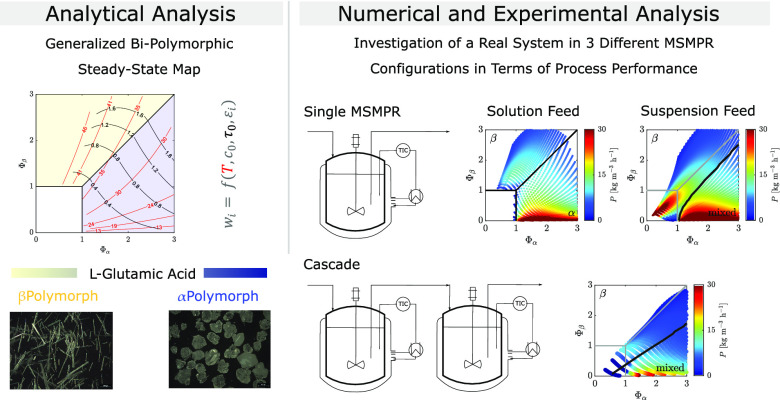
Abstract
The crystallization of the two polymorphs of l-glutamic acid (LGA) is carried out in a continuous crystallization process, and its performance according to different criteria is evaluated. The study aims at identifying suitable operating conditions for producing either αLGA or βLGA with a high polymorphic purity. To this end, we investigate the process both from a theoretical perspective and through experiments using either a single stirred-tank crystallizer or a cascade of two stirred-tank crystallizers in series. In terms of theory, we extend the MSMPR-based steady-state stability analysis of Farmer et al. (Farmer, T. C. et al. AIChE J. 2016, 62, 3505–3514) by accounting for the possibility of a nonrepresentative withdrawal of the solid phase from the crystallizer. Additionally, the process is simulated using population balance equations, thereby investigating the effect of operating conditions on polymorphic purity, yield, and productivity. Guided by the model-based conclusions, we identified suitable operating conditions and experimentally tested them. The experimental campaign has demonstrated that βLGA could be successfully and continuously produced in both process configurations according to the theory with performance as expected, whereas that was not possible for αLGA. The difference between the two stems from different operational challenges, whose consequence is that steady-state operation is attained in the case of βLGA but not in that of αLGA. In the former case, the needle-like βLGA crystals, which exhibit no agglomeration, tend to be only slightly oversampled; in the latter case, the prismatic αLGA crystals undergo major agglomeration and hence are very difficult to suspend and effectively withdraw from the crystallizer.
Short abstract
This work investigates the crystallization of the two polymorphs of L-glutamic acid in (non)ideal continuous stirred-tank crystallizer(s). A combination of experiments and modeling is used to evaluate the effect of the operating conditions on the process performance in terms of purity, yield, and productivity.
1. Introduction
Crystallization is an essential purification step in the production of active pharmaceutical ingredients (APIs).2 The majority of APIs can crystallize in various polymorphic forms. A polymorph can exhibit differences in crystal structure and consequently can also have different solubility, crystal shape, density, and other properties, thus affecting processability and bioavailability.3
Control of polymorphism is therefore crucial for designing a robust crystallization process, which produces a pure crystalline product of one polymorphic type while also achieving high yield and productivity. There are different strategies to produce the desired polymorph in a batch process4,5 as well as in a continuous process.6−10 When a continuous crystallization process is performed, e.g., in a mixed-suspension mixed-product removal (MSMPR), one can selectively produce either metastable or stable polymorphs, since the crystallizer is operated at a single solute concentration, thus avoiding solvent-mediated polymorphic transformation (SMPT). Moreover, continuous operation allows a higher flexibility and degree of process control and reduces costs.11 Which polymorph is formed depends on the interplay between thermodynamics and kinetics, i.e., the polymorphic-specific nucleation and growth rates. By manipulating the crystallization kinetics, e.g., by adapting the process operating conditions such as temperature, residence time, and feed concentration, one can produce a polymorph, which is otherwise not stable thermodynamically. This was shown in a landmark work by Farmer et al.,1 who reduced the complexity of dealing with bipolymorphic systems to the values of two dimensionless numbers. The approach has been supported by numerical tests and validated through experiments with different substances.12−15 This model has been extended further by adding agglomeration16 and breakage,17 thus showing that the polymorphic steady-state map can be adapted for more complex systems. Moreover, it has been shown that in a cascade of two MSMPRs, the seeds produced in the first crystallizer can be used to control the polymorphic outcome in the second crystallizer.13,18 By providing enough surface area of the desired form to the second crystallizer, it is possible to sustain its form at steady state.
To improve process control, the effect of a nonrepresentative withdrawal inside an MSMPR was previously studied, and an expression accounting for nonidealities was proposed.19 These nonidealities were modeled using computational fluid dynamics (CFD) tools. Decoupling the CFD and population balance equations (PBEs) model in such a way allows for much faster computation without loss of generality. This approach is especially valuable for the pharmaceutical industry and becomes important for systems such as the α-polymorph of LGA, the model compound studied in this work. LGA can crystallize in two polymorphic forms, (i) the metastable α l-glutamic acid (αLGA) and (ii) the monotropically stable β l-glutamic acid (βLGA), which has a lower solubility in the entire temperature range considered.20 αLGA crystals exhibit a prismatic shape, which is easy to filter and generally preferred for industrial purposes.15,21,22 However, crystals of that polymorph distribute heterogeneously within the crystallizer, and therefore, a representative withdrawal is operationally challenging.19 βLGA forms needle-like crystals, which are difficult to handle industrially and normally require further processing before usage, yet suspension heterogeneity is not an issue.6
The goal of this work is to produce both polymorphs of the model compound LGA with the highest attainable process performance, accounting for operational challenges for which both modeling and experiments are required. This work is divided as follows. section 2 provides the necessary tools needed for (i) the numerical part, which includes all the mathematical formulas of the population balance model, (ii) the dimensionless form of the PBE model used for the steady-state stability analysis, and (iii) the experimental part, describing the materials and methods. In section 3, the results obtained by the different models are discussed, namely, the results of the theoretical steady-state analysis performed with the dimensionless model and the results of the numerically resolved PBE model. The model-based considerations provide practical guidance for maximizing the process performance and serve as a bridge to the experimental campaign. The corresponding experimental results are described in section 4. Lastly, some concluding remarks are drawn in section 5.
2. Methods
Experiments and modeling are intertwined in this paper. The model equations based on the population balance equation framework are described in section 2.1, while section 2.2 defines the key performance indicators (KPIs) of a continuous crystallization process. Finally, the experimental methodology and materials are discussed in section 2.3, also providing some insight into the chosen analytical methods.
2.1. Model Equations
2.1.1. General Population Balance Equations
For the sake of generality, we consider a cascade of well-mixed continuous crystallizers in series, modeled using population balance equations (PBEs), namely one for each polymorph i ∈ {α, β} in each of the stages k ∈ {1, ..., nstages}, with nstages being the total number of stages.
| 1 |
In the equation above, ni,kin is the number-based particle size distribution given in m–4, with ni,k and ni,kin being the corresponding distributions at the outlet and at the inlet, respectively, of crystallizer k; t is time, x the characteristic crystal length, τ0,k the nominal residence time in s (ratio of the suspension volume to the volumetric feed flow rate), and Gi,k the size-independent growth rate in m s–1. The boundary conditions (BCs) for the PBEs in eq 1 are defined as
| 2 |
with Bi,k corresponding to the nucleation rate in m–3s–1. The PBEs are coupled with the solute mass balance (MB) in each stage:
| 3 |
In eq 3, ck is the solute concentration in the liquid phase with the units g kg–1, ρc, and ρs the crystal and solvent density, respectively, kv,i the polymorphic crystal shape factor, and μ3,i,k the third moment of the i-th polymorph distribution, with μ3,i,kout and μ3,i,k being the corresponding moments at the outlet and at the inlet, respectively, of crystallizer k. The jth moment of distribution ni,k is given by μj,i,k = ∫0∞xjni,k dx. Hence, μ0,i,k is the number of crystals per unit solvent volume, and φi,k = kv,iμ3,i,k is the volumetric fraction of the suspension occupied by the crystals, whereby ρckv,iμ3,i,k represents the suspension density.
With neither additions nor withdrawals between crystallizers, the following equalities obviously hold: ni,kin = ni,k–1 for k ≥ 2. The quantity c0 is, in normal cases, a constant. Finally, note that the solute concentration in the liquid phase is mass based, whereas the particle concentration is volume based (hence, the term 1/ρs in the last equation).
To integrate the set of model equations in time, initial conditions (ICs) are needed, which can be written as follows:
| 4 |
| 5 |
Note that as seeds are present at t = 0, nucleation occurs exclusively via a secondary nucleation mechanism. Moreover, the ICs have no influence on the steady-state operation of any number of continuous crystallizers, provided that either a mixed polymorphic seed population is always chosen15 or the secondary nucleation rate expression for both polymorphs include the crystallization of the counter polymorph.
2.1.2. Constitutive Equations
Rate expressions for growth, dissolution, and secondary nucleation for the two polymorphs of LGA were taken and adapted from the work of Hermanto et al.23 In these expressions, supersaturation of the ith polymorph (i ∈ {α, β}) is defined as
| 6 |
In the above equation, csat,i,k denotes the solubility of the ith polymorph under the operating conditions. The solubility expressions were taken from the work of Ono et al.20 The plotted solubility curves can be found in Figure 4.
Figure 4.
(a) Isotherms (in red; values are given in °C) and isolines of residence time (in black; values are given in h) on the dimensionless steady-state map. The effect of residence time on (b) steady-state concentration and (c) productivity, yield at constant temperature and the effect of temperature on (d) steady-state concentration and (e) productivity, yield at constant residence time are shown for a single solution-fed crystallizer. In panels (b) and (d), the solubilities of both polymorphs are provided additionally (csat,β in yellow and csat,α in blue). Note that the inlet concentration is kept constant (c0 = 30 g/kg).
The growth rate of αLGA can be written as
| 7 |
whereas, the growth rate of βLGA is
| 8 |
Note that the unit of temperature in the growth rate expressions is kelvin. Furthermore, the dissolution rate of αLGA is defined as
| 9 |
The secondary nucleation rate of αLGA is defined as
| 10 |
and similarly, the secondary nucleation rate of βLGA is
| 11 |
The values of all the kinetic parameters used in eqs 7–11 are given in Table 1. It is worth noting that a pure steady-state is not attainable anymore once a cross-nucleation term is introduced.14 However, the impact of the cross-nucleation term on purity is negligible for most process conditions considered in this study, and a high-purity steady-state can still be obtained. Finally, some additional considerations regarding the secondary nucleation rate expressions are provided in the Supporting Information (SI).
Table 1. Values of the Model Parameters for the Two Polymorphs of LGA.15,23.
| Mechanism | Parameter | Notation | Value |
|---|---|---|---|
| growth rate of α | pre-exponential factor | kg,α,0 (m s–1) | 6.540 |
| activation energy | Eα (J mol–1) | 4.3088 × 104 | |
| growth rate exponent | gα (−) | 1.859 | |
| dissolution rate of α | pre-exponential factor | kd,α (m s–1) | 3.5006 × 10–5 |
| growth rate of β | pre-exponential factor | kg,β,0 (m s–1) | 3.8387 × 1022 |
| activation energy | Eβ (J mol–1) | 1.7596 × 105 | |
| growth rate exponent | gβ,1 (−) | 1.047 | |
| exponential constant | gβ,2 (−) | 0.778 | |
| nucleation rate of α | cross-nucleation factor | kb,αβ (m–3 s–1) | 1 × 102 |
| homogeneous nucleation factor | kb,α,0 (m–3 s–1) | 3.0493 × 107 | |
| nucleation rate of β | cross-nucleation factor | kb,βα (m–3 s–1) | 7.2826 × 106 |
| homogeneous nucleation factor | kb,β,0 (m–3 s–1) | 4.8517 × 108 | |
| material constants | shape factor α | kv,α (−) | 0.52 |
| shape factor β | kv,β (−) | 0.01 | |
| density α | ρc,α (kg m–3) | 1532 | |
| density β | ρc,β (kg m–3) | 1569 |
2.1.3. Withdrawal Effects
When considering
and modeling MSMPR crystallizers, “mixed-product removal”
is typically assumed, i.e., the particle size distribution (PSD) at
the outlet of the crystallizer is assumed to be identical to the one
inside the crystallizer  . However, depending on the position of
the withdrawal tube and on the characteristics of the suspension,
such representative removal might not be achieved.19 Although taking into account nonrepresentative withdrawal
in a mathematical model adds complexity, it is necessary to enhance
accuracy. To this purpose, the outlet PSD, ni,kout(x), can be defined as
. However, depending on the position of
the withdrawal tube and on the characteristics of the suspension,
such representative removal might not be achieved.19 Although taking into account nonrepresentative withdrawal
in a mathematical model adds complexity, it is necessary to enhance
accuracy. To this purpose, the outlet PSD, ni,kout(x), can be defined as
| 12 |
where εi,k represents the polymorph-specific degree of dilution and δi,k the extent of the sieving effect, which is characterized by the size-dependent function ωi,k(x).19 On the one hand, since different polymorphs can crystallize in different shapes and grow at different rates, their dilution factors can differ as well; the higher its value, the lower the solid suspension density of the outlet stream and the longer the particles’ residence time. This may alter the nature of the steady-state attained. On the other hand, the sieving function describes additional nonidealities in the crystallizer related to a nonhomogeneous suspension density, which deviates from the ideal case, particularly in the case of larger crystals, that tend to settle. The former effect is easier to characterize, whereas the latter requires the definition of the function ωi,k(x); the former case will be considered first.
2.1.4. Examples of Application
Equation 1 is the general formulation of a PBE for a cascade of continuous crystallizers, which can be specialized for application to several specific cases:
-
1.
MSMPR crystallizer: nstages = 1, ni,1in = 0, ni,1 = ni,1;
-
2.
Continuous crystallizer with suspension feed and representative withdrawal: nstages = 1, ni,1in > 0, ni,1 = ni,1;
-
3.
Continuous crystallizer with either solution or suspension feed and nonrepresentative withdrawal: nstages = 1, ni,1in ≥ 0, ni,1 ≠ ni,1;
-
4.
Cascade of continuous crystallizers with or without representative withdrawal: nstages > 1, ni,1in = 0, ni,k = ni,k–1out for k > 1, ni,1 = ni,1 or ni,1out ≠ ni,1 for k ≥ 1.
Some of these applications will be analyzed and discussed in the following.
2.1.5. Numerical Solution
To solve the system of PDEs above, a high-resolution finite volume (HR-FVM) scheme has been implemented together with a van Leer flux limiter.24,25 The numerical solution is validated by comparison with the analytical solution, if possible. The analytical solution is only for cases featuring a single polymorphic crystal distribution at steady-state.19
2.2. Key Performance Indicators
Three KPIs are defined to assess the process performance in both experiments and simulations. They are the polymorphic purity, wi, the solute yield, Y, and the process productivity, P, i.e., a key product specification, and the measures of how well the material to be crystallized and the crystallization equipment are exploited.17,26 Yield and productivity can be considered proxies for operating costs and capital costs, respectively.
Purity is defined as the mass fraction of the target polymorph at the outlet of the cascade of crystallizers:
| 13 |
The yield Y is defined
using the steady-state
concentration of LGA in the liquid phase at the cascade outlet,  , the concentration fed at the inlet of
the first crystallizer c0, and the solubility
of the stable polymorph at the conditions of the last stage
, the concentration fed at the inlet of
the first crystallizer c0, and the solubility
of the stable polymorph at the conditions of the last stage  :
:
| 14 |
The higher the yield, the lower  , i.e., the more desupersaturated the system.17
, i.e., the more desupersaturated the system.17
Finally, the productivity P is defined as the mass of crystals harvested per unit volume and unit time at the cascade outlet (in this definition the masses of both polymorphs are considered):15,17
| 15 |
It is worth noting that, by exploiting the steady-state form of the material balance of eq 3, one can recast the productivity in terms of the steady-state solute concentrations at the inlet and at the outlet of any cascade:
| 16 |
2.3. Materials and Methods
2.3.1. Experimental Setup
The setup used in this study is shown in Figure 1 and includes two MSMPRs in series, both with a working volume of 0.35 L (EasyMax 402, Mettler Toledo). The feed stream was fed using a peristaltic pump after being heated up to a high enough temperature (typically 60 °C in these experiments) to avoid primary nucleation and possible clogging. Depending on the type of experiment performed, either one MSMPR or two MSMPRs in series was used. The two operation protocols adopted in this study are indicated in Figure 1 with A and B, respectively. The suspension transfer from MSMPR1 to MSMPR2 and from MSMPR2 to the final collector tank was pressure-driven and occurred intermittently, as explained in detail elsewhere.19 A check valve was installed at the outlet of the MSMPR1 to avoid the backflow of air during the pressurization of the MSMPR2. A level control was installed in each crystallizer, which triggered the pressurization once a specified fill level height was reached.26 This avoided a suspension overflow in the crystallizer, whereas the pressurization time was kept short enough to keep the variation of the suspension volume below 10%.27 The whole setup was automated by using an Arduino microcontroller. Moreover, to ensure maximum control over the pressurization, i.e., the pressure applied and the flow rate induced), both a pressure reducer valve and a needle valve were installed.
Figure 1.
Flowsheet of the continuous crystallization setup used. For the operation including a single MSMPR, configuration A was employed (shown in green), and for the cascade operation, configuration B was used (shown in blue).
2.3.2. Experimental Protocol
An LGA solution of a given concentration c0 was prepared for each crystallizer as well as for the feed and heated to dissolve any crystals present in the solution. Then, the MSMPR vessels were cooled to the desired temperature, and the first MSMPR was seeded with the desired polymorph, thus avoiding unwanted primary nucleation, which also leads to encrustation.7 Note that a seed loading of less than 10% of the final mass produced was used. In the case of βLGA, the seed material was the one commercially available (Sigma-Aldrich, purity ≥99% HPLC), and in the case of αLGA, the seeds were produced via pH-shift,21,28 whereas particles <100 μm were chosen. After the seed addition, the process was started by activating the pump, which was previously set to the flow rate, corresponding to the desired nominal residence time. The outflow was initiated automatically once a certain fill level was reached. All experiments were repeated at least twice. For the experiments, which produce mostly αLGA, an anchor-shaped stirrer was used at a stirring rate of 250 rpm, resulting in less sedimentation of the particles, whereas for all other experiments a pitched blade was used at 500 rpm. At the end of the process, the shut-down procedure was initiated. Flushing of the feed line was carried out to avoid clogging, while the suspension was filtered, and the crystals were washed with ethanol, and then left to dry in the oven overnight.
2.3.3. Analytical Methods
Due to the occurrence
of fouling/encrustation, i.e., attachment of crystals on the measurement
probes after a few hours, in situ measurements are difficult to achieve.
Thus, the quantities needed to calculate the KPIs were obtained through
offline measurements. The polymorphic purity was obtained via XRD
measurements.15,18 To obtain quantitative information
from the XRD, a calibration step was performed. Details on the construction
of the calibration curve are provided in the Supporting Information. Moreover, optical microscopy images were taken
for each sample. Finally, the evolution of the solute concentration
over time inside the crystallizer, css, and the feed tank, c0, was measured
gravimetrically. Similarly, the third moments of the distribution
inside the crystallizer, 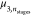 , and in the outlet of the crystallizer,
, and in the outlet of the crystallizer,  were determined, i.e., by weighing the
liquid and dried solid phase. Using these four quantities, one can
determine whether the steady-state is reached by using the mass balance.
At steady-state, a relative error between the solute depleted in the
liquid phase (based on css) and accounted
for in the solid phase (based on
were determined, i.e., by weighing the
liquid and dried solid phase. Using these four quantities, one can
determine whether the steady-state is reached by using the mass balance.
At steady-state, a relative error between the solute depleted in the
liquid phase (based on css) and accounted
for in the solid phase (based on  and
and  ) of less than 5% was deemed acceptable.
Steady-state operation was normally obtained after around 7–10
residence times, whereas it is interesting to note that css approaches a constant value before the steady state
is reached.
) of less than 5% was deemed acceptable.
Steady-state operation was normally obtained after around 7–10
residence times, whereas it is interesting to note that css approaches a constant value before the steady state
is reached.
3. Modeling Results
In this section, we carry out the model-based study of either one continuous crystallizer or two in series for a system where LGA is crystallized from an aqueous solution. To this aim, we solve the corresponding set of population balance equations presented in section 2.1. We report the results in section 3.2, with emphasis on the feasible operating conditions to obtain one polymorph or the other and on the KPIs attained, as defined in section 2.2. To frame it, this simulation-based analysis is preceded by an analytical derivation (see section 3.1), which extends literature results originally presented by Doherty and co-workers1 by accounting for nonrepresentative withdrawal.
3.1. Steady-State Stability Map
In this
section, the analytical solution developed by Farmer et al.1 is extended by studying a single MSMPR (the stage
index k is dropped for the sake of simplicity). The
MSMPR is operated with a solution feed  , under the assumption of a possibly diluted
outlet stream, i.e.,
, under the assumption of a possibly diluted
outlet stream, i.e.,  with εi ≥ 0 (δi = 0 in eq 12). With εi = 0 for both polymorphs, the case studied by Farmer
et al.1 is recovered. Dilution increases
the residence time, and a specific residence time for each polymorph,
namely, τi = τ0/(1 – εi), can be introduced.
with εi ≥ 0 (δi = 0 in eq 12). With εi = 0 for both polymorphs, the case studied by Farmer
et al.1 is recovered. Dilution increases
the residence time, and a specific residence time for each polymorph,
namely, τi = τ0/(1 – εi), can be introduced.
In terms of constitutive equations, the following expressions for the growth and nucleation rates are used, in this subsection only:
| 17 |
| 18 |
Here, μi,2 is the second moment of the ith PSD, and csat,i is the concentration of polymorph i at solubility. The constitutive equations used in this section differ from those presented in section 2.1, but they are consistent with those used by Farmer et al.1 and allow exploitation of a significant simplification of the resulting moment equations that stems from the use of the second moment of the PSD in the expression of the secondary nucleation rate in eq 18.1 The resulting system consists of seven ordinary differential equations (ODEs), instead of nine in the case where the third-order moments were used, as seen below in eqs 20–26, without losing anything in terms of generality. As a consequence, the solute balance of eq 19 is obtained by specializing the general solute balance of eq 3 to the case considered by Farmer et al.,1 which is made possible by the second moment dependence of the nucleation rate. The resulting solute balance, in the case of spherical particles considered here and by Farmer et al.1 where ρc,i = ρc for both polymorphs, reads:
| 19 |
Note that the liquid phase is not diluted at the outlet (i.e., cout = c).
As detailed in the Appendix, section A.1, applying the method of moments to eq 1, by considering eqs 17 and 18,29 and normalizing transforms the set of partial differential equations (PDEs) into the following set of seven ODEs (with higher order moments needed only for a more accurate reconstruction of the PSDs)
| 20 |
| 21 |
| 22 |
| 23 |
| 24 |
| 25 |
| 26 |
where ωi,j is the jth dimensionless moment of the PSD of polymorph i, y the normalized solute concentration (eq 41), and γ the normalized solubility difference between the two polymorphs (eq 41).
The Damköhler number Dai, defined as
| 27 |
includes the characteristic residence time as well as the characteristic crystallization time, which consists of nucleation and growth; it differs from the definition utilized by Farmer et al.1 only in the use of the polymorph-specific residence time. Note that the moment equations above also differ from those reported1 because of the presence of the dimensionless parameter κi, i.e., one for each polymorph, which is the ratio between the nominal residence time τ0 and the residence time of the ith polymorph τi, i.e., the term (1 – εi).
For the general case, where εi ≠ 0 (τ0 ≠ τi), the steady-state analysis is carried out in the same way as in Farmer et al.,1 i.e., by identifying the steady-states and by determining their stability through linearization around the steady-states and application of the Liénard–Chipart criterion to the coefficients of the characteristic polynomial of the associated Jacobian matrix and to the elements of the relevant Hurwitz matrix. The mathematical derivation is carried out in detail in the Appendix, section A.2. Note that, for the analysis, the α-polymorph is assumed to be metastable and the β-polymorph monotropically stable, as it is the case for LGA. The case ε = 0 and consequently τi = τ0 yields a special case of eq 27 that coincides with the definition in Farmer et al.1 and leads obviously to the same results.
Two remarks are worth making. First, the boundaries of the steady-state regions in the steady-state map shown in Figure 2 and in the following figures are the same as those in Farmer et al.1 This is true provided the extended definition of the Damköhler numbers given by eq 27 is used in the definition of the coordinates, Φα and Φβ along the axes of such maps, namely:
| 28 |
| 29 |
Figure 2.

Effect of feed concentration, c0 = 20–40 g/kg, and residence time, τ0 = 0–2 h, on the productivity, P, plotted for five different operating temperatures, i.e., T = 25, 30, 35, 40, 45 °C.
There are four steady-states for a bipolymorphic MSMPR system: (i) the trivial steady-state, whereby 0 < Φα < 1 and 0 < Φβ < 1, where no crystals are present (given by the white square in the left bottom corner in Figure 2); (ii) the α steady-state (colored in a transparent blue), where 1 ≤ Φα and 0 < Φβ < Φα; (iii) the β steady-state (colored in a transparent yellow), where 1 ≤ Φβ and 0 < Φα < Φβ; and (iv) the mixed steady-state, where both polymorphs are present, and 1 < Φα = Φβ; the mixed steady-state is the bifurcation between the two single polymorph steady-states. It is worth noting here that, as demonstrated in the Appendix, yss = Φα–1 and yss = Φβ in the α steady-state region and in the β steady-state region, respectively.
Thus, adding a dilution does not change the general features of the steady-states and their stability. Second, the advantage of this new analysis lies in the fact that accounting for different dilution of the two polymorphs (εα ≠ εβ) is automatically done by using correspondingly different values of the dilution factors εα and εβ in eq 27. This is particularly relevant in the case where the two polymorphs have a different morphology, i.e., the case of LGA; the dilution factor of αLGA may well be larger than that of βLGA, i.e., εα > εβ.
3.2. Performance Analysis
In this section, the three process configurations indicated in the three columns of Table 3, namely, a single solution-fed continuous crystallizer (section 3.2.2), a single suspension-fed continuous crystallizer (section 3.2.3), and a cascade of two continuous crystallizers (section 3.2.4) are investigated.
Table 3. Model Input Parameters Chosen for the Operation of a Solution-Fed Single Crystallizer, a Suspension-Fed Single Crystallizer, and a Cascade.
| Cascade with two crystallizers |
||||
|---|---|---|---|---|
| Single crystallizer solution-fed | Single crystallizer suspension-fed | k = 1 | k = 2 | |
| T (°C) | 10–50 | 10–50 | 45 | 10–40 |
| τ0 (h) | 0–2 | 0–4 | 0–4 | τ0,1 = τ0,2 |
| c0 (g/kg) | 20–40 | 30 | 30 | c0,2 = c0,1out |
| V (mL) | 350 | 350 | 350 | 350 |
| ε (−) | 0, 0.5 | 0 | 0, 0.5 | ε2 = ε1 |
3.2.1. Operating Conditions and Operating Points
The corresponding operating conditions selected, in terms of values of T, τ0, c0, ε, are also reported in Table 3; note that in all simulations for the sake of simplicity the last parameter is kept constant at either ε = 0 (representative withdrawal) or ε = 0.5 (nonrepresentative withdrawal, with dilution of the withdrawn suspension).
Therefore, the three parameters T, τ0, and c0 define the operating conditions, but they are not enough to determine the operating point in the plane with dimensionless coordinates Φα and Φβ. The steady-state concentration css, which is an output of the simulation, is additionally needed, as explained below.
In order to position the numerical results in the dimensionless steady-state map, an algebraic transformation is needed, which is also employed in the literature.16,17 The kinetic expressions of Hermanto et al.23 were chosen. The growth rate expressions are
| 30 |
 |
31 |
In the case of the growth rates Gi, the algebraic transformation step is straightforward. It is noteworthy that kg,β in eq 31 depends on the supersaturation Sβ and thus on the steady-state concentration css, which can only be calculated analytically for simple process configurations and is not known a priori. In the case of the nucleation rates Bi, the third moment μ3,i needs to be replaced by the second moment μ2,i, both being outputs of the simulation. This is done as follows:
 |
32 |
 |
33 |
In this way, the two nucleation rates obtain the same functional form as eq 17 and eq 18 with bα = bβ = 1. Note that for each simulation the kb,α and kb,β would slightly change depending on the moments obtained. Moreover, even though the second and third moment of a polymorph at the steady-state of the counter polymorph is not zero (due to the presence of the cross-nucleation term), its value can be very small. Therefore, it was decided to use an average values for kb,α and kb,β, reported in Table 2, which are based on the output of many simulation runs.16,17 The methodology applied seems to work well.
Table 2. Averaged Values Were Obtained for kb,α and kb,β.
| Parameter | Values |
|---|---|
| kb,α (m–2 s–1) | 1.2e4 |
| kb,β (m–2 s–1) | 6.5e4 |
For the suspension-fed crystallizer, both cases (i.e., feeding either a pure βLGA suspension or a pure αLGA suspension) are studied. For the cascade operation, however, the operating conditions in the first crystallizer are chosen so that a pure βLGA population is obtained and fed into the second crystallizer. It is worth noting that limitations on the maximum operating temperatures exist in a cascade; in general, T2 ≤ T1, with the equality being applied with the only purpose of increasing the residence time of the suspension in the system.
For each operating point, the three KPIs, namely purity, yield, and productivity, are calculated. Results are presented as three distinct heat maps in the dimensionless (Φα, Φβ) plane, i.e., one for each KPI; in all figures the boundaries of the steady-state regions calculated analytically in section 3.1 are also reported (pale gray lines), to help in the analysis of the results. The relevant figures are Figures 2–4 for a single solution-fed crystallizer with representative withdrawal, Figure 5 for a single solution-fed crystallizer with nonrepresentative withdrawal, and Figures 6–8 for a single suspension-fed crystallizer, with the feed being either a βLGA-suspension or an αLGA-suspension.
Figure 5.
Heat maps showing the purity of βLGA wβ (left), yield Y (middle), and productivity P (right) as a function of temperature T and residence time τ0 for a single crystallizer operated with a diluted outlet stream (ε = 0.5, δ = 0). The inlet concentration is kept constant (c0 = 30 g/kg).
Figure 6.
Change in the steady-state map boundaries in the case of continuously feeding a population of pure β crystals (left) and α crystals (right). The highest suspension density fed has an μ3,αin = 0.018 for the α-polymorph and an μ3,β = 0.49 for the β-polymorph, respectively. The other μ3,βin values are 25%, 50%, and 75% of the above reference value. Note that since a polymorphic pure suspension is fed to the MSMPR, only two steady-states are possible, i.e., the mixed steady-state (“mixed”, colored in a transparent green) and the pure steady-state of the polymorph being fed (β or α). The boundaries of the original steady-state map are shown with gray dashed lines for comparison. The green lines denote the 99% purity of the corresponding polymorph.
Figure 8.
Heat maps showing purity of βLGA wβ (left), yield Y (middle), and productivity P (right) as a function of temperature T and residence time τ0 for a single MSMPR fed with a suspension of pure αLGA. The crystallizer is operated with a representative withdrawal (ε = 0, δ = 0). The steady-state map boundaries of a single MSMPR with a solution feed are shown in gray to ease comparison, whereas the black line denotes the 99% purity of αLGA.
3.2.2. Single Solution-Fed Continuous Crystallizer
The effect of changing the operating conditions for a single solution-fed continuous crystallizer with representative withdrawal, i.e., truly an MSMPR, is illustrated in Figure 2. For a given system and its set of physicochemical properties (solubilities, densities, nucleation, and growth rates, which are all function of temperature), the coordinates of the operating point in the steady-state map are calculated after choosing the process operating conditions.
In Figure 2, the operating points corresponding to five sets of operating conditions at five different temperatures, T, are plotted, while for each temperature the feed concentration, c0, and the residence time, τ0, are varied, as the arrows indicate. Each point has a color that indicates the corresponding productivity as to eq 15, according to the color code reported in the figure itself. It is worth noting that the steady-state attained for each operating point coincides with that expected based on the basis of its position in the steady-state map. This is not a trivial result, considering that the model used in the analytical derivation of the boundaries of the steady-state map (section 3.1) and that used in these simulations are not identical. This is, however, a reassuring result as both models are claimed in the literature to provide an accurate description of the LGA system, and therefore, in this context, they should provide very similar results.
We observe that low temperature levels favor the formation of the metastable polymorph, and vice versa. In principle, varying τ0 and/or c0 may drive the operating point from one region, e.g., the αLGA region, to the other, e.g., the βLGA region. In practice, for reasonable ranges of values of these two parameters, this does not happen at all temperatures, in fact, only at T = 35 °C in this case.
It can also be seen in the figure that productivity increases with an increase in c0 at any given temperature. Higher inlet concentrations, c0, led to higher supersaturation levels. Higher supersaturation levels, in turn, lead to higher nucleation and growth rates, which increase productivity. Note that c0 has a minor effect on which polymorph is obtained. Reducing the residence time, τ0, has an obvious positive impact on productivity; it is worth noting, however, that also changing τ0 has a minor effect on the final polymorph obtained. Because in real life operation of a crystallization process changing the feed concentration may be impractical, the following analysis will be carried out for a constant value of c0, in fact for c0 = 30 g/kg (see Table 3); we encourage the interested reader to conceptualize the effect of changing c0 by referring to Figure 2.
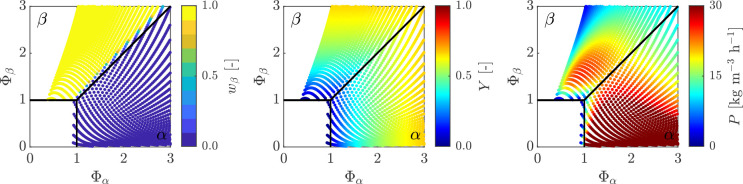
For the case of a single MSMPR with a solution feed at c0 = 30 g/kg, the performance heat maps are presented in Figure 3, showing purity, yield, and productivity for a range of values of T and τ0 (see Table 3).
Figure 3.
Heat maps showing purity of βLGA wβ (left), yield Y (middle), and productivity P (right) as a function of temperature T and residence time τ0 (see Table 3) for a single MSMPR operated with a representative withdrawal (ε = 0), while the inlet concentration is kept constant (c0 = 30 g/kg).
The purity heat map in Figure 3 (left) shows that the steady-state attained for each operating point coincides with that expected based on the basis of its position in the steady-state map obtained through the analytical derivation in section 3.1. The yield heat map (Figure 3, center) confirms that Y increases with increasing τ0, and shows that yield isolines are vertical and horizontal in the α and in the β-polymorph steady-state, respectively. This is a consequence of the property that the definition of yield, i.e., eq 14, can be written as Y = (1 – yss), and of the fact mentioned above and discussed in the Appendix that the dimensionless solution concentration at steady-state is the reciprocal of the horizontal coordinate in the α steady-state (see eq 47) and the reciprocal of the vertical coordinate in the β steady-state (see eq 51). Finally, the productivity heat map in Figure 3 (right) demonstrates that while the βLGA polymorph can not be obtained at high productivity or at high yield, the αLGA polymorph can be obtained at high productivity at low temperatures, but a high yield also can be attained only for a small range of operating points (bottom right corner of the steady-state map).
The grid of operating points, whose corresponding KPIs are shown in Figure 3, are obtained at constant c0 = 30 g/kg by varying the temperature and nominal residence time as shown by the isolines in Figure 4a, namely, red lines at constant T (increasing from the bottom right corner to the top left corner) and black lines at constant τ0 (increasing from the bottom left corner to the top right corner). The effect of changing the residence time (at constant temperature, namely, T = 25 °C) and the temperature (at constant residence time, i.e., τ0 = 1 h) on yield, Y, productivity, P, and steady-state concentration in solution, css is illustrated in Figure 4b–e; note that in Figure 4b,d also the solubilities of the two polymorphs are plotted for reference.
As τ0 increases at T = 25 °C the system goes from the trivial steady-state (no crystals) to the α-polymorph steady-state, while the steady-state concentration decreases and accordingly the yield increases because more supersaturation is consumed. Productivity exhibits in turn a maximum, because both numerator (proportional to c0 – css) and denominator (proportional to τ0) in eq 16 increase with τ0, but the numerator increases more than linearly with τ0 initially and then less than linearly as it asymptotically approaches the solubility of the prevailing polymorph. Note that the behavior would be somewhat different if the bifurcation line were crossed as τ0 varies, as in the case of the isothermal line at T = 35 °C (see below).
As T increases at τ0 = 1 h, the system transitions from the α-polymorph to the β-polymorph steady-state. Consequently, the relevant solubility level (Figure 4d) exhibits a discontinuity at the temperature on the bifurcation line (point B in the figure), corresponding to the switch from the α-polymorph to the β-polymorph solubility. At constant residence time, this leads to the angular point and to the nonmonotonic behavior of the steady-state concentration observed in Figure 4d and as a consequence of the yield and of the productivity (see Figure 4e).
Thus, summarizing, the rather peculiar behavior in the productivity heat map of Figure 3 can now be conceptualized as a combined effect of the decreasing trend of productivity with increasing temperature (at constant τ0) and of the presence of a sharp maximum for varying residence time (at constant T). A correspondingly high productivity island in the α-polymorph steady-state region and a less marked productivity maximum in the β-polymorph steady-state region are consequences of these combined effects.
Such effects are present also in the case of a single solution-fed continuous crystallizer (c0 = 30 g/kg) with nonrepresentative withdrawal (in fact in all cases that follow), whose performance heat maps are presented in Figure 5, for a range of values of T and τ0 (see Table 3; note that a value of ε = 0.5 has been used for all operating points).
The messages given by the purity and the yield heat maps in Figure 5 are the same as those for the case where ε = 0 in Figure 3. The productivity heat map in Figure 5 (right), however, shows that the longer effective residence times due to dilution of the outlet stream enable mid to high productivity levels also in the βLGA polymorph region (though the yield is still low). It is worth underlining (i) that this effect is remarkable, (ii) that it is amplified in the case where there is also a sieving effect, i.e., where ε = 0.5 and δ = 0.5(1 – ε) (the interested reader will find these results in the Supporting Information), and (iii) that our analysis, which includes both dilution and sieving effects in the withdrawal of the suspension, allows prediction of such important effects as well as interpretation of experimental results that could not be explained without considering nonrepresentative withdrawal. Lastly, it is interesting to note that comparing the productivity of different productivity heat maps at a given residence time and temperature provides additional information on the suspension density encountered at the crystallizer outlet, as they are directly proportional. Thus, in the case of dilution or sieving, higher suspension densities are encountered.
3.2.3. Single Suspension-Fed Continuous Crystallizer
In this section, we analyze how the picture given in the previous section changes when the feed to the continuous crystallizer consists of a suspension instead of a solution, i.e., where niin ≠ 0. The strategy here is that of feeding the target polymorph in solid form (feeding both would make attaining a single polymorphic product impossible). This analysis is of interest both in the case of a single crystallizer if the suspension feed leads to better performances than the solution feed and in the case of a cascade where the suspension produced by the first crystallizer is fed to the second.
There are two aspects to this study. First, we will reconsider the steady-state map, as the one obtained for solution-fed continuous crystallizers cannot apply, at least for one reason: only the polymorph that is continuously fed in solid form can be obtained pure in the outlet suspension, and the other not. Second, we will calculate the process performances in the two cases of the feed in solid form of either one or the other polymorph using the detailed model and analyze them.
The PSD of the crystals fed into the continuous crystallizer is an additional operating choice. For the sake of simplicity, but without loss of generality, in this study, we assume the exponential PSD typically obtained in a solution-fed crystallizer, i.e., an MSMPR, at steady-state (which is the case of interest when considering a cascade):
| 34 |
The reference values of ki,j are obtained by using the MSMPR functional dependence on B, G, and τ0, with the feed concentration being c0 = 30 g/kg, and the nominal residence time τ0 = 1 h, whereas T = 45 °C for a suspension feed consisting of a pure population of β-polymorph crystals and T = 25 °C for the pure α-polymorph case. In the following, we keep the values of ki,2 constant, while we vary those of ki,1, namely, by taking 25%, 50%, and 75% of the reference value, so as to explore the effect of different inlet suspension densities on the steady-state behavior of the suspension-fed continuous crystallizer.
In the case of a suspension-fed continuous crystallizer, the analytical steady-state analysis proposed originally by Farmer et al.1 and expanded in section 3.1 of this work cannot, to the best of our capabilities, be carried out, because of the presence of the inlet term in the PBEs. Because of its usefulness, we have calculated the steady-state map also for the suspension-fed continuous crystallizer using the detailed model; we have represented the results in the same (Φα, Φβ) plane, and we have illustrated them in Figure 6, where also the boundaries indicated in the previous figures are again reported for reference.
The two diagrams in Figure 6 demonstrate that feeding a polymorphic pure suspension causes: (i) the disappearance of the steady-state region where the other polymorph is obtained pure as a solid product; (ii) the obvious disappearance of the region where no solids are formed, i.e., the square region with 0 < Φα < 1 and 0 < Φβ < 1; (iii) the expansion beyond the gray straight line borders of the steady-state region, where the polymorph fed is obtained as a solid at high purity (such new boundaries in Figure 6 separate the high purity polymorph steady-state region—either yellow or blue—from the mixed state region—or green—where the final solid is a mixture of the two polymorphic forms; high purity here means that the purity of the target polymorph is at least 99%); and (iv) a clear effect whereby the larger the inlet suspension density, the larger the steady-state region, where the polymorph fed is obtained as pure solid product (note that the analytical case represented by the gray lines corresponds to the limit case where a suspension with zero solid density is fed to the crystallizer). The underlying mechanism leading to a change in the steady-state map lies in the fact that a suspension feed increases the surface and the volume of the crystals of the target polymorph, thus enhancing the secondary nucleation and the growth of its crystals. In other words, a suspension feed leads to a more pronounced depletion of supersaturation, hence css is lower compared to the solution-fed crystallizer. A comparison between the solution-fed and the suspension-fed crystallizer therefore highlights identical qualitative trends of css, Y, and P in dependence on temperature and residence time. As Figure 4 shows, the difference in the quantitative trends, highlighting improved performance when feeding a suspension, is ultimately only on the density of the inlet suspension feed.
The main difference between the solution-fed and the suspension-fed crystallizer can be summarized as follows: with respect to the effect of temperature, namely, as explained in Figure 4, the steady-state map changes on the basis of the type of inlet suspension. In case the feed stream is a suspension of α, the temperature associated with the change of steady-state rises and the pure α region expands toward higher temperatures. Vice versa and simultaneously more applicable, when feeding a suspension of β, the stable β steady-state region expands toward lower temperatures.
The KPI heat maps for the cases of a βLGA polymorph and of an αLGA polymorph suspension-fed continuous crystallizer are presented in Figures 7 and 8, respectively, for a fixed inlet suspension density. In all six diagrams, the black solid line is the corresponding boundary separating the region where the polymorph fed is obtained at 99% purity from the mixed-state region. The purity heat maps in the diagrams on the left of both figures illustrate the results presented in Figure 6 from another perspective, i.e., by highlighting the purity level in the mixed region. The yield heat maps in the diagrams in the center of the two figures show that the isolines in the mixed steady-state regions and in the polymorphic pure steady-states are tilted. Further explanation and illustration of this effect can be found in the Appendix, section A.4).
Figure 7.
Heat maps showing purity of βLGA wβ (left), yield Y (middle), and productivity P (right) as a function of temperature T and residence time τ0 for a single MSMPR fed with a suspension of pure βLGA. The crystallizer is operated with a representative withdrawal (ε = 0, δ = 0). The steady-state map boundaries of a single MSMPR with a solution feed are shown in gray to ease comparison, whereas the black line denotes the 99% purity of βLGA.
The most interesting heat maps are those for productivity (diagrams on the right in Figures 7 and 8), which clearly show that the region of operating conditions where productivity is high expands away from the horizontal axis (compare Figures 7 and 8 with Figure 3). Moreover, in the case of the βLGA polymorph suspension-fed continuous crystallizer, a high productivity region appears, though at rather low yield, which is absent in the corresponding map in Figure 3. Note that higher productivities are a consequence of higher encountered suspension densities at the outlet, which operationally are more challenging to handle.
3.2.4. A Cascade of Two Continuous Crystallizers
The cascade is a suspension-fed crystallizer realized in practice. The conclusions from the previous section regarding the suspension-fed crystallizer are thus fully applicable to the cascade configuration. Namely, apart from the expansion of the steady-state region in the second crystallizer of the polymorph being fed, a substantial improvement in yield accompanied by lower productivity levels is encountered as a consequence of an additional crystallizer, hence the higher total residence time (results are shown in the Supporting Information). Therefore, the suspension-fed single crystallizer clearly outperforms the cascade and the solution-fed single MSMPR in terms of productivity, whereas the cascade drastically favors operation in a higher yield.
4. Experimental Results
This section aims to fulfill multiple objectives. First, the experiments are designed to investigate the influence of different operating conditions and experimental configurations on the three KPIs, thus potentially validating the results of the theoretical analysis from section 3. Second, the experiments are used to quantify the degree of nonidealities in the suspension withdrawal step, i.e., one of the elements of novelty introduced in this work. Third, some of the operational challenges encountered along the way are discussed as well, while simultaneously providing possible solutions for some of these challenges.
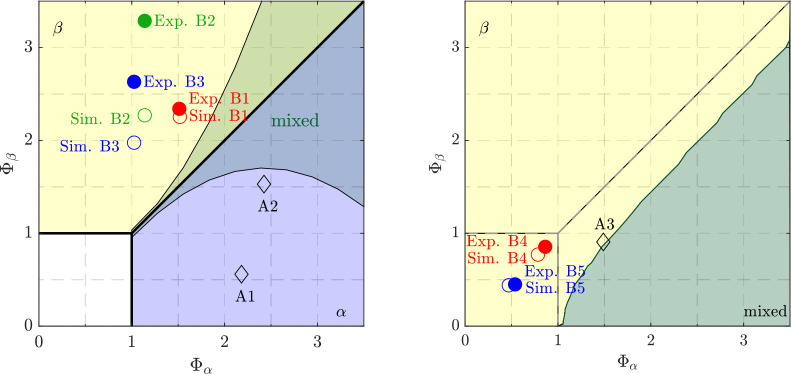
The operational challenges are polymorph-specific since the two polymorphs of LGA differ significantly in their physicochemical properties and are therefore discussed separately. To this end, Experiments A1–A3, which result in the production of either pure αLGA or a mixed product, are evaluated first. However, these experiments exhibit difficulties in reaching steady-state. Then, Experiments B1–B5, leading to a stable and pure βLGA steady-state, are discussed next. In Figure 9, all of the experimental runs are positioned on the dimensionless steady-state map (left panel for the single solution-fed crystallizer operation and right panel for the cascade), with the colored filled and empty circles indicating the experiments and simulations for the βLGA trials, respectively, and the black empty diamond corresponding to runs, which lead to the production of αLGA (i.e., xperiment A3 was aimed at producing βLGA but resulted experimentally in a mixed steady-state). Note that for the latter, only the simulation runs are shown.
Figure 9.
Experiments (filled markers) and simulations (empty markers) operated in a single MSMPR (left) and a cascade (right) were added to the dimensionless Damköhler plot. While the colored circles indicate experimental runs producing pure βLGA, the black empty diamonds indicate simulation runs where the production of αLGA was experimentally observed. The green shaded area, extracted from Farmer et al.16 indicates the boundary of a mixed steady-state region occurring when particles of LGA agglomerate. The boundary between the pure β-polymorph steady-state and the mixed steady-state is obtained when a suspension with μ3,β,2in = 0.44 is fed.
Some further considerations regarding Figure 9 should be made. The left panel of Figure 9, which includes the single crystallizer experiments, considers not only the nonrepresentative withdrawal but also the influence of agglomeration, extracted from the previous study conducted by Farmer et al.16 for LGA. Agglomeration was shown to lead to a modification of the steady-state map; i.e., the mixed steady-state close to the bifurcation expands as indicated by the green shaded region. The right panel of Figure 9 shows the experimental runs performed in a cascade; hence, it is constructed in the same way as Figure 6, namely looking only at the second crystallizer of a cascade, which is fed with the crystal population coming out of the first crystallizer (c2in = c1 and μ3,β,2in = 0.44).
A summary of all of the experimental
results is provided in Tables 4 and 5, which list the operating conditions
for experiments resulting
in the production of the α- and β-polymorph, respectively.
While a feed concentration of about c0 = 30 g kg–1 is used for all experiments, the temperature
and residence time are varied. Furthermore, the tables also report
the experimentally measured state variables, namely, the concentration
in the liquid phase, c, for αLGA and at steady-state, css, for βLGA and the third moments inside
the crystallizer,  , and in the outlet of the crystallizer,
, and in the outlet of the crystallizer,  . For all of the measured state variables,
the average and its standard deviation are provided. Finally, the
three KPIs, namely, purity given with reference to βLGA, denoted
with wβ, productivity, P, and yield, Y, are provided as well. Note that
one can determine the supersaturation in the crystallizer by dividing
the (steady-state) concentration by the solubility of βLGA provided
in the caption of the corresponding table. Due to the distinct shapes
of the two polymorphs, conclusions regarding the purity and the occurring
crystallization phenomena of the obtained product can also be verified
by examining the microscope images of the crystals. These microscope
images are shown in Figure 10 for the experiments producing mostly αLGA and Figure 11 for the experiments
producing βLGA.
. For all of the measured state variables,
the average and its standard deviation are provided. Finally, the
three KPIs, namely, purity given with reference to βLGA, denoted
with wβ, productivity, P, and yield, Y, are provided as well. Note that
one can determine the supersaturation in the crystallizer by dividing
the (steady-state) concentration by the solubility of βLGA provided
in the caption of the corresponding table. Due to the distinct shapes
of the two polymorphs, conclusions regarding the purity and the occurring
crystallization phenomena of the obtained product can also be verified
by examining the microscope images of the crystals. These microscope
images are shown in Figure 10 for the experiments producing mostly αLGA and Figure 11 for the experiments
producing βLGA.
Table 4. Summary of Operating Conditions and Experimental Results (Average Value ± Standard Deviation) For Three Experiments Used for Producing αLGA (Exps. A1 and A2) or Aimed at Producing βLGA, but Resulting in a Mixed Steady-State (Experiment A3)a.
| Single
solution-fed crystallizer |
Cascade with two crystallizers | ||
|---|---|---|---|
| Exp. A1 | Exp. A2 | Exp. A3 | |
| Operating conditions | |||
| T (°C) | 25 | 30 | 30 |
| τ0 (min) | 35 | 70 | 70 |
| c0 (g/kg) | 29.6 ± 1.9 | 29.7 ± 0.1 | 29.9 ± 0.7 |
| State variables | |||
| c (g/kg) | 12.5 ± 0.1 | 14.2 ± 0.2 | 14.4 ± 0.5 |
| μ3,nstages (−) | 0.12 ± 0.007 | 0.09 ± 0.02 | 0.56 ± 0.01 |
| μ3,nstagesout (−) | 0.0069 ± 0.001 | 0.0056 ± 0.002 | 0.74 ± 0.03 |
| KPI | |||
| wβ (−) | 0.04 ± 0.01 | 0.05 ± 0.02 | 0.82 ± 0.02 |
| Y (−) | 0.81 ± 0.01 | 0.79 ± 0.03 | 0.78 ± 0.02 |
| P [kg/(m3h)] | 0.54 ± 0.03 | 0.25 ± 0.05 | 7.5 ± 0.4 |
Note that in the cascade experiment (Experiment A3), the temperature T and residence time τ0 are provided for the second crystallizer. The temperature in the first crystallizer was set to T1 = 45 °C, while the residence time was τ1 = τ2 = 70 min. The solubilities of βLGA at 25 °C and 30 °C are 8.4 g kg–1 and 9.9 g kg–1, respectively.
Table 5. Summary of Operating Conditions and Experimental Results (Average Value ± Standard Deviation) For Five Experiments Yielding a βLGA Steady-State (Experiments B1–B5)a.
| Single
crystallizer solution-fed |
Cascade
with two crystallizers |
|||||
|---|---|---|---|---|---|---|
| Exp B1 | Exp B2 | Exp B3 | Exp B4 | Exp B5 | ||
| Operating conditions | ||||||
| T (°C) | 40 | 45 | 45 | 40 | 45 | |
| τ0 (min) | 70 | 90 | 70 | 70 | 70 | |
| c0 (g/kg) | 30.4 ± 0.4 | 29.8 ± 0.1 | 29.4 ± 0.1 | 29.2 ± 0.1 | 29.6 ± 0.2 | |
| State variables | ||||||
| css (g/kg) (exp) | 20.8 ± 0.3 | 23.3 ± 0.1 | 23.7 ± 0.1 | 18.1 ± 0.3 | 20.4 ± 0.2 | |
|
|
0.61 ± 0.01 | 0.39 ± 0.02 | 0.32 ± 0.05 | 0.66 ± 0.05 | 0.51 ± 0.02 | |
|
|
0.64 ± 0.03 | 0.42 ± 0.03 | 0.34 ± 0.02 | 0.70 ± 0.06 | 0.55 ± 0.02 | |
| css (g/kg) (sim) | 20.8 | 22.0 | 22.5 | 18.9 | 20.5 | |
|
|
0.551 | 0.451 | 0.398 | 0.596 | 0.525 | |
|
|
0.606 | 0.496 | 0.438 | 0.655 | 0.578 | |
| Characterizing withdrawal | ||||||
| εβ (−) (exp) | –0.05 ± 0.04 | –0.04 ± 0.08 | –0.06 ± 0.03 | –0.09 ± 0.05 | –0.07 ± 0.03 | |
| εβ (−) (sim) | –0.1 | –0.1 | –0.1 | –0.1 | –0.1 | |
| KPI | ||||||
| wβ (−) (exp) | 0.97 ± 0.01 | 0.98 ± 0.01 | 0.97 ± 0.01 | 0.98 ± 0.01 | 0.97 ± 0.01 | |
| Y (−) (exp) | 0.58 ± 0.02 | 0.48 ± 0.02 | 0.47 ± 0.01 | 0.73 ± 0.02 | 0.71 ± 0.01 | |
| P [kg/(m3h)] (exp) | 8.59 ± 0.36 | 4.92 ± 0.89 | 4.78 ± 0.14 | 4.73 ± 0.42 | 3.68 ± 0.16 | |
| wβ (−) (sim) | 1 | 1 | 1 | 1 | 1 | |
| Y (−) (sim) | 0.58 | 0.59 | 0.54 | 0.68 | 0.70 | |
| P [kg/(m3h)] (sim) | 8.15 | 5.19 | 5.89 | 4.41 | 3.88 | |
Experiments (denoted with “exp”) are directly compared to the simulation results (denoted with “sim”). For the simulations, a constant dilution factor value of ε = −0.1 was assumed. Note that in the cascade experiments (experiments B4 and B5) the temperature T and residence time τ0 are provided for the second crystallizer, whereas temperature and residence time in the first crystallizer are set to conditions in Exp. B3. The solubilities of βLGA at 40 °C and at 45 °C are 14.0 g kg–1 and 16.6 g kg–1, respectively.
Figure 10.

Top: Microscope images of the continuously produced αLGA in Exp. A1 (left) and Exp. A2 (right). Bottom: Microscope images of Exp. A3 for the first (left) and second (right) crystallizer. For each image, a scale corresponding to 250 μm is provided in the bottom right corner.
Figure 11.
Top: Microscope images of the continuously produced βLGA in Exp. B1 (left) and Exp. B2 (right). Bottom: Microscope images of Exp. B4 for the first (left) and second (right) crystallizer. On the right-hand side, two microscope images showing a cross-nucleation event (Exp. B3) are captured, where a prismatic-shaped α-polymorph nucleates on the surface of a needle-shaped β-polymorph. For each image, a scale corresponding to 250 μm is provided in the bottom right corner.
4.1. Experimental Runs Producing αLGA
αLGA exhibits a few operational challenges preventing stable
production at steady-state (at least in the employed experimental
setup). Operational challenges include a high degree of agglomeration,
a nonrepresentative withdrawal, and heavy encrustation (unwanted deposition
of solid particles, especially due to a higher nucleation and growth
rate at lower temperatures compared to the β-polymorph; further
information is provided in the SI). The
same conclusion is supported by the microscope images in Figure 10, where it is obvious
that both experiments A1 and A2 produce agglomerates. The first two
effects are coupled: as soon as particles reach a certain size, they
settle, grow, and agglomerate. As a consequence, large and round crystals
of millimeter size are obtained, which tend to remain in the crystallizer,
as indicated by  in Table 4. Despite numerous attempts, including, e.g., changing
the impeller blade, withdrawal remains highly nonrepresentative. Particles
remain so long in the crystallizer that the value of the solute concentration
in the crystallizer c approaches the solubility csat,α, leading to a strong increase in
the yield. This is, however, accompanied by a strong decrease in productivity,
since particles cannot be withdrawn. Such a configuration resembles
a batch operation and is not feasible in the context of continuous
crystallization. Hence, further experiments producing αLGA were
not considered.
in Table 4. Despite numerous attempts, including, e.g., changing
the impeller blade, withdrawal remains highly nonrepresentative. Particles
remain so long in the crystallizer that the value of the solute concentration
in the crystallizer c approaches the solubility csat,α, leading to a strong increase in
the yield. This is, however, accompanied by a strong decrease in productivity,
since particles cannot be withdrawn. Such a configuration resembles
a batch operation and is not feasible in the context of continuous
crystallization. Hence, further experiments producing αLGA were
not considered.
For experiment A3, the model-based experimental design when assuming a representative withdrawal results in a pure β steady state, as shown in the right panel of Figure 9. However, after performing the experiment, a mixed steady-state was obtained, which can also be seen in the microscope images in Figure 10, wherein the second crystallizer crystals of the α polymorphic form are observable. Such a process is not useful in practice due to a lack of purity. Therefore, the focus is entirely switched to experiments producing pure βLGA.
4.2. Experimental Runs Producing βLGA
As opposed to experiments producing αLGA, multiple experiments producing pure βLGA result in a stable steady-state with reproducible outcomes in different repetitions (at least 2 repetitions for each type of experiment have been performed with very minor deviations between repeated experiments). High reproducibility is reflected in the low values of the standard deviation, most notably in css, μ3, and μ3out, which are all reported in Table 5. For instance, the highest standard deviation of css is observed in experiment B1 and amounts to (only) 0.3 g/kg. A higher variability is observed for measurements of μ3 and μ3, which are more prone to experimental errors.
As
shown in the microscope images in Figure 11, βLGA crystallizes in a needle-like
morphology and therefore poses a significant risk of process disruption
in a continuous crystallizer. In such conditions, clogging is a prominent
issue in the setup, which is amplified by adding additional elements
to the pipelines, such as valves or pumps. In order to limit clogging,
using a pressure-driven withdrawal with a large diameter tube is identified
as the most effective measure. Nonetheless, clogging still occurs
in some experimental tests; thus, manual flushing and declogging of
the process lines remain necessary, especially in the cascade operation,
where additionally higher suspension densities are encountered (see
the values of  and
and  reported in Table 5). Even though the measured purity is very
high for all of the experimental runs, it is interesting to note that
cross-nucleation events are often observed. Cross-nucleation is a
form of heterogeneous nucleation, where one polymorph nucleates in
the presence of crystals of another polymorph, possibly on them (more
information on this topic can be found in the Supporting Information).2,30 Direct evidence of
such a cross-nucleation event is shown in the right-most microscope
images of Figure 11, where a prismatic αLGA crystal nucleated on the surface of
a needle-shaped βLGA crystal.
reported in Table 5). Even though the measured purity is very
high for all of the experimental runs, it is interesting to note that
cross-nucleation events are often observed. Cross-nucleation is a
form of heterogeneous nucleation, where one polymorph nucleates in
the presence of crystals of another polymorph, possibly on them (more
information on this topic can be found in the Supporting Information).2,30 Direct evidence of
such a cross-nucleation event is shown in the right-most microscope
images of Figure 11, where a prismatic αLGA crystal nucleated on the surface of
a needle-shaped βLGA crystal.
To continue the discussion
with yield and productivity, one must
take a closer look at the measured state variables in Table 5. From the two moments,  and
and  , one can determine the polymorph-specific
dilution factor εi, which characterizes
how representative the withdrawal is19
, one can determine the polymorph-specific
dilution factor εi, which characterizes
how representative the withdrawal is19
| 35 |
From Table 5, one can see that a negative dilution factor is measured (i.e., corresponding to the concentration of the suspension in the withdrawal stream), meaning that βLGA crystals are slightly oversampled. In other words, the needlelike βLGA crystals remain in the crystallizer for less time than τ0, therefore lowering the yield compared to the ideal MSMPR operation, where withdrawal is representative, i.e., where εβ = 0. However, due to the variations of the experimental measurements, a value of εβ ≈ – 0.1 was determined and used in the simulations. Moreover, both the suspension density inside the crystallizer and in the outlet are also lower as compared with the ideal MSMPR, which leads to a reduction in productivity. Similar trends, which might point to the same operational challenges, and oversampling of βLGA crystals are also observable in the literature,18 though the analysis, rationalization, and characterization of the phenomena involved are novel in this work.
However, comparing the single crystallizer with the cascade operation, in which the second crystallizer is operated at lower temperatures, shows a clear improvement of yield by roughly 55% (experiment B3 vs experiment B4). The advantage of operating at lower temperatures and lower residence times is also apparent in the single crystallizer when comparing experiment B1 to experiment B2 and finally to experiment B3: The third moments and thus also the yield and productivity all decrease in the same order as in these three experiments. Nevertheless, despite the improvement of yield and productivity when decreasing the operating temperature and residence time in experiment B3, the operating point can approach the mixed steady-state region in the case of small process disturbances (see the position of experiment B3 in Figure 9). Hence, purity can be affected under these conditions, and therefore, operating the crystallizer further away from the mixed steady-state region is recommended.
5. Concluding Remarks
In this work, a performance evaluation of a continuous crystallization process aimed at selectively producing one of the LGA polymorphs in different operating configurations is conducted. The model-based analysis, which accounts for a nonrepresentative size-independent solid withdrawal in a continuous stirred tank crystallizer, provides a sound understanding of the relationship between polymorphic purity and a certain set of process parameters, i.e., temperature, residence time, inlet concentration, and polymorphic dilution factor. Moreover, including the polymorphic dilution factor in the analytical steady-state analysis, a novelty of this work, leads to a more general formulation of the polymorphic steady-state map, thus expanding its applicability. In this updated map, the Damköhler number, Dai (i = α, β), becomes a function of the polymorphic residence time τi, which varies in response to the polymorph-specific nonrepresentative withdrawal, associated with the heterogeneity of the suspension density within the crystallizer. A nonhomogeneous suspension density stems both from hydrodynamic considerations and from different flowabilities of individual polymorphs (related to their morphology).
Furthermore, the conclusions of the steady-state analysis are further supported with a PBE model, which was developed to assess process performance not only based on the basis of purity but also with respect to two other KPIs, namely, yield and productivity. The developed concepts and conclusions are general and applicable to any substance exhibiting polymorphism; however, in the scope of this work, they are applied to l-glutamic acid. Moreover, three process configurations, namely, a solution-fed crystallizer, a suspension-fed crystallizer, and a cascade, were evaluated. The simulation results demonstrate that the suspension-fed crystallizer maximizes productivity, whereas the cascade operation improves yield due to an additional crystallizer. Considering a nonrepresentative withdrawal though has a more complex effect on the process performance. Depending on the type of nonrepresentative withdrawal, the performance might be improved if the particles are undersampled and thus remain longer in the crystallizer (i.e., higher effective residence time). Conversely, in the case of oversampling, productivity and yield actually decrease since particles have less time to grow.
These conclusions were then investigated experimentally. First of all, polymorph-specific operational challenges were identified. In experiments aimed at producing αLGA heavy agglomeration and encrustation prevented achieving a stable steady-state operation, whereas stable steady-states, where pure βLGA crystals were produced with high reproducibility, were demonstrated. In the case of βLGA experiments, conclusions closely correspond to the model predictions; namely, increasing the residence time leads to a depleted supersaturation and increased suspension density. Handling needle-like crystals and coping with clogging were deemed the main operational challenges associated with βLGA, which was successfully mitigated with a pressure-driven withdrawal.
Finally, the developed methodology allows quantification of the nonrepresentative withdrawal. In the βLGA experimental trials, the dilution factor was found to be εβ ≈ −0.1 for all experiments, indicating a slight oversampling of the needlelike βLGA crystals, which closely follow the flow field in the crystallizer. Hence, the developed approach represents a useful tool to capture and describe a more realistic continuous crystallization process, which can be employed for the purposes of process design, optimization, troubleshooting, and intensification.
Acknowledgments
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program under grant agreement No 788607. The authors would like to express their gratitude to Daniel Trottmann for his constant support with the continuous setup as well as Sarah Kienast and Markus Huber for the XRD analysis. Moreover, the authors would like to thank Paola Brenni, Eduard Meier, and Panagiota Iliopoulou for their contribution to the experimental part.
Appendix
A.1. Method of Moments and Nondimensionalization
Introducing a specific residence time for each polymorph (τi = τ0/(1 – εi)) leads to the following PBEs:
| 36 |
Applying the method of moments to eq 36,29 where the general form of the jth moment of the polymorph i is μi,j = ∫0∞xjni(x,t) dx, transforms the set of PDEs into ODEs.
| 37 |
| 38 |
The first three moment equations are needed to fully characterize the steady-state of a system. As a next step, the above equations will be made dimensionless. For this the following variables are introduced
| 39 |
| 40 |
| 41 |
| 42 |
| 43 |
| 44 |
| 45 |
| 46 |
with  . The resulting dimensionless sets of ODEs
are given by eqs 20–26. These model equations differ from the original
ones presented and analyzed by Farmer et al.1 in two aspects. Firstly, in this work, the withdrawal effects are
considered by introducing κα and κβ, and secondly, the definition of the Damköhler
numbers in eq 27 is
slightly different, again due to the consideration of withdrawal effects.
. The resulting dimensionless sets of ODEs
are given by eqs 20–26. These model equations differ from the original
ones presented and analyzed by Farmer et al.1 in two aspects. Firstly, in this work, the withdrawal effects are
considered by introducing κα and κβ, and secondly, the definition of the Damköhler
numbers in eq 27 is
slightly different, again due to the consideration of withdrawal effects.
A.2. Steady-State Analysis
A steady-state is defined when the system does not change anymore with respect to time. The steady-state map, given by Figure 12, is obtained for each of the four steady-states as follows.
Figure 12.
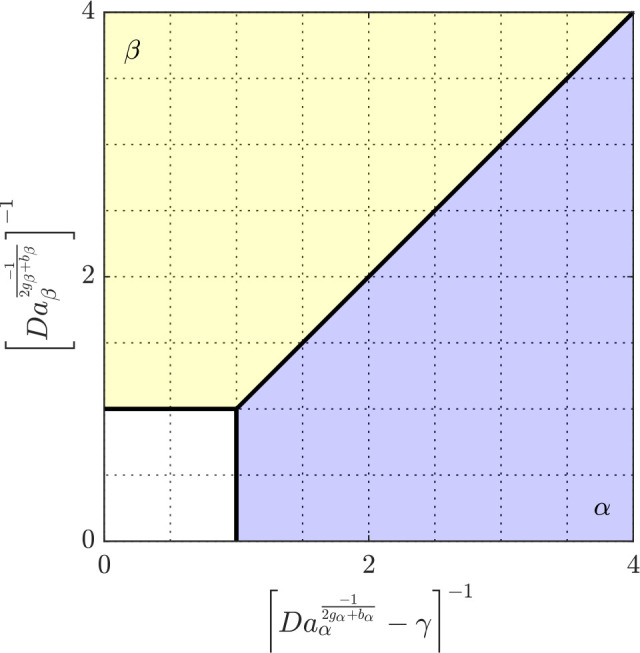
Polymorphic steady-state map operated under an outlet stream, which is diluted (0 ≤ εi ≤ 0.5, δ = 0) or concentrated (−0.5 ≤ εi ≤ 0, δ = 0) in solid particles.
Trivial Steady-State
The trivial steady-state, where ωα = ωβ = 0 and y = 1 corresponds to no nucleation and growth (either under-saturated solution or very fast residence times). Seed crystals, which were initially present, are completely washed out after several residence times.
α Steady-State
In the α steady-state (ωα ≥ 0, ωβ = 0, y ≤ 1), eqs 20–26 can be simplified to the following four steady-state values:
| 47 |
| 48 |
| 49 |
| 50 |
β Steady-State
Analogously, in the β steady-state (ωα = 0, ωβ ≥ 0, y ≤ 1), eqs 20–26 simplify as follows:
| 51 |
| 52 |
| 53 |
| 54 |
Mixed Steady-State
The mixed steady-state lies on a bifurcation at which the yss of both polymorphs are equal. This line is described by the following equation:
| 55 |
The general Jacobian matrix of the system is given below:
 |
To analyze the stability of each steady-state,
the matrix problem is first converted into a polynomial problem (eq 56). The characteristic
polynomial of the matrix is shown in eq 56 with n = 7 and λ
being the eigenvectors. Then, the Liénord–Chipart criterion
is applied, which states that all even Hurwitz determinants  of that matrix as well as all even constants
of the characteristic polynomial equation an have to be positive.31,32
of that matrix as well as all even constants
of the characteristic polynomial equation an have to be positive.31,32
| 56 |
For the trivial steady-state, the following seven constants of the characteristic polynomial equation an and three determinants are obtained:
| 57 |
| 58 |
| 59 |
| 60 |
| 61 |
| 62 |
| 63 |
| 64 |
D4 and D6 are unsuitable for print, and thus, omitted here. However, one can see that in case τα = τβ = τ0, the above expressions (including the expressions obtained for D4 and D6) become identical to the one stated in Farmer et al.1 Thus, it comes at no surprise that the same steady-state conditions are obtained. In order to have positive values for an and Dk (an > 0, Dk > 0) and a stable trivial steady-state, the following two inequalities need to be fulfilled:
| 65 |
| 66 |
The region within the steady-state map for which eqs 65 and 66 are fulfilled is indicated in the bottom left part in Figure 12 by the white square.
For the two pure crystal steady-states, i.e., the α and β steady-state, the following four variables for the two polymorphs are introduced:
 |
67 |
 |
68 |
With this, the seven constants of the characteristic polynomial for both polymorphs as well as the second Hurwitz determinant D2 are presented. Note that, since the expressions for the different values of ak are the same for both polymorphs, they are written with the general indices i, whereas an index j refers to the other polymorph.
| 69 |
| 70 |
| 71 |
| 72 |
| 73 |
| 74 |
| 75 |
| 76 |
| 77 |
The other two Hurwitz determinants D4 and D6 are not depicted as they do not qualitatively change the outcome.1 In case, these two tests are not fulfilled, the solution oscillates around the steady-state, which was predicted to be stable by the other tests.1 To obtain a stable steady-state, all the above tests have to be positive. a1–a7 become positive when both ηi > 0 and ϕi > 0. This is given when the following four conditions, provided by eqs 78 and 79, are fulfilled:
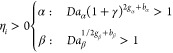 |
78 |
In eq 78, the inequality for α is shown on the right side of the trivial steady-state and the condition for β above the steady-state region, as can be seen in Figure 12.
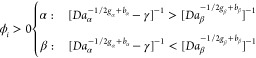 |
79 |
The condition for the α-polymorph in eq 79 corresponds to the area below the α/β bifurcation (indicated with a transparent blue in Figure 12) and the condition for the β-polymorph to the area above the bifurcation (indicated with a transparent yellow in Figure 12). Knowing that Dai > 0, ζi > 0 and 0 < ϕi < 1 means that D2,i > 0.
Note that the boundaries of the steady-state regions in the steady-state map shown in Figure 12 are the same as in Farmer et al.;1 this is true provided the extended definition of the Damköhler numbers given by eq 27 is used.
A.3. Influence of Dilution
The influence of dilution on the bipolymorphic steady-state map is shown in Figure 13.
Figure 13.

Bipolymorphic steady-state map. The operating points associated to different sets of operating conditions are plotted as gray-shaded round markers for varying values of τ0/(1 – ε) (see the color bar). Along the gray arc, T = 34 °C and c0 = 30 g/kg are constant.
Looking at the arc with gray-shaded symbols, these are obtained by keeping T and c0 constant (T = 34 °C and c0 = 30 g/kg) and changing either τ0 or ε (we are considering ε = εα = εβ here; see the color bar for the values of the ratio τ0/(1 – ε)). Increasing either τ0 or ε increases Dai, hence causing the operating point in the steady-state map to move along the arc from the region where the α-polymorph is formed to where the β-polymorph is obtained. Increasing the residence time either by changing the nominal residence time or the withdrawal dilution increases the effective residence time of the crystals in the crystallizer, thus favoring the more stable polymorph. The whole steady-state analysis performed is also valid for εα ≠ εβ.
A.4. Feeding a Suspension
When feeding a suspension of the β-polymorph, the expression for the dimensionless solute concentration yss at the β steady-state becomes a complex functions of the polymorphic’s corresponding moments:
| 80 |
| 81 |
| 82 |
| 83 |
The same problem occurs in the α steady-state when feeding the α-polymorph
| 84 |
| 85 |
| 86 |
| 87 |
with ωi,jin representing the jth dimensionless moment of the PSD of polymorph i at the inlet of the crystallizer.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.cgd.2c01424.
Additional details regarding the population balance equation (PBE) model; simulation results and discussion of a single solution-fed crystallizer when sieving is considered; simulation results and discussion of a cascade operation; details regarding the XRD calibration; considerations regarding process risks associated with fouling (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Xu S.; Hou Z.; Chuai X.; Wang Y. Overview of Secondary Nucleation: From Fundamentals to Application. Ind. Eng. Chem. Res. 2020, 59, 18335–18356. 10.1021/acs.iecr.0c03304. [DOI] [Google Scholar]
- Garside J.; Davey R.. From Molecules to Crystallizers: An Introduction to Crystallization; Oxford Chemistry Primers; Oxford University Press, 2000. [Google Scholar]
- Mangin D.; Puel F.; Veesler S. Polymorphism in Processes of Crystallization in Solution: A Practical Review. Org. Process Res. Dev. 2009, 13, 1241–1253. 10.1021/op900168f. [DOI] [Google Scholar]
- Nicoud L.; Licordari F.; Myerson A. S. Polymorph control in batch seeded crystallizers. A case study with paracetamol. CrystEngComm 2019, 21, 2105–2118. 10.1039/C8CE01428K. [DOI] [Google Scholar]
- Ni X.; Liao A. Effects of mixing, seeding, material of baffles and final temperature on solution crystallization of l-glutamic acid in an oscillatory baffled crystallizer. Chem. Eng. J. 2010, 156, 226–233. 10.1016/j.cej.2009.10.045. [DOI] [Google Scholar]
- Briggs N. E.; Schacht U.; Raval V.; McGlone T.; Sefcik J.; Florence A. J. Seeded Crystallization of β-l-Glutamic Acid in a Continuous Oscillatory Baffled Crystallizer. Org. Process Res. Dev. 2015, 19, 1903–1911. 10.1021/acs.oprd.5b00206. [DOI] [Google Scholar]
- Powell K. A.; Bartolini G.; Wittering K. E.; Saleemi A. N.; Wilson C. C.; Rielly C. D.; Nagy Z. K. Toward Continuous Crystallization of Urea-Barbituric Acid: A Polymorphic Co-Crystal System. Cryst. Growth Des 2015, 15, 4821–4836. 10.1021/acs.cgd.5b00599. [DOI] [Google Scholar]
- Zhang D.; Xu S.; Du S.; Wang J.; Gong J. Progress of Pharmaceutical Continuous Crystallization. Engineering 2017, 3, 354–364. 10.1016/J.ENG.2017.03.023. [DOI] [Google Scholar]
- Lin M.; Wu Y.; Rohani S. Identifying the Polymorphic Outcome of Hypothetical Polymorphs in Batch and Continuous Crystallizers by Numerical Simulation. Cryst. Growth Des. 2020, 20, 7312–7319. 10.1021/acs.cgd.0c00986. [DOI] [Google Scholar]
- Rogers L.; et al. Continuous Production of Five Active Pharmaceutical Ingredients in Flexible Plug-and-Play Modules: A Demonstration Campaign. Org. Process Res. Dev. 2020, 24, 2183–2196. 10.1021/acs.oprd.0c00208. [DOI] [Google Scholar]
- Farmer T. C.; Carpenter C. L.; Doherty M. F. Polymorph selection by continuous crystallization. AIChE J. 2016, 62, 3505–3514. 10.1002/aic.15343. [DOI] [Google Scholar]
- Lai T.-T. C.; Ferguson S.; Palmer L.; Trout B. L.; Myerson A. S. Continuous Crystallization and Polymorph Dynamics in the l-Glutamic Acid System. Org. Process Res. Dev. 2014, 18, 1382–1390. 10.1021/op500171n. [DOI] [Google Scholar]
- Lai T.-T. C.; Cornevin J.; Ferguson S.; Li N.; Trout B. L.; Myerson A. S. Control of Polymorphism in Continuous Crystallization via Mixed Suspension Mixed Product Removal Systems Cascade Design. Cryst. Growth Des. 2015, 15, 3374–3382. 10.1021/acs.cgd.5b00466. [DOI] [Google Scholar]
- Nicoud L.; Licordari F.; Myerson A. S. Polymorph Control in MSMPR Crystallizers. A Case Study with Paracetamol. Org. Process Res. Dev. 2019, 23, 794–806. 10.1021/acs.oprd.8b00351. [DOI] [Google Scholar]
- Köllges T.; Vetter T. Polymorph Selection and Process Intensification in a Continuous Crystallization–Milling Process: A Case Study on l-Glutamic Acid Crystallized from Water. Org. Process Res. Dev. 2019, 23, 361–374. 10.1021/acs.oprd.8b00420. [DOI] [Google Scholar]
- Farmer T. C.; Schiebel S. K.; Chmelka B. F.; Doherty M. F. Polymorph Selection by Continuous Precipitation. Cryst. Growth Des. 2018, 18, 4306–4319. 10.1021/acs.cgd.8b00116. [DOI] [Google Scholar]
- Li Y.; O’Shea S.; Yin Q.; Vetter T. Polymorph Selection by Continuous Crystallization in the Presence of Wet Milling. Cryst. Growth Des. 2019, 19, 2259–2271. 10.1021/acs.cgd.8b01894. [DOI] [Google Scholar]
- Gong W.; Wu Y.; Lin M.; Rohani S. Polymorphism control of l-Glutamic acid in a single-stage and a two-stage MSMPR crystallizer by different seeding strategies. Chem. Eng. Res. Des. 2021, 170, 23–33. 10.1016/j.cherd.2021.03.032. [DOI] [Google Scholar]
- Achermann R.; Wiedmeyer V.; Hosseinalipour M. S.; Güngör S.; Mazzotti M. Model-based design of pressure-driven product removal from stirred suspensions. Chem. Eng. Res. Des. 2021, 174, 57–70. 10.1016/j.cherd.2021.07.001. [DOI] [Google Scholar]
- Ono T.; Kramer H. J. M.; ter Horst J. H.; Jansens P. J. Process Modeling of the Polymorphic Transformation of l-Glutamic Acid. Cryst. Growth Des. 2004, 4, 1161–1167. 10.1021/cg0497694. [DOI] [Google Scholar]
- Perini G.; Salvatori F.; Ochsenbein D. R.; Mazzotti M.; Vetter T. Filterability prediction of needle-like crystals based on particle size and shape distribution data. Sep. Purif. Technol. 2019, 211, 768–781. 10.1016/j.seppur.2018.10.042. [DOI] [Google Scholar]
- Turner T. D.; Gajjar P.; Fragkopoulos I. S.; Carr J.; Nguyen T. T. H.; Hooper D.; Clarke F.; Dawson N.; Withers P. J.; Roberts K. J. Measuring the Particle Packing of l-Glutamic Acid Crystals through X-ray Computed Tomography for Understanding Powder Flow and Consolidation Behavior. Cryst. Growth Des. 2020, 20, 4252–4263. 10.1021/acs.cgd.9b01515. [DOI] [Google Scholar]
- Hermanto M. W.; Kee N. C.; Tan R. B. H.; Chiu M.-S.; Braatz R. D. Robust Bayesian estimation of kinetics for the polymorphic transformation of l-glutamic acid crystals. AIChE J. 2008, 54, 3248–3259. 10.1002/aic.11623. [DOI] [Google Scholar]
- LeVeque R. J.Finite Volume Methods for Hyperbolic Problems; Cambridge University Press, 2002. [Google Scholar]
- Qamar S.; Elsner M.; Angelov I.; Warnecke G.; Seidel-Morgenstern A. A comparative study of high resolution schemes for solving population balances in crystallization. Comput. Chem. Eng. 2006, 30, 1119–1131. 10.1016/j.compchemeng.2006.02.012. [DOI] [Google Scholar]
- Ostergaard I.; de Diego H. L.; Qu H.; Nagy Z. K. Risk-Based Operation of a Continuous Mixed-Suspension-Mixed-Product-Removal Antisolvent Crystallization Process for Polymorphic Control. Org. Process Res. Dev. 2020, 24, 2840–2852. 10.1021/acs.oprd.0c00368. [DOI] [Google Scholar]
- Mersmann A.Crystallization technology handbook, 2nd ed.; Marcel Dekker: Basel, 2001. [Google Scholar]
- Ochsenbein D. R.; Schorsch S.; Vetter T.; Mazzotti M.; Morari M. Growth rate estimation of βl-glutamic acid from online measurements of multidimensional particle size distributions and concentration. Ind. Eng. Chem. Res. 2014, 53, 9136–9148. 10.1021/ie4031852. [DOI] [Google Scholar]
- Marchisio D. L.; Pikturna J. T.; Fox R. O.; Vigil R. D.; Barresi A. A. Quadrature method of moments for population-balance equations. AIChE J. 2003, 49, 1266–1276. 10.1002/aic.690490517. [DOI] [Google Scholar]
- Chen S.; Xi H.; Yu L. Cross-Nucleation between ROY Polymorphs. J. Am. Chem. Soc. 2005, 127, 17439–17444. 10.1021/ja056072d. [DOI] [PubMed] [Google Scholar]
- Mansour M. Stability as the Fundamental Problem of Control Systems. Appl. Mech. Rev. 1999, 52, R11–R15. 10.1115/1.3098929. [DOI] [Google Scholar]
- Daud A. A. M. A note on lienard-chipart criteria and its application to epidemic models. Math. Stat. 2021, 9, 41–45. 10.13189/ms.2021.090107. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.