol. 2014;32(9):927–34. doi: 10.1200/JCO.2013.51.1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman RA, Vaz-Luis I, Barry WT, Lii H, Lin NU, Winer EP, et al. Patterns of chemotherapy, toxicity, and short-term outcomes for older women receiving adjuvant trastuzumab-based therapy. Breast Cancer Res Treat. 2014;145(2):491–501. doi: 10.1007/s10549-014-2968-9 [DOI] [PubMed] [Google Scholar]
- 13.Schreiber AR, Kagihara J, Eguchi M, Kabos P, Fisher CM, Meyer E, et al. Evaluating anthracycline + taxane versus taxane-based chemotherapy in older women with node-negative triple-negative breast cancer: a SEER-Medicare study. Breast Cancer Res Treat. 2022;191(2):389–99. doi: 10.1007/s10549-021-06424-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeder-Hayes KE, Meyer AM, Hinton SP, Meng K, Carey LA, Dusetzina SB. Comparative Toxicity and Effectiveness of Trastuzumab-Based Chemotherapy Regimens in Older Women With Early-Stage Breast Cancer. J Clin Oncol. 2017;35(29):3298–305. doi: 10.1200/JCO.2016.71.4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnuson A, Sedrak MS, Gross CP, Tew WP, Klepin HD, Wildes TM, et al. Development and Validation of a Risk Tool for Predicting Severe Toxicity in Older Adults Receiving Chemotherapy for Early-Stage Breast Cancer. J Clin Oncol. 2021;39(6):608–18. doi: 10.1200/JCO.20.02063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barcenas CH, Niu J, Zhang N, Zhang Y, Buchholz TA, Elting LS, et al. Risk of hospitalization according to chemotherapy regimen in early-stage breast cancer. J Clin Oncol. 2014;32(19):2010–7. doi: 10.1200/JCO.2013.49.3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biganzoli L, Battisti NML, Wildiers H, McCartney A, Colloca G, Kunkler IH, et al. Updated recommendations regarding the management of older patients with breast cancer: a joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol. 2021;22(7):e327–e40. doi: 10.1016/s1470-2045(20)30741-5 [DOI] [PubMed] [Google Scholar]
- 18.Brouwers B, Hatse S, Dal Lago L, Neven P, Vuylsteke P, Dalmasso B, et al. The impact of adjuvant chemotherapy in older breast cancer patients on clinical and biological aging parameters. Oncotarget. 2016;7(21):29977–88. doi: 10.18632/oncotarget.8796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muss HB, Berry DA, Cirrincione CT, Theodoulou M, Mauer AM, Kornblith AB, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055–65. doi: 10.1056/NEJMoa0810266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Minckwitz G, Reimer T, Potenberg J, Conrad B, Schurer U, Eldtmann H, et al. Abstract S3–04. The Phase III ICE study: Adjuvant Ibandronate with or without capecitabine in elderly patients with moderate or high risk early breast cancer. Cancer Res. 2015;75(9_Supplement): S3–04. [Google Scholar]
- 21.Jones S, Holmes FA, O’Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, et al. Docetaxel With Cyclophosphamide Is Associated With an Overall Survival Benefit Compared With Doxorubicin and Cyclophosphamide: 7-Year Follow-Up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27(8):1177–83. doi: 10.1200/JCO.2008.18.4028 [DOI] [PubMed] [Google Scholar]
- 22.Jones SE, Erban J, Overmoyer B, Budd GT, Hutchins L, Lower E, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23(24):5542–51. doi: 10.1200/JCO.2005.02.027 [DOI] [PubMed] [Google Scholar]
- 23.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(20):2105–22. doi: 10.1200/JCO.2018.77.8738 [DOI] [PubMed] [Google Scholar]
- 24.von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747–56. doi: 10.1016/S1470-2045(14)70160-3 [DOI] [PubMed] [Google Scholar]
- 25.Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13–21. doi: 10.1200/jco.2014.57.0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alba E, Chacon JI, Lluch A, Anton A, Estevez L, Cirauqui B, et al. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting. Results from the GEICAM/2006–03, multicenter study. Breast Cancer Res Treat. 2012;136(2):487–93. doi: 10.1007/s10549-012-2100-y [DOI] [PubMed] [Google Scholar]
- 27.Sharma P, Lopez-Tarruella S, Garcia-Saenz JA, Ward C, Connor CS, Gomez HL, et al. Efficacy of Neoadjuvant Carboplatin plus Docetaxel in Triple-Negative Breast Cancer: Combined Analysis of Two Cohorts. Clin Cancer Res. 2017;23(3):649–57. doi: 10.1158/1078-0432.CCR-16-0162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang L, Chen S, Yao L, Liu G, Wu J, Shao Z. Phase II trial of weekly nab-paclitaxel and carboplatin treatment with or without trastuzumab as nonanthracycline neoadjuvant chemotherapy for locally advanced breast cancer. Int J Nanomedicine. 2015;10:1969–75. doi: 10.2147/IJN.S77000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vernieri C, Milano M, Mennitto A, Maggi C, Ferrari B, Rinaldi L, et al. Antitumor activity and safety profile of weekly carboplatin plus paclitaxel in metastatic breast cancer: a ten-year, monocentric, retrospective study. Breast Cancer Res Treat. 2017;165(2):365–73. doi: 10.1007/s10549-017-4336-z [DOI] [PubMed] [Google Scholar]
- 30.Quoix E, Zalcman G, Oster JP, Westeel V, Pichon E, Lavole A, et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet. 2011;378(9796):1079–88. doi: 10.1016/S0140-6736(11)60780-0 [DOI] [PubMed] [Google Scholar]
- 31.Socinski MA, Okamoto I, Hon JK, Hirsh V, Dakhil SR, Page RD, et al. Safety and efficacy analysis by histology of weekly nab-paclitaxel in combination with carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24(9):2390–6. doi: 10.1093/annonc/mdt235 [DOI] [PubMed] [Google Scholar]
- 32.Maemondo M, Inoue A, Sugawara S, Harada T, Minegishi Y, Usui K, et al. Randomized phase II trial comparing carboplatin plus weekly paclitaxel and docetaxel alone in elderly patients with advanced non-small cell lung cancer: north japan lung cancer group trial 0801. Oncologist. 2014;19(4):352–3. doi: 10.1634/theoncologist.2013-0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu KD, Ye FG, He M, Fan L, Ma D, Mo M, et al. Effect of Adjuvant Paclitaxel and Carboplatin on Survival in Women With Triple-Negative Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6(9):1390–6. doi: 10.1001/jamaoncol.2020.2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lichtman SM, Hurria A, Cirrincione CT, Seidman AD, Winer E, Hudis C, et al. Paclitaxel efficacy and toxicity in older women with metastatic breast cancer: combined analysis of CALGB 9342 and 9840. Ann Oncol. 2012;23(3):632–8. doi: 10.1093/annonc/mdr297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26(10):1642–9. doi: 10.1200/JCO.2007.11.6699 [DOI] [PubMed] [Google Scholar]
- 36.Sparano JA, Zhao F, Martino S, Ligibel JA, Perez EA, Saphner T, et al. Long-Term Follow-Up of the E1199 Phase III Trial Evaluating the Role of Taxane and Schedule in Operable Breast Cancer. J Clin Oncol. 2015;33(21):2353–60. doi: 10.1200/jco.2015.60.9271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358(16):1663–71. doi: 10.1056/NEJMoa0707056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21(8):1431–9. doi: 10.1200/JCO.2003.09.081 [DOI] [PubMed] [Google Scholar]
- 39.Masuda N, Nakayama T, Yamamura J, Kamigaki S, Taguchi T, Hatta M, et al. Phase I study of combination therapy with weekly paclitaxel and cyclophosphamide for advanced or recurrent breast cancer. Cancer Chemother Pharmacol. 2010;66(1):89–94. doi: 10.1007/s00280-009-1137-z [DOI] [PubMed] [Google Scholar]
- 40.Kutomi G, Ohmura T, Satomi F, Maeda H, Shima H, Kameshima H, et al. A phase I study of combination therapy with nanoparticle albumin-bound paclitaxel and cyclophosphamide in patients with metastatic or recurrent breast cancer. Int J Clin Oncol. 2015;20(3):474–9. doi: 10.1007/s10147-014-0725-z [DOI] [PubMed] [Google Scholar]
- 41.Pagani O, Sessa C, Martinelli G, Cerny T, de Jong J, Goldhirsch A, et al. Dose-finding study of paclitaxel and cyclophosphamide in advanced breast cancer. Ann Oncol. 1997;8(7):655–61. [DOI] [PubMed] [Google Scholar]
- 42.Cyclophosphamide and Paclitaxel With or Without Trastuzumab in Stage I-II Breast Cancer Who Have Undergone Surgery. https://clinicaltrials.gov/ct2/show/results/NCT01106898?sect=X70156&view=results#outcome1-NCT01106898.
- 43.Hurria A, Cirrincione CT, Muss HB, Kornblith AB, Barry W, Artz AS, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29(10):1290–6. doi: 10.1200/JCO.2010.30.6985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hurria A, Lichtman SM, Gardes J, Li D, Limaye S, Patil S, et al. Identifying vulnerable older adults with cancer: integrating geriatric assessment into oncology practice. J Am Geriatr Soc. 2007;55(10):1604–8. doi: 10.1111/j.1532-5415.2007.01367.x [DOI] [PubMed] [Google Scholar]
- 45.Hurria A, Lachs MS, Cohen HJ, Muss HB, Kornblith AB. Geriatric assessment for oncologists: rationale and future directions. Crit Rev Oncol Hematol. 2006;59(3):211–7. doi: 10.1016/j.critrevonc.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 46.Hurria A, Gupta S, Zauderer M, Zuckerman EL, Cohen HJ, Muss H, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104(9):1998–2005. doi: 10.1002/cncr.21422 [DOI] [PubMed] [Google Scholar]
- 47.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–7. doi: 10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 48.Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). https://healthcaredelivery.cancer.gov/pro-ctcae/ Last accessed March 18, 2022. [DOI] [PMC free article] [PubMed]
- 49.Colleoni M, Price K, Castiglione-Gertsch M, Goldhirsch A, Coates A, Lindtner J, et al. Dose-response effect of adjuvant cyclophosphamide, methotrexate, 5-fluorouracil (CMF) in node-positive breast cancer. International Breast Cancer Study Group. Eur J Cancer. 1998;34(11):1693–700. doi: 10.1016/s0959-8049(98)00209-3 [DOI] [PubMed] [Google Scholar]
- 50.Stewart AL, Hays RD, Wells KB, Rogers WH, Spritzer KL, Greenfield S. Long-term functioning and well-being outcomes associated with physical activity and exercise in patients with chronic conditions in the Medical Outcomes Study. J Clin Epidemiol. 1994;47(7):719–30. doi: 10.1016/0895-4356(94)90169-4 [DOI] [PubMed] [Google Scholar]
- 51.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36(4):428–34. doi: 10.1093/geronj/36.4.428 [DOI] [PubMed] [Google Scholar]
- 52.Loprinzi CL, Laurie JA, Wieand HS, Krook JE, Novotny PJ, Kugler JW, et al. Prospective evaluation of prognostic variables from patient-completed questionnaires. North Central Cancer Treatment Group. J Clin Oncol. 1994;12(3):601–7. doi: 10.1200/JCO.1994.12.3.601 [DOI] [PubMed] [Google Scholar]
- 53.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 54.Stewart AL, & Ware JE Jr. Measuring functioning and well-being: The Medical Outcomes Study approach. Durham, NC: Duke University Press; 1992. [Google Scholar]
- 55.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–14. [DOI] [PubMed] [Google Scholar]
- 56.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140(6):734–9. doi: 10.1176/ajp.140.6.734 [DOI] [PubMed] [Google Scholar]
- 57.Sharma P, Kimler BF, O’Dea A, Nye L, Wang YY, Yoder R, et al. Randomized Phase II Trial of Anthracycline-free and Anthracycline-containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-negative Breast Cancer (NeoSTOP). Clin Cancer Res. 2021;27(4):975–82. doi: 10.1158/1078-0432.CCR-20-3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma P, Lopez-Tarruella S, Garcia-Saenz JA, Khan QJ, Gomez HL, Prat A, et al. Pathological Response and Survival in Triple-Negative Breast Cancer Following Neoadjuvant Carboplatin plus Docetaxel. Clin Cancer Res. 2018;24(23):5820–9. doi: 10.1158/1078-0432.CCR-18-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yardley DA, Coleman R, Conte P, Cortes J, Brufsky A, Shtivelband M, et al. nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: results from the tnAcity trial. Ann Oncol. 2018;29(8):1763–70. doi: 10.1093/annonc/mdy201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan Y, Lee JS, Yost SE, Li SM, Frankel PH, Ruel C, et al. Phase II Trial of Neoadjuvant Carboplatin and Nab-Paclitaxel in Patients with Triple-Negative Breast Cancer. Oncologist. 2021;26(3):e382–e93. doi: 10.1002/onco.13574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laurent M, Paillaud E, Tournigand C, Caillet P, Le Thuaut A, Lagrange JL, et al. Assessment of solid cancer treatment feasibility in older patients: a prospective cohort study. Oncologist. 2014;19(3):275–82. doi: 10.1634/theoncologist.2013-0351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sud S, Lai P, Zhang T, Clemons M, Wheatley-Price P. Chemotherapy in the oldest old: The feasibility of delivering cytotoxic therapy to patients 80 years old and older. J Geriatr Oncol. 2015;6(5):395–400. doi: 10.1016/j.jgo.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 63.Shulman LN, Berry DA, Cirrincione CT, Becker HP, Perez EA, O’Regan R, et al. Comparison of doxorubicin and cyclophosphamide versus single-agent paclitaxel as adjuvant therapy for breast cancer in women with 0 to 3 positive axillary nodes: CALGB 40101 (Alliance). J Clin Oncol. 2014;32(22):2311–7. doi: 10.1200/JCO.2013.53.7142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382(9):810–21. doi: 10.1056/NEJMoa1910549 [DOI] [PubMed] [Google Scholar]
- 65.Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kummel S, et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med. 2022;386(6):556–67. doi: 10.1056/NEJMoa2112651 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the authors, upon reasonable request.


