This randomized clinical trial examines whether the addition of metastasis-directed therapy (MDT) to intermittent hormone therapy in men with oligometastatic prostate cancer improves progression-free survival.
Key Points
Question
Does the addition of metastasis-directed therapy to intermittent hormone therapy improve progression-free survival for men with oligometastatic prostate cancer?
Findings
In this phase 2 randomized clinical trial enrolling 87 men with oligometastatic prostate cancer, time to disease progression was significantly improved in men randomized to combined metastasis-directed therapy and intermittent hormone therapy compared with men randomized to hormone therapy only.
Meaning
In men with oligometastatic prostate cancer wishing to prolong hormone therapy cessation, a strategy incorporating metastasis-directed therapy and intermittent hormones may be warranted.
Abstract
Importance
Despite evidence demonstrating an overall survival benefit with up-front hormone therapy in addition to established synergy between hormone therapy and radiation, the addition of metastasis-directed therapy (MDT) to hormone therapy for oligometastatic prostate cancer, to date, has not been evaluated in a randomized clinical trial.
Objective
To determine in men with oligometastatic prostate cancer whether the addition of MDT to intermittent hormone therapy improves oncologic outcomes and preserves time with eugonadal testosterone compared with intermittent hormone therapy alone.
Design, Setting, Participants
The External Beam Radiation to Eliminate Nominal Metastatic Disease (EXTEND) trial is a phase 2, basket randomized clinical trial for multiple solid tumors testing the addition of MDT to standard-of-care systemic therapy. Men aged 18 years or older with oligometastatic prostate cancer who had 5 or fewer metastases and were treated with hormone therapy for 2 or more months were enrolled to the prostate intermittent hormone therapy basket at multicenter tertiary cancer centers from September 2018 to November 2020. The cutoff date for the primary analysis was January 7, 2022.
Interventions
Patients were randomized 1:1 to MDT, consisting of definitive radiation therapy to all sites of disease and intermittent hormone therapy (combined therapy arm; n = 43) or to hormone therapy only (n = 44). A planned break in hormone therapy occurred 6 months after enrollment, after which hormone therapy was withheld until progression.
Main Outcomes and Measures
The primary end point was disease progression, defined as death or radiographic, clinical, or biochemical progression. A key predefined secondary end point was eugonadal progression-free survival (PFS), defined as the time from achieving a eugonadal testosterone level (≥150 ng/dL; to convert to nanomoles per liter, multiply by 0.0347) until progression. Exploratory measures included quality of life and systemic immune evaluation using flow cytometry and T-cell receptor sequencing.
Results
The study included 87 men (median age, 67 years [IQR, 63-72 years]). Median follow-up was 22.0 months (range, 11.6-39.2 months). Progression-free survival was improved in the combined therapy arm (median not reached) compared with the hormone therapy only arm (median, 15.8 months; 95% CI, 13.6-21.2 months) (hazard ratio, 0.25; 95% CI, 0.12-0.55; P < .001). Eugonadal PFS was also improved with MDT (median not reached) compared with the hormone therapy only (6.1 months; 95% CI, 3.7 months to not estimable) (hazard ratio, 0.32; 95% CI, 0.11-0.91; P = .03). Flow cytometry and T-cell receptor sequencing demonstrated increased markers of T-cell activation, proliferation, and clonal expansion limited to the combined therapy arm.
Conclusions and Relevance
In this randomized clinical trial, PFS and eugonadal PFS were significantly improved with combination treatment compared with hormone treatment only in men with oligometastatic prostate cancer. Combination of MDT with intermittent hormone therapy may allow for excellent disease control while facilitating prolonged eugonadal testosterone intervals.
Trial Registration
ClinicalTrials.gov Identifier: NCT03599765
Introduction
Multiple randomized clinical trials have demonstrated improved overall survival (OS) with immediate up-front hormone therapy for nonmetastatic prostate cancer1,2,3 and with up-front systemic therapy intensification for metastatic disease.4,5 Current clinical guidelines recommend up-front hormone therapy for metastatic prostate cancer regardless of disease volume.6,7 Metastasis-directed therapy (MDT) alone for oligometastatic prostate cancer, in which all sites of metastatic disease receive definitive local therapy, has shown promise for impeding metastatic spread and deferring hormone therapy compared with observation in 2 phase 2 randomized clinical trials.8,9,10 There also exists significant preclinical and clinical evidence of synergy between hormone therapy and radiation therapy, the predominate form of MDT.11,12,13,14 However, despite this rationale and that hormone therapy is often combined with MDT to treat oligometastatic prostate cancer in clinical practice,6,15,16 combining these modalities, to our knowledge, has not been tested in a randomized clinical trial.
With these considerations, we tested in a randomized clinical trial whether the addition of MDT to hormone therapy would improve progression-free survival (PFS) and prolong time from testosterone recovery to disease progression (eugonadal PFS) in men with oligometastatic prostate cancer. Given the relatively indolent nature of oligometastatic prostate cancer and the ability of MDT to defer systemic therapy,8,9,17 we used an intermittent hormone therapy strategy.18,19,20,21 We reasoned that intermittent hormone therapy would leverage the potential of hormone therapy to enhance radiation benefit while limiting medical castration. In addition, given the potential for immune stimulation with radiation-based MDT, we investigated whether MDT modulates peripheral T-cell immune subset distribution and receptor expression.
Methods
Trial Design and Conduct
The External Beam Radiation to Eliminate Nominal Metastatic Disease (EXTEND) trial (NCT03599765) is a phase 2, investigator-initiated, controlled, open-label, multicenter, basket randomized clinical trial to test whether adding MDT to standard-of-care systemic therapy improves PFS for patients with a variety of solid tumor types (trial protocol in Supplement 1).22 The primary end point was prespecified to be independently assessed and reported for each basket. The prostate intermittent hormone therapy basket met the prespecified events number to trigger the primary analysis and is reported. The analysis used an intention-to-treat approach. This protocol was approved by the institutional review board at each participating institution, and all patients provided written informed consent. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Patients and Interventions
In the overarching EXTEND trial, patients were enrolled from September 2018 to November 2020 if they had oligometastatic disease from 1 of 12 solid tumors, were aged 18 years or older, had an Eastern Cooperative Oncology Group performance status of 0 to 2, and had 5 or fewer sites of metastatic disease amenable to MDT. Race and ethnicity categories included Black, Hispanic, White, and other (consisting of Middle Eastern and not otherwise specified) and were ascertained by self-report. Race and ethnicity were included in the study because they include standard demographics that are of interest to our institutions and granting agencies (Cancer Prevention & Research Institute of Texas). Upon enrollment of a patient with prostate cancer, the treating medical oncologist was queried whether they planned intermittent or continuous hormone therapy, which resulted in enrollment into either the intermittent or continuous hormone therapy basket. Additional inclusion criteria for the prostate intermittent hormone therapy basket were pathologically confirmed prostate cancer and hormone therapy use 2 or more months prior to randomization. Men with castrate-sensitive or castrate-resistant disease were eligible with or without prior definitive treatment to the prostate. Hormone therapy consisted of a luteinizing hormone-releasing hormone agonist or antagonist with or without a second-generation androgen receptor–targeting (SART) agent. In both treatment groups, untreated primary prostates received definitive radiation.23 A planned hormone therapy break occurred 6 months (±2 months) after enrollment, after which hormone therapy was withheld until progression.
Baseline images were obtained by computed tomography (CT) of the chest, abdomen, and pelvis with contrast and a bone scan or, alternatively, with fluciclovine F 18 positron emission tomography (PET)/CT. Baseline images were compared with pre–hormone therapy images, which were available for all patients, and all metastatic lesions present before hormone therapy and emerging during hormone therapy were identified and targeted with MDT. Follow-up evaluation included repeated prostate-specific antigen (PSA) measurements every 12 weeks after randomization for the first 2 years and then every 18 weeks thereafter. Imaging was repeated when PSA measurements increased by 1.0 ng/mL or more (to convert to micrograms per liter, multiply by 1.0) above the nadir or when any criteria for disease progression were met. Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1)24 was assessed using central review (the Quantitative Imaging Analysis Core at The University of Texas MD Anderson Cancer Center). Enrollment occurred at The University of Texas MD Anderson Cancer Center (Texas Medical Center and all Houston-area locations), Banner MD Anderson Cancer Center, and The University of Texas Health Science Center at San Antonio.
Randomization
Patients were randomly assigned 1:1 to receive MDT to all sites of metastatic disease with hormone therapy or hormone therapy only using a dynamic randomization method by Pocock and Simon.25 Randomization was stratified by the number of metastatic lesions (1-2 vs 3-5), the number of prior systemic therapies (0-1 vs >1), use of a SART (yes vs no), and hormone therapy duration before enrollment (<12 vs ≥12 weeks).
End Points
The primary end point was PFS, defined as the time from randomization until radiographic (RECIST 1.1), clinical, or PSA (ie, biochemical) progression, or death. Consistent with other phase 2 randomized clinical trials evaluating oligometastatic prostate cancer,8,9 biochemical progression was defined as a PSA level increase of 25% or more and 2 ng/mL or more above the nadir. The primary end point was prespecified to be independently assessed and reported for each basket, with the primary analysis at 41 events. Secondary end points were eugonadal PFS time (ie, time from eugonadal testosterone level ≥150 ng/dL until disease progression [to convert to nanomoles per liter, multiply by 0.0347]), OS, time to subsequent systemic therapy, time to appearance of new lesions, time to local treatment failure, safety, and quality of life. Common Terminology Criteria for Adverse Events, version 4.026 was used, and only grade 2 or greater adverse events were recorded. Longitudinal quality of life surveys assessed for depression (Center for Epidemiological Studies Depression Scale),27 health-related quality of life (12-Item Short Form Health Survey),28 and symptom burden (MD Anderson Symptom Inventory).29
Flow Cytometry and T-Cell Receptor Sequencing
Peripheral blood samples for analyses of immune cell subsets and T-cell receptors (TCRs) were collected at baseline and first follow-up.30 Details regarding flow cytometry processing are provided in the eAppendix in Supplement 2, and antibodies are listed in eTable 1 in Supplement 2. Gating strategies are shown in eFigure 1 in Supplement 2. T-cell receptor–β complementarity-determining region 3 regions were sequenced with an immunoSEQ assay (Adaptive Biotechnologies).31
Statistical Analysis
The cutoff date for the primary analysis was January 7, 2022. The study was designed to have 80% power to detect an improvement in median PFS from 18 to 36 months in the combined therapy arm using a 1-sided log-rank test with a type I error of 0.10. The Kaplan-Meier method was used to estimate the survival function, and stratified log-rank tests were used to compare arms. Cox proportional hazards regression was used to estimate hazard ratios (HRs) between treatments adjusting for baseline randomization stratification factors. All reported P values are 2-sided, and significance was declared at P < .05. A linear mixed model was used to assess the association of the randomization arm with each quality of life questionnaire item. The model allowed for the examination of repeated measures over time while considering missing observations.32 Differences in clone abundance (to quantify the number of productive expanded and contracted TCR clones between baseline and 3 months) were calculated with a beta-binomial model with a Benjamini-Hochberg correction to control false discovery rates.33 Mann-Whitney U tests were used to compare metrics between groups, Wilcoxon matched-pair signed rank tests were used to evaluate changes over time, and Spearman rank correlation was used to assess associations. For secondary, subgroup, and exploratory analyses, multiplicity adjustments were not made, and thus these results could not be used to infer effects. Analyses were conducted with Stata/MP, version 17.0 (StataCorp LLC) and SAS, version 9.4 (SAS Institute Inc).
Results
Patients
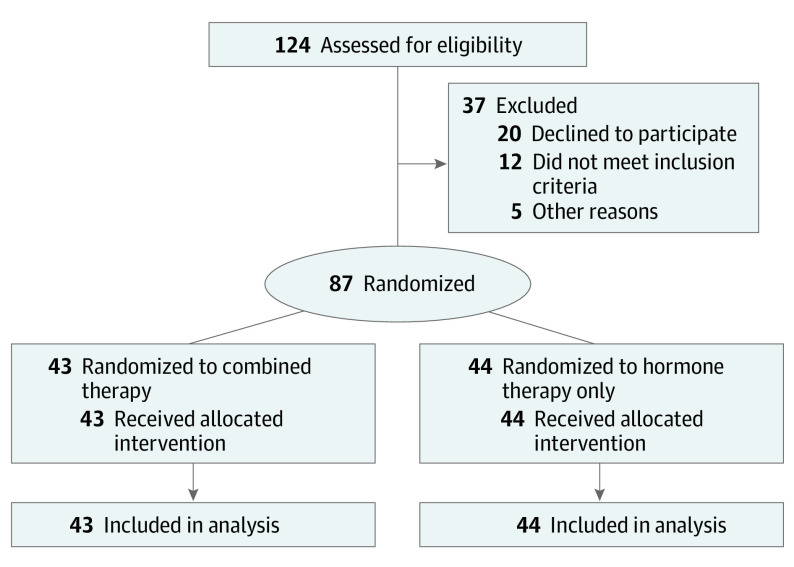
Between September 2018 and November 2020, 124 patients were assessed for eligibility, of whom 37 were excluded. A total of 87 men (median age, 67 years [IQR, 63-72 years]) were randomly assigned to the combined therapy arm (n = 43) or hormone therapy only arm (n = 44). In the combined therapy arm, 4 patients (9%) were Black; 4 (9%), Hispanic; 33 (77%), White; and 2 (5%), other race and ethnicity. In the hormone therapy only arm, 3 patients (7%) were Black; 2 (5%), Hispanic; and 39 (89%), White. All patients received the allocated treatment and were included in the final analysis (Figure 1). Patient baseline characteristics were balanced between arms (Table), including stratification factors (eTable 2 in Supplement 2). All patients were castration sensitive except for 7 (8%; 4 in the combined therapy arm and 3 in the hormone therapy only arm). All patients had disease stage M1a, M1b, or M1c except 6 patients with N1 disease stage (7%; 3 in the combined therapy arm and 3 in the hormone therapy only arm). Most patients (63 [72%]) had received prior definitive local therapy to the prostate (Table).
Figure 1. CONSORT Diagram.
Table. Baseline Patient Characteristics.
| Characteristic | Participants, No. (%)a | |
|---|---|---|
| Combined therapy (n = 43) | Hormone therapy only (n = 44) | |
| Age, median (IQR) [range], y | 67 (63-72) [49-84] | 67 (63-72) [51-81] |
| Race and ethnicity | ||
| Black | 4 (9) | 3 (7) |
| Hispanic | 4 (9) | 2 (5) |
| White | 33 (77) | 39 (89) |
| Otherb | 2 (5) | 0 |
| Enrollment site | ||
| MDACC | ||
| TMC campus | 32 (74) | 34 (77) |
| HAL campus | 10 (23) | 7 (16) |
| Network | 1 (2) | 3 (7) |
| Prior local treatment for prostate | ||
| None | 12 (28) | 12 (27) |
| Surgery | 26 (60) | 21 (48) |
| Radiation | 5 (12) | 8 (18) |
| Cryotherapy | 0 | 3 (7) |
| Castration sensitivity status | ||
| Sensitive | 39 (91) | 41 (93) |
| Resistant | 4 (9) | 3 (7) |
| Disease stage at enrollmentc | ||
| N1, M0 | 3 (7) | 3 (7) |
| N (any), M1a | 11 (26) | 11 (25) |
| N (any), M1b | 28 (65) | 29 (66) |
| N (any), M1c | 1 (2) | 1 (2) |
| Duration of hormone use before randomization, mo | ||
| 2-3 | 16 (37) | 18 (41) |
| 4-12 | 25 (58) | 22 (50) |
| >12 | 2 (5) | 4 (9) |
| Prior lines of systemic therapy, No. | ||
| 0 | 31 (72) | 31 (70) |
| 1 | 10 (23) | 12 (27) |
| 2 | 2 (5) | 1 (2) |
| PSA level at enrollment, ng/mL | ||
| ≤0.2 | 23 (53) | 27 (61) |
| >0.2 to <2.0 | 15 (35) | 14 (32) |
| ≥2.0 | 5 (12) | 3 (7) |
| Metastatic lesions, No. | ||
| 1 | 12 (28) | 21 (48) |
| 2 | 18 (42) | 13 (30) |
| 3 | 8 (19) | 4 (9) |
| 4-5 | 5 (12) | 6 (14) |
| Baseline imaging modality | ||
| CT CAP and bone scan | 33 (77) | 33 (75) |
| Fluciclovine F 18 PET/CT | 10 (23) | 11 (25) |
| Second-generation androgen receptor agent use | ||
| None | 24 (56) | 27 (61) |
| Abiraterone | 15 (35) | 13 (30) |
| Apalutamide | 3 (7) | 3 (7) |
| Enzalutamide | 1 (2) | 1 (2) |
Abbreviations: CT CAP, computed tomography of the chest, abdomen, and pelvis; HAL, all Houston-area locations; MDACC, The University of Texas MD Anderson Cancer Center; PET, positron emission tomography; PSA, prostate-specific antigen; TMC, Texas Medical Center.
SI conversion: To convert PSA level to micrograms per liter, multiply by 1.0.
Percentages may not add to 100% because of rounding.
Other includes Middle Eastern and not otherwise specified.
According to American Joint Committee on Cancer criteria.
Staging was conducted using fluciclovine F 18 PET/CT in 10 patients (23%) in the combined therapy arm and 11 patients (25%) in the hormone therapy only arm. Thirty-six patients (41%) received a SART, most commonly abiraterone (28 [78%]). The MDT in the study was exclusively radiation therapy. The median study follow-up time was 22.0 months (range, 11.6-39.2 months).
Primary End Point
Thirteen progression events (30%) occurred in the combined therapy arm and 28 (64%) in the hormone therapy only arm (eTable 3 in Supplement 2). The median PFS time for all participants was 22.4 months (95% CI, 17.7 months to not estimable) and was significantly longer in the combined therapy arm (not estimable) than in the hormone therapy only arm (15.8 months; 95% CI, 13.6-21.2 months) (HR, 0.25; 95% CI, 0.12-0.55; P < .001) (Figure 2A).
Figure 2. Primary and Key Secondary End Points.
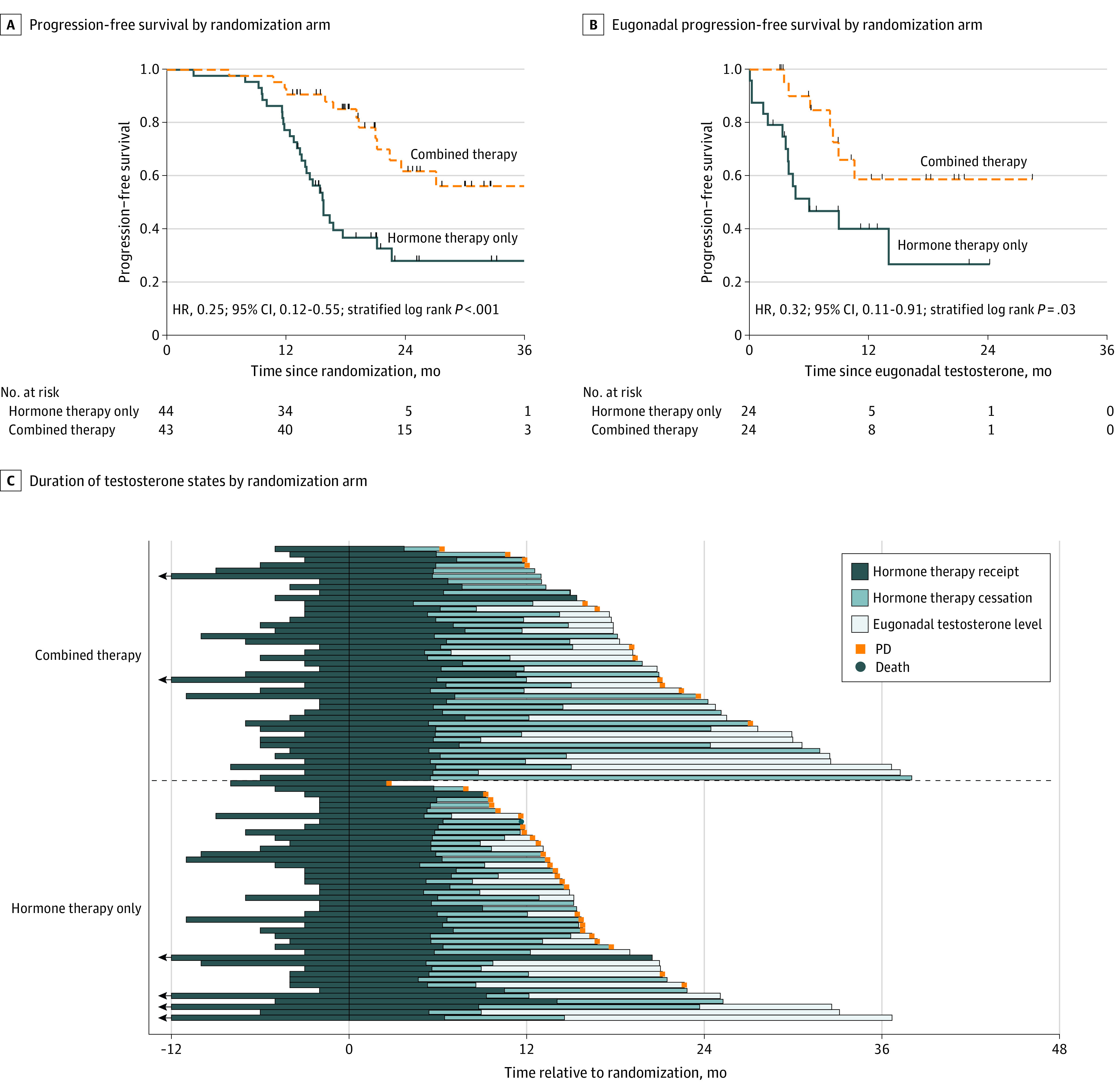
B, Eugonadal progression-free survival (testosterone level ≥150 ng/dL; to convert to nanomoles per liter, multiply by 0.0347) was defined as the time from eugonadal status to disease progression. HR indicates hazard ratio; PD, progressive disease.
The median duration of total hormone therapy administration, including before and after randomization, was similar between arms (combined therapy: 11.2 months [range, 8.0-46.7 months]; hormone therapy only: 10.6 months [range, 7.3-31.6 months]). The swimmer plot in Figure 2C shows timetables for all trial participants in relation to hormone status. Deviations from protocol (hormone therapy administration >8 months after randomization) were noted for 7 patients (16%) in the hormone therapy only arm and 2 patients (5%) in the combined therapy arm. At disease progression, all patients resumed hormone therapy. Post hoc sensitivity analysis limited to the castration-sensitive patients (80 [92%]) confirmed significant improvement in PFS with combined therapy (HR, 0.22; 95% CI, 0.10-0.51; P < .001) (eFigure 2 in Supplement 2).
Secondary Efficacy End Points and Exploratory Subgroup Analyses
Recovery to eugonadal testosterone levels (≥150 ng/dL) occurred in 52 men. Eugonadal PFS was significantly longer for men randomized to combined therapy (median not estimable) vs hormone therapy only (median, 6.1 months; 95% CI, 3.7 months to not estimable) (HR, 0.32; 95% CI, 0.11-0.91; P = .03) (Figure 2B). Time to new lesion failure was longer for patients randomized to combined therapy (2-year incidence, 0.33; 95% CI, 0.18-0.55) than for those receiving hormone therapy only (2-year incidence, 0.41; 95% CI, 0.22-0.68) (HR, 0.33; 95% CI, 0.11-1.0; P = .04) (eFigure 3 in Supplement 2). Overall survival data were immature, and time to subsequent line of systemic therapy was similar between arms. Two cases of local treatment failure (5%) occurred in the combined therapy arm.
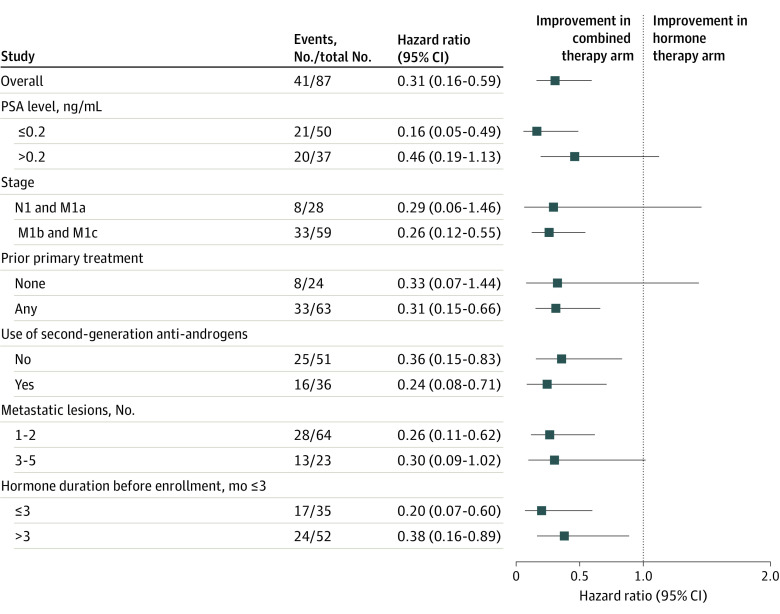
The HRs for combined therapy and improved PFS were similar across subgroups (Figure 3). Notably, MDT prolonged PFS in patients who did (HR, 0.24; 95% CI, 0.08-0.71) and did not (HR, 0.36; 95% CI, 0.15-0.83) receive a SART.
Figure 3. Analysis of Progression-Free Survival According to Key Patient Subgroups.
Data are from an exploratory, unstratified Cox proportional hazards regression. Stages are according to American Joint Committee on Cancer criteria. To convert prostate-specific antigen (PSA) level to micrograms per liter, multiply by 1.0. Squares indicate hazard ratios, with horizontal lines indicating 95% CIs.
Safety
Treatment-emergent adverse events, defined as grade 2 or greater adverse events occurring after enrollment regardless of attribution, are presented in eTable 4 in Supplement 2. There were no grade 4 or 5 adverse events. Six grade 3 events were reported: 3 in the combined therapy arm (in 3 patients) and 3 in the hormone therapy only arm (in 2 patients). Sixteen grade 2 events were reported: 12 in the combined therapy arm (in 7 patients) and 4 in the hormone therapy only arm (in 1 patient).
Quality of Life
Baseline quality of life surveys were analyzed from 37 men (43%; 22 in the combined therapy arm and 15 in the hormone therapy only arm). No differences were found in baseline demographic or disease characteristics between men who answered the surveys and men who did not. Linear mixed-effects modeling did not identify significant associations between randomization arm and test scores (eTable 5 in Supplement 2).
Flow Cytometry and TCR Sequencing
Flow cytometry data from 54 patients (62%) are presented in Figure 4. No significant changes in marker expression among CD4+ and CD8+ T cells were noted between baseline and first follow-up for men in the hormone therapy only arm (eFigure 4 in Supplement 2). For men in the combined therapy arm, increases in the proportion of CD4+ and CD8+ T cells with high and intermediate Ki-67 expression and programmed cell death protein 1 expression, a checkpoint inhibitor expressed in T cells that were active previously, were noted at follow-up (Figure 4A and eFigure 5 in Supplement 2).
Figure 4. Immune Cell Subsets and T-Cell Receptor (TCR) Sequencing.
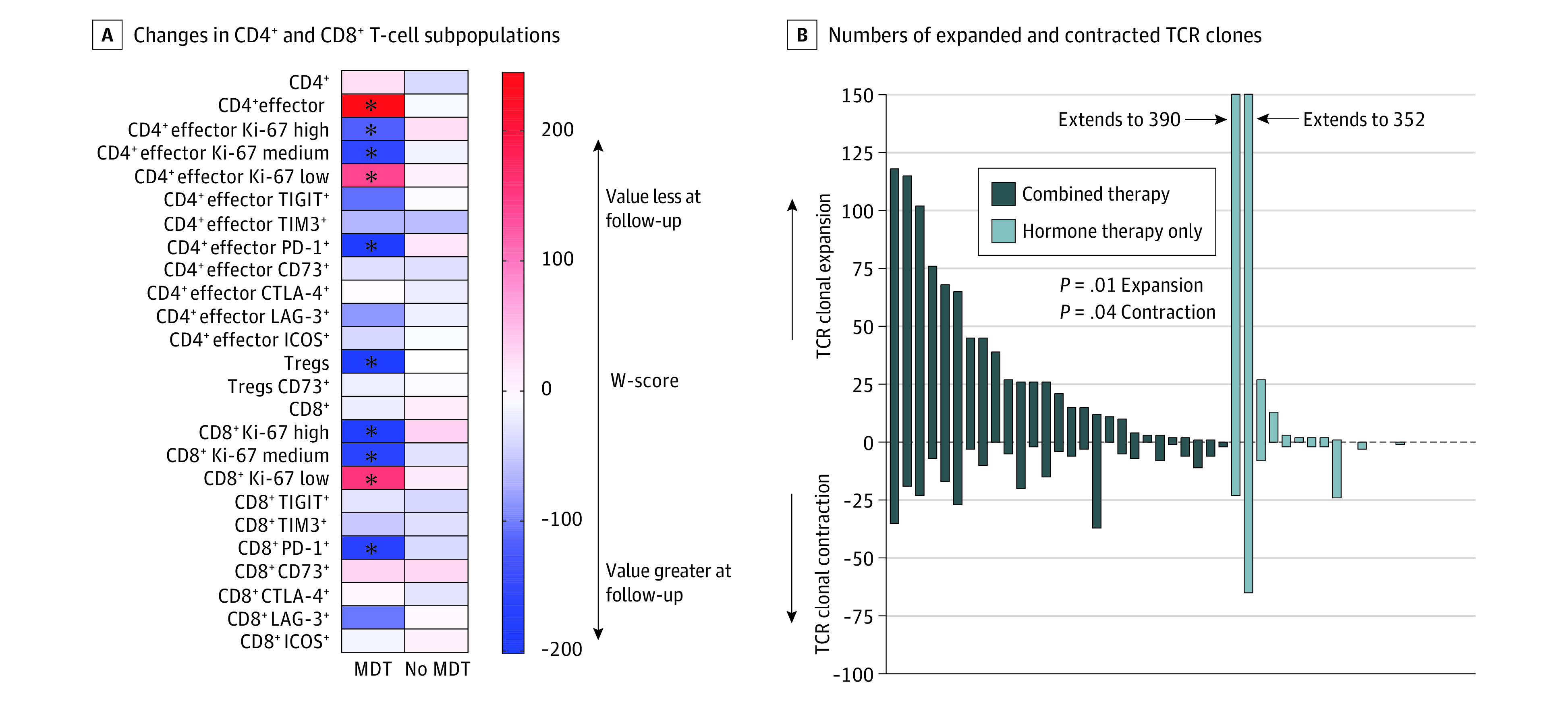
Peripheral blood samples were obtained at baseline and at first follow-up (3 months). A, For flow cytometry gating, parent populations are the CD3+ population for both CD4+ and CD8+, the CD4+ population for all markers on CD4+ cells including T-regulatory cells (Tregs), the CD8+ population for all markers on CD8+ cells, and the Treg population for the Treg CD73+ population. Asterisks indicate P < .05 for the W-score comparing the population between the 3-month follow-up timepoint and the baseline timepoint. CTLA-4 indicates cytotoxic T-lymphocyte associated protein 4; ICOS, inducible costimulator; LAG-3, lymphocyte activation gene 3; MDT, metastasis-directed therapy; PD-1, programmed cell death protein 1; TIGIT, T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domains; TIM3, T-cell immunoglobulin and mucin domain 3.
T-cell receptor sequencing of 4 598 988 T cells from 52 patients (60%) demonstrated 2 738 855 productive rearrangements. Men randomized to combined therapy exhibited higher numbers of expanded and contracted TCR clones at first follow-up (Figure 4B) and a lower Morisita overlap index34 compared with those randomized to hormone therapy only, suggesting greater T-cell clonotype modifications in the combined therapy arm. In the combined therapy arm, a positive association was found at first follow-up between the number of expanded T-cell clones with metrics indicative of a clonal expansion, specifically, the sum of the top 10 clones and productive clonality (eFigure 6 in Supplement 2). Moreover, productive clonality was positively associated with CD8+ T cells and negatively associated with CD4+ T cells, suggesting expansion predominantly within the CD8+ T-cell population. These associations were not observed in the hormone therapy only arm (eFigure 6 in Supplement 2).
Discussion
In this phase 2 randomized clinical trial, the addition of MDT to intermittent hormone therapy improved PFS and the secondary end points of time to new lesion failure and eugonadal PFS. As all men resumed hormone therapy at progression, MDT facilitated a longer time with eugonadal testosterone as part of an intermittent hormone therapy strategy. Adverse event frequencies were modest, with no difference in grade 3 or greater toxic effects or quality of life measures between arms; however, additional follow-up will be required to evaluate long-term toxic effects.
Two phase 2 randomized clinical trials8,9,10 have demonstrated a benefit from MDT vs observation for men with oligometastatic (≤3 metastases) disease recurrent after prior definitive treatment of the prostate. The current study differed in several important ways. First, neither the Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence trial9,10 nor the Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer trial8,10 incorporated hormone therapy. Because hormone therapy is the standard-of-care treatment for metastatic prostate cancer (regardless of disease burden) and downregulates androgen receptor transcriptional programs regulating DNA repair,11 a rationale exists for combining hormone therapy with radiation. Second, our study enrolled men with previously untreated prostates, men receiving SART, men with castrate resistance, and men with up to 5 sites of metastatic disease. In other studies,8,9,10 there was a lack of prospective data regarding the influence of these factors on outcomes in patients with oligometastatic prostate cancer. Owing to multiple studies of prostate cancer and other diseases,17,35,36 there has been a drive for use of MDT for prostate cancer based primarily on the number of metastatic sites, as noted by expert consensus opinions, surveys, and national guidelines.6,7,16,37,38 Our inclusion criteria were designed for generalizability to reflect the clinical setting in which patients present to clinics predominantly based on the number of metastases. Ultimately, the findings from this study are consistent with those of prior studies8,9,10 in highlighting the benefit of MDT in a more generalizable clinical population and in combination with hormone therapy.
Our study was also the first, to our knowledge, to test intermittent hormone therapy for patients with oligometastatic disease. Although phase 3 randomized clinical trials such as the PR.7 trial39 have established intermittent hormone therapy for patients with biochemically recurrent prostate cancer, its routine use in metastatic disease has been controversial.21 The Southwest Oncology Group (SWOG) 9346 trial40 represents the largest randomized clinical trial to date to investigate intermittent hormone therapy for metastatic prostate cancer and could not demonstrate noninferiority. As a result, many practitioners are reluctant to offer intermittent hormone therapy to patients with metastatic disease.19,40 Our study used a similar duration of induction hormone therapy as the SWOG 934640 and PR.739 trials but used a more conservative PSA threshold of a 25% increase and a 2.0 ng/mL increase above the nadir to resume hormone therapy to mitigate the risk of symptomatic progression and emergence of castration resistance. In conjunction with evidence that intermittent hormone therapy can improve mental health, preserve erectile function, and reduce the incidence of medical comorbidities compared with continuous hormone therapy,21,39 our results demonstrated that intermittent hormone therapy combined with MDT allows for excellent disease control while further minimizing hormone therapy exposure.
The mechanism by which MDT prolongs PFS is not completely understood.41 Although the most substantial contribution is likely from tumor debulking,42 a secondary mechanism may be immune stimulation.43 Although studies43 irradiating a single site of disease to induce abscopal responses in other macroscopic sites have not yielded clear efficacy signals, whether radiation to all sites of macroscopic disease induces a clinically significant immune response is unknown. In concordance with recent literature,8,44,45 our data suggest that MDT using radiation stimulates a clonal peripheral T-cell response characterized by increased T-cell proliferation in addition to expansion and remodeling of the peripheral TCR repertoire within the CD8+ population. As recent analyses have shown that peripheral TCR alterations reflect intratumoral changes,46 our exploratory analysis supported the hypothesis that radiation produces immunologic stimulation that contributes to systemic disease control.41
Limitations
This study has several limitations. First, this study was conducted before prostate-specific membrane antigen (PSMA) PET imaging was routinely available. Given data supporting enhanced lesion detection with PSMA over fluciclovine F 18 PET,8,47 we suspect that the observed PFS improvement with MDT would be further enhanced with use of PSMA PET. Second, as this study was conducted during the COVID-19 pandemic, temporary closure of laboratories and reduced willingness of trial participants to attend in-person follow-up visits reduced blood sample and survey recovery. This was more pronounced in patients randomized to hormone therapy only, resulting in an imbalanced correlative collection between arms. Third, this study was conducted in an era of shifting frontline hormone therapies, which resulted in a near-even split between patients who received a SART and those who did not. Of note, the benefit associated with MDT was observed in both subgroups. Finally, our study was heterogeneous and included a limited number of patients with disease stage N1 (6 [7%]) and patients with castrate-resistant disease (7 [8%]). We believe that the study heterogeneity is reflective of current clinical treatment paradigms for oligometastatic prostate cancer treatment. We acknowledge that fundamental biological differences may exist between subgroups, specifically patients with de novo oligometastatic disease at initial diagnosis and those with recurrence following treatment for initially localized disease. Further analyses are warranted to evaluate such differences. Nonetheless, despite the modest sample size and study heterogeneity, randomization balanced patient characteristics between arms and the significant improvement in PFS associated with the combined therapy arm did not deviate substantially among subgroups. Additional inferences will be made upon the completion of the continuous hormone therapy basket, at which time a prespecified joint analysis with the intermittent hormone therapy basket will occur.
Conclusions
In this phase 2 randomized clinical trial, PFS was significantly improved with use of combination MDT and hormone therapy vs hormone therapy only for oligometastatic prostate cancer. The results support the safety and effectiveness of adding MDT to intermittent hormone therapy to prolong hormone therapy cessation of hormone therapy in men with oligometastatic prostate cancer. This therapeutic strategy leveraged the noninvasiveness of radiation and ability of hormone therapy to enhance radiation benefit while limiting the duration of medical castration. Additional studies in a more homogeneous patient population will be necessary to identify predictive biomarkers, optimize systemic therapy regimens and imaging, and identify candidate immunotherapies to exploit the favorable immunologic stimulation with MDT.
Trial Protocol
eAppendix. Expanded Flow Cytometry Methods
eFigure 1. Flow Cytometry Gating Displaying Gating Strategy for T Cells
eFigure 2. Progression-Free Survival in Castration-Sensitive Patients
eFigure 3. Secondary Endpoints
eFigure 4. Change in CD4+ and CD8+ T Cell Populations
eFigure 5. Analysis of Ki-67 Expression in CD8+ and CD4+ T Cell Populations
eFigure 6. Association of TCR Sequencing Metrics and Flow Cytometry Metrics
eTable 1. T Cell Flow Cytometry Panel Design
eTable 2. Stratification Factors
eTable 3. Initial Progression Diagnosis
eTable 4. Adverse Events
eTable 5. LMM Analysis Assessing Association of Quality-of-Life Metrics With Treatment Variables and Time
Data Sharing Statement
References
- 1.Duchesne GM, Woo HH, Bassett JK, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016;17(6):727-737. doi: 10.1016/S1470-2045(16)00107-8 [DOI] [PubMed] [Google Scholar]
- 2.Studer UE, Whelan P, Albrecht W, et al. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) trial 30891. J Clin Oncol. 2006;24(12):1868-1876. doi: 10.1200/JCO.2005.04.7423 [DOI] [PubMed] [Google Scholar]
- 3.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999;341(24):1781-1788. doi: 10.1056/NEJM199912093412401 [DOI] [PubMed] [Google Scholar]
- 4.James ND, Sydes MR, Clarke NW, et al. ; STAMPEDE investigators . Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi: 10.1016/S0140-6736(15)01037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fizazi K, Foulon S, Carles J, et al. ; PEACE-1 investigators . Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399(10336):1695-1707. doi: 10.1016/S0140-6736(22)00367-1 [DOI] [PubMed] [Google Scholar]
- 6.Gillessen S, Armstrong A, Attard G, et al. Management of patients with advanced prostate cancer: report from the Advanced Prostate Cancer Consensus Conference 2021. Eur Urol. 2022;82(1):115-141. doi: 10.1016/j.eururo.2022.04.002 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer. Version 3.2022. January 10, 2022. Accessed May 4, 2022. https://isotopia-global.com/wp-content/uploads/2022/04/NCCN-guidlines-prostate-cancer-2022.pdf
- 8.Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6(5):650-659. doi: 10.1001/jamaoncol.2020.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453. doi: 10.1200/JCO.2017.75.4853 [DOI] [PubMed] [Google Scholar]
- 10.Deek MP, Van der Eecken K, Sutera P, et al. Long-term outcomes and genetic predictors of response to metastasis-directed therapy versus observation in oligometastatic prostate cancer: analysis of STOMP and ORIOLE trials. J Clin Oncol. 2022;40(29):3377-3382. doi: 10.1200/JCO.22.00644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polkinghorn WR, Parker JS, Lee MX, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3(11):1245-1253. doi: 10.1158/2159-8290.CD-13-0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodwin JF, Schiewer MJ, Dean JL, et al. A hormone–DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3(11):1254-1271. doi: 10.1158/2159-8290.CD-13-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Amico AV, Chen MH, Renshaw A, Loffredo M, Kantoff PW. Long-term follow-up of a randomized trial of radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2015;314(12):1291-1293. doi: 10.1001/jama.2015.8577 [DOI] [PubMed] [Google Scholar]
- 14.Locke JA, Dal Pra A, Supiot S, Warde P, Bristow RG. Synergistic action of image-guided radiotherapy and androgen deprivation therapy. Nat Rev Urol. 2015;12(4):193-204. doi: 10.1038/nrurol.2015.50 [DOI] [PubMed] [Google Scholar]
- 15.Deek MP, Yu C, Phillips R, et al. Radiation therapy in the definitive management of oligometastatic prostate cancer: the Johns Hopkins experience. Int J Radiat Oncol Biol Phys. 2019;105(5):948-956. doi: 10.1016/j.ijrobp.2019.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogowski P, Trapp C, von Bestenbostel R, et al. Radiotherapy in oligometastatic prostate cancer—a pattern of care survey among members of the German Society for Radiation Oncology (DEGRO). Strahlenther Onkol. 2022;198(8):727-734. doi: 10.1007/s00066-022-01925-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang C, Msaouel P, Hara K, et al. Definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma: a single-arm, single-centre, feasibility, phase 2 trial. Lancet Oncol. 2021;22(12):1732-1739. doi: 10.1016/S1470-2045(21)00528-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niraula S, Le LW, Tannock IF. Treatment of prostate cancer with intermittent versus continuous androgen deprivation: a systematic review of randomized trials. J Clin Oncol. 2013;31(16):2029-2036. doi: 10.1200/JCO.2012.46.5492 [DOI] [PubMed] [Google Scholar]
- 19.Perera M, Roberts MJ, Klotz L, et al. Intermittent versus continuous androgen deprivation therapy for advanced prostate cancer. Nat Rev Urol. 2020;17(8):469-481. doi: 10.1038/s41585-020-0335-7 [DOI] [PubMed] [Google Scholar]
- 20.Ross RW, Small EJ. Osteoporosis in men treated with androgen deprivation therapy for prostate cancer. J Urol. 2002;167(5):1952-1956. doi: 10.1016/S0022-5347(05)65060-4 [DOI] [PubMed] [Google Scholar]
- 21.Tsai HT, Pfeiffer RM, Philips GK, et al. Risks of serious toxicities from intermittent versus continuous androgen deprivation therapy for advanced prostate cancer: a population based study. J Urol. 2017;197(5):1251-1257. doi: 10.1016/j.juro.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherry AD, Bathala TK, Liu S, et al. Definitive local consolidative therapy for oligometastatic solid tumors: results from the lead-in phase of the randomized basket trial EXTEND. Int J Radiat Oncol Biol Phys. 2022;114(5):910-918. doi: 10.1016/j.ijrobp.2022.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker CC, James ND, Brawley CD, et al. ; Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators . Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366. doi: 10.1016/S0140-6736(18)32486-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours; revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 25.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103-115. doi: 10.2307/2529712 [DOI] [PubMed] [Google Scholar]
- 26.Division of Cancer Treatment & Diagnosis, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.0. April 19, 2021. Accessed February 24, 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40
- 27.Cohen L, Cole SW, Sood AK, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PLoS One. 2012;7(8):e42324. doi: 10.1371/journal.pone.0042324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandek B, Ware JE, Aaronson NK, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA Project—International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1171-1178. doi: 10.1016/S0895-4356(98)00109-7 [DOI] [PubMed] [Google Scholar]
- 29.Fisch MJ, Lee JW, Weiss M, et al. Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol. 2012;30(16):1980-1988. doi: 10.1200/JCO.2011.39.2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deboever N, McGrail DJ, Lee Y, et al. Surgical approach does not influence changes in circulating immune cell populations following lung cancer resection. Lung Cancer. 2022;164:69-75. doi: 10.1016/j.lungcan.2022.01.001 [DOI] [PubMed] [Google Scholar]
- 31.Reuben A, Gittelman R, Gao J, et al. TCR repertoire intratumor heterogeneity in localized lung adenocarcinomas: an association with predicted neoantigen heterogeneity and postsurgical recurrence. Cancer Discov. 2017;7(10):1088-1097. doi: 10.1158/2159-8290.CD-17-0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. Springer; 2000. [Google Scholar]
- 33.Rytlewski J, Deng S, Xie T, et al. Model to improve specificity for identification of clinically-relevant expanded T cells in peripheral blood. PLoS One. 2019;14(3):e0213684. doi: 10.1371/journal.pone.0213684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morisita M. Measuring of the dispersion and analysis of distribution patterns: memoires of the Faculty of Science, Kyushu University, Series E. Biology (Basel). 1959;2:215-235. [Google Scholar]
- 35.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830-2838. doi: 10.1200/JCO.20.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558-1565. doi: 10.1200/JCO.19.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poon DM, Chan CK, Chan TW, et al. Hong Kong Urological Association-Hong Kong Society of Uro-Oncology consensus statements on the management of advanced prostate cancer—2019 updates. Asia Pac J Clin Oncol. 2021;17(suppl 3):12-26. doi: 10.1111/ajco.13580 [DOI] [PubMed] [Google Scholar]
- 38.Morgan SC, Morton GC, Berlin A, et al. Current topics in radiotherapy for genitourinary cancers: consensus statements of the Genitourinary Radiation Oncologists of Canada. Can Urol Assoc J. 2020;14(11):E588-E593. doi: 10.5489/cuaj.6649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367(10):895-903. doi: 10.1056/NEJMoa1201546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368(14):1314-1325. doi: 10.1056/NEJMoa1212299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katipally RR, Pitroda SP, Juloori A, Chmura SJ, Weichselbaum RR. The oligometastatic spectrum in the era of improved detection and modern systemic therapy. Nat Rev Clin Oncol. 2022;19(9):585-599. doi: 10.1038/s41571-022-00655-9 [DOI] [PubMed] [Google Scholar]
- 42.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13(1):8-10. doi: 10.1200/JCO.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- 43.Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol. 2019;16(2):123-135. doi: 10.1038/s41571-018-0119-7 [DOI] [PubMed] [Google Scholar]
- 44.Tang C, Lee WC, Reuben A, et al. Immune and circulating tumor DNA profiling after radiation treatment for oligometastatic non-small cell lung cancer: translational correlatives from a mature randomized phase II trial. Int J Radiat Oncol Biol Phys. 2020;106(2):349-357. doi: 10.1016/j.ijrobp.2019.10.038 [DOI] [PubMed] [Google Scholar]
- 45.Chow J, Hoffend NC, Abrams SI, Schwaab T, Singh AK, Muhitch JB. Radiation induces dynamic changes to the T cell repertoire in renal cell carcinoma patients. Proc Natl Acad Sci U S A. 2020;117(38):23721-23729. doi: 10.1073/pnas.2001933117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu TD, Madireddi S, de Almeida PE, et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature. 2020;579(7798):274-278. doi: 10.1038/s41586-020-2056-8 [DOI] [PubMed] [Google Scholar]
- 47.Calais J, Ceci F, Eiber M, et al. 18F-Fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20(9):1286-1294. doi: 10.1016/S1470-2045(19)30415-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Expanded Flow Cytometry Methods
eFigure 1. Flow Cytometry Gating Displaying Gating Strategy for T Cells
eFigure 2. Progression-Free Survival in Castration-Sensitive Patients
eFigure 3. Secondary Endpoints
eFigure 4. Change in CD4+ and CD8+ T Cell Populations
eFigure 5. Analysis of Ki-67 Expression in CD8+ and CD4+ T Cell Populations
eFigure 6. Association of TCR Sequencing Metrics and Flow Cytometry Metrics
eTable 1. T Cell Flow Cytometry Panel Design
eTable 2. Stratification Factors
eTable 3. Initial Progression Diagnosis
eTable 4. Adverse Events
eTable 5. LMM Analysis Assessing Association of Quality-of-Life Metrics With Treatment Variables and Time
Data Sharing Statement