Abstract
After spinal cord injury (SCI), endogenous neural stem cells are activated and migrate to the injury site where they differentiate into astrocytes, but they rarely differentiate into neurons. It is difficult for brain-derived information to be transmitted through the injury site after SCI because of the lack of neurons that can relay neural information through the injury site, and the functional recovery of adult mammals is difficult to achieve. The development of bioactive materials, tissue engineering, stem cell therapy, and physiotherapy has provided new strategies for the treatment of SCI and shown broad application prospects, such as promoting endogenous neurogenesis after SCI. In this review, we focus on novel approaches including tissue engineering, stem cell technology, and physiotherapy to promote endogenous neurogenesis and their therapeutic effects on SCI. Moreover, we explore the mechanisms and challenges of endogenous neurogenesis for the repair of SCI.
Keywords: Spinal cord injury, Endogenous neurogenesis, Tissue engineering, Stem cells, Biomaterials
INTRODUCTION
Spinal cord injury (SCI) usually leads to permanent sensory, motor and autonomic dysfunction downstream of the injury level, often accompanied by complications such as muscle spasm, sexual dysfunction, and neuropathic pain [1]. SCI seriously affects the patient’s physical and mental health and life expectancy, which brings a heavy economic burden to the patient’s family and society [2]. To date, treatments of SCI focus on surgical decompression for stabilizing the lesion level and preventing further damage to the adjacent spinal cord, combined with postoperative rehabilitation to teach patients how to effectively cope with their disability [3,4]. In sum, there is no clinically effective therapy for neural restoration and regeneration for SCI and the recovery from SCI has always been a global concern [5].
In the acute phase of SCI, ischemia, necrosis, edema, and oxidative stress can result in direct neuronal and glial cell necrosis and apoptosis [6]. With disease progression, the lesion area continues to expand with infiltration of inflammatory factors, glial scaring, fibrous scarring, and formation of cavities, providing an unsuitable microenvironment for nerve regeneration [7,8]. Additionally, neurons of the adult mammalian central nervous system have demonstrated poor spontaneous regeneration and self-repair ability after SCI [9,10]. In brief, the main difficulty of brain-derived descending corticospinal tracts (CST) passing across an injured area lies in the insufficient regeneration potential of endogenous nerve cells and the adverse microenvironment [11]. In tetraplegics and paraplegics with complete SCI, brain-derived descending nerve fibers barely regenerate and traverse through the lesion area; thus, brain-derived descending neural information can hardly retransmit to target neurons to regulate limb movement [12].
To promote CST regeneration, a large number of studies have been carried out using animal models of complete SCI, focusing on enhancing the intrinsic axonal regeneration kinetics of neurons, cell transplantation, and gene therapy [13,14]. Of these, enhancing the regeneration of endogenous neural stem cell (NSC) has the potential to form a neural network, restricting tissue damage and neural loss after SCI [15]. Due to the self-renewal and multipotent properties, NSCs can serve as reliable cell resources for the repair of SCI. After SCI, injury-activated NSCs migrate into the lesion area, where they may proliferate and differentiate into neurons [16]. Some studies have proposed novel tissue engineering strategies involving growth-related factors, biomaterials and physiotherapy, achieved axon regeneration in CST in small rodent models and improvements in the microenvironment at site of injury [17,18]. However, in adult mammals, CST regeneration is unable to penetrate the SCI area and reconnect target neurons which are caudal to the injury site [19]. Therefore, a promising strategy is to trigger the neuronal differentiation of endogenous NSCs into interneurons at the injury site and to form a neural network that receives the neural information from CST and transmits this information to the caudal end of the injury site to restore the voluntary motor function [20]. In this review, we focus on novel approaches and their mechanisms that trigger endogenous neurogenesis in SCI repair to achieve functional recovery.
ENDOGENOUS NEUROGENESIS IN SCI
NSCs transplantation is considered one of the most promising cell therapy for SCI repair [21]. NSCs are primitive cells with self-renewal and multidirectional differentiation capabilities in the central nervous system and have the potential to proliferate and differentiate into neurons, astrocytes, and oligodendrocytes [22]. However, the apoptosis of neurons is one of the main pathological mechanisms of secondary SCI injury [23]. Therefore, researchers expect that inducing NSC proliferation and differentiation into neurons to compensate for the loss of neurons is an effective approach to reduce pathological damage and promote nerve regeneration [24].
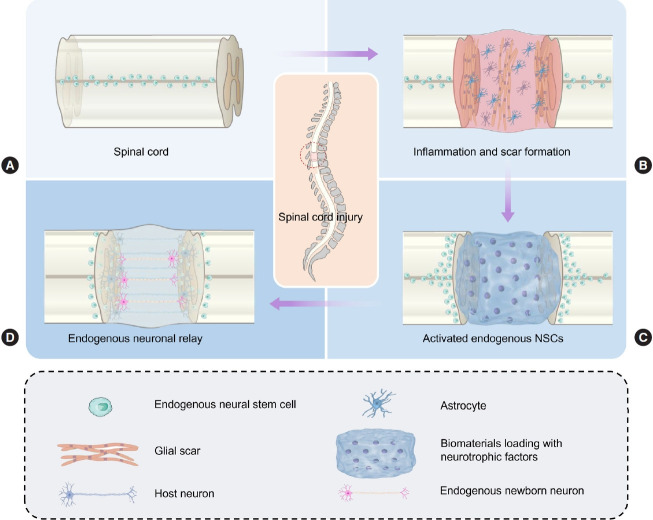
Ependymal cells (ECs), which line in the central canal of the spinal cord, have NSC-like potential (Fig. 1A). Normally, ECs function as a barrier to the brain and spinal cord and rarely undergo cell division [25]. Following SCI, the injury-activated ECs massively migrate out of the central canal and demonstrate NSClike potential during their migration to the injury epicenter [26]. However, most injury-activated ECs start to divide rapidly and generate oligodendrocytes that myelinate axons and astrocytes at the site of the glial scar, but not neuron [27,28]. Whereas astrocytes accumulate primarily at the edge of the lesion area, forming a dense glial scar. Although astrocytes in glial scar can generate inhibitory factors, such as chondroitin sulfate proteoglycans that prevent axons from penetrating the scar, numerous beneficial effects of the scar have been discovered [29,30]. Astrocytes in the glial scar restrict secondary enlargement of the lesion, infiltration of inflammation-associated cells, and prevent further cell death (Fig. 1B) [31]. In addition, the neuronal differentiation of endogenous ECs into oligodendrocytes may guide neural fiber regeneration and remyelination, playing a crucial role in the repair of motor and sensory function [32]. Apart from preserving spinal cord integrity, restricting inflammatory cell infiltration, and providing neurotrophic support for neurons, endogenous NSCs also have the potential to differentiate into functional interneurons [33]. Lin showed that the interneuronal networks formed by NSCs can effectively transmit neural information across the injury site, restoring motor functions [34]. Therefore, how to effectively induce the ECs as abundant and reliable neuronal sources is a major concern in SCI repairing.
Fig. 1.
Activation of endogenous neurogenesis after spinal cord injury. (A) Spinal cord. (B) Inflammation and scar formation after spinal cord injury. (C) Activation of endogenous neurogenesis by transplanting functional biomaterials. (D) Formation of endogenous neuronal relay. NSC, neural stem cell.
Some studies have been designed to promote endogenous neurogenesis (Table 1), in which neurons can transmit ascending and descending impulses, and transfer neural information to propriospinal nerve endings [35,36]. Although descending nerve fibers cannot regenerate and penetrate across the lesion area, it may be feasible to trigger endogenous neurogenesis by transplantation of functional biomaterials which provides a neuronal network able to transmit neural information across the lesion area, improving both motor and sensory functions (Fig. 1C) [37]. Previously, endogenous neurogenesis was defined as the activation of endogenous NSCs, and the generation of new neurons [38,39]. Recently, some researchers supplemented the endogenous neurogenesis as follows: injury-activated endogenous NSCs migrated to the lesion area and then differentiated into mature neurons. The mature neurons then were able to connect with host spinal cord, forming functional neuronal relay (Fig. 1D) [15,40].
Table 1.
Summary of endogenous neurogenesis strategies and their effectiveness for spinal cord injury repair
| Publication | Species | Treatment | Autonomous locomotor recovery | Electrophysiological improvement | Proposed mechanism | Synapselike structure in the injured site | Pathway | |
|---|---|---|---|---|---|---|---|---|
| Transplanting biomaterials mimicking the mechanical property of spinal cord | ||||||||
| Li et al. [45] 2020 | Rat | Injectable nanofiber-hydrogel composite | Yes | NR | Supporting proregenerative macrophage polarization, angiogenesis, axon growth, and neurogenesis in the injured tissue | NR | NR | |
| Zhao et al. [48] 2021 | Rat | Gelatin and hyaluronic acid-based hydrogels made of principle components of extracellular matrix | Yes | NR | Improving endogenous NSC migration and neurogenesis, neuron maturation and axonal regeneration. | NR | NR | |
| Zhu et al. [49] 2021 | Mouse | Mg/Al layered double hydroxide nanoparticles | Yes | Yes | Accelerating NSCs migration, neural differentiation, Ca(2+) channel activation, and inducible action potential generation | NR | Inhibiting inflammation through transforming growth factor-β receptor 2 | |
| Zhou et al. [51] 2018 | Biocompatible conducting polymer hydrogel | Yes | NR | Activating endogenous NSC neurogenesis in the lesion in vivo | NR | NR | ||
| Luo et al. [52] 2022 | Rat | Injectable, self-healing and electroconductive hydrogels | Yes | NR | Activating endogenous NSC neurogenesis, and inducing myelinated axon regeneration into the lesion | NR | Activation of the Pl3k/Akt and MEK/ERK pathways | |
| Ma et al. [53] 2021 | Rat | Poly (lactic-co-glycolic acid) shellensheathed decellularized spinal cord scaffolds | Yes | NR | Creating a favorable microenvironment for migration, residence, and neuronal differentiation of endogenous NSCs and presenting mild immunogenic property, polarizing macrophages to the M2 phenotype | NR | NR | |
| Growth factor-loaded biomaterials | ||||||||
| Yang et al. [40] 2015 | Rat | NT-3-coupled chitosan biomaterial | Yes | NR | Attracted NSCs to migration, differentiation, and formation of functional neural networks | Yes | NR | |
| Li et al. [64] 2016 | Rat and canine | NT-3/fibroin coated gelatin sponge scaffold | Yes | NR | Improved tissue regeneration, reduced cavity areas and abrogated the inflammatory response | NR | Eliciting inflammatory response by reducing TNF-α and CD68 positive cells | |
| Xie et al. [66] 2018 | Rat | Sodium hyaluronate-CNTF scaffold | Yes | Yes | Facilitate NSCs migration, differentiation, forming synaptic contact, and receiving glutamatergic excitatory synaptic input | Yes | NR | |
| Shang et al. [67] 2019 | Rat | bFGF controlled release system | Yes | NR | Reduce microglial activation, promote revascularization, elicit endogenous neurogenesis and promote regrowth of transected axons | Yes | NR | |
| Biomaterials releasing drugs | ||||||||
| Li et al. [71] 2017 | Rat and canine | Cetuximab in modified linear ordered collagen scaffolds | Yes | NR | Neuronal regeneration, including neuronal differentiation, maturation, myelination, and synapse formation | Yes | NR | |
| Fan et al. [36] 2017 | Rat | EGFR antibody with a collagenbinding domain | Yes | NR | Promoted neuronal differentiation and neurite outgrowth under myelin | NR | NR | |
| Yin et al. [72] 2018 | Canine | Taxol-modified collagen scaffold | Yes | Yes | Increased neurogenesis, axon regeneration and reduce glial scar formation | NR | NR | |
| Yang et al. [73] 2021 | Rat | LDN193189, SB431542, CHIR99021 and P7C3-A20 in an injectable collagen hydrogel | Yes | NR | Induced neurogenesis, increase neuronal differentiation of spinal cord NSCs and inhibited astrogliogenesis | NR | NR | |
| Biomaterials with exogenous stem cells | ||||||||
| Yuan et al. [80] 2021 | Rat | DNA hydrogel-carrying exogenous NSCs | Yes | Yes | Enabling sufficient migration, proliferation, and differentiation of both implanted and endogenous NSCs | NR | NR | |
| Li et al. [71] 2017 | Canine | Collagen-based biomaterial loading with human umbilical cordderived mesenchymal stem cells | Yes | NR | Fascinated newborn neurons matured into 5-HT positive neurons and the regenerated axon with remyelination and synapse connection | Yes | NR | |
| Wang et al. [60] 2021 | Rat | Modified scaffolds loading NSCs overexpressing NGF | Yes | NR | Modulating the microenvironment and enhancing endogenous neurogenesis | NR | Activating TrkA, upregulating CREB and microRNA-132 around the lesion focus. | |
| Physiotherapy | ||||||||
| Xu et al. [85] 2021 | Rat | Electroacupuncture on Governor Vessel acupoints | Yes | NR | Activating the intrinsic growth ability of injured spinal neuron | Yes | GV-EA activating CGRP/ RAMP1/alphaCaMKII pathway | |
| Xu et al. [86] 2019 | Rat | Fire needle | Yes | NR | Promoting endogenous NSCs proliferation differentiating into neurons | NR | Activation of Wnt/β-catenin and inhibiting the overexpression of ERK. | |
| Combinatorial treatments | ||||||||
| Li et al. [65] 2021 | Rat | TrkC-modified NSC-derived neural network tissue in the NF-GS | Yes | NR | Establishing favorable microenvironment and supporting the long-time survival of both exogenous neurons and endogenous newborn neurons | Yes | NR | |
| Liu et al. [43] 2021 | Rat | Combining thermosensitive polymer electroactive hydrogel loaded with NGF with electrical stimulation | Yes | NR | Promoted the neuronal differentiation of NSCs and axonal growth | NR | NR | |
NR, not reported; NSCs, neural stem cells; MEK/ERK, mitogen-activated protein kinase kinase/extracellular signal-regulated kinase; TNF-α; tumor necrosis factor-α; CNTF, ciliary neurotrophic factor; NT-3, neurotrophin-3; bFGF, basic fibroblast growth factor; EGFR, epidermal growth factor receptor; 5-HT, 5-hydroxytryptamine; NGF, nerve growth factors; CREB, cAMP-response element binding protein; GV-EA, electroacupuncture on Governor Vessel acupuncture points; NF-GS, NT-3/fibroin coated gelatin sponge scaffold; TrkC, tropomyosin receptor kinase C.
Although the endogenous ECs have been reported to differentiate into neurons which is an appealing candidate for SCI repairing, the efficiency of neuronal differentiation is far from satisfactory [41]. Physiologically, endogenous NSCs mostly differentiate into oligodendrocytes and astrocytes and the number of differentiated interneurons is relatively small, especially in adult mammals [35]. To improve the efficiency of designed neuronal differentiation of ECs, a variety of bioactive materials has been applied to stimulate SCI repair.
BIOMATERIALS FOR TRIGGERING ENDOGENOUS NEUROGENESIS
After SCI, a cascade of pathophysiological progress, such as inflammation, neural death and reactive astrocytes may result in cystic cavity and glial scar formation [42]. The cavity and glial scar block the transduction of electrical signal and stimulation of spinal cord tissue, inhibiting the proliferation and neuronal differentiation of endogenous NSCs [43]. Considering the rapid achievements in biomaterials, scientists have developed novel biomaterials which mimic the mechanical properties of the spinal cord targeting SCI repair [28,44]. The hybrid hydrogels, with highly porous structure, facilitate the transportation of nutrients and in particular, some hydrogels seamlessly integrate with the host tissue by filling the lesion cavity and conforming to the shape of the defect [45]. Moreover, the designed hydrogel promotes axon regeneration via remodeling of the extracellular matrix (ECM) through minimally invasive injection to prevent secondary damage [46]. These biomaterials have achieved therapeutic effects in repairing SCI, improving the microenvironment and eliminating secondary damage. However, some studies suggest that better recovery may be obtained if the biomaterial can activate and guide endogenous NSC differentiation into neurons while improving the microenvironment for nerve regeneration [47].
Recent studies have reported that a series of novel hydrogels could trigger endogenous neurogenesis without the need for additional therapeutic agents and lead to the recovery of motor function. Li et al. [45] designed an injectable nanofiber-hydrogel with interfacial bonding properties, providing mechanical strength and porosity at the lesion area. In addition to providing mechanical support to the constrained spinal cord, the composite material also promoted neurogenesis, proregenerative macrophage polarization, and angiogenesis. After treating with nanofiber-hydrogels, the immature neurons at the injury site gradually increased over times and at 28 days the number of immature neurons was 2-fold higher than in controls. Therefore, the designed nanofiber-hydrogel was able to promote the harsh microenvironment at the injury site, which supported the neuronal differentiation and survival of NSCs.
Zhao et al. [48] developed gelatin and hyaluronic acid-based hydrogels which were constituted of principal ECM. The transplantation of the hybrid hydrogel eliminated the inflammatory responses and suppressed the formation of glial scar. Moreover, the hybrid hydrogel effectively contributed to endogenous neurogenesis, improving NSC migration, neuron maturation, and axonal regeneration. Zhu et al. [49] developed Mg/Al layered double hydroxide nanoparticles to repair the completely transected SCI. They found that the application of nanoparticles accelerated NSC migration and neuronal differentiation, activated the L-Ca (2+) channel, and induced action potentials. By implantation of the layered double hydroxide, they observed BrdU-labeled endogenous NSCs and neurons in the injured area, with an improved electrophysiological and behavior performance in the SCI rat. Further analysis demonstrated that layered double hydroxide inhibited inflammation through the transforming growth factor-β receptor 2 and activated neural cell proliferation.
Apart from mimicking the physical strength, researchers were also interested in developing high-conductivity biomaterials which somehow contributed to endogenous neurogenesis [50]. Zhou et al. [51] developed a biocompatible conducting polymer hydrogel, mimicking mechanical properties of the spinal cord and demonstrating high conductivity. In vitro, conducting polymer hydrogel promoted NSCs differentiation into neurons and suppressed the differentiation into astrocytes. In vivo, the conducting polymer hydrogel could trigger endogenous neurogenesis at the lesion site, improving locomotor functions in rats. Luo et al. [52] also employed an injectable, self-healing, and electro-conductive hydrogels to treat SCI. These hydrogels, composed of natural ECM and polypyrrole, exhibit similar mechanical and electrical properties to the natural spinal cord. In vitro, conductive injectable hydrogels effectively promoted neuronal differentiation, axonal growth, and inhibited astrocyte differentiation. In vivo, the conductive hydrogels activated endogenous NSC neurogenesis and triggered the regeneration of myelinated axon at the site of lesion through the Pl3k/Akt and MEK/ERK (mitogen-activated protein kinase kinase/extracellular signal-regulated kinase) pathways.
Recently, Ma et al. [53] investigated novel tissue scaffolds, which not only met the mechanical properties of pathological spinal cord tissue but also comprised a proregenerative matrix. To construct poly (lactic-co-glycolic acid) shell-ensheathed decellularized spinal cord scaffolds (PLGA-DSCS), researchers removed the inhibitory components and preserved the permissive matrix by electrospinning and chemical extraction strategies. The decellularized spinal cord (DSC) scaffold was mechanically enhanced with a thin PLGA. In vitro, the DSC scaffolds allowed robust neurogenesis and promoted NSC differentiation into neurons. In vivo, the PLGA-DSCS implanted at the injury area created a favorable microenvironment for migration, residence, and neuronal differentiation of endogenous NSCs. Furthermore, PLGA-DSCS presented a mild immunogenic property, polarizing macrophages into the M2 phenotype. Therefore, the PLGADSCS could have significant therapeutic effects on neural regeneration and function recovery.
The biomaterials discussed above could integrate with the host tissue by filling the lesion cavity and somehow promoting the endogenous neurogenesis [37]. For example, the hybrid hydrogels were able to provide mechanical support and high conductivity for the lesion area and the physical characteristics of hydrogels endured in the harsh microenvironment, eliminated inflammatory processes and glial scar formation to facilitate generation of NSC progeny leading to significant recovery of motor function [47]. The modified DSC, which is composed of a proregenerative matrix was able to create a favorable microenvironment for neural regeneration [53]. However, the efficiency of neuronal differentiation of ECs is currently unsatisfactory [54]. To better modulate the microenvironment and guide the directional differentiation in the injury site, functional neurotrophic factors may be a promising strategy.
BIOMATERIALS LOADED WITH NEUROTROPHIC FACTORS TO ACTIVATE ENDOGENOUS NEUROGENESIS
Neurotrophic factors have been reported to modulate various aspects of neural activity [55]. Numerous neurotrophic factors, such as neurotrophin-3 (NT-3), brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), ciliary neurotrophic factor (CNTF) and basic fibroblast growth factor (bFGF), play a crucial role in neural proliferation, migration, neuronal differentiation and synaptogenesis [56]. In vitro, NT-3 promotes the neuronal differentiation and formation of synapses from NSC-derived neurons, demonstrating the potency of synaptic transmission [57,58]. Similarly, NGFs have been reported to promote axonal sprouting, guide axon regeneration and myelination after nerve injury and the upregulated NGF at the perilesion site contributing to repair and synaptic plasticity after SCI [59-60]. Furthermore, CNTF is able to promote the survival and axonal growth of neurons and achieved a promyelinating effect in vitro [61]. These bioactive factors could ameliorate the harsh microenvironment and improve the NSC-like potential of ECs after SCI. Transplantation of the mentioned neurotrophic factors may create a proregenerative microenvironment, thus increasing endogenous repair in both rats and nonhuman primates with SCI [62,63]. However, the release of neurotrophic factors suffers from the short half-lives observed under physiological conditions and limited administration in vivo. Normally, neurotrophic factors diffuse rapidly at the site but are unable to maintain a suitable concentration [64]. To improve the adverse microenvironment conditions, it is necessary to elicit endogenous neurogenesis and to promote axon growth, scientists have designed numerous functional biomaterials combined with neurotrophic factors to achieve long-term release (Fig. 2B). The novel strategies are focused not only on supporting NSC-derived neuron differentiation and increasing remyelination, but also on improving the formation of synaptic connections between propriospinal nerve fibers and neurons at the injury site [9,64].
Fig. 2.
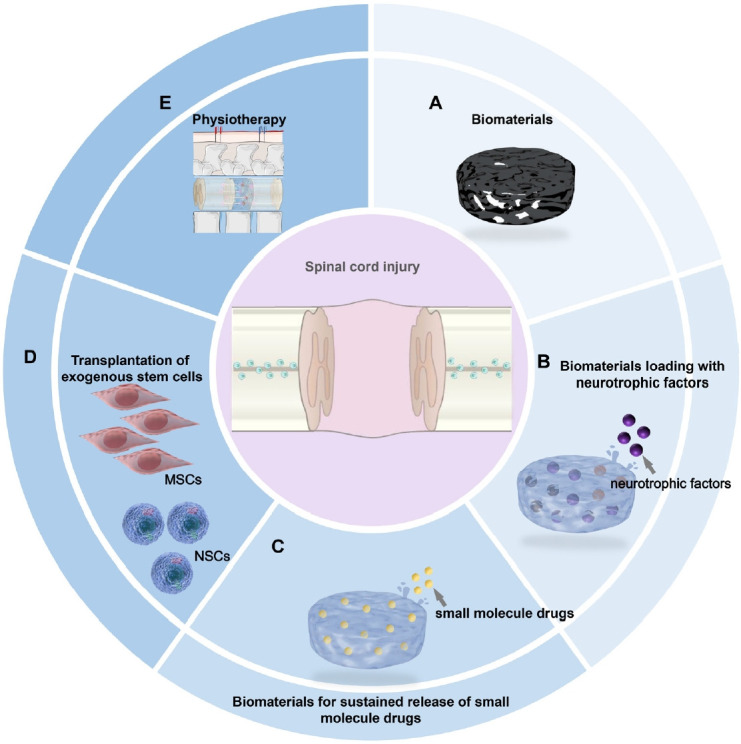
Therapeutic strategies to trigger endogenous neurogenesis. (A) Transplantation of biomaterials that mimic the mechanical properties of the spinal cord. (B) Transplantation of functional biomaterials loaded with neurotrophic factors. (C) Transplanting functional biomaterials for sustained small molecule drugs release. (D) Transplantation of exogenous stem cells. (E) Physiotherapy. MSC, mesenchymal stem cell; NSC, neural stem cell.
NT-3 is a crucial growth factor, distributed in both the central and peripheral nervous systems [56]. In vitro, NT-3 could activate the tropomyosin receptor kinase C (TrkC) receptor, facilitating NSCs proliferation. In vivo, NT-3 has been confirmed to promote neurogenesis in the spinal cord and axonal growth in the CST [65]. Thus, researchers have developed innovative and functional biomaterials able to load NT-3 for the repair of SCI. Yang et al. [40] constructed a 14-week slow-release preparation of NT-3 in a biodegradable chitosan material. After transplanting this material to the injury area, the slow release of NT-3 improved the harsh microenvironment and attracted endogenous NSCs able to migrate into the SCI site and differentiate into neurons. Most importantly, endogenous neurons derived from NSCs connected with propriospinal neurons and formed functional neural networks, leading to both sensory and motor recovery in experimental animals. Similarly, Li et al. [64] developed a gelatin sponge scaffold coated with NT-3/fibroin (NF-GS) to achieve a controlled artificial release lasting 28 days. In vivo, NF-GS improved the concentration of NT-3 and exhibited proper biocompatibility. NF-GS improved tissue regeneration and reduced cavity areas in the lesion area. The axon extensions with myelin sheath penetrated the glial scar and some of the cells traversed the NF-GS. Furthermore, NF-GS abrogated the inflammatory response by reducing tumor necrosis factor-α and CD68-positive cells.
CNTF is a potent survival factor for neurons and oligodendrocytes and it promotes neurotransmitter synthesis and neurite outgrowth. Xie et al. [66] developed a sodium hyaluronate-CNTF scaffold that was capable of releasing CNTF for up to 105 days. The designed scaffold could activate endogenous NSCs from the ependymal layer and promote migration of the NSCs to the injury site. Furthermore, the endogenous NSCs could differentiate into mature neurons, forming synaptic connections and receiving excitatory input from the glutamatergic synapse. The electrophysiological results of the regenerated neural network, recorded by a planar multielectrode dish system, suggest that functional synapses could be established between endogenous NSC-derived neurons and the host spinal cord.
bFGF plays a crucial role in modulating neuronal differentiation and repairing damage. Thus, Shang et al. [64] also investigated bFGF controlled release system for spinal cord regeneration. Under physiological conditions, these scaffolds had proper mechanical properties, enabling the release of bFGF for up to 6 weeks. After implantation, these scaffolds could facilitate revascularization, stimulate endogenous neurogenesis and axon growth and inactivate microglia. Similarly, endogenous neurons connected to each other or with propriospinal neurons through a synapses-like connection. The functional neural networks established between the lesion area and the host spinal cord eventually resulted in recovery from locomotion.
In summary, the above studies demonstrate that functional biomaterials were able to achieve slow release of neurotrophic factors in vivo. Furthermore, the loading of designed functional biomaterials with neurotrophic factors could trigger endogenous neurogenesis of NSCs by creating a regenerative microenvironment, reducing inflammation, improving the migration of NSCs, promoting neuronal differentiation and neurite outgrowth, and generating functional synapses with the propriospinal nerve fibers of the host [68]. Without transplanting exogenous stem cells, endogenous NSCs differentiated into interneurons and functioned as neuronal relays that reconnect with the original downstream targets. Although neurotrophic factors could effectively promote the microenvironment in the area of the injury and modulate the activity of endogenous NSCs, it has proven difficult for neurotrophic factors to fulfill slow-release and maintain long-term activity. Therefore, researchers are interested in finding bioactive materials that intelligently control the release of factors.
BIOMATERIALS WITH SUSTAINED SMALL MOLECULE DRUGS RELEASE THAT TRIGGER ENDOGENOUS NEUROGENESIS
In addition to growth factors, a series of small molecule drugs are associated with modulating the survival, proliferation, and neuronal differentiation of NSCs, inhibiting inflammation, and promoting angiogenesis in vivo. For example, the anticancer drug taxol has the potential to reduce scar formation, decrease axonal degeneration and stabilize microtubule [69]. Cetuximab, an epidermal growth factor receptor (EGFR) antagonist, induces significant neuronal differentiation, generating de novo neuron formations, and reducing astrocytic differentiation of neural progenitor cells in acute SCI [70]. To achieve sustained-release of small molecule drugs that promote endogenous neurogenesis after SCI, researchers have developed functional collagen scaffolds that have demonstrated good biodegradability and excellent biocompatibility (Fig. 2C).
Li et al. [71] implanted cetuximab, an EGFR signaling antagonist, in modified linear ordered collagen scaffolds. After transplantation to injury sites in canine, low-dose cetuximab effectively improved migration and encouraged the neuronal production of endogenous NSCs at the site of injury and inhibited the expression of chondroitin sulfate proteoglycans in the glial scar. Furthermore, neurons derived from endogenous NSCs demonstrated myelination and synapse formation which could connect with host spinal neurons to restore locomotion. In summary, cetuximab-modified linear ordered collagen scaffolds may provide a suitable microenvironment for endogenous neurogenesis and enable neuronal relays after acute SCI. In one study, Fan et al. [36] engineered a collagen-binding EGFR antibody by fusing the EGFR antibody with a collagen-binding domain to achieve its sustained-release from the collagen scaffold. In vitro, the engineered collagens promoted neuronal differentiation and neurite outgrowth under myelin. After transplantation into rat models with SCI, endogenous NSCs for injury-activated neurogenesis were observed, and endogenous NSCs could differentiate into functional neurons and reconnect the 2 injured stumps.
In other studies, the same researchers also investigated the taxol-modified collagen scaffold for SCI repair in canine models [72]. After the complete transection of 1 cm of spinal cord, a linear-order collagen scaffold was implanted that allowed the slow release of taxol into the injured area. In addition to stabilizing microtubules, taxol demonstrated therapeutic effects in restricting scar formation and significantly promoting neurogenesis and axon regeneration after severe spinal cord transection in a canine model. In vitro, taxol promoted the neuronal differentiation of NSCs through the p38 MAPK signaling pathway. Therefore, the taxol-modified scaffold provided a suitable microenvironment for neuronal differentiation of endogenous NSCs and the extension of neuronal axons resulted in significant promotion of locomotion and motor-evoked potentials.
Other attempts, including small molecules, have great therapeutic potential for repairing the spinal cord. Yang et al. [72] loaded functional small molecules including LDN193189, SB431542, CHIR99021, and P7C3-A20 into an injectable collagen hydrogel. The small molecules could induce neurogenesis, increase neuronal differentiation of spinal cord NSCs and inhibit astrogliogenesis at the injury site. Neuronal regeneration at lesion sites leads to recovery from locomotion.
Altogether, the above studies demonstrate that biodegradable and biocompatible materials loaded with small molecule drugs are able to achieve the slow-release of growth factors in vivo. Furthermore, these designed functional biomaterials could create a suitable microenvironment, promoting neuronal differentiation and synapse formation with the propriospinal nerve fibers of the host, leading to better locomotion and electrophysiology. However, for severe SCI or extensive defects, mammals may suffer massive neuron apoptosis within the area of the lesion and the transplantation of biomaterials may not trigger quantitative endogenous neurons to repair impaired neuron circuits [74,75]. A promising solution is represented by the transplantation of exogenous stem cells. These strategies may induce many more endogenous stem cells to participate in the repair process and restore the defected neural circuit through the synergistic effects of both endogenous and exogenous stem cells [58,76,77].
EXOGENOUS STEM CELLS THAT TRIGGER ENDOGENOUS NEUROGENESIS
Transplantation of exogenous stem cells is an attractive strategy for repairing SCI. In the 1980s, transplantation of embryonic spinal cord tissue at the injured site promoted the regeneration of the descending nerve fibers of the brain, resulting in locomotion recovery [78,79]. In this section, we focus on the therapeutic effects of exogenous stem cells on endogenous NSCs migration and neuronal differentiation and on generating functional synapses with host neurons (Fig. 2D).
Yuan et al. [80] designed a DNA hydrogel with high permeability, self-healing, and proper mechanical support for repairing a completely transected spinal cord in rat models. The DNA hydrogel-carrying exogenous NSCs promoted the formation of a renascent neural network, enabling sufficient migration, proliferation, and neuronal differentiation of both implanted and endogenous NSCs. After 8 weeks of transplantation the rats showed better hindlimb function and detectable motor-evoked potentials through synapses of the regenerated neural networks. Furthermore, the hydrogel DNA network offered a regenerative microenvironment by expressing quantitative growth factors including BDNF, GDNF, NGF, and NT-3.
Focusing on chronic SCI in large animals, Li et al. [71] investigated a collagen-based biomaterial loaded with human umbilical cord-derived mesenchymal stem cells in a chronic SCI canine model. Two months after SCI, the glial scar tissue was removed and the biomaterials named the “NeuroRegen scaffold” were transplanted into the lesion area. The implantation of the “NeuroRegen scaffold” facilitated locomotor recovery and endogenous neurogenesis in the center of the lesion area. Additionally, some of the de novo neurons matured into 5-hydroxytryptamine positive neurons and the regenerated axon fibers demonstrated remyelination and synapse connections in the injured area at 1 year after injury. The implantation of the “NeuroRegen scaffold” also reduced the formation of glial scar at the lesion level one year after implantation.
Previous studies have showed that NGF was a crucial growth factor regulating neuronal regeneration [60]. Wang et al. [81] designed modified scaffolds loading NSCs overexpressing NGF for targeted delivery of NGF to the site of the injury. Four weeks after transplantation, NGF-NSCs attenuated damage in the center of the lesion and NGF-NSCs that survived in the core of the lesion maintained high levels of NGF release. The NGF-NSC graft modulated the microenvironment around the lesion core by reducing oligodendrocyte loss, reducing astrocytosis and demyelination, protecting neurons, and increasing the expression of multiple growth factors. Most importantly, in the subacute stage of traumatic SCI, the neuroprotective effect of NGF-NSCs may be mediated by activating TrkA, upregulating cAMP-response element binding protein, and microRNA-132 expression around the epicenter of the injury site. In summary, the exogenous stem cells achieved functional recovery by modulating the microenvironment and enhancing endogenous neurogenesis in rats.
In summary, exogenous stem cell-seeded biomaterials promoted the migration, proliferation, and neuronal differentiation of endogenous NSCs. The researchers also observed the formation of synaptic connections and the formation of neural circuits through implanted and endogenous stem cells. The therapeutic effects of exogenous stem cells targeting endogenous neurogenesis may be attributed to the creation of a favorable microenvironment for axon regeneration, by secreting multiple growth factors to guide migration [58]. To endow host propriospinal neurons with better integration capability with exogenousderived and endogenous-derived neurons, combination with physiotherapy strategies can improve intrinsic growth capacity, activate endogenous neurogenesis and improve the rigid microenvironment.
PHYSIOTHERAPY STRATEGIES FOR ACTIVATING ENDOGENOUS NEUROGENESIS
Physiotherapy has previously been regarded as a symptomatic treatment for SCI. Current advanced physiotherapy strategies which include epidural electrical stimulation (EES) and brain-spine interface can restore leg motor functions after SCI [84,85]. EES following activity-specific stimulation protocols can mimic the natural activation of motor neurons by multielectrode paddle. Three patients following an activity-specific stimulation program were able to complete standing, walking, swimming, and controlling trunk movements in a single day [84]. Although physiotherapy has shown sustained progress in improving motor skills, little is known about the functional effects of physiotherapy on neuroprotection, modulating environment and triggering endogenous neurogenesis.
To improve the hostile microenvironment and poor intrinsic growth capacity, Xu et al. [85] have reported that the application of electroacupuncture on Governor Vessel acupuncture points (GV-EA) could promote neuronal survival and axonal regeneration after SCI. In GV-EA, needles are inserted at GV acupuncture points where a small low-frequency pulsed current can be delivered, ventilating the meridians to promote blood flow. The study suggests that GV-EA could stimulate cells in the dorsal root ganglion to release calcitonin gene-related peptide (CGRP) from the afferent terminals in the spinal cord. However, in vivo and in vitro results demonstrated that CGRP could trigger NT-3 synthesis and secretion by CGRP/receptor activity-modifying protein (RAMP)/calcium/calmodulin-dependent protein kinase (alphaCaMKII) pathway. Furthermore, the mentioned effect could be interrupted by dorsal rhizotomy and blocking the CGRP/RAMP1/alphaCaMKII pathway. Therefore, GV-EA could activate intrinsic growth and promote the survival, axonal growth, and synaptic maintenance of spinal cord neurons in the injured area by increasing NT-3 production (Fig. 2E).
Fire needle acupuncture, known as fire needle, is a physiotherapy technique that combines acupuncture and cauterization with heated needle therapy. Xu et al. [86] found that fire needle improved locomotor function in SCI rats and increased nestin, Gal-C expression while inhibited glial fibrillary acidic protein expression after SCI. These findings indicated that fire needle promoted endogenous NSC migration, proliferation, and differentiation into neurons at the injured site and inhibited differentiated NSCs into astrocytes. Increased Wnt3a, GSK3β, β-catenin, and ngn1 expression and down regulation of ERK1/2 and of cyclinD1 gene and protein expression were observed in the fire needle group. Therefore, endogenous neurogenesis could be mediated by activation of Wnt/β-catenin and inhibition of the ERK pathway.
In conclusion, electrical stimulation has the potential to promote the survival and neuronal differentiation of endogenous NSCs, and contributes to the activation of the propriospinal neuronal network, axonal growth and formation of synaptic connections at the injured site. Electrical stimulation also triggers the synthesis and secretion of neurotrophic factors by cells in the injured spinal cord [87,88]. Other physiotherapies including optogenetic stimulation and magnetic stimulation have been reported to improve the aversive microenvironment, generate bioelectricity, modulate neural plasticity, and promote CST regeneration [89], but these functional effects with respect to endogenous neurogenesis remain uncertain. In the future, the endogenous neurogenesis effects of various physiotherapies should be fully investigated. Furthermore, recent studies have reported that a combination of multiple strategies, including exogenous cell transplantation, the delivery of neurotrophic factors or small molecule drugs and neuromodulation by physiotherapy can achieve more effective repair. The combination of multiple treatments is considered an ideal approach that may provide new insights for clinical treatments.
COMBINATION TREATMENTS
In the literature, some studies describe the therapeutic effects aimed at repairing SCI. For example, biomaterials mimicking the physical characteristics of the spinal cord can compensate for tissue loss, create a better microenvironment, and eliminate secondary damage [52]. Neurotrophic factors and small molecule drugs overcome the harsh microenvironment and promote synapse formation with the propriospinal nerve fibers of the host [64,71]. The transplantation of exogenous stem cells has offered cell sources, and secreted growth factors able to guide cell migration and to create a favorable microenvironment. [76,91] Physiotherapy strategies can activate the propriospinal neuronal network, and integrate regenerated neurons with the spinal cord fiber [88,91]. Most importantly, all these strategies somehow trigger endogenous neurogenesis, promoting the migration, proliferation, and neuronal differentiation of endogenous stem cells at injury site. In fact, the efficiency of repairing SCI using a single treatment without combining other therapeutic agents was low [92].
The following issues or limitations should be carefully considered: (1) improvement of the microenvironment of the impaired spinal cord; (2) triggering of the migration, proliferation and neuronal differentiation of endogenous or exogenous stem cells in the replacement of dead neurons; and (3) promotion the integration of regenerated cells.90 Therefore, the combination of multiple therapeutic strategies may be the key to SCI repair.
To promote tissue repair efficacy, Li et al. [65] combined slow neurotrophic factors release and exogenous cell transplantation strategies, and transplanted a TrkC-modified NSC-derived neural network tissue in the NF-GS. The NF-GS created an NT-3-enriched microenvironment and the NSCs overexpress TrkC, NT-3 receptor, thus creating a functional neuronal populationdominated neural network. In addition to providing a regenerative niche for long-term survival of the exogenous neural network at the injured site, the novel strategy allowed for the sustained differentiation of endogenous NSCs into neurons. Transplantation of the NT-3-releasing scaffold at the lesion site established a favorable microenvironment and supported long-term survival of exogenous neurons and endogenous de novo neurons. This could compensate for the loss of neurons and could lead to an increase in neuronal population at the injury site bringing structural repair to the sensorimotor pathways.
A combination of scaffold-based biochemical and electrical stimulation signals may be useful to repair SCI. Liu et al. [43] investigated a novel approach that combined thermosensitive polymer electroactive hydrogel (TPEH) loaded with NGF with electrical stimulation. The designed hydrogel was able to achieve the sustained-release of NGF for 24 days and demonstrated high conductance on electrical stimulation. In vitro, the TPEH with NGF improved the neuronal differentiation of NSCs and axon growth. In vivo, electrical stimulation and TPEH with NGF promoted endogenous neurogenesis and led to improved motor function.
Current combinatorial treatments focus on providing a regenerative environment to support the long-time survival, proliferation and neuronal differentiation of NSCs. However, we still have limited knowledge about the remodeling and integration of synapses with propriospinal neurons [93]. The application of neuroregulatory technology, including EES, and transcutaneous spinal cord stimulation, can excite the spinal neural network and regulate synaptic plasticity [94]. Therefore, tapping its full potential for better integration with host nerve tracts will require a combination of neuroregulatory techniques with biomaterials.
CHALLENGES AND FUTURE PERSPECTIVES
With recent advances in the understanding of the repair mechanism of tissue engineering along with physiotherapy, endogenous neurogenesis has become a significant mechanism for SCI research [47,95]. Despite some attempts to achieve functional recovery by targeting endogenous neurogenesis, challenges and obstacles remain. For large mammals, it is still challenging to ensure that endogenous NSCs survive, proliferate, and differentiate into neurons in adequate quantities [96]. Considering the obstacles mentioned above, combination therapy may be the most appropriate approach. In the future, combination treatments will be designed as selective approaches that may interact to catalyze with each other [97]. For instance, transplantation of biomaterials creates a favorable microenvironment for axon regeneration, promotes exogenous neuronal differentiation, and facilitates synapse formation with the endogenous newborn neurons that contribute to neurological recovery. In turn, neuromodulation techniques could theoretically excite spinal neural networks, strengthen synaptic connections, promote plasticity, and facilitate integration into the central nervous system [98]. To reach their full potential, combinatorial treatments must adhere to the strict temporal window and select an appropriate SCI model.
Apart from revealing the mechanisms and effects underlying endogenous neurogenesis through combination treatments, efforts should be made to further expand the applications in clinical practice: (1) Differences in neuroanatomy (distribution of CST) and size gaps between humans and rodents hinder clinical trials. In rodents, the lesion site is about a few millimeters long, whereas human injuries can span centimeters [99]. To conduct neural information through lesion area, the functional recovery of SCI patients needs more endogenous newborn neurons and longer-distance axon growth. Despite being costly and time-consuming, SCI models of non–human-primate and large mammals provide numerous advantages for evaluating treatment efficacy before clinical trials due to their similar neuroanatomical and functional characteristics [100]. (2) It is important to note that SCI patients exhibit variability in the neurological level of injury, lesion severity, treatment duration, and types of early treatment, making injuries unreproducible [101]. Possible solutions may be aligning the animal models closely with clinical conditions [5]. (3) Immunosuppression is often required in SCI patients who receive NSCs transplantation [102]. The application of immunosuppressants increases the risks of malignancies, infection, and other side effects in humans. The possible solutions include the use of low-immunogenic biomaterials or endogenous NSCs. (4) The exogenous stem cells have the potential to form ectopic aggregates as stem cells migrate and proliferate in the central nervous system [103]. Engineered neural network tissues provide terminally differentiated cells and a stable matrix that successfully mitigates the migration of stem cells [104]. Although no severe adverse events including cancer, infections, and allergic reaction have been identified in some Phase I-II clinical trials, long-term side effects require constant monitoring [105]. (5) The majority of SCI patients can be classified as chronic SCI which is still understudied and its treatments remain more challenging than acute/subacute SCI [1]. In the future, clinical trials will shift their focus to the treatment of chronic SCI.
CONCLUSION
Currently, triggering endogenous neurogenesis represents a potentially practical and feasible strategy for SCI repair [15]. In this review, promising therapeutic strategies, including implantation of biomaterials alone, implantation of biomaterials loaded with neurotrophic factors or small molecule drugs, transplantation of exogenous stem cells, physiotherapy, and combination treatments have been proposed. The current evidence suggests that these strategies may provide a more supportive microenvironment and trigger the migration, proliferation and neuronal differentiation of endogenous NSCs. However, the efficiency and therapeutic effects of single strategies for SCI repair are relatively low and functional recovery is currently unsatisfactory [106]. To better cope with SCI repair, combinatory strategies may be the optimal choice. The combination of physiotherapy with bioactive materials loaded with exogenous stem cells (Fig. 3) can be a promising approach which can trigger endogenous neurogenesis to reconstruct the neural circuits and regulate neuroplasticity for better integration with host nerve tracts. In the future, more clinical studies will be required to ensure the safety of combination therapy and to translate this combination treatment modality into a wide range of clinical settings.
Fig. 3.
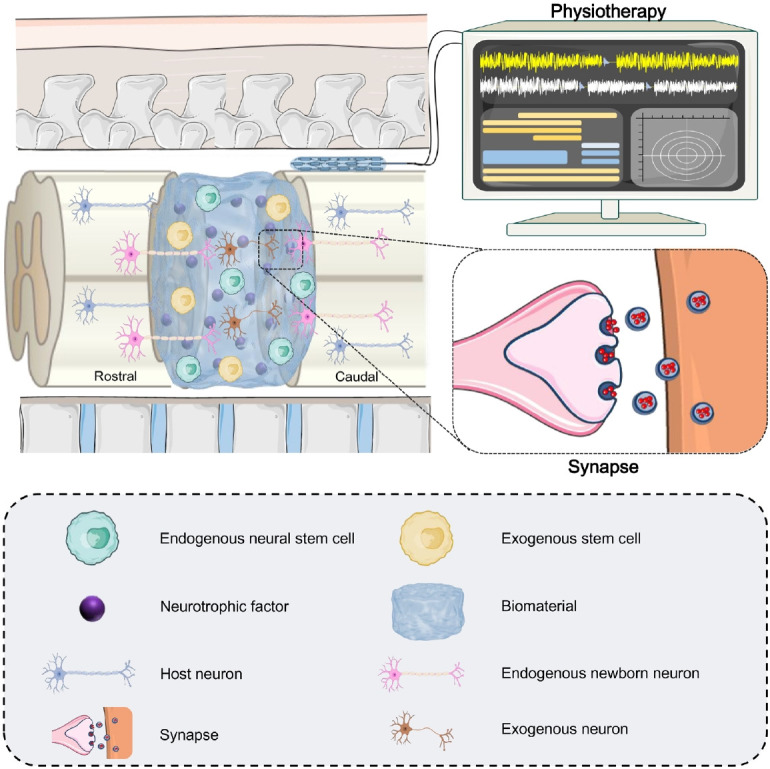
Schematic illustration of combined strategies using exogenous stem cell-seeded biomaterials and physiotherapy for the repair of spinal cord injury.
Acknowledgments
We thank all the researchers who generously shared their data.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Funding/Support
The work was supported by National Natural Science Foundation of China (81971157).
Author Contribution
Conceptualization: HY, SY, BL; Data curation: HY, SY, HL, RW; Formal analysis: HY, SY; Funding acquisition: BL, QZ; Visualization: HY, SY, HL, RW; Writing - original draft: HY, SY; Writing - review & editing: BL, QZ.
REFERENCES
- 1.Ahuja CS, Wilson JR, Nori S, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87. doi: 10.1016/S1474-4422(18)30415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramer LM, Ramer MS, Bradbury EJ. Restoring function after spinal cord injury: towards clinical translation of experimental strategies. Lancet Neurology. 2014;13:1241–56. doi: 10.1016/S1474-4422(14)70144-9. [DOI] [PubMed] [Google Scholar]
- 4.James ND, McMahon SB, Field-Fote EC, et al. Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol. 2018;17:905–17. doi: 10.1016/S1474-4422(18)30287-4. [DOI] [PubMed] [Google Scholar]
- 5.Assinck P, Duncan GJ, Hilton BJ, et al. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20:637–47. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Lu P, McKay HM, et al. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J Neurosci. 2006;26:2157–66. doi: 10.1523/JNEUROSCI.4070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grumbles RM, Thomas CK. Motoneuron death after human spinal cord injury. J Neurotrauma. 2017;34:581–90. doi: 10.1089/neu.2015.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer NR, Wilems TS, Sakiyama-Elbert SE. Stem cells for spinal cord injury: strategies to inform differentiation and transplantation. Biotechnol Bioeng. 2017;114:245–59. doi: 10.1002/bit.26074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson MA, O'Shea TM, Burda JE, et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature. 2018;561:396–400. doi: 10.1038/s41586-018-0467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Tian Z, He L, et al. Exosomes derived from miR-26a-modified MSCs promote axonal regeneration via the PTEN/AKT/mTOR pathway following spinal cord injury. Stem Cell Res Ther. 2021;12:224. doi: 10.1186/s13287-021-02282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sofroniew MV. Dissecting spinal cord regeneration. Nature. 2018;557:343–50. doi: 10.1038/s41586-018-0068-4. [DOI] [PubMed] [Google Scholar]
- 12.Hou S. Relay strategies combined with axon regeneration: a promising approach to restore spinal cord injury. Neural Regen Res. 2014;9:1177–9. doi: 10.4103/1673-5374.135322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohori Y, Yamamoto S, Nagao M, et al. Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J Neurosci. 2006;26:11948–60. doi: 10.1523/JNEUROSCI.3127-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadoya K, Lu P, Nguyen K, et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat Med. 2016;22:479–87. doi: 10.1038/nm.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan H, Song W, Zhao W, et al. Endogenous neurogenesis in adult mammals after spinal cord injury. Science China. Life Sci. 2016;59:1313–8. doi: 10.1007/s11427-016-0205-2. [DOI] [PubMed] [Google Scholar]
- 16.O’Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J Clin Invest. 2017;127:3259–70. doi: 10.1172/JCI90608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okano H, Sakaguchi M, Ohki K, et al. Regeneration of the central nervous system using endogenous repair mechanisms. J Neurochem. 2007;102:1459–65. doi: 10.1111/j.1471-4159.2007.04674.x. [DOI] [PubMed] [Google Scholar]
- 18.Tai W, Wu W, Wang LL, et al. In vivo reprogramming of NG2 glia enables adult neurogenesis and functional recovery following spinal cord injury. Cell Stem Cell. 2021;28:923–37. doi: 10.1016/j.stem.2021.02.009. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Xu C, Xu Y, et al. Decreased regional homogeneity in insula and cerebellum: a resting-state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry Res. 2010;182:211–5. doi: 10.1016/j.pscychresns.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Wu GH, Shi HJ, Che MT, et al. Recovery of paralyzed limb motor function in canine with complete spinal cord injury following implantation of MSC-derived neural network tissue. Biomaterials. 2018;181:15–34. doi: 10.1016/j.biomaterials.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa Y, Sawamoto K, Miyata T, et al. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res. 2002;69:925–33. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton LK, Truong MK, Bednarczyk MR, et al. Cellular organization of the central canal ependymal zone, a niche of latent neural stem cells in the adult mammalian spinal cord. Neuroscience. 2009;164:1044–56. doi: 10.1016/j.neuroscience.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Silva NA, Sousa N, Reis RL, et al. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Sabelstrom H, Stenudd M, Reu P, et al. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342:637–40. doi: 10.1126/science.1242576. [DOI] [PubMed] [Google Scholar]
- 25.Ferretti P. Neural stem cell plasticity: recruitment of endogenous populations for regeneration. Curr Neurovasc Res. 2004;1:215–29. doi: 10.2174/1567202043362397. [DOI] [PubMed] [Google Scholar]
- 26.Goritz C, Dias DO, Tomilin N, et al. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–42. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 27.Barnabe-Heider F, Goritz C, Sabelstrom H, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–82. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Mothe AJ, Tator CH. Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int J Dev Neurosci. 2013;31:701–13. doi: 10.1016/j.ijdevneu.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 29.McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136:32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- 30.Stenudd M, Sabelstrom H, Frisen J. Role of endogenous neural stem cells in spinal cord injury and repair. JAMA Neurol. 2015;72:235–7. doi: 10.1001/jamaneurol.2014.2927. [DOI] [PubMed] [Google Scholar]
- 31.Dickendesher TL, Baldwin KT, Mironova YA, et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci. 2012;15:703–12. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabelstrom H, Stenudd M, Frisen J. Neural stem cells in the adult spinal cord. Exp Neurol. 2014;260:44–9. doi: 10.1016/j.expneurol.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 33.Lai BQ, Che MT, Feng B, et al. Tissue-engineered neural network graft relays excitatory signal in the completely transected canine spinal cord. Adv Sci (Weinh) 2019;6:1901240. doi: 10.1002/advs.201901240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin XY, Lai BQ, Zeng X, et al. Cell transplantation and neuroengineering approach for spinal cord injury treatment: a summary of current laboratory findings and review of literature. Cell Transplant. 2016;25:1425–38. doi: 10.3727/096368916X690836. [DOI] [PubMed] [Google Scholar]
- 35.Picard-Riera N, Nait-Oumesmar B, Baron-Van Evercooren A. Endogenous adult neural stem cells: limits and potential to repair the injured central nervous system. J Neurosci Res. 2004;76:223–31. doi: 10.1002/jnr.20040. [DOI] [PubMed] [Google Scholar]
- 36.Fan C, Li X, Xiao Z, et al. A modified collagen scaffold facilitates endogenous neurogenesis for acute spinal cord injury repair. Acta Biomater. 2017;51:304–16. doi: 10.1016/j.actbio.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Liu D, Xiao Z, et al. Scaffold-facilitated locomotor improvement post complete spinal cord injury: motor axon regeneration versus endogenous neuronal relay formation. Biomaterials. 2019;197:20–31. doi: 10.1016/j.biomaterials.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Caviness VS, Jr, Takahashi T, Nowakowski RS. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 1995;18:379–83. doi: 10.1016/0166-2236(95)93933-o. [DOI] [PubMed] [Google Scholar]
- 39.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–84. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Z, Zhang A, Duan H, et al. NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2015;112:13354–9. doi: 10.1073/pnas.1510194112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin Y, Zhang W, Yang P. Current states of endogenous stem cells in adult spinal cord. J Neurosci Res. 2015;93:391–8. doi: 10.1002/jnr.23480. [DOI] [PubMed] [Google Scholar]
- 42.Liu H, Xu X, Tu Y, et al. Engineering microenvironment for endogenous neural regeneration after spinal cord injury by reassembling extracellular matrix. ACS Appl Mater Interfaces. 2020;12:17207–19. doi: 10.1021/acsami.9b19638. [DOI] [PubMed] [Google Scholar]
- 43.Liu W, Luo Y, Ning C, et al. Thermo-sensitive electroactive hydrogel combined with electrical stimulation for repair of spinal cord injury. J Nanobiotechnol. 2021;19:286. doi: 10.1186/s12951-021-01031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou Y, Ma D, Shen H, et al. Aligned collagen scaffold combination with human spinal cord-derived neural stem cells to improve spinal cord injury repair. Biomater Sci. 2020;8:5145–56. doi: 10.1039/d0bm00431f. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Zhang C, Haggerty AE, et al. The effect of a nanofiber-hydrogel composite on neural tissue repair and regeneration in the contused spinal cord. Biomaterials. 2020;245:119978. doi: 10.1016/j.biomaterials.2020.119978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong LTA, Kim YM, Park HH, et al. An injectable hydrogel enhances tissue repair after spinal cord injury by promoting extracellular matrix remodeling. Nat Commun. 2017;8:533. doi: 10.1038/s41467-017-00583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X, Dai J. Bridging the gap with functional collagen scaffolds: tuning endogenous neural stem cells for severe spinal cord injury repair. Biomater Sci. 2018;6:265–71. doi: 10.1039/c7bm00974g. [DOI] [PubMed] [Google Scholar]
- 48.Zhao X, Wang H, Zou Y, et al. Optimized, visible light-induced crosslinkable hybrid gelatin/hyaluronic acid scaffold promotes complete spinal cord injury repair. Biomed Mater. 2022;17 doi: 10.1088/1748-605X/ac45ec. doi: 10.1088/1748-605X/ac45ec. [DOI] [PubMed] [Google Scholar]
- 49.Zhu R, Zhu X, Zhu Y, et al. Immunomodulatory layered double hydroxide nanoparticles enable neurogenesis by targeting transforming growth factor-β receptor 2. ACS Nano. 2021;15:2812–30. doi: 10.1021/acsnano.0c08727. [DOI] [PubMed] [Google Scholar]
- 50.Benfenati V, Toffanin S, Bonetti S, et al. A transparent organic transistor structure for bidirectional stimulation and recording of primary neurons. Nat Mater. 2013;12:672–80. doi: 10.1038/nmat3630. [DOI] [PubMed] [Google Scholar]
- 51.Zhou H, Xu X, Liu X, et al. Evaluations of gray and white matter volume of brain changes in patients with acute spinal cord injury using voxel-based morphometry. Chin J MedImaging Technol. 2018;34:1337–41. [Google Scholar]
- 52.Luo Y, Fan L, Liu C, et al. An injectable, self-healing, electroconductive extracellular matrix-based hydrogel for enhancing tissue repair after traumatic spinal cord injury. Bioact Mater. 2022;7:98–111. doi: 10.1016/j.bioactmat.2021.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma YH, Shi HJ, Wei QS, et al. Developing a mechanically matched decellularized spinal cord scaffold for the in situ matrix-based neural repair of spinal cord injury. Biomaterials. 2021;279:121192. doi: 10.1016/j.biomaterials.2021.121192. [DOI] [PubMed] [Google Scholar]
- 54.Meletis K, Barnabe-Heider F, Carlen M, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hagg T. From neurotransmitters to neurotrophic factors to neurogenesis. Neuroscientist. 2009;15:20–7. doi: 10.1177/1073858408324789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li X, Yang Z, Zhang A. The effect of neurotrophin-3/chitosan carriers on the proliferation and differentiation of neural stem cells. Biomaterials. 2009;30:4978–85. doi: 10.1016/j.biomaterials.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 57.Lai BQ, Wang JM, Duan JJ, et al. The integration of NSCderived and host neural networks after rat spinal cord transection. Biomaterials. 2013;34:2888–901. doi: 10.1016/j.biomaterials.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 58.Lai BQ, Bai YR, Han WT, et al. Construction of a nichespecific spinal white matter-like tissue to promote directional axon regeneration and myelination for rat spinal cord injury repair. Bioact Mater. 2022;11:15–31. doi: 10.1016/j.bioactmat.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mortazavi Y, Sheikhsaran F, Khamisipour GK, et al. The evaluation of nerve growth factor over expression on neural lineage specific genes in human mesenchymal stem cells. Cell J. 2016;18:189–96. doi: 10.22074/cellj.2016.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Lai X, Wu D, et al. Umbilical mesenchymal stem cell-derived exosomes facilitate spinal cord functional recovery through the miR-199a-3p/145-5p-mediated NGF/ TrkA signaling pathway in rats. Stem Cell Res Ther. 2021;12:117. doi: 10.1186/s13287-021-02148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang SS, Keasey MP, Hagg T. P2X7 receptor inhibition increases CNTF in the subventricular zone, but not neurogenesis or neuroprotection after stroke in adult mice. Transl Stroke Res. 2013;4:533–45. doi: 10.1007/s12975-013-0265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin Bauknight W, Chakrabarty S, Hwang BY, et al. Convection enhanced drug delivery of BDNF through a microcannula in a rodent model to strengthen connectivity of a peripheral motor nerve bridge model to bypass spinal cord injury. J Clin Neurosci. 2012;19:563–9. doi: 10.1016/j.jocn.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 63.Wang N, Zhang S, Zhang AF, et al. Sodium hyaluronateCNTF gelatinous particles promote axonal growth, neurogenesis and functional recovery after spinal cord injury. Spinal Cord. 2014;52:517–23. doi: 10.1038/sc.2014.54. [DOI] [PubMed] [Google Scholar]
- 64.Li G, Che MT, Zhang K, et al. Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials. 2016;83:233–48. doi: 10.1016/j.biomaterials.2015.11.059. [DOI] [PubMed] [Google Scholar]
- 65.Li G, Zhang B, Sun JH, et al. An NT-3-releasing bioscaffold supports the formation of TrkC-modified neural stem cell-derived neural network tissue with efficacy in repairing spinal cord injury. Bioact Mater. 2021;6:3766–81. doi: 10.1016/j.bioactmat.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie Y, Song W, Zhao W, et al. Application of the sodium hyaluronate-CNTF scaffolds in repairing adult rat spinal cord injury and facilitating neural network formation. Science China. Life Sci. 2018;61:559–68. doi: 10.1007/s11427-017-9217-2. [DOI] [PubMed] [Google Scholar]
- 67.Shang J, Qiao H, Hao P, et al. bFGF-sodium hyaluronate collagen scaffolds enable the formation of nascent neural networks after adult spinal cord injury. J Biomed Nanotechnol. 2019;15:703–16. doi: 10.1166/jbn.2019.2732. [DOI] [PubMed] [Google Scholar]
- 68.Hachem LD, Mothe AJ, Tator CH. Positive modulation of AMPA receptors promotes survival and proliferation of neural stem/progenitor cells from the adult rat spinal cord. Stem Cells Dev. 2017;26:1675–81. doi: 10.1089/scd.2017.0182. [DOI] [PubMed] [Google Scholar]
- 69.Hellal F, Hurtado A, Ruschel J, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–31. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li XR, Xiao ZF, Han J, et al. Promotion of neuronal differentiation of neural progenitor cells by using EGFR antibody functionalized collagen scaffolds for spinal cord injury repair. Biomaterials. 2013;34:5107–16. doi: 10.1016/j.biomaterials.2013.03.062. [DOI] [PubMed] [Google Scholar]
- 71.Li X, Tan J, Xiao Z, et al. Transplantation of hUC-MSCs seeded collagen scaffolds reduces scar formation and promotes functional recovery in canines with chronic spinal cord injury. Sci Rep. 2017;7:43559. doi: 10.1038/srep43559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin H, Shen L, Xu C, Liu J. Lentivirus-mediated overexpression of miR-29a promotes axonal regeneration and functional recovery in experimental spinal cord injury via PI3K/Akt/mTOR pathway. Neurochem Res. 2018;43:2038–46. doi: 10.1007/s11064-018-2625-5. [DOI] [PubMed] [Google Scholar]
- 73.Yang Y, Fan Y, Zhang H, et al. Small molecules combined with collagen hydrogel direct neurogenesis and migration of neural stem cells after spinal cord injury. Biomaterials. 2021;269:120479. doi: 10.1016/j.biomaterials.2020.120479. [DOI] [PubMed] [Google Scholar]
- 74.Nishimura S, Yasuda A, Iwai H, et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain. 2013;6:3. doi: 10.1186/1756-6606-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng Y, Mao YR, Yuan TF, et al. Multimodal treatment for spinal cord injury: a sword of neuroregeneration upon neuromodulation. Neural Regen Res. 2020;15:1437–50. doi: 10.4103/1673-5374.274332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lai BQ, Che MT, Du BL, et al. Transplantation of tissue engineering neural network and formation of neuronal relay into the transected rat spinal cord. Biomaterials. 2016;109:40–54. doi: 10.1016/j.biomaterials.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 77.Lai BQ, Zeng X, Han WT, et al. Stem cell-derived neuronal relay strategies and functional electrical stimulation for treatment of spinal cord injury. Biomaterials. 2021;279:121211. doi: 10.1016/j.biomaterials.2021.121211. [DOI] [PubMed] [Google Scholar]
- 78.Reier PJ, Bregman BS, Wujek JR. Intraspinal transplantation of embryonic spinal cord tissue in neonatal and adult rats. J Comp Neurol. 1986;247:275–96. doi: 10.1002/cne.902470302. [DOI] [PubMed] [Google Scholar]
- 79.Houle JD, Reier PJ. Regrowth of calcitonin gene-related peptide (CGRP) immunoreactive axons from the chronically injured rat spinal cord into fetal spinal cord tissue transplants. Neurosci Lett. 1989;103:253–8. doi: 10.1016/0304-3940(89)90108-0. [DOI] [PubMed] [Google Scholar]
- 80.Yuan T, Shao Y, Zhou X, et al. Highly permeable DNA supramolecular hydrogel promotes neurogenesis and functional recovery after completely transected spinal cord injury. Adv Mater. 2021;33:e2102428. doi: 10.1002/adma.202102428. [DOI] [PubMed] [Google Scholar]
- 81.Wang L, Gu S, Gan J, et al. Neural stem cells overexpressing nerve growth factor improve functional recovery in rats following spinal cord injury via modulating microenvironment and enhancing endogenous neurogenesis. Front Cell Neurosci. 2021;15:773375. doi: 10.3389/fncel.2021.773375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van den Brand R, Heutschi J, Barraud Q, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–5. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- 83.Capogrosso M, Milekovic T, Borton D, et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;539:284–8. doi: 10.1038/nature20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rowald A, Komi S, Demesmaeker R, et al. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat Med. 2022;28:260–71. doi: 10.1038/s41591-021-01663-5. [DOI] [PubMed] [Google Scholar]
- 85.Xu H, Yang Y, Deng QW, et al. Governor vessel electro-acupuncture promotes the intrinsic growth ability of spinal neurons through activating calcitonin gene-related peptide/alpha-calcium/calmodulin-dependent protein kinase/neurotrophin-3 pathway after spinal cord injury. J Neurotrauma. 2021;38:734–45. doi: 10.1089/neu.2020.7155. [DOI] [PubMed] [Google Scholar]
- 86.Xu J, Cheng S, Jiao Z, et al. Fire needle acupuncture regulates Wnt/ERK multiple pathways to promote neural stem cells to differentiate into neurons in rats with spinal cord injury. CNS Neurol Disord Drug Targets. 2019;18:245–55. doi: 10.2174/1871527318666190204111701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang YT, Jin H, Wang JH, et al. Tail nerve electrical stimulation and electro-acupuncture can protect spinal motor neurons and alleviate muscle atrophy after spinal cord transection in rats. Neural Plast. 2017;2017:7351238. doi: 10.1155/2017/7351238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zeng YS, Ding Y, Xu HY, et al. Electro-acupuncture and its combination with adult stem cell transplantation for spinal cord injury treatment: a summary of current laboratory findings and a review of literature. CNS Neurosci Ther. 2022;28:635–47. doi: 10.1111/cns.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ceto S, Sekiguchi KJ, Takashima Y, et al. Neural stem cell grafts form extensive synaptic networks that integrate with host circuits after spinal cord injury. Cell Stem Cell. 2020;27:430–40. doi: 10.1016/j.stem.2020.07.007. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lai BQ, Wang JM, Ling EA, et al. Graft of a tissue-engineered neural scaffold serves as a promising strategy to restore myelination after rat spinal cord transection. Stem Cells Dev. 2014;23:910–21. doi: 10.1089/scd.2013.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeng X, Qiu XC, Ma YH, et al. Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials. 2015;53:184–201. doi: 10.1016/j.biomaterials.2015.02.073. [DOI] [PubMed] [Google Scholar]
- 92.Sun JH, Li G, Wu TT, et al. Decellularization optimizes the inhibitory microenvironment of the optic nerve to support neurite growth. Biomaterials. 2020;258:120289. doi: 10.1016/j.biomaterials.2020.120289. [DOI] [PubMed] [Google Scholar]
- 93.Lai BQ, Feng B, Che MT, et al. A modular assembly of spinal cord-like tissue allows targeted tissue repair in the transected spinal cord. Adv Sci (Weinh) 2018;5:1800261. doi: 10.1002/advs.201800261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wenger N, Moraud EM, Gandar J, et al. Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat Med. 2016;22:138–45. doi: 10.1038/nm.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maeda Y, Nakagomi N, Nakano-Doi A, et al. Potential of adult endogenous neural stem/progenitor cells in the spinal cord to contribute to remyelination in experimental autoimmune encephalomyelitis. Cells. 2019;8:1025. doi: 10.3390/cells8091025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fischer I, Dulin JN, Lane MA. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nature reviews. Neuroscience. 2020;21:366–83. doi: 10.1038/s41583-020-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Courtine G, Sofroniew MV. Spinal cord repair: advances in biology and technology. Nat Med. 2019;25:898–908. doi: 10.1038/s41591-019-0475-6. [DOI] [PubMed] [Google Scholar]
- 98.Bonizzato M, Pidpruzhnykova G, DiGiovanna J, et al. Brain-controlled modulation of spinal circuits improves recovery from spinal cord injury. Nat Commun. 2018;9:3015. doi: 10.1038/s41467-018-05282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Strnadel J, Carromeu C, Bardy C, et al. Survival of syngeneic and allogeneic iPSC-derived neural precursors after spinal grafting in minipigs. Sci Transl Med. 2018;10:eaam6651. doi: 10.1126/scitranslmed.aam6651. [DOI] [PubMed] [Google Scholar]
- 100.Yamane J, Nakamura M, Iwanami A, et al. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. J Neurosci Res. 2010;88:1394–405. doi: 10.1002/jnr.22322. [DOI] [PubMed] [Google Scholar]
- 101.Aarabi B, Sansur CA, Ibrahimi DM, et al. Intramedullary lesion length on postoperative magnetic resonance imaging is a strong predictor of ASIA Impairment Scale grade conversion following decompressive surgery in cervical spinal cord injury. Neurosurgery. 2017;80:610–20. doi: 10.1093/neuros/nyw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ma YH, Zeng X, Qiu XC, et al. Perineurium-like sheath derived from long-term surviving mesenchymal stem cells confers nerve protection to the injured spinal cord. Biomaterials. 2018;160:37–55. doi: 10.1016/j.biomaterials.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 103.Steward O, Sharp KG, Yee KM, et al. Characterization of ectopic colonies that form in widespread areas of the nervous system with neural stem cell transplants into the site of a severe spinal cord injury. J Neurosci. 2014;34:14013–21. doi: 10.1523/JNEUROSCI.3066-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosenzweig ES, Brock JH, Lu P, et al. Restorative effects of human neural stem cell grafts on the primate spinal cord. Nat Med. 2018;24:484–90. doi: 10.1038/nm.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang Y, Pang M, Chen YY, et al. Human umbilical cord mesenchymal stem cells to treat spinal cord injury in the early chronic phase: study protocol for a prospective, multicenter, randomized, placebo-controlled, single-blinded clinical trial. Neural Regen Res. 2020;15:1532–8. doi: 10.4103/1673-5374.274347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu Y, Tan B, Wang L, et al. Endogenous neural stem cells in central canal of adult rats acquired limited ability to differentiate into neurons following mild spinal cord injury. Int J Clin Exp Pathol. 2015;8:3835–42. [PMC free article] [PubMed] [Google Scholar]