Abstract
HIV care engagement is a dynamic process. We employed group-based trajectory modeling to examine longitudinal patterns in care engagement among people who were newly diagnosed with HIV and enrolled in the Ryan White program in Florida (n = 9,755) between 2010 and 2015. Five trajectories were identified (47.9% “in care” with 1–2 care visit(s) per 6 months, 18.0% “frequent care” with 3 or more care visits per 6 months, 11.0% “re-engage”, 11.0% “gradual drop out”, 12.6% “early dropout”) based on the number of care attendances (including outpatient/case management visits, viral load or CD4 test) for each six-month during the first five years since diagnosis. Relative to “in care”, people in the “frequent care” trajectory were more likely to be Hispanic/Latino and older at HIV diagnosis, whereas people in the three suboptimal care retention trajectories were more likely to be younger. Area deprivation index, rurality, and county health rankings were also strongly associated with care trajectories. Individual- and community-level factors associated to the three suboptimal care retention trajectories, if confirmed to be causative and actionable, could be prioritized to improve HIV care engagement.
Keywords: Care engagement and retention, HIV care continuum, Trajectory analysis, Community factors, Ryan White
Introduction
Inadequate HIV care engagement and retention is closely linked to negative HIV-related health outcomes, including high viremia, drag resistance, onward disease transmission, and progression to AIDS (1-4). Sustained care engagement is clinically beneficial to control infection and increase the quality of life of people with HIV (PWH) while also being important for preventing HIV transmission in the community. Care engagement of PWH is typically measured cross-sectionally, at a particular time point or at yearly evaluations, and dichotomized into “engaged in care” and “not engaged care” (5-9). This classification is effective in identifying gaps in the HIV care continuum; however, it neglects to consider that care engagement is a dynamic process, in which PWH may be out of care and re-enroll between time points.
Given these nuances in care engagement over time, more studies are examining longitudinal engagement data to understand the characterization of suboptimal care among PWH. Powers and colleagues (10) identified five distinct care engagement trajectories among newly diagnosed PWH in North Carolina enhanced HIV/AIDS Reporting System (eHARS), ranging from constantly-high to consistently-low care attendance over time. Likewise, a recent study by Enns et al. (11) found similar results from a public, hospital-based HIV clinic in Minneapolis, MN.
HIV care outcomes have been associated with care trajectory patterns. An estimated 89% of PWH constantly in care for six years achieve sustained viral suppression; this proportion is reduced to 61% for PWH that gradually decrease their care visits and to 3.4% for people who had rapid attrition in the first year (11). Another study in Zambia showed a gradient increase in risk of mortality as level of engagement in care decreased, with early care attrition representing the greatest mortality risk.
Previous studies have modeled HIV care trajectories using binary indicators (any care vs. no care attendance in the observation periods) (10-12), but have failed to consider the frequency of care visits. Certain care engagement patterns that vary by visit frequency may have been missed by these studies. There is a positive relationship between greater frequency of care visits and faster viral suppression (13). Identifying these care trajectories can enhance the understanding of the dynamics in care seeking behavior and associated HIV care outcomes.
Moreover, baseline-individual factors, including age at diagnosis, sex, HIV transmission risk, race, and baseline CD4 have been found to be predictive of HIV care trajectories (10). Community-level factors, such as rural geography and neighborhood deprivation, can also influence differential access to HIV testing and prevention, cross-sectionally measured care retention, medication adherence, and viral suppression (14-18). The relationship between community-level factors and longitudinal care engagement trajectories is still poorly understood.
Florida is one of the epicenters of the HIV epidemic in the US. In 2019, Florida identified over 4,500 new HIV diagnoses, with more than 116,000 diagnosed PWH living in the state (19). Despite high HIV rates, Florida shows a slightly higher HIV care engagement and retention rate than the national average. The estimated proportions of PWH in Florida who received some HIV care and who were retained in care –defined as having 2 or more HIV lab tests at least 3 months apart in a year– were 76.5% and 62.8%, respectively, while the national estimates in the same year were 75.7% and 57.9% (20). A better understanding of the longitudinal care retention patterns among PWH can facilitate tailored re-engagement strategies by identifying underserved individuals and appropriately timing interventions designed to minimize dropout. Therefore, in this study, we (1) expand on prior HIV care engagement trajectory studies using a dynamic approach that incorporates the frequency of care visits into the trajectory estimations, (2) examine association of HIV care trajectories with individual- and community-level sociodemographic factors, and (3) characterize HIV viral suppression by identified care trajectories among PWH in the Florida Ryan White program.
Methods
Study population and data sources
We conducted a retrospective cohort study of recipients of the Florida Ryan White program Part B (21) –a comprehensive statewide system of health care and support services for low-income people diagnosed with HIV that is managed by Florida Department of Health. To be eligible for the Florida Ryan White Part B program, individuals must be diagnosed with HIV, live in Florida, have an income at or below 400% of the Federal Poverty Level, and unable to receive the same services from Medicaid or other insurance. Ryan White Part B support is provided in all areas of the state, and Part B eligibility is independent from other Ryan White services such as Part A (funding provide to county governments in eligible metropolitan and transitional areas) or Parts C and D (funding provided directly to certain clinics or community-based organizations). De-identified demographic, electronic health data, including service and lab records, were extracted from CAREWare, an electronic health information system for the Ryan White program Part B, and from the Florida Department of Health HIV Surveillance enhanced HIV/AIDS Reporting System (eHARS) between 2010 and 2019. To ensure all persons could contribute at least four years of longitudinal data before December 2019, we only included people who were diagnosed with HIV between January 2010 and December 2015 (n = 11,273). People with perinatal HIV exposure (n = 56) or diagnosed outside of Florida (n = 1,462) were excluded, yielding a final sample size of 9,755.
Longitudinal design and study variables
HIV care engagement measurements
The frequencies of HIV care visits within the first five years from HIV diagnosis were used as longitudinal indicators to determine the trajectory of HIV care engagement. Retention in HIV care is typically measured as having two or more laboratory tests or clinical visits, at least three months apart, within a year (22, 23). The current recommendation is that PWH should attend at least one HIV medical care visit every six months (24). We thus divided the five-year follow-up period into ten six-month time intervals. We counted the number of HIV care visits (including any viral load test, CD4 test, or Ryan White service, including HIV-related outpatient and case management visit) within each time interval.
Covariates
Individuals’ demographic information, including age, sex at birth, race/ethnicity, and mode of transmission, were collected at the time of HIV diagnosis as documented in eHARS. Age at HIV diagnosis was treated continuously and then categorized into groups (< 25 years, 25–44 years and 45 + years). Race/ethnicity was grouped into Hispanic, non-Hispanic Black, non-Hispanic White, and other. HIV transmission was categorized as persons who inject drags (PWID), men who have sex with men (MSM), heterosexual contact, and other based on the Centers for Disease Control & Prevention transmission hierarchy. Baseline (i.e., at HIV diagnosis date) CD4 counts were grouped into > = 500, 350–499, 200–349, and < 200 T cells/mL.
In addition, individuals were linked with community-level factors, namely the Rural-Urban Commuting Area (RUCA) (25) and the Area Deprivation Index (ADI) (26) using the residential zip-code at HIV diagnosis. The RUCA measures population density and urbanization based on the 2010 Census and American Community Survey. The RUCA codes ranged from 1-Metropolitan area to 10-Rural areas, which we collapsed into a dichotomous variable of Urban (codes 1–3) or Rural (codes 4–10) (27). ADI is an estimate of neighborhood socioeconomic disadvantage and considers a wide range of indicators, including employment, education, median family income, income disparity, home value, etc. The ADI was calculated using the 2014–2018 five-year average American Community Survey indices. Zip-code level ADI rankings were scored from 1 (the least disadvantaged) to 10 (the most disadvantaged). Additional health-related community-level factors not covered by ADI were selected from the American Community Survey and included: county-level health outcomes rank, clinical care rank, percent of the population with poor/fair health, number of physically unhealthy days, and number of mentally unhealthy days.
HIV care outcomes
We determined whether each individual achieved viral suppression (viral load < 200 copies/mL) during each of the time intervals and overall (five years since HIV diagnosis). For each time interval, the proportion of people with suppressed viral loads was estimated based on the most recent viral load test (28). The length of time between diagnosis and first viral suppression was calculated and log-transformed due to skewness. We ran a sensitivity analysis with viral suppression defined as viral load < 50 copies/ml and the results were practically unchanged. Lastly, year of death was documented in eHARS, and we calculated a binary variable to indicate if the patient died within the first five years of HIV diagnosis.
Statistical Analysis
Group-based trajectory modeling (GBTM) was used to assess longitudinal HIV care engagement patterns. GBTM is a type of finite mixture model similar to latent class analysis, capable of identifying population subgroups with distinct developmental trajectories over time (29).
Time zero was defined as the date of HIV diagnosis. Individuals’ observation times were censored at death (assumed to occur at the end of the year) or at the end of the follow-up, i.e., Jan 2020. A dropout extension was added to the GBTM implementation to estimate the average number of care attendances among people with non-censored values only, accounting for the changing size of the trajectory groups over time (18). Indicators representing the number of HIV care visits during each of the ten-time intervals were included in the model. Given that indicators are count variables, a Poisson regression model was used.
Predictor-free GBTMs were fitted with an increasing number of trajectories, up to five. The maximum number of five was based on prior literature (10-12) and to ease model interpretability. The best number of trajectories was then decided by evaluating the Bayesian Information Criterion (BIC), the Akaike Information Criterion (AIC), the average posterior probability of each trajectory, and a 5% minimum membership requirement (30). The Log Bayes factor for the differences in BIC values between a model with n trajectories and n + 1 trajectories was also evaluated (31). The average posterior probability of group membership of each identified trajectory is an approximation of internal reliability (32).
Associations between the individual-level and community-level factors and trajectory group were determined via Chi-square and ANOVA analyses. Multinomial logistic regressions were also performed in which “in care”, the largest trajectory group, was the reference group. The model was first fitted with only baseline individual-level variables. A second model was fitted with both baseline individual- and community-level variables.
Lastly, we examined the HIV care outcomes (viral suppression and death) by identified care engagement trajectories. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA), and the SAS procedure PROC TRAJ (31) was used for GBTM.
Results
There were 9,755 people in the Ryan White program newly diagnosed with HIV between 2010 and 2015. The mean age was 36 years old (SD 12.5), 73.7% were male, 49.4% were non-Hispanic black, 25.5% were non-Hispanic white, and 22.9% were Hispanic; over half of the sample (51.6%) identified as MSM and 37.3% as heterosexual (Table 1).
Table 1.
Baseline individual and community characteristics by HIV care engagement trajectory
| Total | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
Chi-
Square/ F value |
|
|---|---|---|---|---|---|---|---|
| In care | Frequent care |
Re-engage | Gradual drop out |
Early drop out |
|||
| n = 9,755 | n = 4,812 | n = 1,696 | n = 1,037 | n = 1,191 | n = 1,019 | ||
| Baseline individual level characteristics | |||||||
| Age at HIV diagnosis, mean (SD) | 36.0 (12.5) | 36.6 (12.7) | 39.0 (12.1) | 34.2 (12.2) | 33.8 (11.8) | 33.0 (11.9) | F = 58.3* |
| Age group at HIV diagnosis (years) | |||||||
| < 25 | 2,226 (22.8) | 1,052 (21.9) | 222 (13.1) | 303 (29.2) | 325 (27.3) | 324 (31.8) | χ2 = 240.8* |
| 25–44 | 4,796 (49.2) | 2,309 (48.0) | 876 (51.7) | 498 (48.0) | 609 (51.1) | 504 (49.5) | |
| 45+ | 2,733 (28.0) | 1,451 (30.1) | 598 (35.3) | 236 (22.8) | 257 (21.6) | 191 (18.7) | |
| Sex at birth | |||||||
| female | 2,567 (26.3) | 1,271 (26.4) | 490 (29.9) | 270 (26.0) | 294 (24.7) | 242 (23.8) | χ2 = 10.9* |
| male | 7,188 (73.7) | 3,541 (73.6) | 1,206 (71.1) | 767 (74.0) | 897 (75.3) | 777 (76.3) | |
| Race/Ethnicity | |||||||
| Hispanic | 2,231 (22.9) | 1,077 (22.4) | 479 (28.2) | 204 (19.7) | 252 (21.2) | 219 (21.5) | χ2 = 63.6* |
| non-Hispanic black | 4,815 (49.4) | 2,356 (49.0) | 747 (44.0) | 579 (55.8) | 597 (50.1) | 536 (52.6) | |
| non-Hispanic white | 2,491 (25.5) | 1,281 (26.6) | 421 (24.8) | 237 (22.9) | 310 (26.0) | 242 (23.8) | |
| other | 218 (2.2) | 98 (2.0) | 49 (2.9) | 17 (1.6) | 32 (2.7) | 22 (2.2) | |
| HIV transmission group | |||||||
| PWID | 778 (8.0) | 381 (7.9) | 147 (8.7) | 72 (6.9) | 106 (8.9) | 72 (7.1) | χ2 = 44.7* |
| MSM only | 5,037 (51.6) | 2,475 (51.4) | 782 (46.1) | 553 (53.3) | 670 (56.3) | 557 (55.7) | |
| heterosexual contact only | 3,634 (37.3) | 1,806 (37.5) | 707 (41.7) | 380 (36.6) | 388 (32.6) | 353 (34.6) | |
| unknown/other | 306 (3.1) | 150 (3.1) | 60 (3.5) | 32 (3.1) | 27 (2.3) | 37 (3.6) | |
| Baseline CD4 count | |||||||
| >=500 | 3,251 (33.4) | 1,635 (34.1) | 448 (26.5) | 401 (38.7) | 416 (35.1) | 351 (34.5) | χ2 = 114.4* |
| 350–499 | 1,812 (18.6) | 929 (19.4) | 284 (16.8) | 188 (18.2) | 242 (20.4) | 169 (16.6) | |
| 200–349 | 1,607 (16.5) | 778 (16.2) | 274 (16.2) | 192 (18.6) | 200 (16.9) | 163 (16.0) | |
| < 200 | 3,054 (31.4) | 1,452 (30.3) | 684 (40.5) | 254 (24.5) | 329 (27.7) | 335 (32.9) | |
| Year of diagnosis | |||||||
| 2010 | 1,775 (18.2) | 825 (17.1) | 172 (10.1) | 334 (32.2) | 146 (12.3) | 298 (29.2) | χ2 = 841.2* |
| 2011 | 1,658 (17.0) | 837 (17.4) | 225 (13.3) | 275 (26.5) | 129 (10.8) | 192 (18.8) | |
| 2012 | 1,658 (17.0) | 887 (18.4) | 281 (16.7) | 189 (18.2) | 152 (12.8) | 149 (14.6) | |
| 2013 | 1,570 (16.1) | 792 (16.5) | 364 (21.5) | 112 (10.8) | 180 (15.1) | 122 (12.0) | |
| 2014 | 1,601 (16.4) | 799 (16.6) | 386 (22.8) | 81 (7.8) | 209 (17.6) | 126 (12.4) | |
| 2015 | 1,493 (15.3) | 672 (14.0) | 268 (15.8) | 46 (4.4) | 375 (31.5) | 132 (13.0) | |
| Baseline community level characteristics | |||||||
| Rural/Urban | |||||||
| rural | 310 (3.2) | 161 (3.4) | 50 (3.0) | 27 (2.6) | 39 (3.3) | 33 (3.3) | χ2 = 1.9 |
| urban | 9,399 (96.8) | 4,627 (96.6) | 1,637 (97.0) | 1,006 (97.4) | 1,147 (96.7) | 982 (96.8) | |
| Area deprivation index, mean (SD) | 6.3 (2.0) | 6.3 (2.0) | 6.4 (6.5) | 6.1 (2.0) | 6.3 (1.9) | 6.4 (2.0) | F = 3.0* |
| Health outcomes rank | 21.8 (14.9) | 22.4 (15.5) | 21.1 (12.6) | 20.1 (14.9) | 21.9 (15.1) | 21.7 (14.9) | F = 6.4* |
| Clinical care rank | 27.3 (16.3) | 27.9 (16.8) | 24.8 (14.3) | 29.1 (17.4) | 27.2 (15.5) | 26.4 (16.7) | F = 15.9* |
| Percent poor/fair health | 15.9 (3.0) | 15.9 (3.0) | 15.8 (2.8) | 15.7 (2.9) | 16.0 (3.0) | 15.8 (3.2) | F = 2.6* |
| physically unhealthy days | 3.7 (0.5) | 3.7 (0.6) | 3.7 (0.5) | 3.6 (0.5) | 3.7 (0.5) | 3.7 (0.6) | F = 5.4* |
| mentally unhealthy days | 3.7 (0.6) | 3.8 (0.6) | 3.6 (0.5) | 3.7 (0.6) | 3.7 (0.6) | 3.7 (0.6) | F = 20.5* |
p < 0.05
All BIC, AIC, and log Bayes improved as the number of trajectory groups increased from one to five, and therefore we chose to include five groups (fit statistics shown in supplementary Table 1; alternative group solutions shown in the supplementary Fig. 1.).
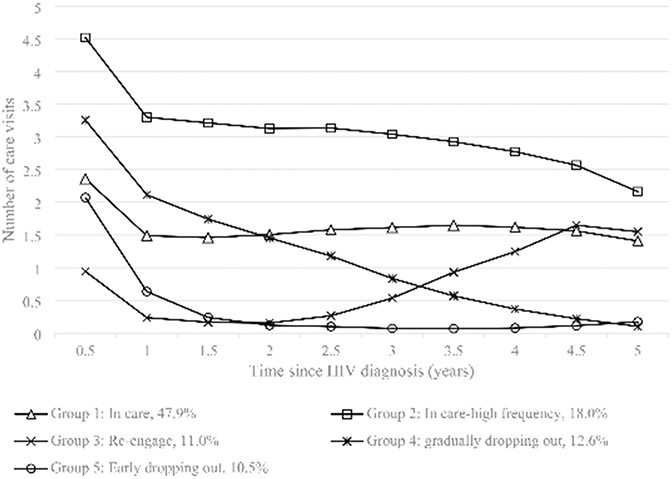
The five HIV care engagement trajectories are illustrated in Fig. 1. Trajectory 1, the largest, included 47.9% of the sample. Individuals in this group – labeled “in care”– had on average 1–2 care visits every six months after HIV diagnosis. Trajectory 2 (18.0% of the sample) was similar to trajectory 1, but individuals had a higher care attendance frequency during follow-up periods, so this group was labeled as “frequent care”. Trajectory 3 included 11.0% of the sample; this group was characterized by individuals who tended to drop out of care after the initial visit and then re-engage in care around three years after diagnosis. This group was labeled as “re-engage”. Trajectories 4 and 5 represented people who dropped out of care and did not re-engage. Individuals in group 4 gradually decreased their frequency of care attendance around two years after diagnosis (“gradual drop out”, 12.6%), whereas people in group 5 quickly dropped out of care one year after diagnosis (“early drop out”, 10.5%).
Fig. 1.
Trajectories of care engagement within 5 years of HIV diagnosis
Table 1 shows the individual- and community-level characteristics of people assigned to their most probable HIV care engagement trajectory. Significant differences were observed across the identified care trajectories for all factors except for urbanicity.
In the multinomial regression model (Table 2), the adjusted R-square for the regression model with only individual-level factors was 0.11. The value increased to 0.15 when community-level factors were added. The magnitude of associations between most of the individual-level variables and HIV care trajectories remained when community-level factors entered the model, except for race/ethnicity for which the affect was slightly attenuated.
Table 2.
Association between individual- and community- level baseline characteristics and HIV care engagement trajectory membership (reference: group 1 “in care”) a
| Group 2: Frequent care |
Group 3: Re-engage |
Group 4: Gradual drop out |
Group 5: Early drop out |
|
|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | OR (95%CI) | |
| Individual-level variables only enter the model a | ||||
| Age group at HIV diagnosis b | 1.26 (1.15, 1.37) | 0.77 (0.70,0.86) | 0.79 (0.71, 0.87) | 0.64 (0.57, 0.71) |
| Male vs. female | 0.90 (0.76, 1.05) | 1.08 (0.87, 1.32) | 0.92 (0.75, 1.12) | 1.13 (0.92, 1.40) |
| Hispanic vs. non-Hispanic white | 1.33 (1.14, 1.56) | 1.06 (0.86, 1.31) | 0.91 (0.75, 1.10) | 1.04 (0.85, 1.27) |
| Non-Hispanic black vs. white | 0.92 (0.80, 1.07) | 1.33 (0.11, 1.59) | 1.04 (0.88, 1.23) | 1.10 (0.92, 1.32) |
| Other vs. non-Hispanic white | 1.53 (1.05, 2.19) | 1.00 (0.56, 1.67) | 1.11 (0.71, 1.69) | 1.10 (0.66, 1.76) |
| PWID vs. heterosexual | 1.01 (0.81, 1.27) | 0.97 (0.72, 1.29) | 1.33 (1.02, 1.73) | 0.98 (0.73, 1.31) |
| MSM only vs. heterosexual | 0.89 (0.76, 1.05) | 1.06 (0.86, 1.30) | 1.17 (0.96, 1.43) | 0.96 (0.79, 1.18) |
| Other vs. heterosexual | 0.89 (0.76, 1.05) | 1.19 (0.78, 1.76) | 0.88 (0.56, 1.33) | 1.36 (0.92, 1.98) |
| Baseline CD4 groups c | 1.19 (1.34, 1.25) | 0.90 (0.85, 0.96) | 1.01 (0.96, 1.07) | 1.05 (0.99, 1.11) |
| HIV diagnosis year | 1.16 (1.13, 1.20) | 0.70 (0.67, 0.73) | 1.28 (1.23, 1.33) | 0.86 (0.82, 0.89) |
| Both individual- and community- level variables enter the model a | ||||
| Age group at HIV diagnosis b | 1.30 (1.19, 1.42) | 0.76 (0.69, 0.84) | 0.79 (0.71, 0.87) | 0.65 (0.58, 0.72) |
| Male vs. female | 0.89 (0.75, 1.05) | 1.08 (0.88, 1.33) | 0.92 (0.75, 1.13) | 1.15 (0.93, 1.42) |
| Hispanic vs. non-Hispanic white | 1.42 (1.20, 1.69) | 0.93 (0.74, 1.16) | 0.86 (0.71, 1.05) | 1.07 (0.86, 1.69) |
| Non-Hispanic black vs. white | 0.85 (0.73, 0.99) | 1.32 (1.10, 1.60) | 0.99 (0.83, 1.17) | 1.11 (0.92, 1.34) |
| Other vs. non-Hispanic white | 1.43 (0.98, 2.07) | 1.01 (0.57, 1.70) | 1.04 (0.66, 1.60) | 1.12 (0.67, 1.80) |
| PWID vs. heterosexual | 1.00 (0.80, 1.26) | 1.01 (0.75, 1.35) | 1.33 (1.02, 1.73) | 0.99 (0.73, 1.32) |
| MSM only vs. heterosexual | 0.92 (0.78, 1.09) | 1.06 (0.87, 1.31) | 1.17 (0.96, 1.44) | 0.97 (0.79, 1.20) |
| Other vs. heterosexual | 0.90 (0.64, 1.24) | 1.17 (0.76, 1.75) | 0.88 (0.56, 1.34) | 1.32 (0.88, 1.92) |
| Baseline CD4 groups c | 1.18 (1.13, 1.24) | 0.90 (0.85, 0.96) | 1.01 (0.96, 1.06) | 1.04 (0.98, 1.11) |
| HIV diagnosis year | 1.17 (1.13, 1.21) | 0.70 (0.67, 0.74) | 1.28 (1.23, 1.34) | 0.86 (0.82, 0.89) |
| Rural vs. Urban | 1.47 (1.02, 2.10) | 0.81 (0.51, 1.26) | 1.37 (0.92, 2.01) | 1.08 (0.68, 1.65) |
| Area deprivation index | 1.03 (1.00, 1.07) | 0.98 (0.94, 1.01) | 1.01 (0.97, 1.05) | 1.02 (0.98, 1.06) |
| Health outcomes rank (5 units change) | 0.94 (0.90, 0.97) | 0.98 (0.94, 1.03) | 0.97 (0.93, 1.01) | 0.95 (0.91, 0.99) |
| Clinical care rank (5 units change) | 0.85 (0.82, 0.87) | 1.03 (0.99, 1.06) | 0.96 (0.93, 0.98) | 0.94 (0.91, 0.97) |
| Percent poor/fair health (5 units change) | 1.60 (1.33, 1.93) | 0.95 (0.75, 1.18) | 1.35 (1.10, 1.65) | 1.21 (0.97, 1.50) |
| Physically unhealthy days | 2.26 (1.80, 2.83) | 0.93 (0.69, 1.25) | 1.05 (0.80, 1.37) | 1.35 (1.03, 1.77) |
| Mentally unhealthy days | 0.27 (0.23, 0.33) | 0.98 (0.78, 1.23) | 0.71 (0.58, 0.87) | 0.73 (0.59, 0.91) |
Bold: statistically significant (p < 0.05)
Adjusted R-square for the model with only individual-level factors (age, sex, race/ethnicity, HIV transmission group, baseline CD4, and HIV diagnosis year): 0.11. Adjusted R-square for the model with both individual and community level factors: 0.15
per increase in age group: 1 = age < 25, 2 = age 25–44, 3 = age > = 45
per increase in CD4 group: 1 = CD4 > = 500, 2 = CD4 350–499, 3 = CD4 200–349, 4 = CD4 < 200
In the full model, age group at HIV diagnosis and HIV diagnosis year were the only two predictors associated with all trajectories identified. Compared to the youngest age group (< 25 years), persons who were in the two older age groups at HIV diagnosis were less likely to be in one of the three suboptimal care trajectories (ORs ranged from 0.65, 95%CI 0.58–0.72 to 0.79, 95%CI 0.69–0.84) and more likely to be in the “frequent care” trajectory (OR=1.30, 95% CI 1.19–1.42). Also, people who were diagnosed with HIV in more recent years were more likely to be in the trajectories of “gradual drop out” and “frequent care” than being “in care” (1.28 and 1.17 times the odds respectively); and less likely to be in the “re-engage” and “early drop out” groups (0.70 and 0.86 times lower odds respectively).
Being Hispanic (vs. White; OR=1.42, 95%CI 1.20–1.69), having worse baseline CD4 count (OR = 1.18, 95%CI 1.13–1.24), and living in rural (OR = 1.47, 95%CI 1.02–2.10) or high area deprivation areas (OR = 1.03, 95%CI 1.00-1.07) were associated with increased odds of having “frequent care”. Being Non-Hispanic black (OR = 1.32 95%CI 1.10–1.60) and having better baseline CD4 cell count (OR = 0.90, 95%CI 0.85–0.96) were predictive of being in the “re-engage” trajectory. Being a PWID was uniquely predictive of “gradual drop out” trajectory (OR = 1.33, 95%CI 1.02–1.73).
Additionally, county-level health indicators were found to be associated with care engagement trajectories. For example, people diagnosed with HIV in a county with a higher average number of physically unhealthy days had increased odds of being in the “early drop out” and “frequent care” groups (OR = 1.35, 95%CI 1.03–1.77; OR = 2.26, 95%CI 1.80–2.83, respectively); while PWH diagnosed in a county with a higher number of mentally unhealthy days had lower odds of being in these two groups (OR = 0.73, 95%CI: 0.59–0.91; OR = 0.27, 95%CI 0.23–0.33, respectively).
For HIV treatment outcomes (Table 3), the “early drop out” group had the lowest proportion of people ever achieving viral suppression. Conditional on ever achieving viral suppression, time to first viral suppression ranged from 288 days in the “frequent care” trajectory to 1354 days in the “early drop out: trajectory. The rate of having a suppressed viral load increased over time for those “in care”, “frequent care”, and “re-engage” groups, and decreased for the “gradual drop out” and “early drop out” groups. Around 8% of people died during the five-year follow-up, and this rate was stable across trajectories.
Table 3.
HIV care outcome characteristics by HIV care engagement trajectory
| Total | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
Chi-Square/
F value |
|
|---|---|---|---|---|---|---|---|
| In care | Frequent care |
Re-engage | Gradual drop out |
Early drop out | |||
| n = 9,755 | n = 4,812 | n = 1,696 | n = 1,037 | n = 1,191 | n = 1,019 | ||
| Ever achieved viral suppression | 9,049 (92.8) | 4,654 (96.7) | 1,676 (98.8) | 973 (93.8) | 1,085 (91.1) | 661 (64.9) | χ2 = 1392.5* |
| Time to viral suppression a (days, mean/median/SD) | 522/243/576 | 424/267/414 | 288/181/296 | 1,162/1,227/583 | 352/203/451 | 1,135/800/1,024 | |
| Log time to viral suppression (mean/median/SD) | 5.7//5.7/1.1 | 5.6/5.6/1.0 | 5.3/5.2/0.9 | 6.8/7.1/0.9 | 5.4/5.3/1.0 | 6.3/6.9/1.5 | F = 451.7* |
| Having a suppressed viral load at: b | NA | ||||||
| T0.5 | 3,030 (31.1) | 1,530 (31.8) | 766 (46.2) | 90 (8.7) | 458 (38.5) | 186 (18.3) | |
| T1 | 4,042 (41.4) | 2,195 (45.6) | 1,036 (61.1) | 63 (6.1) | 588 (49.4) | 160 (15.7) | |
| T1.5 | 4,225 (43.3) | 2,412 (50.1) | 1,087 (64.1) | 44 (4.2) | 602 (50.6) | 80 (7.9) | |
| T2 | 4,419 (45.3) | 2,608 (54.2) | 1,168 (68.9) | 31 (3.0) | 577 (48.5) | 35 (3.4) | |
| T2.5 | 4,670 (47.9) | 2,865 (59.5) | 1,189 (70.1) | 88 (8.5) | 504 (42.3) | 24 (2.4) | |
| T3 | 4,833 (49.5) | 2,977 (61.9) | 1,246 (73.5) | 203 (19.6) | 380 (31.9) | 27 (2.7) | |
| T3.5 | 4,933 (50.6) | 3,060 (63.6) | 1,263 (74.5) | 319 (30.8) | 276 (23.2) | 15 (1.5) | |
| T4 | 4,985 (51.1) | 3,073 (63.9) | 1,243 (73.3) | 432 (41.7) | 203 (17.0) | 34 (3.3) | |
| T4.5 | 4,991 (51.2) | 3,090 (64.2) | 1,220 (71.9) | 551 (53.1) | 88 (7.4) | 42 (4.1) | |
| T5 | 4,512 (46.3) | 2,729 (56.7) | 1,098 (64.7) | 582 (56.1) | 34 (2.9) | 69 (46.8) | |
| Death during follow-up | 783 (8.0) | 404 (8.4) | 123 (7.3) | 77 (7.4) | 106 (8.9) | 73 (7.2) | χ2 = 5.0 |
Conditional on ever achieved viral suppression
The rate was calculated not conditional on having at least one viral load test. If no test was conducted for the time interval, it was coded as not having a suppressed viral load. The denominator was the n of each trajectory group. Chi-square tests were not performed for these outcomes
p < 0.05
Discussion
We conducted a secondary data analysis to examine the longitudinal patterns in HIV care engagement among a retrospective cohort of newly diagnosed people with HIV enrolled in the Florida Ryan White program. This study aims to address the dearth of literature regarding the longitudinal care retention patterns in Florida, an epicenter of the HIV epidemic in the US, by examining the heterogeneity and dynamics in the frequency of care attendance over time.
Using group-based trajectory analysis, five trajectories were identified. According to the trajectories we identified, PWH may follow one of the three primary pathways after HIV diagnosis: constant care retention (groups 1&2), re-engaging in care after initial attrition (group 3), and dropping out of care and never re-engaging (groups 4&5). The first and third pathways each correspond to two trajectories that present different care attendance frequencies and different timing for attrition (year 1 and 3), respectively, which are similar to the patterns identified in past studies (10-12).
This analysis uniquely identified the “frequent care” trajectory, which distinguishes high-frequency care users from any care users. Relative to the “in care” group, individuals in the “frequent care” group had worse baseline CD4 count but were more likely to achieve viral suppression quickly after diagnosis. These results suggest that more frequent care within the first few years of diagnosis is beneficial for attaining better HIV treatment outcomes. Previous studies were likely to miss this variation in care attendance frequency as they used binary indicators of care engagement, i.e., had any visit or no visit during the time interval. We also found the proportion of people with suppressed viral load between years 2 and 5 remained relatively stable among the “frequent care” group even during the later years which witnessed a gradual decrease in care visit frequency. A reduced frequency of visits among clinically stable PWH may help reduce the burden of care for them and for the healthcare system without jeopardizing their treatment outcomes (33, 34). Additionally, although the care attendance trajectories are relatively homogenous within the group, the reason behind the frequent care visits may still be diverse. Some people may have a high awareness of their health and are motivated to attend care, while others may have a suboptimal prognosis and need more frequent monitoring. Thus, their pathways in achieving and sustaining viral suppression may vary. Future studies could consider exploring the trajectories of viral suppression or CD4 count (20, 21) and their intersections with the care attendance trajectories to explore their dynamics in the HIV care continuum (35, 36).
This study also identified three suboptimal care engagement trajectories with substantive dropout and lower re-engagement in care. Interventions should focus on reducing the proportion of people in the early dropout class as this class had the lowest percentage of people ever achieving viral suppression and a longer time to reach first viral suppression. Encouragingly, people in more recent diagnosis years were less likely to follow this trajectory, likely owing to increased success in Florida’s Test & Treat program which links PWH to antiretroviral therapy and case management soon after diagnosis (37-39). The two dropouts of care trajectories also provided insights on the appropriate timing of future re-engagement efforts. Supported also by a previous analysis (10), interventions should start within the first year of HIV diagnosis to re-engage people with rapid attrition. Three years after diagnosis was identified as another critical time to promote care retention to reduce attrition for people who gradually decrease their care attendance.
Additionally, we found that better CD4 results or early achievement of viral suppression were not predictive of better care engagement trajectories, but instead were associated with a higher risk of attrition. Specifically, our results demonstrated that relative to the “in care” group, the group with early care disengagement (the re-engage trajectory) had a better baseline CD4 characteristic. The “gradual drop out” group had a higher proportion of people with a suppressed viral load at the first two time intervals. This result was supported by past research (8, 10) as other studies have also found that better baseline CD4 counts were predictive of poor HIV care retention. However, others have found an opposite association and suggested worse baseline CD4 counts or viral loads were predictive of suboptimal future care retention (11, 40, 41). Furthermore, although the “re-engage” trajectory reached a comparable proportion of people with a suppressed viral load compared to the “in care” group four to five years after diagnosis, it still represents a suboptimal care trajectory. The mean and the median times for achieving first viral suppression more than doubled relative to people in the “in care” trajectory. Delayed viral suppression poses an increased risk of suboptimal individual clinical outcomes, in addition to onward transmission in the community (42, 43). Interventions are needed to prevent the initial attrition and advance the timing for re-engagement.
Both individual- and community-level factors have been found to be associated with HIV care retention (23, 41, 44). In our analysis, we examined baseline individual- and community- characteristics predictive of trajectory membership. These factors could better characterize the identified trajectories and help inform future interventions to identity high-risk populations. As in other studies, older age at HIV diagnosis was protective against the three suboptimal trajectories (10). Non-Hispanic blacks were less likely to be in the “frequent care” group and more likely to be in the “re-engage” group. Similarly, past research has found that black PWH was more likely to experience suboptimal care retention (44). Other than age, we did not identity other demographic factors predictive of the two dropout trajectories. However, several community-level factors such as county health rankings and county average number of physically and mentally unhealthy days were associated with these trajectories. Additionally, we noticed that when community-level factors were added to the model, the association between race/ethnicity and care trajectories changed in direction or in strength, though not all statistically significantly, while the associations for other individual-level variables remained stable. These results suggest that racial/ethnic disparities in care are largely influenced by community-level factors and consistent with the current understanding about the social determinants of health (18, 45).
Some limitations should be noted. First, the study population is people newly diagnosed with HIV enrolled in the Florida Ryan White program, which requires certain eligibility (such as being low-income). Thus, this sample may not represent all PWH in Florida. In addition, people enrolled in the Ryan White program may get access to better care services and have better care retention relative to people with similar income levels not receiving Ryan White benefits (11, 46, 47). Additionally, our sample consisted of Ryan White Part B recipients only. People who received services exclusively from other parts of the Ryan White program (e.g., PWH who only received Part A services in metropolitan areas) were not included. Second, we used only the presence of viral load or CD4 lab or Ryan White services to proxy care attendance, as the receipts of HIV treatment medication were not captured. This may lead to an underestimation of the actual care attendance frequency. Third, some people may re-locate out of state, and Florida eHARS may no longer track their lab records. As a result, the proportion of people in the two dropping out of care trajectories may be slightly overestimated. Overall, individual-level factors in our analysis explained 11% of the variance for the trajectory membership; the value increased to 15% when community-level factors were added, but the value remained relatively low. We suspect the low R-square might be due to uncertainties in class membership. The identified trajectories represent a group average, and not everyone within each class follows precisely the same trajectory. Secondly, the low R-square may be explained by the lack of available data on some important baseline characteristics, such as substance use and comorbidities (8, 48).
A critical future step is to develop a predictive tool to forecast future HIV care attrition trajectories to guide early interventions (11, 41). In addition to baseline characteristics, future research could additionally consider adding time-varying factors, such as the change in disease progression and health status and perceptions of past clinic experiences, that may trigger changes in care attendance behavior. Lastly, telehealth is quickly becoming a common way to seek care. Future studies could examine whether implementing technology-based adaptive care models that allow flexibility in visit modality could positively impact HIV care trajectories.
In summary, trajectory analysis is a helpful tool to better understand the dynamics in HIV care engagement since HIV diagnosis. The identified trajectory could provide insights for future HIV intervention and re-engagement programs to prioritize their efforts and better determine the timing of tailored interventions. With the ultimate goal of improving the HIV care continuum and health outcomes, future studies should consider building a predictive tool that incorporates both baseline and time-varying factors to forecast future HIV care attrition to guide early interventions.
Supplementary Material
Funding (information that explains whether and by whom the research was supported)
This work was supported by the Florida Department of Health (contract CODRU) and the National Institute of Allergy and Infectious Diseases (NIAID) under Award Number R01AI145552 (Co-PIs: Salemi, Prosperi) and Award Number 5P30AI073961-13 (PI: Pahwa).
Footnotes
Conflicts of interest/Competing interests No conflicts of interest to disclose.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10461-022-03659-9.
Ethics approval (include appropriate approvals or waivers) The study has been approved by the University of Florida Institutional Review Board (IRB) and Florida Department of Health IRB.
Consent for publication (consent statement regarding publishing an individual?s data or image) All authors have approved the manuscript for publication.
References
- 1.Crawford T, Sanderson W, Thornton A. Impact of poor retention in HIV medical care on time to viral load suppression. Journal of the International Association of Providers of AIDS Care. 2014;13(3):242–249. [DOI] [PubMed] [Google Scholar]
- 2.Mohammed D, Koumoulos L, Martin E, Slim J. Annual and durable HIV retention in care and viral suppression among patients of Peter Ho Clinic. 2013–2017. PloS one. 2020;15(12):e0244376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stricker SM, Fox KA, Baggaley R, Negussie E, de Pee S, Grede N, et al. Retention in care and adherence to ART are critical elements of HIV care interventions. AIDS Behav. 2014;18(Suppl 5):465–75. [DOI] [PubMed] [Google Scholar]
- 4.Amstutz A, Brown JA, Ringera I, Muhairwe J, Lejone TI, Klimkait T, et al. Engagement in Care, Viral Suppression, Drug Resistance, and Reasons for Nonengagement After Home-Based Same-Day Antiretroviral Therapy Initiation in Lesotho: A Two-Year Follow-up of the CASCADE Trial. Clin Infect Dis. 2020;71(10):2608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brantley AD, Burgess S, Bickham J, Wendell D, Gruber D. Using Financial Incentives to Improve Rates of Viral Suppression and Engagement in Care of Patients Receiving HIV Care at 3 Health Clinics in Louisiana: The Health Models Program, 2013–2016. Public Health Rep. 2018;133(2_suppl):75S–86S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baguso GN, Turner CM, Santos GM, Raymond HF, Dawson-Rose C, Lin J, et al. Successes and final challenges along the HIV care continuum with transwomen in San Francisco. J Int AIDS Soc. 2019;22(4):e25270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogler IH, Alfieri DF, Gianjacomo HDB, Almeida ERD, Reiche EMV. Cascade of care for people living with HIV infection in Southern Brazil: results from a public health network. Cad Saude Publica. 2018;34(12):e00009718. [DOI] [PubMed] [Google Scholar]
- 8.Giordano TP, Hartman C, Gifford AL, Backus LI, Morgan RO. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials. 2009;10(5):299–305. [DOI] [PubMed] [Google Scholar]
- 9.Wiewel EW, Braunstein SL, Xia Q, Shepard CW, Torian LV. Monitoring outcomes for newly diagnosed and prevalent HIV cases using a care continuum created with New York city surveillance data. J Acquir Immune Defic Syndr. 2015;68(2):217–26. [DOI] [PubMed] [Google Scholar]
- 10.Powers KA, Samoff E, Weaver MA, Sampson LA, Miller WC, Leone PA, et al. Longitudinal HIV Care Trajectories in North Carolina. J Acquir Immune Defic Syndr. 2017;74(Suppl 2):88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enns E, Reilly C, Horvath K, Baker-James K, Henry K. HIV Care Trajectories as a Novel Longitudinal Assessment of Retention in Care. AIDS and behavior. 2019;23(9):2532–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mody A, Eshun-Wilson I, Sikombe K, Schwartz SR, Beres LK, Simbeza S, et al. Longitudinal engagement trajectories and risk of death among new ART starters in Zambia: A group-based multi-trajectory analysis. PLoS Med. 2019:16(10):e1002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall HI, Tang T, Westfall AO, Mugavero MJ. HIV care visits and time to viral suppression, 19 U.S. jurisdictions, and implications for treatment, prevention and the national HIV/AIDS strategy. PLoS ONE. 2013;8(12):e84318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edun B, Iyer M, Albrecht H, Weissman S. The South Carolina rural-urban HIV continuum of care. AIDS Care. 2017;29(7):817–22. [DOI] [PubMed] [Google Scholar]
- 15.Benbow ND, Aaby DA, Rosenberg ES, Brown CH. County-level factors affecting Latino HIV disparities in the United States. PLoS ONE. 2020;15(8):e0237269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandran A, Edmonds A, Benning L, Wentz E, Adedimeji A, Wilson TE, et al. Longitudinal Associations Between Neighborhood Factors and HIV Care Outcomes in the WIHS. AIDS Behav. 2020;24(10):2811–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiewel EW, Borrell LN, Jones HE, Maroko AR, Torian LV. Neighborhood Characteristics Associated with Achievement and Maintenance of HIV Viral Suppression Among Persons Newly Diagnosed with HIV in New York City. AIDS Behav. 2017;21(12):3557–66. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan AS, Rosenberg E, Shouse RL, Sullivan PS. Connecting race and place: a county-level analysis of White, Black, and Hispanic HIV prevalence, poverty, and level of urbanization. Am J Public Health. 2014;104(7):e77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Florida Department of Health. HIV Data Center: HIV Care Data 2020. [Available from: http://www.floridahealth.gov/diseases-and-conditions/aids/surveillance/index.html.
- 20.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2018. HIV Surveillance Supplemental Report 2021. [Google Scholar]
- 21.Florida Department of Health. HIV Patient Care. Ryan White Part B 2021. [Available from: http://www.floridahealth.gov/diseases-and-conditions/aids/patient-care/index.html. [Google Scholar]
- 22.U.S. Department of Health and Human Services. HIV National Strategic Plan for the United States: A Roadmap to End the Epidemic 2021–2025. Washington, DC.; 2021. [Google Scholar]
- 23.Sheehan DM, Fennie KP, Mauck DE, Maddox LM, Lieb S, Trepka MJ. Retention in HIV Care and Viral Suppression: Individual- and Neighborhood-Level Predictors of Racial/Ethnic Differences, Florida. 2015. AIDS Patient Care STDS. 2017;31(4):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valdiserri R, Forsyth A, Yakovchenko V, Koh H. Measuring what matters: development of standard HIV core indicators across the U.S. Department of Health and Human Services. Public health reports (Washington. DC: 1974). 2013:128(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.USDA. United States Department of Agriculture Economic Research Service-. Rural-Urban Commuting Area Codes 2021. [Available from: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx.
- 26.Kind A, Buckingham W. Making Neighborhood-Disadvantage Metrics Accessible - The Neighborhood Atlas. The New England journal of medicine. 2018;378(26):2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rural Health Information Hub. What is Rural? 2021. [Available from: https://www.ruralhealthinfo.org/topics/what-is-rural. [Google Scholar]
- 28.Centers for Disease Control and Prevention NCfHA. Selected National HIV, Prevention and Care Outcomes, https://www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-prevention-and-care-out-comes-2018.pdf2018[.
- 29.Nagin D Group-Based Trajectory Modeling: An Overview. Annals of Nutrition and Metabolism. 2014:65(2–3):205–10. [DOI] [PubMed] [Google Scholar]
- 30.Nagin D, Odgers C. Group-based trajectory modeling in clinical research. Annual review of clinical psychology. 2010;6:109–138. [DOI] [PubMed] [Google Scholar]
- 31.Jones B, DS N. K R. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociol Methods Res. 2001;29(3):374–93. [Google Scholar]
- 32.Andruff F, Carraro N, Thompson A, Gaudreau F. Latent Class Growth Modelling: A Tutorial. Tutorials in Quantitative Methods for Psychology. 2009:5(1): 11–24. [Google Scholar]
- 33.Reekie J, Mocroft A, Sambatakou H, Machala L, Chiesi A, van Lunzen J, et al. Does less frequent routine monitoring of patients on a stable, fully suppressed cART regimen lead to an increased risk of treatment failure? AIDS. 2008;22(17):2381–90. [DOI] [PubMed] [Google Scholar]
- 34.Mutasa-Apollo T, Ford N, Wiens M, Socias ME, Negussie E, Wu P, et al. Effect of frequency of clinic visits and medication pick-up on antiretroviral treatment outcomes: a systematic literature review and meta-analysis. J Int AIDS Soc. 2017;20(Suppl 4):21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Platt L, Xu A, Giddy J, Bogart LM, Boulle A, Parker RA, et al. Identifying and predicting longitudinal trajectories of care for people newly diagnosed with HIV in South Africa. PLoS ONE. 2020;15(9):e0238975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duff P, Shannon K, Braschel M, Ranville F, Kestler M, Elwood Martin R, et al. HIV viral load trajectories of women living with HIV in Metro Vancouver, Canada. Int J STD AIDS. 2021;32(4):322–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford N, Migone C, Calmy A, Kerschberger B, Kanters S, Nsanzimana S, et al. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS. 2018;32(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koenig SP, Dorvil N, Dévieux JG, Hedt-Gauthier BL, Riviere C, Faustin M, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomized unblinded trial. PLoS Med. 2017;14(7):e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Florida Department of Health. HIV/AIDS- Florida’s Plan to Eliminate HIV Transmission and Reduce HIV-Related Deaths http://www.floridahealth.gov/diseases-and-conditions/aids/index.html#Provide_Rapid_Access2022 [Available from: http:/www.floridahealth.gov/diseases-and-conditions/aids/index.html#Provide_Rapid_Access.
- 40.Tripathi A, Youmans E, Gibson JJ, Duffus WA. The impact of retention in early HIV medical care on viro-immunological parameters and survival: a statewide study. AIDS Res Hum Retroviruses. 2011;27(7):751–8. [DOI] [PubMed] [Google Scholar]
- 41.Gebrezgi MT, Fennie KP, Sheehan DM, Ibrahimou B, Jones SG, Brock P, et al. Developing a triage tool for use in identifying people living with HIV who are at risk for non-retention in HIV care. Int J STD AIDS. 2020;31(3):244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samandari T, Wiener J, Huang YA, Hoover KW, Siddiqi AE. Impact of viral suppression among persons with HIV upon estimated HIV incidence between 2010 and 2015 in the United States. PLoS ONE. 2020;15(10):e0240727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasin F, Rizk C, Taylor B, Barakat LA. Substantial gap in primary care: older adults with HIV presenting late to care. BMC Geriatr. 2020:20(1):438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gebrezgi MT, Sheehan DM, Mauck DE, Fennie KP, Ibanez GE, Spencer EC, et al. Individual and neighborhood predictors of retention in care and viral suppression among Florida youth (aged 13–24) living with HIV in 2015. Int J STD AIDS. 2019;30(11):1095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hogan JW, Galai N, Davis WW. Modeling the Impact of Social Determinants of Health on HIV. AIDS Behav. 2021;25(Suppl 2):215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan PS, Denniston M, Mokotoff E, Buskin S, Broyles S, McNaghten AD. Quality of care for HIV infection provided by Ryan White Program-supported versus Non-Ryan White Program-supported facilities. PLoS ONE. 2008;3(9):e3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valverde E, Del Rio C, Metsch L, Anderson-Mahoney P, Krawczyk CS, Gooden L, et al. Characteristics of Ryan White and non-Ryan White funded HIV medical care facilities across four metropolitan areas: results from the Antiretroviral Treatment and Access Studies site survey. AIDS Care. 2004;16(7):841–50. [DOI] [PubMed] [Google Scholar]
- 48.Xavier Hall CD, Morgan E, Bundy C, Foran JE, Janulis P, Newcomb ME, et al. Substance Use Predicts Sustained Viral Suppression in a Community Cohort of Sexual and Gender Minority Youth Living with HIV. AIDS Behav. 2021;25(10)3303–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.