Abstract

Some members of the human gut microbiota profoundly influence their host’s physiology, health, and therapeutic responses, but the responsible molecules and mechanisms are largely unknown. As part of a project to identify immunomodulators produced by gut microbes, we analyzed the metabolome of Collinsella aerofaciens, an actinomycete that figures prominently in numerous association studies. The associations are typically positive correlations of C. aerofaciens with pro-inflammatory responses and undesirable outcomes, but an association with favorable responses to PD-1/PD-L1 cancer immunotherapy is a notable exception. A phenotypic assay-guided screen using dendritic cells (mBMDCs) and cytokine readouts identified the active compound, which was structurally characterized as a lysoglycoglycerolipid with an acetal-bearing β-galactofuranose head group (CaLGL-1, 1). The structural assignment was confirmed through total synthesis. Assays with tlr2–/–, tlr4–/–, and wt mBMDCs revealed TLR2-dependent signaling. CaLGL-1 is produced by a conversion of a bacterially biosynthesized plasmalogen (CaPlsM, 3) to CaLGL-1 (1) in a low-pH environment.
Cancer immunotherapies have produced promising but disparate outcomes: dramatic responses in a significant minority and little to no response in the majority.1−3 Pursuing the source of this variability led researchers to identify several commensal bacteria in the human gut whose increased presence correlated with favorable therapeutic outcomes in checkpoint therapies.1,2,4,5 One of the notable contributors is Collinsella aerofaciens, a Gram-positive obligate anaerobe that is the most abundant actinobacterium in a healthy human gut.6−8 In addition to its identification in metastatic melanoma patients undergoing PD-1/PD-L1 cancer immunotherapy, it has also been identified in studies of rheumatoid arthritis, psoriasis, Crohn’s disease, IBD (inflammatory bowel disease), atherosclerosis, NASH (non-alcoholic steatohepatitis), type 2 diabetes, and COVID-19.8−15 Here we report the structures, cellular target, and mixed biosynthetic and environmental formation of unusual lipid-derived immunogens that activate TLR2 to produce pro-inflammatory cytokines.
We used assay-guided fractionation to interrogate the metabolome of C. aerofaciens for immune regulators.16 The assay uses dendritic cells (DCs) from the innate immune system that detect potential pathogens and alert the adaptive immune system through the release of cytokines. DCs from mouse bone marrow (mBMDC) were used, and their response to bacterial extracts was monitored by measuring TNFα, a pro-inflammatory cytokine, with an enzyme-linked immunosorbent assay (ELISA). The butanol extract of anaerobic monocultures of C. aerofaciens ATCC 25986 showed strong pro-inflammatory TNFα inducing activity, and the cell pellet of a large (175 L) culture was subjected to multiple rounds of reversed-phase chromatography that identified 1a (1.6 mg) as the active compound (Figure 1A, Figure S1).
Figure 1.
Structures and relationships of C. aerofaciens glycolipids.
Compound 1a was assigned the molecular formula C35H66O9 based on a [M+Na]+ ion at m/z 653.4605 (see the Supporting Information (SI)). A combination of 1H, 13C, and HSQC NMR data analysis identified one carbonyl signal, two anomeric signals, eight oxygen-bound methine/methylene signals, a large envelope of aliphatic methylene groups, and two overlapped methyl groups. Additional NMR analysis established the planar structure of 1a as a modified 2-lysoglycoglycerolipid (2-lyso-GL), bearing a β-d-galactofuranose (Galf) with an acetal chain head group at the sn-3 position and a dodecanoyl ester at the sn-1 position (Figure 1A). The relative and absolute configurations at sn-2 and the anomeric carbon of Galf were established through a combination of J-based configuration, ROESY correlations, diastereomeric derivatization, acetylation, and esterification for GC-MS analysis (see SI). During our analysis it became clear that 1a interconverts with 1b through a 1,2-diacyl shift (Figure 1B). These shifts are well known in lysoglycerolipids, and their kinetics and equilibria depend on structure, solvent, temperature, and pH.17−19 Compounds 1a and 1b are difficult to separate and convert to a 90:10 mixture of 1a:1b rapidly at 37 °C in PBS solution (Figure 1B). In addition to 1a and 1b, we also characterized the structurally related but inactive compounds 2a, 2b, and 3 (C14(Plasm)-C12:0 PE) and analogs with varying 10- and 12-C lipid chains at the sn-1 and sn-2 positions (see Figure 1B, Figures S3–S19, and Tables S1–S3).
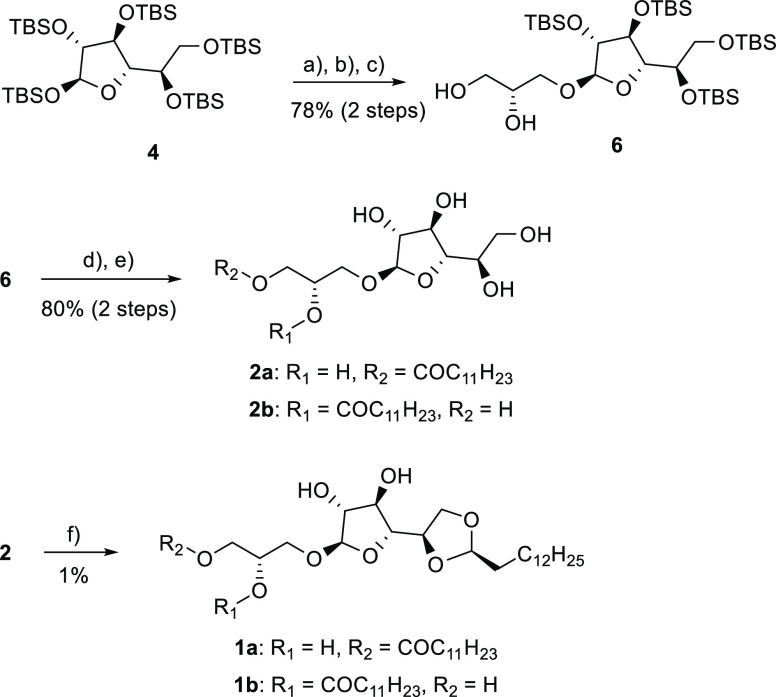
To confirm the structural assignment of 1a, to rule out the possibility of immunogenic contaminants, and to probe the specificity of the acetal-forming step, we synthesized the proposed structure for 1a (Scheme 1, Figure S2). Penta-TBS-protected β-d-galactofuranose was used to glycosylate (S)-(+)-solketal to give compound 6 after selective deprotection of the ketal.20,21 Regiospecific esterification of 6 with dodecyl chloride led to the sn-1 ester 7 (Figure S2). After TBS deprotection, compound 2 was isolated as an inseparable mixture of isomers 2a and 2b. We were able to isolate the acetal 1 as an inseparable mixture of the isomers 1a and 1b by acetalization of 2 with tetradecanal and extensive chromatographic purifications. The low yield and difficult purification were expected since the acetalization leads to a complex mixture of regio- and stereoisomers as described by others.22,23 Comparison of the NMR spectra of the synthesized and the isolated compound confirmed the structural identity of 1 (CaLGL-1).
Scheme 1. Synthesis of CaLGL-1.
Reagents and conditions: (a) TMSI, 4 Å mol. sieve, DCM, 15 min, 0 °C, (b) DIPEA, (S)-(+)-solketal, 3 h, rt (79%); (c) TFA/water/DCM, 10 min, rt (99%); (d) dodecyl chloride, 2,4,6-collidine, DCM, 1 h, −78 °C (85%); (e) TBAF, THF, 30 min, 4 °C (94%, inseparable mixture of sn-1 (2a) and sn-2 ester (2b) (84:16)); (f) tetradecanal, pTsOH·H2O, THF, 24 h, rt then 7 h, 50 °C (1% after prep. HPLC, inseparable mixture of sn-1 (1a) and sn-2 ester (1b)). See Figures S20–S29.
Compound 1a’s unusual structure reflects its formation by both enzyme-catalyzed and spontaneous reactions. C. aerofaciens produces 3, a specialized lipid called a plasmalogen, from a diacylglyceride by the recently identified two-gene pathway in anaerobic bacteria.24 In C. aerofaciens, one ORF (GXM19_05965) has the four domains that were identified as responsible for plasmalogen production.7,24 Plasmalogens, which contain a vinyl ether rather than an ester at sn-1, are widespread in both animals and anaerobic bacteria, although the two kingdoms use very different biosynthetic pathways to produce them.24−27 Their roles are not completely defined, but the susceptibility of the electron-rich vinyl ether that characterizes them to electrophilic attack by reactive oxygen species (ROS), HOCl from activated T cells, or acids is thought to be important.28,29 A hydrolysis reaction at the sn-1 position of 3 releases the aldehyde that generates an acetal on the Galf head group. With the sn-1 position open, the acyl chain at sn-2 migrates to the sn-1 position (Figure 1B).9 The pathway from 3 to 1 would go through 2, with one possible sequence being 3 to 2b to 2a to 1a, but other pathways are possible (Figure 1B). The general correctness of an intramolecular acid-catalyzed conversion in which the masked aldehyde forming the plasmalogen migrates to the sugar head group is strengthened by the clean conversion of 3 to 1 under acidic conditions, in contrast to the complex mixture generated in the conversion of 8 to 9 in our synthetic scheme (Scheme 1 and Figure 2). Plasmalogens are long-lived at pH 7.5, but react cleanly at pH 6.5 to form the acetal product. The pH of a typical tumor environment is ∼6.5 due to the Warburg effect, in which rapidly growing cancer cells generate ATP through fermentation rather than respiration.30
Figure 2.
HPLC-MS analysis of intramolecular acid-catalyzed conversion from 3 to 1. The data shown are HPLC-MS extracted ion chromatograms for 1 and 3 (m/z = 653.4).
With the structure and genesis of CaLGL-1 in hand, we focused on its ability to induce an immune response, the release of TNFα and other cytokines. The most likely pathways involve the toll-like receptors TLR2 or TLR4, the key receptors by which the innate immune system detects microbes. Typical TLR2 agonists are lipopeptides, while TLR4 agonists are typically lipopolysaccharides (LPSs).31−33 TLR2/TLR4 activation could be differentiated in our screen by using BMDC from genetically altered mice, either tlr2–/– or tlr4–/–. As shown in Figure 3, CaLGL-1 induces a robust TNFα response in BMDCs lacking TLR4, but no response in BMDCs lacking TLR2. The EC50 of both natural and synthetic CaLGL-1 is 3.2 μM (Figure 3B). This low μM value is a bulk concentration, not the dynamic local concentration relevant to immune signaling between bacteria and receptors.34 For C. aerofaciens the concentration also reflects the local pH of a tumor microenvironment (pH 6.5).
Figure 3.
Proinflammatory activities of 1. (A) TNFα production of 1 in mBMDC assay from tlr2–/– and tlr4–/– mice. (B) Dose–response curve of 1. No detectable activity from 2 and 3. Pam3CSK4 and LPS were used for positive controls of TLR1/2 and TLR4 signaling, respectively.
Our search into the molecular mechanism of C. aerofaciens’ ability to enhance the effectiveness of PD-1/PD-L1-based cancer immunotherapy led to an unexpected finding: the unusual immunogenic lipid CaLGL-1 (1). The lipid is produced in a context-dependent fashion—the low-pH characteristic of tumors—through the generation and intramolecular recapture of a long-chain aldehyde from a plasmalogen precursor (3). More broadly, the study illustrates the ability of plasmalogens to generate immunogenic lipids that function as TLR2 agonists in a context-dependent fashion.
Acknowledgments
This work was funded by NIH R01 AT009708 and NIH R01 ATI72147. We thank the Harvard Medical School’s Analytical Chemistry Core (ACC), East Quad NMR facility, and Institute of Chemistry and Cell Biology (ICCB) facility for their analytical services.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c00250.
Supplementary figures, NMR spectra for synthetic compounds, and detailed experimental methods (PDF)
Author Contributions
∥ J.K., M.B., and D.S. contributed equally to this work.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare the following competing financial interest(s): Some of the authors have filed a patent application related to the research reported in this article.
Supplementary Material
References
- Finlay B. B.; Goldszmid R.; Honda K.; Trinchieri G.; Wargo J.; Zitvogel L. Can We Harness the Microbiota to Enhance the Efficacy of Cancer Immunotherapy?. Nat. Rev. Immunol. 2020, 20, 522–528. 10.1038/s41577-020-0374-6. [DOI] [PubMed] [Google Scholar]
- Routy B.; Le Chatelier E.; Derosa L.; Duong C. P. M.; Alou M. T.; Daillere R.; Fluckiger A.; Messaoudene M.; Rauber C.; Roberti M. P.; Fidelle M.; Flament C.; Poirier-Colame V.; Opolon P.; Klein C.; Iribarren K.; Mondragon L.; Jacquelot N.; Qu B.; Ferrere G.; Clemenson C.; Mezquita L.; Masip J. R.; Naltet C.; Brosseau S.; Kaderbhai C.; Richard C.; Rizvi H.; Levenez F.; Galleron N.; Quinquis B.; Pons N.; Ryffel B.; Minard-Colin V.; Gonin P.; Soria J.-C.; Deutsch E.; Loriot Y.; Ghiringhelli F.; Zalcman G.; Goldwasser F.; Escudier B.; Hellmann M. D.; Eggermont A.; Raoult D.; Albiges L.; Kroemer G.; Zitvogel L. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- Woelk C. H.; Snyder A. Modulating Gut Microbiota to Treat Cancer. Science 2021, 371, 573–574. 10.1126/science.abg2904. [DOI] [PubMed] [Google Scholar]
- Routy B.; Gopalakrishnan V.; Daillère R.; Zitvogel L.; Wargo J. A.; Kroemer G. The Gut Microbiota Influences Anticancer Immunosurveillance and General Health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. 10.1038/s41571-018-0006-2. [DOI] [PubMed] [Google Scholar]
- Derosa L.; Routy B.; Thomas A. M.; Iebba V.; Zalcman G.; Friard S.; Mazieres J.; Audigier-Valette C.; Moro-Sibilot D.; Goldwasser F.; Silva C. A. C.; Terrisse S.; Bonvalet M.; Scherpereel A.; Pegliasco H.; Richard C.; Ghiringhelli F.; Elkrief A.; Desilets A.; Blanc-Durand F.; Cumbo F.; Blanco A.; Boidot R.; Chevrier S.; Daillere R.; Kroemer G.; Alla L.; Pons N.; Le Chatelier E.; Galleron N.; Roume H.; Dubuisson A.; Bouchard N.; Messaoudene M.; Drubay D.; Deutsch E.; Barlesi F.; Planchard D.; Segata N.; Martinez S.; Zitvogel L.; Soria J.-C.; Besse B. Intestinal Akkermansia muciniphila Predicts Clinical Response to PD-1 Blockade in Patients with Advanced Non-Small-Cell Lung Cancer. Nat. Med. 2022, 28, 315–324. 10.1038/s41591-021-01655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourlousse D. M.; Sakamoto M.; Miura T.; Narita K.; Ohashi A.; Uchino Y.; Yamazoe A.; Kameyama K.; Terauchi J.; Ohkuma M.; Kawasaki H.; Sekiguchi Y. Complete Genome Sequence of Collinsella aerofaciens JCM 10188T. Microbiol. Resour. Announc. 2020, 9, e00134–20. 10.1128/MRA.00134-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bag S.; Ghosh T. S.; Das B. Complete Genome Sequence of Collinsella aerofaciens Isolated from the Gut of a Healthy Indian Subject. Genome Announc. 2017, 5, e01361-17. 10.1128/genomeA.01361-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson V.; Fessler J.; Bao R.; Chongsuwat T.; Zha Y.; Alegre M.-L.; Luke J. J.; Gajewski T. F. The Commensal Microbiome Is Associated with Anti-PD-1 Efficacy in Metastatic Melanoma Patients. Science 2018, 359, 104–108. 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J.; Cohen N. A.; Shalev V.; Uzan A.; Koren O.; Maharshak N. Psoriatic Patients Have a Distinct Structural and Functional Fecal Microbiota Compared with Controls. J. Dermatol. 2019, 46, 595–603. 10.1111/1346-8138.14933. [DOI] [PubMed] [Google Scholar]
- Mena-Vázquez N.; Ruiz-Limón P.; Moreno-Indias I.; Manrique-Arija S.; Tinahones F. J.; Fernández-Nebro A. Expansion of Rare and Harmful Lineages Is Associated with Established Rheumatoid Arthritis. J. Clin. Med. 2020, 9, 1044. 10.3390/jcm9041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joossens M.; Huys G.; Cnockaert M.; De Preter V.; Verbeke K.; Rutgeerts P.; Vandamme P.; Vermeire S. Dysbiosis of the Faecal Microbiota in Patients with Crohn’s Disease and Their Unaffected Relatives. Gut 2011, 60, 631. 10.1136/gut.2010.223263. [DOI] [PubMed] [Google Scholar]
- Kassinen A.; Krogius-Kurikka L.; Mäkivuokko H.; Rinttilä T.; Paulin L.; Corander J.; Malinen E.; Apajalahti J.; Palva A. The Fecal Microbiota of Irritable Bowel Syndrome Patients Differs Significantly from That of Healthy Subjects. Gastroenterology 2007, 133, 24–33. 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Karlsson F. H.; Fåk F.; Nookaew I.; Tremaroli V.; Fagerberg B.; Petranovic D.; Bäckhed F.; Nielsen J. Symptomatic Atherosclerosis Is Associated with an Altered Gut Metagenome. Nat. Commun. 2012, 3, 1245. 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astbury S.; Atallah E.; Vijay A.; Aithal G. P.; Grove J. I.; Valdes A. M. Lower Gut Microbiome Diversity and Higher Abundance of Proinflammatory Genus Collinsella Are Associated with Biopsy-Proven Nonalcoholic Steatohepatitis. Gut Microbes 2020, 11, 569. 10.1080/19490976.2019.1681861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama M.; Nishiwaki H.; Hamaguchi T.; Ito M.; Ueyama J.; Maeda T.; Kashihara K.; Tsuboi Y.; Ohno K. Intestinal Collinsella May Mitigate Infection and Exacerbation of COVID-19 by Producing Ursodeoxycholate. PLoS One 2021, 16, e0260451 10.1371/journal.pone.0260451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke M. T.; Kenny D. J.; Cassilly C. D.; Vlamakis H.; Xavier R. J.; Clardy J. Ruminococcus Gnavus, a Member of the Human Gut Microbiome Associated with Crohn’s Disease, Produces an Inflammatory Polysaccharide. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 12672–12677. 10.1073/pnas.1904099116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plueckthun A.; Dennis E. A. Acyl and Phosphoryl Migration in Lysophospholipids: Importance in Phospholipid Synthesis and Phospholipase Specificity. Biochemistry 1982, 21, 1743–1750. 10.1021/bi00537a007. [DOI] [PubMed] [Google Scholar]
- Okudaira M.; Inoue A.; Shuto A.; Nakanaga K.; Kano K.; Makide K.; Saigusa D.; Tomioka Y.; Aoki J. Separation and Quantification of 2-Acyl-1-Lysophospholipids and 1-Acyl-2-Lysophospholipids in Biological Samples by LC-MS/MS. J. Lipid Res. 2014, 55, 2178–2192. 10.1194/jlr.D048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasini D.; Subbaiah P. V. Rate of Acyl Migration in Lysophosphatidylcholine (LPC) Is Dependent upon the Nature of the Acyl Group. Greater Stability of sn-2 Docosahexaenoyl LPC Compared to the More Saturated LPC Species. PLoS One 2017, 12, e0187826 10.1371/journal.pone.0187826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldoni L.; Marino C. Facile Synthesis of Per-O-Tert -Butyldimethylsilyl-β-d-Galactofuranose and Efficient Glycosylation via the Galactofuranosyl Iodide. J. Org. Chem. 2009, 74, 1994–2003. 10.1021/jo8025274. [DOI] [PubMed] [Google Scholar]
- Sauvageau J.; Foster A. J.; Khan A. A.; Chee S. H.; Sims I. M.; Timmer M. S. M.; Stocker B. L. Synthesis and Biological Activity of the Lipoteichoic Acid Anchor from Streptococcus sp. DSM 8747. Chembiochem 2012, 13, 2416–2424. 10.1002/cbic.201200468. [DOI] [PubMed] [Google Scholar]
- Belmessieri D.; Gozlan C.; Duclos M.-C.; Dumitrescu O.; Lina G.; Redl A.; Duguet N.; Lemaire M. Dodecyl Sorbitan Ethers as Antimicrobials against Gram-Positive Bacteria. Bioorg. Med. Chem. Lett. 2017, 27, 4660–4663. 10.1016/j.bmcl.2017.09.015. [DOI] [PubMed] [Google Scholar]
- Gozlan C.; Deruer E.; Duclos M.-C.; Molinier V.; Aubry J.-M.; Redl A.; Duguet N.; Lemaire M. Preparation of Amphiphilic Sorbitan Monoethers through Hydrogenolysis of Sorbitan Acetals and Evaluation as Bio-Based Surfactants. Green Chem. 2016, 18, 1994–2004. 10.1039/C5GC02131F. [DOI] [Google Scholar]
- Jackson D. R.; Cassilly C. D.; Plichta D. R.; Vlamakis H.; Liu H.; Melville S. B.; Xavier R. J.; Clardy J. Plasmalogen Biosynthesis by Anaerobic Bacteria: Identification of a Two-Gene Operon Responsible for Plasmalogen Production in Clostridium perfringens. ACS Chem. Biol. 2021, 16, 6–13. 10.1021/acschembio.0c00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-García A.; Monera-Girona A. J.; Pajares-Martínez E.; Bastida-Martínez E.; Pérez-Castaño R.; Iniesta A. A.; Fontes M.; Padmanabhan S.; Elías-Arnanz M. A Bacterial Light Response Reveals an Orphan Desaturase for Human Plasmalogen Synthesis. Science 2019, 366, 128–132. 10.1126/science.aay1436. [DOI] [PubMed] [Google Scholar]
- Goldfine H. The Appearance, Disappearance and Reappearance of Plasmalogens in Evolution. Prog. Lipid Res. 2010, 49, 493–498. 10.1016/j.plipres.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Werner E. R.; Keller M. A.; Sailer S.; Lackner K.; Koch J.; Hermann M.; Coassin S.; Golderer G.; Werner-Felmayer G.; Zoeller R. A.; Hulo N.; Berger J.; Watschinger K. The TMEM189 Gene Encodes Plasmanylethanolamine Desaturase Which Introduces the Characteristic Vinyl Ether Double Bond into Plasmalogens. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 7792–7798. 10.1073/pnas.1917461117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean J. M.; Lodhi I. J. Structural and Functional Roles of Ether Lipids. Protein Cell 2018, 9, 196–206. 10.1007/s13238-017-0423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braverman N. E.; Moser A. B. Functions of Plasmalogen Lipids in Health and Disease. Biochim. Biophys. Acta 2012, 1822, 1442–1452. 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Corbet C.; Feron O. Tumour Acidosis: From the Passenger to the Driver’s Seat. Nat. Rev. Cancer 2017, 17, 577–593. 10.1038/nrc.2017.77. [DOI] [PubMed] [Google Scholar]
- Akira S.; Takeda K. Toll-like Receptor Signalling. Nat. Rev. Immunol. 2004, 4, 499–511. 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Takeda K.; Akira S. Toll-Like Receptors. Curr. Protoc. Immunol. 2015, 109, 14.12.1–14.12.10. 10.1002/0471142735.im1412s109. [DOI] [PubMed] [Google Scholar]
- Kang J. Y.; Nan X.; Jin M. S.; Youn S.-J.; Ryu Y. H.; Mah S.; Han S. H.; Lee H.; Paik S.-G.; Lee J.-O. Recognition of Lipopeptide Patterns by Toll-like Receptor 2-Toll-like Receptor 6 Heterodimer. Immunity 2009, 31, 873–884. 10.1016/j.immuni.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Shen Y.; Torchia M. L. G.; Lawson G. W.; Karp C. L.; Ashwell J. D.; Mazmanian S. K. Outer Membrane Vesicles of a Human Commensal Mediate Immune Regulation and Disease Protection. Cell Host Microbe 2012, 12, 509–520. 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.