Abstract
Background
Bacillus cereus infections in immunocompetent patients are uncommon and mainly observed in fragile patients. It can cause lethal infections with multiple organ dysfunction syndrome (MODS). However, a patient presenting as venous sinus thrombosis and survival without sequela has not been reported.
Case presentation
A 20-year-old previously healthy male developed gastroenteritis after a meal, followed by fever, convulsions, and severe disturbance of consciousness. The patient had significant leukocytosis with a mildly elevated D-dimer, creatinine level, and respiratory failure. The CT(computed tomography) revealed fatal brain edema and subarachnoid hemorrhage. Previous blood culture in a local hospital revealed B. cereus, which was confirmed by mNGS(metagenomic next-generation sequencing) using blood and urine in our hospital. Accordingly, B. cereus sepsis with MODS were considered. Later, cerebral venous sinus thrombosis was proved. After anti-infection (linezolid 0.6 g, Q12h; and meropenem 1.0 g, Q8h), anti-coagulant (enoxaparin 6000U, Q12h), and other symptomatic treatments, the patient recovered completely without sequela at the 6-month follow-up.
Conclusions
This case suggests that in immunocompetent adults, there is still a risk of infection with B. cereus, causing severe MODS. Special attention should be paid to venous sinus thrombosis and subarachnoid hemorrhage in such cases, while, anti-coagulant is essential therapy.
Keywords: Bacillus cereus, Sepsis, Venous sinus thrombosis
Background
Bacillus cereus (B. cereus) is a common gram-positive, aerobic and facultative anaerobic bacillus widely distributed in soil, water, air and intestine [1]. B. cereus is usually non-pathogenic and considered as a contaminating bacteria in clinical practices, but it can also cause food poisoning with self-limiting symptoms, such as vomiting and diarrhea. In immunosuppressed people, severe infectious symptoms by B. cereus might occur [2]. Several studies have reported that the bacterium can cause severe bacteremia, leading to multiple organ failure, especially in patients with hematological malignancies. The delay in treatment may result in an even poor prognosis [3, 4]. Here, we report a previously healthy young patient with B. cereus sepsis, complicated by central nervous system involvement, multiple organ dysfunction, and venous sinus thrombosis, in addition, a patient presenting as venous sinus thrombosis and survival without sequela has not been reported. A relevant literature review on B. cereus infection in previously healthy individuals has also been provided.
Case presentation
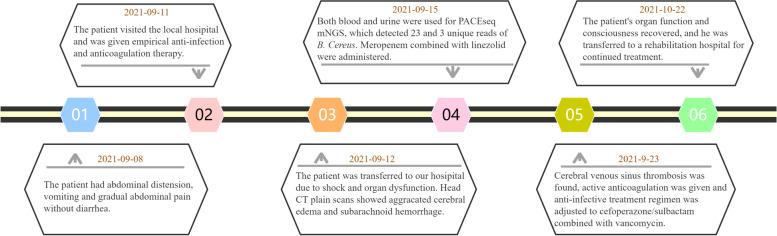
A 20-year-old male college student visited the local hospital due to fever, disturbance of consciousness, and convulsion. He had abdominal distension, vomiting, and gradual abdominal pain without diarrhea after meal three days before. The patient had no meningeal irritation and no history of neurological disorder in his family. Physical examination on admission showed body temperature of 40.2 °C, blood pressure of 90/50 mmHg, and heart rate of 160 bpm. The patient developed a coma and abdominal distension of no reason. He became hypotensive and was commenced on norepinephrine treatment (Maximum dose reached 0.72ug/kg/min). Hematological examination revealed raised white blood cell (WBC) of 30.89 × 109/L and D-dimer level of 13,484 ng/ml (normal value 0–243 ng/ml), slightly decreased blood platelet (PLT) of 75 × 109/L. Coagulation tests suggested abnormal coagulation profile with prothrombin time (PT) of 14.5S (normal value 11.5–14.8S), activated partial thromboplastin time (APTT) of 35.8S (normal value 25.1–36.5S), thrombin time (TT) of 36.5 S (normal value 14-21S), fibrinogen (FIB) of 1.64 g/L (normal value 2.38–4.98 g/L), and fibrin degradation products (FDP) of 71.2ug/ml (normal value 0–2.01ug/ml). There were apparent liver and kidney dysfunction with alanine aminotransferase (ALT) of 131.6 U/L, aspartate aminotransferase (AST) of 111.3 U/L, total bilirubin (TBIL) of 33.8 μmol/L, creatinine of 125 μmol/L, and urea of 8.2 mmol/L. Laboratory investigations showed elevated level of C-reactive protein (CRP 221.85 mg/L) and procalcitonin (PCT 1.76 μg/L). But normal range of serum amylase and lipase, and the results of immunoglobulin lymphocyte subsets and connective tissue diseases are normal. CSF (cerebrospinal fluid) examination showed pale bloody CSF, red blood cells of 12,000 × 106/L, white blood cell of 100 × 106/L, with a pressure of 230cmH2O. Head CT performed at local hospital showed cerebral edema and subarachnoid hemorrhage (Fig. 1A and B). No abnormality was found in digital subtraction angiography (DSA) of skull. Purulent urine was drained from the indwelling urinary catheter. The patient was initially diagnosed with subarachnoid hemorrhage, sepsis, and septic shock. Therefore, meropenem (1.0 g, Q8h) and datomycin (0.5 g per day) were given for anti-infection, and anticoagulant therapy (enoxaparin 6000U, Q12h) was given, blood culture was also performed before antibiotics are used.
Fig. 1.
Radiographic findings of the patient. A-B showed cerebral edema and subarachnoid hemorrhage by cranial CT in local hospital; C-D showed local edema and thickening of the ascending colon wall with peritoneal thickening by abdominal CT plain scans in our hospital; E–F showed aggravated cerebral edema and subarachnoid hemorrhage by CT One day later; G-H showed significantly aggravated cerebral edema and multiple subarachnoid hemorrhages by CT After 12 days; I showed fine contrast in the left transverse sinus, sigmoid sinus and the opposite side of the internal jugular vein, and less uniform local density of transverse sinusAfter 12 days; J ~ K showed alleviated cerebral edema and subarachnoid hemorrhage after 2 weeks
One day later, the patient was transferred to our hospital due to shock and multiple organ dysfunction. Abdominal CTA (computed tomography arteriography) and CTV (computed tomography venography) revealed no abnormalities, but local edema and thickening of the ascending colon wall with peritoneal thickening were found by abdominal CT plain scans (Fig. 1C and D). Head CT plain scans showed aggravated cerebral edema and subarachnoid hemorrhage (Fig. 1E and F). Lumbar puncture was not performed due to severe cerebral edema and high intracranial pressure. Four days later, both blood and urine were used for PACE seq mNGS (pathogen capture engine mutagenic next-generation sequencing), which detected 23 and 3 unique reads of B. cereus, respectively (Fig. 2A and B). Six days later, the blood culture results at the local hospital were reported as Bacillus cereus, and the results of drug sensitivity report that Cefazolin, cefoperazone sulbactam, levofloxacin, vancomycin and linezolid are effective.
Fig. 2.
mNGS results of the patient. A. The DNA reads positions of B. cereus and the content of detected microbes by blood mNGS. The coverage of B. cereus detected by blood mNGS was 0.0361%. B. The DNA reads positions of B. cereus and the content of detected microbes by urine mNGS. The coverage of B. cereus detected by urine mNGS were 0.0081%
Accordingly, the patient was diagnosed with B. cereus sepsis and sepsis-associated encephalopathy, complicated with multiple organ dysfunction, including gastroenteritis, acute kidney injury, urinary tract infection, and acute respiratory distress syndrome. Anticoagulation was promptly discontinued in view of the patient's increased subarachnoid hemorrhage. Sufficient meropenem (1.0 g, Q8h) combined with linezolid (0.6 g, Q12h) was administered for anti-infection. Adjuvant treatments, such as sedative and analgesic anti-sympathetic therapy, body temperature management, cerebral protection, assisted ventilation, hemoperfusion to remove inflammatory mediators, and volume management were also given. The patient's vital signs were gradually stabilized, and the laboratory test indicators showed improvement after a week. The patient had gastrointestinal bleeding during the treatment of patients, and was given symptomatic hemostatic treatment.
After 12 days, the patient developed increased heart rate (> 100 beats/min) and fever (39.2 °C). Reexamination of head CT showed significantly aggravated cerebral edema and multiple subarachnoid hemorrhages (Fig. 1G and H). Head CTA showed bilateral anterior, middle and posterior cerebral arteries with uneven thickness, multiple luminal narrowing, and dilatation changes, suggesting local cerebrovascular inflammation. CTV showed fine contrast in the left transverse sinus, sigmoid sinus and the opposite side of the internal jugular vein, and less uniform local density of transverse sinus (Fig. 1I). Venous sinus thrombosis was considered. Active adequate anticoagulation therapy was given, and because meropenem and linezolid had been anti-infective treatment for 2 weeks, considering the side effects of the drugs, the anti-infective treatment regimen was adjusted to cefoperazone/sulbactam (3.0 g, Q8h) combined with vancomycin (1.0 g, Q12h). Repeated head CT showed alleviated cerebral edema and subarachnoid hemorrhage after 2 weeks. (Fig. 1J and K).
After forty days, the patient's organ function and consciousness recovered, but he was in a minimally conscious state, and his limbs could move spontaneously and still could not communicate. He was transferred to a rehabilitation hospital for continued treatment. During this period, lumbar puncture examination was performed intermittently, and no abnormality was found. After 2 months, the patient was able to communicate and take care of himself, but his cognitive function remained poor. After 4 months, his cognitive function gradually recovered. Six months later, he was able to return to school and continued his education. Clinical course timeline is described in Fig. 3.
Fig. 3.
Timeline for the reported case
Discussion
B. cereus is widely distributed in various environments, such as contaminated meat, rice, and pasta of the living environment. Intravascular catheters, open wounds and contaminated sheets are the major medical environmental sources of B. cereus [5]. B. cereus is often responsible for self-limiting food poisoning, mainly characterized by diarrhea and vomiting, usually causing transient gastroenteritis in immunocompetent patients [6]. For immunosuppressed patients, B. cereus can cause not only gastrointestinal diseases, but also other infectious diseases such as fulminant bacteremia, central nervous system infections [7], endophthalmitis [8], osteomyelitis [9], urinary tract infections [10], skin infections [11], endocarditis [12], and pneumonia [13]. The rapid and fulminant progress of B. cereus caused illnesses seriously threatens the patients' life [14]. Currently, patients with hematological disorders, who received intravascular catheters, have become the high risk population for fulminant bloodstream and even blood-borne disseminated central nervous system infections caused by B. cereus [2, 15]. Severe illnesses with or without gastroenteritis caused by B. cereus in previously healthy individuals are uncommon. We reviewed the reported cases with B. cereus infections, who were previously healthy (Table 1), and found there were only five patients with bloodstream infections. In this case, the patient was previously healthy, who presented with discomfort after eating rice, severe B. cereus sepsis, accompanied by impaired consciousness and multiple organ dysfunction was considered.
Table 1.
Summary of cases of B. Cereus reported in previously healthy individuals
| No | Year | Sex | Age | Profession | Basic disease | Symptoms on presentation | Diagnosis | Pathogen detection method | Treatment (antibiotics) | Outcome | Reference (PMID) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1985 | M | 67 | Engineer | cardiac failure | Retrosternal and right pleural pain | Pleuropulmonary infection | Pleural fluid cultures | GEN, CFZ, ERY | Death | 3,936,280 |
| 2 | 1985 | M | 18 | Student | No | Fever, cough, hemoptysis | Pneumonia | Sputum and blood cultures | CLDM, ERY, AMP | Survival | 3,919,626 |
| 3 | 1991 | M | 21 | Unknown | Bronchiectasia | Fever, cough, chills | Pneumonia | Sputum and BALF cultures | CPFX | Survival | 1,902,995 |
| 4 | 1997 | M | 17 | Student | No | Vomiting, lethargy, jaundice, abdominal pain | Fulminant hepatic failure, rhabdomyolysis, and acute renal failure | Intestinal contents and pan residue cultures | Untreated by antibiotics | Death | 9,099,658 |
| 5 | 1997 | M | 46 | Welder | No | Fever, cough, hemoptysis, vomiting | Bacteremia Pneumonia | Sputum and blood cultures | CPFX, CTX | Death | 9,003,628 |
| 6 | 1997 | M | 41 | Welder | No | Fever, cough, hemoptysis | Bacteremia Pneumonia | Sputum and blood cultures | AMP/SUL, ERY, CLDM, VAN | Death | 9,003,628 |
| 7 | 2002 | M | 29 | Unknown | No | Left leg erythema papules, ulceration | Skin soft-tissue infection | Tissue cultures | CPFX | Recovery | 12140490 [11] |
| 8 | 2003 | M | 76 | Unknown | Abdominal operation | Fever and shock occurred 17 days after surgery | BSI | Blood culture and central venous tip cultures | PIP/TZP | Recovery | 14,616,690 |
| 9 | 2008 | F | 69 | Unknown | Diabetes, hypertension | Difficulty breathing, sweating, weight loss | Endocarditis | Blood cultures | CFZ | Recovery | 18706120 [16] |
| 10 | 2008 | F | 9 | Student | No | Abdominal pain vomiting diarrhea, convulsions, coma, shock | Gastroenteritis, liver failure | Food cultures | MEM, CLDM | Recovery | 18,664,929 |
| 11 | 2011 | M | 20 | Student | No | Headache, nausea, vomiting, abdominal pain, diarrhea | Gastroenteritis, liver necrosis, colon necrosis | Faeces cultures | Untreated | Death | 22,012,017 |
| 12 | 2010 | M | 11 | Student | No | Abdominal cramps, vomiting, lethargy, convulsions | Gastroenteritis, liver failure, encephalopathy | Gastric juice, leftover race and faeces cultures | Untreated by antibiotics | Recovery | 19796886 [17] |
| 13 | 2011 | M | 74 | Unknown | Valvular history, coronary stent history | Fever, dyspnea, night sweats, shock | BSI | Blood and bone marrow cultures | VAN, CPFX | Death | 22173364 [9] |
| 14 | 2016 | M | 59 | Mechanic | No | Fatigue, nausea, indigestion, weight loss | Liver abscess | Puncture drainage fluid cultures | VAN, LEV | Recovery | 28025629 [18] |
| 15 | 2017 | F | 81 | Unknown | Atrial fibrillation, Cardiac failure | Fever, cough, respiratory distress | Pneumonia, BSI | Blood sample cultures | IPM, LEV | Recovery | 29457069 [19] |
| 16 | 2019 | F | 32 | Unknown | Abdominal trauma | Fever 48 h after surgery | Liver abscess, BSI | Drainage fluid and blood cultures | AMP/SUL, VAN | Recovery | 31,791,818 |
| 17 | 2019 | M | 60 | Unknown | Hypertension | Hemoptysis, respiratory distress, chest pain, right shoulder and right abdominal pain, numbness in the right hand | Necrotic hemorrhagic pneumonia | Sputum cultures | Untreated by antibiotics | Death | 30813918 [13] |
| 18 | 2019 | M | 91 | Unknown | End-stage renal disease | High fever, shortness of breath, shock, ARDS | Aortic aneurysm | Blood cultures | VAN, CTRX, | Recovery | 31,711,418 |
| 19 | 2020 | M | 58 | School principal | Diabetes | Intermittent low fever, painless hematuria, oliguria, weight loss, focal epilepsy | Infective endocarditis, cerebral hemorrhage, splenic abscess | Identified by BD Phoenix | CPFX | Recovery | 32655954 [12] |
| 20 | 2020 | F | 40 | Unknown | No | Vomiting, abdominal pain, syncope | Sepsis, multi-organ failure, shock, metabolic acidosis, rhabdomyolysis, coagulopathy, | Mass spectrometry (Bruker) | PIP/TZP, MNZ, AZI, VAN, MEM | Death | 33462030 [20] |
| 21 | 2021 | M | 25 | Chef | No | Nausea, vomiting, diarrhea, abdominal pain | Gastroenteritis, liver failure | Leftover rice cultures | PIP/TZP | Recovery | 35050989 [21] |
| 22 | 2022 | F | 27 | Unknown | No | Fever Abdominal pain vomiting diarrhea | Malaria, Gastroenteritis | Blood cultures | VAN/levofloxacin | Recovery | 36,712,755 |
| 23 | 2022 | No | 80 | Unknown | pituitary tumor | Abdominal pain vomiting diarrhea | meningoencephalitis | Blood cultures, broad-range 16 s rDNA PCR | VAN | Recovery | 35,880,229 |
| 24 | 2022 | F | 33 | Unknown | No | small vesicles, itching | cutaneous infection | broad-range 16 s rDNA PCR | CPFX | Recovery | 35,448,975 |
| 25 | 2023 | F | 40 | Unknown | type 1 bipolar disorder, major depressive disorder (MDD), migraines without aura, hypothyroidism, | severe headache | meningoencephalitis | Blood cultures, cerebrospinal fluid cultures | VAN | Survival | 36,788,826 |
AMP Ampicillin, AZI Azithromycin, AMP&SUL Ampicillin/sulbactam, CFZ Cefazolin, CTX Cefotaxime, CTRX Ceftriaxone, CLDM Clindamycin, CPFX Ciprofloxacin, ERY Erythromycin, GEN Gentamicin, IPM Imipenem, LEV Levofloxacin, MEM Meropenem, MNZ Metronidazole, PIP/TZP Piperacillin/tazobactam, VAN Vancomycin
The pathogenicity and virulence of B. cereus are associated with toxins and enzymes production by this pathogen, including hemolytic, non-hemolytic enterotoxins, emetic toxins, and some enzymes that cause tissue necrosis [22]. The emetic toxin with a lipophilic ring structure belongs to mitochondrial toxins [23] and can resist heating and proteolysis, which can destroy mitochondrial function leading to impaired mitochondrial β oxidation process and then cause cell death and promote multiple organ dysfunction. A case of a chef with B. cereus infection caused by eating rice was recently reported, in which large amounts of B. cereus were detected in his leftover, and the non-ribosomal peptide synthase gene (ces) and the non-hemolytic enterotoxin gene (nhe) of the emetic toxin were detected. The patient's clinical presentation from initial vomiting and diarrhea to acute liver failure and renal tubular necrosis [21]. In addition, enzymes that promote tissue necrosis can also cause liver abscesses [15]. It is reported that the liver may become another target organ for B. cereus infection [18]. Fat droplet deposition is seen in liver biopsies of B. cereus-associated liver dysfunction, causing liver failure in severe cases [17, 21, 24]. In 1997, a 17-year-old boy was reported to have vomiting, diarrhea, and rapid development of liver failure and rhabdomyolysis after eating overnight spaghetti leftovers. Autopsy showed diffuse microbubble steatosis and intermediate zone necrosis in the liver [23]. Our patient presented with elevated liver enzymes and bilirubin after onset, and abdominal ultrasound and CT revealed diffuse abnormalities in the liver.
Emetic toxin of B. cereus can cross the blood–brain barrier to attack the central nervous system in immunocompetent people [20, 25], causing subarachnoid hemorrhage, parenchymal hemorrhage, brain abscess, cerebral edema, and disturbance of consciousness [15, 17]. Patients with hematological diseases combined with central nervous system involvement have a very poor prognosis for B. cereus infection [26]. For example, B. cereus has been reported to cause bacteremia in a patient with a history of neutropenic acute myeloid leukemia following chemotherapy, resulting in poor prognosis, including multiple parenchymal hemorrhages and subarachnoid hemorrhages in the brain, as well as diffuse intraparenchymal and subcortical liquefaction necrosis [27]. In 1995, a 26-year-old man with leukemia died after infection with B. cereus. Cellulose in the meningeal vessels and B. cereus in the meningeal vessels and intracranial necrotic indicated that B. cereus might trigger thrombosis [15]. This patient we reported was previously healthy and had no underlying diseases, such as hematological diseases. Early head CT showed cerebral edema and subarachnoid hemorrhage, but cranial DSA showed no vascular abnormalities. Anticoagulant therapy had been administered for suspected thrombotic disease due to the high level of D-dimer, which was later discontinued due to an expanding extent of subarachnoid hemorrhage. Later, head CTA and CTV examination revealed that there was indeed thrombus in the venous sinus. To our knowledge, this is the first case report of venous sinus thrombosis caused by community-acquired B. cereus infection in healthy adults with good outcomes.
Urinary tract infections caused by B. cereus have rarely been reported (only one case with invasive bladder cancer who underwent radical cystectomy with percutaneous left ureterostomy followed by irrigation of the ureter with saline) [10]. Our patient presented with pyuria drained from the urinary catheter after admission. Urine mNGS revealed B. cereus, consistent with blood mNGS detection, suggesting hematogenous systemic dissemination of bacteria from gastrointestinal infections. However, the patient's coagulation function did not deteriorate significantly, and the platelet count remained at 75 × 109/L. The drained ascites was pale bloody, considering that B. cereus might cause systemic vascular injury, with multiple serous luminal hemorrhage, further observation was required.
B. cereus was highly susceptible to meropenem, linezolid and vancomycin [26, 27]. There are also reports of susceptibility of B. cereus to cefazolin [16], imipenem, levofloxacin [19], and ciprofloxacin [9]. The patient of this report was very young and had no underlying disease, with regular lifestyle. Systemic diseases rather than infection were considered due to the rapid progress and multiple organ dysfunction development. Fortunately, mNGS of blood and urine and blood culture detected the pathogen as B. cereus. Meropenem in combination with linezolid were subsequently given against B. cereus, which was later adjusted to cefoperazone/sulbactam in combination with vancomycin, resulting in good clinical outcome and normal final organ function as well as good recovery of consciousness. In addition, anticoagulation regimen against the definite venous sinus thrombosis of the patient also obtained good clinical effect. No similar treatment (anticoagulation regimen) has been reported at present, and this treatment may provide some reference for other cases.
This patient has severe sepsis with multiple organ dysfunction, it carries a risk for mortality, considerably exceeding that of a mere infection. Therefore, we actively give broad-spectrum antibiotics to fight infection and conduct bedside hemoperfusion treatment. There have been many literature reports on the clearance of inflammatory factors by hemoperfusion, and good results have been achieved[28, 29].
Conclusions
In conclusion, we report a previously healthy, immunocompetent young male patient with community-acquired sepsis and sepsis-associated encephalopathy caused by B. cereus. Rapid and accurate pathogenic diagnosis by mNGS provides a significantly important role for the rapid diagnosis and timely adjustment of antibiotic treatment, saving the patient’s life. Appropriate anticoagulant therapy may help improve the conditions of patients with venous embolism caused by B. cereus. In addition, clinicians should raise vigilance for foodborne infections, fully recognize fulminant diseases caused by B. cereus.
Acknowledgements
None to declare.
Abbreviations
- B.cereus
Bacillus cereus
- MODS
Multiple organ dysfunction syndrome
- mNGS
Metagenomic next-generation sequencing
- CT
Computed tomography
- CSF
Cerebrospinal fluid
- CTA
Computed tomography arteriography
- CTV
Computed tomography venography
- DNA
Deoxyribo nucleic acid
- PACEseq mNGS
Pathogen capture engine metagenomic next-generation sequencing
- DSA
Digital subtraction angiography
Authors’ contributions
Zr W and JC wrote this paper. Zr W, JC, HL, JZ and Ff F contributed to diagnosis and clinical decision for treatment. HX contributed in giving advice from mNGS. All authors reviewed the manuscript. All Authors read and approved the final version of this manuscript.
Funding
This study was supported by Shanxi Provincial Natural Science Foundation (NO.201801D121332), Shanxi Provincial Scholarship Council Foundation (NO.20220039).JC wrote this paper and contributed to diagnosis and clinical decision for treatment.
Availability of data and materials
The authors are ready to provide the data in response to the need.
Declarations
Ethics approval and consent to participate
Informed consent was obtained from the patient after verbal and written information provision. All the procedures have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent for publication
We have confirmed that the patient and his family gave written consent for his personal or clinical details along with any identifying images to be published in this study.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23:382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inoue D, Nagai Y, Mori M, Nagano S, Takiuchi Y, Arima H, et al. Fulminant sepsis caused by Bacillus cereus in patients with hematologic malignancies: analysis of its prognosis and risk factors. Leuk Lymphoma. 2010;51:860e9. doi: 10.3109/10428191003713976. [DOI] [PubMed] [Google Scholar]
- 3.Sunisha Arora, Dhwanee Thakkar, K Upasana, Anjali Yada, Neha Rastogi, Satya Prakash Yadav. Bacillus cereus infection in pediatric oncology patients: A case report and review of literature. IDCases. 2021 Oct 5;26: e01302. doi: 10.1016/j.idcr.2021. e01302 [DOI] [PMC free article] [PubMed]
- 4.Chou Y-L, Cheng S-N, Hsieh K-H, Wang C-C, Chen S-J, Lo W-T. Bacillus cereus septicemia in a patient with acute lymphoblastic leukemia: A case report and review of the literature. J Microbiol Immunol Infect. 2016;49(3):448–451. doi: 10.1016/j.jmii.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Sasahara T, Hayashi S, Morisawa Y, Sakihama T, Yoshimura A, Hirai Y. Bacillus Cereus bacteremia outbreak due to contaminated hospital linens. Eur J Clin Microbiol Infect Dis. 2011;30(2):219–226. doi: 10.1007/s10096-010-1072-2. [DOI] [PubMed] [Google Scholar]
- 6.Edward Bottone J. Bacillus Cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23(2):382–98. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drobniewski FA. Bacillus cereus and related species. Clin Microbiol Rev. 1993;6(4):324–338. doi: 10.1128/CMR.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Fang, Alvin Kwok KH, Bernard Cheung MY. The efficacy of intravitreal vancomycin and dexamethasone in the treatment of experimental bacillus cereus endophthalmitis. Curr Eye Res. 2008;33(9):761–8. doi: 10.1080/02713680802344690. [DOI] [PubMed] [Google Scholar]
- 9.Lede I, Vlaar A, Roosendaal R, Geerlings S, Spanjaard L. Fatal outcome of Bacillus cereus septicaemia. Neth J Med. 2011;69(11):514–516. [PubMed] [Google Scholar]
- 10.Sato K, Ichiyama S, Ohmura M, Takashi M, Agata N, Ohta M, Nakashima N. A case of urinary tract infection caused by Bacillus cereus. J Infect. 1998;36(2):247–248. doi: 10.1016/s0163-4453(98)80032-7. [DOI] [PubMed] [Google Scholar]
- 11.Boulinguez S, Viraben R. Cutaneous Bacillus cereus infection in an immunocompetent patient. J Am Acad Dermatol. 2002;47(2):324–325. doi: 10.1067/mjd.2002.121349. [DOI] [PubMed] [Google Scholar]
- 12.Nallarajah J, Mujahieth MI. Bacillus Cereus Subacute Native Valve Infective Endocarditis and Its Multiple Complications. Case Rep Cardiol. 2020;2020:8826956. doi: 10.1155/2020/8826956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishida R, Ueda K, Kitano T. Fatal community-acquired Bacillus cereus pneumonia in an immunocompetent adult man: a case report. BMC Infect Dis. 2019;19(1):197. doi: 10.1186/s12879-019-3836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaur AH, Patrick CC, McCullers JA. Bacillus cereus bacteremia and meningitis in immunocompromised children. Clin Infect Dis. 2001;32(10):1456–1462. doi: 10.1086/320154. [DOI] [PubMed] [Google Scholar]
- 15.Marley EF, Saini NK, Venkatraman C, Orenstein JM. Fatal Bacillus cereus meningoencephalitis in an adult with acute myelogenous leukemia. South Med J. 1995;88(9):969–972. doi: 10.1097/00007611-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Abusin S, Bhimaraj A, Khadra S. Bacillus Cereus Endocarditis in a permanent pacemaker: a case report. Cases J. 2008;1(1):95. doi: 10.1186/1757-1626-1-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichikawa K, Gakumazawa M, Inaba A, Shiga K, Takeshita S, Mori M, Kikuchi N. Acute encephalopathy of Bacillus cereus mimicking Reye syndrome. Brain Dev. 2010;32(8):688–690. doi: 10.1016/j.braindev.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Alessandri-Bonetti M, Vespasiani-Gentilucci U, Luppi G. The liver as another possible target organ for Bacillus Cereus infection. Case Rep Infect Dis. 2016;2016:7438972. doi: 10.1155/2016/7438972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimoyama Y, Umegaki O, Ooi Y. Bacillus cereus pneumonia in an immunocompetent patient: a case report. JA Clin Rep. 2017;3(1):25. doi: 10.1186/s40981-017-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colaco CMG, Basile K, Draper J, Ferguson PE. Fulminant Bacillus cereus food poisoning with fatal multi-organ failure. BMJ Case Rep. 2021;14(1):e238716. doi: 10.1136/bcr-2020-238716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schreiber N, Hackl G, Reisinger AC. Acute liver failure after ingestion of fried rice balls: a case series of bacillus cereus food poisonings. Toxins (Basel) 2021;14(1):12. doi: 10.3390/toxins14010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.StenforsArnesen LP, Fagerlund A, Granum PE. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol Rev. 2008;32(4):579–606. doi: 10.1111/j.1574-6976.2008.00112. [DOI] [PubMed] [Google Scholar]
- 23.Mahler H, Pasi A, Kramer JM. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N Engl J Med. 1997;336(16):1142–1148. doi: 10.1056/NEJM199704173361604. [DOI] [PubMed] [Google Scholar]
- 24.Tschiedel E, Rath P-M, Steinmann J. Lifesaving liver transplantation for multi-organ failure caused by Bacillus cereus food poisoning. Pediatr Transplant. 2015;19(1):E11–E14. doi: 10.1111/petr.12378. [DOI] [PubMed] [Google Scholar]
- 25.Bauer T, Sipos W, Stark TD. First insights into within host translocation of the bacillus cereus toxin cereulide using a porcine model. Front Microbiol. 2018;9:2652. doi: 10.3389/fmicb.2018.02652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koizumi Y, Okuno T, Minamiguchi H. Survival of a case of Bacillus cereus meningitis with brain abscess presenting as immune reconstitution syndrome after febrile neutropenia - a case report and literature review. BMC Infect Dis. 2020;20(1):15. doi: 10.1186/s12879-019-4753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horii T, Notake S, Tamai K, Yanagisawa H. Bacillus cereus from blood cultures: virulence genes, antimicrobial susceptibility and risk factors for blood stream infection. FEMS Immunol Med Microbiol. 2011;63(2):202–209. doi: 10.1111/j.1574-695X.2011.00842.x. [DOI] [PubMed] [Google Scholar]
- 28.Hellman T, Uusalo P, Järvisalo MJ. Renal Replacement Techniques in Septic Shock. Int J Mol Sci. 2021;22(19):10238. doi: 10.3390/ijms221910238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ronco C, Chawla L, Husain-Syed F, Kellum JA. Rationale for sequential extracorporeal therapy in sepsis. Crit Care. 2023;27(1):50. doi: 10.1186/s13054-023-04310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors are ready to provide the data in response to the need.