Abstract
Laparoscopic cholecystectomy is one of the most frequently performed procedures in gastrointestinal surgery worldwide. Bleeding complications due to vascular injuries represent an important cause of morbidity and mortality, especially when facing major bleeding during laparoscopy, where bleeding control can be technically challenging in inexperienced hands. Interestingly, the reported incidence rate of conversion to open surgery due to vascular lesions is approximately 0%-1.9%, with a mortality rate of approximately 0.02%. The primary aim of this article was to perform an up-to-date overview regarding the incidence and surgical management of vascular injuries during laparoscopic cholecystectomy according to the available scientific evidence.
Keywords: Laparoscopic cholecystectomy, Vascular injury, Vascular anomalies, Surgical management, Specialized hepatobiliary centers
Core Tip: The theme of biliary injuries in laparoscopic cholecystectomy and the prevention and management of bile duct lesions have been extensively exanimated. However, little attention has been given to vascular injuries. Bleeding complications due to vascular injuries represent an important cause of morbidity and mortality, as well as the negative outcomes of biliary reconstruction when associated with biliary injuries. The vascular lesions should be correctly identified, and surgeons must choose the best therapeutic option to quickly repair the vascular lesion, depending on their own surgical experience and medical center resources. Currently, the management of referrals to specialized hepatobiliary centers for multidisciplinary approaches is mandatory.
INTRODUCTION
Laparoscopic cholecystectomy (LC) is one of the most frequently performed procedures in general surgery worldwide[1]. Iatrogenic vascular and bile duct injuries still represent a major public health problem related to both medico-legal implications and health care costs[1,2]. The theme of biliary injuries in laparoscopic cholecystectomy and the prevention and management of bile duct lesions have been extensively exanimated. However, little attention has been given to vascular lesions[3]. Bleeding complications due to vascular injuries are an important cause of morbidity and mortality, especially during laparoscopy, where bleeding control can be technically challenging in inexperienced hands. Vascular injuries during laparoscopic cholecystectomy may mainly occur during trocar or Veress needle insertion or during dissection of the hepato-cystic triangle. Interestingly, the reported incidence rate of conversion to open surgery due to intra-operative vascular lesions is approximately 0%-1.9%, with a mortality rate of approximately 0.02%[3-5]. The aim of this article was to analyze and explore the incidence, diagnosis and surgical management of vascular injuries in laparoscopic cholecystectomy according to the available scientific evidence. MEDLINE and PubMed searches were performed using the MeSH terms “vascular injury”, “vascular lesion”, “vasculo-biliary injury”, and “laparoscopic cholecystectomy” to identify relevant articles (cohort studies, systematic reviews, case reports, multicenter studies) published in English, French, Spanish, and Italian over the last twenty years.
INCIDENCE AND RISK FACTORS
Several risk factors may contribute to vascular injuries during laparoscopic cholecystectomy: Anatomical factors, including vascular anomalies, patient-related factors, the gallbladder pathology and surgeon’s experience, as summarized in Table 1. Concerning the anatomical factors, the different variants of vascular anatomy may represent a possible cause of bile duct injuries, particularly anomalies of the cystic artery and right hepatic artery (RHA). If surgeons are not aware of possible variations of the RHA, such as in the case of acute and chronic cholecystitis with unclear anatomy of Calot’s triangle, the RHA may be accidentally injured or mistaken for the cystic artery and actively cut off. In a study assessing the frequency of anatomical variations of biliary and vascular systems from Singh et al[6], the operative findings revealed 197 (26.62%) vascular anomalies, mostly related to cystic artery and RHA anatomy. Arterial anomalies are more common, occurring in at least 50% of individuals, and can be recognized only by careful dissection[7]. Based on the classification proposed by Smadja and Blumgart[8], the cystic artery is considered normally positioned when located in the center of the hepato-cystic triangle. In a recent Spanish study performed on a sample of 2000 Laparoscopic cholecystectomy procedures, Noguera et al[9] found an origin of the cystic artery from the RHA in 91.5% of cases. These data are similar to those of Bergamaschi et al[10], where, in a study of 90 consecutive human cadavers, a single artery was found in 59 of 70 specimens (84.3% of all cases).The incidence of third structures within the hepato-cystic triangle was found to be arteries in 36.2% of cases, with a reported incidence of caterpillar hump of the RHA in 12.9% of all cases and double cystic arteries in 5.7%. The most common variations of cystic artery are shown in Figure 1, according to the literature data[7-10]. Among the patient-related factors, overweight and pathological obesity, a history of biliary surgery or endoscopic procedures, and hepatic cirrhosis or chronic liver disease appear to be factors correlated with the development of perioperative complications, both for biliary and vascular structures. However, emergent cholecystectomy for acute cholecystitis increases the risk of iatrogenic lesions, as gallbladder inflammation causes a series of anatomical changes that are associated with an increased risk of iatrogenic injury. Ultimately, the surgeon’s experience plays an important role, and for this reason the importance of a correct “learning curve” for young surgeons should be stressed.
Table 1.
Summary of risk factors associated to vascular injuries
|
Anatomical factors
|
Description
|
| Common vascular variants of cystic artery and right hepatic artery | Single cystic artery[6] |
| Two arteries (superficial and deep)[6] | |
| Single short cystic artery originated from caterpillar right hepatic artery[4,5,10] | |
| Long single cystic artery not from right hepatic artery crossing anterior to the common hepatic duct[7,8] | |
| Double cystic artery/accessory cystic artery[9] | |
| Cystic artery seen more anteriorly than posteriorly in relation to Mascagni’s lymph node[7,9] | |
| A constant vessel found on the postero-lateral margin of gallbladder bed[6,9] | |
| Cystic artery coming from gastroduodenal artery, passing outside Calot’s triangle[6,9] | |
| Patient-related factors | Overweight and pathological obesity[1] |
| History of biliary surgery or endoscopic procedures[1] | |
| Underlying liver disease[1] | |
| Gallbladder pathology | Acute or chronic cholecystitis[1-3] |
| Gallbladder anomalies (gallbladder duplication, gallbladder agenesia, left-side gallbladder)[1-3] | |
| Surgical experience | Learning curve[1,2] |
| Inadequate exposure[1,2,6] | |
| Failure to recognize anatomical landmarks[2,6] |
Figure 1.
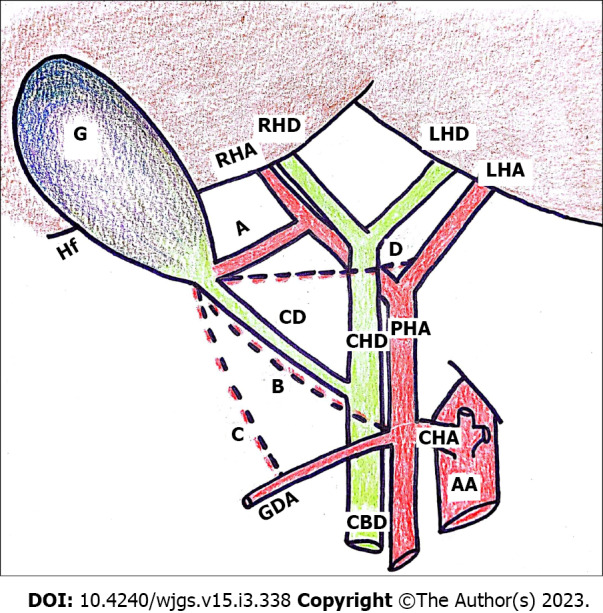
Anatomic illustration of the most common variants of cystic artery. Common variants of cystic artery. A: Cystic artery originating from the right hepatic artery in the classical position[6-8]; B: Long cystic artery seen anteriorly to the cystic duct[6,7]; C: Cystic artery coming from the gastroduodenal artery, passing outside Calot’s triangle[6,9]; D: Long single cystic artery not from the right hepatic artery crossing anterior to the common hepatic duct[6,9]. RHA: Right hepatic artery; LHA: Left hepatic artery; PHA: Proper hepatic artery; CHA: Common hepatic artery; GDA: Gastroduodenal artery; G: Gallbladder; CHD: Common hepatic duct; CD: Cystic duct; CBD: Common bile duct; RHD: Right hepatic duct; LHD: Left hepatic duct; AA: Abdominal aorta.
HEPATIC ARTERY INJURIES
Intra-operative bleeding is certainly the most common and feared complication of arterial injury during laparoscopic procedures, followed by ligation. Among hepatic artery injuries, lesions of the RHA are the most described complication that may occur during laparoscopic cholecystectomy. Hepatic artery closure is usually well tolerated without any particular consequences due to the portal flow and a dense series of collateral arterial branches coming from the hepatic hilum. However, in such cases, hepatic artery ligation can sometimes cause ischemic hepatic necrosis or liver atrophy. RHA injury is frequently encountered in association with bile duct injury, even if the true incidence of RHA injury without concomitant bile duct injury is not clear[11]. In a cadaveric study, Halasz[12] reported that the incidence of injury to the RHA or its branches was only 7%. All patients survived at least one year after cholecystectomy, and they had normal livers. For this reason, the decision to immediately repair the lesion remains controversial. RHA injuries always occur in two ways: the first is when the fundus-first approach for laparoscopic cholecystectomy is performed in the presence of severe inflammation[13]; the second is in the presence of vascular anomalies, as in the case of caterpillar hump of the RHA, where the hepatic artery may be wrongly mistaken for the cystic artery[4,5]. The most important complication of RHA injury is massive bleeding during dissection, which always leads to conversion to open surgery in inexperienced hands. In some cases, laparoscopic repair is feasible and safe[11] by suturing in cases of intra-operative bleeding or direct end-to-end anastomosis when possible in cases of ligation or clipping of the RHA.
Hepatic artery pseudoaneurysm represents another important complication after RHA injury, and it may occur in the early or late postoperative course after LC. Approximately 10% of all the reported cases of hemobilia are secondary to iatrogenic cystic artery or hepatic artery pseudoaneurysms as a consequence of an opening of cystic or RHA pseudoaneurysm within the biliary tree[14]. The exact mechanism of hepatic pseudoaneurysm formation is yet unclear: The most accredited hypotheses concern the mechanical or thermal damage during surgical dissection. Transarterial embolization represents the best therapeutic solution for hepatic artery aneurysm with a high success rate, and surgical repair should be performed in cases where the embolization attempt has failed. In many patients, an open approach in an emergency setting is performed[14], even if a laparoscopic approach is used in some cases[15].
HEPATIC VEINS, PORTAL VEIN AND MAJOR RETROPERITONEAL VESSEL INJURIES
Venous bleeding is less common than arterial bleeding. Bleeding from hepatic vein injury commonly comes from the liver bed during detachment of the gallbladder. Between 10% and 15% of patients may present a large branch of the middle hepatic vein adherent to the liver bed, leading to an increased risk of venous injury during cholecystectomy. In 1999, Misawa et al[16] first proposed an ultrasonographic assessment of the risk of injury to branches of the middle hepatic vein during laparoscopic cholecystectomy by analyzing the middle hepatic vein distance from the gallbladder bed before laparoscopy. Currently, there is controversy about the risk of injury to the branch of the middle hepatic vein during LC[17]. In a previous study, Zhang et al[18] analyzed the anatomical relationship between the gallbladder bed and the branches of the middle hepatic vein in 143 healthy volunteers by color Doppler ultrasound and found that, in most subjects, the branch of the middle hepatic vein and the gallbladder bed were well separated. Only patients with large branches of the middle hepatic vein running very close to the gallbladder bed are at risk of bleeding during laparoscopic cholecystectomy. The diameter of the distal branch of the middle hepatic vein close to the gallbladder bed is reported to vary from 0.9 mm to 3.2 mm and in some cases over 5 mm[17,19,20]. Moreover, according to Ball et al[19], the presence of chronic cholecystitis and fibrous tissue may increase the risk of significant bleeding from the liver bed. Concerning the treatment of venous injuries, bleeding from the middle hepatic vein branch during the operation can only be stopped by direct hemostatic stitches; it can be performed by laparoscopy in experienced hands or can often require conversion to open surgery[21]. In general, we strongly recommend careful dissection during the final steps of laparoscopic cholecystectomy, especially for training surgeons, when dissection becomes easier, and the surgeon may relax.
Portal vein injuries are frequently associated with biliary and RHA injuries. Compared with arterial injury after cholecystectomy, there are very few reports of isolated portal vein injury without associated biliary lesions[22]. Furthermore, as a result of its rarity, the pathogenesis of this type of injury remains unclear. The surgical repair of portal vein lesions is very difficult, often complicated by massive hemorrhage, and seldom successfully managed. When the portal vein is injured during surgery, it should be reconstructed immediately by an experienced hepato-biliary surgeon if the patient is hemodynamically stable. However, the most important complication after surgical repair of a portal vein injury is represented by acute portal thrombosis, often leading to liver infarction. For this reason, anticoagulation therapy should be started as soon as possible to avoid the progression of acute portal vein thrombosis. Liver transplantation is a salvage therapy that should only be considered in end-stage liver disease[22].
Injuries of major retroperitoneal vascular structures are uncommon but potentially life-threatening complications of laparoscopy[23-25]. Inferior vena cava and aorta injuries are frequently associated with trocar or Veress needle insertion during laparoscopic surgery. Early diagnosis and immediate conversion are mandatory for the proper management of these important injuries to minimize morbidity and mortality. Some authors have also described occasional injuries to the right renal artery with the formation of a pseudoaneurysm and consequent renal-vena cava fistula[26,27]. This often represents a late complication following laparoscopic cholecystectomy.
Another important medical complication associated with vena cava injury is venous air embolism[28]; the cardiovascular, pulmonary, and central nervous systems may all be affected, with severity ranging from no symptoms to immediate cardiovascular collapse.
CLASSIFICATION OF VASCULAR INJURIES
Several attempts have been made to uniformly classify vascular lesions, so they are always underreported, as summarized in Table 2. In early 2000, Schäfer et al[29] proposed a working classification from the Swiss Association of Laparoscopic and Thoracoscopic Surgery (SALTS) by defining vascular injuries during laparoscopy into intraoperative and postoperative bleeding complications. In 2007, Bektas et al[30] proposed the Hannover classification by underlining the importance of including additional vascular injury and the location of the biliary lesion at or above the bifurcation of the hepatic duct, as they were found to have a major impact on the extent of surgical intervention for iatrogenic bile duct injury. The Neuhaus and Strasberg-Bismuth classifications for biliary injuries do not consider vascular involvement.
Table 2.
Classifications of vascular injuries during laparoscopic cholecystectomy
|
Ref.
|
Definition of vascular injury
|
| Schäfer et al[29], 2000 | Major injury: Injury to any of the following vessels: Aorta, vena cava, portal vein, hepatic artery and splenic artery, iliac vessels, mesenteric, omental and renal vessels; the vascular injury is classified in: Intra-operative; local haemorrhage within the abdominal cavity, retroperitoneum or abdominal wall; post-operative: Bleeding occurring within 24 h after surgery |
| Bektas et al[30], 2007 | Vascular involvement in different biliary injuries grades (types C and D): Type C tangential injury of the common bile duct: with or without vascular lesion; Type D complete transection of the common bile duct: with or without vascular lesion |
| Kaushik[31], 2010 | Major injury: Any bleeding involving cystic artery, right hepatic artery, portal vein, superior mesenteric vein, suprahepatic veins, inferior vena cava, aorta that requires conversion to open surgery to control/repair; additional surgical procedures; need for blood transfusions |
| Fingerhut et al[32], 2013 | Vasculo-biliary involvement by reporting the type of injured vessel |
| Our study | Major vascular injury: Any bleeding involving right hepatic artery, portal vein, suprahepatic veins, inferior vena cava that always requires conversion to open surgery for control/repair; need for blood transfusions; associated biliary injury; need for transfer to tertiary center |
In 2010, Kaushik[31] suggested a new classification system, wherein vascular injuries were divided into major and minor injuries based on the need for conversion, additional surgical procedures, or blood transfusions.
The European Association for Endoscopic Surgery (EAES) recently proposed a new classification named ATOM, including the anatomy of damage and vascular injury (A), timing of detection (To), and mechanism of damage (M)[32].
VASCULO-BILIARY INJURY
Vascular injury, in particular to the RHA, is found in approximately 12% to 61% of iatrogenic bile duct lesions, leading to high morbidity and mortality associated with altered quality of life[33-35]. It is very important to know preoperatively if a vascular lesion occurs along with a biliary lesion because the poor vascularization of the common bile duct may result in anastomotic strictures after surgical biliary tract repair, recurrent cholangitis and secondary biliary cirrhosis[33]. For these reasons, appropriate knowledge of the vascular lesion represents an important condition when the patient is referred to a specialized hepato-biliary center. In most cases, the surgical repair of biliary and vascular injuries is performed simultaneously. There are no guidelines for the timing of repair, whereas few studies have compared early vs late repair of bile duct injury[34,36,37]. In a multicenter study of the European-African Hepato Pancrea to Biliary Association (E-AHPBA), the timing of biliary reconstruction after bile duct injury with hepaticojejunostomy was not correlated with the occurrence of severe postoperative complications, re-intervention or liver-related mortality[34]. In another multicenter study, the most favorable outcomes were more frequently observed in the immediate (within the first 72 h) and (after 6 wk) reconstruction of biliary injury, and type E4 injury was found to be an independent factor of worse outcome[36]. In general, if a major bile duct transection occurs (types E1-E2 according to the Strasberg classification), the integrity of the hepatic artery, especially the RHA, should always be examined meticulously to plan early vascular reconstruction if technically possible. If revascularization is not technically feasible, biliary reconstruction close to the hilar plate is mandatory in order to minimize the possibility of anastomotic complications[38]. If the vascular lesion is discovered late, in a minority of cases, hepatectomy is needed as a salvage strategy[39,40].
DIAGNOSIS AND SURGICAL MANAGEMENT
The diagnosis of intra-operative bleeding during dissection may be obvious, but it should be correctly identified, and surgeons must choose the best therapeutic option to quickly repair the vascular lesion, depending on their own experience and medical center resources. At present, there is no clear consensus on the most suitable type or time to perform the repair, especially when vascular injury occurs in centers not specialized in complex hepato-biliary surgery; thus, the management of these complications is still a much debated topic[33]. In a recent multicenter retrospective study, the authors analyzed the management of vascular injuries during laparoscopic cholecystectomy, focusing on referral to specialized centers, time to perform the repair, and different treatment option outcomes[33]. In a cohort of 104 patients with vascular injuries, 29 patients underwent vascular repair (27.9%), 13 (12.5%) liver resection, and 1 liver transplant as a first treatment. The majority of vascular and biliary injuries occurred in non-specialized centers, and more than half were immediately transferred. The authors concluded that the management of complex vascular and biliary lesions should be mandatory in specialized centers and that late vascular repair is not necessarily associated with worse outcomes[33]. Another interesting study by Li et al[38] analyzed the effects of vascular reconstruction and hepatic re-arterialization when technically possible. In this study, successful early arterial reconstruction with or without a vascular graft (within 4 d) allowed recovery from hepatic ischemia, without any evidence of hepatic atrophy or necrosis during follow-up.
CONCLUSION
Vascular injuries represent a life-threatening complication, and they should be carefully evaluated along with biliary lesions during laparoscopic cholecystectomy. The recognition of these lesions must occur as early as possible, and the surgeon must choose the best therapeutic option for the patient according to available medical resources. Currently, the management of referrals to specialized hepato-biliary centers for multidisciplinary approaches is mandatory.
Footnotes
Conflict-of-interest statement: There are no conflicts of interest to report.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: November 27, 2022
First decision: December 26, 2022
Article in press: February 22, 2023
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chuang SH, Taiwan; Cochior D, Romania S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
Contributor Information
Antonio Pesce, Department of Surgery, University of Ferrara, Azienda USL of Ferrara, Azienda USL of Ferrara, Lagosanto 44023, Ferrara, Italy. antonio.pesce@ausl.fe.it.
Nicolò Fabbri, Department of Surgery, University of Ferrara, Azienda USL of Ferrara, Azienda USL of Ferrara, Lagosanto 44023, Ferrara, Italy.
Carlo Vittorio Feo, Department of Surgery, University of Ferrara, Azienda USL of Ferrara, Azienda USL of Ferrara, Lagosanto 44023, Ferrara, Italy.
References
- 1.Pesce A, Palmucci S, La Greca G, Puleo S. Iatrogenic bile duct injury: impact and management challenges. Clin Exp Gastroenterol. 2019;12:121–128. doi: 10.2147/CEG.S169492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pesce A, Diana M. Critical View of Safety During Laparoscopic Cholecystectomy: From the Surgeon's Eye to Fluorescent Vision. Surg Innov. 2018;25:197–198. doi: 10.1177/1553350618763200. [DOI] [PubMed] [Google Scholar]
- 3.Tzovaras G, Dervenis C. Vascular injuries in laparoscopic cholecystectomy: an underestimated problem. Dig Surg. 2006;23:370–374. doi: 10.1159/000097951. [DOI] [PubMed] [Google Scholar]
- 4.Marano L, Bartoli A, Polom K, Bellochi R, Spaziani A, Castagnoli G. The unwanted third wheel in the Calot's triangle: Incidence and surgical significance of caterpillar hump of right hepatic artery with a systematic review of the literature. J Minim Access Surg. 2019;15:185–191. doi: 10.4103/jmas.JMAS_75_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pesce A, Fabbri N, Labriola R, Barbara SJ, Feo C. Moynihan's hump of the right hepatic artery: the surgeon's eye cannot recognize what mind doesn't know. ANZ J Surg. 2022 doi: 10.1111/ans.18120. [DOI] [PubMed] [Google Scholar]
- 6.Singh K, Singh R, Kaur M. Clinical reappraisal of vasculobiliary anatomy relevant to laparoscopic cholecystectomy. J Minim Access Surg. 2017;13:273–279. doi: 10.4103/jmas.JMAS_268_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott-Conner CE, Hall TJ. Variant arterial anatomy in laparoscopic cholecystectomy. Am J Surg. 1992;163:590–592. doi: 10.1016/0002-9610(92)90563-7. [DOI] [PubMed] [Google Scholar]
- 8.Smadja C, Blumgart LH. The biliary tract and the anatomy of biliary exposure. In: Blumgart LH. Surgery of the liver and biliary tract. Edinburgh: Churchill Livingstone, 1988. [Google Scholar]
- 9.Noguera MA, Romero CA, Martinez AG, Diaz SRH, Rotger M, Espeche F. Findings and proposal for systematization of surgically important variations of the cystic artery based on an in vivo study of 2000 outpatient laparoscopic cholecystectomies. Int J Morphol . 2020;38:30–34. [Google Scholar]
- 10.Bergamaschi R, Ignjatovic D. More than two structures in Calot's triangle. A postmortem study. Surg Endosc. 2000;14:354–357. doi: 10.1007/s004640000154. [DOI] [PubMed] [Google Scholar]
- 11.Fujioka S, Fuke A, Funamizu N, Nakayoshi T, Okamoto T, Yanaga K. Laparoscopic repair for intraoperative injury of the right hepatic artery during cholecystectomy. Asian J Endosc Surg. 2015;8:75–77. doi: 10.1111/ases.12151. [DOI] [PubMed] [Google Scholar]
- 12.Halasz NA. Cholecystectomy and hepatic artery injuries. Arch Surg. 1991;126:137–138. doi: 10.1001/archsurg.1991.01410260021002. [DOI] [PubMed] [Google Scholar]
- 13.Strasberg SM, Gouma DJ. 'Extreme' vasculobiliary injuries: association with fundus-down cholecystectomy in severely inflamed gallbladders. HPB (Oxford) 2012;14:1–8. doi: 10.1111/j.1477-2574.2011.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alrajraji M, Nawawi A, Jamjoom R, Qari Y, Aljiffry M. Delayed hemobilia due to hepatic artery pseudo-aneurysm: a pitfall of laparoscopic cholecystectomy. BMC Surg. 2016;16:59. doi: 10.1186/s12893-016-0175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panda N, Narasimhan M, Gunaraj A, Ardhanari R. Laparoscopic management of post-cholecystectomy sectoral artery pseudoaneurysm. J Minim Access Surg. 2014;10:37–39. doi: 10.4103/0972-9941.124471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Misawa T, Koike M, Suzuki K, Unemura Y, Murai R, Yoshida K, Kobayashi S, Yamazaki Y. Ultrasonographic assessment of the risk of injury to branches of the middle hepatic vein during laparoscopic cholecystectomy. Am J Surg. 1999;178:418–421. doi: 10.1016/s0002-9610(99)00202-0. [DOI] [PubMed] [Google Scholar]
- 17.Shen BY, Li HW, Chen M, Zheng MH, Zang L, Jiang SM, Li JW, Jiang Y. Color Doppler ultrasonographic assessment of the risk of injury to major branch of the middle hepatic vein during laparoscopic cholecystectomy. Hepatobiliary Pancreat Dis Int. 2003;2:126–130. [PubMed] [Google Scholar]
- 18.Zhang WZ, Shen J, Xie JX, Zhu H. Color Doppler ultrasonographic examination on the relationship between the gallbladder bed and major branch of the middle hepatic vein. Hepatobiliary Pancreat Dis Int. 2005;4:299–301. [PubMed] [Google Scholar]
- 19.Ball CG, MacLean AR, Kirkpatrick AW, Bathe OF, Sutherland F, Debru E, Dixon E. Hepatic vein injury during laparoscopic cholecystectomy: the unappreciated proximity of the middle hepatic vein to the gallbladder bed. J Gastrointest Surg. 2006;10:1151–1155. doi: 10.1016/j.gassur.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Levi Sandri GB, Eugeni E, Bufo A, Dominici E. Unexpected bleeding during laparoscopic cholecystectomy: a hepatic vein injury. Surg Radiol Anat. 2017;39:1061–1062. doi: 10.1007/s00276-017-1845-8. [DOI] [PubMed] [Google Scholar]
- 21.Santivañez JJ, Velásquez ME, Cadena M, Vergara A. Management of Middle Hepatic Vein Injury during Laparoscopic Cholecystectomy: A Case Report. Surg J (N Y) 2020;6:e47–e48. doi: 10.1055/s-0040-1701695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z, Yu L, Wang W, Xia J, Li D, Lu Y, Wang B. Therapeutic strategies of iatrogenic portal vein injury after cholecystectomy. J Surg Res. 2013;185:934–939. doi: 10.1016/j.jss.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Guloglu R, Dilege S, Aksoy M, Alimoglu O, Yavuz N, Mihmanli M, Gulmen M. Major retroperitoneal vascular injuries during laparoscopic cholecystectomy and appendectomy. J Laparoendosc Adv Surg Tech A. 2004;14:73–76. doi: 10.1089/109264204322973826. [DOI] [PubMed] [Google Scholar]
- 24.Thompson JE Jr, Bock R, Lowe DK, Moody WE 3rd. Vena cava injuries during laparoscopic cholecystectomy. Surg Laparosc Endosc. 1996;6:221–223. [PubMed] [Google Scholar]
- 25.Alcázar MT, Ornaque I, Delgado MA, Martí C, Gil A, Montero A. [Abdominal aortic injury as a complication of laparoscopic cholecystectomy] Rev Esp Anestesiol Reanim. 2004;51:452–455. [PubMed] [Google Scholar]
- 26.Lemmo G, Marrocco-Trischitta MM, Manni R, Snider F. Renal artery pseudoaneurysm and arteriovenous fistula to the inferior vena cava: a late complication following laparoscopic cholecystectomy. Am Surg. 2002;68:143–145. [PubMed] [Google Scholar]
- 27.Di Stasi C, Pedicelli A, Manfredi R, Sallustio G. Renocaval arteriovenous fistula as a complication of laparoscopic cholecystectomy. AJR Am J Roentgenol. 2001;176:261–262. doi: 10.2214/ajr.176.1.1760261. [DOI] [PubMed] [Google Scholar]
- 28.Abut YC, Eryilmaz R, Okan I, Erkalp K. Venous air embolism during laparoscopic cholecystectomy. Minim Invasive Ther Allied Technol. 2009;18:366–368. doi: 10.3109/13645700903384443. [DOI] [PubMed] [Google Scholar]
- 29.Schäfer M, Lauper M, Krähenbühl L. A nation's experience of bleeding complications during laparoscopy. Am J Surg. 2000;180:73–77. doi: 10.1016/s0002-9610(00)00416-5. [DOI] [PubMed] [Google Scholar]
- 30.Bektas H, Schrem H, Winny M, Klempnauer J. Surgical treatment and outcome of iatrogenic bile duct lesions after cholecystectomy and the impact of different clinical classification systems. Br J Surg. 2007;94:1119–1127. doi: 10.1002/bjs.5752. [DOI] [PubMed] [Google Scholar]
- 31.Kaushik R. Bleeding complications in laparoscopic cholecystectomy: Incidence, mechanisms, prevention and management. J Minim Access Surg. 2010;6:59–65. doi: 10.4103/0972-9941.68579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fingerhut A, Dziri C, Garden OJ, Gouma D, Millat B, Neugebauer E, Paganini A, Targarona E. ATOM, the all-inclusive, nominal EAES classification of bile duct injuries during cholecystectomy. Surg Endosc. 2013;27:4608–4619. doi: 10.1007/s00464-013-3081-6. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Lopez V, Kuemmerli C, Cutillas J, Maupoey J, López-Andujar R, Ramos E, Mils K, Valdivieso A, Valero AP, Martinez PA, Paterna S, Serrablo A, Reese T, Oldhafer K, Brusadin R, Conesa AL, Valladares LD, Loinaz C, Garcés-Albir M, Sabater L, Mocchegiani F, Vivarelli M, Pérez SA, Flores B, Lucena JL, Sánchez-Cabús S, Calero A, Minguillon A, Ramia JM, Alcazar C, Aguilo J, Ruiperez-Valiente JA, Grochola LF, Clavien PA, Petrowsky H, Robles-Campos R. Vascular injury during cholecystectomy: A multicenter critical analysis behind the drama. Surgery. 2022;172:1067–1075. doi: 10.1016/j.surg.2022.06.020. [DOI] [PubMed] [Google Scholar]
- 34.A European-African HepatoPancreatoBiliary Association (E-AHPBA) Research Collaborative Study management group; Other members of the European-African HepatoPancreatoBiliary Association Research Collaborative. Post cholecystectomy bile duct injury: early, intermediate or late repair with hepaticojejunostomy - an E-AHPBA multi-center study. HPB (Oxford) 2019;21:1641–1647. doi: 10.1016/j.hpb.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Strasberg SM, Helton WS. An analytical review of vasculobiliary injury in laparoscopic and open cholecystectomy. HPB (Oxford) 2011;13:1–14. doi: 10.1111/j.1477-2574.2010.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Nakeeb A, Sultan A, Ezzat H, Attia M, Abd ElWahab M, Kayed T, Hassanen A, AlMalki A, Alqarni A, Mohammed MM. Impact of referral pattern and timing of repair on surgical outcome after reconstruction of post-cholecystectomy bile duct injury: A multicenter study. Hepatobiliary Pancreat Dis Int. 2021;20:53–60. doi: 10.1016/j.hbpd.2020.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Conde Monroy D, Torres Gómez P, Rey Chaves CE, Recamán A, Pardo M, Sabogal JC. Early versus delayed reconstruction for bile duct injury a multicenter retrospective analysis of a hepatopancreaticobiliary group. Sci Rep. 2022;12:11609. doi: 10.1038/s41598-022-15978-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Frilling A, Nadalin S, Paul A, Malagò M, Broelsch CE. Management of concomitant hepatic artery injury in patients with iatrogenic major bile duct injury after laparoscopic cholecystectomy. Br J Surg. 2008;95:460–465. doi: 10.1002/bjs.6022. [DOI] [PubMed] [Google Scholar]
- 39.Furtado R, Yoshino O, Muralidharan V, Perini MV, Wigmore SJ. Hepatectomy after bile duct injury: a systematic review. HPB (Oxford) 2022;24:161–168. doi: 10.1016/j.hpb.2021.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Moldovan C, Cochior D, Gorecki G, Rusu E, Ungureanu FD. Clinical and surgical algorithm for managing iatrogenic bile duct injuries during laparoscopic cholecystectomy: A multicenter study. Exp Ther Med. 2021;22:1385. doi: 10.3892/etm.2021.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]


