This randomized clinical trial investigates whether first-line treatment with nivolumab plus ipilimumab vs nivolumab alone improves objective response rate in adult patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN).
Key Points
Question
Does first-line nivolumab plus ipilimumab provide clinical benefit vs nivolumab in patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN)?
Findings
In this randomized clinical trial of 425 adults with platinum-refractory or platinum-eligible R/M SCCHN, the primary end point of objective response rate benefit with nivolumab plus ipilimumab vs nivolumab was not met; results were generally similar in the population with platinum-eligible disease. Nivolumab plus ipilimumab had a manageable safety profile.
Meaning
In this trial, nivolumab plus ipilimumab had no clinical benefit over nivolumab alone as first-line treatment for R/M SCCHN.
Abstract
Importance
There remains an unmet need to improve clinical outcomes in patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN).
Objective
To evaluate clinical benefit of first-line nivolumab plus ipilimumab vs nivolumab alone in patients with R/M SCCHN.
Design, Setting, and Participants
The CheckMate 714, double-blind, phase 2 randomized clinical trial was conducted at 83 sites in 21 countries between October 20, 2016, and January 23, 2019. Eligible participants were aged 18 years or older and had platinum-refractory or platinum-eligible R/M SCCHN and no prior systemic therapy for R/M disease. Data were analyzed from October 20, 2016 (first patient, first visit), to March 8, 2019 (primary database lock), and April 6, 2020 (overall survival database lock).
Interventions
Patients were randomized 2:1 to receive nivolumab (3 mg/kg intravenously [IV] every 2 weeks) plus ipilimumab (1 mg/kg IV every 6 weeks) or nivolumab (3 mg/kg IV every 2 weeks) plus placebo for up to 2 years or until disease progression, unacceptable toxic effects, or consent withdrawal.
Main Outcomes and Measures
The primary end points were objective response rate (ORR) and duration of response between treatment arms by blinded independent central review in the population with platinum-refractory R/M SCCHN. Exploratory end points included safety.
Results
Of 425 included patients, 241 (56.7%; median age, 59 [range, 24-82] years; 194 males [80.5%]) had platinum-refractory disease (nivolumab plus ipilimumab, n = 159; nivolumab, n = 82) and 184 (43.3%; median age, 62 [range, 33-88] years; 152 males [82.6%]) had platinum-eligible disease (nivolumab plus ipilimumab, n = 123; nivolumab, n = 61). At primary database lock, the ORR in the population with platinum-refractory disease was 13.2% (95% CI, 8.4%-19.5%) with nivolumab plus ipilimumab vs 18.3% (95% CI, 10.6%-28.4%) with nivolumab (odds ratio [OR], 0.68; 95.5% CI, 0.33-1.43; P = .29). Median duration of response for nivolumab plus ipilimumab was not reached (NR) (95% CI, 11.0 months to NR) vs 11.1 months (95% CI, 4.1 months to NR) for nivolumab. In the population with platinum-eligible disease, the ORR was 20.3% (95% CI, 13.6%-28.5%) with nivolumab plus ipilimumab vs 29.5% (95% CI, 18.5%-42.6%) with nivolumab. The rates of grade 3 or 4 treatment-related adverse events with nivolumab plus ipilimumab vs nivolumab were 15.8% (25 of 158) vs 14.6% (12 of 82) in the population with platinum-refractory disease and 24.6% (30 of 122) vs 13.1% (8 of 61) in the population with platinum-eligible disease.
Conclusions and Relevance
The CheckMate 714 randomized clinical trial did not meet its primary end point of ORR benefit with first-line nivolumab plus ipilimumab vs nivolumab alone in platinum-refractory R/M SCCHN. Nivolumab plus ipilimumab was associated with an acceptable safety profile. Research to identify patient subpopulations in R/M SCCHN that would benefit from nivolumab plus ipilimumab over nivolumab monotherapy is warranted.
Trial Registration
ClinicalTrials.gov Identifier: NCT02823574
Introduction
Patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) not amenable to curative therapy have substantial morbidity and high mortality.1,2,3 Anti–programmed death 1 (PD-1) immunotherapy with or without platinum–5-fluorouracil–based chemotherapy has improved outcomes vs the EXTREME regimen4 (cisplatin or carboplatin plus 5-fluorouracil in combination with cetuximab) or chemotherapy in first- and second-line settings and is the standard of care for R/M SCCHN.5,6,7,8,9,10,11 In patients with platinum-refractory R/M SCCHN, nivolumab improved overall survival (OS) vs chemotherapy, with OS benefit also noted in patients with disease progression within 6 months of platinum-based therapy for locally advanced disease.8,9,12,13 Pembrolizumab also showed OS benefit vs chemotherapy in platinum-refractory disease in first- and later-line settings.6 In patients with platinum-eligible R/M SCCHN, first-line pembrolizumab provided OS benefit vs cetuximab plus chemotherapy in programmed death ligand 1 (PD-L1)–positive disease measured by combined positive score (CPS), and first-line pembrolizumab plus chemotherapy provided OS benefit in patients regardless of the PD-L1 CPS; however, neither pembrolizumab nor pembrolizumab plus chemotherapy showed OS improvement vs cetuximab plus chemotherapy in patients with a CPS lower than 1, and 4-year survival rates were less than 20% in the total population.5,7,14 Despite recent advances, there persists an unmet need for efficacious therapies and novel biomarkers for predicting response to immunotherapy in patients with R/M SCCHN.
Nivolumab (an anti–PD-1 antibody) and ipilimumab (an anti–cytotoxic T-lymphocyte antigen 4 [CTLA-4] antibody) are immune checkpoint inhibitors with distinct but complementary mechanisms of action.15 Nivolumab plus ipilimumab at various doses and/or schedules has shown long-term, durable survival benefit for several cancer types, including non–small cell lung cancer (NSCLC), melanoma, renal cell carcinoma, esophageal squamous cell carcinoma, and malignant pleural mesothelioma.16,17,18,19,20 However, first-line nivolumab plus ipilimumab did not result in a statistically significant improvement in OS vs the EXTREME regimen for R/M SCCHN in the phase 3 CheckMate 651 trial21: median OS was 13.9 months (95% CI, 12.1-15.8 months) vs 13.5 months (95% CI, 12.6-15.2 months) in the full randomized population and 17.6 months (95% CI, 13.8-22.0 months) vs 14.6 months (95% CI, 12.3-16.0 months) in the population with a CPS of 20 or higher.
Here, we report results from the CheckMate 714 randomized clinical trial, which evaluated nivolumab plus ipilimumab vs nivolumab monotherapy, thereby assessing the contribution of each component of dual immunotherapy as first-line treatment for patients with platinum-refractory or platinum-eligible R/M SCCHN. We also report an exploratory analysis of efficacy outcomes by tumor immune assessment gene expression profile (TIA-GEP), a measure of tumor inflammation.
Methods
Design
CheckMate 714 was a double-blind, phase 2 randomized clinical trial designed to assess whether nivolumab plus ipilimumab improved the objective response rate (ORR) and duration of response (DOR) vs nivolumab monotherapy as first-line treatment for platinum-refractory R/M SCCHN (NCT02823574). The trial was conducted between October 20, 2016, and January 23, 2019, at 83 sites in 21 countries (eAppendix in Supplement 2) in accordance with the Declaration of Helsinki22 and the International Conference on Harmonization Good Clinical Practice guidelines. Local institutional review board or ethical committee approval was obtained at each site before study initiation. All patients provided written informed consent. The trial protocol is available in Supplement 1. This report followed the Consolidated Standards of Reporting Trials (CONSORT) guideline.
Patients and Treatment
Eligible patients were 18 years of age or older with histologically confirmed R/M SCCHN not amenable to curative therapy; Eastern Cooperative Oncology Group performance status of 0 or 1; measurable disease per Response Evaluation Criteria in Solid Tumors, version 1.1; documentation of tumor PD-L1 expression (defined as the percentage of tumor cells with membrane immunohistochemistry staining at any intensity, ie, tumor proportion score)23; documentation of human papillomavirus (HPV) (determined by p16 for oropharyngeal cancer [OPC]) status; and no prior systemic therapy in the R/M setting. Patients were randomized 2:1 to receive nivolumab (3 mg/kg intravenously [IV] every 2 weeks) plus ipilimumab (1 mg/kg IV every 6 weeks) or nivolumab (3 mg/kg IV every 2 weeks) plus placebo. Stratification factors were platinum-refractory status (yes vs no), tumor PD-L1 expression (<1%, nonevaluable, or indeterminate vs ≥1%), and HPV p16 status (OPC p16 positive vs OPC p16 negative or non-OPC). Treatment continued up to 24 months or until disease progression, unacceptable toxic effects, or withdrawal of consent. Crossover between treatment arms was not allowed.
The population with platinum-refractory R/M SCCHN included patients with SCCHN who had recurring disease less than 6 months after completion of platinum-based chemotherapy (adjuvant or neoadjuvant or as part of multimodal treatment [chemotherapy, surgery, and/or radiotherapy]). The population with platinum-eligible R/M SCCHN included patients who were platinum-based chemotherapy naive or had disease recurrence 6 or more months after completion of platinum-based chemotherapy (adjuvant or neoadjuvant or as part of multimodal treatment). Both populations could have received prior platinum-based chemotherapy for locally advanced disease but not for R/M disease.
Outcomes and Assessments
The primary end point was ORR by blinded independent central review (BICR) in the population with platinum-refractory R/M SCCHN. Response was further characterized by DOR and time to response (TTR). Secondary end points included ORR and DOR by BICR in the population with platinum-eligible R/M SCCHN and progression-free survival (PFS) by BICR, OS, and efficacy (ORR, DOR, PFS, and OS) by baseline tumor PD-L1 expression (<1% or ≥1%) in both populations. Exploratory end points included safety and tolerability, subgroup analyses to assess the effect of baseline characteristics on OS, and efficacy by baseline TIA-GEP score (<10 or ≥10, indicating lower or higher T cell–inflamed phenotype, respectively). The TIA-GEP assessment was performed on a set of formalin-fixed, paraffin-embedded tumor sections by next-generation sequencing using an inflammation gene panel of 95 genes. Within this panel, a 16-gene signature that highly correlates with CD8 immunohistochemistry was assessed using a validated assay.24
Statistical Analysis
Data were analyzed from October 20, 2016 (first patient, first visit), to March 8, 2019 (primary database lock for the analysis of primary end points), and April 6, 2020 (OS database lock for the analyses of other end points). For the primary end point, a target sample size of 216 patients (nivolumab plus ipilimumab, 144; nivolumab, 72) in the population with platinum-refractory R/M SCCHN provided 84% power for testing the odds ratio (OR) of nivolumab plus ipilimumab vs nivolumab with a 2-sided significance level of P < .05, assuming an ORR of 35% and 15%, respectively (OR, 3.05). For the population with platinum-eligible R/M SCCHN, a sample size of 180 patients (nivolumab plus ipilimumab, 120 patients; nivolumab, 60 patients) was targeted to ensure a maximum width of 18.6% for the exact 2-sided 95% CI for an ORR in the nivolumab plus ipilimumab arm in the 10% to 55% range.
Comparison of ORR between treatment arms in the population with platinum-refractory R/M SCCHN (primary end point) was performed using a 2-sided Cochran-Mantel-Haenszel (CMH) test stratified by tumor PD-L1 and HPV p16 status. Following a preplanned interim analysis at which the study did not stop, the final adjusted type I error (α) level was 4.5%; therefore, 95.5% CIs were assessed for the ORRs. An estimate of the difference in ORRs between treatment arms and the corresponding 95% CIs were calculated using the CMH method with adjustment for stratification factors. The DOR was estimated using the Kaplan-Meier product-limit method. There was no additional adjustment for missing covariate data. Analyses of efficacy (in all randomized patients) and safety (in all treated patients) were conducted separately in each population.
Analyses were conducted using SAS, version 9.2 (SAS Institute Inc). Additional details on treatment, assessments, and statistical analyses are included in the eMethods in Supplement 2.
Results
Patients and Treatment
Of 425 patients overall, the 241 patients in the population with platinum-refractory R/M SCCHN (56.7%; median age, 59 [range, 24-82] years; 47 females [19.5%], 194 males [80.5%]) were randomized to nivolumab plus ipilimumab (n = 159) or nivolumab (n = 82); 158 (99.4%) and 82 (100%), respectively, received treatment (Figure 1A). Of the 184 patients with platinum-eligible R/M SCCHN (43.3%; median age, 62 [range, 33-88] years; 32 females [17.4%] and 152 males [82.6%]), 122 of 123 randomized patients (99.2%) received nivolumab plus ipilimumab and 61 of 61 (100%) received nivolumab (Figure 1C).
Figure 1. CONSORT Diagram.
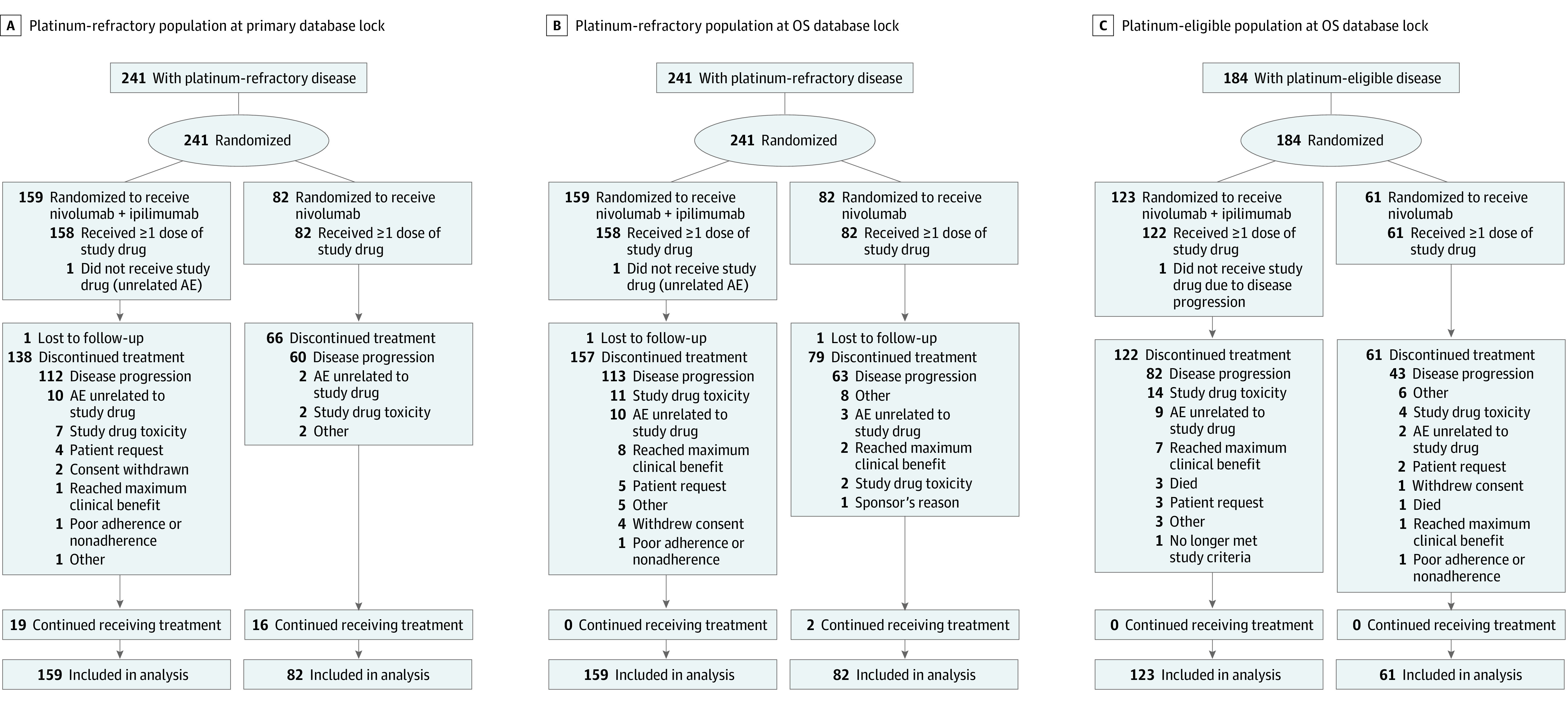
Primary database lock was March 8, 2019; overall survival (OS) database lock was April 6, 2020. AE indicates adverse event.
Population With Platinum-Refractory R/M SCCHN
Baseline characteristics were well balanced between treatment arms (Table 1). Drug exposure is summarized in eTable 1 in Supplement 2. At primary database lock (minimum follow-up, 9.6 months), 19 of 158 treated patients (12.0%) in the nivolumab plus ipilimumab arm and 16 of 82 in the nivolumab arm (19.5%) continued to receive treatment (Figure 1A); at OS database lock (minimum follow-up, 22.2 months), 0 of 158 patients in the nivolumab plus ipilimumab arm and 2 of 82 patients in the nivolumab arm (2.4%) continued to receive treatment (Figure 1B). The primary reason for treatment discontinuation was disease progression in both arms (nivolumab plus ipilimumab: 113 of 158 [71.5%]; nivolumab: 63 of 82 [76.8%]). Subsequent systemic therapy (mainly taxanes) was received by 38 of 159 patients (23.9%) in the nivolumab plus ipilimumab arm vs 21 of 82 (25.6%) in the nivolumab arm; subsequent immunotherapy included anti–PD-1 (nivolumab plus ipilimumab: 7 of 159 [4.4%]; nivolumab: 0 of 82), anti–PD-L1 (nivolumab plus ipilimumab: 0 of 159; nivolumab: 1 of 82 [1.2%]), and anti–CTLA-4 (nivolumab plus ipilimumab: 1 of 159 [0.6%]; nivolumab: 1 of 82 [1.2%]) (eTable 2 in Supplement 2).
Table 1. Baseline Characteristics of Patients With Platinum-Refractory or Platinum-Eligible Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck.
| Characteristic | Patients, No. (%) | |||
|---|---|---|---|---|
| Platinum-refractory disease | Platinum-eligible disease | |||
| Nivolumab plus ipilimumab (n = 159) | Nivolumab (n = 82) | Nivolumab plus ipilimumab (n = 123) | Nivolumab (n = 61) | |
| Age, median (range), y | 59.0 (24-82) | 58.0 (36-77) | 61.0 (37-88) | 62.0 (33-79) |
| Sex | ||||
| Female | 29 (18.2) | 18 (22.0) | 18 (14.6) | 14 (23.0) |
| Male | 130 (81.8) | 64 (78.0) | 105 (85.4) | 47 (77.0) |
| Disease status | ||||
| Locally recurrent | 62 (39.0) | 36 (43.9) | 43 (35.0) | 24 (39.3) |
| Locally recurrent or metastatic | 48 (30.2) | 21 (25.6) | 36 (29.3) | 11 (18.0) |
| Metastatic | 49 (30.8) | 25 (30.5) | 44 (35.8) | 26 (42.6) |
| Primary site | ||||
| Oral cavity | 53 (33.3) | 29 (35.4) | 34 (27.6) | 14 (23.0) |
| Larynx | 29 (18.2) | 12 (14.6) | 25 (20.3) | 13 (21.3) |
| Oropharynx | 54 (34.0) | 26 (31.7) | 49 (39.8) | 21 (34.4) |
| Hypopharynx | 16 (10.1) | 10 (12.2) | 9 (7.3) | 8 (13.1) |
| Unknown or other | 7 (4.4) | 5 (6.1) | 6 (4.9) | 5 (8.2) |
| HPV p16 statusa | ||||
| OPC p16+ | 28 (17.6) | 13 (15.9) | 28 (22.8) | 15 (24.6) |
| OPC p16− | 25 (15.7) | 13 (15.9) | 21 (17.1) | 6 (9.8) |
| Non-OPC or not reportedb | 106 (66.7) | 56 (68.3) | 74 (60.2) | 40 (65.6) |
| Smoking status | ||||
| Current or former | 121 (76.1) | 67 (81.7) | 93 (75.6) | 49 (80.3) |
| Never | 32 (20.1) | 14 (17.1) | 27 (22.0) | 12 (19.7) |
| Unknown | 6 (3.8) | 1 (1.2) | 3 (2.4) | 0 |
| Alcohol use | ||||
| Current | 41 (25.8) | 19 (23.2) | 53 (43.1) | 17 (27.9) |
| Former | 76 (47.8) | 35 (42.7) | 34 (27.6) | 31 (50.8) |
| Never or unknown | 42 (26.4) | 28 (34.1) | 36 (29.3) | 13 (21.3) |
| ECOG PS | ||||
| 0 | 38 (23.9) | 18 (22.0) | 41 (33.3) | 23 (37.7) |
| 1 | 120 (75.5) | 62 (75.6) | 82 (66.7) | 37 (60.7) |
| 2 | 1 (0.6) | 2 (2.4) | 0 | 1 (1.6) |
| Tumor PD-L1 expression, %a | ||||
| <1 or Nonevaluablec | 67 (42.1) | 36 (43.9) | 58 (47.2) | 28 (45.9) |
| ≥1 | 92 (57.9) | 46 (56.1) | 65 (52.8) | 33 (54.1) |
| TIA-GEP | ||||
| <10 | 61 (38.4) | 26 (31.7) | 58 (47.2) | 14 (23.0) |
| ≥10 | 60 (37.7) | 35 (42.7) | 37 (30.1) | 29 (47.5) |
| Not reported | 38 (23.9) | 21 (25.6) | 28 (22.8) | 18 (29.5) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; HPV, human papillomavirus; OPC, oropharyngeal cancer; PD-L1, programmed death ligand 1; TIA-GEP, tumor immune assessment gene expression profile.
Per interactive voice response system.
Non-OPC includes unknown site (positive) and other site (positive, negative, or no HPV test available).
Platinum-refractory: nivolumab plus ipilimumab, n = 15; nivolumab, n = 5; platinum-eligible: nivolumab plus ipilimumab, n = 7 patients; nivolumab, n = 5.
Population With Platinum-Eligible R/M SCCHN
Baseline characteristics were generally well balanced between treatment arms (Table 1). At OS database lock, all 183 treated patients in the population with platinum-eligible R/M SCCHN had discontinued treatment (Figure 1C). The primary reason for treatment discontinuation was disease progression (nivolumab plus ipilimumab: 82 of 122 [67.2%]; nivolumab: 43 of 61 [70.5%]). Drug exposure is summarized in eTable 1 in Supplement 2. Subsequent systemic therapy (mainly platinum-based chemotherapy) was received by 28 of 123 patients (22.8%) in the nivolumab plus ipilimumab arm vs 18 of 61 (29.5%) in the nivolumab arm; 3 of 123 patients (2.4%) vs 2 of 61 (3.3%), respectively, received subsequent anti–PD-1 immunotherapy (eTable 2 in Supplement 2).
Efficacy
Population With Platinum-Refractory R/M SCCHN
The study did not meet its primary end point of ORR in the population with platinum-refractory R/M SCCHN. At the primary database lock, there was no ORR benefit with nivolumab plus ipilimumab (13.2%; 95% CI, 8.4%-19.5%) vs nivolumab (18.3%; 95% CI, 10.6%-28.4%) (OR, 0.68; 95.5% CI, 0.33-1.43; CMH P = .29) (Table 2). The ORR difference between the treatment arms was estimated to be −5.1% (95.5% CI, −15.0 to 4.8). Median TTR was 2.6 months (range, 1.1-6.6 months) with nivolumab plus ipilimumab vs 1.5 months (range, 1.2-7.7 months) with nivolumab. Median DOR was not reached (NR) (95% CI, 11.0 months to NR) for nivolumab plus ipilimumab vs 11.1 months (95% CI, 4.1 months to NR) for nivolumab; 90.5% (95% CI, 67.0%-97.5%) in the nivolumab plus ipilimumab arm vs 66.7% (95% CI, 37.5%-84.6%) in the nivolumab arm had ongoing responses for more than 6 months.
Table 2. Objective Response Rate and Duration of Response per Blinded Independent Central Review in the Populations With Platinum-Refractory and Platinum-Eligible Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck.
| End point | Platinum-refractory disease | Platinum-eligible disease | ||||
|---|---|---|---|---|---|---|
| Primary database locka | OS database lockb | OS database lockb | ||||
| Nivolumab plus ipilimumab (n = 159) | Nivolumab (n = 82) | Nivolumab plus ipilimumab (n = 159) | Nivolumab (n = 82) | Nivolumab plus ipilimumab (n = 123) | Nivolumab (n = 61) | |
| Best overall response, No. (%) | ||||||
| Complete response | 6 (3.8) | 2 (2.4) | 8 (5.0) | 2 (2.4) | 5 (4.1) | 4 (6.6) |
| Partial response | 15 (9.4) | 13 (15.9) | 13 (8.2) | 14 (17.1) | 20 (16.3) | 14 (23.0) |
| Stable disease | 62 (39.0) | 28 (34.1) | 55 (34.6) | 26 (31.7) | 41 (33.3) | 16 (26.2) |
| Progressive disease | 55 (34.6) | 32 (39.0) | 61 (38.4) | 33 (40.2) | 43 (35.0) | 21 (34.4) |
| Not evaluable | 21 (13.2) | 7 (8.5) | 22 (13.8) | 7 (8.5) | 14 (11.4) | 6 (9.8) |
| ORR, No./total No. (%) [95% CI]c,d | 21/159 (13.2) [8.4 to 19.5] | 15/82 (18.3) [10.6 to 28.4] | 21/159 (13.2) [8.4 to 19.5] | 16/82 (19.5) [11.6 to 29.7] | 25/123 (20.3) [13.6 to 28.5] | 18/61 (29.5) [18.5 to 42.6] |
| TTR, median (range), moe | 2.6 (1.1 to 6.6) | 1.5 (1.2 to 7.7) | 2.6 (1.1 to 16.5) | 1.6 (1.2 to 7.7) | 2.7 (1.2 to 6.9) | 2.6 (1.1 to 11.1) |
| DOR, median (95% CI) [range], moe,f,g | NR (11.0 to NR) [2.9 to 21.9] | 11.1 (4.1 to NR) [2.7 to 15.0] | 26.7 (26.7 to NR) [3.1 to 33.7] | 11.1 (4.9 to NR) [2.7 to 26.0] | 27.0 (11.1 to NR) [2.6 to 34.7] | 24.6 (5.5 to NR) [2.5 to 30.4] |
| Probability of ongoing response >6 mo, % (95% CI)e,f | 90.5 (67.0 to 97.5) | 66.7 (37.5 to 84.6) | 95.2 (70.7 to 99.3) | 68.8 (40.5 to 85.6) | 91.3 (69.5 to 97.8) | 76.7 (49.2 to 90.6) |
Abbreviations: DOR, duration of response; NR, not reached; ORR, objective response rate; OS, overall survival; TTR, time to response.
Primary database lock: March 8, 2019.
Overall survival database lock: April 6, 2020.
In the primary analysis for platinum-refractory disease, the difference in ORR was −5.1% (95.5% CI, −15.0% to 4.8%).
For platinum-refractory disease, the Cochran-Mantel-Haenszel estimate of common odds ratio was 0.68 (95.5% CI, 0.33-1.43; P = .29) in the primary analysis and 0.63 (95.5% CI, 0.30-1.30) in the OS analysis.
Among responders.
Computed using the Kaplan-Meier method.
The higher end of the range is a censored value.
At the OS database lock, an additional patient in the nivolumab arm had a response, resulting in an ORR of 19.5% (95% CI, 11.6%-29.7%) with nivolumab; 8 of 159 patients (5.0%) showed complete response with nivolumab plus ipilimumab vs 2 of 82 (2.4%) with nivolumab (Table 2). Median TTR was 2.6 months (range, 1.1-16.5 months) with nivolumab plus ipilimumab vs 1.6 months (range, 1.2-7.7 months) with nivolumab. Median DOR was 26.7 months (95% CI, 26.7 months to NR) for nivolumab plus ipilimumab vs 11.1 months (95% CI, 4.9 months to NR) for nivolumab; 95.2% (95% CI, 70.7%-99.3%) in the nivolumab plus ipilimumab arm vs 68.8% (95% CI, 40.5%-85.6%) in the nivolumab arm had ongoing responses for more than 6 months.
Nivolumab plus ipilimumab did not improve PFS or OS vs nivolumab in the population with platinum-refractory R/M SCCHN. Median PFS was 2.6 months (95% CI, 1.4-2.8 months) for nivolumab plus ipilimumab vs 2.6 months (95% CI, 1.5-3.4 months) for nivolumab (hazard ratio [HR], 1.02; 95% CI, 0.76-1.37); 12-month PFS rates were 16% vs 16%, respectively, and 18-month PFS rates were 15% vs 10%, respectively (Figure 2A). Median OS was 10.0 months (95% CI, 6.5–11.4 months) for nivolumab plus ipilimumab vs 9.6 months (95% CI, 7.1–14.3 months) for nivolumab (HR, 1.08; 95% CI, 0.80-1.46); 12-month OS rates were 42% vs 47%, respectively, and 18-month OS rates were 27% vs 33%, respectively (Figure 2B). When analyzed by baseline characteristics, OS was similar between treatment arms in most subgroups except in patients with primary tumor location in the oral cavity or in patients who had never smoked; in these subgroups, OS benefit seemed to favor nivolumab plus ipilimumab vs nivolumab (eFigure 1 in Supplement 2). However, these subgroup comparisons were not statistically powered and should be interpreted with caution owing to small sample sizes.
Figure 2. Progression-Free Survival (PFS) and Overall Survival (OS) in the Populations With Platinum-Refractory and Platinum-Eligible Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (R/M SCCHN).
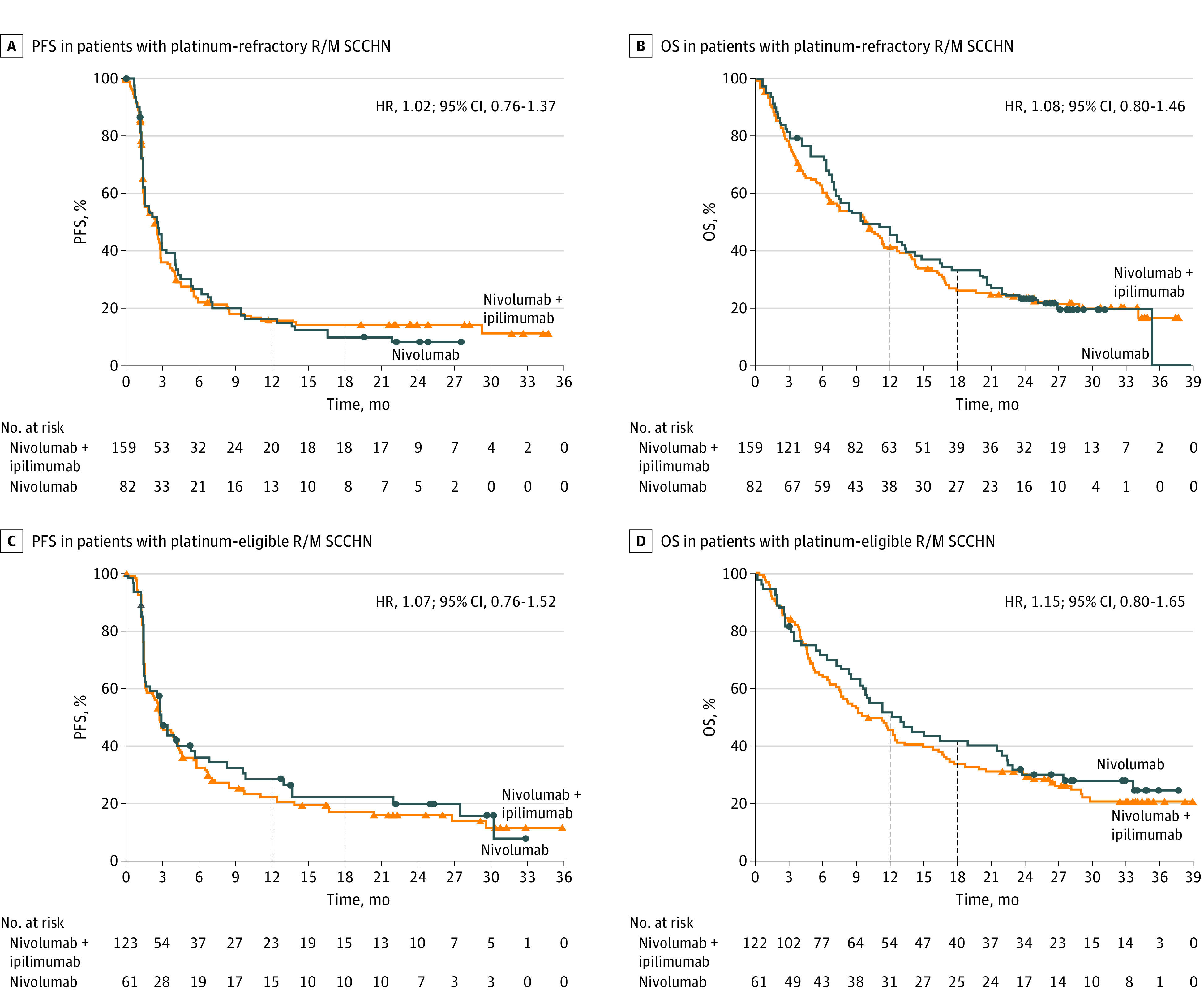
Progression-free survival was assessed by blinded independent central review. The minimum follow-up was 22.2 months for the population with platinum-refractory disease and 22.7 months for the population with platinum-eligible disease. Outcomes were assessed at OS database lock (April 6, 2020). Triangles and circles indicate censored values. HR indicates hazard ratio.
Population With Platinum-Eligible R/M SCCHN
There was no ORR benefit with nivolumab plus ipilimumab vs nivolumab (ORR, 20.3% [95% CI, 13.6%-28.5%] vs 29.5% [95% CI, 18.5%-42.6%]) in the population with platinum-eligible R/M SCCHN (Table 2). Median TTR was 2.7 months (range, 1.2-6.9 months) for nivolumab plus ipilimumab vs 2.6 months (range, 1.1-11.1 months) for nivolumab. Median DOR was 27.0 months (95% CI, 11.1 months to NR) vs 24.6 months (95% CI, 5.5 months to NR); 91.3% (95% CI, 69.5%-97.8%) in the nivolumab plus ipilimumab arm vs 76.7% (95% CI, 49.2%-90.6%) in the nivolumab arm had ongoing responses for more than 6 months.
Nivolumab plus ipilimumab did not improve PFS or OS vs nivolumab in the population with platinum-eligible R/M SCCHN. Median PFS was 2.8 months (95% CI, 1.6-4.2 months) for nivolumab plus ipilimumab vs 2.9 months (95% CI, 1.5-5.6 months) for nivolumab (HR, 1.07; 95% CI, 0.76-1.52); 12-month PFS rates were 22% vs 29%, respectively, and 18-month PFS rates were 17% vs 22%, respectively (Figure 2C). Median OS was 10.0 months (95% CI, 7.5-12.6 months) vs 12.9 months (95% CI, 9.3-22.0 months) (HR, 1.15; 95% CI, 0.80-1.65); 12-month OS rates were 46% vs 52%, respectively, and 18-month OS rates were 34% vs 42%, respectively (Figure 2D). In a subgroup analysis of OS by baseline characteristics, there were no notable differences across treatment arms in patient subgroups (eFigure 2 in Supplement 2).
Efficacy by Biomarker Status
Efficacy was analyzed by tumor PD-L1 expression (≥1% or <1%) and baseline TIA-GEP score (≥10 or <10). There were no significant differences in ORR, PFS, or OS between treatment arms by tumor PD-L1 subgroups in either population (eTable 3 in Supplement 2).
Baseline characteristics by TIA-GEP score are summarized in eTable 4 in Supplement 2. The proportion of patients with a baseline TIA-GEP score of 10 or higher was similar in the nivolumab plus ipilimumab and the nivolumab arms (60 of 159 [37.7%] vs 35 of 82 [42.7%]) in the population with platinum-refractory R/M SCCHN but lower in the nivolumab plus ipilimumab arm in the population with platinum-eligible R/M SCCHN (37 of 123 [30.1%] vs 29 of 61 [47.5%]) (Table 1; eFigure 3 in Supplement 2). Although there were no substantial differences in efficacy between treatment arms by TIA-GEP subgroups in either population, efficacy outcomes were numerically better in the subgroup with a TIA-GEP score of 10 or higher vs lower than 10 (eTable 5 in Supplement 2).
Safety
In both populations, the rates of any grade and grade 3 or 4 any-cause adverse events (AEs) and treatment-related AEs (TRAEs) were generally similar across treatment arms, with a modestly higher incidence of grade 3 or 4 toxic effects with nivolumab plus ipilimumab vs nivolumab in the population with platinum-eligible R/M SCCHN (Table 3). In the population with platinum-refractory R/M SCCHN, rates of any grade and grade 3 or 4 serious TRAEs were 8.2% (13 of 158 patients) and 5.7% (9 of 158), respectively, with nivolumab plus ipilimumab vs 9.8% (8 of 82) and 3.7% (3 of 82), respectively, with nivolumab; rates of any grade and grade 3 or 4 TRAEs leading to discontinuation of any component of the regimen were 5.1% (8 of 158) and 2.5% (4 of 158), respectively, with nivolumab plus ipilimumab vs 1.2% (1 of 82) and 0% (0 of 82), respectively, with nivolumab. In the population with platinum-eligible R/M SCCHN, the rates of any grade and grade 3 or 4 serious TRAEs were 14.8% (18 of 122) and 13.1% (16 of 122), respectively, with nivolumab plus ipilimumab vs 4.9% (3 of 61) and 3.3% (2 of 61), respectively, with nivolumab, and rates of any grade and grade 3 or 4 TRAEs leading to discontinuation of any component of the regimen were 9.8% (12 of 122) and 8.2% (10 of 122), respectively, with nivolumab plus ipilimumab vs 3.3% (2 of 61) and 3.3% (2 of 61), respectively, with nivolumab. No treatment-related deaths were reported in either population.
Table 3. Incidence of AEs in the Populations With Platinum-Refractory and Platinum-Eligible Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Necka.
| AE | Patients, No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Platinum-refractory disease | Platinum-eligible disease | |||||||
| Nivolumab plus ipilimumab (n = 158) | Nivolumab (n = 82) | Nivolumab plus ipilimumab (n = 122) | Nivolumab (n = 61) | |||||
| Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 | Any grade | Grade 3 or 4 | |
| Any-cause AEs | 153 (96.8) | 91 (57.6) | 80 (97.6) | 50 (61.0) | 122 (100) | 67 (54.9) | 59 (96.7) | 31 (50.8) |
| TRAEs | ||||||||
| Any | 98 (62.0) | 25 (15.8) | 53 (64.6) | 12 (14.6) | 81 (66.4) | 30 (24.6) | 42 (68.9) | 8 (13.1) |
| Leading to discontinuation of any component of the regimenb | 8 (5.1) | 4 (2.5) | 1 (1.2) | 0 | 12 (9.8) | 10 (8.2) | 2 (3.3) | 2 (3.3) |
| Serious | 13 (8.2) | 9 (5.7) | 8 (9.8) | 3 (3.7) | 18 (14.8) | 16 (13.1) | 3 (4.9) | 2 (3.3) |
| Treatment-related deaths, No. | 0 | NA | 0 | NA | 0 | NA | 0 | NA |
Abbreviations: AE, adverse event; NA, not applicable; TRAE, treatment-related adverse event.
At OS database lock (April 6, 2020).
In the event of discontinuation of ipilimumab treatment, nivolumab treatment could continue; however, continuation of ipilimumab after discontinuation of nivolumab was not allowed.
The most common any grade nonendocrine immune-mediated AE (IMAE) was rash (platinum-refractory: 16 of 158 patients [10.1%] with nivolumab plus ipilimumab vs 5 of 82 [6.1%] with nivolumab; platinum-eligible: 11 of 122 [9.0%] with nivolumab plus ipilimumab vs 6 of 61 [9.8%] with nivolumab), and the most common any grade endocrine IMAE was hypothyroidism or thyroiditis (platinum-refractory: 28 of 158 [17.7%] with nivolumab plus ipilimumab vs 7 of 82 [8.5%] with nivolumab; platinum-eligible: 23 of 122 [18.9%] with nivolumab plus ipilimumab vs 10 of 61 [16.4%] with nivolumab) (eTable 6 in Supplement 2). The incidence of grade 3 or 4 IMAEs except for rash was less than 2% with nivolumab plus ipilimumab in the population with platinum-refractory R/M SCCHN (3.2% [5 of 158]) and the population with platinum-eligible R/M SCCHN (4.9% [6 of 122]); the incidence of hepatitis with nivolumab in the population with platinum-eligible disease was 3.3% (2 of 61).
Discussion
To our knowledge, the phase 2 CheckMate 714 randomized clinical trial was the first study to evaluate nivolumab plus ipilimumab vs nivolumab monotherapy as first-line treatment for R/M SCCHN and the largest study designed to specifically assess dual immunotherapy in patients with platinum-refractory SCCHN, a population with high unmet need. The study did not meet its primary end point of ORR in the platinum-refractory population (OR, 0.68; 95.5% CI, 0.33-1.43; CMH P = .29). No ORR benefit with nivolumab plus ipilimumab vs nivolumab was reported in the population with platinum-eligible R/M SCCHN either. With a minimum follow-up of over 20 months, there was no OS or PFS benefit in either population. Subgroup analyses of OS by baseline characteristics were exploratory; patient numbers were small, and the analyses lacked statistical power, preventing definitive conclusions about OS benefit with nivolumab plus ipilimumab vs nivolumab in patient subgroups. There was a modest increase in the incidence of grade 3 or 4 toxic effects in the nivolumab plus ipilimumab arm of the population with platinum-eligible R/M SCCHN; however, the combination regimen had a manageable safety profile in both populations.
Although cross-trial comparisons should be made with caution owing to differences in patient populations and study designs and changes in the treatment landscape during the course of the studies, it should be noted that a wide range of OS results with immunotherapy have been reported across studies of R/M SCCHN.5,9,12,21 The poorer OS outcomes with nivolumab plus ipilimumab in CheckMate 714 vs CheckMate 65121 may partly be due to a potential enrollment bias in CheckMate 651 as randomization to the EXTREME arm could have influenced patient selection, although no apparent differences in baseline characteristics of patients were noted between the studies. Furthermore, rates of subsequent systemic therapy in CheckMate 714 (nivolumab plus ipilimumab, 23.9%) were lower vs CheckMate 65121 (nivolumab plus ipilimumab, 49%) or KEYNOTE 0485 (pembrolizumab, 49%; pembrolizumab plus chemotherapy, 41%), which may have also impacted OS outcomes.
Several potential predictive biomarkers for immunotherapy have been explored in R/M SCCHN.25,26 Of these, PD-L1 CPS appears to be predictive of response regardless of the PD-1 inhibitor used.5,21 In CheckMate 714, no clinical benefit with nivolumab plus ipilimumab vs nivolumab was observed in patients with tumor PD-L1 expression less than 1% or 1% or greater; however, analysis by CPS was not protocol defined. In many tumor types, the association of inflammatory gene signatures in the tumor microenvironment with response to immunotherapy has been investigated. Nivolumab plus ipilimumab has been associated with improved OS in patients with high vs low inflammation as assessed by a 4-gene inflammatory signature score in various tumors.27,28,29 In R/M SCCHN, an interferon γ–based signature has been shown to correlate with response to immunotherapy based on PD-L1 status.30,31 In an exploratory analysis of efficacy by the 16-gene signature TIA-GEP score in CheckMate 714, patients with a TIA-GEP score of 10 or higher showed numerically better survival outcomes than those with a TIA-GEP score lower than 10, suggesting that efficacy benefit with immunotherapy was associated with higher tumor inflammation; however, within TIA-GEP subgroups, no notable differences were observed between treatment arms in either the population with platinum-refractory R/M SCCHN or the population with platinum-eligible R/M SCCHN. In the population with platinum-refractory disease, the TIA-GEP score was balanced between arms; however, in the population with platinum-eligible disease, there was an imbalance in TIA-GEP scores, with a higher proportion of patients with a TIA-GEP score of 10 or higher in the nivolumab arm (47.5%) vs the nivolumab plus ipilimumab arm (30.1%).
Limitations
This study has limitations. In CheckMate 714, the dosage of ipilimumab (1 mg/kg IV every 6 weeks) was based on results from the CheckMate 012 study in advanced NSCLC,32 in which this dose, in combination with nivolumab 3 mg/kg IV every 2 weeks, was associated with tolerable safety and promising efficacy. While ipilimumab was well tolerated in CheckMate 714, the dosage and/or treatment schedule may not have been optimal for patients with R/M SCCHN. Additionally, recent studies have shown that a high PD-L1 CPS can be a predictive biomarker for response to immunotherapy in patients with R/M SCCHN.5,21 However, treatment effect by CPS could not be analyzed in Checkmate 714 due to lack of a validated CPS assay at the time of histologic analyses. Furthermore, while exploratory efficacy analyses by the TIA-GEP score were promising, it should be noted that these subgroups were small, and these results should be interpreted with caution due to potential imbalances in treatment arms, as TIA-GEP was not a stratification factor in the study.
Conclusions
The CheckMate 714 randomized clinical trial did not meet its primary end point of ORR benefit with first-line nivolumab plus ipilimumab vs nivolumab monotherapy in patients with platinum-refractory R/M SCCHN. Nivolumab plus ipilimumab was not associated with clinical benefit in either the population with platinum-refractory R/M SCCHN or the population with platinum-eligible R/M SCCHN. Treatment was associated with a manageable safety profile. Patient subpopulations that would benefit from nivolumab plus ipilimumab over nivolumab for R/M SCCHN are yet to be identified, and further research to identify biomarkers to optimize patient selection and improve patient outcomes is warranted. In summary, the role of dual immunotherapy in the first-line treatment of R/M SCCHN remains unclear and needs further investigation.
Trial Protocol
eMethods
eAppendix. List of Participating Sites by Country
eTable 1. Median Duration of Therapy and Cumulative Dose in the Platinum-refractory and Platinum-eligible Populations
eTable 2. Subsequent Therapy in the Platinum-refractory and Platinum-eligible Populations
eTable 3. Efficacy by Tumor PD-L1 Expression in the Platinum-refractory and Platinum-eligible Populations
eTable 4. Baseline Characteristics by Tumor Immune Assessment Gene Expression Profile Score in the Platinum-refractory and Platinum-eligible Populations
eTable 5. Efficacy by Tumor Immune Assessment Gene Expression Profile Score in the Platinum-refractory and Platinum-eligible Populations
eTable 6. Incidence of Nonendocrine and Endocrine IMAEs in the Platinum-refractory and Platinum-eligible Populations
eFigure 1. Overall Survival Subgroup Analyses by Baseline Characteristics in the Platinum-refractory Population
eFigure 2. Overall Survival Subgroup Analyses by Baseline Characteristics in the Platinum-eligible Population
eFigure 3. Tumor Immune Assessment Gene Expression Profile Score Distribution Between Treatment Arms in the Platinum-refractory and Platinum-eligible Populations
eReference
Data Sharing Statement
References
- 1.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695-1709. doi: 10.1016/S0140-6736(08)60728-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):92. doi: 10.1038/s41572-020-00224-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 4.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116-1127. doi: 10.1056/NEJMoa0802656 [DOI] [PubMed] [Google Scholar]
- 5.Burtness B, Harrington KJ, Greil R, et al. ; KEYNOTE-048 Investigators . Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915-1928. doi: 10.1016/S0140-6736(19)32591-7 [DOI] [PubMed] [Google Scholar]
- 6.Cohen EEW, Soulières D, Le Tourneau C, et al. ; KEYNOTE-040 Investigators . Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156-167. doi: 10.1016/S0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 7.Harrington KJ, Burtness B, Greil R, et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the phase III KEYNOTE-048 study. J Clin Oncol. 2023;41(4):790-802. doi: 10.1200/JCO.21.02508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillison ML, Blumenschein G Jr, Fayette J, et al. CheckMate 141: 1-year update and subgroup analysis of nivolumab as first-line therapy in patients with recurrent/metastatic head and neck cancer. Oncologist. 2018;23(9):1079-1082. doi: 10.1634/theoncologist.2017-0674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Food & Drug Administration. Nivolumab for SCCHN. Accessed February 23, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/nivolumab-scchn
- 11.US Food and Drug Administration. FDA approves pembrolizumab for first-line treatment of head and neck squamous cell carcinoma. Accessed February 23, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-first-line-treatment-head-and-neck-squamous-cell-carcinoma
- 12.Gillison ML, Blumenschein G Jr, Fayette J, et al. Long-term outcomes with nivolumab as first-line treatment in recurrent or metastatic head and neck cancer: subgroup analysis of CheckMate 141. Oncologist. 2022;27(2):e194-e198. doi: 10.1093/oncolo/oyab036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45-51. doi: 10.1016/j.oraloncology.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burtness B, Rischin D, Greil R, et al. Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. J Clin Oncol. 2022;40(21):2321-2332. doi: 10.1200/JCO.21.02198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2019;381(16):1535-1546. doi: 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 17.Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079. doi: 10.1136/esmoopen-2020-001079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paz-Ares LG, Ramalingam SS, Ciuleanu TE, et al. First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 CheckMate 227 part 1 trial. J Thorac Oncol. 2022;17(2):289-308. doi: 10.1016/j.jtho.2021.09.010 [DOI] [PubMed] [Google Scholar]
- 19.Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397(10272):375-386. doi: 10.1016/S0140-6736(20)32714-8 [DOI] [PubMed] [Google Scholar]
- 20.Doki Y, Ajani JA, Kato K, et al. ; CheckMate 648 Trial Investigators . Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449-462. doi: 10.1056/NEJMoa2111380 [DOI] [PubMed] [Google Scholar]
- 21.Haddad RI, Harrington K, Tahara M, et al. Nivolumab plus ipilimumab versus EXTREME regimen as first-line treatment for recurrent/metastatic squamous cell carcinoma of the head and neck: the final results of CheckMate 651. J Clin Oncol. Published online December 6, 2022. doi: 10.1200/JCO.22.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 23.Dako North America Inc . PD-L1 IHC 28-8 pharmDx. Accessed June 23, 2022. https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150027c.pdf
- 24.Szabo PM, Pant S, Ely S, et al. Development and performance of a CD8 gene signature for characterizing inflammation in the tumor microenvironment across multiple tumor types. J Mol Diagn. 2021;23(9):1159-1173. doi: 10.1016/j.jmoldx.2021.06.002 [DOI] [PubMed] [Google Scholar]
- 25.Hanna GJ, Lizotte P, Cavanaugh M, et al. Frameshift events predict anti-PD-1/L1 response in head and neck cancer. JCI Insight. 2018;3(4):e98811. doi: 10.1172/jci.insight.98811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rischin D. Biomarkers for immune modulatory treatment in head and neck squamous cell carcinoma (HNSCC). In: Vermorken JB, Budach V, Leemans CR, Machiels JP, Nicolai P, O’Sullivan B, eds. Critical Issues in Head and Neck Oncology. Springer Nature; 2021:83-91. doi: 10.1007/978-3-030-63234-2_6 [DOI] [Google Scholar]
- 27.Hodi FS, Wolchok JD, Schadendorf D, et al. TMB and inflammatory gene expression associated with clinical outcomes following immunotherapy in advanced melanoma. Cancer Immunol Res. 2021;9(10):1202-1213. doi: 10.1158/2326-6066.CIR-20-0983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei M, Siemers NO, Pandya D, et al. Analyses of PD-L1 and inflammatory gene expression association with efficacy of nivolumab ± ipilimumab in gastric cancer/gastroesophageal junction cancer. Clin Cancer Res. 2021;27(14):3926-3935. doi: 10.1158/1078-0432.CCR-20-2790 [DOI] [PubMed] [Google Scholar]
- 29.Peters S, Scherpereel A, Cornelissen R, et al. LBA56 first-line nivolumab (NIVO) plus ipilimumab (IPI) vs chemotherapy (chemo) in patients (pts) with unresectable malignant pleural mesothelioma (MPM): 3-year update from CheckMate 743. Ann Oncol. 2021;32(suppl 5):S1341-S1342. doi: 10.1016/j.annonc.2021.08.2146 [DOI] [PubMed] [Google Scholar]
- 30.Chow LQM, Mehra R, Haddad RI, et al. Biomarkers and response to pembrolizumab (pembro) in recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). J Clin Oncol. 2016;34(15)(suppl):6010. doi: 10.1200/JCO.2016.34.15_suppl.6010 [DOI] [Google Scholar]
- 31.Haddad RI, Seiwert TY, Chow LQM, et al. Influence of tumor mutational burden, inflammatory gene expression profile, and PD-L1 expression on response to pembrolizumab in head and neck squamous cell carcinoma. J Immunother Cancer. 2022;10(2):e003026. doi: 10.1136/jitc-2021-003026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31-41. doi: 10.1016/S1470-2045(16)30624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods
eAppendix. List of Participating Sites by Country
eTable 1. Median Duration of Therapy and Cumulative Dose in the Platinum-refractory and Platinum-eligible Populations
eTable 2. Subsequent Therapy in the Platinum-refractory and Platinum-eligible Populations
eTable 3. Efficacy by Tumor PD-L1 Expression in the Platinum-refractory and Platinum-eligible Populations
eTable 4. Baseline Characteristics by Tumor Immune Assessment Gene Expression Profile Score in the Platinum-refractory and Platinum-eligible Populations
eTable 5. Efficacy by Tumor Immune Assessment Gene Expression Profile Score in the Platinum-refractory and Platinum-eligible Populations
eTable 6. Incidence of Nonendocrine and Endocrine IMAEs in the Platinum-refractory and Platinum-eligible Populations
eFigure 1. Overall Survival Subgroup Analyses by Baseline Characteristics in the Platinum-refractory Population
eFigure 2. Overall Survival Subgroup Analyses by Baseline Characteristics in the Platinum-eligible Population
eFigure 3. Tumor Immune Assessment Gene Expression Profile Score Distribution Between Treatment Arms in the Platinum-refractory and Platinum-eligible Populations
eReference
Data Sharing Statement


