Abstract
Background
General anaesthesia in the neonatal period has detrimental effects on the developing mammalian brain. The impact of underlying inflammation on anaesthesia-induced developmental neurotoxicity remains largely unknown.
Methods
Postnatal day 7 (PND7) rats were randomly assigned to receive sevoflurane (3 vol% for 3 h) or carrier gas 12 h after bacterial lipopolysaccharide (LPS; 1 μg g−1) or vehicle injection. Pharmacological inhibition of caspase-1 by Vx-765 (two doses of 50 μg g−1 body weight) was used to investigate mechanistic pathways of neuronal injury. Histomorphological injury and molecular changes were quantified 2 h after the end of anaesthesia. Long-term functional deficits were tested at 5–8 weeks of age using a battery of behavioural tests in the memory and anxiety domains.
Results
Sevoflurane or LPS treatment increased activated caspase-3 and caspase-9 expression in the hippocampal subiculum and CA1, which was greater when sevoflurane was administered in the setting of LPS-induced inflammation. Neuronal injury induced by LPS+sevoflurane treatment resulted in sex-specific behavioural outcomes when rats were tested at 5–8 weeks of age, including learning and memory deficits in males and heightened anxiety-related behaviour in females. Hippocampal caspase-1 and NLRP1 (NLR family pyrin domain containing 1), but not NLRP3, were upregulated by LPS or LPS+sevoflurane treatment, along with related proinflammatory cytokines, interleukin (IL)-1β, and IL-18. Pretreatment with Vx-765, a selective caspase-1 inhibitor, led to reduced IL-1β in LPS and LPS+sevoflurane groups. Caspase-1 inhibition by Vx-765 significantly decreased activated caspase-3 and caspase-9 immunoreactivity in the subiculum.
Conclusions
Systemic inflammation promotes developmental neurotoxicity by worsening anaesthesia-induced neuronal damage with sex-specific behavioural outcomes. This highlights the importance of studying anaesthesia-induced neurotoxicity in more clinically relevant settings.
Keywords: apoptosis, caspase-1, hippocampus, inflammasome, inflammation, lipopolysaccharide, neonatal anaesthesia
Editor's key points.
-
•
General anaesthetics alone can enhance apoptotic neuronal and glial death and produce long-term behavioural deficits in animal models, but whether this is modified by underlying inflammation is unclear.
-
•
Systemic inflammation induce with bacterial lipopolysaccharide (LPS) worsened neuroapoptosis and sex-specific behavioural deficits produced by sevoflurane anaesthesia exposure in neonatal rats.
-
•
These findings demonstrate effects of both sex and systemic inflammation on developmental anaesthetic neurotoxicity in rats.
-
•
These findings highlight the potential impact of underlying pathological conditions on developmental anaesthetic neurotoxicity, which will require clinical validation.
General anaesthesia is required in more than 4 million procedures performed annually in children in the USA. Compelling preclinical evidence suggests that anaesthesia is neurotoxic to developing mammalian brains. By triggering neuroapoptosis through activation of caspase-3, it kills many neurones in the developing brain of rodents1, 2, 3, 4, 5 and non-human primates.6, 7, 8 Especially vulnerable to anaesthetics is the subiculum, a hippocampal region that plays a key role in learning and memory. Hence, damage to this region could underly observed long-lasting cognitive impairments.1,9 Particularly concerning is the growing clinical evidence suggesting an association between early anaesthesia exposure and learning disability, and/or socio-affective disorders later in life.10, 11, 12
Anaesthesia-induced developmental neurotoxicity has largely been studied in isolation from the disease processes that necessitate anaesthesia such as malrotation with volvulus13 or appendicitis.14 Additional pathological states may require extended hospitalisation with prolonged sedation and repeated exposures to anaesthesia for procedures and imaging. Therefore, systemic inflammation preceding anaesthesia is likely common in many clinical scenarios.
Although activation of inflammatory pathways is intended to restore homeostasis, it frequently causes collateral cell death that exacerbates damage caused by the original insult. Rampant inflammation can cause activation of caspase-1-dependent programmed cell death (apoptosis), resulting in release of interleukins (e.g. IL-1β and IL-18) while also activating pro-apoptotic caspase-9 and caspase-3.15,16 As neuroinflammation plays a significant role in brain pathologies,17, 18, 19 we asked whether systemic inflammation preceding general anaesthesia worsens developmental anaesthetic neurotoxicity.
To address this question, we induced systemic inflammation with lipopolysaccharide (LPS) before exposure to the commonly used inhaled anaesthetic sevoflurane. We treated rat pups at the peak of synaptogenesis (postnatal day 7 [PND7]) and assessed acute histomorphological endpoints as well as neurobehaviour later in life, with special focus on neuroapoptotic pathways and their role in systemic inflammation-propagated developmental anaesthetic neurotoxicity.
Methods
Animals
We used PND7 Sprague Dawley (Envigo, Indianapolis, IN, USA) rat pups for all experiments. Rats were housed under a 14/10-h light–dark cycle with ad libitum access to food and water. Animals were acclimated for at least 36 h before experimental procedures.
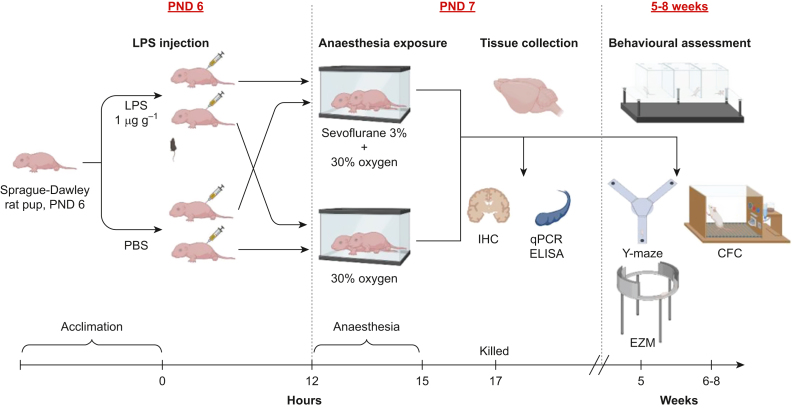
The experimental design summary is shown in Fig 1. Details of the specific procedures are outlined in the Supplementary materials.
Fig 1.
Summary of experimental design, with behavioural timepoints. Postnatal day 6 (PND6) rat pups were randomly assigned to receive either LPS or vehicle injections i.p., and 12 h later, pups were exposed to 3 vol% sevoflurane or 30% oxygen carrier gas mixture for 3 h. Animals were killed and tissue was collected 2 h after the end of anaesthesia. For behavioural assessments, animals from a different cohort were returned to their home cage after anaesthesia, weaned at PND28, and aged to 5 weeks (Y-maze, EZM) or 6–8 weeks (CFC). CFC, contextual fear conditioning; EZM, elevated zero maze; IHC, immunohistochemistry; LPS, lipopolysaccharide; PBS, phosphate-buffered saline; qPCR, quantitative polymerase chain reaction.
Results
Assessment of neuroapoptosis in the subiculum and CA1 regions of hippocampus
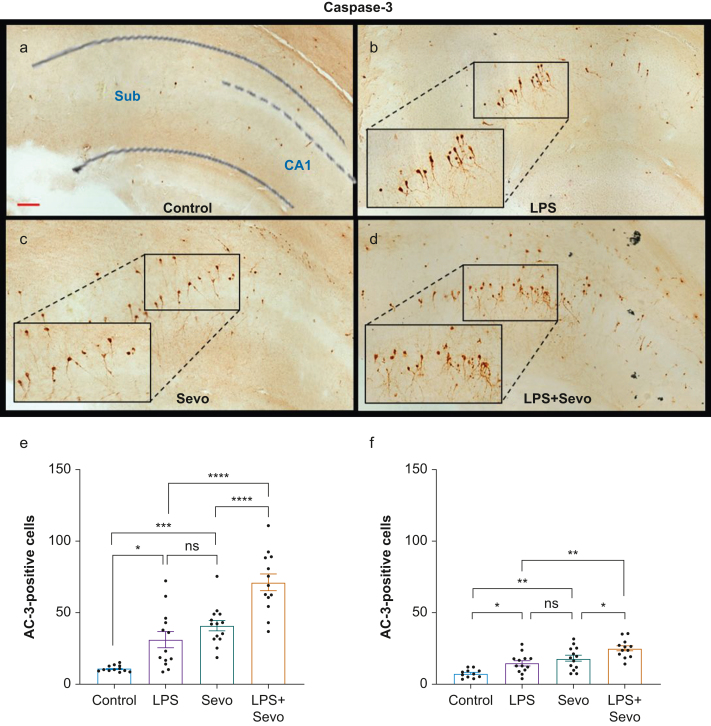
We examined whether LPS-induced systemic inflammation exacerbates sevoflurane-induced neuroapoptosis by focusing on two vulnerable brain regions: the subiculum and the CA1 hippocampal area. We used activated caspase-3 (AC-3) immunohistochemistry as an established marker of developmental neuroapoptosis (Fig. 2a–d). LPS caused a three-fold increase in AC-3-positive cells in the subiculum compared with control (P=0.016, Cohen's F=0.910) (Fig 2e). Similarly, sevoflurane exposure caused a four-fold increase in AC-3-positive neurones compared with control (P<0.001, Cohen's F=1.883). Sevoflurane-induced neuroapoptosis was similar to that caused by LPS (P=0.416). Sevoflurane exposure in the setting of LPS-induced systemic inflammation resulted in a two-fold increase in AC-3-positive cells in the subiculum when compared with LPS (P<0.001, Cohen's F=0.973) or sevoflurane (P<0.001, Cohen's F=0.872). Apoptosis was almost seven-fold higher in the LPS+sevoflurane group compared with control (P<0.001, Cohen's F=2.608).
Fig 2.
Histomorphological analysis of neuronal apoptosis in subiculum and CA1 hippocampal region. (a–d) Representative images of activated caspase-3 (AC-3) staining in subiculum in control group, and after treatment with LPS, sevoflurane or LPS+sevoflurane, respectively. The number of AC-3-positive cells was increased in the LPS+sevoflurane group (d) compared with other groups (a–c). Scale bar is 200 μm. Bar graphs show the apoptogenic action of LPS, sevoflurane, or both as the number of AC-3 positive cells per square millimetre in subiculum (e) and CA1 hippocampal region (f). Both LPS and sevoflurane alone increased AC-3 positive neurones compared with the control group. The proapoptotic effect of sevoflurane and LPS was similar. However, the numbers of AC-3 positive cells were greater compared with either treatment alone. Results are presented as mean (sem). One-way anova + Tukey's post hoc, ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001, ∗∗∗∗P<0.0001. anova, analysis of variance; LPS, lipopolysaccharide; sem, standard error of the mean; Sevo, sevoflurane.
To assess whether increased apoptosis in the setting of LPS-induced systemic inflammation is unique to the subiculum, we examined the CA1 region of hippocampus. Both LPS and sevoflurane increased neuroapoptosis compared with control (P=0.044, Cohen's F=0.740 and P<0.001, Cohen's F=0.977, respectively) (Fig 2f). As in the subiculum, sevoflurane-induced neuroapoptosis was similar to that observed with LPS (P=0.517). LPS+sevoflurane treatment resulted in higher AC-3 positive cells when compared with control (P<0.001, Cohen's F=1.883), LPS (P=0.001, Cohen's F=0.791), or sevoflurane (P=0.045, Cohen's F=0.484).
Taken together, these data suggest that sevoflurane-induced neuroapoptosis is similar to that observed with LPS, and that there is an overall worsening of neuroapoptosis in the subiculum and CA1 in the setting of LPS-induced systemic inflammation.
Long-term behavioural deficits after sevoflurane in the setting of systemic inflammation
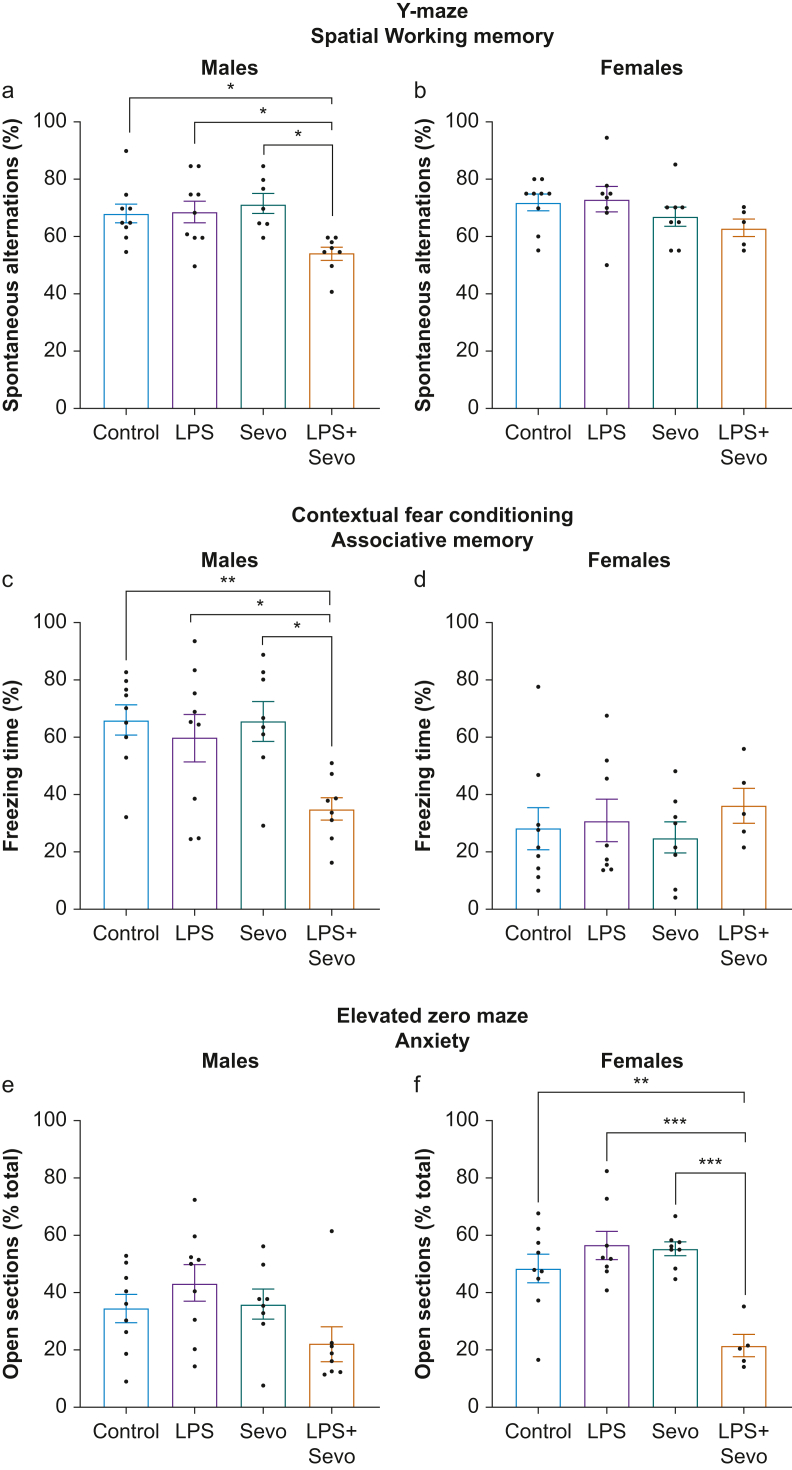
We tested whether hippocampal injury stemming from the neonatal exposure to LPS, sevoflurane, or both alters long-term functional outcomes. We performed two hippocampal-dependent behavioural tasks: Y-maze (spatial working memory) and contextual fear conditioning (CFC; associative memory) at 5 and 6–8 weeks of age, respectively, and one anxiety-related paradigm (elevated zero maze), at 5 weeks of age.
Y-maze. In male mice (Fig 3a), neither LPS nor sevoflurane treatment changed spontaneous alternations compared with control (P=0.998 and P=0.893, respectively). In contrast, LPS+sevoflurane treatment resulted in fewer spontaneous alternations compared with control (P=0.034, Cohen's F=0.837), LPS (P=0.025, Cohen's F=0.781), or sevoflurane (P=0.010, Cohen's F=1.108). Interestingly, females exhibited no difference in spontaneous alternations (P=0.265) (Fig 3b), suggesting sex-specific deficits in hippocampus-dependent spatial working memory.
Fig 3.
Long-term deficits in behavioural task performance after neonatal exposure to LPS, sevoflurane, or both. (a, b) Bar graphs show performance in Y-maze behavioural task in 5-week-old male and female rats, respectively, treated as neonates with LPS, sevoflurane, or both. There were persistent spatial working memory deficits in LPS+sevoflurane treated male, but not in female, rats. (c, d) Bar graphs showing performance of contextual fear conditioning behavioural task in 6- to 8-week-old male and female rats, respectively, after neonatal treatment with LPS, sevoflurane, or both. These data indicate impaired associative memory after neonatal LPS+sevoflurane treatment in male, but not in female, rats. (e, f) In the elevated zero maze, an anxiety-related paradigm, no significant differences were noted in duration of open arm exploration in males (e), whereas LPS+sevoflurane treated females spent much less time in the open sections of the arena (f), indicative of heightened anxiety-related behaviour in females only. No differences were observed in LPS or sevoflurane treated animals. Data shown as mean (sem). One-way anova + Tukey's post hoc, ∗P<0.05, ∗∗P<0.01. anova, analysis of variance; LPS, lipopolysaccharide; sem, standard error of the mean; Sevo, sevoflurane.
Contextual fear conditioning (CFC). In males, neither LPS nor sevoflurane altered freezing time compared with control (P=0.891 and P>0.999, respectively) (Fig 3c). However, LPS+sevoflurane treatment resulted in less freezing compared with control (P=0.010, Cohen's F=1.135), LPS (P=0.049, Cohen's F=0.678), or sevoflurane (P=0.014, Cohen's F=1.004) (Fig 3c). These findings indicate that LPS+sevoflurane treatment in males, unlike either individual treatment, resulted in long-term deficits in learning and memory in the CFC paradigm. In females we observed less freezing in all experimental groups compared with males. We found no differences in freezing time between control, LPS, sevoflurane, and LPS+sevoflurane groups (P=0.752) (Fig 3d).
Elevated zero maze. In males (Fig 3e), we observed no differences between treatment groups in the time spent exploring open sections (P=0.079). In contrast, females treated with LPS+sevoflurane spent less time exploring open sections of the maze compared with control (P=0.002, Cohen's F=1.108), LPS (P<0.001, Cohen's F=1.540), or sevoflurane (P<0.001, Cohen's F=2.197) group (Fig 3f). No differences were found when LPS or sevoflurane were compared with control (P=0.510 and P=0.662, respectively) or to each other (P=0.995).
Taken together, although neither LPS- nor sevoflurane-induced histological injury translated into measurable neurobehavioural deficits, there was sex-specific vulnerability to LPS+sevoflurane treatment that manifested as learning and memory deficits in males, and heightened anxiety-related behaviour in females.
Quantification of inflammasome signalling after LPS and sevoflurane exposure
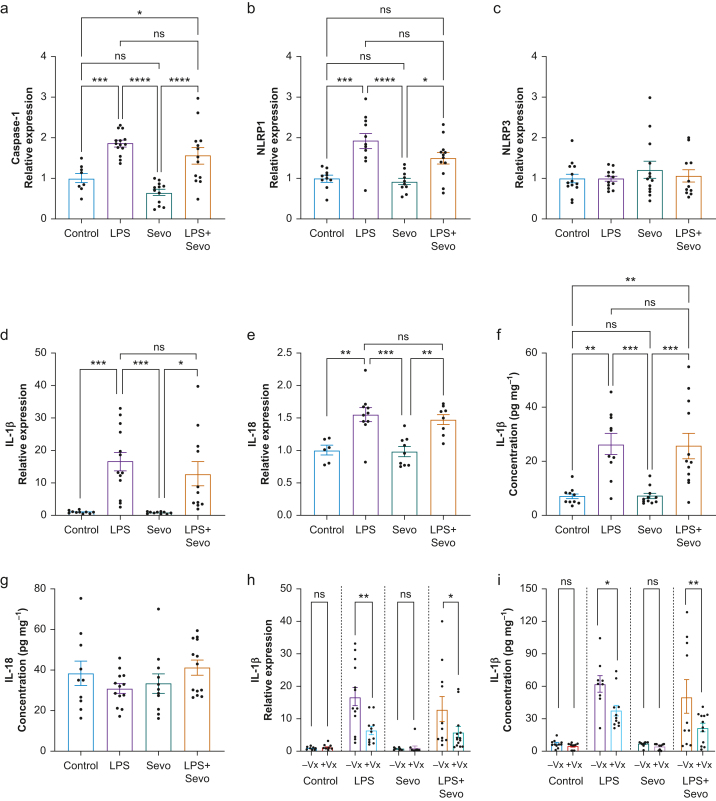
We measured the relative expression of caspase-1 in the hippocampus (Fig 4a) using quantitative polymerase chain reaction (qPCR). We found no difference in caspase-1 mRNA relative expression with sevoflurane treatment vs control (P=0.321). However, there was an 86% increase in caspase-1 expression after LPS injection compared with control (P<0.001) and almost three-fold increase compared with sevoflurane (P<0.001). LPS+sevoflurane treatment resulted in an increase in caspase-1 mRNA levels compared with control (P=0.038) and a 2.4-fold increase compared with sevoflurane (P<0.001). There was no difference in caspase-1 mRNA expression between LPS and LPS+sevoflurane treatment (P=0.327), suggesting that caspase-1 upregulation was primarily driven by LPS-induced systemic inflammation.
Fig 4.
Quantification of inflammasome signalling after treatment with LPS, sevoflurane, or both. (a) LPS alone or in combination with sevoflurane induced higher levels of caspase-1 mRNA compared with control or sevoflurane. Caspase-1 mRNA did not differ between LPS alone or LPS+sevoflurane, indicating that caspase-1 induction is primarily LPS-driven. (b) Hippocampal NLRP1 mRNA followed a similar pattern of induction, although LPS+sevoflurane was marginally insignificant compared with control (P=0.063). (c) Conversely, we observed no differences in NLRP3 mRNA across treatments. Analysis of IL-1β and IL-18 mRNA (d, e) and protein (f, g) levels in hippocampus revealed that upregulation of these cytokines was primarily LPS-driven, whereas sevoflurane did not differ from the control. Whereas mRNA was upregulated after LPS treatment, IL-18 protein levels were not different at this time point. (h, i) Pretreatment with Vx-765 (+Vx), a selective caspase-1 inhibitor, reduced IL-1β mRNA (h) and protein (i) levels. Vx-765 had no effect on baseline IL-1β levels, nor in the sevoflurane group. In each pair, 10% DMSO was administered as vehicle control (–Vx). (a–g) One-way anova+Tukey's post hoc; (h, i) two-way anova+Sidak's post hoc. ∗P<0.05, ∗∗P<0.01, ∗∗∗P<0.001, ∗∗∗∗P<0.0001. anova, analysis of variance; DMSO, dimethyl sulfoxide; IL, interleukin; LPS, lipopolysaccharide; NLRP1, NLR family pyrin domain containing 1; Sevo, sevoflurane.
As proteolytic activity of caspase-1 occurs on cytoplasmic molecular platforms known as inflammasomes, we further characterised this interaction by measuring the mRNA levels of NLRP1 (NLR family pyrin domain containing 1) and NLRP3 using qPCR (Fig 4b and c). Sevoflurane caused no change in NLRP1 mRNA levels (P=0.982) compared with control. Consistent with caspase-1 activation, LPS upregulated NLRP1 compared with control and sevoflurane (P<0.001 for both). LPS+sevoflurane induced NLRP1 to a similar level compared with LPS (P=0.096) and higher compared with sevoflurane (P=0.025), but the difference was insignificant when compared with control (P=0.063). These data suggest that NLRP1 upregulation was driven primarily by LPS, and that the NLRP1 inflammasome might be involved in enabling proteolytic activity of caspase-1. We found no difference in NLRP3 levels between any of the experimental groups (P=0.650; Fig 4c).
We analysed IL-1β (Fig 4d) and IL-18 (Fig 4e) because they are proteolytically cleaved by caspase-1 before secretion. Sevoflurane did not change expression of IL-1β (Fig 4d) or IL-18 (Fig 4e) compared with control (P>0.999 for both). However, LPS increased IL-1β mRNA levels vs control and sevoflurane groups (by 17-fold and 21-fold, respectively; P<0.001 for both). Similarly, LPS+sevoflurane induced a 13-fold increase in mRNA levels of IL-1β compared with control (P=0.014) and 16-fold increase compared with sevoflurane (P=0.012), but no difference compared with LPS (P=0.692) (Fig 4b).
Similar findings were observed with IL-18 (Fig 4c). Specifically, we found that LPS treatment induced a 45% increase in IL-18 mRNA levels compared with control (P=0.002) and a 47% increase compared with sevoflurane (P<0.001). LPS+sevoflurane induced a 48% increase in IL-18 mRNA levels compared with control (P=0.012) and 50% increase compared with sevoflurane (P=0.003), but no difference compared with LPS (P=0.930). These findings suggest that the observed upregulation of the proinflammatory cytokines IL-1β and IL-18 was driven mainly by LPS, and that there was no significant worsening in the presence of sevoflurane.
To validate our qPCR findings, we measured hippocampal levels of IL-1β and IL-18. Sevoflurane did not alter IL-1β levels (Fig 4e) compared with control (P>0.999 for both). LPS increased IL-1β levels compared with control and sevoflurane (P=0.001, Cohen's F=1.251 and P=0.001, Cohen's F=1.258, respectively). Similarly, LPS+sevoflurane increased levels of IL-1β compared with control (P=0.001, Cohen's F=0.966) and sevoflurane (P<0.001, Cohen's F=0.975), with no difference compared with LPS (P=0.998) (Fig 4e). Although IL-18 was detectable in all groups (Fig 4f), no change in levels was observed 17 h after LPS treatment (P=0.257).
To evaluate the role of caspase-1 activation in LPS-induced interleukin release, we examined hippocampal mRNA and protein levels of IL-1β in animals treated with Vx-765, a selective caspase-1 inhibitor. We found a significant effect of Vx-765 pretreatment on hippocampal IL-1β mRNA and protein levels (P<0.001 for both) (Fig 4g and h). There was no effect of Vx-765 in control or sevoflurane group (P>0.999 both for mRNA; P=0.998 and P=0.999 for protein, respectively). However, there was a reduction in hippocampal IL-1β mRNA in the LPS group (62% reduction, P<0.001, Cohen's F=0.706) and in the LPS+sevoflurane group (54% reduction, P=0.043, Cohen's F=0.374). Although the protein concentrations of hippocampal IL-1β measured in this cohort were higher (presumably driven by the DMSO vehicle that was given to all experimental groups),20 there was a reduction with Vx-765 in hippocampal IL-1β levels in the LPS group (P=0.030, Cohen's F=0.613) and in the LPS+sevoflurane group (P=0.005, Cohen's F=0.457). These data show that AC-1 plays an important role in LPS-induced release of IL-1β.
Role of caspase-1, -9, and -3 axis in sevoflurane-induced systemic inflammation and developmental neuroapoptosis
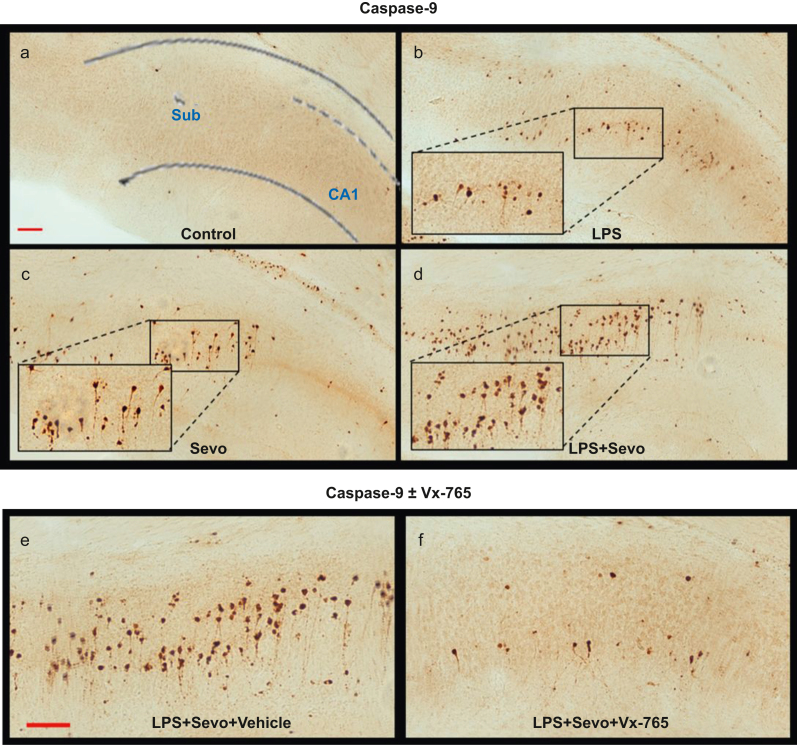
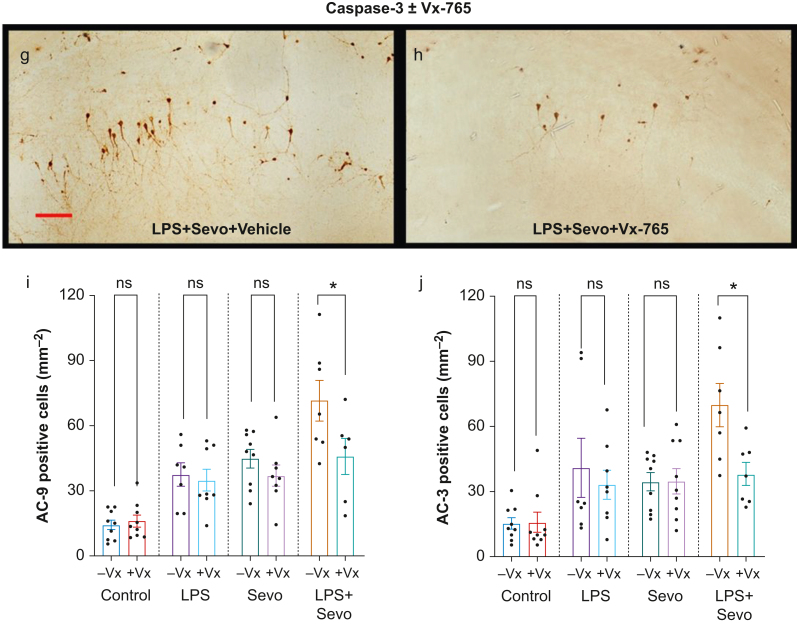
Because inflammation-induced caspase-3-dependent apoptosis relies on activation of the caspase-1/-9 axis,15 we examined whether increased caspase-3 activation caused by sevoflurane in the setting of LPS-induced systemic inflammation is driven by caspase-1 activation. We administered Vx-765, and quantified AC-9 and AC-3 immunoreactivity in the subiculum (Fig. 5a–f). Both LPS (P=0.014, Cohen's F=1.299) and sevoflurane (P=0.001, Cohen's F=1.573) treatments upregulated AC-9 compared with control (Fig 5i). In the LPS+sevoflurane group, the number of AC-9 positive cells was higher than in sevoflurane (P=0.008, Cohen's F=0.705), LPS (P=0.005, Cohen's F=0.823), or control (P<0.001, Cohen's F=1.812) group. The LPS vs sevoflurane groups showed no difference in caspase-9 activation (P=0.941) (Fig 5i), confirming that the sevoflurane effect was comparable with the LPS-induced effect. Vx-765 pretreatment significantly decreased the number of AC-9 positive cells in the LPS+sevoflurane group (P=0.011, Cohen's F=0.571) (Fig 5i) but had no effect in vehicle control (P=0.998), sevoflurane (P=0.718), or LPS (P=0.995) group.
Fig 5.
Histomorphological analysis of activated caspase-9 (AC-9)-positive cells in subiculum and effects of Vx-765 pretreatment. (a–d) Representative images of AC-9 staining in subiculum. Images show AC-9 staining profile similar to that observed with AC-3. Scale bar is 200 μm. (e–h) Side-by-side comparison of representative images of AC-9 (e, f) and AC-3 (g, h) staining in subiculum of animals treated with LPS+sevoflurane, with vehicle vs Vx-765 pretreatment, respectively. Vx-765 pretreatment reduced the number of AC-9 (f) and AC-3 (h) -positive cells compared with vehicle (e and g, respectively) in combined LPS and sevoflurane treatment. Scale bar=200 μM. Bar graphs showing the effect of Vx-765 (+Vx) vs vehicle (10% DMSO, –Vx) pretreatment on subicular AC-9 (i) and AC-3 (j) cell counts. In addition to being non-apoptogenic to controls compared with vehicle, pretreatment with Vx-765 was able to prevent a significant number of AC-3-positive neurones from undergoing apoptosis in subiculum, but had no statistically significant effect when animals were challenged with LPS or sevoflurane alone. Data shown as mean (sem). Two-way anova + Sidak's post hoc, ∗P<0.05. anova, analysis of variance; LPS, lipopolysaccharide; sem, standard error of the mean; Sevo, sevoflurane.
Finally, we examined the effect of Vx-765 pretreatment on caspase-3 activation (Fig 5g and h). Vx-765 pretreatment resulted in reduced AC-3 with LPS+sevoflurane group in the subiculum (P=0.014, Cohen's F=0.784) (Fig 5j) but not in control (P=0.999), sevoflurane (P>0.999), or LPS group (P=0.908).
We conclude that, unlike sevoflurane alone, sevoflurane-induced neuroapoptosis in the setting of LPS-induced systemic inflammation is, at least in part, caspase-1 mediated.
Discussion
We show that sevoflurane-induced developmental neurotoxicity is worsened by systemic inflammation. This was evident in the activation of the caspase-1/-9/-3 axis in the subiculum and CA1 hippocampal region and upregulation of the caspase-1 modulated inflammatory interleukins, IL-1β and IL-18. There were lasting sex-specific behavioural impairment with spatial working and associative memory deficits in males and greater anxiety in females. These findings suggest that anaesthesia in the setting of systemic inflammation during early brain development may be more detrimental to the neonatal brain than previously considered. Our experiments with Vx-765, a selective and potent inhibitor of caspase-1 with virtually no direct effects on caspases-3 and -9,21 implicate caspase-1 in the activation of the caspase-9/-3 axis and upregulation of hippocampal IL-1β and IL-18, which together exacerbate sevoflurane-induced developmental neurotoxicity in the setting of systemic inflammation.
Although a single exposure of 6 h of sevoflurane in rodents caused profound neuroapoptosis and long-lasting behavioural deficits,22 our results suggest that neuroapoptosis after 3 h of sevoflurane might not translate into functional deficits later in life. Conversely, an equivalent duration of sevoflurane exposure compounded by underlying systemic inflammation led to (1) more neuroapoptosis, (2) significant long-term deficits, with (3) sex-specific vulnerabilities, in which males exhibited learning and memory deficits whereas females were affected in the anxiety domain. This dichotomy in behavioural outcomes is consistent with a previous report,23 and highlights the importance of studying sex as a biological variable in anaesthesia-induced developmental neurotoxicity.
There are limited and conflicting studies of the importance of inflammation in anaesthesia-induced developmental neurotoxicity.24, 25, 26 Using the models of skin incision and paw formalin injection, isoflurane+nitrous oxide exposure in young rats worsened both neurotoxicity and long-lasting impairment of cognitive development compared with isoflurane+nitrous oxide alone.27 In contrast, intraplantar injection of complete Freund's adjuvant at the time of anaesthesia ameliorated the neurotoxic effect of ketamine in young rats.26 We used LPS 12 h before anaesthesia, not as a model of sepsis (with its inherent limitations including interspecies differences in endotoxin sensitivity and dynamics of cytokine induction upon challenge28,29), but rather as an established model of systemic inflammation.30 This provided insight into the role of acute illness, often complicated by systemic inflammation, which frequently precedes anaesthesia in clinical settings.
Caspase-1 is widely regarded as an inflammatory caspase. Our data suggest that upregulation of caspase-1 is driven primarily by LPS-induced systemic inflammation, and that caspase-1 activation plays a significant role in activation of proapoptotic caspase-9 and -3, which in turn worsens sevoflurane-induced neuronal damage. Consistent with this finding, caspase-1 knock-down reduced caspase-3 activation in bone marrow-derived macrophages, implicating caspase-1 as an initiator caspase in the apoptotic cascade.16 Moreover, caspase-1-deficient cortical neurones were resistant to ischaemia-induced apoptosis, evidenced by downregulation of both caspase-9 and -3 activation.31 This is in accordance with a study that showed that depletion of caspase-9 (but not caspases-2, -6, -7, or -8) in the setting of caspase-1 driven apoptosis led to a reduction in caspase-3 activation in CL26-iCasp1 cells.15 Caspase-1 is also implicated in other forms of cell death, such as pyroptosis and necroptosis.32 Although our study focuses on caspase-3-mediated apoptosis, our findings warrant future investigation of other cell death mechanisms that may play a role in anaesthesia-induced neuroapoptosis in the setting of systemic inflammation.
Upregulation of NLRP1 in the hippocampus after LPS treatment provides further insight into inflammasome assembly. Inflammasomes are multiprotein cytosolic complexes that serve as molecular platforms for caspase-1 proteolytic activity. This could be the molecular basis for processing and secretion of IL-1β and IL-18, which are downstream of the caspase-1/inflammasome complex. Dysregulation of these proinflammatory cytokines correlates with neurodegenerative diseases and cognitive impairment.33,34 Although the best-studied NLRP3-inflammasome is commonly associated with microglia,35,36 caspase-1/inflammasome complex activity, particularly the NLRP1 inflammasome, are similarly detected in neurones.21,37,38 Although hippocampal NLRP3 levels were unchanged in our study, NLRP1 upregulation in response to LPS suggests that neurones might be, at least in part, involved in inflammasome signalling in the CNS.
Our results support antiapoptotic effects of Vx-765 in the LPS+sevoflurane but not in the LPS group. Although similarly efficient in suppressing hippocampal IL-1β levels in either treatment, Vx-765 did not reduce the number of immunoreactive cells with LPS treatment in contrast with the marked reduction in AC-9 and AC-3 cell counts with LPS+sevoflurane treatment. Notwithstanding this observation, Vx-765 has good oral bioavailability and blood–brain barrier penetration,39 which – together with its established safety profile in phase IIb clinical trials for drug-resistant epilepsy40 – makes it a promising neuroprotective strategy for children undergoing anaesthesia for surgical procedures.
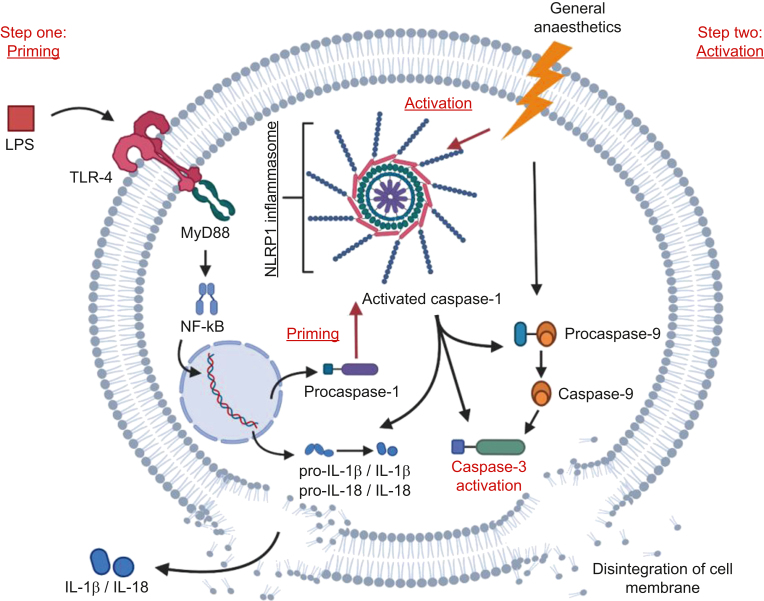
Based on our findings, we propose a model of anaesthesia-induced developmental neuroapoptosis in the setting of systemic inflammation as shown in Fig 6. Once LPS crosses the blood–brain barrier and binds to TLR-4, intracellular signal transduction, possibly via the MyD88–Nf-kB pathway, is activated. This increases transcription of proinflammatory cytokines, switches the brain microenvironment towards an inflammatory state, and primes the caspase-1/NLRP1 inflammasome complex (Step one: Priming). Anaesthesia causes neuronal injury and triggers activation of primed inflammasomes (Step two: Activation). Proteolytic activity of AC-1 leads to cleavage of IL-1β and IL-18, contributing to the inflammatory milieu, while increasing caspase-3 activation, either through direct proteolysis or via cleavage of caspase-9.
Fig 6.
Proposed two-hit hypothesis of worsened anaesthetic neurotoxicity when combined with inflammation. The first hit is provided when LPS crosses the blood–brain barrier and binds to TLR-4 receptors, activating the MyD88–Nf-kB axis. This leads to upregulation of genes that code for the pro-forms of IL-1β and IL-18, as well as pro-caspase-1 and other inflammasome components such as NLRP1, and also inducing the assembly of caspase-1/NLRP1 inflammasome complex. With inflammasome assembled, pro-caspase-1 undergoes auto-proteolytic activation to caspase-1. The second hit is provided by the general anaesthetic, which activates the previously primed inflammasome, in addition to triggering the apoptotic cascade via caspase-9/caspase-3 cleavage. Primed and activated inflammasome and caspase-1 lead to further cleavage of caspase-9 and caspase-3, therefore exacerbating the apoptotic death of neurones. Secretion of IL-1β and IL-18 processed by caspase-1 could promote neuronal apoptosis, thus facilitating neuronal death. IL, interleukin; LPS, lipopolysaccharide; MyD88, myeloid differentiation primary response 88; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP1, NLR family pyrin domain containing 1; TLR, Toll-like receptor.
For more than three decades, we have investigated anaesthesia-induced developmental apoptosis largely in isolation from pathological processes. Here we show that systemic inflammation can worsen anaesthesia-induced neuronal damage in the young brain and potentiate cognitive-affective impairments. These findings reveal potentially detrimental effects of inflammation in worsening anaesthesia-induced neurotoxicity, suggesting that future research should include more in-depth mechanistic studies of neuronal responses to anaesthesia in disease-relevant models.
Authors' contributions
Study design: NU, OHC, NQ, VJ-T
Conduct of experiments: NU, SM, CL
Data analysis: NU, SM, CL, OHC
Data interpretation: NU, SM, OHC, NQ, VJ-T
Manuscript preparation: NU, SM, OHC, NQ, VJ-T
Overall project supervision: NQ, VJ-T
Declaration of interest
The authors declare that they have no conflicts of interest.
Funding
Department of Anesthesiology, University of Colorado, Anschutz Medical Campus (Aurora, CO, USA); US National Institutes of Health (Bethesda, MD, USA: R01 HD097990, R01 GM118197) to VJ-T; F32HD101357 to OHC; CU Medicine Endowment to VJ-T.
Handling editor: Hugh C Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2022.05.008.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jevtovic-Todorovic V., Hartman R.E., Izumi Y., et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabrera O.H., O’Connor S.D., Swiney B.S., et al. Caffeine combined with sedative/anesthetic drugs triggers widespread neuroapoptosis in a mouse model of prematurity. J Matern Fetal Neonatal Med. 2017;30:2734–2741. doi: 10.1080/14767058.2016.1261400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzi S., Carter L.B., Ori C., Jevtovic-Todorovic V. Clinical anesthesia causes permanent damage to the fetal Guinea pig brain. Brain Pathol. 2008;18:198–210. doi: 10.1111/j.1750-3639.2007.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olney J.W., Tenkova T., Dikranian K., et al. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis. 2002;9:205–219. doi: 10.1006/nbdi.2001.0475. [DOI] [PubMed] [Google Scholar]
- 5.Yon J.H., Daniel-Johnson J., Carter L.B., Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi K.K., Johnson S.A., Dissen G.A., et al. Isoflurane exposure for three hours triggers apoptotic cell death in neonatal macaque brain. Br J Anaesth. 2017;119:524–531. doi: 10.1093/bja/aex123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schenning K.J., Noguchi K.K., Martin L.D., et al. Isoflurane exposure leads to apoptosis of neurons and oligodendrocytes in 20- and 40-day old rhesus macaques. Neurotoxicol Teratol. 2017;60:63–68. doi: 10.1016/j.ntt.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slikker W., Jr., Zou X., Hotchkiss C.E., et al. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 9.Paule M.G., Li M., Allen R.R., et al. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilder R.T., Flick R.P., Sprung J., et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu D., Flick R.P., Zaccariello M.J., et al. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology. 2017;127:227–240. doi: 10.1097/ALN.0000000000001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ing C., Hegarty M.K., Perkins J.W., et al. Duration of general anaesthetic exposure in early childhood and long-term language and cognitive ability. Br J Anaesth. 2017;119:532–540. doi: 10.1093/bja/aew413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alani M., Rentea R.M. StatPearls Publishing LLC; 2021. Midgut malrotation. Treasure island (FL) [PubMed] [Google Scholar]
- 14.Marzuillo P., Germani C., Krauss B.S., Barbi E. Appendicitis in children less than five years old: a challenge for the general practitioner. World J Clin Pediatr. 2015;4:19–24. doi: 10.5409/wjcp.v4.i2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuchiya K., Nakajima S., Hosojima S., et al. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat Commun. 2019;10:2091. doi: 10.1038/s41467-019-09753-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagulenko V., Vitak N., Vajjhala P.R., Vince J.E., Stacey K.J. Caspase-1 is an apical caspase leading to caspase-3 cleavage in the AIM2 inflammasome response, independent of caspase-8. J Mol Biol. 2018;430:238–247. doi: 10.1016/j.jmb.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 17.Qin L., Wu X., Block M.L., et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardoso F.L., Herz J., Fernandes A., et al. Systemic inflammation in early neonatal mice induces transient and lasting neurodegenerative effects. J Neuroinflammation. 2015;12:82. doi: 10.1186/s12974-015-0299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badshah H., Ali T., Kim M.O. Osmotin attenuates LPS-induced neuroinflammation and memory impairments via the TLR4/NFkappaB signaling pathway. Sci Rep. 2016;6 doi: 10.1038/srep24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing L., Remick D.G. Mechanisms of dimethyl sulfoxide augmentation of IL-1 beta production. J Immunol. 2005;174:6195–6202. doi: 10.4049/jimmunol.174.10.6195. [DOI] [PubMed] [Google Scholar]
- 21.Flores J., Noel A., Foveau B., Lynham J., Lecrux C., LeBlanc A.C. Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer’s disease mouse model. Nat Commun. 2018;9:3916. doi: 10.1038/s41467-018-06449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satomoto M., Satoh Y., Terui K., et al. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 23.Rothstein S., Simkins T., Nuñez J.L. Response to neonatal anesthesia: effect of sex on anatomical and behavioral outcome. Neuroscience. 2008;152:959–969. doi: 10.1016/j.neuroscience.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anand K.J., Garg S., Rovnaghi C.R., Narsinghani U., Bhutta A.T., Hall R.W. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res. 2007;62:283–290. doi: 10.1203/PDR.0b013e3180986d2f. [DOI] [PubMed] [Google Scholar]
- 25.Liu J., Li Y., Xia X., et al. Propofol reduces microglia activation and neurotoxicity through inhibition of extracellular vesicle release. J Neuroimmunol. 2019;333 doi: 10.1016/j.jneuroim.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J.R., Liu Q., Li J., et al. Noxious stimulation attenuates ketamine-induced neuroapoptosis in the developing rat brain. Anesthesiology. 2012;117:64–71. doi: 10.1097/ALN.0b013e31825ae693. [DOI] [PubMed] [Google Scholar]
- 27.Shu Y., Zhou Z., Wan Y., et al. Nociceptive stimuli enhance anesthetic-induced neuroapoptosis in the rat developing brain. Neurobiol Dis. 2012;45:743–750. doi: 10.1016/j.nbd.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Copeland S., Warren H.S., Lowry S.F., et al. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12:60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nemzek J.A., Hugunin K.M., Opp M.R. Modeling sepsis in the laboratory: merging sound science with animal well-being. Comp Med. 2008;58:120–128. [PMC free article] [PubMed] [Google Scholar]
- 30.Hoogland I.C., Houbolt C., van Westerloo D.J., van Gool W.A., van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W.H., Wang X., Narayanan M., et al. Fundamental role of the Rip2/caspase-1 pathway in hypoxia and ischemia-induced neuronal cell death. Proc Natl Acad Sci U S A. 2003;100:16012–16017. doi: 10.1073/pnas.2534856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertheloot D., Latz E., Franklin B.S. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell Mol Immunol. 2021;18:1106–1121. doi: 10.1038/s41423-020-00630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alboni S., Cervia D., Sugama S., Conti B. Interleukin 18 in the CNS. J Neuroinflammation. 2010;7:9. doi: 10.1186/1742-2094-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Z.B., Sheng G.Q. Interleukin-1β with learning and memory. Neurosci Bull. 2010;26:455–468. doi: 10.1007/s12264-010-6023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tejera D., Mercan D., Sanchez-Caro J.M., et al. Systemic inflammation impairs microglial Abeta clearance through NLRP3 inflammasome. EMBO J. 2019;38 doi: 10.15252/embj.2018101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y., Lu M., Du R.H., et al. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson's disease. Mol Neurodegener. 2016;11:28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaushal V., Dye R., Pakavathkumar P., et al. Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ. 2015;22:1676–1686. doi: 10.1038/cdd.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman W.R., de Rivero Vaccari J.P., Locovei S., et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boxer M.B., Quinn A.M., Shen M., et al. A highly potent and selective caspase 1 inhibitor that utilizes a key 3-cyanopropanoic acid moiety. ChemMedChem. 2010;5:730–738. doi: 10.1002/cmdc.200900531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bialer M., Johannessen S.I., Levy R.H., Perucca E., Tomson T., White H.S. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI) Epilepsy Res. 2013;103:2–30. doi: 10.1016/j.eplepsyres.2012.10.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.