Abstract
Pancreatic ductal adenocarcinoma is speculated to become the second leading cause of cancer-related mortality by 2030, a high mortality rate considering the number of cases. Surgery and chemotherapy are the main treatment options, but they are burdensome for patients. A clear histological diagnosis is needed to determine a treatment plan, and endoscopic ultrasound (EUS)-guided tissue acquisition (TA) is a suitable technique that does not worsen the cancer-specific prognosis even for lesions at risk of needle tract seeding. With the development of personalized medicine and precision treatment, there has been an increasing demand to increase cell counts and collect specimens while preserving tissue structure, leading to the development of the fine-needle biopsy (FNB) needle. EUS-FNB is rapidly replacing EUS-guided fine-needle aspiration (FNA) as the procedure of choice for EUS-TA of pancreatic cancer. However, EUS-FNA is sometimes necessary where the FNB needle cannot penetrate small hard lesions, so it is important clinicians are familiar with both. Given these recent dev-elopments, we present an up-to-date review of the role of EUS-TA in pancreatic cancer. Particularly, technical aspects, such as needle caliber, negative pressure, and puncture methods, for obtaining an adequate specimen in EUS-TA are discussed.
Keywords: Endoscopic ultrasound-guided fine needle biopsy, Endoscopic ultrasound-guided tissue acquisition, Personalized medicine, Genomic profiling test, Pancreatic cancer, Puncture procedure
Core Tip: Endoscopic ultrasound (EUS)-guided tissue acquisition (TA) began in 1992 as EUS-guided fine-needle aspiration (FNA). Recently, with the development of personalized medicine and precision treatment, the fine-needle biopsy (FNB) needle was developed. EUS-FNB is rapidly replacing EUS-FNA for pancreatic cancer. The EUS-TA strategy with three or more punctures, the stylet retraction method, the torque or fanning technique, and a 22-G or thicker FNB needle may be effective in patients with solid pancreatic tumors scheduled for treatment, including personalized medicine. It is also important clinicians are familiar with both procedures, as EUS-FNA is occasionally necessary when FNB is unsuccessful.
INTRODUCTION
Approximately 80%-85% of patients with pancreatic cancer are borderline-resectable (BR) requiring neoadjuvant treatment and unresectable with metastases. International guidelines for pancreatic neoplasms recommend pathological diagnosis of pancreatic adenocarcinoma prior to chemotherapy. Therefore, endoscopic ultrasound (EUS)-guided tissue acquisition (TA) is usually performed in patients with BR requiring neoadjuvant therapy, or in metastatic disease requiring palliative therapy[1]. The remaining 15%-20% of patients may benefit from EUS-TA prior to surgery; this can confirm pancreatic neoplasms, and reduce the number of unnecessary operations for other similar conditions (e.g., autoimmune pancreatitis, local chronic pancreatitis, and lymphomas). Before the development of EUS-TA, 5%-10% of patients were misdiagnosed with pancreatic cancer and underwent surgery for pancreatic lesions[2-4].
EUS-TA can be divided into EUS-fine-needle aspiration (FNA) using Menghini needles, and EUS-fine-needle biopsy (FNB) using Franseen or fork-tip needles. FNB has equal or better diagnostic accuracy than FNA, with increased tissue volume and decreased number of punctures for diagnosis[5-8]. Moreover, EUS-FNB may provide higher diagnostic yield for peri-hepatic lymph nodes[9]. FNB increases the number of cases for which ancillary tests such as immunohistochemistry and tumor molecular profiling can be performed[9,10]. Therefore, with the advent of personalized medicine and precision therapy, EUS-FNB is rapidly becoming the procedure of choice for EUS-TA of pancreatic cancer. However, EUS-FNA may be used where the FNB needle cannot penetrate small hard lesions; therefore, there is a need for familiarity with both procedures. Conti et al[11] also recommend EUS-FNA and FNB in difficult cases of puncture and sampling. Needle size, the negative pressure applied to the needle, and puncture techniques are important for obtaining an adequate specimen in EUS-TA.
Considering recent developments, we present an up-to-date review of the role of EUS-TA in pancreatic cancer. In particular, technical aspects and novel applications for obtaining appropriate specimens during EUS-TA are discussed.
EUS-FNA
EUS-FNA has 87%-92% sensitivity and 96%-98% specificity for the pathological diagnosis of solid pancreatic malignancy[12-15]. FNA needles with calibers ranging from 19-gauge (G)–25G are available, with many studies comparing the different calibers and technical aspects of the EUS-FNA technique. We present a table summarizing the respective characteristics of FNA and FNB, focusing on key points (Table 1).
Table 1.
The respective characteristics of fine-needle aspiration and fine-needle biopsy
|
|
EUS-FNA
|
EUS-FNB
|
| Recommended needle caliber | 22-25G | 22G or thicker |
| Recommended negative pressure | Suction or wet suction | Stylet retraction |
| Recommended puncture technique | Fanning or DKM | Fanning or torque |
| Recommended number of needle passes | 1-3 times for histological diagnosis, depending on site and size | Three times for NGS |
| Diagnostic accuracy | FNB ≥ FNA | |
| Tissue volume | FNB ≥ FNA | |
| Suitability for NGS | FNB ≥ FNA | |
| MOSE | Recommended for use in combination | Recommended for use in combination |
| Complications | Rare | Rare |
| Cost-effectiveness | FNB ≥ FNA |
DKM: Door knocking method; G: Gauge; EUS: Endoscopic ultrasound; FNA: Fine-needle aspiration; FNB: Fine-needle biopsy; MOSE: Macroscopic on-site evaluation; NGS: Next-generation sequencing.
FNA needle caliber
The small 25G FNA needle may have a technical advantage over larger bore needles when sampling very fibrous, solid lesions. Moreover, its superior flexibility improves operability, especially when sampling pancreatic head and apex masses from the duodenum[1]. Three meta-analyses compared 25G FNA needles with 22G FNA needles. One of them showed increased diagnostic sensitivity (with similar specificity), while the other two showed no significant differences. Therefore, both calibers (25G and 22G needles) can be used when sampling solid pancreatic lesions[16]. Compared with thinner needles, 19G FNA needles are stiffer and harder to manipulate, increasing technical failure when sampling pancreatic head lesions[17].
Negative pressure during EUS-FNA
The conventional aspiration method, where 10 mL of negative pressure is applied using a syringe attached to a needle, is recommended because of its higher sensitivity and diagnostic rate[18,19]. In addition, EUS-FNA with 50 mL negative pressure is superior to 10 mL during tissue collection[20]. The wet aspiration method involves precleaning the needle with saline solution, replacing the air column with liquid, and applying negative pressure. Theoretically, this method improves the cell count and sample quality compared with the conventional aspiration and suction methods, as the negative pressure applied from the syringe is better transmitted to the needle tip[21]. In the stylet-throw-pull method, the stylet is slowly and gradually removed (without using a syringe) while the needle is moved within the lesion to create minimal negative pressure. The diagnostic results for solid pancreatic lesions sampled using stylet-throw-pull method are comparable to the conventional aspiration method[22,23].
EUS-FNA puncture technique
The fanning technique is used to collect specimens from multiple sites with a single puncture by fanning the needle back and forth using the elevator of the endoscope and the up/down dial control. The fanning technique improves diagnostic yield, especially in cancerous tumors with necrotic centers. In a study of pancreatic masses, fanning reduced the number of passes required for diagnosis and resulted in a high first-pass diagnostic rate[24]. The door knocking method (DKM), in which the needle is moved forward within the mass so quickly and powerfully that a sound is emitted when a part of the handle hits the needle stopper, did not improve the accuracy of histological diagnosis. However, it enabled the acquisition of a larger amount of tissue specimen compared with the conventional puncture[25]. Thus, DKM may be advantageous for genomic profiling tests; however, to our knowledge, no study has verified this.
Uehara et al[26] reported the optimal number of needle passes for accuracy of histological diagnosis: 3 where the head was < 15 mm; 2 where the head was ≥ 15 mm; 2 where the body and tail were ≤ 15 mm; and 1 where the body and tail were ≥ 15 mm. In total, 93% of pancreatic lesions were correctly diagnosed using these numbers of passes. In addition, size of ≤ 15 mm, head location, and neuroendocrine tumors independently required 2 or more needle passes[26].
EUS-FNB FOR HISTOLOGICAL DIAGNOSIS
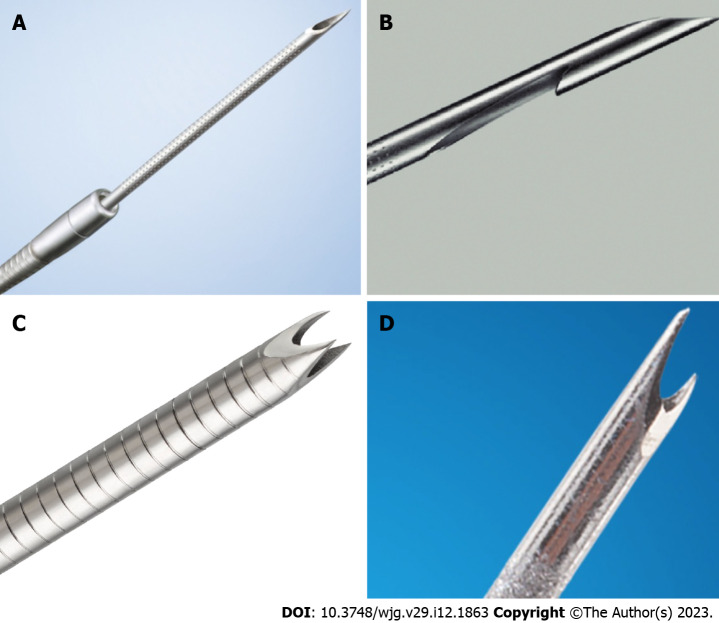
The desire to collect samples with increased cell counts and preserved tissue structures led to the development of a new type of needle, the FNB needle. This needle is specifically designed to collect core tissue samples. To date, three generations of FNB needles exist. The first generation, the Tru-Cut 19G needle (QuickCore, Cook Endoscopy, Limerick, Ireland), became rapidly outdated due to inflexibility and technical problems[27]. In a randomized controlled trial (RCT) comparing a 19G Tru-Cut needle with a conventional 22G FNA needle in transduodenal EUS-TA of pancreatic head lesions, Sakamoto et al[28] reported that the 22G FNA needle group had significantly higher diagnostic accuracy for malignancy and technical success rate than the 19G Tru-Cut needle group. The second generation of FNB needles, Procore needles (Cook Endoscopy, Limerick, Ireland), were created with a reverse bevel design. More than 10 years after the Tru-Cut needle was introduced, the third generation EUS-FNB needles for core biopsy were developed. They could collect more tissue than the conventional FNA needle, thus improving diagnostic results. Various types of needles have been developed, including the fork type (SharkCore; Medtronic, Minneapolis, MN, United States), flanged type (Acquire; Boston Scientific, Marlborough, MA, United States) (Top-gain; Medi-Globe, Achenmühle, Germany), and 20G FNB needles with forward-facing core traps (ProCore 20G; Cook Medical)[27] (Figure 1). A meta-analysis demonstrated the diagnostic performance of the new generation FNB needles, namely the crown and fork tip needles. They showed a remarkable diagnostic accuracy of 96% for solid pancreatic lesions, with no significant difference between the crown and fork-tip needles (97% and 95%, respectively, P = 0.8)[29]. Renelus et al[7] conducted a meta-analysis of RCTs published between 2012 and 2019; they found that FNA demonstrated significantly reduced diagnostic accuracy compared with FNB (81% and 87%, respectively, P = 0.005). In addition, FNA required an increased number of mean passes compared with FNB (2.3 and 1.6, respectively, P < 0.0001). Furthermore, there was no significant difference in the rate of adverse events between FNA and FNB needles (1.8% and 2.3%, respectively, P = 0.64)[7].
Figure 1.
Examples of needle designs. A: Menghini needle (EZ shot 3, Olympus medical systems, Tokyo, Japan); B: Reverse-beveled needle (ProCore, Cook Medical, Bloomington, IN, United States); C: Franseen needle (AcquireTM, Boston Scientific, Marlborough, MA, United States); D: Fork-tip needle (SharkCore, Medtronic, Minneapolis, MN, United States).
An RCT analysing cost-effectiveness found that pancreatic mass EUS-FNB (two passes without on-site cytopathology evaluation) was more cost-effective than EUS-FNA (number of passes dictated by on-site cytopathology evaluation). Variables with the largest impact were EUS procedure and sedation cost, specimen adequacy, and diagnostic yield associated with EUS-FNB[30].
THE ERA OF PERSONALIZED AND PRECISION MEDICINE
Several studies have reported increased tissue volume with EUS-FNB samples compared with EUS-FNA. One study reported a 20-fold increase in tissue volume[9,31,32]. Elhanafi et al[10] reported that FNB resulted in a higher proportion of sufficient samples for targeted next-generation sequencing (NGS) than FNA (90.9% vs 66.9%; P = 0.02). In multivariable modeling, only FNB [odds ratio = 4.95, 95% confidence interval (CI): 1.11-22.05, and P = 0.04] was associated with sufficient sampling capacity for genomic testing[10].
Pancreatic ductal adenocarcinoma is anticipated to become the second leading cause of cancer-related mortality by 2030, with high mortality considering the incidence. Given the low median survival time of 12 mo for patients with advanced metastatic pancreatic cancer (ductal adenocarcinoma in particular)[33,34], urgent new treatments are warranted. Molecular profiling data has recently revelated that up to 25% of pancreatic cancers have actionable molecular changes[35-42], defined as changes with strong clinical or preclinical evidence suggesting specific treatment efficacy[34]. Actionable molecular changes were found in 5.5%-21.7% of EUS-FNA/B specimens from pancreatic ductal adenocarcinoma patients[39,43]. Pishvaian et al[34] reported that patients with actionable molecular alterations treated with matched therapy (n = 46) had significantly longer median overall survival times than those treated with unmatched therapies [n = 143; 2.58 years vs 1.51 years; hazard ratio (HR) 0.42 (95%CI: 0.26-0.68), P < 0.001][34]. Therefore, EUS-TA can improve diagnostic accuracy and help ensure successful personalized medicine, with EUS-FNB expected to be particularly useful.
Advances have been made in both tissue collection methods and devices, and genetic analysis technology. The most common genomic alterations noted in metastatic pancreatic cancer specimens are in the KRAS, TP53, CDKN2A, andSMAD4 genes. The first genetic analysis reported using EUS-FNA samples was KRAS mutation analysis. This driver mutation is found in more than 90% of pancreatic cancers, and drugs targeting KRAS mutations, such as Sotrasib, may be effective for pancreatic cancer[27,44]. The DNA mismatch repair (MMR) pathway is an important function that identifies and corrects base pair mismatches in DNA. Loss of MMR function leads to elevated microsatellite instability (MSI)-high, making the MSI test useful for evaluating pembrolizumab response. Sugimoto et al[45] reported that in unresectable pancreatic ductal adenocarcinoma, EUS-FNB with the Franseen 22G needle had a higher success rate in MSI analysis than EUS-FNA [FNB, 88.9% (8/9) vs FNA, 35.7% (5/14); P = 0.03].
Targeted genome sequencing (TGS) applies polymerase chain reaction technology to construct and sequence a library of specific regions. Currently available multigene NGS systems, such as MSK-IMPACT (Memorial Sloan Kettering Cancer Center, New York, NY, United States) and FoundationOne CDx (F1CDx; Foundation Medicine, Cambridge, MA, United States), screen several hundred cancer-related genes simultaneously as companion diagnostic tests[46,47]. Whole genome sequencing (WGS) can detect various genomic structural alterations (translocations, inversions, duplications, chromosomal aberrations, chromosomal breaks) by obtaining sequence information of the entire genome, including non-coding regions. WGS may be more useful than TGS, but it is significantly more costly, requires more samples, and its superiority in therapeutic selection has not yet been proven[48]. RNA sequencing is a newly developed technology that can identify fusion genes faster, with greater sensitivity, and more efficiently than DNA panels. It can also detect new genes and genetic mutations.
Agents for actionable molecular changes are still being investigated. For example, evidence suggests trametinib in GNAS alterations[49], sotorasib in KRAS G12C alterations[44], olaparib in germline BRCA1/2 mutation[50], entrectinib in NTRK gene fusion[51], pembrolizumab in MSI-high[52,53], and afatinib in NRG1 fusions[54,55] may be effective treatment options. In malignancies such as pancreatic cancer, it is likely that multiple genomic drivers may be present. A combination of compatible drugs with targeting multiple genomic alterations at once may overcome this; however, evidence is lacking, and further studies are needed[56,57]. The application of precision medicine for treating pancreatic ductal adenocarcinoma has just begun, and further development of genetic testing equipment, drugs, and tissue collection devices is required[27].
EUS-FNB FOR THE ERA OF PERSONALIZED AND PRECISION MEDICINE
FNB needle type and caliber
Since EUS-FNB is more suitable than EUS-FNA for personalized medicine, further investigation into needle size and type has been conducted. The abovementioned meta-analysis evaluating crown and fork-tip needles, showed equivalent sample adequacy, diagnostic accuracy, optimal histological core procurement, mean number of needle passes, pooled specificity, and sensitivity of these two FNB needles[29]. Oh et al[58] compared 22G with 25G Franseen needles in the three needle passes technique, with suction using 10 mL syringe, and movement within the target lesion at least 10 times using the fanning technique. They reported no diagnostic accuracy difference between the two needles; however, the 22G Franseen needle was superior to the 25G needle in histologic core facilitated easier collection of appropriate specimens for the OncoGuide NCC Oncopanel System [70.0% (49/70) vs 28.6% (20/70), respectively; P < 0.001]. The 19G FNB needle was reported to have the potential for easier collection of appropriate specimens for the OncoGuide NCC Oncopanel System compared with the 22G FNB needle, however, concerns were expressed about risks such as bleeding and pancreatic juice leakage[59,60]. For successful personalized medicine, 22G FNB needles are better than 25G ones. However, usage of 19G FNB needles raises concerns regarding accidental injury and harder to manipulate compared with 22G ones.
Negative pressure during EUS-FNB
A randomized controlled trial of tissue samples from patients with pancreatic masses on imaging compared collection techniques using Menghini-tip, reverse-bevel, Franseen, and fork-tip needles. A second randomized control, patient-by-patient analysis, compared the use of suction, no suction, and stylet retraction during biopsies. The biopsy samples collected by fork-tip or Franseen needles had significantly higher cellularity than those collected by Menghini-tip needles or reverse-bevels (P < 0.001). The predictors of high cellularity for pancreatic masses showed that Franseen needles, fork-tip needles, not applying suction, stylet retraction, and pancreatic mass size of > 3 cm were associated with high cellularity. Therefore, they recommended using a negative pressure technique with every EUS-TA needle for an optimal outcome, as shown in Table 2[61]. Stylet retraction and standard suction technique may be equivalent when using the 20G reverse-beveled needle[62]; however, the stylet retraction technique is considered equally or more useful than the standard suction technique when performing EUS-FNB.
Table 2.
Recommended negative pressure procedural technique for each endoscopic ultrasound-guided tissue acquisition needle to achieve optimal outcomes[61]
|
Needle type
|
Best cellularity
|
Best diagnostic accuracy
|
Best overall outcome (specimen)
|
| Menghini | Stylet retraction = suction = no suction | Suction | Suction |
| Reverse-bevel | Stylet retraction = suction | Suction | Suction |
| Franseen | Stylet retraction | Stylet retraction = suction | Stylet retraction |
| Fork-tip | Stylet retraction = no suction | Stylet retraction = suction | Stylet retraction |
EUS-FNB puncture technique
Sample quality scores were significantly higher when obtained by the torque and fanning techniques rather than the standard one (P < 0.001)[63]. The new torque technique consists of turning the echoendoscope counterclockwise or clockwise while repeated to-and-fro movements are performed to reach multiple areas within the lesion; this is obtained by maneuvering the "up-down" articulating dial of the echoendoscope. Conversely, in the standard technique, the mass is pierced by the tip of the needle in a unidirectional movement. Studies on FNA needles suggest that the DKM may be useful for increasing cell volume[25]; however, there are no specific studies on DKM in FNB.
The European Society of Gastrointestinal Endoscopy recommends that 2-3 needle passes are sufficient to ensure a sensitivity of at least 90% for the diagnosis of malignancy[16]. However, evidence on the number of needle passes required for successful personalized medicine is lacking. In a few recent retrospective studies, a median number of 3 FNB needle passes (interquartile range 3-4) yielded sufficient tissue for targeted NGS in 91% of patients[10].
Based on these findings, we believe that the EUS-TA strategy with three or more punctures, the stylet retraction method, the torque or fanning technique, and a 22-G or thicker FNB needle may be effective in patients with solid pancreatic tumors scheduled for treatment, including personalized medicine.
RAPID ON-SITE PATHOLOGIST EVALUATION AND MACROSCOPIC ON-SITE QUALITY EVALUATION
It is unclear whether an on-site pathologist improves the diagnostic accuracy of EUS-FNA for solid pancreatic lesions; however, Rapid On-Site Pathologist Evaluation (ROSE) can reduce the number of needle passes required to obtain a pathological diagnosis[64,65]. The subsequent introduction of the FNB needle has made it possible to collect large quantities of macroscopic white samples. A recent global, randomized trial showed that ROSE did not affect the diagnostic accuracy of EUS-FNB (96.4% with ROSE vs 97.4% without ROSE, P = 0.396)[66]. In addition, the application of ROSE is limited by the availability and additional cost of trained cytopathologists at each facility; therefore, ROSE is unlikely to be recommended for future diagnostic practice[67].
Direct observation of specimens obtained by FNA/B, macroscopic on-site evaluation (MOSE), is more feasible and readily available alternative to ROSE[68,69]. Kaneko et al[70] showed that the macroscopic visible core (MVC) length and histological sample quantity were positively correlated in EUS-FNB using a 22G Franseen needle. Multivariate analysis showed that MVC length ≥ 30 mm on MOSE was a significant factor affecting suitability for NGS (odds ratio 6.19; 95%CI: 2.72-14.10).
NEEDLE TRACT SEEDING
Theoretically, cancer seeding through the needle tract may occur more frequently in the body or tail of the lesion when using the transgastric approach, than in the head of the lesion using the transduodenal approach[71]. This is because the EUS-FNA tract of pancreatic head cancers is later resected in a blocked fashion along with the pancreatic head in curative surgery. In contrast, the EUS-FNA tract of lesions in the body or tail lies beyond the surgical resection margin[72,73].
Ngamruengphong et al[73] reported that EUS-FNA for pancreatic head, body, and tail cancers was marginally associated with improved overall survival (HR 0.84, 95%CI: 0.72-0.99) but did not affect cancer-specific survival (HR 0.87, 95%CI: 0.74-1.03). Park et al[74] reported 528 patients with distal pancreatic cancer who underwent distal pancreatectomy. Among these, 193 were treated using EUS-FNA, and 335 were not. The between-group recurrence rates were comparable (EUS-FNA, 72.7%; non-EUS-FNA, 75%; P = 0.58) at follow-up (median, 21.7 mo), with similar cancer-free survival (P = 0.58), showing that preoperative EUS-FNA does not reduce cancer-specific or overall survival[74]. However, as gastric wall recurrence only occurred in patients treated using EUS-FNA (n = 2), clinicians must consider the potential risks of needle tract seeding, and patients should be carefully selected[74]. Yane et al[75] also reported that the 5-year cumulative needle tract seeding rate, estimated using Fine and Gray's method, was 3.8% (95%CI: 1.6%-7.8%). They concluded that, although preoperative EUS-FNA for pancreatic body and tail cancers has no negative effect on recurrence-free survival or overall survival, needle tract seeding after EUS-FNA was observed to have a non-negligible rate[75]. Sakamoto reported that among the six cases with needle tract seeding occurred, it was revealed by upper gastrointestinal endoscopy in four. They suggested upper gastrointestinal endoscopy follow-up, as well as computed tomography, in patients who undergo gastric EUS–FNA before distal pancreatectomy to identify needle tract seeding at soon as possible[76].
CONCLUSION
Since pancreatic cancer treatment is highly invasive and EUS-TA does not worsen cancer-specific prognosis, histological diagnosis should be made to establish effective treatment options. Furthermore, with genomic profiling, a large volume of tissue are needed from each biopsy; Franseen and Fork-tip needles should be the first choice for this purpose. EUS-TA involving three or more punctures using the stylet retraction method and the torque or fanning technique with a 22-G FNB or thicker needle is the most effective tissue-sampling method for patients with solid pancreatic tumors scheduled for treatment, including those receiving personalized medicine. The prediction of MOSE histological specimen volume by measuring MVC length may have clinical significance, especially when submitting specimens for NGS. The application of precision medicine for treating pancreatic ductal adenocarcinoma has just begun, and further development of genetic testing equipment, drugs, tissue collection devices, and puncture technique is required.
ACKNOWLEDGEMENTS
Table 2 in this paper is adapted from Figure 5 of a report titled “Comparing Needles and Methods of Endoscopic Ultrasound-Guided Fine-Needle Biopsy to Optimize Specimen Quality and Diagnostic Accuracy for Patients With Pancreatic Masses in a Randomized Trial”. We thank Young Bang et al and “Clinical Gastroenterology and Hepatology” for granting us permission to use it on December 26, 2022.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Japanese Society of Gastroenterology; Japan Biliary Association; Japan Gastroenterological Endoscopy Society; Japan Pancreas Society; The Japan Society of Hepatology; The Japanese Society of Internal Medicine.
Peer-review started: December 27, 2022
First decision: January 22, 2023
Article in press: March 15, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kaneko J, Japan; Tan B, China S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
Contributor Information
Sakue Masuda, Department of Gastroenterology, Shonan Kamakura General Hospital, Kanagawa 247-8533, Japan. sakue.masuda@tokushukai.jp.
Kazuya Koizumi, Department of Gastroenterology, Shonan Kamakura General Hospital, Kanagawa 247-8533, Japan.
Kento Shionoya, Department of Gastroenterology, Shonan Kamakura General Hospital, Kanagawa 247-8533, Japan.
Ryuhei Jinushi, Department of Gastroenterology, Shonan Kamakura General Hospital, Kanagawa 247-8533, Japan.
Makomo Makazu, Department of Gastroenterology, Shonan Kamakura General Hospital, Kanagawa 247-8533, Japan.
Takashi Nishino, Department of Gastroenterology, Shonan Kamakura General Hospital, Kanagawa 247-8533, Japan.
Karen Kimura, Department of Gastroenterology, Shonan Kamakura General Hospital, Kanagawa 247-8533, Japan.
Chihiro Sumida, Department of Gastroenterology, Shonan Kamakura General Hospital, Kanagawa 247-8533, Japan.
Jun Kubota, Department of Gastroenterology, Shonan Kamakura General Hospital, Kanagawa 247-8533, Japan.
Chikamasa Ichita, Department of Gastroenterology, Shonan Kamakura General Hospital, Kanagawa 247-8533, Japan.
Akiko Sasaki, Department of Gastroenterology, Shonan Kamakura General Hospital, Kanagawa 247-8533, Japan.
Masahiro Kobayashi, Department of Gastroenterology, Shonan Kamakura General Hospital, Kanagawa 247-8533, Japan.
Makoto Kako, Department of Gastroenterology, Shonan Kamakura General Hospital, Kanagawa 247-8533, Japan.
Uojima Haruki, Department of Gastroenterology, Internal Medicine, Kitasato University School of Medicine, Kanagawa 252-0375, Japan.
References
- 1.Marques S, Bispo M, Rio-Tinto R, Fidalgo P, Devière J. The Impact of Recent Advances in Endoscopic Ultrasound-Guided Tissue Acquisition on the Management of Pancreatic Cancer. GE Port J Gastroenterol. 2021;28:185–192. doi: 10.1159/000510730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith CD, Behrns KE, van Heerden JA, Sarr MG. Radical pancreatoduodenectomy for misdiagnosed pancreatic mass. Br J Surg. 1994;81:585–589. doi: 10.1002/bjs.1800810435. [DOI] [PubMed] [Google Scholar]
- 3.Thompson JS, Murayama KM, Edney JA, Rikkers LF. Pancreaticoduodenectomy for suspected but unproven malignancy. Am J Surg. 1994;168:571–3; discussion 573. doi: 10.1016/s0002-9610(05)80124-2. [DOI] [PubMed] [Google Scholar]
- 4.Abraham SC, Wilentz RE, Yeo CJ, Sohn TA, Cameron JL, Boitnott JK, Hruban RH. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: are they all 'chronic pancreatitis'? Am J Surg Pathol. 2003;27:110–120. doi: 10.1097/00000478-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 5.van Riet PA, Larghi A, Attili F, Rindi G, Nguyen NQ, Ruszkiewicz A, Kitano M, Chikugo T, Aslanian H, Farrell J, Robert M, Adeniran A, Van Der Merwe S, Roskams T, Chang K, Lin F, Lee JG, Arcidiacono PG, Petrone M, Doglioni C, Iglesias-Garcia J, Abdulkader I, Giovannini M, Bories E, Poizat F, Santo E, Scapa E, Marmor S, Bucobo JC, Buscaglia JM, Heimann A, Wu M, Baldaque-Silva F, Moro CF, Erler NS, Biermann K, Poley JW, Cahen DL, Bruno MJ. A multicenter randomized trial comparing a 25-gauge EUS fine-needle aspiration device with a 20-gauge EUS fine-needle biopsy device. Gastrointest Endosc. 2019;89:329–339. doi: 10.1016/j.gie.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Bang JY, Kirtane S, Krall K, Navaneethan U, Hasan M, Hawes R, Varadarajulu S. In memoriam: Fine-needle aspiration, birth: Fine-needle biopsy: The changing trend in endoscopic ultrasound-guided tissue acquisition. Dig Endosc. 2019;31:197–202. doi: 10.1111/den.13280. [DOI] [PubMed] [Google Scholar]
- 7.Renelus BD, Jamorabo DS, Boston I, Briggs WM, Poneros JM. Endoscopic Ultrasound-Guided Fine Needle Biopsy Needles Provide Higher Diagnostic Yield Compared to Endoscopic Ultrasound-Guided Fine Needle Aspiration Needles When Sampling Solid Pancreatic Lesions: A Meta-Analysis. Clin Endosc. 2021;54:261–268. doi: 10.5946/ce.2020.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Facciorusso A, Bajwa HS, Menon K, Buccino VR, Muscatiello N. Comparison between 22G aspiration and 22G biopsy needles for EUS-guided sampling of pancreatic lesions: A meta-analysis. Endosc Ultrasound. 2020;9:167–174. doi: 10.4103/eus.eus_4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine I, Trindade AJ. Endoscopic ultrasound fine needle aspiration vs fine needle biopsy for pancreatic masses, subepithelial lesions, and lymph nodes. World J Gastroenterol. 2021;27:4194–4207. doi: 10.3748/wjg.v27.i26.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elhanafi S, Mahmud N, Vergara N, Kochman ML, Das KK, Ginsberg GG, Rajala M, Chandrasekhara V. Comparison of endoscopic ultrasound tissue acquisition methods for genomic analysis of pancreatic cancer. J Gastroenterol Hepatol. 2019;34:907–913. doi: 10.1111/jgh.14540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conti CB, Cereatti F, Grassia R. Endoscopic ultrasound-guided sampling of solid pancreatic masses: the fine needle aspiration or fine needle biopsy dilemma. Is the best needle yet to come? World J Gastrointest Endosc. 2019;11:454–471. doi: 10.4253/wjge.v11.i8.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banafea O, Mghanga FP, Zhao J, Zhao R, Zhu L. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: a meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016;16:108. doi: 10.1186/s12876-016-0519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319–331. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass? Pancreas. 2013;42:20–26. doi: 10.1097/MPA.0b013e3182546e79. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Yang R, Lu Y, Xia Y, Zhou H. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: a systematic review. J Cancer Res Clin Oncol. 2012;138:1433–1441. doi: 10.1007/s00432-012-1268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polkowski M, Jenssen C, Kaye P, Carrara S, Deprez P, Gines A, Fernández-Esparrach G, Eisendrath P, Aithal GP, Arcidiacono P, Barthet M, Bastos P, Fornelli A, Napoleon B, Iglesias-Garcia J, Seicean A, Larghi A, Hassan C, van Hooft JE, Dumonceau JM. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline - March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 17.Song TJ, Kim JH, Lee SS, Eum JB, Moon SH, Park DY, Seo DW, Lee SK, Jang SJ, Yun SC, Kim MH. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739–1745. doi: 10.1038/ajg.2010.108. [DOI] [PubMed] [Google Scholar]
- 18.Tarantino I, Di Mitri R, Fabbri C, Pagano N, Barresi L, Granata A, Liotta R, Mocciaro F, Maimone A, Baccarini P, Fabio T, Curcio G, Repici A, Traina M. Is diagnostic accuracy of fine needle aspiration on solid pancreatic lesions aspiration-related? Dig Liver Dis. 2014;46:523–526. doi: 10.1016/j.dld.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Lee JK, Choi JH, Lee KH, Kim KM, Shin JU, Lee JK, Lee KT, Jang KT. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745–751. doi: 10.1016/j.gie.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Kudo T, Kawakami H, Hayashi T, Yasuda I, Mukai T, Inoue H, Katanuma A, Kawakubo K, Ishiwatari H, Doi S, Yamada R, Maguchi H, Isayama H, Mitsuhashi T, Sakamoto N Japan EUS-FNA Negative Pressure Suction Study Group. High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: a multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014;80:1030–7.e1. doi: 10.1016/j.gie.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Attam R, Arain MA, Bloechl SJ, Trikudanathan G, Munigala S, Bakman Y, Singh M, Wallace T, Henderson JB, Catalano MF, Guda NM. "Wet suction technique (WEST)": a novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015;81:1401–1407. doi: 10.1016/j.gie.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Kin T, Katanuma A, Yane K, Takahashi K, Osanai M, Takaki R, Matsumoto K, Gon K, Matsumori T, Tomonari A, Maguchi H, Shinohara T, Nojima M. Diagnostic ability of EUS-FNA for pancreatic solid lesions with conventional 22-gauge needle using the slow pull technique: a prospective study. Scand J Gastroenterol. 2015;50:900–907. doi: 10.3109/00365521.2014.983155. [DOI] [PubMed] [Google Scholar]
- 23.Saxena P, El Zein M, Stevens T, Abdelgelil A, Besharati S, Messallam A, Kumbhari V, Azola A, Brainard J, Shin EJ, Lennon AM, Canto MI, Singh VK, Khashab MA. Stylet slow-pull versus standard suction for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic lesions: a multicenter randomized trial. Endoscopy. 2018;50:497–504. doi: 10.1055/s-0043-122381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–450. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukai S, Itoi T, Ashida R, Tsuchiya T, Ikeuchi N, Kamada K, Tanaka R, Umeda J, Tonozuka R, Fukutake N, Hoshi K, Moriyasu F, Gotoda T, Irisawa A. Multicenter, prospective, crossover trial comparing the door-knocking method with the conventional method for EUS-FNA of solid pancreatic masses (with videos) Gastrointest Endosc. 2016;83:1210–1217. doi: 10.1016/j.gie.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Uehara H, Sueyoshi H, Takada R, Fukutake N, Katayama K, Ashida R, Ioka T, Takenaka A, Nagata S, Tomita Y. Optimal number of needle passes in endoscopic ultrasound-guided fine needle aspiration for pancreatic lesions. Pancreatology. 2015;15:392–396. doi: 10.1016/j.pan.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Ashida R, Kitano M. Endoscopic ultrasound-guided tissue acquisition for pancreatic ductal adenocarcinoma in the era of precision medicine. Dig Endosc. 2022;34:1329–1339. doi: 10.1111/den.14344. [DOI] [PubMed] [Google Scholar]
- 28.Sakamoto H, Kitano M, Komaki T, Noda K, Chikugo T, Dote K, Takeyama Y, Das K, Yamao K, Kudo M. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384–390. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 29.Facciorusso A, Del Prete V, Buccino VR, Purohit P, Setia P, Muscatiello N. Diagnostic yield of Franseen and Fork-Tip biopsy needles for endoscopic ultrasound-guided tissue acquisition: a meta-analysis. Endosc Int Open. 2019;7:E1221–E1230. doi: 10.1055/a-0982-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aadam AA, Wani S, Amick A, Shah JN, Bhat YM, Hamerski CM, Klapman JB, Muthusamy VR, Watson RR, Rademaker AW, Keswani RN, Keefer L, Das A, Komanduri S. A randomized controlled cross-over trial and cost analysis comparing endoscopic ultrasound fine needle aspiration and fine needle biopsy. Endosc Int Open. 2016;4:E497–E505. doi: 10.1055/s-0042-106958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bang JY, Hebert-Magee S, Navaneethan U, Hasan MK, Hawes R, Varadarajulu S. EUS-guided fine needle biopsy of pancreatic masses can yield true histology. Gut. 2018;67:2081–2084. doi: 10.1136/gutjnl-2017-315154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukai S, Itoi T, Yamaguchi H, Sofuni A, Tsuchiya T, Tanaka R, Tonozuka R, Honjo M, Fujita M, Yamamoto K, Matsunami Y, Asai Y, Kurosawa T, Nagakawa Y. A retrospective histological comparison of EUS-guided fine-needle biopsy using a novel franseen needle and a conventional end-cut type needle. Endosc Ultrasound. 2019;8:50–57. doi: 10.4103/eus.eus_11_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 34.Pishvaian MJ, Blais EM, Brody JR, Lyons E, DeArbeloa P, Hendifar A, Mikhail S, Chung V, Sahai V, Sohal DPS, Bellakbira S, Thach D, Rahib L, Madhavan S, Matrisian LM, Petricoin EF 3rd. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020;21:508–518. doi: 10.1016/S1470-2045(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aguirre AJ, Nowak JA, Camarda ND, Moffitt RA, Ghazani AA, Hazar-Rethinam M, Raghavan S, Kim J, Brais LK, Ragon D, Welch MW, Reilly E, McCabe D, Marini L, Anderka K, Helvie K, Oliver N, Babic A, Da Silva A, Nadres B, Van Seventer EE, Shahzade HA, St Pierre JP, Burke KP, Clancy T, Cleary JM, Doyle LA, Jajoo K, McCleary NJ, Meyerhardt JA, Murphy JE, Ng K, Patel AK, Perez K, Rosenthal MH, Rubinson DA, Ryou M, Shapiro GI, Sicinska E, Silverman SG, Nagy RJ, Lanman RB, Knoerzer D, Welsch DJ, Yurgelun MB, Fuchs CS, Garraway LA, Getz G, Hornick JL, Johnson BE, Kulke MH, Mayer RJ, Miller JW, Shyn PB, Tuveson DA, Wagle N, Yeh JJ, Hahn WC, Corcoran RB, Carter SL, Wolpin BM. Real-time Genomic Characterization of Advanced Pancreatic Cancer to Enable Precision Medicine. Cancer Discov. 2018;8:1096–1111. doi: 10.1158/2159-8290.CD-18-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM Australian Pancreatic Cancer Genome Initiative, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 37.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, Chang DK, Cowley MJ, Gardiner BB, Song S, Harliwong I, Idrisoglu S, Nourse C, Nourbakhsh E, Manning S, Wani S, Gongora M, Pajic M, Scarlett CJ, Gill AJ, Pinho AV, Rooman I, Anderson M, Holmes O, Leonard C, Taylor D, Wood S, Xu Q, Nones K, Fink JL, Christ A, Bruxner T, Cloonan N, Kolle G, Newell F, Pinese M, Mead RS, Humphris JL, Kaplan W, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chou A, Chin VT, Chantrill LA, Mawson A, Samra JS, Kench JG, Lovell JA, Daly RJ, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N Australian Pancreatic Cancer Genome Initiative, Kakkar N, Zhao F, Wu YQ, Wang M, Muzny DM, Fisher WE, Brunicardi FC, Hodges SE, Reid JG, Drummond J, Chang K, Han Y, Lewis LR, Dinh H, Buhay CJ, Beck T, Timms L, Sam M, Begley K, Brown A, Pai D, Panchal A, Buchner N, De Borja R, Denroche RE, Yung CK, Serra S, Onetto N, Mukhopadhyay D, Tsao MS, Shaw PA, Petersen GM, Gallinger S, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL, Morgan RA, Lawlor RT, Capelli P, Corbo V, Scardoni M, Tortora G, Tempero MA, Mann KM, Jenkins NA, Perez-Mancera PA, Adams DJ, Largaespada DA, Wessels LF, Rust AG, Stein LD, Tuveson DA, Copeland NG, Musgrove EA, Scarpa A, Eshleman JR, Hudson TJ, Sutherland RL, Wheeler DA, Pearson JV, McPherson JD, Gibbs RA, Grimmond SM. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, Cooc J, Weinkle J, Kim GE, Jakkula L, Feiler HS, Ko AH, Olshen AB, Danenberg KL, Tempero MA, Spellman PT, Hanahan D, Gray JW. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lowery MA, Jordan EJ, Basturk O, Ptashkin RN, Zehir A, Berger MF, Leach T, Herbst B, Askan G, Maynard H, Glassman D, Covington C, Schultz N, Abou-Alfa GK, Harding JJ, Klimstra DS, Hechtman JF, Hyman DM, Allen PJ, Jarnagin WR, Balachandran VP, Varghese AM, Schattner MA, Yu KH, Saltz LB, Solit DB, Iacobuzio-Donahue CA, Leach SD, O'Reilly EM. Real-Time Genomic Profiling of Pancreatic Ductal Adenocarcinoma: Potential Actionability and Correlation with Clinical Phenotype. Clin Cancer Res. 2017;23:6094–6100. doi: 10.1158/1078-0432.CCR-17-0899. [DOI] [PubMed] [Google Scholar]
- 40.Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung AH, Smyla JK, Anderson JM, Kim HJ, Bentrem DJ, Talamonti MS, Iacobuzio-Donahue CA, Hollingsworth MA, Yeh JJ. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47:1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MC, Robertson AJ, Fadlullah MZ, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Wilson PJ, Markham E, Cloonan N, Anderson MJ, Fink JL, Holmes O, Kazakoff SH, Leonard C, Newell F, Poudel B, Song S, Taylor D, Waddell N, Wood S, Xu Q, Wu J, Pinese M, Cowley MJ, Lee HC, Jones MD, Nagrial AM, Humphris J, Chantrill LA, Chin V, Steinmann AM, Mawson A, Humphrey ES, Colvin EK, Chou A, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Pettitt JA, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, Graham JS, Niclou SP, Bjerkvig R, Grützmann R, Aust D, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Falconi M, Zamboni G, Tortora G, Tempero MA Australian Pancreatic Cancer Genome Initiative, Gill AJ, Eshleman JR, Pilarsky C, Scarpa A, Musgrove EA, Pearson JV, Biankin AV, Grimmond SM. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witkiewicz AK, McMillan EA, Balaji U, Baek G, Lin WC, Mansour J, Mollaee M, Wagner KU, Koduru P, Yopp A, Choti MA, Yeo CJ, McCue P, White MA, Knudsen ES. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. doi: 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semaan A, Bernard V, Lee JJ, Wong JW, Huang J, Swartzlander DB, Stephens BM, Monberg ME, Weston BR, Bhutani MS, Chang K, Scheet PA, Maitra A, Jakubek YA, Guerrero PA. Defining the Comprehensive Genomic Landscapes of Pancreatic Ductal Adenocarcinoma Using Real-World Endoscopic Aspiration Samples. Clin Cancer Res. 2021;27:1082–1093. doi: 10.1158/1078-0432.CCR-20-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong DS, Kuo J, Sacher AG, Barlesi F, Besse B, Kuboki Y, Dy GK, Dembla V, Krauss JC, Burns TF, Kim J, Henary H, Ngarmchamnanrith G, Li BT. CodeBreak 100: Phase I study of AMG 510, a novel KRASG12C inhibitor, in patients (pts) with advanced solid tumors other than non-small cell lung cancer (NSCLC) and colorectal cancer (CRC) J Clin Orthod. 2020;38:3511–3511. [Google Scholar]
- 45.Sugimoto M, Irie H, Takagi T, Suzuki R, Konno N, Asama H, Sato Y, Nakamura J, Takasumi M, Hashimoto M, Kato T, Kobashi R, Kobayashi Y, Hashimoto Y, Hikichi T, Ohira H. Efficacy of EUS-guided FNB using a Franseen needle for tissue acquisition and microsatellite instability evaluation in unresectable pancreatic lesions. BMC Cancer. 2020;20:1094. doi: 10.1186/s12885-020-07588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, Chandramohan R, Liu ZY, Won HH, Scott SN, Brannon AR, O'Reilly C, Sadowska J, Casanova J, Yannes A, Hechtman JF, Yao J, Song W, Ross DS, Oultache A, Dogan S, Borsu L, Hameed M, Nafa K, Arcila ME, Ladanyi M, Berger MF. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allegretti M, Fabi A, Buglioni S, Martayan A, Conti L, Pescarmona E, Ciliberto G, Giacomini P. Tearing down the walls: FDA approves next generation sequencing (NGS) assays for actionable cancer genomic aberrations. J Exp Clin Cancer Res. 2018;37:47. doi: 10.1186/s13046-018-0702-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Golan T, O'Kane GM, Denroche RE, Raitses-Gurevich M, Grant RC, Holter S, Wang Y, Zhang A, Jang GH, Stossel C, Atias D, Halperin S, Berger R, Glick Y, Park JP, Cuggia A, Williamson L, Wong HL, Schaeffer DF, Renouf DJ, Borgida A, Dodd A, Wilson JM, Fischer SE, Notta F, Knox JJ, Zogopoulos G, Gallinger S. Genomic Features and Classification of Homologous Recombination Deficient Pancreatic Ductal Adenocarcinoma. Gastroenterology. 2021;160:2119–2132.e9. doi: 10.1053/j.gastro.2021.01.220. [DOI] [PubMed] [Google Scholar]
- 49.Parish AJ, Nguyen V, Goodman AM, Murugesan K, Frampton GM, Kurzrock R. GNAS, GNAQ, and GNA11 alterations in patients with diverse cancers. Cancer. 2018;124:4080–4089. doi: 10.1002/cncr.31724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pishvaian MJ, Garrido-Laguna I, Liu SV, Multani PS, Chow-Maneval E, Rolfo C. Entrectinib in TRK and ROS1 Fusion-Positive Metastatic Pancreatic Cancer. JCO Precis Oncol. 2018;2:1–7. doi: 10.1200/PO.18.00039. [DOI] [PubMed] [Google Scholar]
- 52.Zhao L, Singh V, Ricca A, Lee P. Survival Benefit of Pembrolizumab for Patients With Pancreatic Adenocarcinoma: A Case Series. J Med Cases. 2022;13:240–243. doi: 10.14740/jmc3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 54.Jones MR, Williamson LM, Topham JT, Lee MKC, Goytain A, Ho J, Denroche RE, Jang G, Pleasance E, Shen Y, Karasinska JM, McGhie JP, Gill S, Lim HJ, Moore MJ, Wong HL, Ng T, Yip S, Zhang W, Sadeghi S, Reisle C, Mungall AJ, Mungall KL, Moore RA, Ma Y, Knox JJ, Gallinger S, Laskin J, Marra MA, Schaeffer DF, Jones SJM, Renouf DJ. NRG1 Gene Fusions Are Recurrent, Clinically Actionable Gene Rearrangements in KRAS Wild-Type Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2019;25:4674–4681. doi: 10.1158/1078-0432.CCR-19-0191. [DOI] [PubMed] [Google Scholar]
- 55.Laskin JJ, Cadranel J, Renouf DJ, Weinberg BA, Goto Y, Duruisseaux M, Tolba K, Branden E, Doebele RC, Heining C, Schlenk RF, Cheema PK, Jones MR, Trombetta D, Muscarella LA, Cseh A, Solca F, Liu SV. Afatinib as a novel potential treatment option for NRG1 fusion-positive tumors. J Glob Oncol. 2019;5:110–110. [Google Scholar]
- 56.Sicklick JK, Kato S, Okamura R, Schwaederle M, Hahn ME, Williams CB, De P, Krie A, Piccioni DE, Miller VA, Ross JS, Benson A, Webster J, Stephens PJ, Lee JJ, Fanta PT, Lippman SM, Leyland-Jones B, Kurzrock R. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med. 2019;25:744–750. doi: 10.1038/s41591-019-0407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shaya J, Kato S, Adashek JJ, Patel H, Fanta PT, Botta GP, Sicklick JK, Kurzrock R. Personalized matched targeted therapy in advanced pancreatic cancer: a pilot cohort analysis. NPJ Genom Med. 2023;8:1. doi: 10.1038/s41525-022-00346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh D, Kong J, Ko SW, Hong SM, So H, Hwang JS, Song TJ, Lee SK, Kim MH, Lee SS. A comparison between 25-gauge and 22-gauge Franseen needles for endoscopic ultrasound-guided sampling of pancreatic and peripancreatic masses: a randomized non-inferiority study. Endoscopy. 2021;53:1122–1129. doi: 10.1055/a-1369-8610. [DOI] [PubMed] [Google Scholar]
- 59.Ikeda G, Hijioka S, Nagashio Y, Maruki Y, Ohba A, Hisada Y, Yoshinari M, Harai S, Kitamura H, Koga T, Murashima Y, Maehara K, Okada M, Yamashige D, Okamoto K, Hara H, Hagiwara Y, Agarie D, Takasaki T, Takeshita K, Kawasaki Y, Kondo S, Morizane C, Ueno H, Hiraoka N, Yatabe Y, Saito Y, Iwakiri K, Okusaka T. Fine-needle biopsy with 19G needle is effective in combination with endoscopic ultrasound-guided tissue acquisition for genomic profiling of unresectable pancreatic cancer. Dig Endosc. 2023;35:124–133. doi: 10.1111/den.14423. [DOI] [PubMed] [Google Scholar]
- 60.Hisada Y, Hijioka S, Ikeda G, Maehara K, Hashimoto T, Kitamura H, Harai S, Yoshinari M, Kawasaki Y, Murashima Y, Koga T, Takeshita K, Maruki Y, Ohba A, Nagashio Y, Kondo S, Morizane C, Ueno H, Saito Y, Yatabe Y, Okusaka T. Proportion of unresectable pancreatic cancer specimens obtained by endoscopic ultrasound-guided tissue acquisition meeting the OncoGuide™ NCC Oncopanel System analysis suitability criteria: a single-arm, phase II clinical trial. J Gastroenterol. 2022;57:990–998. doi: 10.1007/s00535-022-01926-z. [DOI] [PubMed] [Google Scholar]
- 61.Young Bang J, Krall K, Jhala N, Singh C, Tejani M, Arnoletti JP, Navaneethan U, Hawes R, Varadarajulu S. Comparing Needles and Methods of Endoscopic Ultrasound-Guided Fine-Needle Biopsy to Optimize Specimen Quality and Diagnostic Accuracy for Patients With Pancreatic Masses in a Randomized Trial. Clin Gastroenterol Hepatol. 2021;19:825–835.e7. doi: 10.1016/j.cgh.2020.06.042. [DOI] [PubMed] [Google Scholar]
- 62.Di Mitri R, Mocciaro F, Antonini F, Scimeca D, Conte E, Bonaccorso A, Scibetta N, Unti E, Fornelli A, Giorgini S, Binda C, Macarri G, Larghi A, Fabbri C. Stylet slow-pull vs. standard suction technique for endoscopic ultrasound-guided fine needle biopsy in pancreatic solid lesions using 20 Gauge Procore™ needle: A multicenter randomized trial. Dig Liver Dis. 2020;52:178–184. doi: 10.1016/j.dld.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 63.Yang MJ, Park SW, Lee KJ, Koh DH, Lee J, Lee YN, Park CH, Shin E, Kim S. EUS-guided tissue acquisition using a novel torque technique is comparable with that of the fanning technique for solid pancreatic lesions: A multicenter randomized trial. J Hepatobiliary Pancreat Sci. 2022 doi: 10.1002/jhbp.1255. [DOI] [PubMed] [Google Scholar]
- 64.Wani S, Mullady D, Early DS, Rastogi A, Collins B, Wang JF, Marshall C, Sams SB, Yen R, Rizeq M, Romanas M, Ulusarac O, Brauer B, Attwell A, Gaddam S, Hollander TG, Hosford L, Johnson S, Kushnir V, Amateau SK, Kohlmeier C, Azar RR, Vargo J, Fukami N, Shah RJ, Das A, Edmundowicz SA. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: a prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015;110:1429–1439. doi: 10.1038/ajg.2015.262. [DOI] [PubMed] [Google Scholar]
- 65.Lee LS, Nieto J, Watson RR, Hwang AL, Muthusamy VR, Walter L, Jajoo K, Ryou MK, Saltzman JR, Saunders MD, Suleiman S, Kadiyala V. Randomized Noninferiority Trial Comparing Diagnostic Yield of Cytopathologist-guided versus 7 passes for EUS-FNA of Pancreatic Masses. Dig Endosc. 2016;28:469–475. doi: 10.1111/den.12594. [DOI] [PubMed] [Google Scholar]
- 66.Crinò SF, Di Mitri R, Nguyen NQ, Tarantino I, de Nucci G, Deprez PH, Carrara S, Kitano M, Shami VM, Fernández-Esparrach G, Poley JW, Baldaque-Silva F, Itoi T, Manfrin E, Bernardoni L, Gabbrielli A, Conte E, Unti E, Naidu J, Ruszkiewicz A, Amata M, Liotta R, Manes G, Di Nuovo F, Borbath I, Komuta M, Lamonaca L, Rahal D, Hatamaru K, Itonaga M, Rizzatti G, Costamagna G, Inzani F, Curatolo M, Strand DS, Wang AY, Ginès À, Sendino O, Signoretti M, van Driel LMJW, Dolapcsiev K, Matsunami Y, van der Merwe S, van Malenstein H, Locatelli F, Correale L, Scarpa A, Larghi A. Endoscopic Ultrasound-guided Fine-needle Biopsy With or Without Rapid On-site Evaluation for Diagnosis of Solid Pancreatic Lesions: A Randomized Controlled Non-Inferiority Trial. Gastroenterology. 2021;161:899–909.e5. doi: 10.1053/j.gastro.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Pausawasdi N, Cheirsilpa K, Chalermwai W, Asokan I, Sriprayoon T, Charatcharoenwitthaya P. Endoscopic Ultrasound-Guided Fine-Needle Biopsy Using 22G Franseen Needles without Rapid On-Site Evaluation for Diagnosis of Intraabdominal Masses. J Clin Med. 2022;11 doi: 10.3390/jcm11041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iwashita T, Yasuda I, Mukai T, Doi S, Nakashima M, Uemura S, Mabuchi M, Shimizu M, Hatano Y, Hara A, Moriwaki H. Macroscopic on-site quality evaluation of biopsy specimens to improve the diagnostic accuracy during EUS-guided FNA using a 19-gauge needle for solid lesions: a single-center prospective pilot study (MOSE study) Gastrointest Endosc. 2015;81:177–185. doi: 10.1016/j.gie.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 69.Ishiwatari H, Sato J, Fujie S, Sasaki K, Kaneko J, Satoh T, Matsubayashi H, Kishida Y, Yoshida M, Ito S, Kawata N, Imai K, Kakushima N, Takizawa K, Hotta K, Ono H. Gross visual inspection by endosonographers during endoscopic ultrasound-guided fine needle aspiration. Pancreatology. 2019;19:191–195. doi: 10.1016/j.pan.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Kaneko J, Ishiwatari H, Sasaki K, Yasuda I, Takahashi K, Imura J, Iwashita T, Uemura S, Hatano Y, Miyazaki T, Satoh T, Sato J, Ishikawa K. Macroscopic visible core length can predict the histological sample quantity in endoscopic ultrasound-guided tissue acquisition: Multicenter prospective study. Dig Endosc. 2022;34:622–631. doi: 10.1111/den.14116. [DOI] [PubMed] [Google Scholar]
- 71.Beane JD, House MG, Coté GA, DeWitt JM, Al-Haddad M, LeBlanc JK, McHenry L, Sherman S, Schmidt CM, Zyromski NJ, Nakeeb A, Pitt HA, Lillemoe KD. Outcomes after preoperative endoscopic ultrasonography and biopsy in patients undergoing distal pancreatectomy. Surgery. 2011;150:844–853. doi: 10.1016/j.surg.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 72.Cosgrove ND, Yan L, Siddiqui A. Preoperative endoscopic ultrasound-guided fine needle aspiration for diagnosis of pancreatic cancer in potentially resectable patients: Is this safe? Endosc Ultrasound. 2015;4:81–84. doi: 10.4103/2303-9027.156708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ngamruengphong S, Swanson KM, Shah ND, Wallace MB. Preoperative endoscopic ultrasound-guided fine needle aspiration does not impair survival of patients with resected pancreatic cancer. Gut. 2015;64:1105–1110. doi: 10.1136/gutjnl-2014-307475. [DOI] [PubMed] [Google Scholar]
- 74.Park JS, Lee JH, Song TJ, Lee JS, Jo SJ, Oh DW, Song KB, Hwang DW, Park DH, Lee SS, Kim SC, Seo DW, Lee SK, Kim MH. The impact of preoperative EUS-FNA for distal resectable pancreatic cancer: Is it really effective enough to take risks? Surg Endosc. 2022;36:3192–3199. doi: 10.1007/s00464-021-08627-3. [DOI] [PubMed] [Google Scholar]
- 75.Yane K, Kuwatani M, Yoshida M, Goto T, Matsumoto R, Ihara H, Okuda T, Taya Y, Ehira N, Kudo T, Adachi T, Eto K, Onodera M, Sano I, Nojima M, Katanuma A. Non-negligible rate of needle tract seeding after endoscopic ultrasound-guided fine-needle aspiration for patients undergoing distal pancreatectomy for pancreatic cancer. Dig Endosc. 2020;32:801–811. doi: 10.1111/den.13615. [DOI] [PubMed] [Google Scholar]
- 76.Sakamoto U, Fukuba N, Ishihara S, Sumi S, Okada M, Sonoyama H, Ohshima N, Moriyama I, Kawashima K, Kinoshita Y. Postoperative recurrence from tract seeding after use of EUS-FNA for preoperative diagnosis of cancer in pancreatic tail. Clin J Gastroenterol. 2018;11:200–205. doi: 10.1007/s12328-018-0822-z. [DOI] [PubMed] [Google Scholar]