Abstract
The evening chronotype is strongly associated with greater alcohol use, though mechanisms underlying this association are not well understood. The current study evaluated emotion regulation as a potential mechanism linking evening chronotype and alcohol use. Participants were 81 undergraduate students. Chronotype was assessed using the Composite Scale of Morningness (CSM). Alcohol use disorder severity was assessed using the Alcohol Use Disorder Identification Test (AUDIT). Participants recorded daily sleep patterns using an online diary for seven days. Participants then completed a standardized laboratory emotion regulation task. Self-reported affect, high-frequency heart rate variability (HF-HRV), and pre-ejection period (PEP) were measured throughout the task. Sleep duration on non-free days (defined as days when sleep was restricted by morning obligations such as work or school) was evaluated as a moderator. Thirty-one evening chronotypes (CSM scores ≤ 26) were compared to 50 non-evening chronotypes (CSM scores >26). Evening chronotypes reported significantly greater symptoms of alcohol use disorder (F = 4.399, p = .039). In the full sample, emotion regulation was successful for altering affective but not autonomic reactivity to emotional stimuli. There were no chronotype differences in self-reported affect, HF-HRV, or PEP during the emotion regulation task. Longer sleep duration on non-free days was associated with increased HF-HRV during negative emotion regulation among non-evening chronotypes. Moderated mediation revealed that emotion regulation did not mediate the association between evening chronotype and alcohol use, irrespective of sleep duration on non-free days. This study is consistent with the literature on chronotype and substance use, demonstrating that undergraduate evening chronotypes endorse greater severity of alcohol use disorder. Given that emotion regulation did not successfully alter autonomic reactivity to emotional stimuli, emotion regulation as a potential mechanism linking chronotype and alcohol use remains inconclusive. Longer sleep duration appears to be protective for non-evening chronotypes in terms of parasympathetic control during the regulation of negative emotions.
Keywords: Chronotype, alcohol, emotion regulation, HRV, autonomic
Introduction
The “evening chronotype”, as defined by a preference for later sleep/wake or circadian timing, is associated with greater alcohol consumption (Prat and Adan 2011), binge drinking (Watson et al. 2013), and greater levels of alcohol dependence (Hasler et al. 2013). Emotion regulation may be one potential mechanism linking chronotype and alcohol use. Emotion regulation is a multifaceted construct, composed of both spontaneous and volitional processes. These processes are involved in the generation, appraisal, and modification of emotional states. Volitional emotion regulation is often defined in terms of the ability to down-regulate unpleasant mood states or to up-regulate or maintain positive mood states (Ochsner and Gross 2004). Difficulty regulating emotions “at will” may cause individuals to seek out external means of reducing negative and enhancing positive emotions (Aurora and Klanecky 2016; Beseler et al. 2011; Cooper et al. 1995; Grayson and Nolen-Hoeksema 2005). Emotion regulation difficulties, such as impulse control difficulties and limited emotion regulation strategies, have been associated with greater alcohol consumption in numerous studies (Aurora and Klanecky 2016; Dvorak et al. 2014; Klanecky et al. 2015; Radomski and Read 2016). Therefore, if evening chronotypes use alcohol as an emotion regulation strategy, it is plausible that emotion regulation may constitute a critical mechanism underlying the evening chronotype-alcohol consumption association.
Greater eveningness on continuous measures has been associated with emotion regulation difficulties (Azad-Marzabadi and Amiri 2017). Evening chronotypes also report greater use of emotional suppression (i.e., inhibiting the expression of emotion), while morning chronotypes report greater use of cognitive reappraisal (i.e., reinterpreting an emotion-eliciting stimuli in order to change its emotional impact) (Watts and Norbury 2017). Related literature also suggests that evening chronotypes may have less spontaneous or implicit control of emotions. For instance, research has shown that evening chronotypes score lower on trait measures of emotional stability, emotional control, and self-regulation (Cavallera et al. 2014; Ottoni et al. 2012; Owens et al. 2016) and score higher on measures of emotional problems and emotional distress (Gau et al. 2007; Giannotti et al. 2002; Jeong Jeong et al. 2015). Moreover, morning chronotypes have been found to have more protective qualities, such as resilience (defined as the ability to adapt to various situations, psychological states, and physical health conditions), relative to evening chronotypes (Lee et al. 2016). Taken together, the literature suggests that evening chronotypes may employ less adaptive emotion regulation strategies and may have less successful spontaneous or implicit regulation of emotions; however, the majority of studies in this area have used self-report measures which may be subject to demand characteristics and self-perception bias.
Only one study has examined emotion regulation across chronotypes using an objective, measurement. Horne and Norbury (2018) examined neural activation in response to fearful and happy faces and found that evening chronotypes demonstrated significantly greater amygdala activation in response to fearful versus happy faces (Horne and Norbury 2018). The findings of this study suggest that emotion regulation may vary by chronotype at the physiological level. To date, no studies have examined volitional emotion regulation across chronotypes and, more broadly, the use of physiological signals to measure psychological constructs is scarce within the chronotype literature. Activation of the autonomic nervous system (ANS) is the most common physiological index of emotion regulation (Purves et al. 2001; Thayer et al. 2012) and has not been used to examine emotion regulation across chronotype groups.
The current study examined self-report and autonomic physiology as measures of emotional response during a standardized laboratory emotion regulation task (described in detail below). We hypothesized: 1) evening chronotypes would endorse more symptoms of alcohol use disorder; 2) evening chronotypes would demonstrate poorer emotion regulation on measures of autonomic physiology and self-reported affect; and 3) emotion regulation would statistically account for the associations between chronotype and alcohol use disorder symptoms. Finally, self-reported and autonomic measures of emotion regulation appear to be sensitive to insufficient sleep duration (Baum et al. 2014; Franzen et al. 2011; Mezick et al. 2014; Racine et al. 2013); therefore, sleep duration may modulate chronotype differences in emotion regulation. Sleep duration is often curtailed in evening chronotypes (Jafar et al. 2017; Monk and Buysse 2014), especially on non-free days (i.e., work/school days) (Soehner et al. 2011). Therefore, we evaluated sleep duration on non-free days as a moderator of associations between chronotype and emotion regulation. To our knowledge, no studies have examined the moderating influence of sleep duration on non-free days in associations between chronotype and alcohol use or emotion regulation; however, it is possible that associations between eveningness and greater alcohol consumption and/or poorer emotion regulation may only occur within the context of shorter sleep duration. Thus, exploratory moderation analyses were conducted to evaluate the role of habitual sleep duration on non-free days in predicted associations.
Methods
Participants
Eighty-four undergraduate males and females met inclusion/exclusion criteria (i.e., undergraduate student status, between the ages of 18 and 25 y, free from bipolar disorder, post-traumatic stress disorder, severe depression, alcohol dependence, self-reported pregnancy, and self-reported use of medications that affect autonomic physiology: beta-blockers, tricyclics, clozapine or thioridazine). Three participants did not complete the emotion regulation task. Three participants had missing data in outcome variables. All available data were used and sample sizes for autonomic and affective emotion regulation outcomes were 80 and 79, respectively.
Procedure
After signing informed consent, data were collected using the Qualtrics Survey Service (Qualtrics, Provo, UT). Participants recorded sleep-wake times in an online diary for seven days and completed an emotion regulation task within 2 h of their habitual wake-time to standardize time of day while avoiding the artificial inflation of chronotype group differences. This study was carried out in accordance with international guidelines for chronobiology research (Portaluppi et al. 2010).
Measures
Composite scale of morningness (CSM) (Smith et al. 1989)
The CSM is a 13-item measure of chronotype on a continuum from eveningness to morningness, with current suggested cutoff criteria as follows: ≤ 26 evening type, 27–41 intermediate type, and ≥ 42 morning type (Natale and Alzani 2000). In our sample, 38.3% (N = 31) of participants were evening, 56.8% (N = 46) were intermediate, and 4.9% (N = 4) were morning chronotypes. To form comparable groups, CSM scores ≤ 26 (N = 31) denoted evening chronotypes and CSM scores > 26 (N = 50) denoted non-evening chronotypes. We present results using both categorical chronotype groups and continuous CSM scores.
The alcohol use disorder identification test (Babor AUDIT: the alcohol use disorders identification test: guidelines for use in primary health care)
The Alcohol Use Disorder Identification Test (AUDIT) is a 10-item questionnaire probing three domains of alcohol use disorders: harmful alcohol use (e.g., consequences of drinking such as blackouts or alcohol-related injuries), hazardous alcohol use (e.g., the frequency and quantity of alcohol consumption), and dependence symptoms (e.g., impaired control over drinking). Higher scores indicate more problematic alcohol use. Scores ≥ 20 indicate probable alcohol dependence.
Demographics
Participants reported age, sex, gender, pregnancy, race, ethnicity, and medication use that can affect autonomic functioning. There was concordance between self-reported sex and gender; thus, sex is used throughout and can be considered synonymous with gender in this study.
Seven-day diary
Using the Qualtrics Survey Service (Qualtrics, Provo, UT), participants recorded daily bed and wake times, sleep latency (SL), and minutes spent awake after initial sleep onset (WASO). Habitual sleep duration was measured as the minutes between bed and wake times minus SL and WASO. Each day, participants indicated if they had a “free day”, meaning they were able to sleep in if they wanted to, or a “non-free day”, meaning they needed to wake up at a specific time in order to meet an obligation such as attending a morning class or going to work.
The 16-item quick inventory of depressive symptomatology (Rush et al. 2003)
The 16-Item Quick Inventory of Depressive Symptomatology (QIDS SR-16) is a self-report measure of depressive symptom severity. Total QIDS SR-16, minus sleep items (i.e., difficulty falling asleep, staying asleep, waking up too early, or sleeping too much), were controlled for in analyses due to the relevance of depression for alcohol use and emotion regulation.
Laboratory emotion regulation protocol
Participants were scheduled for a laboratory emotion regulation protocol within 2 h of their habitual diary-assessed wake-time. Participants were asked to abstain from drinking alcohol or caffeine and exercising 12 h prior to their testing session and abstain from smoking 4 h prior to their testing session. Compliance was assessed via self-report at the time of testing.
Baseline
Participants viewed a series of colored blocks for 10 min and were asked to count the first color shown. This baseline task has been shown to be effective for washing-out individual differences in physiological arousal prior to lab-based tasks (Jennings et al. 1992).
Emotion regulation task
The emotion regulation task was adapted from widely-used emotion regulation testing paradigms (Greening et al. 2014; Lee et al. 2014; Moutsiana et al. 2014). Participants viewed a series of neutral, negative, and positive images, selected from the International Affective Picture System (IAPS) (Bradley and Lang 2017). In a pseudorandom order, images were presented in 10 blocks, each comprised of 12 positive, 12 negative, or 12 neutral images (Figure 1). Each image was preceded by one of three cues: “look”, “reframe”, or “enhance”. Prior to the task, participants were trained on each cue: “look” cued participants to view the image while experiencing their emotions naturally; “reframe” cued participants to reappraise the image’s meaning in a more positive way; “enhance” cued participants to increase their positive response to the image by thinking about it in a more personally relevant manner. Participants practiced “reframing” and “enhancing” their emotions out-loud with a study staff member and then again during a computer practice session. The training and practice sessions were not intended to improve participants’ emotion regulation abilities but were rather intended to ensure that participants could perform the task correctly.
Figure 1.

Emotion regulation task.
Using e-Prime software (Psychology Software Tools, Pittsburgh, PA), participants viewed cues for 1500 milliseconds, an image for 6000 milliseconds, and then rated how they felt on a scale ranging from “extremely negative” to “extremely positive” (for example, see Figure 2).
Figure 2.

Slide presentation.
Operationalization of emotion regulation
Emotion regulation was operationalized as the difference between “look” trials and emotion regulation trials (see key in Table 1).
Table 1.
Emotion regulation variable key.
| Type of Emotion Regulation | Outcome | Calculation | Interpretation | |
|---|---|---|---|---|
|
| ||||
| Negative | ||||
| Self-Report Affect | Affect “Reframe” | Affect “Look” | Greater values = more positive affect when regulating negative emotion | |
| HF-HRV | HRV “Reframe” | HRV “Look” | Greater values = increased HF-HRV when regulating negative emotion | |
| PEP | PEP “Reframe” | PEP “Look” | Greater values = increased PEP when regulating negative emotion | |
| Positive | ||||
| Self-Report Affect | Affect “Enhance” | Affect “Look” | Greater values = more positive affect when regulating positive emotion | |
| HF-HRV | HRV “Enhance” | HRV “Look” | Greater values = increased HF-HRV when regulating positive emotion | |
| PEP | PEP “Enhance” | PEP “Look” | Greater values = increased PEP when regulating positive emotion | |
Physiology
High-frequency variability (HF-HRV) and pre-ejection period (PEP) were used as physiological markers of emotion regulation. Spectral power in the high frequency band of HRV (0.15 to 0.40 Hz) can be obtained from short-term recordings (e.g., 1–5 min) (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996; Pinna et al. 2007) and higher HF-HRV is correlated with greater self-reported emotion regulation (Christou-Champi et al. 2015; De Witte et al. 2016; Smith et al. 2011; Volokhov and Demaree 2010; Williams et al. 2015). Greater HF-HRV during the “reframe” or “enhance” blocks relative to the “look” blocks reflected better emotion regulation. PEP, the time between depolarization of the left ventricular myocardium and the opening of the aortic valve, indicates sympathetic nervous system activation with longer PEPs indicating lower sympathetic activation (Schachinger et al. 2001) and lower emotional reactivity (Clark et al. 2016). Thus, longer PEPs during the “reframe” or “enhance” blocks relative to the “look” blocks reflected better emotion regulation.
HF-HRV and PEP data acquisition and processing.
Data were collected using Mindware Bionex hardware. Simultaneous electrocardiogram (ECG) and impedance cardiogram (ICG) signals were collected. Mindware HRV 3.1 and IMP 3.1 software were used to calculate HF-HRV and PEP values. HF-HRV was derived through automatic detection of R-peaks, visual inspection, and manual correction. Spectral power in the 0.15 Hz to 0.40 Hz range was calculated using fast Fourier transformation of the inter-beat interval. A Hamming window was used to de-trend, center, and taper the inter-beat interval series. Mindware software IMP 3.1 automatically detected the ECG Q point (ventricular depolarization onset) and the dZ/dt B point (opening of aortic valve) to calculate PEP. Ensemble averages produced by the software were manually inspected, and Q and B points were adjusted in < 10% of cases in accordance with established correction methods (Berntson et al. 2004; Lozano et al. 2007).
Statistical analysis
A natural log transformation was used to correct the skewed distribution of PEP. A general linear model was used to compare chronotype group means on AUDIT scores, controlling for age and depressive symptoms (Hypothesis 1). Emotion regulation variables were compared between evening and non-evening chronotypes using a multivariate analysis of covariance (MANCOVA), controlling for age and depressive symptoms (Hypothesis 2). Due to marked sex differences in chronotype, potentially due to higher conscientiousness among females (Rahafar et al. 2017), sex was evaluated as a potential covariate but had no influence on the results and was not included in the analyses. The percentile bootstrapping method (Shrout & Bolger 2002) was used to test the indirect effect of chronotype on alcohol use disorder severity via emotion regulation. Mediation was only tested when statistically significant pathways were identified between chronotype and emotion regulation (Hypothesis 3). In an exploratory fashion, we tested whether the mediation pathway (the effect of chronotype on emotion regulation) was moderated by sleep duration.
Results
Among the 81 participants who completed the study, the average age was 19.63 ± 1.17 y, 67.9% (N = 55) were female, 69.1% (N = 56) were Caucasian, and 97.5% (N = 79) identified as Non-Hispanic or Latino (Table 2). Average sleep duration was 8.12 h ± 70 min on free days and 6.74 h ± 79 min on non-free days. Evening and non-evening chronotypes did not differ in terms of age, sex, race, or screening measures. Sleep duration on non-free (but not free days) was significantly shorter among evening chronotypes (F = 10.555, p = .002). Evening chronotypes had 2 h less sleep on non-free days relative to free days, whereas non-evening chronotypes had 1 h less sleep on non-free days.
Table 2.
Descriptive characteristics.
| Total N = 81 | Evening Chronotypes N = 30 | Non-Evening Chronotypes N = 51 | Sig | |
|---|---|---|---|---|
|
| ||||
| Demographics | ||||
| Agea | 19.63 ± 1.17 | 19.65 ± 1.25 | 19.62 ± 1.12 | .926 |
| Sex, Femaleb | 55 (67.9%) | 21 (67.7%) | 34 (61.8%) | .981 |
| Race Whiteb | 56 (69.1%) | 19 (61.3%) | 37 (74.0%) | .682 |
| Blackb | 11 (13.6%) | 5 (16.1%) | 6 (12.0%) | |
| Asianb | 10 (12.3%) | 5 (16.1%) | 5 (10.0%) | |
| Otherb | 4 (4.9%) | 2 (6.5%) | 2 (4.0%) | |
| Sleep | ||||
| SMP Freea | 6:04 am ± 95 min | 7:02 am ± 83 min | 5:29 am ± 85 min | <0.001 |
| SMP NonFreea | 4:53 am ± 62 min | 5:24 am ± 53 min | 4:34 am ± 61 min | <0.001 |
| TST Freea | 8.12 hrs ± 70 min | 8.19 hrs ± 65 min | 8.09 hrs ± 73 min | 0.762 |
| TST NonFreea | 6.74 hrs ± 79 min | 6.15 hrs ± 82 min | 7.10 hrs ± 70 min | 0.002 |
| Alcohol Use Severity | ||||
| AUDITa | 5.19 ± 3.83 | 6.35 ± 4.05 | 4.46 ± 3.54 | 0.029 |
| Psychological | ||||
| IDSa | 13.49 ± 2.24 | 13.73 ± 2.41 | 13.34 ± 2.15 | 0.451 |
means ± standard deviation
N(%)
SMP = sleep midpoint; TST = total sleep time; AUDIT = Alcohol Use Disorder Identification Test; IDS = Inventory of Depressive Symptoms.
Impact of task on emotion regulation
Manipulation checks revealed that emotion regulation successfully altered affective but not autonomic responses to negative and positive stimuli (Table 3). While viewing negative images, self-reported affect was significantly more negative during “look” trials relative to “reframe” trials (t (78) = −15.274, p < .001) and significantly more positive during “enhance” trials relative to “look” trials (t (78) = −13.671, p < .001). There were no differences in HF-HRV or PEP between “look” and “reframe” or “look” and “enhance” trials.
Table 3.
Impact of task on emotion regulation.
| Negative Images “Look” versus “Reframe” |
Positive Images “Look” versus “Enhance” |
|||
|---|---|---|---|---|
| t | sig | t | sig | |
|
| ||||
| SRA | −15.274 | <0.001 | −13.671 | <0.001 |
| HF-HRV | 1.187 | 0.239 | 0.809 | 0.421 |
| PEP | 1.832 | 0.071 | −0.875 | 0.385 |
SRA = self-reported affect; HF-HRV = high frequency heart rate variability; PEP = pre-ejection period.
Chronotype and alcohol use disorder symptoms
Scores on the AUDIT ranged from 0 to 18 (mean = 5.19 ± 3.83) and were significantly higher among evening chronotypes (Table 1), after controlling for age and depressive symptoms (F(1,77) = 4.399, p = .039; Table 4). Scores on the AUDIT were all below the threshold for alcohol use disorder (i.e., AUDIT scores ≥ 20).
Table 4.
Chronotype predicting alcohol use disorder symptoms.
| AUDIT Scores Regressed on CSM Groups |
AUDIT Scores Regressed on Continuous CSM Scores |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| β | ΔR2 | β | ΔR2 | ||||||
|
| |||||||||
| Step 1 | Age | .025 | .023 | .025 | .023 | ||||
| Depression | .150 | .150 | |||||||
| Step 2 | Chronotype | −.232 | * | .054 | * | −.281 | * | .079 | * |
AUDIT = Alcohol Use Disorder Identification Test; CSM = Composite Scale of Morningness
p < 0.05.
Chronotype and emotion regulation
Multivariate tests revealed no effect of chronotype on changes in self-reported affect, HF-HRV, and PEP during negative or positive emotion regulation trials (Pillai’s Trace = 0.51, F(6,67) = 0.599, p = .730; Table 5). Emotion regulation was also unrelated to AUDIT scores.
Table 5.
Chronotype and emotion regulation.
| Negative Emotion Regulation (“reframe”) |
Positive Emotion Regulation (“enhance”) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SRA |
HF-HRV |
PEP |
SRA |
HF-HRV |
PEP |
||||||||
| Categorical1 | F | sig | F | sig | F | sig | F | sig | F | sig | F | sig | |
|
| |||||||||||||
| Univariate | 0.173 | 0.679 | 0.239 | 0.626 | 0.914 | 0.342 | 0.283 | 0.597 | 0.998 | 0.321 | 0.000 | 0.991 | |
| Adjusted* | 0.180 | 0.673 | 0.203 | 0.654 | 1.156 | 0.286 | 0.206 | 0.651 | 1.055 | 0.308 | 0.030 | 0.863 | |
| Continuous2 | |||||||||||||
| Univariate | 0.009 | 0.923 | 0.062 | 0.803 | 1.445 | 0.233 | 1.915 | 0.171 | 1.050 | 0.309 | 0.061 | 0.806 | |
| Adjusted* | 0.010 | 0.920 | 0.054 | 0.817 | 1.620 | 0.207 | 1.704 | 0.196 | 1.067 | 0.305 | 0.151 | 0.699 | |
SRA = self-reported affect; HF-HRV = high frequency heart rate variability; PEP = pre-ejection period
Adjusted for age and depressive symptoms
evening chronotypes compared to non-evening chronotypes
predicted by continuous Composite Scale of Morningness scores.
Emotion regulation as a mediator
Given the lack of difference in emotion regulation between evening and non-evening chronotypes, there was no justification to test the mediation model (Baron & Kenny 1986; Zhao et al. 2010).
Exploratory moderation by sleep duration on non-free days
Moderation analyses revealed a significant interaction between sleep duration on non-free days and chronotype on HF-HRV during negative emotion regulation trials (β = 0.365, p = .039; Table 6). Specifically, longer sleep duration on non-free days was associated with increases in HF-HRV from “look” to “reframe” trials for non-evening chronotypes but not for evening chronotypes (Figure 3). This interaction was robust to adjustment for age and depressive symptoms (β = 0.369, p = .05). Sleep duration on non-free days did not moderate associations between chronotype and any other emotion regulation outcome.
Table 6.
Interactions between sleep duration on non-free days and chronotype predicting emotion regulation.
| Negative Emotion Regulation (“reframe”) |
Positive Emotion Regulation (“enhance”) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SRA |
HF-HRV |
PEP |
SRA |
HF-HRV |
PEP |
|||||||
| β | sig | β | sig | β | sig | β | sig | β | sig | β | sig | |
|
| ||||||||||||
| Chronotype × Sleep Duration Non-Free | −0.272 | 0.126 | 0.367 | 0.039 | 0.215 | 0.240 | −0.062 | 0.729 | −0.023 | 0.899 | −0.134 | 0.472 |
| Adjusted for Age and Depressive Symptoms | −0.280 | 0.128 | 0.369 | 0.050 | 0.171 | 0.371 | −0.077 | 0.676 | 0.015 | 0.938 | −0.063 | 0.742 |
SRA = self-reported affect; HF-HRV = high frequency heart rate variability; PEP = pre-ejection period; significant results are in bold.
Figure 3.
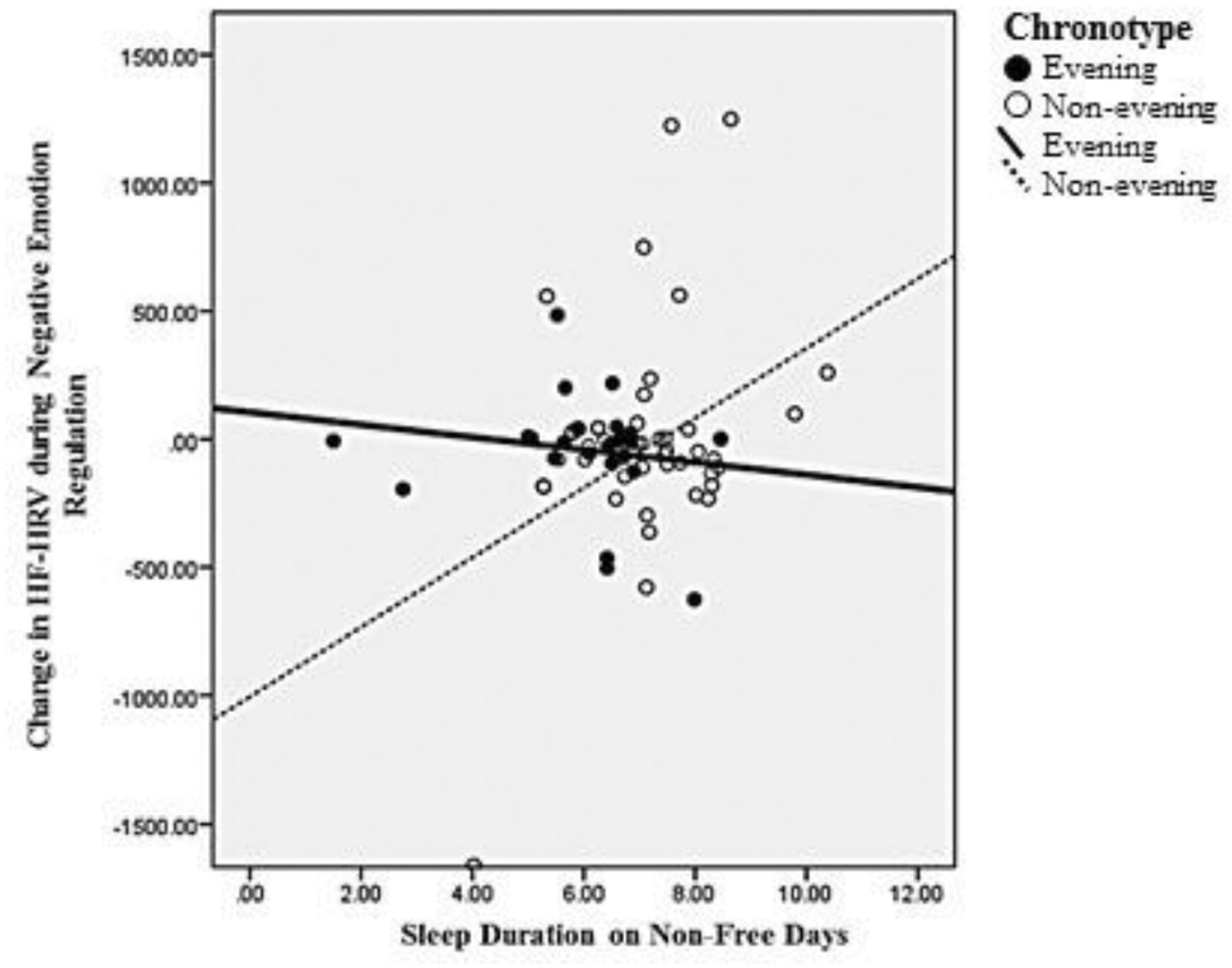
Sleep duration by chronotype interaction.
Discussion
The primary goal of the current study was to evaluate emotion regulation as a pathway linking chronotype to alcohol use. Our data supported Hypothesis 1: evening chronotypes endorsed greater symptoms of alcohol use disorder compared to non-evening chronotypes. Hypothesis 2 was not confirmed: chronotype was unrelated to changes in self-reported affect, high frequency HF-HRV, and PEP during negative and positive emotion regulation. However, in the sample as a whole, the emotion regulation task had an effect on self-reported affect but no effect on HF-HRV or PEP. Alternative emotion regulation tasks using different emotion regulation strategies, longer time to practice emotion regulation strategies, and different emotional stimuli may be needed to observe emotion regulation using physiological measures. Because self-reported affect during the emotion regulation task was subject to demand characteristics, chronotype differences in emotion regulation remain unclear. Finally, given the lack of associations between emotion regulation and either chronotype or alcohol use, we were unable to test our conceptual mediation model. Future studies using alternate experimental designs are needed to evaluate if emotion regulation could be a mechanism linking chronotype and alcohol use.
Numerous studies have shown that cognitive reappraisal strategies are effective for regulating physiology (Christou-Champi et al. 2015; Di Simplicio et al. 2012; Wu et al. 2015); however, other studies have also found effects for self-reported outcomes but not for physiology (Bernat et al. 2011; De Witte et al. 2017; Hampton et al. 2015; Pedersen and Larson 2016). Differences in manipulation success across studies may be due to slight variations in protocol and length of time participants were given to practice emotion regulation strategies. For instance, in one study, one group of participants was given three training sessions on three separate days, prior to the laboratory emotion regulation task (Christou-Champi et al. 2015). Those who participated in training sessions successfully increased heart rate variability while “reappraising” negative images, while those in a control group who received no training, exhibited no change in heart rate variability. In addition, lack of change in autonomic measures may have been due to our choice of emotional stimuli. While the IAPS are highly validated, they have also been criticized for being somewhat outdated (Gruhn and Sharfian 2016). Thus, it is unclear if the lack of change in autonomic measures was due to a lack of emotional arousal or lack of success regulating emotional arousal.
Despite lack of main effects, exploratory analyses revealed a significant interaction between chronotype and sleep duration on non-free days in relation to negative emotion regulation as measured by changes in HF-HRV. Among non-evening chronotypes, longer sleep duration on non-free days was associated with increases in HF-HRV during negative “reframe” trials relative to negative “look” trials, which may reflect dampened emotional arousal while employing the emotion regulation strategy. Consistent with the broader literature, this finding suggests that longer sleep duration may be protective for autonomic regulation of emotion. Short habitual sleep duration has been associated with lower resting HF-HRV (Castro-Diehl et al. 2016), while sleep deprivation has been shown to result in acute decreases in overall heart rate variability (Tobaldini et al. 2013; Virtanen et al. 2015; Zhong et al. 2005). ANS reactivity to stress has also been shown to be sensitive to short sleep duration (Franzen et al. 2011; Mezick et al. 2014). Interestingly, evening chronotypes demonstrated no association between sleep duration on non-free days and change in HF-HRV during the negative emotion regulation task, indicating that longer sleep duration may not be as beneficial for evening chronotypes as non-evening chronotypes. Alternatively, this finding may suggest that non-evening chronotypes are more sensitive to the effects of shorter sleep duration on parasympathetic regulation of emotions. A prospective study of nightly sleep and nightly emotion regulation may allow us to further understand the chronotype differences seen here. Multilevel modeling could then be employed to evaluate interactions between sleep duration and chronotype on emotion regulation at the between-and within-person levels.
In our sample, eveningness was associated with greater alcohol use disorder symptoms but chronotype was unrelated to changes in affective and autonomic variables during emotion regulation. Therefore, we were unable to test our proposed mediation model. Three studies have identified emotion regulation as mediator between belonging to an at-risk group and substance use; however, all used self-report measures of emotion regulation. Khosravani and colleagues showed that difficulty with emotion regulation, as measured by the Difficulties in Emotion Regulation Scale (DERS) (Gratz and Roemer 2004), mediated the association between positive/negative affect and alcohol cravings in a sample of alcoholic adults (Khosravani et al. 2017). Radomski and Read (2016) have also shown that scores on the DERS mediated associations between PTSD and alcohol use (Radomski and Read 2016). Finally, positive affect during a “savor” condition of an emotion regulation task mediated the association between opioid misuse status and current cravings for opioids (Garland et al. 2017). Emotion regulation strategies are not all equivalent and may vary in their relevance to situations and populations. Future studies may seek to test multiple regulatory strategies and dimensions of emotion regulation to fully understand the role of emotion regulation the conceptual model proposed here.
The current study had several limitations worth addressing. Morning chronotypes were under-represented in our sample (n = 4); thus, evening chronotypes were compared to a group composed mostly of intermediate chronotypes. Studies comparing morning and evening chronotype groups have used a variety of approaches to categorizing continuous measures such as the CSM and MEQ (e.g., a priori cutoffs, 10th and 90th percentiles, 20th and 80th percentiles, tertiles, and median splits). Our limited sample size prevented us from obtaining adequate comparison groups and the lack of group differences may have been due to our lack of morning chronotypes. The homogeneity of chronotype in the sample decreases our ability to make strong conclusions regarding chronotype group differences. In spite of this, we were able to find chronotype differences in alcohol use disorder severity while likely being underpowered to detect chronotype differences in emotion regulation. In addition, the current study was limited by the lack of effect emotion regulation had on autonomic physiology. Interestingly, previous studies using highly similar, single-session protocols have shown that autonomic activity (i.e., HF-HRV) is sensitive to both negatively and positively rated IAPS images (Garland et al. 2017). Despite this, more emotionally salient images that are consistent with modern standards or film clips that may better approximate real-life images may be necessary to evoke acute changes in PNS and SNS activation and fully assess emotion regulation across chronotype. Finally, no participants met the threshold for alcohol use disorder, and a lack of individuals on the extreme end of the alcohol use disorder spectrum may be another reason for the lack of conclusive results. Despite this, to our knowledge our results are consistent with the vast majority of studies in this area, with the exception of only one study (Nowakowska-Domagala et al. 2016).
Our study also had several notable strengths. This is the first experimental study to examine volitional emotion regulation in relation to chronotype. The current study extends the literature by demonstrating that chronotypes do not differ in affective or autonomic measures when asked to regulate emotional responses to visual stimuli. While numerous studies have demonstrated an association between chronotype and alcohol use, few studies have tested moderators or mediators of this association. Here, we showed that emotion regulation, as operationalized in the current study, was unrelated to alcohol use disorder severity and, therefore, could not mediate the chronotype-alcohol use association. Future studies may investigate other strategies for regulating emotions, such as acceptance or distraction. Future studies should also systematically investigate our exploratory finding that longer sleep duration may be protective for negative emotion regulation among non-evening chronotypes.
The current study has implications for public health given the predominance of an evening-oriented culture in colleges and universities and the greater endorsement of alcohol use disorder symptoms among evening chronotypes. However, due to some limitations, including our small sample size, the role of emotion regulation as a potential mechanism underlying the chronotype-alcohol use association is still unclear. Further work using stronger more impactful stimuli to elicit positive and negative emotions and larger study samples are needed to evaluate if emotion regulation is compromised in evening chronotypes and if emotion regulation contributes to the increased risk for alcohol use disorder among evening chronotypes.
Footnotes
Disclosure of interest
The authors report no conflict of interest.
References
- Aurora P, Klanecky AK. 2016. Drinking motives mediate emotion regulation difficulties and problem drinking in college students. Am J Drug Alcohol Abuse. 42:341–350. doi: 10.3109/00952990.2015.1133633 [DOI] [PubMed] [Google Scholar]
- Azad-Marzabadi E, Amiri S. 2017. Morningness-eveningness and emotion dysregulation incremental validity in predicting social anxiety dimensions. Int J Gen Med. 10:275–279. doi: 10.2147/IJGM.S144376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. 2001. AUDIT: the alcohol use disorders identification test: guidelines for use in primary health care. World Health Organization DoMHaSD, ed.. Geneva (Switzerland): World Health Organization. [Google Scholar]
- Baron RM, Kenny DA. 1986. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 51:1173–1182. doi: 10.1037//0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW. 2014. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry. 55:180–190. doi: 10.1111/jcpp.12125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat EM, Cadwallader M, Seo D, Vizueta N, Patrick CJ. 2011. Effects of instructed emotion regulation on valence, arousal, and attentional measures of affective processing. Dev Neuropsychol. 36:493–518. doi: 10.1080/87565641.2010.549881 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Lozano DL, Chen YJ, Cacioppo JT. 2004. Where to q in pep. Psychophysiology. 41:333–337. doi: 10.1111/j.1469-8986.2004.00156.x [DOI] [PubMed] [Google Scholar]
- Beseler CL, Aharonovich E, Hasin DS. 2011. The enduring influence of drinking motives on alcohol consumption after fateful trauma. Alcohol Clin Exp Res. 35:1004–1010. doi: 10.1111/j.1530-0277.2010.01431.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. 2017. International Affective Picture System. In: V ZH, T S, editors. Encyclopedia of Personality and Individual Differences. Springer, Cham. doi: 10.1007/978-3-319-28099-8_42-1 [DOI] [Google Scholar]
- Castro-Diehl C, Diez Roux AV, Redline S, Seeman T, McKinley P, Sloan R, Shea S. 2016. Sleep duration and quality in relation to autonomic nervous system measures: the multi-ethnic study of atherosclerosis (mesa). Sleep. 39:1927–1940. doi: 10.5665/sleep.6218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallera GM, Gatto M, Boari G. 2014. Personality, cognitive styles and morningness-eveningness disposition in a sample of yoga trainees. Med Sci Monit. 20:238–246. doi: 10.12659/MSM.889030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou-Champi S, Farrow TF, Webb TL. 2015. Automatic control of negative emotions: evidence that structured practice increases the efficiency of emotion regulation. Cogn Emot. 29:319–331. doi: 10.1080/02699931.2014.901213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CA, Skowron EA, Giuliano RJ, Fisher PA. 2016. Intersections between cardiac physiology, emotion regulation and interpersonal warmth in preschoolers: implications for drug abuse prevention from translational neuroscience. Drug Alcohol Depend. 163(Suppl 1):S60–9. doi: 10.1016/j.drugalcdep.2016.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. 1995. Drinking to regulate positive and negative emotions: A motivational model of alcohol use. J Pers Soc Psychol. 69:990–1005. doi: 10.1037//0022-3514.69.5.990 [DOI] [PubMed] [Google Scholar]
- De Witte NA, Sutterlin S, Braet C, Mueller SC. 2016. Getting to the heart of emotion regulation in youth: the role of interoceptive sensitivity, heart rate variability, and parental psychopathology. PLoS One. 11:e0164615. doi: 10.1371/journal.pone.0164615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Witte NA, Sutterlin S, Braet C, Mueller SC. 2017. Psychophysiological correlates of emotion regulation training in adolescent anxiety: evidence from the novel pier task. J Affect Disord. 214:89–96. doi: 10.1016/j.jad.2017.03.012 [DOI] [PubMed] [Google Scholar]
- Di Simplicio M, Costoloni G, Western D, Hanson B, Taggart P, Harmer CJ. 2012. Decreased heart rate variability during emotion regulation in subjects at risk for psychopathology. Psychol Med. 42:1775–1783. doi: 10.1017/S0033291711002479 [DOI] [PubMed] [Google Scholar]
- Dvorak RD, Sargent EM, Kilwein TM, Stevenson BL, Kuvaas NJ, Williams TJ. 2014. Alcohol use and alcohol-related consequences: associations with emotion regulation difficulties. Am J Drug Alcohol Abuse. 40:125–130. doi: 10.3109/00952990.2013.877920 [DOI] [PubMed] [Google Scholar]
- Franzen PL, Gianaros PJ, Marsland AL, Hall MH, Siegle GJ, Dahl RE, Buysse DJ. 2011. Cardiovascular reactivity to acute psychological stress following sleep deprivation. Psychosom Med. 73:679–682. doi: 10.1097/PSY.0b013e31822ff440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Bryan CJ, Nakamura Y, Froeliger B, Howard MO. 2017. Deficits in autonomic indices of emotion regulation and reward processing associated with prescription opioid use and misuse. Psychopharmacology (Berl). 234:621–629. doi: 10.1007/s00213-016-4494-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gau SS, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng AT. 2007. Association between morningness-eveningness and behavioral/emotional problems among adolescents. J Biol Rhythms. 22:268–274. doi: 10.1177/0748730406298447 [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. 2002. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 11:191–199. doi: 10.1046/j.1365-2869.2002.00302.x [DOI] [PubMed] [Google Scholar]
- Gratz KL, Roemer L. 2004. Multidimensional assessment of emotion regulation and dysregulation: development, factor structure, and initial validation of the difficulties in emotion regulation scale. J Psychopathol Behav Assess. 26:41–54. doi: 10.1023/B:JOBA.0000007455.08539.94 [DOI] [Google Scholar]
- Grayson CE, Nolen-Hoeksema S. 2005. Motives to drink as mediators between childhood sexual assault and alcohol problems in adult women. J Trauma Stress. 18:137–145. doi: 10.1002/jts.20021 [DOI] [PubMed] [Google Scholar]
- Greening SG, Osuch EA, Williamson PC, Mitchell DG. 2014. The neural correlates of regulating positive and negative emotions in medication-free major depression. Soc Cogn Affect Neurosci. 9:628–637. doi: 10.1093/scan/nst027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruhn D, Sharfian N. 2016. Lists of emotional stimuli. In: Meiselman HL, editor. Emotion measurement. Duxford (CB22 4QH, UK): Woodhead Publishing. doi: 10.1016/B978-0-08-100508-8.00007-2 [DOI] [Google Scholar]
- Hampton AJ, Hadjistavropoulos T, Gagnon MM, Williams J, Clark D. 2015. The effects of emotion regulation strategies on the pain experience: A structured laboratory investigation. Pain. 156:868–879. doi: 10.1097/j.pain.0000000000000126 [DOI] [PubMed] [Google Scholar]
- Hasler BP, Sitnick SL, Shaw DS, Forbes EE. 2013. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Res. 214:357–364. doi: 10.1016/j.pscychresns.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne CM, Norbury R. 2018. Late chronotype is associated with enhanced amygdala reactivity and reduced fronto-limbic functional connectivity to fearful versus happy facial expressions. Neuroimage. 171:355–363. doi: 10.1016/j.neuroimage.2018.01.025 [DOI] [PubMed] [Google Scholar]
- Jafar NK, Tham EK, Eng DZ, Goh DY, Teoh OH, Lee YS, Shek LP, Yap F, Chong YS, Meaney MJ, et al. 2017. The association between chronotype and sleep problems in preschool children. Sleep Med. 30:240–244. doi: 10.1016/j.sleep.2016.11.015 [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, Johnson P. 1992. Alternate cardiovascular baseline assessment techniques: vanilla or resting baseline. Psychophysiology. 29:742–750. doi: 10.1111/j.1469-8986.1992.tb02052.x [DOI] [PubMed] [Google Scholar]
- Jeong Jeong H, Moon E, Min Park J, Dae Lee B, Min Lee Y, Choi Y, In Chung Y. 2015. The relationship between chronotype and mood fluctuation in the general population. Psychiatry Res. 229:867–871. doi: 10.1016/j.psychres.2015.07.067 [DOI] [PubMed] [Google Scholar]
- Khosravani V, Sharifi Bastan F, Ghorbani F, Kamali Z. 2017. Difficulties in emotion regulation mediate negative and positive affects and craving in alcoholic patients. Addict Behav. 71:75–81. doi: 10.1016/j.addbeh.2017.02.029 [DOI] [PubMed] [Google Scholar]
- Klanecky AK, Woolman EO, Becker MM. 2015. Child abuse exposure, emotion regulation, and drinking refusal self-efficacy: an analysis of problem drinking in college students. Am J Drug Alcohol Abuse. 41:188–196. doi: 10.3109/00952990.2014.998365 [DOI] [PubMed] [Google Scholar]
- Lee S, Park JE, Cho SJ, Cho IH, Lee YJ, Kim SJ. 2014. Association between morningness-eveningness and temperament and character in community-dwelling korean adults. Asia Pac Psychiatry. 6:77–82. doi: 10.1111/j.1758-5872.2012.00214.x [DOI] [PubMed] [Google Scholar]
- Lozano DL, Norman G, Knox D, Wood BL, Miller BD, Emery CF, Berntson GG. 2007. Where to b in dz/dt. Psychophysiology. 44:113–119. doi: 10.1111/j.1469-8986.2006.00468.x [DOI] [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall MH, Richard Jennings J, Kamarck TW. 2014. Sleep duration and cardiovascular responses to stress in undergraduate men. Psychophysiology. 51:88–96. doi: 10.1111/psyp.12144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ. 2014. Chronotype, bed timing and total sleep time in seniors. Chronobiol Int. 31:655–659. doi: 10.3109/07420528.2014.885981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsiana C, Fearon P, Murray L, Cooper P, Goodyer I, Johnstone T, Halligan S. 2014. Making an effort to feel positive: insecure attachment in infancy predicts the neural underpinnings of emotion regulation in adulthood. J Child Psychol Psychiatry. 55:999–1008. doi: 10.1111/jcpp.12198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale V, Alzani A. 2000. Additional validity evidence for the composite scale of morningness. Pers Individ Dif. 30:293–301. doi: 10.1016/S0191-8869(00)00046-5 [DOI] [Google Scholar]
- Nowakowska-Domagala K, Mokros L, Jablkowska-Gorecka K, Grzelinska J, Pietras T. 2016. The relationship between chronotype and personality among patients with alcohol dependence syndrome: pilot study. Chronobiol Int. 33:1351–1358. doi: 10.1080/07420528.2016.1213738 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. 2004. Thinking makes it so: A social cognitive neuroscience approach to emotion regulation. In: Baumeister RF, Vohs KD, editors. Handbook of self-regulation: research, theory, and applications. NJ: Erlbaum. [Google Scholar]
- Ottoni GL, Antoniolli E, Lara DR. 2012. Circadian preference is associated with emotional and affective temperaments. Chronobiol Int. 29:786–793. doi: 10.3109/07420528.2012.679329 [DOI] [PubMed] [Google Scholar]
- Owens JA, Dearth-Wesley T, Lewin D, Gioia G, Whitaker RC. 2016. Self-regulation and sleep duration, sleepiness, and chronotype in adolescents. Pediatrics. 138. doi: 10.1542/peds.2016-1406 [DOI] [PubMed] [Google Scholar]
- Pedersen WS, Larson CL. 2016. State anxiety carried over from prior threat increases late positive potential amplitude during an instructed emotion regulation task. Emotion. 16:719–729. doi: 10.1037/emo0000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna GD, Maestri R, Torunski A, Danilowicz-Szymanowicz L, Szwoch M, La Rovere MT, Raczak G. 2007. Heart rate variability measures: A fresh look at reliability. Clin Sci (Lond). 113:131–140. doi: 10.1042/CS20070055 [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. 2010. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 27:1911–1929. doi: 10.3109/07420528.2010.516381 [DOI] [PubMed] [Google Scholar]
- Prat G, Adan A. 2011. Influence of circadian typology on drug consumption, hazardous alcohol use, and hangover symptoms. Chronobiol Int. 28:248–257. doi: 10.3109/07420528.2011.553018 [DOI] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia AS, McNamara JO, Williams SM. 2001. Neuroscience, 2nd edition. Sunderland (MA): Sinauer Associates. [Google Scholar]
- Racine C, Kalra K, Ceide M, Williams NJ, Zizi F, Mendlowicz MV, Jean-Louis G. 2013. Sleep duration, insomnia symptoms, and emotion regulation among black women. J Sleep Disord Ther. 2. doi: 10.4172/2167-0277.1000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski SA, Read JP. 2016. Mechanistic role of emotion regulation in the ptsd and alcohol association. Traumatology (Tallahass Fla). 22:113–121. doi: 10.1037/trm0000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahafar A, Castellana I, Randler C, Antúnez JM. 2017. Conscientiousness but not agreeableness mediates females’ tendency toward being a morning person. Scan J Psychol. 58:249–253. doi: 10.1111/sjop.12362 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, et al. 2003. The 16-item quick inventory of depressive symptomatology (qids), clinician rating (qids-c), and self-report (qids-sr): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 54:573–583. doi: 10.1016/s0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- Schachinger H, Weinbacher M, Kiss A, Ritz R, Langewitz W. 2001. Cardiovascular indices of peripheral and central sympathetic activation. Psychosom Med. 63:788–796. doi: 10.1097/00006842-200109000-00012 [DOI] [PubMed] [Google Scholar]
- Shrout PE, Bolger N. 2002. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods. 7:422–445. doi: 10.1037/1082-989X.7.4.422 [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. 1989. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 74:728–738. doi: 10.1037/0021-9010.74.5.728 [DOI] [PubMed] [Google Scholar]
- Smith TW, Cribbet MR, Nealey-Moore JB, Uchino BN, Williams PG, Mackenzie J, Thayer JF. 2011. Matters of the variable heart: respiratory sinus arrhythmia response to marital interaction and associations with marital quality. J Pers Soc Psychol. 100:103–119. doi: 10.1037/a0021136 [DOI] [PubMed] [Google Scholar]
- Soehner AM, Kennedy KS, Monk TH. 2011. Circadian preference and sleep-wake regularity: associations with self-report sleep parameters in daytime-working adults. Chronobiol Int. 28:802–809. doi: 10.3109/07420528.2011.613137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. 1996. Hear rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 93:1043–1065. doi: 10.1161/01.CIR.93.5.1043 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ 3rd, Wager TD. 2012. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 36:747–756. doi: 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Tobaldini E, Nobili L, Strada S, Casali KR, Braghiroli A, Montano N. 2013. Heart rate variability in normal and pathological sleep. Front Physiol. 4:294. doi: 10.3389/fphys.2013.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen I, Kalleinen N, Urrila AS, Leppanen C, Polo-Kantola P. 2015. Cardiac autonomic changes after 40 hours of total sleep deprivation in women. Sleep Med. 16:250–257. doi: 10.1016/j.sleep.2014.10.012 [DOI] [PubMed] [Google Scholar]
- Volokhov RN, Demaree HA. 2010. Spontaneous emotion regulation to positive and negative stimuli. Brain Cogn. 73:1–6. doi: 10.1016/j.bandc.2009.10.015 [DOI] [PubMed] [Google Scholar]
- Watson NF, Buchwald D, Harden KP. 2013. A twin study of genetic influences on diurnal preference and risk for alcohol use outcomes. J Clin Sleep Med. 9:1333–1339. doi: 10.5664/jcsm.3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AL, Norbury R. 2017. Reduced effective emotion regulation in night owls. J Biol Rhythms. 32:369–375. doi: 10.1177/0748730417709111 [DOI] [PubMed] [Google Scholar]
- Williams DP, Cash C, Rankin C, Bernardi A, Koenig J, Thayer JF. 2015. Resting heart rate variability predicts self-reported difficulties in emotion regulation: A focus on different facets of emotion regulation. Front Psychol. 6:261. doi: 10.3389/fpsyg.2015.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Winkler MH, Wieser MJ, Andreatta M, Li Y, Pauli P. 2015. Emotion regulation in heavy smokers: experiential, expressive and physiological consequences of cognitive reappraisal. Front Psychol. 6:1555. doi: 10.3389/fpsyg.2015.01555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Lynch JG, Chen Q. 2010. Reconsidering baron and kenny: myths and truths about mediation analysis. J Consum Res. 37:197–206. doi: 10.1086/651257 [DOI] [Google Scholar]
- Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, Bartels MN, Demeersman RE, Basner RC. 2005. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol. 98:2024–2032. doi: 10.1152/japplphysiol.00620.2004 [DOI] [PubMed] [Google Scholar]


