Abstract
Patients with hematological malignancies are at risk for poor outcomes when diagnosed with Coronavirus Disease 2019 (COVID-19). It remains unclear whether cytopenias and specific leukemia subtypes play a role in the clinical course of COVID-19 infection. Here we report outcomes and their clinical/laboratory predictors for 65 patients with acute and chronic leukemias diagnosed with COVID-19 between March 8, 2020 and May 19, 2020 at Memorial Sloan Kettering Cancer Center in New York City. Most patients had CLL (48%) or AML (26%). A total of 14 (22%) patients required high flow nasal cannula or were intubated for mechanical ventilation and 11 patients (17%) died. A diagnosis of AML (OR 4.7, p=0.028), active treatment within the last 3 months (OR 5.22, p=0.047), neutropenia within seven days prior and up to 28 days after SARS-CoV-2 diagnosis (11.75, p=0.001) and ≥ 3 comorbidities (OR 6.55, p=0.019) were associated with increased odds of death.
Keywords: COVID-19, SARS-CoV-2, leukemia, AML, ALL, CML, CLL, MDS, MPN
Introduction:
Cancer patients appear especially susceptible to a worse Coronavirus Disease 2019 (COVID-19) course, making the pandemic of urgent concern to oncologists and patients with cancer[1]. Multiple studies have identified patients with hematologic cancer as having especially high risk of respiratory complications and death[2–7]. Leukemia patients may be at especially high risk of severe COVID-19 due to the potential for severe immune dysregulation[5–12]. However, although published cohort studies suggest patients with leukemia represent a population broadly vulnerable to COVID-19 infection related adverse outcomes, it is unclear whether some leukemia subtypes have worse outcomes compared to others and whether treatment-related cytopenias impact outcomes. Therefore, we analyzed the outcomes and predictors for adverse outcomes in a cohort of 65 patients with acute and chronic leukemia, who were diagnosed with COVID-19 during the height of the pandemic in New York City.
Methods:
Patients:
This retrospective study included all adult patients followed at Memorial Sloan Kettering Cancer Center (MSKCC) for a diagnosis of acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), chronic lymphocytic leukemia (CLL), myelodysplastic syndromes (MDS) and myeloproliferative neoplasm (MPN) who developed COVID-19 in the inpatient or outpatient setting between March 8, 2020 and May 19, 2020. SARS-CoV-2 status was determined using a nasopharyngeal swab to determine the presence of virus specific RNA (MSKCC FDA EUA-approved assay and Cephied®). Clinical outcomes were monitored until July 9, 2020. All patient data was obtained from electronic medical records (EMR). The institutional review board approved the study and waived the requirement for informed consent.
Data sources:
Patient demographics, laboratory data, and outcomes (i.e. use of noninvasive or invasive mechanical ventilation and death) were automatically curated from the EMR. Diagnosis, disease status, and timing of recent treatment were manually extracted from the EMR by licensed physicians. Relevant comorbidities and hematologic cancer status were extracted from International Classification of Disease (ICD)-9 and ICD-10 codes using a process cross-validated with manual curation. Disease status was defined as ‘receiving active treatment’ if patients were on treatment within 3 months of their COVID-19 diagnosis.
Statistical Analysis:
The following variables were considered as predictors of outcomes: gender, BMI over 30, age greater than 60, history of current or former smoking, disease-type (AML vs other), treatment within the last 3 months of COVID-19 diagnosis, history of allogeneic transplant and presence of three or more pre-defined comorbidities. These comorbidities included history of hypertension, diabetes, hyperlipidemia, chronic hepatic or renal disease, coronary artery disease, asthma, thromboembolism, chronic obstructive lung disease, congestive heart failure, valvular heart disease, stroke, HIV, or peripheral artery disease. Additional laboratory variables of interest were neutropenia (any absolute neutrophil count, ANC < 1000/uL within seven days prior and up to 28 days after SARS-CoV-2 diagnosis) and lymphopenia (any absolute lymphocyte count, ALC < 500/uL within seven days prior and up to 28 days after SARS-CoV-2 diagnosis), creatinine > 1.3 mg/dL, ALT > upper limit of normal (ULN), AST > ULN, total bilirubin > 1.2 mg/dL, Albumin > 3.5 g/dL. The severity of neutropenia and lymphocytopenia were further categorized based on the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0)[13]. Moderate/grade 3 neutropenia and lymphocytopenia were defined as ANC 500–1000/uL and ALC 200–500/uL, respectively[13]. Severe/grade 4 neutropenia and lymphocytopenia were defined as ANC 0–500/uL and ALC 0–200/uL, respectively[13]. The lab value obtained closest to the time of SARS-CoV-2 diagnosis, within a 7-day window, was selected. Outcomes analyzed included (1) death (all causes and at any time point post COVID-19 diagnosis), and (2) combined death or requirement of high flow nasal cannula or intubation (advanced respiratory support). Associations between continuous laboratory measurements and the outcome were tested by the Wilcoxon rank-sum test and associations between the outcome and discrete patient characteristics were tested by Fisher’s exact test. Univariate logistic regression was used to estimate odds ratios (ORs) with 95% confidence intervals (CI) for these risk factors associated with the outcome. In general, the absence of risk factor under study was used as the reference group.
Results:
Patients characteristics:
A total of 65 patients were diagnosed with COVID-19 at MSKCC; 57% were female. Median age at the time of COVID-19 diagnosis was 65 years (range 20–83 years). The majority of patients had either CLL (38%) or AML (26%) with fewer patients carrying a diagnosis of ALL, CML, MDS or MPN (Table 1). While 52% of patients had received treatment within the last 3 months, 48% of patients had not received any treatment during the prior 3 months being diagnosed with COVID-19. Treatment characteristics for all patients, who received active treatment within 3 months prior to COVID-19 diagnosis, are summarized in Supplemental Table 1. Neutropenia at time of COVID-19 diagnosis was noted in 14 patients (22%). Additionally, 16 patients (25%) had received a prior allogeneic stem cell transplant (allo-SCT). Transplant characteristics for all patients, who had received an allo-SCT prior to COVID-19 diagnosis, are summarized in Supplemental Table 2. Of the 16 patients, who received an allo-SCT, 4 had acute graft versus host disease (GVHD) and 3 had chronic GVHD at time of COVID-19 diagnosis.
Table 1:
Patient characteristics and outcomes
| Patient characteristics | N, % | Death | Death/Intubation/High Flow nasal cannula |
|---|---|---|---|
|
Gender Female Male |
28, 43% 37, 57% |
3, 11% 8, 22% |
7, 25% 12, 32% |
|
Age median, range > 60 years old < 60 years old |
65, 20–83 36, 55% 29, 45% |
9, 25% 2, 7% |
15, 42% 4, 14% |
|
Disease
AML ALL CML CLL MDS MPN |
17, 26% 6, 9% 7, 11% 25, 38% 3, 5% 7, 11% |
6, 35% 1, 17% 0, 0% 2, 8% 0, 0% 2, 29% |
8, 47% 1, 17% 1, 14% 6, 24% 1, 33% 2, 29% |
|
Treatment prior to COVID-19 treatment < 3 months ago treatment > 3 months ago/never treated prior allogeneic stem cell transplant |
34, 52% 31, 48% 16, 25% |
9, 26% 2, 6% 4, 25% |
11,32% 8, 26% 7, 44% |
|
Neutropenia: ANC < 1000/uL Lymphocytopenia: ALC < 500/uL |
14, 22% 21, 38% |
7, 50% 5, 24% |
8, 57% 10, 21% |
|
COVID-19 directed therapy
No treatment required Hydroxychloroquine Convalescent plasma Tocilizumab Remdesivir Azithromycin Dexamethasone |
34, 52% 21, 32% 12, 19% 5, 8% 3, 5% 16, 25% 5, 8% |
2, 5% 7, 33% 2, 17% 3, 60% 1, 33% 4, 25% 2, 40% |
3, 9% 13, 62% 5, 41% 4, 80% 2, 67% 8, 50% 3, 60% |
| Administration of G-CSF | 8, 12% | 2, 25% | 3, 38% |
Outcomes:
Clinical trajectories and outcomes of all patients are shown in Figure 1 and Table 1. While 26 patients (40%) received all their care on outpatient basis, 39 patients (60%) received inpatient care. A total of 31 (48%) patients received COVID-19 directed therapies. Most commonly, patients received hydroxychloroquine (32%), azithromycin (25%), convalescent plasma (18%), tocilizumab (8%) and remdesivir (5%). Dexamethasone was administered to 5 patients (8%) and G-CSF was administered to 8 (12%) patients after their diagnosis of COVID-19. A total of 34 (52%) of patients did not receive any COVID-19 directed therapy. A total of 2 (3%) and 12 (18%) patients required high flow nasal cannula and were intubated for mechanical ventilation, respectively; 11 patients (17%) died because of COVID-19 related complications. Median time from diagnosis to the combined endpoint of death or respiratory failure requiring advanced respiratory support was 7 days. Median time to death was 12 days.
Figure 1:
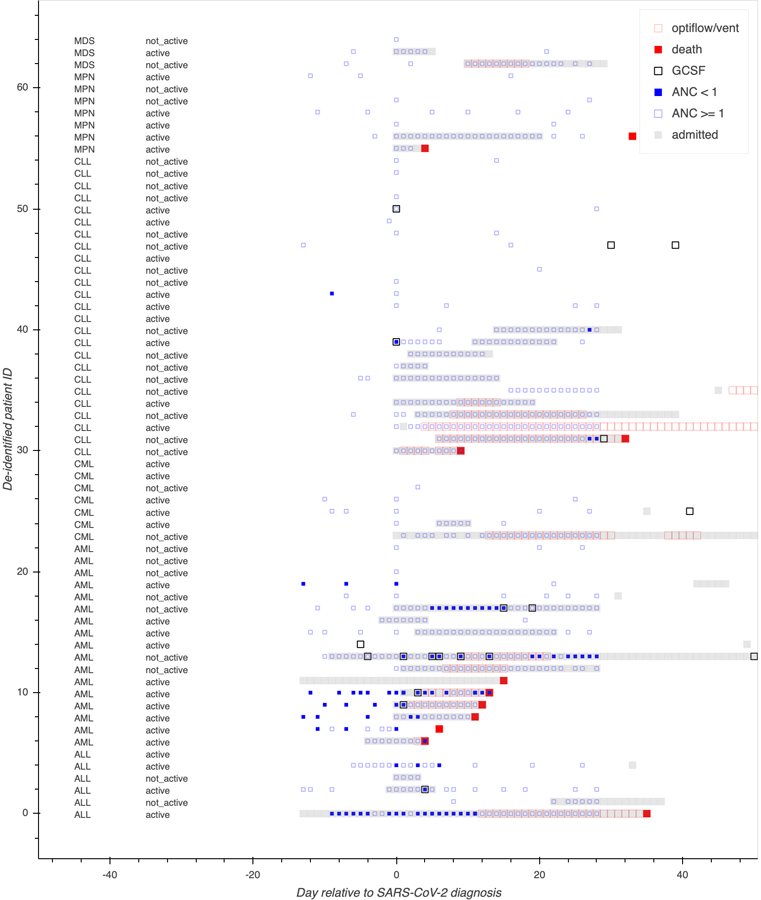
Swimmers plot
Clinical predictors of outcomes:
Clinical predictors of adverse outcomes are shown in Table 2. Age > 60 years was associated with experiencing a combined event of death/advanced respiratory support (OR 4.46, p=0.027) and presence of above defined comorbidities was associated with increased odds of dying because of COVID-19 (OR 6.55, p=0.019, Table 2, Figure 2A). BMI and a history of prior or current smoking did not statistically significantly impact odds of dying because of COVID-19 (Table 2). However, a history of prior or current smoking did significantly increase the odds of experiencing a combined event of death/advanced respiratory support (OR 3.9, p=0.022, Table 2).
Table 2:
Associations with adverse outcomes
| Predictor | Death | Death/Intubation/High Flow nasal cannula | ||
|---|---|---|---|---|
| OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Gender: Male vs. female | 2.3 (0.55,9.61) | 0.33 | 1.44 (0.48,4.32) | 0.59 |
| BMI: > 30 vs < 30 | 2.0 (0.5,8) | 0.44 | 1.66 (0.5,5.49) | 0.53 |
| History of current/former smoking: Yes vs. No | 2.6 (0.7,9.8) | 0.18 | 3.9 (1.27,11.98) | 0.022 |
| Age: >60 vs. < 60 years | 4.5 (0.89,22.79) | 0.09 | 4.46 (1.28,15.52) | 0.027 |
| ≥ 3 comorbidities: Yes vs. No | 6.55 (1.29,33.26) | 0.019 | 3.37 (1.08,10.48) | 0.055 |
| Disease: AML vs. all others | 4.7 (1.2,18.3) | 0.028 | 2.99 (0.93,9.6) | 0.072 |
| Treatment prior to COVID-19: – Last treatment < 3 months vs. > 3 months – Prior allo-SCT vs. no prior allo-SCT |
5.22 (1.03,26.45) 2.0 (0.5,8) |
0.047 0.44 |
1.37 (0.47,4.04) 2.4 (0.73,7.83) |
0.60 0.21 |
| Laboratory values: – Neutropenia: ANC < 1000/uL vs. > 1000/uL – Lymphocytopenia: ALC < 500/uL vs. > 500/uL – Albumin: Alb < 3.5 vs. > 3.5 – Creatinine 1.3: Yes vs. No – ALT > 55: Yes vs. No – AST > 37: Yes vs. No – Total bilirubin > 1.2: Yes vs. No |
11.75 (2.72,50.74) 0.55 (0.14,2.22) 9.0 (1.92,42.14) 0.72 (0.13,3.94) 4.43 (0.88,22.17) 9.0 (1.92,42.14) 4.0 (0.68,23.67) |
0.001* 0.48 0.004 1.0 0.079 0.004 0.13 |
4.85 (1.39,16.94) 0.24 (0.1–1.1) 8.8 (2.2,35.15)) 1.1 (0.27,4.45) 3.61 (0.74,17.64) 5.48 (1.46,20.48) 1.86 (0.33,10.45) |
0.018 0.081 0.003* 1.0 0.13 0.012 0.66 |
remains statistically significant after Bonferroni correction (p < 0.0033)
Figure 2:
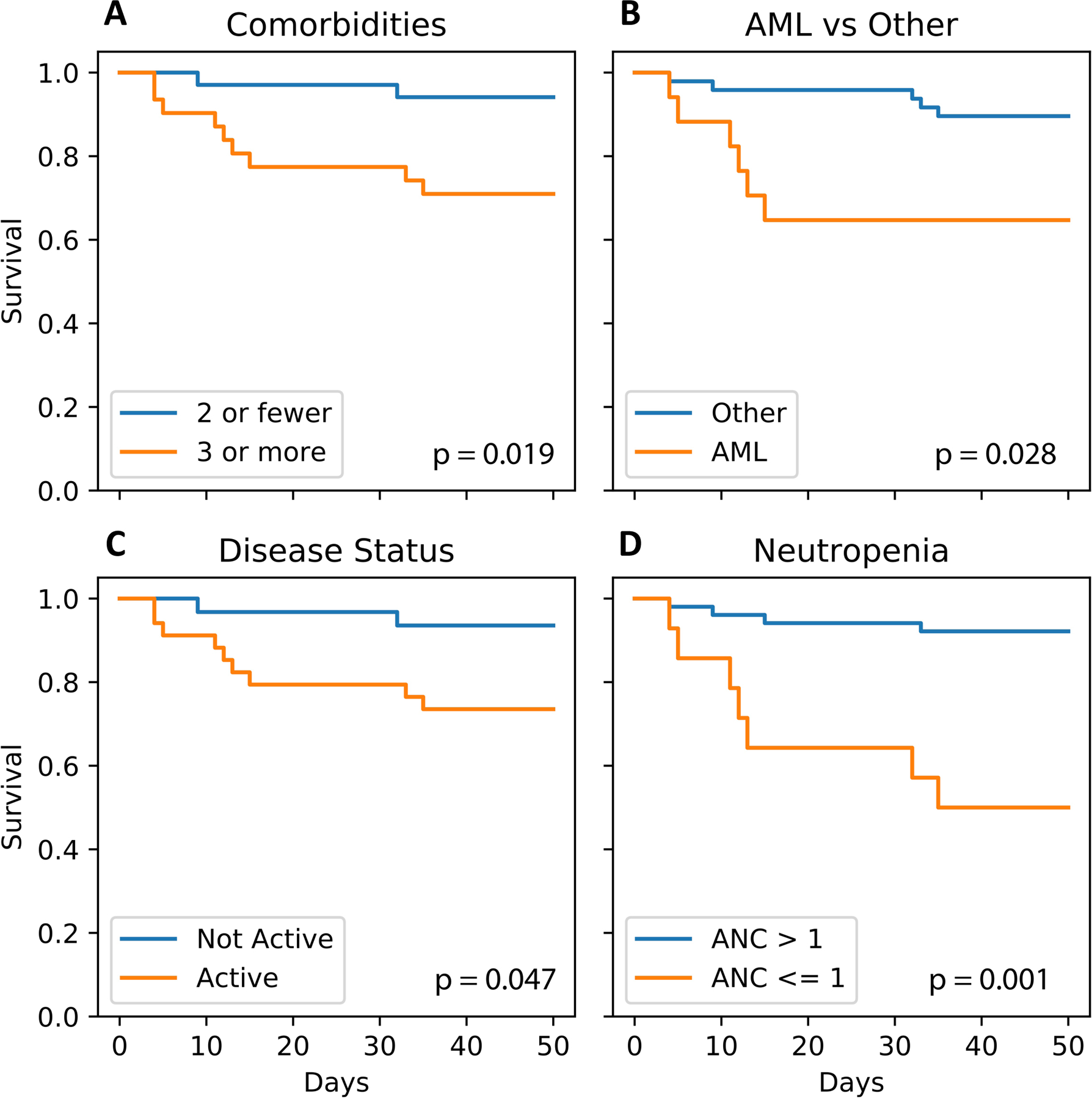
Overall survival
A) by presence of comorbidities vs. not
B) by type of disease (AML vs. all other)
C) by disease treatment status (active vs. inactive)
D) by presence of neutropenia vs. not
Survival after COVID-19 diagnosis differed based on the specific hematological malignancy. AML patients were more likely to die because of COVID-19 compared to non-AML patients (OR 4.7, p=0.028, Table 2, Figure 2B). While 35% of AML patients died from complications related to COVID-19 infection, 17% of ALL, 8% of CLL and 29% of MPN patients died because of COVID-19. None of the 10 patients with MDS (n=3) or CML (n=7) died because of COVID-19. Furthermore, patients with active treatment during the last 3 months prior to COVID-19 diagnosis had higher odds of dying because of COVID-19 (OR 5.22, p=0.047, Table 2, Figure 2C). Of the 4 patients, who had received an allo-SCT prior to COVID-19 diagnosis and died, all had relapsed active AML or ALL and 2 also had chronic GVHD (Supplemental Table 2).
Laboratory predictors of outcomes:
Distribution of lab parameters at time of COVID-19 diagnosis are shown in Figure 3. Compared to patients who survived COVID-19, patients who died of complications of COVID-19 had statistically significantly lower platelets and neutrophil count, lower albumin, higher ferritin, procalcitonin, troponin, AST and total bilirubin. Neutropenia (ANC < 1000/uL) was associated with increased odds of experiencing a combined event of death/advanced respiratory support (OR 4.85, p=0.018) and dying of COVID-19 (OR 11.75, p=0.001, Supplemental Figure 1D and Figure 2D). Lymphocytopenia (ALC < 500/uL) was not statistically significantly associated with experiencing the combined event of death/advanced respiratory support (OR = 0.24; 95%CI 0.1–1.1; p=0.081) or death (OR = 0.55; 95%CI 0.14–2.22; p=0.48). We found that there was no difference between moderate/grade 3 neutropenia/lymphocytopenia and severe/grade 4 neutropenia/lymphocytopenia in the likelihood of death (p= 1.0 for neutropenia; p=1.0 for lymphocytopenia) or the combined event of death/intubation/high flow cannula (p= 1.0 for neutropenia; p=1.0 for lymphocytopenia).
Figure 3:
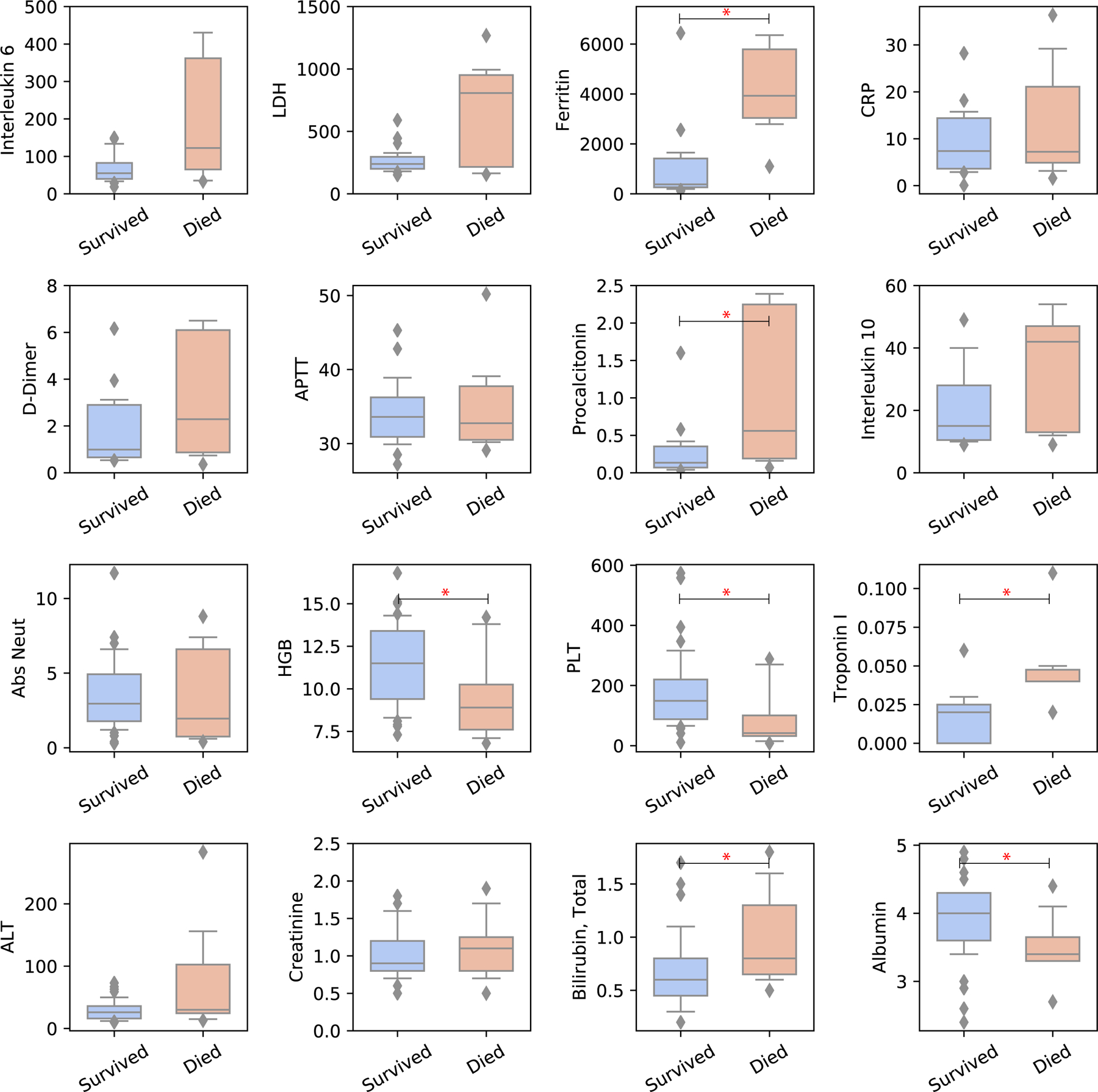
Lab characteristics in patients who died because of COVID-19 vs. patients who survived
* indicates p < 0.05
An albumin level < 3.5 g/dL predicted both higher rates of having a combined event of death/advanced respiratory support (OR 8.8, p=0.003) and death (OR 9.0, p=0.004). Lastly, AST > ULN was associated with increased odds of having a combined event of death/advanced respiratory support (OR 5.48, p=0.012) and death (OR 4.2, p=0.049) (Table 2). After applying a Bonferroni correction for multiple comparisons, only the impact of neutropenia on death and the impact of an albumin level < 3.5 g/dL on having a combined event of death/advanced respiratory support remained statistically significant (Table 2). As patients with AML who were diagnosed with COVID-19 had particularly poor outcomes, we focused our attention on the clinical and laboratory characteristics of these patients (Figure 4). Poor outcomes were driven by high rates of COVID-19 related death in AML patients with neutropenia (5/6 patients, 83%) and active treatment during the last 3 months prior to COVID-19 diagnosis (6/6 patients 100%).
Figure 4:
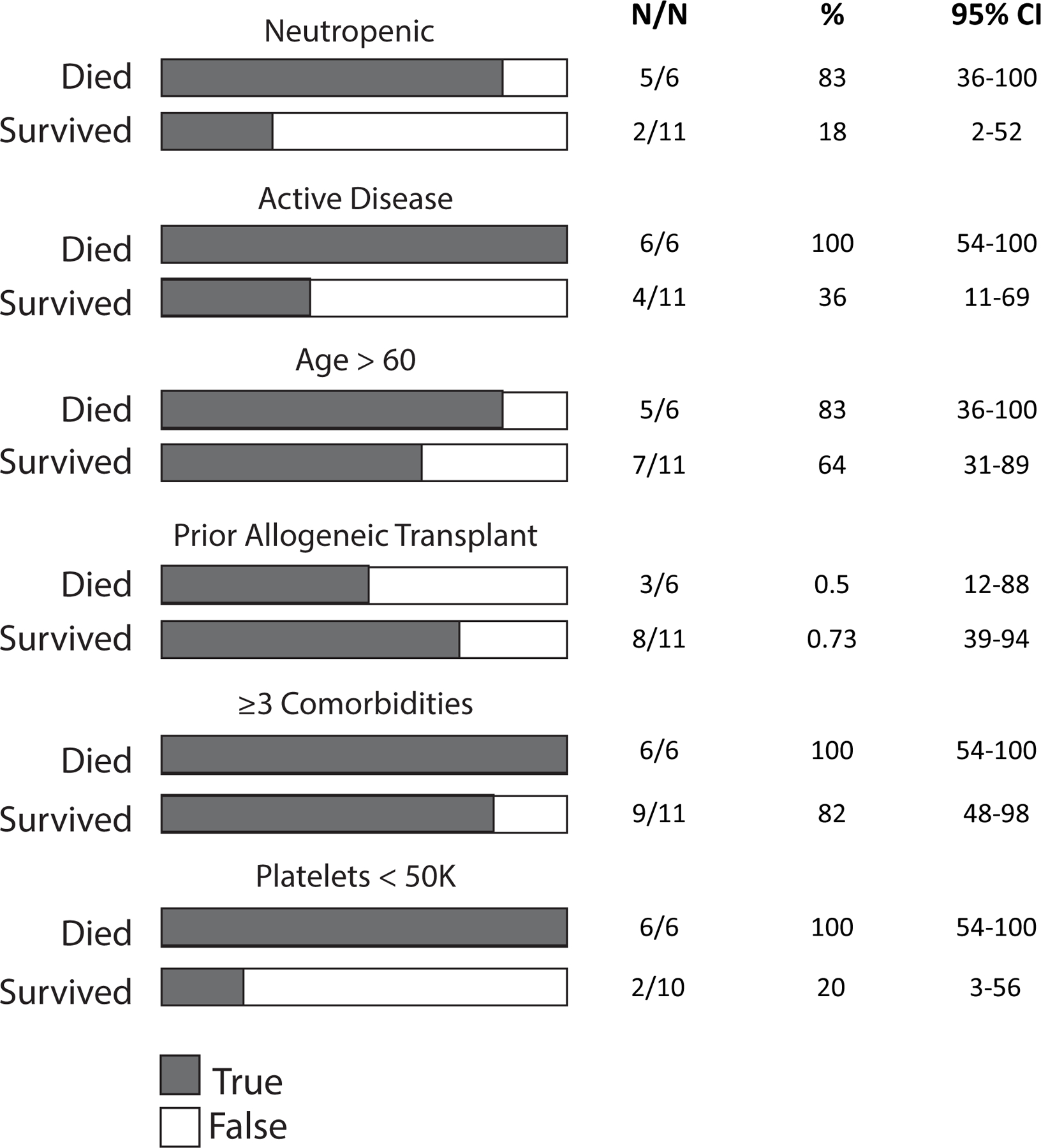
Characteristics for AML patients who died because of COVID-19 vs. patients who survived COVID-19
Discussion:
Here we present the clinical outcomes and predictors of these outcomes of patients with acute and chronic leukemia, who were diagnosed with COVID-19 in the largest private cancer center at the height and in the initial epicenter of the pandemic in the United States. We show that patients with AML, neutropenia within seven days prior and up to 28 days after SARS-CoV-2 diagnosis and active treatment during the last 3 months prior to COVID-19 diagnosis have poor outcomes compared to leukemia patients without these characteristics. Importantly, while our data detailing the New York experience of COVID-19 in patients with leukemia are in line with recently published results from a retrospective Italian study, which showed that progressive disease status and AML were associated with increased risk of COVID-19 related mortality [5], we also demonstrate that neutropenia in leukemia patients is a significant and powerful risk factor for COVID-19 related mortality (OR 11.75, p<0.001). In our cohort, AML patients who were neutropenic at the time of COVID-19 diagnosis or developed neutropenia within 28 days after the COVID-19 diagnosis had a mortality rate of 83%.
Our results have implications for practicing hematologists, as many commonly used AML treatment regimens induce neutropenia. Given the powerful association of neutropenia with adverse outcomes following COVID-19 infection in leukemia patients, we suggest that all patients who are scheduled to receive potentially myelosuppressive treatment should be routinely screened for SARS-CoV-2 prior to treatment initiation. If tested positive for SARS-CoV-2, delay of treatment should be considered if clinically appropriate [14]. In this context, it is important to point out that in a large real-world data set from the German Study Alliance Leukemia–Acute Myeloid Leukemia (SAL-AML) registry, a delay of treatment of up to or more than 15 days did not result in worse 2-year OS, even in patients who delayed treatment after being diagnosed with a high initial WBC of > 50 × 109/L [15]. To potentially reduce the risk of severe COVID-19, the practicing hematologist can therefore strongly consider delaying the initiation of anti-leukemic therapy if a positive COVID-19 test coincides with the diagnosis of AML.
More investigation is needed to evaluate the impact of different AML treatment regimens on outcomes in areas of high COVID-19 transmission rates. Notably, although our data show that neutropenia is a risk factor for COVID-19 mortality in AML patients, we would advise caution in administering granulocyte-colony stimulating factor (G-CSF) in the context of COVID-19 infection. Data on the effects of G-CSF in COVID-19 are mixed, but some case series suggest a potential risk for promoting lung inflammation and severe respiratory failure [16–18]. In contrast, in a randomized clinical trial of 200 Chinese patients with COVID-19, lymphopenia, and no comorbidities, recombinant human G-CSF treatment did not lead to clinical improvement but was also not associated with an increased risk of serious adverse events [19]. More investigation is needed to better define the role of G-CSF in the treatment of neutropenia in cancer patients with COVID-19.
Our data have important limitations. While more deeply annotated than other published cohorts of leukemia patients diagnosed with COVID-19, our data are representative of a single center, based on a relatively small number of patients, and some of the variables predicting outcomes are not independent of one another. The powerful adverse impact of AML, neutropenia, and active treatment on outcomes following COVID-19 infection should be evaluated in larger studies. Notably, as these data are from the initial height of the pandemic, few patients treated at this time received dexamethasone (8%) or remdesivir (5%), both of which are interventions that we now know to benefit hospitalized patients with COVID-19 [20,21]. In addition, as a reflection of prevailing treatment practices at the initial height of the pandemic in New York City, 32% of patients in our cohort received hydroxychloroquine, an intervention that data now suggest provides no benefit over placebo in patients with COVID-19, and may be associated with QT prolongation and serious cardiac adverse events [22,23].
In summary, our experience in New York City shows that patients with AML, active disease and neutropenia have a high COVID-19 mortality rate, arguing for aggressive infection prevention strategies in this vulnerable patient population.
Supplementary Material
Supplemental Table 1: Treatment characteristics of patients who received active treatment within 3 months prior to COVID-19 infection
Supplemental Table 2: Transplant characteristics of patients who received an allo-SCT prior to COVID-19 infection
Supplemental Figure 1: Event free survival for a combined event of death/advanced respiratory support
A) by presence of comorbidities vs. not
B) by type of disease (AML vs. all other)
C) by disease treatment status (active vs. inactive)
D) by presence of neutropenia vs. not
Acknowledgments:
Maximilian Stahl received funding from the MSKCC Clinical Scholars T32 Program under award number T32 CA009512–31 and support from an ASCO/Conquer Cancer Foundation Young Investigator Award. Varun Narendra received funding from the MSKCC Clinical Scholars T32 Program under award number T32-CA009512. Justin Jee received funding from the MSKCC T32 Program under award number T32 CA009207. Research reported in this publication was supported by the Cancer Center Support Grant/Core Grant to Memorial Sloan Kettering Cancer Center (P30 CA008748) The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Aaron Goldberg received funding from an American Society of Hematology (ASH) Fellow Scholar Award in Clinical Research.
Footnotes
Declaration of interest statement:
Maximilian Stahl: No conflict of interests
Varun Narendra: No conflict of interests
Justin Jee: MDSeq Inc: Patent licensed
Andriy Derkach: No conflict of interests
Molly Maloy: No conflict of interests
References:
- 1.Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol 2020;21:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol 2020;21:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai M, Liu D, Liu M, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov 2020;10:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med 2020. [DOI] [PMC free article] [PubMed]
- 5.Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol 2020. [DOI] [PMC free article] [PubMed]
- 6.Lee LYW, Cazier JB, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol 2020. [DOI] [PMC free article] [PubMed]
- 7.Cattaneo C, Daffini R, Pagani C, et al. Clinical characteristics and risk factors for mortality in hematologic patients affected By COVID-19. Cancer 2020. [DOI] [PubMed]
- 8.Pinana JL, Martino R, Garcia-Garcia I, et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol 2020;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Suarez J, de la Cruz J, Cedillo A, et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol 2020;13:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jee J, Foote MB, Lumish M, et al. Chemotherapy and COVID-19 Outcomes in Patients With Cancer. J Clin Oncol 2020:JCO2001307. [DOI] [PMC free article] [PubMed]
- 11.Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood 2020;136:1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scarfo L, Chatzikonstantinou T, Rigolin GM, et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia 2020;34:2354–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SERVICES USDOHAH. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 2017.
- 14.Zeidan AM, Boddu PC, Patnaik MM, et al. Special considerations in the management of adult patients with acute leukaemias and myeloid neoplasms in the COVID-19 era: recommendations from a panel of international experts. Lancet Haematol 2020;7:e601–e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rollig C, Kramer M, Schliemann C, et al. Does time from diagnosis to treatment affect the prognosis of patients with newly diagnosed acute myeloid leukemia? Blood 2020;136:823–830. [DOI] [PubMed] [Google Scholar]
- 16.Nawar T, Morjaria S, Kaltsas A, et al. Granulocyte-colony stimulating factor in COVID-19: Is it stimulating more than just the bone marrow? Am J Hematol 2020;95:E210–E213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths EA, Alwan LM, Bachiashvili K, et al. Considerations for Use of Hematopoietic Growth Factors in Patients With Cancer Related to the COVID-19 Pandemic. J Natl Compr Canc Netw 2020:1–4. [DOI] [PMC free article] [PubMed]
- 18.Lasagna A, Zuccaro V, Ferraris E, Rizzo G, Tancredi RJ, Pedrazzoli P. How to Use Prophylactic G-CSF in the Time of COVID-19. JCO Oncol Pract 2020:OP2000484. [DOI] [PubMed]
- 19.Cheng LL, Guan WJ, Duan CY, et al. Effect of Recombinant Human Granulocyte Colony-Stimulating Factor for Patients With Coronavirus Disease 2019 (COVID-19) and Lymphopenia: A Randomized Clinical Trial. JAMA Intern Med 2020. [DOI] [PMC free article] [PubMed]
- 20.Group RC, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med 2020.
- 21.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Preliminary Report. N Engl J Med 2020. [DOI] [PubMed]
- 22.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med 2020. [DOI] [PMC free article] [PubMed]
- 23.FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or. U.S. Food and Drug administration (FDA); 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Treatment characteristics of patients who received active treatment within 3 months prior to COVID-19 infection
Supplemental Table 2: Transplant characteristics of patients who received an allo-SCT prior to COVID-19 infection
Supplemental Figure 1: Event free survival for a combined event of death/advanced respiratory support
A) by presence of comorbidities vs. not
B) by type of disease (AML vs. all other)
C) by disease treatment status (active vs. inactive)
D) by presence of neutropenia vs. not


