Summary:
Mechanosensitive processes often rely on adhesion structures to strengthen, or mature, in response to applied loads. However, limited understanding of how molecular tensions experienced by a particular protein affect the recruitment of other proteins represents a major obstacle towards deciphering molecular mechanisms that underlie mechanosensitive processes. Here, we describe an imaging-based technique, termed Fluorescence Tension Co-localization (FTC), for studying molecular tension-sensitive protein recruitment inside cells. Guided by discrete time Markov Chain simulations of protein recruitment, we integrate immunofluorescence labeling, molecular tension sensors, and machine learning to determine the sensitivity, specificity, and context-dependence of molecular tension sensitive protein recruitment. Application of FTC to the mechanical linker protein vinculin in mouse embryonic fibroblasts reveals constitutive and context specific molecular tension-sensitive protein recruitment that varies with adhesion maturation. FTC overcomes limitations associated with the alteration of myriad proteins during manipulation of cell contractility to provide molecularly specific insight into tension sensitive protein recruitment.
eTOC Blurb:
Tao and LaCroix et al. describe FTC, a microscopy methodology that employs FRET-based biosensors and immunofluorescence to study how molecular tension on one protein influences the recruitment of other proteins. This technique identifies that during focal adhesion maturation, vinculin mediates constitutive and context dependent tension sensitive protein recruitment mechanisms.
Graphical Abstract

Introduction:
The abilities of cells to detect and adapt to mechanical loads are increasingly recognized as critical drivers of many fundamentally important biological processes, including migration, proliferation, differentiation, and morphogenesis1–4. In response to either internally-generated or externally-applied mechanical loads, many adhesion structures strengthen or mature to maintain pertinent linkages to the environment and ensure tissue integrity5. Adhesion maturation is most well-studied in focal adhesions (FAs), which link the extracellular matrix (ECM) to force-generating actomyosin networks6. This process is driven by mechanotransduction, where the deformation of load-bearing proteins alters protein function to regulate the direct and indirect recruitment of a plethora of structural and signaling proteins5,7.
While conceptually simple, the regulatory complexity of mechanotransduction is likely substantial. In some systems, recruitment of up to 50% of the approximately 900 proteins detected in FAs is dependent on cell force-generation8. The exact mechanisms mediating this complexity are poorly understood, as only several tension sensitive protein-protein interactions have been demonstrated4. Furthermore, to our knowledge, there are no techniques capable of probing the relationship between the mechanical loading of a specific protein and the recruitment of other proteins in cells, where key post-translational modifications and other spatiotemporal-varying regulatory mechanisms can be maintained. Most cellular techniques for studying mechanosensitive protein recruitment rely on either large increases in applied forces through the use of deformable membranes, magnets, or large decreases in cell contractility induced by actomyosin inhibitors8–14. These studies have been instrumental in identifying proteins that change localization in response to applied loads, but such manipulations affect the loading and localization of large numbers of protein simultaneously. This makes it challenging to identify pertinent mechanical linkages and specific recruitment mechanisms. Therefore, determining the effects of molecular tensions experienced by specific proteins on the recruitment of other proteins to load-bearing sub-cellular structures remains a critical step towards a mechanistic understanding of mechanotransduction and adhesion maturation.
In this work, we developed an imaging-based technique for studying molecular tension-sensitive protein recruitment inside cells. Inspired by fluorescence co-localization, where the correlation of the local concentrations of two proteins is used to infer potential regulatory relationships, we reasoned, and verified with discrete time Markov Chain simulations of protein recruitment to FAs, that local measurements of molecular tension experienced by a specific protein and the concentration of another protein could be used to reveal tension-sensitive recruitment. Initial efforts were focused on the mechanical linker protein vinculin, which has previously been shown to mediate a tension-sensitive switch that controls adhesion maturation15. In our experimental approach, termed fluorescence-tension co-localization (FTC), we integrated immunofluorescence labeling, Forster Resonance Energy Transfer (FRET)-based molecular tension sensors, biophysical knowledge of key residues required for protein loading, and automated image analysis to create a procedure for determining if the recruitment of candidate proteins was dependent on molecular tension experienced by vinculin. Additionally, we developed a biochemical approach for studying the role of vinculin tension in protein recruitment to FAs by adapting promiscuous biotin ligase-based proximity labeling and observed similar responses. Furthermore, we trained a machine learning algorithm to identify FAs at various maturation states and performed FTC in these sub-populations. Excitingly, we find instances of vinculin tension sensitive protein recruitment that are either constitutive or dependent on FA maturation. This suggests a previously unappreciated layer of regulation in adhesion maturation involving a single load-bearing protein generating both common and context-specific mechanosensitive signals to mediate protein recruitment and alter FA composition. As FTC is generalizable to any FRET-based molecular tension sensor, we expect this technique to be useful in the study of many mechanosensitive processes.
Design:
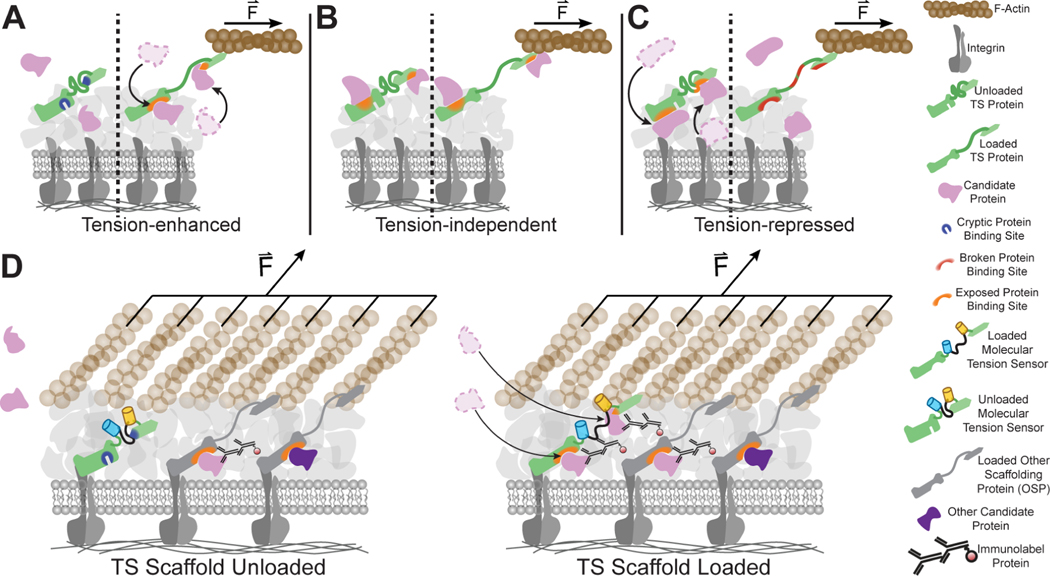
Inspired by fluorescence co-localization16,17, a microscopy-based approach for studying biochemically regulated protein recruitment in cells, we update this approach to instead, detect molecular tension-sensitive protein recruitment. This is achieved by correlating the molecular tension across a scaffolding protein containing a tension sensor (TS), which leverages distance-dependent changes in FRET to optically report the mechanical load across a scaffolding protein, with the concentration of a candidate protein (CP) (Figure 1).
Figure 1. Fluorescence tension co-localization enables studies on tension-sensitive recruitment of proteins to the FA.
(A) Schematic of tension-enhanced protein recruitment. In response to applied loads, binding sites of TS are exposed, and CP is recruited to the FA. (B) Schematic of tension-independent protein recruitment. Binding sites on TS are constitutively exposed and accessible to the CP, regardless of application of load. (C) Schematic of tension-repressed protein recruitment. In response to applied loads, binding sites of TS are broken, and CP is repressed from the FA. For simplicity, schematics only show direct interactions. (D) Schematic of FTC, which combines FRET-based molecular tension sensors with labelling of candidate proteins, in this example, using immunofluorescence, to study tension-sensitive recruitment of proteins to the FA in response to the mechanical loading of the tension sensor. When TS is unloaded, molecular-tension sensitive recruitment of candidate proteins does not occur. When TS is loaded, molecular-tension sensitive protein recruitment of candidate proteins occurs and can be detected optically through a relationship between FRET and CP-labelling intensity. Note that when comparing the TS scaffold loading, the only differences are candidate proteins recruited specifically due to changes in the tension experienced by the TS scaffold.
To inform design and determine limitations of the FTC approach, we created a simple model of tension-sensitive protein recruitment using simulations of discrete time Markov Chains (Supplemental Methods I). This formalism is well suited to aid in the creation of new techniques, as different scenarios are simulated by specifying the probability of transitions between key states. This enables evaluation of a large variety of scenarios without detailed knowledge of specific kinetic parameters. Specifically, we assessed CP recruitment to TS in response to various strength of interactions and multiple types of protein recruitment (molecular tension independent, enhanced, and repressed). In direct analogy to the proposed FTC approach, measurable outputs include: the number of bound CP molecules, the number of bound TS molecules, and the average molecular tension across TS. We first used simulations to evaluate a variety of quantification metrics to detect various types of protein recruitment. (Supplemental Methods II). Of the evaluated metrics, only the tension recruitment index (TRI), defined as the log2 ratio of normalized intensities of protein labeling in areas of high versus low molecular tension, was found to accurately identify the specified type of protein recruitment, independently of FA size. Therefore, it was chosen as a quantification metric. Large positive values of TRI suggest molecular tension-enhanced CP recruitment, values near zero suggest molecular tension-independent recruitment, and large negative values suggest molecular tension-repressed CP recruitment.
Next, we used the simulations to probe whether TRI can accurately detect tension sensitive CP recruitment to TS in the presence of additional tension insensitive or sensitive recruitment mechanisms involving another scaffolding protein (OSP) (Supplemental Methods III). TRI robustly detected tension sensitive recruitment of CP in the presence of additional tension insensitive and tension sensitive recruitment mechanisms involving uncorrelated molecular tensions between TS and OSP. However, the accuracy of TRI was reduced, and false positives arose, if strong tension sensitive CP recruitment to OSP occurred in the presence of coordinated tensions between TS and OSP. Thus, the TRI metric can detect if CPs are recruited in a molecular tension-sensitive manner with high sensitivity, but cannot determine whether that recruitment is specific to the molecular tension across TS.
We next sought to develop an approach for detecting CP recruitment that was specifically due to tensions experienced by TS (Supplemental Methods IV). As various point mutations, domain removals, or other alterations that unload scaffolding proteins have been identified15,18,19, we performed simulations with a zero tension control (ZTC) that is conceptualized as retaining all characteristics of TS, except the abilities to bear load and mediate any load dependent functions. As the ZTC does not experience tension, we focused on other outputs of FTC, like the amounts of TS and CP. Simulations revealed that the Pearson correlation coefficient (PCC) between the amounts of CP and TS change with molecular tension dependent recruitment of CP to TS. Additionally, disruption of molecular tension sensitive protein recruitment with a ZTC led to a distinct value of the PCC for CP and ZTC. A substantial difference between these two metrics (ΔPCC) was indicative of TS tension being the dominant recruitment mechanism of CP. Specifically, ΔPCC is highly positive in dominant molecular tension-enhanced recruitment, highly negative in dominant molecular tension-repressed protein recruitment, and close to zero in molecular tension-independent protein recruitment. Furthermore, ΔPCC detected these interactions in the presence of coordinated loading of TS and OSP, while TRI reported false positives. However, in comparison to TRI, ΔPCC detected molecular tension sensitive recruitment only in the presence of weak alternative recruitment mechanisms. Therefore, we regard TRI as measure of molecular tension sensitivity and ΔPCC as measure of molecular tension specificity.
We then evaluated the performance of simultaneously using both metrics, TRI and ∆PCC, to detect molecular tension sensitive protein recruitment (Supplemental Methods V). We observed that highly positive (or negative) values of both TRI and ΔPCC are indicative of molecular tension enhanced (or repressed) protein recruitment, that is both a dominant mechanism and specific for the tensions experienced by TS. We term this “molecular tension dependent”. Highly positive (or negative) values of TRI, coupled with small values of ΔPCC, are indicative of CP being recruited through molecular tension sensitive mechanisms that are not specific to TS. We term this “molecular tension associated” as it likely occurs when TS and another scaffolding protein (OSP) have coordinated molecular tensions, but OSP, instead of TS, drives the recruitment of CP. Simulations revelated that small TRI values are challenging to interpret regardless of ΔPCC values, and we refer to these cases as unclassified. See Supplemental Methods VI for a further discussion of the simulations. Overall, these simulations demonstrate that, conceptually, FTC can be used to probe, evaluate, and classify molecular tension sensitive protein recruitment.
Results:
Experimental quantification of molecular tension-sensitive protein recruitment
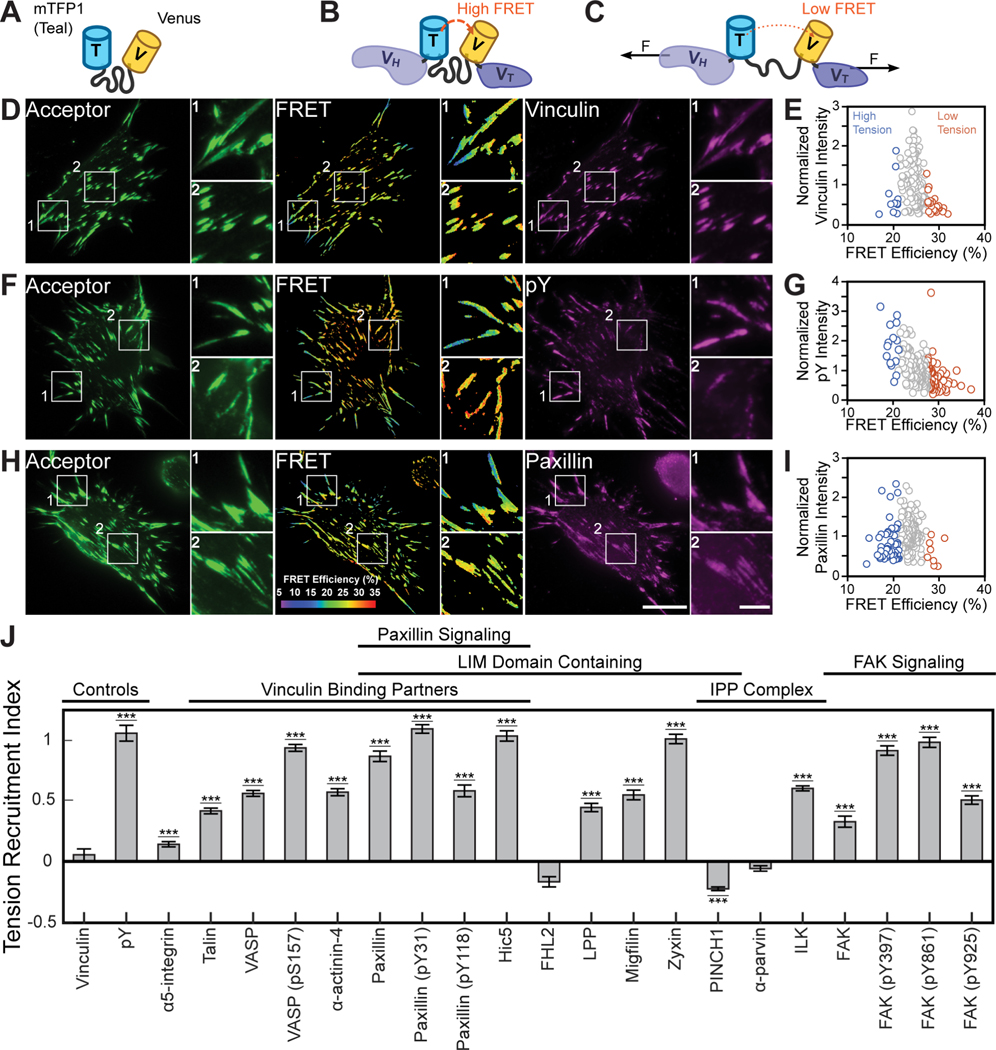
Vinculin was selected as an archetypal tension-bearing scaffolding protein to first study with FTC, as it bears substantial spatiotemporally varying mechanical loads15,20, forms the most interactions with other proteins in the consensus integrin adhesome21, and is a key regulator of FA maturation10,22. Furthermore, a FRET-based molecular tension sensor for vinculin (VinTS) has already been developed and used in different contexts15,19,20 (Figure 2A–2C). Given the tremendous molecular diversity within FAs, pertinent CPs were identified as members of the consensus adhesome that had more than one interaction partner and a commercially/readily available antibody that labeled FAs in our system21. Given the known roles of VASP, paxillin, and FAK in mediating FA and actin dynamics23, we also probed their phosphorylated states. The final CP set was comprised of 14 FA proteins and 6 protein phosphorylation states.
Figure 2. Quantifying molecular tension-sensitive protein recruitment.
(A-F) Representative images of acceptor intensity, masked FRET efficiency, and candidate protein staining, along with a scatter plot of the relationship between normalized candidate protein expression and FRET efficiency for all FAs within the depicted cell. Images and data shown for a negative control, vinculin (A, B), positive control for molecular tension-sensitive recruitment, pY (C, D), and protein expected to exhibit molecular tension-sensitive recruitment based on the literature, paxillin (E, F). Zoom-ins of FAs under high (1) and low (2) tension are provided for indicated regions within the representative images. Scale bars for full and zoom-in images are 20 μm and 5 μm, respectively. (G) TRIs for assessing vinculin tension-sensitive recruitment to FAs across probed candidate proteins and candidate protein states. Data represents TRI ± s.e (***p < 0.001, evaluated by two-tailed one sample t-test with Bonferroni correction). See Table S1 for detailed statistical parameters and number of biological replicates.
To detect molecular tension sensitive protein recruitment to FAs with FTC, vinculin −/− mouse embryonic fibroblasts (MEFs)24 stably expressing VinTS at close to physiological levels19 were indirectly immunolabeled for individual CPs. TS concentrations were probed through direct excitation of the acceptor fluorophore, while the tension across vinculin was quantified through changes in FRET Efficiency15,19,20. Proteins were visualized with far-red dyes to minimize the possibility of intermolecular FRET between VinTS and labeled CPs25. Use of standard immunofluorescence-based detection methods allows for a high-content approach where many proteins and cells can be imaged quickly, avoids protein over-expression artifacts, and prevents delays associated with the generation of many engineered cell lines. An object-oriented colocalization method was used to maintain information about the FA state26. To account for heterogeneity in sub-cellular structures and across cell populations, we assembled a dataset comprised of over 300,000 FAs imaged from over 3,000 cells, with at least 60 cells per probed CP or CP phosphorylation state. Representative VinTS images, CP images, and single cell data for all probed CPs are provided (Supplemental Methods VII). As a negative control, we immunolabeled VinTS with a vinculin antibody. As expected, vinculin tension-sensitivity was not observed (TRI = 0.05, Figure 2D and 2E). As a positive control, we immunolabeled phospho-tyrosine (pY), which is a classic marker for mechanosensitive signaling27. This resulted in a large, 2-fold enrichment in FAs where vinculin bears high tension (TRI = 1.06, Figure 2F and 2G). Paxillin, a known vinculin binding partner28, also exhibited significant vinculin tension-sensitive recruitment (TRI = 0.87, Figure 2H and 2I).
Most CPs (12 of 14) and all CP phosphorylation states exhibited statistically significant vinculin tension-sensitive recruitment (Figure 2J). However, consistent with complex mechanisms mediating FA maturation, there is substantial variation in TRIs. For instance, α5-integrin exhibits a small TRI, consistent with it being an upstream mediator of vinculin load. Proteins known to form tension-sensitive interactions with vinculin, including talin, α-actinin-4, VASP, and probed phosphorylation states10,29, exhibit large TRIs. Previous studies using blebbistatin to inhibit myosin activity demonstrated that many LIM domain containing proteins are enriched in loaded FAs8,9. The majority of LIM domain containing proteins probed here exhibit high TRIs, including known vinculin regulators paxillin, Hic5, and LPP30,31. Phospho-paxillin had a particularly high TRI, likely consistent with the established role of myosin-activity dependent paxillin phosphorylation in vinculin recruitment to FAs32. A notable exception was the LIM domain containing protein, PINCH1, which appears to be weakly excluded from high vinculin tension FAs. Furthermore, members of ternary IPP complexes, ILK, PINCH1, and α-parvin, show differential response to vinculin tension, suggesting that vinculin tension influences formation or maintenance of the complex. FAK and, particularly, phosphorylated FAK have large TRIs, consistent with the established roles of FAK in vinculin recruitment32 and the ability of tensed vinculin to promote FAK activity33. In total, the data and connections to existing literature demonstrate that the FTC method and TRI metric can be used to identify vinculin tension-sensitive recruitment of CPs.
Probing specificity of molecular tension-sensitive protein recruitment to assess molecular tension association or dependence
We next sought to identify a ZTC for vinculin. We employed the vinculin I997A mutation, as it has substantially reduced actin affinity and bears no significant loads in living cells19,34. Furthermore, previous work has shown that when compared to WT vinculin, vinculin I997A supports normal lipid binding, FA assembly, and conformational dynamics35,36. Thus, the mutation has little effect on the tension independent functions of vinculin and is suitable as a ZTC (see further discussion in Supplemental Methods VI).
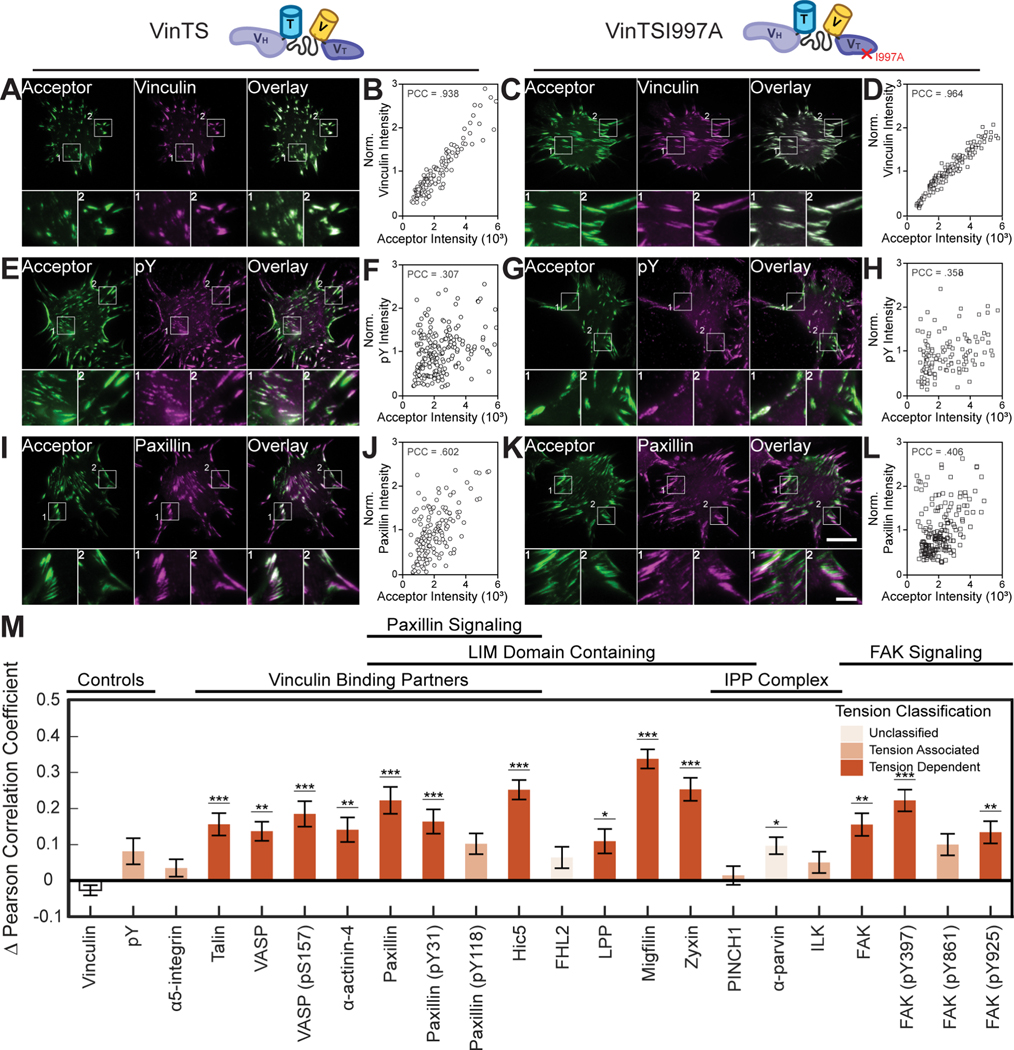
To create the ZTC, VinTSI997A was stably expressed in vinculin −/− MEFs. Western blot analysis demonstrated similar CP expression between VinTS and VinTSI997A containing cells (Figure S1). To probe the specificity of vinculin tension-sensitive recruitment, VinTSI997A expressing vinculin −/− MEFs were subject to the same staining, imaging, and analysis as VinTS expressing cells, yielding a dataset of over 200,000 FAs from over 2,000 cells with at least 60 cells per CP or CP phosphorylation state. Representative VinTSI997A images, CP images, and single cell data for all CPs are provided (Supplemental Methods VII). As expected, the ΔPCC for vinculin immunolabeling was close to zero (Figure 3A–3D and 3M). Further, pY, a classic marker for general tension-dependent signaling27, independent of vinculin tension, also had a small ΔPCC (Figure 3E–3H and 3M). In contrast, many vinculin binding partners and regulators exhibited a large ΔPCC (Figure 3I–3M). Overall, these data demonstrate that VinTS I997A can serve as a ZTC to probe specificity in vinculin tension sensitive protein recruitment.
Figure 3. Assessing specificity of molecular tension-sensitive protein recruitment.
(A-L) Representative images of acceptor intensity, candidate protein staining, and overlay, along with a scatter plot of the correlation between normalized candidate protein stain intensity and acceptor intensity. Images and data shown for a negative control, vinculin in VinTS- (A, B) and VinTSI997A- (C, D) expressing cells, example of non-specific molecular tension-sensitive recruitment, pY in VinTS- (E, F) and VinTSI997A- (G, H) expressing cells, and protein expected to exhibit specific molecular tension-sensitive recruitment based on the literature, paxillin in VinTS- (I, J) and VinTSI997A- (K, L) expressing cells. Zoom-ins of FAs are provided for indicated regions within the representative images. Scale bars for full and zoomed in images are 20 μm and 5 μm, respectively. (M) ΔPCC and protein recruitment tension classification, based on combined analyses of vinculin tension sensitivity (TRI) and specificity (ΔPCC), across probed candidate proteins and candidate proteins states. Data represents mean ΔPCC ± s.e (*p < 0.05, **p < 0.01, ***p < 0.001, evaluated by one-way ANOVA, Dunnett’s post-hoc test). See Table S1 for detailed statistical parameters and number of biological replicates.
Combining the analyses of vinculin tension sensitivity (Figure 2J) and specificity (Figure 3M) enabled classification of protein recruitment as molecular tension-dependent or tension-associated (Table S1). All known vinculin binding partners21, along with a subset of LIM domain containing proteins, exhibited vinculin tension-dependent protein recruitment. Notably, migfilin localization to FAs was almost completely lost in VinTS I997A expressing cells (Supplemental Methods VII), suggesting particularly dominant vinculin tension dependent recruitment. Vinculin tension-associated recruitment was observed in α5-integrin and some members of the IPP complex, broadly consistent with the role of integrins as upstream regulators of vinculin tension and the IPP complex as a parallel force-transmission pathway37. Differences between the protein, versus phosphorylation state, for FAK and paxillin, suggest that vinculin tension could differentially affect aspects of downstream signaling. Overall, these data demonstrate the ability of FTC to identify and characterize the effects of loading a specific protein on the recruitment of other proteins.
Migfilin is a tension-dependent vinculin proximal binding partner
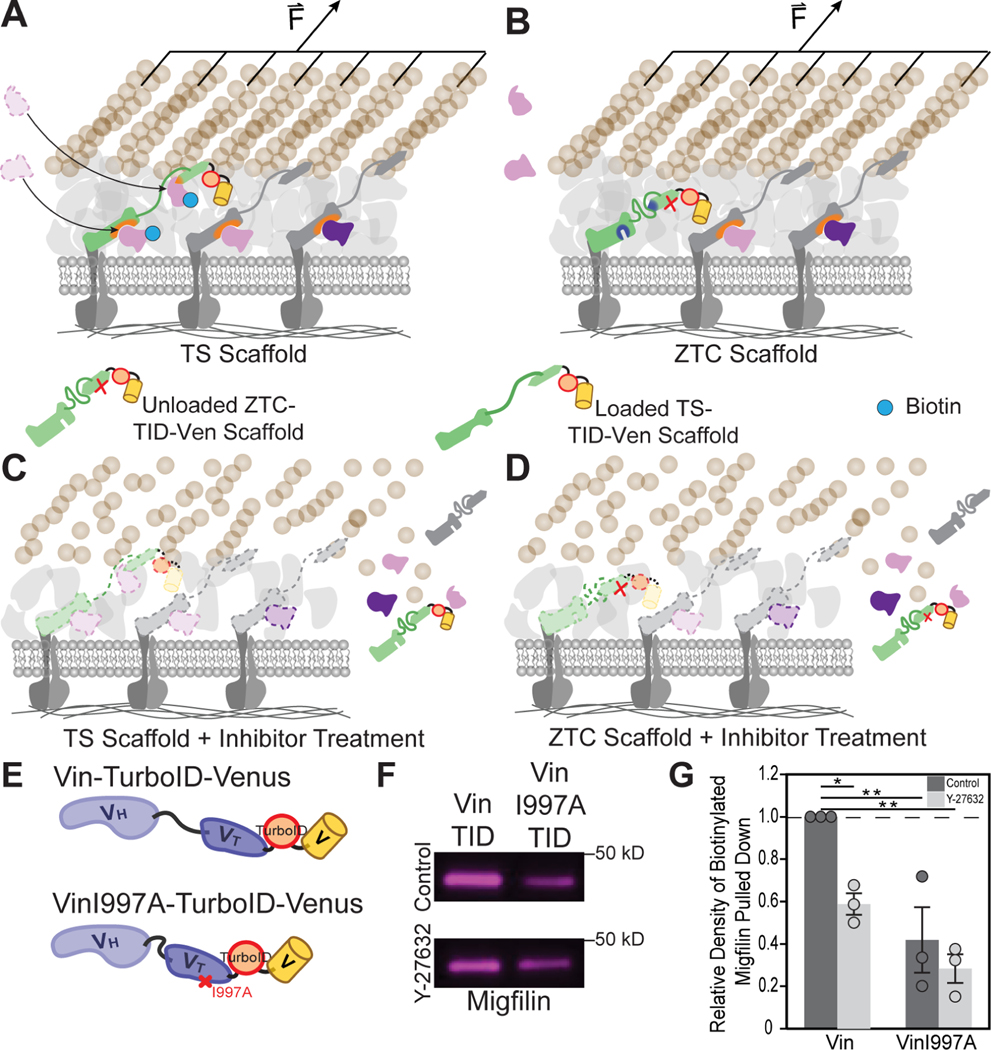
Next, we sought to probe vinculin tension dependent protein recruitment in a manner that was independent of imaging and FRET-based tension sensors, as well as readily compatible with actomyosin inhibitors. Specifically, we adapted proximity based labeling techniques, focusing on those using promiscuous biotin ligases like TurboID38. In this approach TurboID is attached to a protein of interest, and, upon the addition of exogenous biotin, proteins proximal to TurboID (typically with a 10 nm radius) will be labeled with biotin38,39. Thus, the approach is molecularly-specific and can be readily integrated with a tension sensitive scaffold protein of interest and its associated ZTC to probe the tension dependence of proximal interactors (Figure 4A and 4B). Furthermore, as the labelling is temporally controlled, the approach can also be readily combined with an actomyosin inhibitor (Figure 4C and 4D).
Figure 4. Proximity-dependent biotinylation reveals that migfilin is a tension-dependent vinculin proximal binding partner.
(A, B) Schematic of proximity-dependent biotinylation of nearby proteins that are present upon recruitment in a tension-sensitive manner when TS scaffold is loaded (A), but absent when TS scaffold is unloaded (B). (C, D) Schematic of proximity-dependent biotinylation of nearby proteins upon inhibitor treatment, resulting in the unloading and departure of scaffolding and FA proteins, alike, regardless of when TS scaffold is originally loaded (C) or unloaded (D). This results in minimal labeling as proteins are absent. (E) Schematic of the biotin ligase, TurboID, inserted between either vinculin-venus, or a zero-tension control variant, vinculinI997A-venus for probing proximal protein interactions. (F, G) Biotinylated migfilin pulldowns (n=3 biological replicates) from vinculin −/− MEFs expressing either Vin-TurboID-Venus or VinI997A-TurboID-Venus, treated with ROCK inhibitor Y-27632, were run on a western blot (F) and quantified by normalizing to biotinylated migfilin in untreated Vin-TurboID-Venus expressing samples (G). Data represents mean normalized relative density of western blot bands ± s.e.m with exact data points plotted (*p < 0.05, **p < 0.01, evaluated by one-way ANOVA, Tukey’s HSD).
To adapt proximity-dependent biotin labeling for studying vinculin tension-dependent protein proximal binding, we integrate TurboID into either vinculin venus (Vin-TurboID-Venus) or the ZTC variant, vinculin I997A venus (VinI997A-TurboID-Venus) (Figure 4E). Constructs were validated by demonstrating the expected localization to, and biotin labelling within FAs, (Figure S2A) and significant labeling in Western blots (Figure S2B). We investigated the recruitment of migfilin, as its localization to FAs was substantially reduced in the absence of vinculin tension (Supplemental Methods VII), and it has not been previously identified as a vinculin binding partner21,40. Streptavidin pulldown assays revealed that there was more biotinylated migfilin in Vin-TurboID-Venus than in VinI997A-TurboID-Venus (Figure 4F and 4G). Furthermore, reduction of actomyosin contractility with the ROCK inhibitor Y-27632, which has previously been shown to reduce cell force generation and unload VinTS19, reduced the amount of biotinylated migfilin in Vin-TurboID-Venus, but not VinI997A-TurboID-Venus (Figure 4F and 4G). Together, this data demonstrates that the proximal interaction of vinculin and migfilin requires actomyosin-mediated tension across vinculin.. Additionally, it validates both the overall FTC approach and using a ZTC to study tension-dependent protein recruitment.
Vinculin tension and conformation change during FA maturation
FA maturation involves changes in the forces exerted by FAs, as well as the amount of, type of, and modifications to proteins within these structures9,23. Therefore, we sought to determine if similar or distinct forms of molecular tension sensitive protein recruitment exist during different stages of FA maturation. Since vinculin recruitment and tension are both altered in different FA maturation states15,41, we again focused on this load-bearing scaffolding protein.
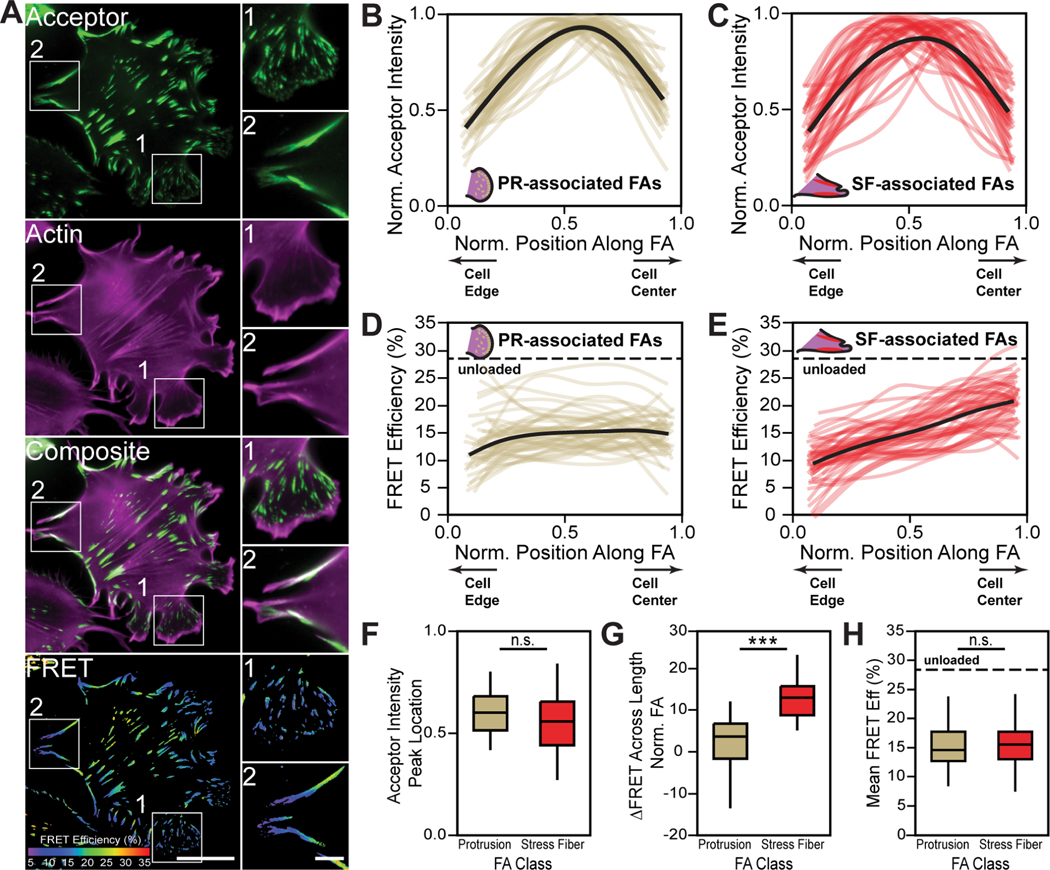
We examined patterns of vinculin tension associated with maturation state, as defined by FA proximity to pertinent actin structures23. Specifically, FAs in lamellipodia and protrusive (PR) regions were classified as immature and FAs associated with stress fibers (SF) were classified as mature (Figure 5A). Line scans of acceptor intensity show that VinTS distributions within individual PR- and SF- associated FAs were similar (Figure 5B, 5C, 5F). However, line scans of FRET efficiency revealed spatial uniformity in PR-associated FAs and spatial gradients in SF-associated FAs (Figure 5D, 5E, 5G). Despite differences in the spatial distribution of tension between the two groups, the average tensions are similar (Figure 5H). To further probe the relationship between vinculin tension and FA maturation, we investigated two other cell perturbations. First, constitutively active forms of Rac1 and RhoA were used to enhance immature PR-associated and mature SF-associated FAs, respectively (Figure S3). Second, we evaluated spreading cells which form more mature FAs with increased spreading time23 (Figure S3). In both cases, the distributions of VinTS were equivalent in immature and mature FAs, despite the distributions of tension varying with FA maturation state.
Figure 5. Spatial gradients in vinculin tension change during FA maturation.
(A) Representative images of acceptor intensity, F-actin-labeling, and masked FRET efficiency for VinTS expressing cells. Zoom-ins of immature PR- (1) and mature SF- (2) associated FAs are provided for indicated regions within the representative images. Scale bars for full and zoomed in images are 20 μm and 5 μm, respectively. (B-E), Line scans for normalized acceptor intensity in PR- (B) and SF- (C) associated FAs and FRET efficiency in PR- (D) and SF- (E) associated FAs. (F) VinTS localization, measured via peak acceptor intensity location, for PR- (n=45) and SF-associated (n=45) FAs (n.s., evaluated by two-tailed unpaired t test). (G) Change in FRET efficiency across length of a normalized FA for PR- (n=45) and SF-associated FAs (n=45 biological replicates) (***p < 0.001, evaluated by two-tailed unpaired t test). (H) Mean FRET efficiency for PR- (n=45) and SF- (n=45) associated FAs (n.s., evaluated by two-tailed unpaired t test). Data represents median, middle line, and 75th and 25th percentile, top and bottom lines, respectively. Whiskers extend to data within 1.5x interquartile range. n represents biological replicates.
Vinculin undergoes conformational regulation and can exist in a variety of conformations with varying abilities to bind actin and bear tension36. We assessed the relationship between vinculin conformation and FA maturation using a FRET-based vinculin conformation sensor (VinCS)42 and the actin structure-based classification scheme (Figure S4). Vinculin is in a more closed, but not completely closed, conformation in PR-associated FAs and a more open conformation in SF-associated FAs. Since these are ensemble measurements of VinCS, it is impossible to determine if changes in VinCS FRET efficiency are due to a mix of fully-opened and fully-closed populations and/or changes in intermediary states.
Altogether, these data demonstrate that the spatial distribution of vinculin tension and conformation change during FA maturation, and further, suggest a basis for differential molecular tension mediated protein recruitment during FA maturation.
Vinculin tension-sensitive protein recruitment varies with FA maturation state
To detect and classify vinculin tension sensitive protein recruitment in immature and mature FA contexts, we developed a decision tree-based machine learning model that is compatible with the data produced by FTC. Unlike most previous efforts, metrics associated with FA intensity were not used for classification to avoid potential issues with similar average intensities between small and large FAs, as well as small variations in expression level (Supplemental Methods VIII). Also, FRET efficiency-based metrics were not used to ensure generalizability to the variety of biosensors used here. Specifically, physical characteristics (e.g. area, axis ratio, orientation, proximity of other FAs, relative position in the cell) were used as the basis for classification. As we detected differences in vinculin tension between PR- and SF-associated FAs (Figure 5), we focused our initial classification analysis on FAs encompassed in these two classes that are likely transitioning between focal contacts and FAs23. Fibrillar adhesions could not be analyzed due to immunolabelling of some CPs in the nucleus.
The developed machine learning model classifies analyzed FAs as being either PR-associated, SF-associated, or unclassified (UC). To test the overall performance of the machine learning model, several analyses were performed (Supplemental Methods VIII). First, the model performs substantially better than a random guessing scenario for classification of three different groups. Additionally, PR- and SF-associated FAs that were classified by this model retained key physical FA characteristics, as well as key characteristics of vinculin tension and conformation identified in manual analyses. These analyses show that the machine learning based classification functions like manual classification, but with substantially greater speed and reproducibility, and thus is appropriate for use with the large FTC dataset.
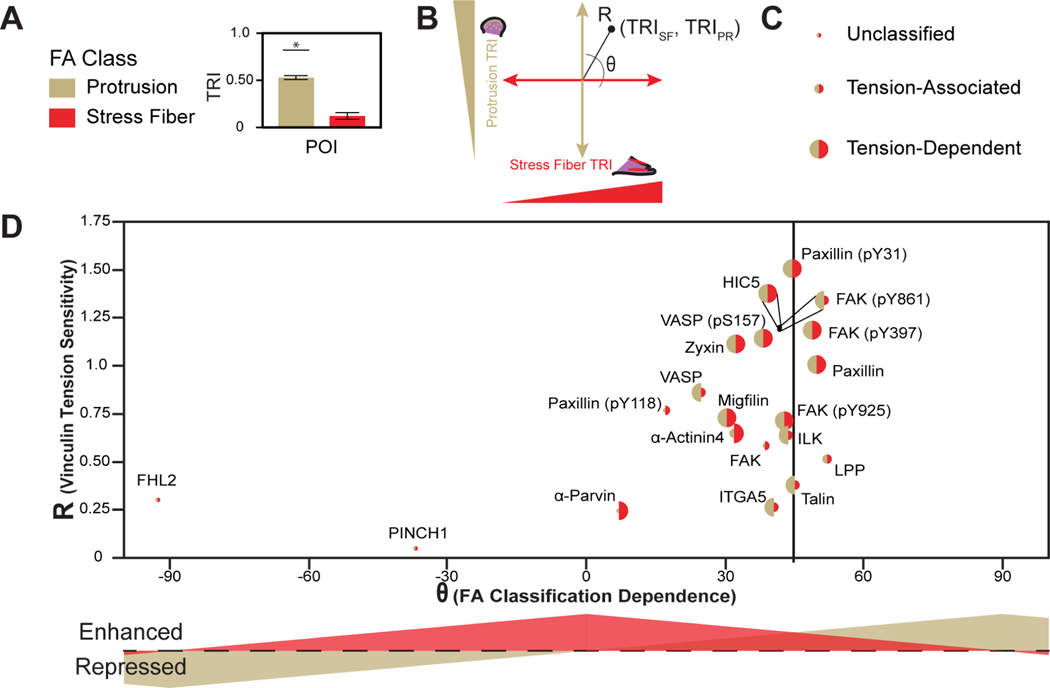
We sought to develop a formalism for displaying and interpreting tension sensitive recruitment of probed CPs in an adhesion maturation dependent manner. To directly compare tension sensitivity in both immature and mature FA classes, TRIs for both PR- and SF-associated FAs (Table S1) were converted to polar coordinates (Figure 6A and 6B). In this representation, R indicates the overall degree of tension sensitivity. Theta indicates the preference, as well as whether recruitment is tension-enhanced or tension-repressed, for a given FA class. To compare the classification of tension sensitive recruitment (tension dependent, tension associated, or unclassified) in each FA class, we varied the size of the hemispheres for each CP, with PR-associated FAs colored gold, left, and SF-associated FAs colored red, right (Figure 6C). To demonstrate the interpretability of this formalism, we compared it to some existing models of adhesion maturation (Figure S5). Physical models of FA maturation often focus on the ability of loaded scaffolding proteins to recruit additional mechanical reinforcement proteins4,15,43. We term this phenomenon constitutive tension dependent protein recruitment, as no differences in tension sensitive protein recruitment classification between FA classes are expected, although differences in relative recruitment strength are possible (Figure S5A and S5B). Additionally, several studies have also demonstrated that FA compositions and mechanical functions can change with maturation12,44–46, which we term context dependent tension sensitive protein recruitment. Early ideas focused on how CPs selectively localized to a single FA class can mediate these differential effects, which leads to a clear separation, as well as different size hemispheres, for a given CP (Figure S5C). More recent studies have shown that CPs localize to diverse classes of FAs, but have distinct mechanical functions in these various classes, analogous to the concept of molecular tension-associated and tension-dependent protein recruitment described above. This type of behavior breaks the clear spatial separation of points, and further contains differences in hemisphere size (Figure S5D).
Figure 6. Classification of tension sensitive protein recruitment in PR- and SF-associated FAs.
(A) Schematic representing TRIs for PR- and SF-associated FAs. (B) Representation of TRI data for recruitment classifications in cartesian coordinates and associated representation in polar coordinates. (C) Size of points indicating unclassified, molecular tension-associated, or molecular tension-dependent protein recruitment for PR- and SF-associated FAs. (D) Polar plot of vinculin tension sensitivity (R) and the preference (θ), as well as degree of molecular tension - enhanced or -repressed recruitment, for a given CP in PR- and SF-associated FAs. The size of the point indicates tension classification. See Table S1 for detailed statistical parameters.
We applied this formalism to measures of vinculin tension sensitivity, specificity, and classification of CP recruitment for PR- and SF-associated FAs. Like the global analysis of the entire data set (Figure 2J), various proteins and protein states exhibit a wide degree of differential vinculin tension sensitivities (Figure 6D). For example, Hic5, paxillin (pY31), and FAK (pY397, pY861) exhibit high vinculin tension sensitivity (high R) while FHL2 and PINCH1 exhibit low vinculin tension sensitivity (low R). Most proteins exhibit vinculin tension sensitivity that is stronger in mature SF-associated FAs (0°<θ<45°), particularly α-parvin and paxillin (pY118). Talin and paxillin (pY31) do not exhibit FA class preferences (θ~45°). LPP, paxillin, and FAK (pY397) exhibit greater vinculin tension sensitivity in immature, PR-associated FAs (45°<θ<90°). The differences in R and θ between proteins and their phosphorylation states for FAK and paxillin suggest that vinculin tension has distinct roles in recruitment that are separate from the post-translational modification of these proteins during FA maturation. Several proteins (paxillin, HIC5, migfilin, and zyxin) and protein states (VASP (pS157), paxillin (pY31), FAK (pY397), and FAK (pY925)) exhibit constitutive vinculin tension-dependent recruitment in both the global and FA-classification specific analyses. Excitingly, several proteins and protein states exhibited context-dependent vinculin tension sensitive recruitment (Figure 6D and S6). For instance, α5-integrin, talin, VASP, ILK, and FAK (pY861) exhibit vinculin tension-dependent recruitment in PR-associated FAs, but exhibit vinculin tension-associated recruitment in SF-associated FAs. On the other hand, α-actinin-4 and α-parvin exhibit vinculin tension-dependent recruitment in SF-associated FAs, but not in PR-associated FAs. Interestingly, LPP exhibited vinculin tension-associated recruitment in both SF- and PR-associated FAs, despite exhibiting vinculin tension-dependent recruitment globally, which potentially indicates a role for vinculin tension dependent recruitment of LPP in FAs that are transitioning between immature and mature states. Overall, these data show how pairing FTC and machine learning based classification approaches reveal the context dependence of molecular tension sensitive protein recruitment during FA maturation.
Discussion:
We develop, validate, and utilize a microscopy-based methodology, termed FTC, to study molecular tension-sensitive protein recruitment in cells. This approach provides a powerful means of elucidating the broad consequences of mechanical loading of a single protein in living cells. When used with a ZTC, it enables determination of the sensitivity, specificity, and context-dependence of molecular tension dependent protein recruitment. The ability to resolve the consequences of loading a specific scaffolding protein is the primary advantage of FTC over previous approaches that study protein recruitment through applying, manipulating, or observing mechanical stresses that affect a plethora of proteins simultaneously.
Focusing on the mechanical linker protein vinculin, we find that vinculin tension affects the recruitment of only a select group of FA proteins and protein phosphorylation states. To validate the approach, we used proximity-dependent biotin labeling to demonstrate that proximal vinculin-migflin interactions are dependent on vinculin tension and actomyosin contractility. To more broadly compare changes in FA composition due to manipulation of cell contractility versus vinculin tension sensitive effects, we compiled previous observations of blebbistatin sensitivity in mass spectrometry-based studies for the CP set (Table S2)8,9. Only 43% (6/14) of the CP set show the same blebbistatin sensitivities across previous studies. Only 2 of these 6 proteins, zyxin and Hic5, also show constitutive vinculin tension dependent recruitment. This analysis highlights the distinction between studies ablating cell contractility and the protein-specific FTC- and Turbo-ID-based approaches developed here. Key questions for future work will include exploring the prominent role in mechanosensitive processes for tension dependent vinculin-zyxin and vinculin-Hic5 interactions, as well as assessing molecular tension sensitive protein recruitment at FAs in patterned cells, where vinculin experiences compressive forces47.
Through the integration of FTC with machine learning based classification approaches to identify mature and immature FAs, we observe that a single protein, vinculin, mediates both constitutive and context-dependent mechanosensitive protein recruitment. CPs that exhibit constitutive vinculin tension-dependent recruitment include paxillin, Hic-5, migfilin, and zyxin. Interestingly, these are all LIM-domain containing proteins, consistent with the established role of this protein class in sensing mechanical stress at FAs and the actin cytoskeleton8,9,48. Context-dependent vinculin tension sensitive protein recruitment seems related to vinculin’s ability to both localize to integrin-rich or actin-rich layers within FAs, as well as recruit specifically to immature, but not mature, adhesions within mechanical force-driven contexts36,41. Broadly, in immature PR-associated FAs, vinculin tension specifically recruits integrin-associated proteins, while in mature SF-associated FAs, vinculin tension specifically recruits actin-associated proteins. This multi-faceted ability of vinculin to regulate constitutive tension sensitive protein recruitment of some mechanosensitive LIM-domain containing proteins, integrin-associated processes in immature FAs, as well as actin-associated process in mature FAs likely underlies the critical switch-like regulatory role of vinculin tension in adhesion strengthening and FA dynamics5,15. One key question for future work is to determine what aspect of vinculin enables multi-context tension sensitive recruitment mechanisms. Some possibilities include differential loading, conformation, and/or positioning within the FA. An important target of future work will be determining if these behaviors are either specific to vinculin or common to many scaffolding proteins. We note that although the ILK-PINCH-PARVIN (IPP) is traditionally regarded as a tripartite complex, we found that each component actually has a distinct, and often context-dependent, relationship to vinculin tension. This suggests that molecular tension sensitive IPP recruitment to, or rearrangement within, during FA maturation could be a particularly interesting area of future study.
The compositions of a wide variety of other sub-cellular structures, including fibrillar adhesions, cell-cell contacts, stress fibers, the plasma membrane, and the nuclear membrane, are all thought to be mechanosensitive and subject to drastically different loads in physiological versus pathological conditions and/or in in vitro versus in vivo contexts3,7. Additionally, FRET-based tension sensors have been constructed for key load-bearing proteins that reside within all these structures49,50, further suggesting that FTC could be used to study molecular tension dependent protein recruitment in diverse contexts. Therefore, we anticipate that FTC will enable a wide range of novel studies of tension sensitive protein recruitment by providing key information regarding new mechanisms of mechanotransduction that are currently inaccessible with other techniques.
Limitations of the Study:
Our study introduces a versatile approach for assessing the effects of molecular tension on one protein in the recruitment of another. Key assumptions and limitations of the FTC design using discrete time Markov Chains simulations are discussed in Supplemental Methods VI. This particular implementation of the FTC approach relies on the use of standard immunofluorescence antibody labeling within fixed cellular systems. Thus, it is limited by the quality of available antibodies that can bind to a specific loaded protein of interest and may be subject to fixation artifacts. These issues could be bypassed by using FP-tagged proteins of interest, but then would be hampered by potential overexpression artifacts or slowed by the production of edited cell lines. Like the fluorescence co-localization approaches that inspired FTC, care must be taken not to overinterpret the data. FTC is incapable of distinguishing between direct and indirect protein interactions. While detection of tension sensitive recruitment of a candidate protein can be achieved with a molecular tension sensor, further classification into tension-associated or tension-dependent recruitment relies on having a high quality ZTC that completely unloads the scaffolding protein. In cases where a ZTC is not completely unloaded, FTC will instead reveal “high tension” -associated and -dependent recruitment. To test the overall premise of FTC, we performed experiments in the simplest possible system for imaging, enabling the large datasets needed for machine learning. It will be an interesting question to determine if these tension-sensitive relationships occur in other mechanical microenvironments.
Resource Availability
Lead contact:
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Brenton D. Hoffman (Brenton.hoffman@duke.edu)
Materials Availability:
Plasmids generated in this study will be shared upon request to the lead contact, Brenton D. Hoffman, until made publicly available on Addgene.
Data and Code Availability:
All data reported in this paper will be shared by the lead contact, Brenton D. Hoffman, upon reasonable request.
All original code has been deposited at, https://gitlab.oit.duke.edu/HoffmanLab-Public, and is publicly available as of the date of publication.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request.
Experimental Model and Subject Details
Cell Culture:
Vinculin −/− mouse embryonic fibroblasts (MEFs) and derived cell lines expressing various FRET-based biosensors and TurboID constructs were maintained in high-glucose DMEM with sodium pyruvate (Sigma-Aldrich) supplemented with 10% FBS (HyClone), 1% v/v non-essential amino acids (Invitrogen), and 1% v/v antibiotic-antimycotic solutions (Sigma-Aldrich). HEK293 cells were maintained in high-glucose DMEM (Sigma-Aldrich) supplemented with 10% FBS (HyClone) and 1% v/v antibiotic-antimycotic solution (Sigma-Aldrich). Cells were grown at 37 °C in a humidified 5% CO2 atmosphere.
For transient transfections, Lipofectamine 2000 (Thermo Fisher Scientific) was utilized according to manufacturer’s instructions.
To generate cell lines that stably express a given construct, second generation viral packaging plasmids psPax2 (Addgene Plasmid #12260) and pMD2.G (Addgene Plasmid #12259) were used. To create viral particles, pRRL-VinTS, pRRLVinTSI997A, pRRL-Vin-TurboID-Ven, pRRL-VinI997A-TurboID-Ven, or pRRL-VinCS along with psPax2 and pMD2.G plasmids were co-transfected into HEK293-T cells. Three days post-transfection, media containing viral particles was harvested and stored at −80 °C. One day prior to viral transduction, Vin−/− MEFs were plated in 6-well dishes at a density of 100,000 cells per dish. Cells were transduced with 500 μL of viral mixture in full media supplemented with 2 μg/mL Polybrene (Sigma-Aldrich) to enhance viral uptake. After 3 passages, transduced cells were sorted with flow cytometry into several groups based on intensity of the stable construct’s fluorescent signal expression before being frozen down. As previously described, an immunofluorescence-based procedure was used to select vinculin −/− MEFs expressing the vinculin biosensor at levels comparable to endogenous vinculin in unaltered MEFs (Rothenberg et al., 2018). More specifically, immunofluorescence labeling intensity of vinculin in WT MEFs was taken as a standard for endogenous vinculin expression and matched in VinTS, VinTSI997A, Vin-TurboID-Venus, VinI997A-TurboID-Venus, and VinCS expressing vinculin −/− MEFs.
Method Details
Generating Viral Expression Constructs:
Construction of pRRL-VinTS (Rothenberg et al., 2018), pRRL-VinTSI997A (Rothenberg et al., 2018), and pcDNA3.1-VinCS (Grashoff et al., 2010) have been described previously.
Construction of pcDNA3.1-Vin-TurboID-Ven transient expression construct was achieved by taking pcDNA3.1-Vin-Venus (Addgene #27300) (Grashoff et al., 2010) and inserting the TurboID construct, derived from pcDNA3-V5-TurboID-NES (Addgene #07169) (Branon et al., 2018), in between the vinculin and Venus protein via PCR. Specifically, the TurboID construct was isolated via PCR using forward primer 5’- AAA AGA ATT CAT GAA AGA CAA TAC TGT GCC TCT GAA GCT −3’, reverse primer 5’- TTT TGG TAC CCT TTT CGG CAG ACC GCA GAC T −3’, and template DNA pcDNA3-V5-TurboID-NES (Branon et al., 2018). The Venus protein was modified to introduce a compatible cloning site via PCR using forward primer 5’- AAA AGG TAC CAT GGT GAG CAA GGG CGA GG −3’, reverse primer 5’- TTT TAG AAG GCA CAG TCG AGG CTG AT −3’, and template DNA pcDNA3.1-Vin-Venus (Grashoff et al., 2010). The TurboID construct was isolated from PCR product using 5’-EcoRI/ 3’-KpnI and the Venus construct was isolated from PCR product using 5’-KpnI/ 3’-NotI. Both constructs were inserted into pcDNA3.1-Vin-Venus (Grashoff et al., 2010) digested with 5’-EcoRI/ 3’-NotI, yielding pcDNA3.1-Vin-TurboID-Ven. Gibson cloning was used to generate the tension insensitive construct, pcDNA3.1-VinI997A-TurboID-Ven, with the forward primer 5’- GCA GCT CAA AGC TCT TTC CAC AGT GAA AGC TAC CAT GCT G −3’, reverse primer 5’- CTG TGG AAA GAG CTT TGA GCT GCG TGC TGA TGG TTG −3’, and template DNA pcDNA3.1-Vin-TurboID-Venus.
To facilitate stable lentiviral expression of Vin-TurboID-Ven, and VinI997A-TurboID-Ven, and VinCS, these constructs and the upstream CMV promoter were extracted from pcDNA3.1 using 5’-NruI/ 3’-XbaI. Afterwards constructs were ligated into pRRL vector, digested with 5’-EcoRV/3’-XbaI using standard cloning protocols.
Cell Seeding:
For all imaging and spreading assays, prior to cell seeding, 24-well glass bottom dishes (World Precision Instruments) were incubated with 10 μg/mL fibronectin (Thermo Fisher Scientific) diluted in PBS at 4 ºC overnight. Dishes were rinsed twice with PBS immediately prior to cell seeding. For imaging, cells were seeded in each well at a density of 20,000 cells per well, and then allowed to spread in cell culture media for 4 hours. For spreading assays, cells were allowed to spread in complete cell culture media for 15, 30, 60, and 120 minutes.
For all pulldown assays, cells were seeded in 100 mm tissue culture dishes (Sarstedt) and grown to 80% confluency before harvesting.
Cell Staining and Immunofluorescence:
Cells were fixed for 10 min with 4% EM grade, methanol-free paraformaldehyde (Electron Microscopy Sciences). Cells were then rinsed twice with PBS and subject to permeabilization in 0.1% Triton-X100 for 5 min at room temperature. After a subsequent round of washing, cells were blocked in 2% bovine serum albumin (Sigma-Aldrich) diluted in PBS for 1 hour and then labeled with primary antibody for 1 hour at room temperature per the dilutions listed (Table S3). Cells were rinsed twice in PBS and subjected to another 1 hour blocking step at room temperature. Secondary staining using either donkey anti-mouse AlexaFluor-647 (Life Technologies) or donkey anti-rabbit AlexaFluor-647 (Life Technologies) at a 1:500 dilution, depending on the species of the primary antibody, was carried out for 1 hour at room temperature before cells were rinsed twice and left immersed in PBS for imaging. Previous work has shown that this fixation processes maintains FRET signals in live MEFs expressing TSMod, VinTS, or VinTS I997A (Gates et al., 2019).
Cells were labeled for biotin using Streptavidin-AF647 (Thermo Fisher Scientific), diluted 1:500 in 2% bovine serum albumin for 1 hour. Cells were subsequently washed twice with PBS and left immersed in PBS for imaging.
Cells were labeled for actin using Phalloidin-AF647 (Thermo Fisher Scientific), diluted 1:100 in 2% bovine serum albumin for 1 hour. Cells were subsequently washed twice with PBS and left immersed in PBS for imaging.
Biotin and Inhibitor Treatment:
500 μM biotin, diluted in cell culture media, was added to the plated cell sample. Cells were biotin-labeled for 30 minutes in the cell incubator before being take out, transferred on to ice, and washed with cold PBS to stop the biotinylation reaction. Cells were harvested immediately afterwards. For cells that were treated with inhibitor, 10 μM Y-27632, diluted in 500 μM biotin-containing cell culture media, was added to cells and biotin-labeling was allowed to proceed for 30 minutes before cells were transferred to ice, washed with cold PBS, and subsequently harvested.
Biotinylated Protein Pulldown:
This protocol is adapted from a previously published protocol on proximity labeling using TurboID (Cho et al., 2020). Cells grown in 100 mm cell culture dishes to 80% confluency were harvested by first removing the biotin-containing cell culture media and then washing twice with cold PBS to remove residual biotin and media. Afterwards, cells are lysed with 100 μl of RIPA buffer (50 mM Tris, 150 mM NaCl, 0.1% (wt/vol) SDS, 0.5% (wt/vol) sodium deoxycholate, 1% (vol/vol) Triton X-100 in dH2O, adjust to pH of 7.5) supplemented with 1x protease inhibitor cocktail (Sigma) and 1 mM PMSF (ThermoFisher). Lysed cells are scraped from the cell culture dish and collected. The collected cell lysate is clarified by centrifugation at 13,000 g at 4 °C for 10 minutes. Protein concentration in clarified cell lysates were measured with a Pierce BCA Protein Assay Kit (ThermoFisher) following manufacture protocols. Equal amounts of clarified cell lysate were harvested for samples and stored at −80 °C for western blot running.
For each biotinylated protein pulldown sample, 25 μl of streptavidin agarose beads (Thermo Fisher) were taken and equilibrated by washing twice for 2 minutes at 1,000 g with 1 mL of RIPA lysis buffer, not supplemented with 1x protease inhibitor cocktail or 1x PMSF. Equal amounts of clarified cell lysate were added to the equilibrated streptavidin agarose beads and samples were rotated overnight at 4 °C.
On the next day, samples were spun at 1,000 g for 5 minutes to collect beads and remove supernatant. Afterwards, samples are washed twice for 2 minutes at 1,000 g with 1 mL of RIPA lysis buffer, once for 2 minutes at 1,000 g with 1 mL of 1 M KCl, once for 15 seconds at 1,000 g with 1 mL of 0.1 M Na2CO3, once for 15 seconds at 1,000 g with 1 mL of 2 M urea in 10 mM Tris-HCL (pH 8.0), and then twice for 2 minutes at 1,000 g with 1 mL of RIPA lysis buffer. Wash times were decreased for Na2CO3 and urea to prevent bead denaturation and aggregation.
After the final wash, all beads were pelleted by centrifugation at 13,000 g for 1 minute. To elute protein from beads, 30 μL of 2x protein loading buffer, supplemented with 2 mM biotin and 20 mM DTT, was added and then all samples were boiled at 95 °C for 10 minutes. Afterwards, beads were again pelleted via centrifugation at 13,000 g for 1 minute and eluted protein samples were collected.
Western Blots:
To harvest lysate for comparing candidate protein expression in VinTS- and VinTSI997A-expressing cells, cells were washed, lysed with lysis buffer [250 mM NaCl, 10% glycerol, 2mM EDTA, 0.5% IGEPAL (Sigma-Aldrich), 50 mM Hepes (Sigma-Aldrich)], and then centrifuged at 13,000 RPM for 5 minutes. Afterwards, 2x Laemmli sample buffer (Bio-Rad Laboratories) was added to the lysate for a 1:1 dilution and the sample was boiled at 95 °C for 10 minutes.
Protein samples from harvested cell lysates and biotin-streptavidin pulldowns were loaded onto a 4–20% gradient mini protean tgx pre-cast gel (Bio-Rad Laboratories) and ran at 100 V for 70 minutes before being transferred to a PVDF membrane (Bio-Rad Laboratories) via wet-transfer. Membranes were blocked with 3% BSA diluted in PBS for 1 hour and then incubated with primary antibodies per the dilution listed (Table S3) overnight at 4 °C. Afterwards, the membrane was rinsed 3 times in TBST and incubated with the appropriate enzyme conjugated secondary antibody (Life Technologies), depending on the animal species, for 1 hour at room temperature. Membranes were later developed using Supersignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). The resulting signal was detected on either x-ray film (Kodak) or using ChemiDoc Touch Imaging System (Bio-rad Laboratories).
Western blots for biotin-streptavidin pulldown assays were quantified on ImageJ (NIH) by first converting all images into 8-bit gray scale images. Afterwards, bands from lanes of interest are selected and the density (intensity) of each band is analyzed. Relative densities are taken by normalizing the density of different bands of interest, as denoted in the paper and corresponding figures.
FRET Imaging:
An Olympus IX83 inverted epifluorescent microscope (Olympus) equipped with a LambdaLS 300W ozone-free xenon bulb (Sutter Instruments), sCMOS ORCA-Flash4.0 V2 camera (Hamamatsu), motorized filter wheels (Sutter Instruments, 10–3), and automated stage (Prior Scientific, H117EIX3) were used to image samples. MetaMorph Advanced software (Olympus) was used to control the image acquisition. Unless otherwise indicated, all samples were imaged at 60X magnification (Olympus, UPlanSApo 60X/NA1.35 objective, 108nm/pix), with a three-image sensitized emission acquisition sequence(Chen et al., 2006). To image mTFP1-Venus sensors, the filter set used includes mTFP1 excitation (Chroma, ET450/30x), mTFP1 emission (Chroma, ET485/20m), Venus excitation (Chroma, ET514/10x), Venus emission (Semrock, FF01–571/72) filters, and a dichroic mirror (Chroma, T450/514rpc). Images were acquired across the acceptor channel (Venus excitation, Venus emission, 1000 ms exposure), FRET channel (mTFP1 excitation, Venus emission, 1500 ms exposure), and donor channel (mTFP1 excitation, mTFP1 emission, 1500 ms exposure). The filter set for imaging of co-localized proteins utilized Cy5 filters (Semrock, DA/FI/TR/Cy5–4X4 M-C Brightline Sedat) and the associated dichroic mirror (Semrock, FF410/504/582/669-Di01). Images of protein co-localization were acquired in the stain channel (Cy5 excitation, Cy5 emission, 1000 ms exposure).
Quantification and Statistical Analysis
Quantitative FRET Efficiency Measurements from Sensitized Emission:
FRET was detected through measurement of sensitized emission and calculated on a pixel-by-pixel basis using custom written code in MATLAB (Mathworks) (Chen et al., 2006; Rothenberg et al., 2018). Prior to these FRET calculations, all images were corrected for dark current, uneven illumination, registered, and background-subtracted. FRET-imaging of donor-only and acceptor-only samples (cells expressing either a single donor or acceptor FP) were used to calculate spectral bleed-through coefficients. Donor bleed-through coefficients (dbt) were calculated for mTFP1 as:
where is the FRET-channel intensity and is the donor-channel intensity. Data was binned by donor-channel intensity. Accordingly, acceptor bleed-through coefficients (abt) were calculated for Venus as:
where is the acceptor-channel intensity. In this case, data was binned by acceptor-channel intensity. To correct for spectral bleed-through in experimental data, pixel-by-pixel corrected FRET images were generated according to the equation:
Afterwards, FRET efficiency (E) calculations were made according to the following equation:
where is a constant describing the increase in acceptor intensity, due to sensitized emission, relative to the decrease in donor intensity, due to quenching (Chen et al., 2006). A separate metric, the donor per acceptor (DPA) concentration ratio that describes the relative concentrations of donor and acceptor FPs, is calculated as:
where , is a constant that describes the ratio of donor fluorescence intensity relative to acceptor fluorescence intensity under equimolar fluorophore concentrations, in the absence of FRET. Both constants were calculated for mTFP1-Venus biosensors through imaging donor-acceptor fusion constructs of differing, but constant, FRET efficiencies (Chen et al., 2006; Gates et al., 2019).
Image Segmentation, FA Analysis, Normalization Schemes, and Data Selection Criteria:
Segmentation and quantification of the characteristics of individual FAs were performed using custom-written code in MATLAB involving the water algorithm and blob analysis (Zamir et al., 1999). Single cells were identified based on closed boundaries, drawn by the user, using unmasked acceptor channel images.
As often occurs in large datasets, non-physical values FRET were rarely observed (Gates et al., 2019). The basis of these outliers can be statistical (e.g. due to non-physiological under expression of the sensor leading to insufficient intensity in the various channels to accurately measure FRET) (Krieg et al., 2014), technical (e.g. non-physiology overexpression leading to intermolecular FRET) (Gayrard and Borghi, 2016), or biological (e.g. cell to cell variation in the maturation of fluorescent proteins) (Hebisch et al., 2013). Important for later discussions, data filtering process are also very important when using machine learning procedures, as non-physical outliers can strongly bias the results (Chu et al., 2016). Therefore, to ensure the quality of FTC data, exclusion criteria were set. Specifically, a multi-scale filtering approach and normalization scheme was used. First, FRET images were evaluated on the whole cell level. Cells that were not fully spread, detected as containing less than 20 FAs, were discarded. Additionally, cells with average FRET efficiency outside of the range of 15–40% range and/or average DPA outside the range of 0.6–1.5, were discarded. FRET data was further refined at the FA level by discarding FAs with an average FRET efficiency outside the range of 5–50% and/or DPA outside the range of 0.6–1.5. Additionally, all FAs within a Euclidian distance of 7 μm from the geometric centroid of the cell were discarded to remove artifacts associated with apparent immunolabeling of proteins in the cell nucleus. Overall, this scheme resulted in the exclusion of approximately 5% of all cells and 15% of all FAs (2.5% were removed for misfunctioning tension sensor and 12.5% were removed for proximity to the nucleus) from the dataset.
To account for unavoidable day-to-day variability in immunofluorescent staining, the immunofluorescent signal of segmented FAs was normalized by the average immunofluorescent signal of all FAs in the individual cell in which they resided. To investigate the relationship between vinculin tension and the recruitment of a protein of interest (POI), quartile analysis was used. To identify criteria for high and low tension FAs, all FAs in the dataset were sorted by FRET efficiency and organized into quartiles. The bottom quartile FRET range was used to establish criteria for high tension FAs while the upper quartile FRET range was used to establish criteria for low tension FAs. To evaluate the amount of protein localized to the high and low tension FA populations within a given cell, i, the average stain intensity was calculated as:
where and denote the number of FAs that were classified as high and low tension, respectively and and are the stain intensities of FAs in either the high or low tension population, as labeled. For a given POI, preferential protein recruitment to high, versus low tension FAs, can be described using the tension recruitment index (TRI) metric:
where and denote the number of cells, for a given POI, that contained high and low tension FA populations, respectively.
To directly probe the role of vinculin loading in POI recruitment to FAs, a tension-insensitive sensor, VinTSI997A, was used in FTC and compared to VinTS. To relate changes in protein recruitment between different sensors, a Pearson correlation coefficient (PCC) value between the concentrations of the POI and either VinTS or VinTSI997A at FAs was calculated for each cell:
where and are the sensor intensities for a given FA, , and are the mean sensor intensities across all FAs in the cell, is the POI intensity for a given FA, , and is the POI intensity across all FAs in the cell. To validate tension-dependent interactions for different POIs, the difference in PCC between the VinTS and tension-insensitive VinTSI997A sensor was taken:
where and are the number of cells for a given POI.
In architecture-specific calculations, TRI and ΔPCC were calculated solely for FAs belonging to either the PR- or SF-associated FA class for all cells.
Analysis of Spatial Variation in Vinculin Tension:
To manually detect spatial variations in vinculin tension within individual FAs, line scans of single FAs were performed using ImageJ software (US National Institutes of Health) and JMP Pro software (Statistical Analytical Systems). A cubic spline-based smoothing algorithm was used for noise reduction of the acceptor intensity and FRET efficiency profiles.
Spatial variations in vinculin tension are detected by comparing the geometric centroid, which provides information on the physical location of the FA, to the FRET−1-weighted centroid, which is shifted towards areas of higher tension. The geometric cell centroid is calculated as x- and y-coordinates, and :
where and represent the x- and y-coordinates corresponding to the ith pixel location for all pixels within a given FA. The FRET−1-weighted centroid is calculated as x- and y-coordinates, and :
Where is the FRET index at the ith pixel location for all pixels within a given FA. To maintain a relationship with tension, the inverse of , was used. As FAs are randomly oriented and positioned within an imaging frame, we also calculate a cell centroid to use as a frame of reference. The cell centroid is calculated as:
for a cell of N FAs. A spatial variation index was calculated as:
where represents the FA major axis length. This normalization was used to account for differences in FA size. This index is defined such that more distally skewed (away from the cell center) vinculin tension profiles are represented as larger positive values.
Statistical Analysis:
All statistical analyses were performed using either MATLAB or JMP Pro software. Independent experiments performed on separate days were combined only after statistical tests were used to verify that there were no statistically detectable differences between data from the same experimental groups. To compare means and associated standard errors to either zero in calculations of TRI, or one other mean and associated standard errors in comparisons between PR- and SF-associated FAs, t-tests were performed. Additionally, Bonferroni corrections were used in calculations of TRI statistical significance to account for multiple comparisons. To compare statistical differences between multiple groups in spatial variation index assays, or against another construct, such as in the case of calculations for ΔPCC and comparisons between TurboID constructs, ANOVAs were used. If a statistical difference was detected, either Tukey’s HSD or Dunnett’s post-hoc testing was used for multiple comparisons to assess statistical significance.
Supplementary Material
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| VASP | Cell Signaling | Cat #: 3132S; RRID: AB_2213393 |
| VASP (pS157) | Abcam | Cat #: ab47268; RRID: AB_883418 |
| pY (phosphoTyrosine) | Cell Signaling | Cat #: 9411S; RRID: AB_331228 |
| LPP | Cell Signaling | Cat #: 3389S; RRID: AB_2242711 |
| Zyxin | Gift from Dr. Mary Beckerle, University of Utah School of Medicine | N/A |
| α-Actinin-4 | Abcam | Cat #: Ab108198; RRID: AB_10858236 |
| Vinculin | Sigma | Cat #: V9131; RRID: AB_477629 |
| Talin | Sigma | Cat #: T3287; RRID: AB_477572 |
| FHL2 | Sigma | Cat #: HPA006028; RRID: AB_1078858 |
| Hic5 | BD Biosciences | Cat #: 611165; RRID: AB_398703 |
| Paxillin | Thermo Fisher | Cat #: AHO0492; RRID: AB_2536312 |
| Paxillin (pY31) | Abcam | Cat #: ab32115; RRID: AB_777116 |
| Paxillin (pY118) | Cell Signaling | Cat #: 2541S; RRID: AB_2174466 |
| FAK | BD Biosciences | Cat #: 610088; RRID: AB_397495 |
| FAK (pY397) | Thermo Fisher | Cat #: 44625G; RRID: AB_2533702 |
| FAK (pY861) | Thermo Fisher | Cat #: 44626G; RRID: AB_2533703 |
| FAK (pY925) | Cell Signaling | Cat #: 3284S; RRID: AB_10831810 |
| Migfilin | Novus Biologicals | Cat #: NBP1-86665; RRID: AB_11018045 |
| PINCH1 | Abcam | Cat #: Ab108609; RRID: AB_10862774 |
| ILK | Millipore | Cat #: 04-1149; RRID: AB_11212224 |
| α-Parvin | Thermo Fisher | Cat #: PA5-17185; RRID: AB_10984094 |
| α5-integrin | Millipore | Cat #: AB1928; RRID: AB_11213121 |
| Donkey anti-mouse AlexaFluor-647 | Life Technologies | Cat #: A-31571; RRID: AB_162542 |
| Donkey anti-rabbit AlexaFluor-647 | Life Technologies | Cat #: A-31573; RRID: AB_2536183 |
| Bacterial and virus strains | ||
| NEB 5-alpha competent E. coli | New England Biolabs | C2987U |
| Chemicals, peptides, and recombinant proteins | ||
| Fibronectin | Thermo Fisher | Cat #: 33016015 |
| Phalloidin-AF647 | Thermo Fisher | Cat #: A22287 |
| Streptavidin-AF647 | Thermo Fisher | Cat #: S32357 |
| Biotin | Sigma Aldrich | Cat #: B4639 |
| Y-27362 | Sigma Aldrich | Cat #: Y-0503 |
| Lipofectamine 2000 | Thermo Fisher | Cat #: 11668019 |
| Streptavidin agarose beads | Thermo Fisher | Cat #: 20347 |
| EcoRI-HF | New England Biolabs | Cat #: R310S |
| KpnI-HF | New England Biolabs | Cat #: R3142S |
| NotI-HF | New England Biolabs | Cat #: R3189S |
| NruI-HF | New England Biolabs | Cat #: R3192S |
| XbaI | New England Biolabs | Cat #: R0145S |
| EcoRV-HF | New England Biolabs | Cat #: R3195S |
| Critical commercial assays | ||
| NucleoSpin Plasmid miniprep | Machery-Nagel | Cat #: 740588.250 |
| NucleoBond Xtra Midi Plus | Machery-Nagel | Cat #: 740412.5 |
| Zymoclean Gel DNA Recovery Kit | Genesee Scientific | Cat #: 11-300 |
| Deposited Data | ||
| Unprocessed images of blots | This paper | DOI: 10.17632/nkgwrcs89r.1 |
| Experimental models: Cell lines | ||
| Vinculin −/− Mouse embryonic fibroblasts | Gift from Dr. Ben Fabry and Dr. Wolfgang H. Goldmann, University of Erlangen-Nurnberg | N/A |
| Human embryonic kidney cells (HEK 293) | ATCC | Cat #: CRL-1573 |
| Oligonucleotides | ||
| Primer: TurboID Forward: AAAAGAATTCATGAAAGACAATACTGTGCCTCTGAAGCT | Integrated DNA Technologies | N/A |
| Primer: TurboID Reverse: TTTTGGTACCCTTTTCGGCAGACCGCAGACT | Integrated DNA Technologies | N/A |
| Primer: Venus FP Cloning Site Forward: AAAAGGTACCATGGTGAGCAAGGGCGAGG | Integrated DNA Technologies | N/A |
| Primer: Venus FP Cloning Site Reverse: TTTTAGAAGGCACAGTCGAGGCTGAT | Integrated DNA Technologies | N/A |
| Primer: Gibson Clone pcDNA3.1-VinI997A-TurboID-Ven Forward: GCAGCTCAAAGCTCTTTCCACAGTGAAAGCTACCATGCTG | Integrated DNA Technologies | N/A |
| Primer: Gibson Clone pcDNA3.1-VinI997A-TurboID-Ven Reverse: CTGTGGAAAGAGCTTTGAGCTGCGTGCTGATGGTTG | Integrated DNA Technologies | N/A |
| Recombinant DNA | ||
| pRRL-VinculinTS | Addgene | Cat #: 111830 |
| pRRL-VinculinTS-I997A | Addgene | Cat #: 111832 |
| pRRL-VinculinCS | This paper | N/A |
| pcDNA3.1-Vin-Venus | Addgene | Cat #: 27300 |
| pcDNA3-V5-TurboID-NES | Addgene | Cat #: 07169 |
| pRRL-Vin-TurboID-Ven | This paper | N/A |
| pRRL-VinI997A-TurboID-Ven | This paper | N/A |
| psPax2 | Addgene | Cat #: 12260 |
| pMD2.G | Addgene | Cat #: 12259 |
| Software and algorithms | ||
| Matlab | Mathworks | https://www.mathworks.com/products/matlab.html |
| JMP Pro | SAS Institute | https://www.jmp.com/en_us/home.html |
| MetaMorph Advanced | Molecular Devices | https://www.moleculardevices.com/products/cellular-imaging-systems/acquisition-and-analysis-software/metamorph-microscopy |
| ImageJ | US National Institute of Health | https://imagej.nih.gov/ij/ |
| Image Corrections | Original code developed by LaCroix et al. 20 | https://gitlab.oit.duke.edu/HoffmanLab-Public/image-preprocessing |
| FRET-Colocalization Analysis | This paper | N/A |
| FTC Markov Chain Model | This paper | N/A |
Highlights:
This work develops an approach termed Fluorescence-tension correlation (FTC).
FTC identifies recruitment of one protein due to the molecular tension on another.
Vinculin mediates constitutive and context-dependent tension-sensitive recruitment.
Acknowledgements:
We thank Dr. Mary Beckerle for providing the zyxin primary antibody. This research was supported by the Searle Foundation, National Science Foundation (CAREER 1454257 and GRFP DGE 1644868), Duke Science and Technology Spark Seed Award, and National Institute of Health (5R21EB022166, 1R01GM121739, and T32GM008555).
Inclusion and Diversity:
We support inclusive, diverse, and equitable conduct of research.
Footnotes
Declaration of Interests:
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.DuFort CC, Paszek MJ, and Weaver VM (2011). Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol 12, 308–319. 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engler AJ, Sen S, Sweeney HL, and Discher DE (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 3.Wolfenson H, Yang B, and Sheetz MP (2019). Steps in Mechanotransduction Pathways that Control Cell Morphology. Annual Review of Physiology 81, 585–605. 10.1146/annurev-physiol-021317-121245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elosegui-Artola A, Trepat X, and Roca-Cusachs P. (2018). Control of Mechanotransduction by Molecular Clutch Dynamics. Trends Cell Biol 28, 356–367. 10.1016/j.tcb.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman BD, Grashoff C, and Schwartz MA (2011). Dynamic molecular processes mediate cellular mechanotransduction. Nature 475, 316–323. 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Case LB, and Waterman CM (2015). Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat Cell Biol 17, 955–963. 10.1038/ncb3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CS (2008). Mechanotransduction - a field pulling together? J Cell Sci 121, 3285–3292. 10.1242/jcs.023507. [DOI] [PubMed] [Google Scholar]
- 8.Kuo JC, Han X, Hsiao CT, Yates JR 3rd, and Waterman CM (2011). Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol 13, 383–393. 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiller HB, Friedel CC, Boulegue C, and Fassler R. (2011). Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep 12, 259–266. 10.1038/embor.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carisey A, Tsang R, Greiner AM, Nijenhuis N, Heath N, Nazgiewicz A, Kemkemer R, Derby B, Spatz J, and Ballestrem C. (2013). Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr Biol 23, 271–281. 10.1016/j.cub.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, and Sheetz MP (2006). Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127, 1015–1026. 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaidel-Bar R, Ballestrem C, Kam Z, and Geiger B. (2003). Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci 116, 4605–4613. 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- 13.Sawada Y, and Sheetz MP (2002). Force transduction by Triton cytoskeletons. J Cell Biol 156, 609–615. 10.1083/jcb.200110068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galbraith CG, Yamada KM, and Sheetz MP (2002). The relationship between force and focal complex development. J Cell Biol 159, 695–705. 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, and Schwartz MA (2010). Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266. 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolte S, and Cordelières FP (2006). A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224, 213–232. 10.1111/j.1365-2818.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 17.Dunn KW, Kamocka MM, and McDonald JH (2011). A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol 300, C723–742. 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, and Dunn AR (2012). E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc Natl Acad Sci U S A 109, 12568–12573. 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothenberg KE, Scott DW, Christoforou N, and Hoffman BD (2018). Vinculin Force-Sensitive Dynamics at Focal Adhesions Enable Effective Directed Cell Migration. Biophys J 114, 1680–1694. 10.1016/j.bpj.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaCroix AS, Lynch AD, Berginski ME, and Hoffman BD (2018). Tunable molecular tension sensors reveal extension-based control of vinculin loading. Elife 7. 10.7554/eLife.33927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horton ER, Byron A, Askari JA, Ng DHJ, Millon-Fremillon A, Robertson J, Koper EJ, Paul NR, Warwood S, Knight D, et al. (2015). Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol 17, 1577–1587. 10.1038/ncb3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumbauld DW, Lee TT, Singh A, Scrimgeour J, Gersbach CA, Zamir EA, Fu J, Chen CS, Curtis JE, Craig SW, and Garcia AJ (2013). How vinculin regulates force transmission. Proc Natl Acad Sci U S A 110, 9788–9793. 10.1073/pnas.1216209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons JT, Horwitz AR, and Schwartz MA (2010). Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 11, 633–643. 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mierke CT, Kollmannsberger P, Zitterbart DP, Diez G, Koch TM, Marg S, Ziegler WH, Goldmann WH, and Fabry B. (2010). Vinculin facilitates cell invasion into three-dimensional collagen matrices. J Biol Chem 285, 13121–13130. 10.1074/jbc.M109.087171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gates EM, LaCroix AS, Rothenberg KE, and Hoffman BD (2019). Improving Quality, Reproducibility, and Usability of FRET-Based Tension Sensors. Cytometry A 95, 201–213. 10.1002/cyto.a.23688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, and Kam Z. (1999). Molecular diversity of cell-matrix adhesions. J Cell Sci 112 (Pt 11), 1655–1669. [DOI] [PubMed] [Google Scholar]
- 27.Burridge K, Turner CE, and Romer LH (1992). Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol 119, 893–903. 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner CE, Glenney JR Jr., and Burridge K. (1990). Paxillin: a new vinculin-binding protein present in focal adhesions. J Cell Biol 111, 1059–1068. 10.1083/jcb.111.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benz PM, Blume C, Seifert S, Wilhelm S, Waschke J, Schuh K, Gertler F, Münzel T, and Renné T. (2009). Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J Cell Sci 122, 3954–3965. 10.1242/jcs.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deakin NO, Ballestrem C, and Turner CE (2012). Paxillin and Hic-5 interaction with vinculin is differentially regulated by Rac1 and RhoA. PLoS One 7, e37990. 10.1371/journal.pone.0037990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petit MM, Meulemans SM, and Van de Ven WJ (2003). The focal adhesion and nuclear targeting capacity of the LIM-containing lipoma-preferred partner (LPP) protein. J Biol Chem 278, 2157–2168. 10.1074/jbc.M206106200. [DOI] [PubMed] [Google Scholar]
- 32.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, and Waterman CM (2010). Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol 188, 877–890. 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubashkin MG, Cassereau L, Bainer R, DuFort CC, Yui Y, Ou G, Paszek MJ, Davidson MW, Chen YY, and Weaver VM (2014). Force engages vinculin and promotes tumor progression by enhancing PI3K activation of phosphatidylinositol (3,4,5)-triphosphate. Cancer Res 74, 4597–4611. 10.1158/0008-5472.CAN-13-3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson PM, Tolbert CE, Shen K, Kota P, Palmer SM, Plevock KM, Orlova A, Galkin VE, Burridge K, Egelman EH, et al. (2014). Identification of an actin binding surface on vinculin that mediates mechanical cell and focal adhesion properties. Structure 22, 697–706. 10.1016/j.str.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thievessen I, Thompson PM, Berlemont S, Plevock KM, Plotnikov SV, Zemljic-Harpf A, Ross RS, Davidson MW, Danuser G, Campbell SL, and Waterman CM (2013). Vinculin-actin interaction couples actin retrograde flow to focal adhesions, but is dispensable for focal adhesion growth. J Cell Biol 202, 163–177. 10.1083/jcb.201303129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Case LB, Baird MA, Shtengel G, Campbell SL, Hess HF, Davidson MW, and Waterman CM (2015). Molecular mechanism of vinculin activation and nanoscale spatial organization in focal adhesions. Nat Cell Biol 17, 880–892. 10.1038/ncb3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wickstrom SA, Lange A, Montanez E, and Fassler R. (2010). The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J 29, 281–291. 10.1038/emboj.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Branon TC, Bosch JA, Sanchez AD, Udeshi ND, Svinkina T, Carr SA, Feldman JL, Perrimon N, and Ting AY (2018). Efficient proximity labeling in living cells and organisms with TurboID. Nat Biotechnol 36, 880–887. 10.1038/nbt.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim DI, and Roux KJ (2016). Filling the Void: Proximity-Based Labeling of Proteins in Living Cells. Trends Cell Biol 26, 804–817. 10.1016/j.tcb.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, et al. (2019). STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47, D607–d613. 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massou S, Nunes Vicente F, Wetzel F, Mehidi A, Strehle D, Leduc C, Voituriez R, Rossier O, Nassoy P, and Giannone G. (2020). Cell stretching is amplified by active actin remodelling to deform and recruit proteins in mechanosensitive structures. Nat Cell Biol 22, 1011–1023. 10.1038/s41556-020-0548-2. [DOI] [PubMed] [Google Scholar]
- 42.Chen H, Cohen DM, Choudhury DM, Kioka N, and Craig SW (2005). Spatial distribution and functional significance of activated vinculin in living cells. J Cell Biol 169, 459–470. 10.1083/jcb.200410100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elosegui-Artola A, Oria R, Chen Y, Kosmalska A, Perez-Gonzalez C, Castro N, Zhu C, Trepat X, and Roca-Cusachs P. (2016). Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat Cell Biol 18, 540–548. 10.1038/ncb3336. [DOI] [PubMed] [Google Scholar]
- 44.Schiller HB, Hermann MR, Polleux J, Vignaud T, Zanivan S, Friedel CC, Sun Z, Raducanu A, Gottschalk KE, Thery M, et al. (2013). beta1- and alphav-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat Cell Biol 15, 625–636. 10.1038/ncb2747. [DOI] [PubMed] [Google Scholar]
- 45.Driscoll TP, Ahn SJ, Huang B, Kumar A, and Schwartz MA (2020). Actin flow-dependent and -independent force transmission through integrins. Proc Natl Acad Sci U S A 117, 32413–32422. 10.1073/pnas.2010292117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, and Chen CS (2003). Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci U S A 100, 1484–1489. 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothenberg KE, Neibart SS, LaCroix AS, and Hoffman BD (2015). Controlling Cell Geometry Affects the Spatial Distribution of Load Across Vinculin. Cellular and Molecular Bioengineering 8, 364–382. 10.1007/s12195-015-0404-9. [DOI] [Google Scholar]
- 48.Winkelman JD, Anderson CA, Suarez C, Kovar DR, and Gardel ML (2020). Evolutionarily diverse LIM domain-containing proteins bind stressed actin filaments through a conserved mechanism. Proc Natl Acad Sci U S A 117, 25532–25542. 10.1073/pnas.2004656117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freikamp A, Mehlich A, Klingner C, and Grashoff C. (2017). Investigating piconewton forces in cells by FRET-based molecular force microscopy. J Struct Biol 197, 37–42. 10.1016/j.jsb.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Ham TR, Collins KL, and Hoffman BD (2019). Molecular Tension Sensors: Moving Beyond Force. Curr Opin Biomed Eng 12, 83–94. 10.1016/j.cobme.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact, Brenton D. Hoffman, upon reasonable request.
All original code has been deposited at, https://gitlab.oit.duke.edu/HoffmanLab-Public, and is publicly available as of the date of publication.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request.