Abstract
Background
The novel synthetic neuroactive steroid (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) blocks T-type calcium channels but does not directly modulate neuronal γ-aminobutyric acid type A (GABAA) currents like other anaesthetic neurosteroids. As 3β-OH has sex-specific hypnotic effects in adult rats, we studied the mechanism contributing to sex differences in its effects.
Methods
We used a combination of behavioural loss of righting reflex, neuroendocrine, pharmacokinetic, in vitro patch-clamp electrophysiology, and in vivo electrophysiological approaches in wild-type mice and in genetic knockouts of the CaV3.1 T-type calcium channel isoform to study the mechanisms by which 3β-OH and its metabolite produces sex-specific hypnotic effects.
Results
Adult male mice were less sensitive to the hypnotic effects of 3β-OH compared with female mice, and these differences appeared during development. Adult males had higher 3β-OH brain concentrations despite being less sensitive to its hypnotic effects. Females metabolised 3β-OH into the active GABAA receptor positive allosteric modulator (3α,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3α-OH) to a greater extent than males. The 3α-OH metabolite has T-channel blocking properties with sex-specific hypnotic and pharmacokinetic effects. Sex-dependent suppression of the cortical electroencephalogram is more pronounced with 3α-OH compared with 3β-OH.
Conclusions
The sex-specific differences in the hypnotic effect of 3β-OH in mice are attributable to differences in its peripheral metabolism into the more potent hypnotic metabolite 3α-OH.
Keywords: calcium channels, electroencephalogram, metabolism, neuroactive steroid, pharmacokinetics, sex-specific pharmacology
Editor's key points.
-
•
The novel synthetic neuroactive steroid 3β-OH blocks T-type calcium channels but does not directly modulate neuronal GABAA currents like most other anaesthetics.
-
•
The sex-specific pharmacodynamic and pharmacokinetic effects of 3β-OH were investigated in vivo and in vitro.
-
•
Adult male mice were less sensitive to the hypnotic effects of 3β-OH compared with females, and had higher brain concentrations despite being less sensitive.
-
•
Female mice, to a greater extent than males, metabolised 3β-OH into the active GABAA receptor positive allosteric modulator 3α-OH, which has sex-specific hypnotic and pharmacokinetic effects, including greater suppression of the cortical electroencephalogram.
-
•
The greater hypnotic effect of 3β-OH in female mice is attributable to greater metabolism into the potent hypnotic metabolite 3α-OH, emphasising the importance of investigating sex-specific anaesthetic effects.
The novel neuroactive steroid (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3β-OH) has hypnotic properties, with a primary mechanism of action of blocking low voltage-activated T-type calcium channels (T channels).1,2 In adult rats, 3β-OH has sex-specific hypnotic effects.6 Here we studied the mechanisms contributing to the sex differences in its hypnotic effect.
Addressing sex-specific differences is central to developing new therapeutics, as females may have different pharmacodynamic and pharmacokinetic responses to currently used pharmaceuticals. This includes differences in hepatic metabolism, in drug absorption and distribution, and in circulating hormones.3 The US Food and Drug Administration recommended studying sex differences in clinical trials in 1993, and until recently, there has been a disparity in the use of male and female animal models in translational research despite the importance of understanding the differential effects of new therapeutics in men and women.3
Using a combination of pharmacokinetic, behavioural, neuroendocrine, and electrophysiological approaches, we show that sex differences in the hypnotic effects of 3β-OH are driven by differential metabolism of 3β-OH to a γ-aminobutyric acid type A (GABAA) receptor positive allosteric modulator (GABA-PAM) with hypnotic properties, (3α,5β,17β)-3-hydroxyandrostane-17-carbonitrile (3α-OH).
Methods
Animals
We used adult and adolescent male and female C57Bl/6 wild-type (WT) mice and CaV3.1 global knockouts (KOs) on a C57Bl/6J background. Animals were housed in temperature- and humidity-controlled facilities with food and water ad libitum and maintained on a 14:10 light–dark cycle. Experiments were approved by the University of Colorado Anschutz Medical Campus Institutional Animal Care and Use Committee and comply with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments followed ARRIVE guidelines.
Behaviour and pharmacokinetics
To determine levels of hypnosis, animals were injected intraperitoneally (i.p.) or intravenously (i.v.) with either 3β-OH (Fig 1a) or 3α-OH and assessed for time to loss of righting reflex (LORR) and time to regaining the righting reflex as described.4 We performed pharmacokinetic experiments to measure the concentration of 3β-OH and 3α-OH over time in brain, liver, plasma, and urine. We injected adult mice with 3β-OH 100 mg kg−1 i.p. and collected tissue at 0, 10, 20, 30, 40, 60, 80, 120, 180, 360, 540, or 720 min after injection. This dose of 3β-OH was used as a standard dose at which 100% of male and female mice lost their righting reflex. We also injected adult mice with 3α-OH 60 mg kg−1 i.p. and collected tissue at 40 min after injection. This dose of 3α-OH produced relatively similar hypnotic effect to 100 mg kg−1 3β-OH, and later revealed to be the lowest dose at which all male and female mice lost their righting reflex. Tissue samples were processed for high-performance liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS) as described in supplemental information.
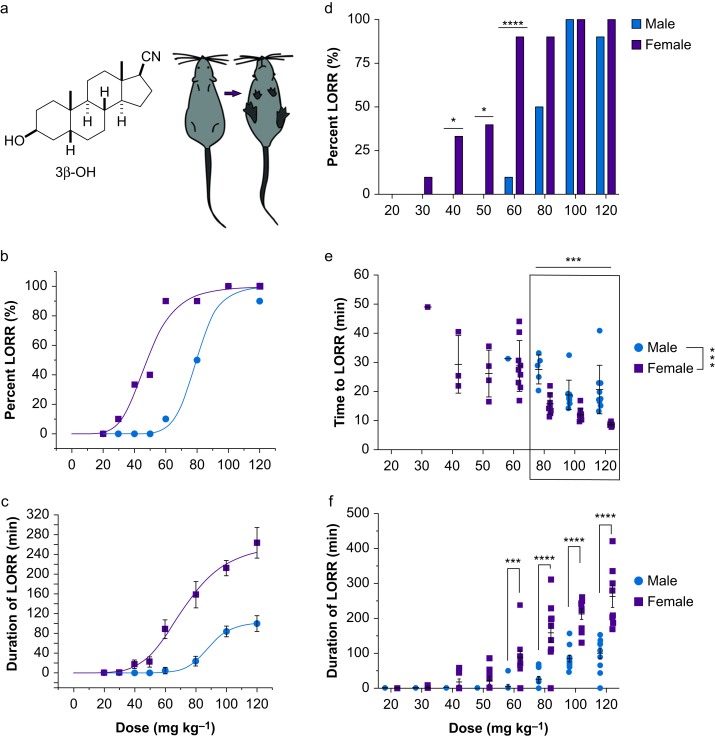
Fig 1.
Hypnotic effects of 3β-OH are more potent in female mice. (a) Male (n=5–10) and female (n=8–10) mice were injected with a range of doses of 3β-OH and tested for LORR. (b) Dose–response results revealed that females lost righting reflex at lower doses of 3β-OH and (c) remained unresponsive longer than males. (d) χ2 analysis showed that males and females did not differ in percentage of LORR at lower doses of 20 mg kg−1 (χ2=0.0, P=1.0) and 30 mg kg−1 (χ2=1.05, P=0.31) or higher hypnotic doses of 80 mg kg−1 (χ2=3.81, P=0.051), 100 mg kg−1 (χ2=0.00, P=1.0), and 120 mg kg−1 (χ2=0.39, P=0.5). At intermediate doses, females were more likely to achieve LORR than males at 40 mg kg−1 (χ2=3.99, ∗P=0.047), 50 mg kg−1 (χ2 =2.81, ∗P = 0.025), and 60 mg kg−1 (χ2=12.80, ∗∗∗∗P<0.0001). (e) At doses between 80 and 120 mg kg−1, indicated by the black box, latency to LORR decreased as dose increased and females lost righting reflex more quickly than males (two-way anova sex by dose, Sidak's multiple comparisons [F2,45 =8.827, ∗∗∗P=0.001 for dose; F1,45=52.346, ∗∗∗P<0.001 for sex]). (f) For duration of LORR, a significant interaction (F7,135=6.477, P<0.001), indicated that at doses at 60 (∗∗∗P<0.001), 80 (∗∗∗∗P<0.0001), 100 (∗∗∗∗P<0.0001), and 120 mg kg–1 (∗∗∗∗P<0.0001), females remained unresponsive longer than males. 3β-OH, (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile; LORR, loss of righting reflex; anova, analysis of variance.
Electrophysiology
To study modulation of T channels, we cultured human embryonic kidney cells (HEK-293) stably transfected with human CaV3.1 channels and conducted in vitro whole-cell patch-clamp electrophysiology in voltage clamp configuration as described.5 We performed in vivo electrophysiology experiments to measure cortical EEG oscillations in adult mice after i.p. administration of 3β-OH or 3α-OH as described.4
Results
Hypnotic effects of 3β-OH are more potent in female mice
A dose–response curve was generated for doses of 20–120 mg kg−1 i.p. to determine the pharmacodynamic properties of 3β-OH. The Hill–Langmuir equation was used to calculate the 50% effective dose (ED50) for percentage and duration of LORR. The ED50 for LORR in female mice was 48 mg kg−1, and the ED50 in male mice was 80 mg kg−1 (Fig 1b). Females also experienced longer duration of hypnosis. Males did not lose righting reflex at doses <60 mg kg−1, whereas LORR was observed in females at doses as low as 30 mg kg−1 (Fig 1c).
Male and female mice did not differ in the percentage of LORR at lower (20–30 mg kg−1) and higher doses (80–120 mg kg−1) of 3β-OH. However, female mice were more likely to achieve LORR than male mice at 40, 50, and 60 mg kg−1 (Fig 1d). For latency to hypnosis, few female and even fewer male mice lost righting reflex at doses <60 mg kg−1. At doses of 80–120 mg kg−1, females lost righting reflex two-fold more quickly than males (Fig 1e), and higher doses produced 3.5-fold longer duration of LORR in females than in males (Fig 1f).
Sex hormones modulate the hypnotic effects of 3β-OH
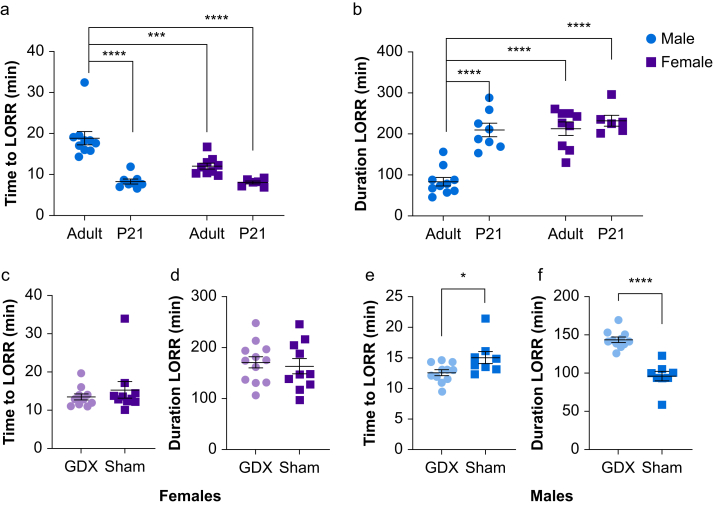
Our previous studies on 3β-OH in neonatal rats found no differences in hypnotic effect between males and females,1 whereas adult rats showed sex differences in EEG power spectra.6 To further explore these age differences, we gave 3β-OH to juvenile male and female mice at P21 and tested for LORR. Similar to previous LORR experiments, adult female mice exhibited LORR sooner than adult male mice. However, P21 male mice lost righting reflex more quickly than adult males, and their LORR values were similar to those of P21 and adult female mice (Fig 2a). Furthermore, P21 male mice lost righting reflex for longer than adult males and had similar duration of LORR as P21 and adult female mice (Fig 2b). To determine the effects of circulating androgens on hypnosis, we gave 3β-OH to gonadectomised and sham-operated female and male mice and tested for LORR. Gonadectomised female mice did not differ from sham-operated females in time to or duration of LORR (Fig 2c and d). However, gonadectomised males lost righting reflex sooner than their sham-operated counterparts (Fig 2e) and LORR lasted longer than for sham-operated males (Fig 2f).
Fig 2.
Sex differences in 3β-OH hypnosis occur after hormonal maturity. (a) We administered 3β-OH 100 mg kg−1 i.p. to juvenile male (n=8) and female (n=6) mice at P21 and tested for LORR in comparison with adult mice (two-way anova sex by age, Sidak's multiple comparisons; F1,29=8.657, P=0.006).Adult (∗∗∗P<0.001) and juvenile (∗∗∗∗P<0.0001) females lost righting reflex faster than adult males, and P21 males achieved LORR more quickly than adult males (∗∗∗∗P<0.0001). (b) For duration of LORR, there was a significant interaction (F1,29=13.38, P=0.001) showing that adult (∗∗∗∗P<0.0001) and juvenile (∗∗∗∗P<0.0001) females were unresponsive longer than adult males. P21 males were unresponsive longer than adult males (∗∗∗∗P<0.0001) and had similar duration of LORR as P21 females and adult females. (c, d) We gave 3β-OH 100 mg kg−1 i.p. to gonadectomised (GDX) and sham-operated females (n=10 and n=12 sham and GDX, respectively) and males (n=8 and n=11 for sham and GDX, respectively) and tested for LORR. Gonadectomised females did not differ from sham females in time to or duration of LORR (unpaired t-test, P=0.385 and P=0.682). (e,f). However, GDX males lost righting reflex sooner than their sham counterparts (t17=2.416, ∗P=0.027) and were unresponsive longer than sham males, (t17=7.071, ∗∗∗∗P<0.0001). 3β-OH, (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile; LORR, loss of righting reflex; anova, analysis of variance.
Sex-specific metabolism modulates the hypnotic effects of 3β-OH
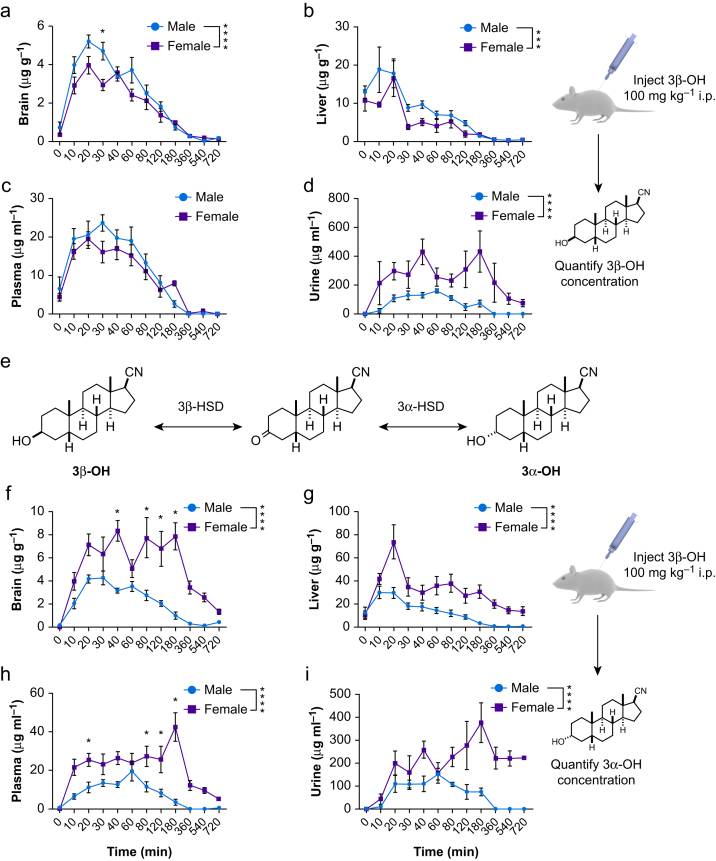
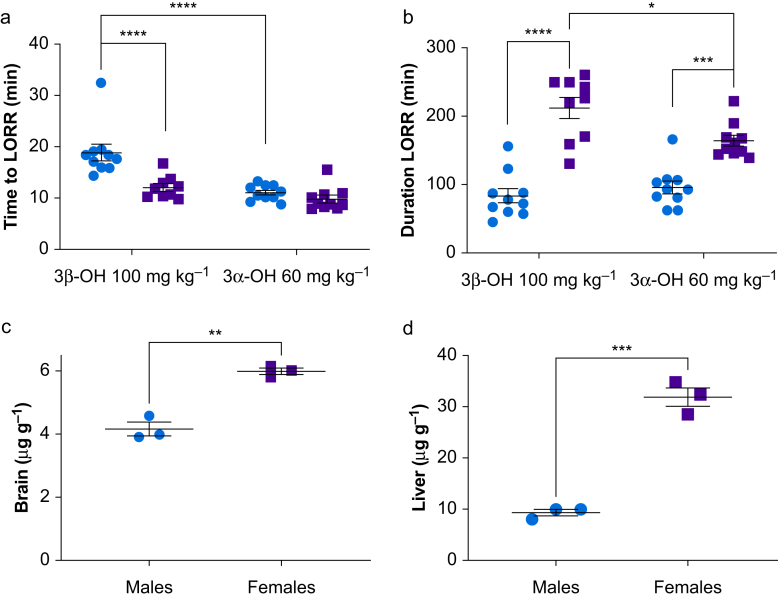
To examine differences in metabolism of 3β-OH, we measured 3β-OH at several times after i.p. administration. Male mice had higher 3β-OH concentrations in brain and liver compared with females (Fig 3a and b). However, there were no significant differences in 3β-OH concentrations in plasma (Fig 3c). Female mice excreted approximately three-fold more 3β-OH in urine compared with males (Fig 3d). Because males were less sensitive to the hypnotic effects of 3β-OH despite having higher brain concentrations, we hypothesised that a metabolite of 3β-OH contributes to hypnosis. We proposed that 3β-OH is metabolised to (3α,5β,17β)-3-hydroxyandrostane 17-carbonitrile (3α-OH) by a combination of 3β- and 3α-hydroxysteroid dehydrogenases (3β- and 3α-HSD, Fig 3e). 3α-OH is a known GABAA receptor positive allosteric modulator and likely a hypnotic.7 We measured 3α-OH concentrations in mice injected with 3β-OH i.p. ,and found that female mice had higher concentrations of 3α-OH in brain, liver, plasma, and urine compared with males (Fig. 3f–i). Moreover, females had higher Cmax and area under the curve (AUC) concentrations of 3α-OH compared with males (Supplementary Table S1).
Fig 3.
Sex-specific metabolism modulates hypnotic effect of 3β-OH. We harvested tissue samples after administration of 100 mg kg−1 of 3β-OH i.p. and measured steroid concentration using HPLC-MS/MS as indicated by cartoon inset on the right (brain and liver, n=2–3 at 0 min and n=5–6 at 10–720 min; plasma and urine, n=1–3 at 0 and 720 min and n=4–6 at 10–540 min). (a–d) In brain, liver, plasma, and urine, concentration of 3β-OH changed across different time points (two-way anova sex by time-point, Sidak's multiple comparisons: F11,108=48.170, P<0.0001; F11,108=16.242, P<0.0001; F1,104=29.355, P<0.0001; F11,94=2.114, P=0.026, respectively). (a) In brain, males had higher 3β-OH concentrations than females (F1,108=12.902, ∗∗∗∗P<0.0001; inset) and the interaction showed that this was specific to 30 (∗P=0.003) min after injection. (b) Males also had higher 3β-OH concentrations in liver (F1,108=9.103, ∗∗∗P=0.003; inset) but no significant interaction. (c) Plasma samples showed no sex effects. (d) In urine, females had higher 3β-OH concentrations compared with males (F1,94=24.982, ∗∗∗∗P<0.0001; inset). (e) Scheme shows that 3β-OH is metabolised into 3α-OH by 3α-HSD in a reversible reaction. (f–i) We found that after injections of 3β-OH i.p., concentrations of 3α-OH changed across different time points in brain, liver, plasma, and urine (F11,108=11.757, P<0.0001; F11,108=10.623, P<0.0001; F11,104=8.911, P<0.0001; F11,94=2.462, P=0.009, respectively). (f) For brain concentrations, females had higher 3α-OH concentrations compared with males (F1,108=85.652, ∗∗∗∗P<0.0001; inset). There was also a significant interaction (F11,108=3.161, P=0.001) showing that this was specific to 40 (∗P<0.0001), 80 (∗P<0.0001), 120 (∗P<0.001), and 180 (∗P<0.0001) min after injection. (g) Females also had higher 3α-OH in liver compared with males (F11,108=64.689, ∗∗∗∗P<0.0001; inset). (h) Similarly, in plasma, females had higher 3α-OH concentrations compared with males (F1,104=83.985, ∗∗∗∗P<0.0001; inset), and there was a significant interaction (F11,104=3.703, P<0.0001), indicating this was specific to 20 (∗P=0.049), 80 (∗P=0.019), 120 (∗P=0.012), and 180 (∗P<0.0001) min after injection of 3β-OH. (i) Females excreted 3α-OH in urine at higher concentrations than males (F1,94=37.160, ∗∗∗∗P<0.0001; inset), and there was no significant interaction. 3β-OH, (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile; LORR, loss of righting reflex; HPLC-MS/MS, high-performance liquid chromatography coupled to tandem mass spectrometry; 3α-OH, (3α,5β,17β)-3-hydroxyandrostane-17-carbonitrile; 3α-HSD, 3α-hydroxysteroid dehydrogenase; 3β-HSD, 3β-hydroxysteroid dehydrogenase; anova, analysis of variance.
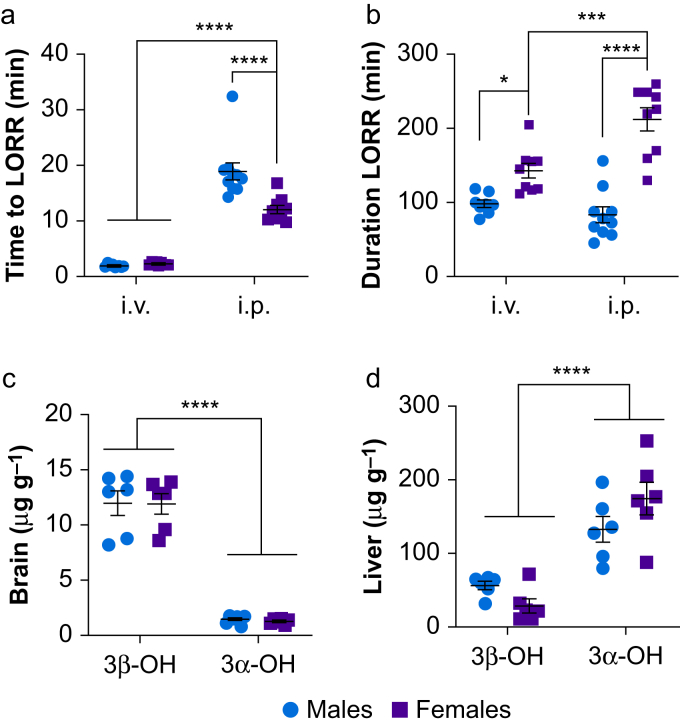
We compared the effects of 3β-OH administered i.v. with the effects of 3β-OH administered i.p. on LORR in dose–response experiments. Both male and female mice lost righting reflex faster after i.v. administration than after i.p. administration. In contrast to i.p. injection, there were no sex differences in time to LORR after i.v. administration (Fig 4a). For duration of LORR, regardless of route of administration, female mice were more sensitive than males. Whereas female mice injected with 3β-OH i.v. lost their righting reflex for a shorter duration compared with mice injected i.p., male mice given 3β-OH i.v. lost righting for a similar duration to males given 3β-OH i.p. (Fig 4b).
Fig 4.
Sex-specific hypnotic effects are less prominent after intravenous than intraperitoneal administration of 3β-OH. We compared the effects of administration of 100 mg kg−1 3β-OH i.v. in adult male (n=8) and female (n=9) mice to animals that received 100 mg kg−1 i.p. in dose–response experiments. (a) A significant interaction (two-way anova sex by route of administration, Sidak's multiple comparisons: F1,32=13.99, P=0.007) revealed that all animals lost righting reflex sooner after i.v. administration compared with i.p. administration (∗∗∗∗P<0.0001 for both), and that females lost righting reflex before males after i.p. administration (∗∗∗∗P<0.0001). However, there were no differences between males and females in time to LORR after i.v. administration (P=0.995). (b) For duration of LORR, a significant interaction (F1,32=13.99, P<0.001) revealed that females were unresponsive longer than males regardless of route of administration (∗∗∗∗P<0.0001 and ∗P=0.045 for i.p. and i.v., respectively). Whereas females were unresponsive for a shorter duration after i.v. administration compared with i.p. administration (∗∗∗P<0.001), males administered 3β-OH i.v. lost LORR for a similar duration as males given 3β-OH i.p. (P=0.788). (c) We measured 3β-OH and 3α-OH brain and liver concentrations after i.v. administration of 3β-OH 100 mg kg−1 at time to LORR (n=6 males, n=6 females). There were no sex differences in 3β-OH or 3α-OH concentrations in the brain at time to LORR (two-way anova sex by steroid, Sidak's multiple comparisons: P>0.999 and P=0.999, respectively). However, 3β-OH concentrations were greater than those of 3α-OH (F1,20=209.6, ∗∗∗∗P<0.0001). (d) At time to loss of righting reflex, a significant interaction revealed (F1,20=5.292, P=0.032) that 3α-OH concentrations were higher in liver than those of 3β-OH across all groups (P<0.001 for all groups). 3β-OH, (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile; LORR, loss of righting reflex; 3α-OH, (3α,5β,17β)-3-hydroxyandrostane-17-carbonitrile; anova, analysis of variance.
We then measured 3β-OH and 3α-OH concentrations in brain and liver at time to LORR after i.v. administration of 3β-OH. We observed no sex differences in 3β-OH concentrations in brain at time of LORR. The brain had notable concentrations of 3α-OH at time to LORR with no differences between males and females. However, 3β-OH concentrations were approximately seven-fold greater than 3α-OH concentrations and exceeded concentrations after i.p. administration in pharmacokinetic experiments (Fig 4c). At the time of LORR, the liver was producing large amounts of 3α-OH, but there were no sex differences for 3β-OH and 3α-OH. However, 3α-OH concentrations were higher than those of 3β-OH across all groups, indicating metabolism occurring early after 3β-OH administration (Fig 4d). These results support the hypothesis that sex differences in time to LORR may be mediated by peripheral metabolism of 3β-OH into 3α-OH.
The metabolite 3α-OH is a potent hypnotic agent
To test whether 3α-OH induces LORR, we generated dose–response curves by measuring LORR between 10 and 80 mg kg−1 i.p.. As the dose increased, time to LORR decreased and duration increased (Supplementary Fig. S1a and b). However, sex differences were only observed for duration of LORR (Supplementary Fig. S1b). To make direct comparisons with 3β-OH, we compared 60 mg kg−1 3α-OH with 100 mg kg−1 3β-OH i.p.. Male and female mice had similar onset of LORR. Moreover, females given 3α-OH lost righting at a similar rate as females given 3β-OH, whereas males given 3α-OH lost righting sooner than males given 3β-OH (Fig 5a). For duration of LORR, sex differences persisted with 3α-OH; females lost their righting reflex approximately two-fold longer than males did (Fig 5b). Whereas 3α-OH i.p. elicited similar duration of LORR in males as 3β-OH, females given 3α-OH lost righting for a shorter duration than females given 3β-OH (Fig 5b).
Fig 5.
3α-OH is a potent hypnotic. We administered 3α-OH 60 mg kg−1 i.p. to adult male (n=10) and female (n=10) mice and measured loss of righting reflex. (a) A significant interaction (two-way anova sex by steroid, Sidak's multiple comparisons: F1,35=7.872, P=0.008) revealed that males and females lost righting reflex at similar times after i.p. injection of 60 mg kg−1 3α-OH (P=0.806). Females given 60 mg kg−1 3α-OH lost righting reflex at a similar time as females given 100 mg kg−1 3β-OH (P=0.391), and more quickly than males given 100 mg kg−1 3β-OH (∗∗∗∗P<0.0001). Males given 3α-OH lost righting reflex sooner than males given 3β-OH (∗∗∗∗P<0.0001). (b) For duration of LORR, a significant interaction (F1,35=7.482, P=0.010) revealed that females lost righting longer than males after injection of 100 mg kg−1 3β-OH (∗∗∗∗P<0.0001) and 60 mg kg−1 3α-OH (∗∗∗P=0.0004). Also, females given 60 mg kg−1 3α-OH lost righting for a shorter duration than females given 100 mg kg−1 3β-OH (∗P=0.022). To determine if sex differences were also attributable to the sex-specific pharmacokinetics of 3α-OH, we injected male (n=3) and female (n=3) mice with 60 mg kg−1 3α-OH and measured brain and liver concentrations of 3α-OH and 3β-OH at 40 min after LORR. (c) Females had higher 3α-OH brain concentrations compared with males (unpaired t-test t4=7.907, ∗∗P=0.0014). (d) Similarly, females had higher liver 3α-OH concentrations than males (t4=11.167, ∗∗∗P=0.0003). 3β-OH, (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile; LORR, loss of righting reflex; 3α-OH, (3α,5β,17β)-3-hydroxyandrostane-17-carbonitrile; anova, analysis of variance.
To determine if sex differences are attributable to sex-specific pharmacokinetic differences, we injected mice with 3α-OH i.p. and measured brain and liver concentrations of 3α-OH and 3β-OH at 40 min after LORR. Females had higher 3α-OH concentrations in both brain and liver compared with males (Fig 5c and d). Only males showed minimal conversion of 3α-OH to 3β-OH for both brain and liver, with liver showing more conversion to 3β-OH than brain (Supplementary Table S2).
Inhibition of CaV3.1 T channels contributes to the hypnotic effects of 3α-OH
We cultured HEK-293 cells expressing the human CaV3.1 subtype to investigate the effects of 3α-OH on T channels. 3α-OH blocked ∼50% of T-channel currents at relevant concentrations (Supplementary Fig. S2a,b). 3α-OH also reduced normalised current after application of 30 and 10 μM and significantly decreased current decay time (τ) compared with baseline (Supplementary Fig. S2c,d). Finally, we plotted activation kinetics (G/Gmax) against steady-state inactivation kinetics (I/Imax) values. 3α-OH at 10 μM produced a hyperpolarising shift in I/Imax V50 of ∼7 mV compared with baseline and shifted G/Gmax V50 to slightly more depolarised potentials ∼3 mV (Supplementary Fig. S2e,f).
Because knockout of T-channel isoform CaV3.1 reduces the hypnotic effect of 3β-OH in male mice,4 we determined the possible role of CaV3.1 channels in the hypnotic effects of 3α-OH. Adult WT and CaV3.1 KO mice were injected with 3α-OH i.p. and tested for LORR. KO mice took ∼40% longer to LORR compared with WT mice, with no sex differences (Supplementary Fig. S3a). KO mice lost righting reflex for a shorter duration compared with WT mice, and females lost righting reflex longer than males (Supplementary Fig. S3b). These results confirm that 3α-OH can partially block T channels at relevant brain concentrations, which contributes to its hypnotic effects.
3β-OH and 3α-OH show sex-specific effects on cortical EEG
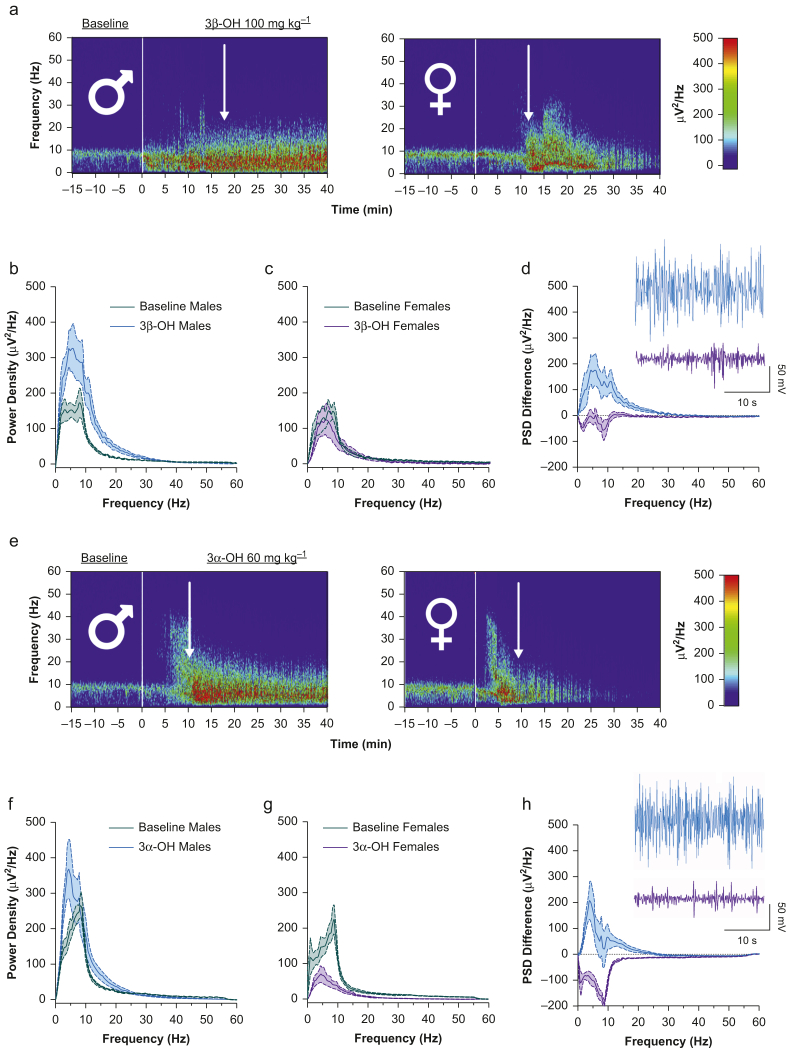
We recently showed that 3β-OH causes sex-specific changes in cortical EEG associated with hypnosis6; however, the mechanism driving these differences is unknown. We analysed power spectral density (PSD) of the EEG in male and female mice before and after receiving either 3β-OH or 3α-OH i.p.. Male mice given 3β-OH showed a sustained increase in PSD in the delta and theta range (1–8 Hz), whereas after an initial increase, PSD in females decreased over time (Fig 6a). To examine this further, we plotted PSD for males and females at baseline and at 40 min after injection (when females achieve Cmax for 3α-OH metabolite). We found that PSD increased in males, but not in females (Fig 6b and c). When we subtracted 3β-OH values from baseline, females showed greater suppression in the EEG compared with males (Fig 6d).
Fig 6.
3β-OH and 3α-OH show sex-specific changes in cortical EEG recordings. We analysed power spectral density (PSD) from male (n=7–9) and female mice (n=7–9) before (baseline) and after receiving either 100 mg kg−1 3β-OH or 60 mg kg−1 3α-OH i.p. (a) Spectral plots showing sustained increase in PSD in males (left panel) and progressive decrease in PSD in females (right panel) after administration of 3β-OH. Arrows indicate average time of LORR from dose–response experiments. Note that females show segments of near-complete suppression over time. (b) At 40 min after injection, males showed increase in PSD compared with baseline, peaking in the delta to theta range (Mann–Whitney U=17 119, P=0.0007). (c) Female PSD is instead suppressed, and does not differ from baseline after 40 min (Mann–Whitney U=12 212, P<0.0001). (d) After subtracting 40 min PSD values from baseline, we found clear sex differences showing that females had suppressed power (Mann–Whitney U=7961, P<0.0001). Representative traces from males in blue and females in pink visually show stark contrast in the raw EEG waveforms. (e) Spectral plots showing sustained increase in PSD in males (left panel) after 3α-OH and profound progressive decrease in female (right panel) PSD with consistent patterns of burst-like suppression. Arrows represent average time to LORR from dose–response curves. (f) 40 min after injection, males showed an increase in PSD values compared with baseline (Mann–Whitney U=16 020, P<0.0001), and the peak appeared to shift from around 10 Hz to slower delta oscillations around 4 Hz. (g) Female PSD also showed a leftward shift toward lower frequency oscillations from baseline. In addition, female EEG showed suppression from baseline at 40 min after injection (Mann–Whitney U=9227, P<0.0001). (h) We subtracted PSD values after 40 min from baseline PSD values and found that females had suppressed power compared with males (t410=8.46, P<0.0001). Representative traces from males in blue and females in pink again highlight the profound suppression in the female EEG waveforms. 3β-OH, (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile; LORR, loss of righting reflex; 3α-OH, (3α,5β,17β)-3-hydroxyandrostane-17-carbonitrile; anova, analysis of variance.
We repeated these experiments with 3α-OH and found even more pronounced effects. After administering 3α-OH i.p., male mice showed a sustained increase in PSD in the delta to theta range, whereas PSD in females decreased over time (Fig 6e). When we plotted the PSD values, PSD in males after 40 min increased from baseline, but PSD in females significantly decreased from baseline (Fig 6f and g). Subtracting 3α-OH values from baselines revealed that females had greater suppression of the EEG compared with males (Fig 6h). Because at 40 min after injection females showed no conversion of 3α-OH to 3β-OH (Supplementary Table S2), we conclude that suppression of EEG in females receiving 3β-OH or 3α-OH may be attributable to the effects of 3α-OH.
Discussion
We found that 3β-OH has sex-specific hypnotic effects in mice. Males become less sensitive to 3β-OH as they age and more sensitive after gonadectomy. Furthermore, sex differences are directly related to sex-specific pharmacokinetic effects, as males had more 3β-OH than females in brain, but females produced higher concentrations of the metabolite 3α-OH, a GABAA receptor positive allosteric modulator. Both pharmacokinetic and EEG data suggest that 3α-OH contributes to the hypnotic and sex-specific effects of 3β-OH. Similar to its pro-compound 3β-OH, 3α-OH blocks the CaV3.1 T-channel subtype in a voltage-dependent manner, which partially contributes to its hypnotic effects.
Sex differences in therapeutic responses is common. For example, women emerge from propofol anaesthesia more quickly than men.8,9 These differences can be attributable to hepatic metabolism, drug absorption, or effects of circulating hormones.3 Previous research on endogenous and synthetic neuroactive steroids have reported that female rodents exhibit longer LORR than their male counterparts, which can be influenced by sex hormones.10, 11, 12 Similar to work by others, we found that female gonadectomy did not diminish neuroactive steroid induced hypnosis, but that male gonadectomy made males more sensitive to hypnosis.12 Our results suggest circulating androgens may contribute to duration of hypnosis induced by 3β-OH in sexually mature mice, but more experiments manipulating oestrogens and androgens are needed.
We found that the observed sex differences are directly related to sex-specific pharmacokinetic effects, specifically drug metabolism, as females produced more of the metabolite 3α-OH compared with males, indicating that sex differences in 3β–OH–induced hypnosis may be attributable to increased concentrations of 3α-OH. This is supported by studies showing that there were no sex differences in hypnotic effects or brain concentrations of 3β-OH or 3α-OH administered i.v. in time to LORR. Sex differences appear when measuring duration of LORR after sufficient time for drug metabolism has passed.
3α-OH is a potent hypnotic, and lower doses of 3α-OH are required to achieve similar hypnotic effects as 3β-OH. The potent hypnotic effects of 3α-OH are likely attributable to its effect as a potent GABAA receptor modulator.7 Female mice do not convert 3α-OH to 3β-OH after administration of 3α-OH, supporting the conclusion that the hypnotic effects, at least in females, are solely attributable to 3α-OH. These results indicate that sex differences in 3β–OH–induced hypnosis may be caused by increased concentrations of its active metabolite 3α-OH.
Our data suggest that 3β-OH is metabolised by 3β-hydroxysteroid dehydrogenase into a 3-keto steroid that is further reduced by 3α-hydroxysteroid dehydrogenase into 3α-OH. We speculate that circulating androgens in adult animals inhibit conversion of 3β-OH to 3α-OH. Mouse 3β-hydroxysteroid dehydrogenase groups I, II, III, and VI convert 3β-reduced steroids into active 3-keto compounds, such as the conversion of pregnenolone to progesterone. Mouse 3β-hydroxysteroid dehydrogenase groups IV and V act as keto-reductases.13 Unlike adult male mice, adult females do not express liver 3β-hydroxysteroid dehydrogenase V, the expression of which increases during sexual maturity.13 Liver 3β-hydroxysteroid dehydrogenase V may be necessary for back conversion of 3β-OH. In humans, 3β-hydroxysteroid dehydrogenase isoforms 1 and 2 are primarily expressed in reproductive organs and adrenal glands as opposed to liver. Various 3α-hydroxysteroid dehydrogenase isoforms are expressed in liver in both humans and mice.14, 15, 16, 17 Although there is sex-specific distribution in some tissues, there are no reported sex differences in liver expression between mouse and humans.17, 18, 19 Furthermore, 3α and 3β reduction are often initial steps in steroid metabolism with sulphation and glucuronidation likely second steps.14 Further studies are warranted to identify the isoforms of 3β-hydroxysteroid dehydrogenase, 3α-hydroxysteroid dehydrogenase, and other liver enzymes that might mediate sex differences in neurosteroid-induced hypnosis.
Our studies are in line with previous work showing that GABAA positive allosteric modulator anaesthetics are T-channel blockers.20,21 3α-OH is a partial T-channel blocker at relevant brain concentrations, and knockout of CaV3.1 reduces its hypnotic effects. After intravenous administration of 3β-OH, its concentrations are eight times higher than those of 3α-OH, and twice as high after intraperitoneal administration at the time to LORR. We posit that T channels play a role in induction of hypnosis by decreasing thalamocortical excitability.4,22, 23, 24 We have shown previously that after intravenous administration of 3β-OH, male and female rats had similar PSD patterns soon after they became sedated.6
Our current findings confirm that 3β-OH increases PSD in the delta and theta frequency range and that EEG power is suppressed in females.4,6 We hypothesise that suppression of the EEG in females is driven by the active metabolite 3α-OH. Burst suppression activity may be caused by changes in cortical metabolism resulting in inhibition of the EEG with periods of excitatory bursts.25,26 This suppression is similar to that of other GABAA receptor positive allosteric modulator anaesthetics, including propofol, isoflurane, and alfaxalone,27,28 but not of the N-methyl-d-aspartate (NMDA) antagonist ketamine.29 Because females do not convert 3α-OH to 3β-OH, we conclude that suppression in the EEG is not attributable to 3β-OH. Alternatively, 3α-OH metabolism may be faster in females than in males, causing the increased power of delta and theta to return to baseline faster in females. However, this is less likely given that female mice exhibited longer LORR and greater suppression of EEG power after injection of 3α-OH compared with males.
Conclusions
Current GABAA receptor modulating general anaesthetics are associated with neurotoxicity in the developing brain and have low therapeutic indexes, highlighting the need for novel, safer anaesthetics.30,31 Our results show that neuroactive steroids act as potent hypnotics that should be investigated for clinical use. We also demonstrate the importance of studying sex-specific effects of anaesthetics, especially pharmacokinetic differences. Because females can have different pharmacodynamic and pharmacokinetic responses to pharmaceuticals, addressing sex-specific differences is central to developing new therapeutics.
Authors' contributions
Conceptualisation of experiments: FMM, DFC, VJT, SMT
Conduct of majority of the experiments: FMM
Data analysis: FMM
Writing of the manuscript: FMM, JK
Pharmacokinetic experiments: OHC, JK
Editing of the manuscript: OHC, DFC, VJT, SMT
Perform loss of righting reflex experiments: DW, BFR
Provided steroid compounds: DFC, KK
Supervision of experiments: SMT
Acknowledgements
We thank the University of Colorado Anschutz Medical Campus In Vivo Neurophysiology Core, which is part of NeuroTechnology Centre, for providing facilities to acquire and review video-EEG data. We thank Charles Adrian Handforth for donating breeding pairs of CaV3.1 knockout mice to our laboratory.
Handling editor: Hugh C Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2022.09.025.
Declaration of interest
The authors have no conflict of interest to disclose.
Funding
US National Institutes of Health (R01GM 123746 to SMT and VJT, and R35GM141802 to SMT); Department of Anesthesiology and School of Medicine at the Anschutz Medical Campus.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Atluri N., Joksimovic S.M., Oklopcic A., et al. A neurosteroid analogue with T-type calcium channel blocking properties is an effective hypnotic, but is not harmful to neonatal rat brain. Br J Anaesth. 2018;120:768–778. doi: 10.1016/j.bja.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Todorovic S.M., Pathirathna S., Brimelow B.C., et al. 5 beta-Reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Mol Pharmacol. 2004;66:1223–1235. doi: 10.1124/mol.104.002402. [DOI] [PubMed] [Google Scholar]
- 3.Soldin P.O., Mattison R.D. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2009;44:143–157. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- 4.Timic Stamenic T., Feseha S., Manzella F.M., et al. The T-type calcium channel isoform Cav 3.1 is a target for the hypnotic effect of the anaesthetic neurosteroid (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile. Br J Anaesth. 2021;126:245–255. doi: 10.1016/j.bja.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joksovic P.M., Brimelow B.C., Murbartián J., Perez-Reyes E., Todorovic S.M. Contrasting anesthetic sensitivities of T-type Ca2+ channels of reticular thalamic neurons and recombinant Ca v3.3 channels. Br J Pharmacol. 2005;144:59–70. doi: 10.1038/sj.bjp.0706020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joksimovic S.M., Sampath D., Krishnan K., et al. Differential effects of the novel neurosteroid hypnotic (3β,5β,17β)-3-hydroxyandrostane-17-carbonitrile on electroencephalogram activity in male and female rats. Br J Anaesth. 2021;127:435–446. doi: 10.1016/j.bja.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covey D.F., Nathan D., Kalkbrenner M., et al. Enantioselectivity of pregnanolone-induced γ-aminobutyric acid(A) receptor modulation and anesthesia. J Pharmacol Exp Ther. 2000;293:1009–1116. [PubMed] [Google Scholar]
- 8.Kodaka M., Suzuki T., Maeyama A., Koyama K., Miyao H. Gender differences between predicted and measured propofol CP50 for loss of consciousness. J Clin Anesth. 2006;18:486–489. doi: 10.1016/j.jclinane.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan F.F., Myles P.S., Cicuttini F. Effect of patient sex on general anaesthesia and recovery. Br J Anaesth. 2011;106:832–839. doi: 10.1093/bja/aer094. [DOI] [PubMed] [Google Scholar]
- 10.Siriarchavatana P., Ayers J.D., Kendall L.V. Anesthetic activity of alfaxalone compared with ketamine in mice. J Am Assoc Lab Anim Sci. 2016;55:426–430. [PMC free article] [PubMed] [Google Scholar]
- 11.Brewster M.E., Anderson W.R., Webb A., Bodor N., Pop E. In: Proceedings of the eighth international symposium on cyclodextrins. Szejtli J., Szente L., editors. Springer; Dordrecht: 1996. Anesthetic activity and pharmacokinetics of the neurosteroid alfaxalone formulated in 2-hydroxypropyl-β-cyclodextrin in the rat; pp. 499–502. [Google Scholar]
- 12.Fink G., Sarkar D.K., Dow R.C., et al. Sex difference in response to alphaxalone anaesthesia may be oestrogen dependent. Nature. 1982;298:270–272. doi: 10.1038/298270a0. [DOI] [PubMed] [Google Scholar]
- 13.Payne A.H., Abbaszade I.G., Clarke T.R., Bain P.A., Park C.H.J. The multiple murine 3β-hydroxysteroid dehydrogenase isoforms: structure, function, and tissue- and developmentally specific expression. Steroids. 1997;1:169–175. doi: 10.1016/s0039-128x(96)00177-8. [DOI] [PubMed] [Google Scholar]
- 14.Schiffer L., Barnard L., Baranowski E.S., et al. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: a comprehensive review. J Steroid Biochem Mol Biol. 2019;194 doi: 10.1016/j.jsbmb.2019.105439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penning T.M., Wangtrakuldee P., Auchus R.J. Structural and functional biology of aldo-keto reductase steroid-transforming enzymes. Endocr Rev. 2018;40:447–475. doi: 10.1210/er.2018-00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergnes L., Phan J., Stolz A., Reue K. A cluster of eight hydroxysteroid dehydrogenase genes belonging to the aldo-keto reductase supergene family on mouse chromosome 13. J Lipid Res. 2003;44:503–511. doi: 10.1194/jlr.M200399-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Bellemare V., Labrie F., Luu-The V. Isolation and characterization of a cDNA encoding mouse 3α-hydroxysteroid dehydrogenase: an androgen-inactivating enzyme selectively expressed in female tissues. J Steroid Biochem Mol Biol. 2006;98:18–24. doi: 10.1016/j.jsbmb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Mitev Y.A., Darwish M., Wolf S.S. Gender differences in the regulation of 3 alpha-hydroxysteroid dehydrogenase in rat brain and sensitivity to neurosteroid-mediated stress protection. Neuroscience. 2003;120:541–549. doi: 10.1016/s0306-4522(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 19.Pirog E.C., Collins D.C. Metabolism of dihydrotestosterone in human liver: importance of 3α- and 3β-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab. 1999;84:3217–3221. doi: 10.1210/jcem.84.9.5963. [DOI] [PubMed] [Google Scholar]
- 20.Eckle V.S., DiGruccio M.R., Uebele V.N., Renger J.J., Todorovic S.M. Inhibition of T-type calcium current in rat thalamocortical neurons by isoflurane. Neuropharmacology. 2012;63:266–273. doi: 10.1016/j.neuropharm.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pathirathna S., Brimelow B.C., Jagodic M.M., et al. New evidence that both T-type calcium channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5α-reduced neuroactive steroids. Pain. 2005;114:429–443. doi: 10.1016/j.pain.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Joksimovic S.M., Izumi Y., Joksimovic S.L., et al. Novel neurosteroid hypnotic blocks T-type calcium channel-dependent rebound burst firing and suppresses long-term potentiation in the rat subiculum. Br J Anaesth. 2019;122:643–651. doi: 10.1016/j.bja.2019.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timic Stamenic T., Feseha S., Valdez R., Zhao W., Klawitter J., Todorovic S.M. Alterations in oscillatory behavior of central medial thalamic neurons demonstrate a key role of CaV3.1 isoform of T-channels during isoflurane-induced anesthesia. Cereb Cortex. 2019;29:4679–4696. doi: 10.1093/cercor/bhz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreyfus F.M., Tscherter A., Errington A.C., et al. Selective T-type calcium channel block in thalamic neurons reveals channel redundancy and physiological impact of ITwindow. J Neurosci. 2010;30:99–109. doi: 10.1523/JNEUROSCI.4305-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukatch H.S., Kiddoo C.E., MacIver M.B. Anesthetic-induced burst suppression EEG activity requires glutamate-mediated excitatory synaptic transmission. Cereb Cortex. 2005;15:1322–1331. doi: 10.1093/cercor/bhi015. [DOI] [PubMed] [Google Scholar]
- 26.Ching S.N., Purdon P.L., Vijayan S., Kopell N.J., Brown E.N. A neurophysiological-metabolic model for burst suppression. Proc Natl Acad Sci U S A. 2012;109:3095–3100. doi: 10.1073/pnas.1121461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenny J.D., Westover M.B., Ching S.N., Brown E.N., Solt K. Propofol and sevoflurane induce distinct burst suppression patterns in rats. Front Syst Neurosci. 2014;8:1–13. doi: 10.3389/fnsys.2014.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visser S.A.G., Smulders C.J.G.M., Reijers B.P.R., Van Der Graaf P.H., Peletier L.A., Danhof M. Mechanism-based pharmacokinetic-pharmacodynamic modeling of concentration-dependent hysteresis and biphasic electroencephalogram effects of alphaxalone in rats. J Pharmacol Exp Ther. 2002;302:1158–1167. doi: 10.1124/jpet.302.3.1158. [DOI] [PubMed] [Google Scholar]
- 29.Cavazzuti M., Porro C.A., Biral G.P., Benassi C., Barbieri G.C. Ketamine effects on local cerebral blood flow and metabolism in the rat. J Cereb Blood Flow Metab. 1987;7:806–811. doi: 10.1038/jcbfm.1987.138. [DOI] [PubMed] [Google Scholar]
- 30.Jevtovic-Todorovic V., Hartman R.E., Izumi Y., et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanley T.H. Anesthesia for the 21st century. Baylor Univ Med Cent Proc. 2000;13:7–10. doi: 10.1080/08998280.2000.11927635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.