Abstract
Perioperative complications and deaths occurred while developing a novel surgical model of pediatric kyphosis in 10 to 12 kg male farm-raised Yorkshire piglets. All piglets appeared clinically normal preoperatively. Intraoperative complications included tachycardia, respiratory acidosis, and death. Postoperatively, clinical signs included posterior paresis, head pressing, prolonged anesthetic recovery, difficulty rising, and sudden death. Necropsies were performed on all piglets. Some morbidity and mortality were accurately attributed to the spinal surgery. However, the index piglet for this report died suddenly approximately 16 to 18 h after surgery. Necropsy of this animal revealed clear, serosanguineous pleural and pericardial effusions along with myocardial hemorrhage and hepatic lesions, consistent with mulberry heart disease and hepatosis dietetica, respectively. Serum vitamin E and selenium levels from this animal were below age-specific lab reference ranges. Clinical signs of vitamin E and selenium deficiency are most common in fast-growing weaner piglets. The added stress of major surgery may exacerbate the condition in young piglets. Resolution of morbidity and mortality in both juvenile and adult pigs occurred upon the use of an alternate vendor able to provide feed analyses meeting industry standards, although serum levels of vitamin E and selenium in similar ages and breed of swine were still occasionally slightly below reference ranges.
Abbreviations: HD, Hepatosis Dietetica; MHD, Mulberry Heart Disease
Selenium is an essential trace element required for human and animal health that is usually acquired in the diet. Selenium is an important component of glutathione peroxidase, which protects the cell and subcellular membrane from peroxidative damage.3,13 Glutathione peroxidase, vitamin E and superoxide dismutase together make up one of the main antioxidant defense systems in animals and humans.6 Therefore, most diseases of selenium deficiency in animals are concomitant with vitamin E deficiency.
Clinical disease resulting from vitamin E-selenium deficiency has been recognized in agricultural animals (cattle, sheep, pigs), laboratory rodents (rats, mice), and humans.1 Commonly reported clinical signs in animals in farm settings include reduced appetite, poor growth, failure to thrive, reproductive infertility, and muscle weakness.6 White muscle disease is a classic manifestation in ruminants, while mulberry heart disease (MHD) and hepatosis dietetica (HD) are common in swine.6 Young animals are at higher risk of deficiency because they do not have the reserves of older animals, and their needs are higher while they are growing. Serum levels of vitamin E and selenium are usually lower in weanlings than adults.5 One study found that 54% of piglets had selenium levels below reference ranges at weaning.33 Animals experiencing stress are also at risk of deficiency, as vitamin E and selenium are consumed when cells undergo stress.23
In most research settings, animals are fed commercially prepared diets verified to meet industry standards for health. However, farm animals, including swine that are sold for research purposes, may have received feed that was grown and mixed on the farm, rather than purchased from a specialized feed vendor. Such feeds will have varying nutritional quality, depending on factors such as nutrient content of the soil, quality of the crops harvested, duration of storage, ratios of mixing, and inclusion or exclusion of mineral supplementation.
A swine project in our facility aimed to develop a model of pediatric kyphosis by inducing premature closure of specific thoracic vertebral body endplates during the growth phase of Yorkshire farm piglets whose diet was mixed on-farm. Animals were young (6 to 8 wk., 10 to 12 kg) at the beginning of the project to allow developmental deformation to occur with growth. In this case study, we describe how suspicions were raised by unusual levels of postoperative mortality and how we obtained a diagnosis of vitamin E-selenium deficiency in the swine used for translational research at our institution.
Case Study
Index case and juvenile animals.
The first case of postoperative mortality retrospectively attributed to vitamin E/selenium deficiency was an animal displaying head pressing and neurologic signs during recovery from the surgical thoracic spinal manipulation. The first case of suspected mulberry heart disease occurred before inhouse processes for collecting and storing serum in all surgical cases were in place. Therefore, serum vitamin E and selenium levels could not be tested in this first case. However, cardiac lesions seen at necropsy were consistent with MHD. Due to the nature of the surgery, we suspected a traumatic etiology for this lesion, secondary to the invasive thoracic manipulation. However, the prolonged time between death and pathologic examination (approximately 12 h) meant that tissue samples for retrospective analysis of tissue vitamin E and selenium values were also not available.
The subsequent death (index case) had classic MHD lesions (Figure 1 and 2) and centrilobular hepatic necrosis suggestive of HD (Figure 3). Serum samples from this second animal were submitted for testing of vitamin E and selenium levels, both of which were below the lower limit of the normal reference range. Histologic findings in the second case included microthrombi in the small vessels of the heart, myocarditis, diffuse hemorrhage with loss and degeneration of cardiomyocytes (Figure 2), and deposition of mineral within cardiomyocytes (Figure 2 B). In the liver, centrilobular coagulative necrosis with hemorrhage was evident histologically (Figure 3). The stomach contained areas of erosion, ulceration, and hyperkeratosis histologically. Gross pathologic findings in subsequent animals included serosanguineous fluid accumulation in the thorax, pericardium, and abdomen, as well as erosion, ulceration or hyperparakeratosis of the gastric mucosa (Figure 4). All thoracic organs observed through a 9th to 10th intercostal space thoracotomy appeared to be grossly normal at the time of surgery.
Figure 1.
(A) Gross image of porcine heart with paintbrush lesions (white arrows) consistent with vitamin E/selenium deficiency (Mulberry Heart Disease). (B) Gross image of cut surface porcine heart with hemorrhagic myocardium consistent with vitamin E/selenium deficiency (Mulberry Heart Disease).
Figure 2.
(A) Microscopic overview image of left ventricle (Hematoxylin and eosin, 2×). (B) Higher magnification image of panel A. Microscopic image of left ventricle (Hematoxylin and eosin, 20×). There is pronounced mineralization (*) evident within cardiomyocytes. There is loss of cells and abundant hemorrhage separating cells (black arrows).
Figure 3.
(A) Microscopic overview image of liver (Hematoxylin and eosin, 4×). There is loss of cell detail surrounding central veins (centrilobular necrosis). (B) Higher magnification image of panel A. Microscopic image of liver (Hematoxylin and eosin, 20×). There is pronounced loss of cellular detail (*) surrounding central veins with shrunken hepatocyte nuclei (arrows) and hemorrhage (centrilobular necrosis).
Figure 4.

Gross image of stomach. The esophagus is at the upper right and pylorus is at the left. There is erosion and ulceration at the pars esophageal region (arrow).
Once a confirmed diagnosis of vitamin E and selenium deficiency was determined in the second case, close evaluation of clinical illnesses in our swine on other projects/protocols around this period identified other derangements attributed to this deficiency. Clinical signs in other animals attributed to vitamin E/selenium deficiencies included melena, head pressing, muscle weakness, wound dehiscence, and purulent discharge from wound sites. Serum levels were not submitted for all cases, but a reevaluation of internal processes during this period resulted in new procedures for long-term storage of serum samples collected from all animals in the preoperative period. Serum samples sent for vitamin E and selenium analysis were submitted to one of 2 veterinary diagnostic laboratories (Table 1). Complete blood counts and serum chemistries were conducted inhouse on a subset of affected animals.
Table 1.
Vitamin E and selenium values from 3 different pigs when aliquots of same sample were sent to 2 separate labs. Asterisks indicate values below reference ranges.
| Lab A | Lab B | |||
|---|---|---|---|---|
| Vitamin E (sent to secondary lab) 2–3.5 ug/mL | Selenium 200–350 ppb | Vitamin E 2–2.5 ppm (based on age) | Selenium 150–300 ppb | |
| Pig 1 | 2.37 | 169 * | 2.2 | 178 |
| Pig 2 | 0.99 * | 144 * | 2.7 | 177 |
| Pig 3 | 1.01 * | 110 * | 2.5 | 147 * |
We began testing all pigs at our facility within 3 to 7 d of arrival for vitamin E and selenium to determine the extent of the problem. Several experimentally naïve animals had serum levels of vitamin E, selenium, or both, that were below reference intervals when measured after arrival to our facility. Upon completion of studies for which the animals were ordered, swine were euthanized and necropsies performed. Necropsy of pigs with vitamin E or selenium deficiencies at study endpoints did not reveal classic clinical or pathologic lesions of MHD/HD. Classic MHD/HD lesions were seen only in 2 of the younger pigs; not all younger pigs with decreased vitamin E and selenium values had classic heart or liver lesions.
Materials and Methods
Animals.
Castrated male Yorkshire pigs (30 to 120 d-old; 10 to 65 kg) were purchased from a regional USDA class A vendor with whom we had worked for over 20 y. The closed herd breeding program ensured all purchased animals were negative for Porcine Reproductive and Respiratory Syndrome, Pseudorabies, Swine Influenza, and Brucellosis. Standard husbandry included tail docking, teeth trimming, castration, iron supplementation (100 mg) on postnatal days 2 and 12 and selenium supplementation on postnatal day 21. Animals were vaccinated for Pasteurella multocida, Bordetella bronchiseptica, Clostridium perfringens A, Parvovirus, Erysipelothrix rhusiopathiae, and Leptospirosis interrogans (5 serovars). Upon arrival at our facility, all pigs received a broad-spectrum antibiotic (ceftiofur crystalline free acid; 5 mg/kg, IM) for the prevention of post-shipping respiratory disease.
Facilities and housing.
All procedures involving animals were approved by the Medical University of South Carolina IACUC and were consistent with federal policies and guidelines. The MUSC animal care and use program is accredited by AAALAC International. Piglets were acclimatized for at least one week. They were pair-housed before surgery and single-housed afterward. Housing included raised, slatted fiberglass pen floors19 and a 12:12 light: dark cycle, consistent with recommendations of the Guide for the Care and Use of Laboratory Animals.14 Animals were fed commercial pelleted chow twice daily (Envigo, Teklad miniswine diet 7037, Madison, WI) in amounts appropriate for their weight. Reverse osmosis water was provided through an automatic watering system with automatic watering systems positioned at the shoulder height of the pigs. Supplemental heat lamps were used to achieve temperatures of 80 to 85°F (26.7 to 29.5°C) at pig level until animals reached 30 pounds body weight (13.6 kg), with humidity maintained between 30 and 70%. Enrichment included rotation of deformable and nondeformable toys, food treats, visual and auditory contact with conspecifics, and regular positive interaction with humans.
Surgical preparation and procedure.
Piglets were bathed 1 to 3 d before surgery using chlorhexidine soap, ensuring contact time was at least 10 min before rinsing. A liquid diet (Ensure, Abbott Laboratories, Abbott Park, IL) was fed the day before surgery. Ensure is a common part of our preoperative procedures in nursery aged piglets to decrease fasting-associated glucose changes. A liquid diet was also used in the kyphosis study before the overnight fasting period to reduce the amount of ingesta in the gastrointestinal tract. This intervention reduced intraoperative cranial displacement of the diaphragm, thus improving the exposure of the surgical site. Pigs were fasted the night before surgery, with constant access to water.
Pigs were premedicated with acepromazine (1.1 mg/kg), atropine (0.04 mg/kg), and ketamine (22 mg/kg) subcutaneously, given carprofen (2 mg/kg) and buprenorphine SR (0.24 mg/kg) subcutaneously for analgesia, induced with isoflurane (2% to 3%), intubated and maintained on isoflurane in oxygen using a mechanical ventilator. Perioperative antibiotics were given (cefazolin, 62.5 mg/kg IV; 40 min before skin incision and every 90 min thereafter until skin closure) due to placement of implants. A left lateral thoracotomy was performed to access the ventral thoracic spine (T9-T11) for partial vertebrectomy of T10 vertebral body and tethering of T9 to T11 using orthopedic screws and wire as described previously.8 A dorsal midline approach to the thoracic vertebral spinous processes allowed surgical release of the interspinous ligaments. Postoperatively, piglets were housed for up to 48 h in a recovery area where they were closely monitored and received carprofen (2 mg/kg) twice daily for 3 d for postoperative analgesia.
Vitamin E and selenium testing.
Serum was separated, placed into a light restrictive container, and shipped on ice to 2 diagnostic laboratories (Lab A, Antech Diagnostics and Lab B Iowa State University Veterinary Diagnostic Laboratory). Lab A aliquoted the samples they received and forwarded them to other labs for analysis (Michigan State University Animal Health Diagnostic Laboratory for Vitamin E and Utah Veterinary Diagnostic Laboratory for selenium (Lab C)). Lab B performed all testing on site. The labs declined to provide further details on how the testing was performed.
Results
Study Population.
The initial study included 20 animals: 8 that had been used prior to the index case but had suspicious clinical signs, the index case, and 11 that occurred after the index case. Of the 8 animals that were used prior to the index case, 3 showed clinical signs that were potentially related to low Vitamin E and/or selenium. One of these died after surgery, one was euthanized due to clinical signs of head pressing prior to the study endpoint, and the others reached the endpoint but had multiple complications that were later attributed to suspected Vitamin E or selenium deficiency. Only 10 of these 20 animals were tested for Vitamin E only (3) or both Vitamin E and selenium (7) as we did not routinely test or bank serum prior to the index case, and testing was stopped after a year, at which time levels had been normal in several tested animals.
After the index case, we tested 18 animals obtained from the same vendor as the index animal. These 18 pigs had been used on other protocols and were either still in house or had banked samples available. This provided an additional 18 samples of different sexes and ages on 4 different protocols to expand our data set.
Necropsy.
Necropsy findings of the case we initially diagnosed with vitamin E/selenium deficiency displayed the following pathology. The left thoracic and dorsal midline incisions from surgery 24 h previously were not considered to be lesions. The eyes, ears, and nasal turbinates were within normal limits. The right pleural space contained 83 mL of flocculent serosanguineous fluid, and the abdominal cavity contained 30 mL of serosanguineous fluid. The right caudal lung and left ventral lobes were very dark red, consistent with hemorrhage and congestion. The heart displayed pale white to tan streaks in the epicardium, which extended through the myo- and endocardium. Red paintbrush lesions were present on the epicardial and endocardial surfaces, and the myocardium was plum red (Figure 1). The liver was dark red with a pale reticular pattern and sharp edges. The spleen and adrenal glands were grossly normal. Both kidneys were minimally pale, relative to normal color. The GI tract was grossly normal. Both stifle joints were grossly normal.
Histologically, the spinal cord and spleen were normal. The lung displayed hemorrhage, congestion, edema, and lymphocytic alveolar inflammation with macrophage infiltrate. The liver had diffuse centrilobular necrosis, bile duct hyperplasia, and mild lymphoplasmacytic hepatitis. Kidneys and lymph nodes were normal histologically. Similar lesions were present throughout the myocardium; these consisted of microthrombi in the small vessels, myocarditis, diffuse hemorrhage with loss and degeneration of cardiomyocytes (Figure 2), and deposition of mineral within cardiomyocytes (Figure 2 B).
Results of vitamin E and selenium testing.
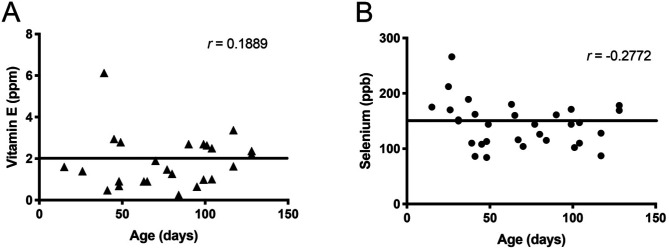
Vitamin E and selenium were both below age-specific lab reference ranges in the pig with MHD and HD. Serum samples for all pigs were collected and submitted within a week of arrival. Of 22 animals tested, 18 were below normal levels for vitamin E and/or selenium; this deficiency was seen in pigs ranging from 40 to 120 d of age (Figure 5).
Figure 5.
(A) Vitamin E values (Lab A) compared with age. Black bar represents regression line. (B) Selenium values (Lab A) compared with age. Black bar represents regression line.
The manufacturer’s analysis of the Envigo miniswine diet used in our facility showed vitamin E and selenium concentrations in the pelleted feed to be higher than those recommended.22 The low serum vitamin E and/or selenium levels in newly arrived pigs, along with evaluation of our commercial diet, led us to conclude that the deficiencies were occurring prior to arrival at our facility. Working closely with the vendor, we were able to develop a system for testing young animals in the vendor’s herd to ensure the delivery of piglets with adequate vitamin E/selenium levels. Although this decreased mortality, some postoperative morbidity continued, and additional testing on banked serum samples taken in the preoperative period were also submitted for testing. Test results showed selenium levels at our facility were lower than they had been when tested at the vendor (Figure 6).
Figure 6.

Difference in selenium levels in blood values. Samples taken from same animals sequentially on farm and upon arrival at facility (Lab B).
For some animals, we performed serial testing of selenium, consisting of a test performed on the farm followed by test at our facility on day of surgery, or a test on the day of surgery followed by another test 2 to 3 wk later. Selenium levels were low in all animals after shipping and in at least one of 3 animals with serial measurements at and after surgery. Of 22 samples tested for both vitamin E and selenium, both were low in 8 animals, only vitamin E was low in 4, and only selenium in 6 (18/22 had either low vitamin E, selenium, or both). After laboratory confirmation of vitamin E/selenium deficiency in the pigs at our facility, all incoming swine received injectable vitamin E supplementation upon arrival at our facility.
Initially serum samples were sent to a diagnostic lab (Lab A) different than that used by the vendor (Lab B). Subsequently, samples were submitted to both labs (Labs A and B). Samples collected at the same time from 3 pigs and handled similarly were tested by both Labs A and B (Table 1). Results from the labs differed. Vitamin E values varied between the labs, whereas selenium values were always lower at lab A relative to lab B. Upon inquiry, Lab A sent the samples to a second lab for testing (Lab C), whereas Lab B performed the tests inhouse.
Vitamin E and selenium values were plotted against age for our cohort, and Spearman correlation test was performed. No correlation was evident with age within the range of 15 to 128 d of age (Vitamin E r = 0.1889 and Selenium r = -0.2722, respectively with P > 0.1 for both) (Figure 5).
Discussion
Deficiencies in either selenium or vitamin E can lead to damage in all cell types. Selenium is an integral part of glutathione peroxidase, which protects cellular membranes from oxidative damage. Vitamin E is also found within the membrane, where it is required to prevent the auto-oxidation of membrane lipids.3,20
Vitamin E/selenium deficiency can result in the sudden death of piglets and gross lesions of the heart and liver. The heart may display extensive ecchymotic and suffusive hemorrhage through all of its layers, leading to a subtle mottling of the myocardium with scattered pale streaks, a condition colloquially called mulberry heart.17,41 The MHD lesions commonly associated with deficiency of vitamin E and selenium consist of epicardial edema with paintbrush hemorrhage throughout the epicardial to myocardial layers of the heart.31 These classic lesions are due to oxidative damage to the endothelium. Histology may show fragmentation of cardiomyocytes, interstitial hemorrhage, focal accumulation of mineral and the infiltration of mononuclear cells.35 Histologic lesions may also include interstitial hemorrhage, swollen cardiomyocytes and loss of cross-striations; the swollen cells are hypereosinophilic, with pyknotic nuclei.26 Cardiomyocyte mineralization may also be seen. Fibrinoid degeneration of the arterioles, capillary thrombosis, and focal fiber necrosis can also occur.35,39 Microangiopathy typically affects pigs that are 2 to 4 mo old.31
The liver of E/selenium deficient animals may also show classic lesions such as massive hepatic necrosis (hepatosis dietetica), and there may be pallor of the skeletal muscle. In HD, the liver is congested with centrilobular necrosis.
Other common gross pathologic lesions associated with E/selenium deficiency consist of accumulation of a serous transudate in the pericardium, thorax, and abdomen; stomach erosions and ulcers; and gastric parakeratosis.4,5,7,10,17,35 Skeletal muscle may display loss of striations, fragmentation and vacuolization.35 Small vessels are also commonly affected, displaying hyalinization of arteriolar walls and thrombosis of small blood vessels.10,35 Although stomach ulcers in pigs have commonly been attributed to stress or small food particle size, they have also been associated with deficiency of vitamin E/Se. In our case, the evidence of vitamin E and selenium deficiency, combined with the potential stress due to transport and surgery, led us to conclude that the gastric ulcers seen in our pigs were related to the E/selenium deficiency. Edema of connective tissues is common, and some affected animals may display leukoencephalomalacia in the cortex and/or the midbrain.31 Pigs may display neurologic signs, or simply be found dead.
Given these data, we suspect that in the year previous to the index case, vitamin E-selenium deficiency contributed to the presence of intermittent morbidities not typical for the surgical lab. Morbidity affected several active projects using adult pigs, where it manifested as poor wound healing, surgical site infection, arthropathy, and septic arthritis. Vitamin E and selenium both play a role in protecting leukocytes and macrophages during phagocytosis. Vitamin E also plays a role as a nonenzymatic antioxidant in vivo by interacting with free radicals. Significant stress on the body and the subsequent creation of reactive oxygen species (ROS) can significantly deplete vitamin E stores at the wound site because vitamin E is converted to an inactive form after a reaction with ROS.12 Selenium plays a key role in the glutathione peroxidase family of enzymatic antioxidants, which reduces H2O2 and switches macrophage polarization from proinflammatory, ROS generating M1 to anti-inflammatory, proliferation stimulating M2 macrophages. Animals with low vitamin E and selenium levels before surgery are susceptible to disruption of the redox homeostasis pathways and delayed wound healing.
MHD is not a new disease and has historically been associated with vitamin E and/or selenium deficiency.28,41 However, as seen in these cases, insufficiency of these nutrients may not always lead to overt disease. MHD can occur in young pigs with normal vitamin E and selenium levels, or in piglets with decreased hepatic vitamin E and normal selenium.25,26 More recent hypotheses are that the disease is multifactorial and results from an imbalance in free radicals and radical scavengers, or that a genetic component contributes to an animal’s predisposition to suffer overt disease.34 Some pigs have an impaired ability to use dietary selenium, a condition which is genetically determined and predisposes these animals to both hyper- and hypo-selenemia.34 Both young and adult pigs require additional selenium in the presence of excess vitamin E.7
Other micronutrient imbalances potentially contribute to MHD. One study found that adding silver, cobalt, and tellurium to the diet of laboratory swine resulted in lesions similar to those reported for vitamin E and selenium deficiency, even with adequate dietary amounts of vitamin E and selenium.39 Zinc, cadmium, and vanadium supplements were also occasionally associated with similar lesions.40 Other heavy metals (such as silver, copper, and arsenic) can modify selenium availability via both direct interactions with selenium and interactions with glutathione peroxidase.3 Vitamin E requirements in pigs can also be affected by dietary iron, copper, sulfur and selenium levels, and vitamin E and selenium may compensate for each other.11,33 MHD lesions have also been induced by increasing calcium and decreasing magnesium in the diet.15 Therefore, this “classic” disease is not a simple deficiency in vitamin E and selenium.
Increased stress, which results in decreased free radical scavengers,32 has also been associated with MHD.2 Stress has been shown to exacerbate lesions in a manner similar to vitamin E and selenium deficiency.2,42 In the cases presented here, the age of the pigs and the surgical stress were likely contributing factors in the development of disease, combined with the low serum levels of vitamin E and selenium. Although MHD lesions are not always consistently associated with vitamin E or selenium deficiency, we hypothesize that the decreased levels of these nutrients in young pigs predispose them to develop lesions when exposed to stressful events, such as transportation and thoracic surgery.
Vitamin E and selenium are both important for wound healing and immune function. The delayed healing of some of the surgical sites, increased prevalence of infection, and infectious arthritis are consistent with perturbation of the immune system. Pigs with low vitamin E or selenium levels have deficient immune status as compared with those with normal levels.11,17 Both cell mediated and humoral immunity depend on vitamin E and selenium.11,17 The immune system of piglets may also be affected by vitamin E/selenium intake of the sow.18
Some researchers have searched for a unifying infectious cause of MHD. Streptococcus suis type 2 and E. coli have both been suspected to contribute to some cases of MHD, but this has yet to be confirmed.21,26,29 Others have suspected a virus as the causative agent of disease, but did not consistently identify any known virus in animals displaying MHD lesions.29 Yet-to-be-identified viruses were not ruled out.29
Selenium and vitamin E in piglets are affected by levels present in the milk of the sows as well as levels in feed. Sow nutritional intake and subsequent tissue and serum levels of both nutrients are critical to maintaining sufficiency of these nutrients in piglets. Selenium crosses the placenta, but vitamin E does not. However, high levels of vitamin E are present in the colostrum.17 The levels of both vitamin E and selenium in sow milk decrease with parity, so older sows have decreased levels.17,18,20,24 The widely adopted practice of early weaning results in lower tissue reserves of both selenium and vitamin E.17 Selenium levels in the feed can be affected by where the foodstuffs were grown (type of soil and amount of rainfall).3,38 The stability of vitamin E in feed is low, and it is susceptible to destruction by many environmental factors including oxygen, heat, sunlight, and moisture.11 Losses of vitamin E during storage can reach up to 50%.11 A 1000-y-flood that occurred in South Carolina in October 2015 after months of drought damaged the nutrient quality of staple feedstuffs used for mixing animal feed.9,37 The index case of this case series was diagnosed in October of 2015. The southeastern United States has also long held a reputation for having geographic pockets of selenium-deficient soil, affecting the nutrient content of crops grown in those locations.
Animals in a state of rapid growth are more often affected by nutrient imbalances.21,25,35 Pigs undergo 3 distinct periods of growth when vitamin E/selenium deficiency is most likely, based on growth rate. These periods, in order of decreasing need, are the postweaning, postnatal, and prenatal periods.17 At weaning, a rapid accumulation of fat occurs, with a decrease in protein and fat in the diet induced by the change from milk or prestarter diet to starter feed. This often results in loss of body weight and depletion of vitamin E stores.11,17 In 1982, the FDA allowed 0.3 ppm of selenium in starter diets, although levels up to 0.5 ppm are not toxic (levels 7 to 10 ppm are toxic).20 Some authors have suggested that the requirement for dietary selenium in 3 to 5 wk old piglets exceeds what is currently allowed in pig feed.16
In piglets, selenium levels fall after weaning with a nadir at 9 to 15 wk.30 The optimal feed level of vitamin E depends on the presence of adequate levels of selenium, as selenium improves digestibility, and selenium requires an excess of vitamin E for adequate absorption.5,10 Blood levels of vitamin E depend on and vary with recent food absorption.27 All of the animals in our study had been fasted prior to surgery, which may have led to the decreased vitamin E levels in the postoperative period. High levels of unsaturated fats in the diet may also result in MHD.31,36
Aliquots of the same serum samples from 3 pigs that were submitted to 2 separate labs yielded different results, leading us to question the validity of testing techniques and sample handling. The 2 labs gave different normal ranges for vitamin E based on age, but their ranges did not differ for selenium. Some reports claim that young pigs normally have low levels of selenium that do not fall into published normal ranges used by commercial laboratories until animals are approximately 3 mo of age.13,34 Selenium levels are also influenced by genetics. Some breeds have higher levels than others, and individual animals with high and low selenium are found within breeds, even when sows and piglets are fed the same diet.34 The question that remains is whether selenium levels must be within the normal range to allow an animal to experience a major stressor such as surgery successfully, or if lower levels that are age-appropriate are adequate.
A confounding factor in this case is the invasive thoracic surgery, which included retraction of the lungs to gain access to the ventral vertebral bodies. For this reason, the thoracic effusion was initially suspected to be related to the procedure and was not viewed as the cause of death. We initially diagnosed vitamin E and selenium deficiency based on the pathologic presentation of the animals, but after detecting animals with low serum levels of vitamin E and selenium that had no associated clinical signs or pathology, we realized that the disease is complicated and multifactorial. The only consistency in these results is that MHD is associated with a nutritional deficiency, either due to diet, genetics, or a combination of both. Much remains to be learned concerning how these micronutrients interact in the body, especially when the genetic component is taken into account.
Conclusion
In this report, we document that young pigs received from a local vendor of swine produced for both biomedical research and agricultural use had inherently low levels of vitamin E and selenium. We hypothesized that the stresses of transportation and surgery exacerbated this nutritional deficiency in some but not all animals. Few nationwide vendors produce agricultural breeds of swine of defined health status that are sold exclusively for research use. Research institutions often obtain animals from local farms that may also produce animals for agricultural purposes. If animals are fed a noncommercial ration that is mixed on the farm, its quality will be susceptible to soil and climatic factors, such as extremes of rainfall. This may result in changes in selenium content of the feed. We recommend that quality control measures when using pigs obtained from agricultural vendors should include periodic feed analysis and consideration of classic agricultural diseases in the event of unusual clinical presentations.
In our investigation of postoperative mortality, we speculate that the stress of transportation and surgery likely exacerbated oxidative damage, resulting in decreased levels of vitamin E and selenium, and contributing to MHD and HD. Vitamin E and selenium levels were below reference ranges, which may have contributed to lesions in these cases, although not all animals with low E/selenium levels displayed lesions associated with MHD. The levels of other micronutrients were not tested and so cannot be excluded as a factor in lesion development. Multiple factors contribute to the occurrence of clinical signs of deficiency, making initial recognition of nutritional deficits difficult. Our experience with these cases highlights the need for vigilance regarding classic agricultural diseases that are not typically seen in research settings, especially when using agricultural species such as swine.
Acknowledgments
The index cases reported herein were on a project was supported by a research grant from the Scoliosis Research Society and a NIH grant P20GM121342. We would also like to recognize and thank the staff members of the Surgical Research Laboratory and the MUSC-VA Veterinary Diagnostic Laboratory.
References
- 1.Beck MA, Levander OA, Handy J. 2003. Selenium deficiency and viral infection. J Nutr 133:1463S–1467S. 10.1093/jn/133.5.1463S. [DOI] [PubMed] [Google Scholar]
- 2.Carlsten J, Bjurström S, Häggendal J, Jönsson L. 1994. Reduction of heart lesions after experimental restraint stress: a study in stress-susceptible pigs. Zentralbl Veterinärmed A 41:722–730. 10.1111/j.1439-0442.1994.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 3.Ekermans LG, Schneider JV. 1982. Selenium in livestock production: a review. J S Afr Vet Assoc 53:223–228. [PubMed] [Google Scholar]
- 4.Ewan RC, Wastell ME, Bicknell EJ, Speer VC. 1969. Performance and deficiency symptoms of young pigs fed diets low in vitamin E and selenium. J Anim Sci 29:912–915. 10.2527/jas1969.296912x. [DOI] [PubMed] [Google Scholar]
- 5.Fontaine M, Valli VE, Young LG, Lumsden JH. 1977. Studies on vitamin E and selenium deficiency in young pigs. I. Hematological and biochemical changes. Can J Comp Med 41:41–51. [PMC free article] [PubMed] [Google Scholar]
- 6.Fordyce FM. 2013. Selenium deficiency and toxicity in the environment. p 375–416. In: Selinus O, editor. Essentials of medical geology: revised edition. New York (NY): Springer. 10.1007/978-94-007-4375-5_16 [DOI] [Google Scholar]
- 7.Glienke LR, Ewan RC. 1977. Selenium deficiency in the young pig. J Anim Sci 45:1334–1340. 10.2527/jas1977.4561334x. [DOI] [PubMed] [Google Scholar]
- 8.Gross RH, Wu Y, Bonthius DJ, Gross V, Smith A, McCrackin MA, Wolfe M, Helke K, Gallien T, Yao H. 2019. Creation of a porcine kyphotic model. Spine Deform 7:213–219. 10.1016/j.jspd.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta M, Gupta S. 2017. An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7:1–14. 10.3389/fpls.2016.02074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakkarainen J, Lindberg P, Bengtsson G, Jonsson L, Lannek N. 1978. Requirement for selenium (as selenite) and vitamin E (as α-tocopherol) in weaned pigs: III. The effect on the development of the VESD syndrome of varying selenium levels in a low-tocopherol diet. J Anim Sci 46:1001–1008. 10.2527/jas1978.4641001x. [DOI] [PubMed] [Google Scholar]
- 11.Hidiroglou N, Cave N, Atwall AS, Farnworth ER, McDowell LR. 1992. Comparative vitamin E requirements and metabolism in livestock. Ann Rech Vet 23:337–359. [PubMed] [Google Scholar]
- 12.Hobson R. 2016.Vitamin E and wound healing: an evidence-based review. Int Wound J 13: 331–335. 10.1111/iwj.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvath CJ, Stowe HD, Miller ER. 1992. Effects of monensin on selenium status and related factors in genetically hypo- and hyperselenemic growing swine. Am J Vet Res 53:2109–2118. [PubMed] [Google Scholar]
- 14.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 15.Korpela H. 1991. Hypothesis: increased calcium and decreased magnesium in heart muscle and liver of pigs dying suddenly of microangiopathy (mulberry heart disease): an animal model for the study of oxidative damage. J Am Coll Nutr 10:127–131. 10.1080/07315724.1991.10718136. [DOI] [PubMed] [Google Scholar]
- 16.Mahan DC. 1985. Effect of inorganic selenium supplementation on selenium retention in postweaning swine. J Anim Sci 61:173–178. 10.2527/jas1985.611173x. [DOI] [PubMed] [Google Scholar]
- 17.Mahan DC. [Internet]. 1996. Are we still having vitamin E and selenium deficiencies in pigs? [Cited 15 December 2018]. Available at: http://livestocktrail.illinois.edu/uploads/porknet/papers/mahan.pdf
- 18.Mavromatis J, Koptopoulos G, Kyriakis SC, Papasteriadis A, Saoulidis K. 1999. Effects of alpha-tocopherol and selenium on pregnant sows and their piglets’ immunity and performance. Zentralbl Veterinärmed A 46:545–553. 10.1046/j.1439-0442.1999.00244.x. [DOI] [PubMed] [Google Scholar]
- 19.McCrackin MA, Swindle MM. 2016. Biology, handling, husbandry, and anatomy, 1–39. In: Swindle MM, Smith AC, editors. Swine in the laboratory: surgery, anesthesia, imaging, and experimental techniques. Boca Raton (FL): CRC Press. [Google Scholar]
- 20.Miller ER, Kornegay ET. 1983. Mineral and vitamin nutrition of swine. J Anim Sci 57 Suppl 2:315–329. [PubMed] [Google Scholar]
- 21.Mortimer DT. 1983. Vitamin E/selenium deficiency syndrome in pigs. Vet Rec 112:278–279. 10.1136/vr.112.12.278. [DOI] [PubMed] [Google Scholar]
- 22.National Research Council. 2012. Nutrient requirements of swine, 11th revised ed. Washington, (DC): National Academies Press. 10.17226/13298. [DOI] [Google Scholar]
- 23.Navarro F, Navas P, Burgess JR, Bello RI, De Cabo R, Arroyo A, Villalba JM.1988.Vitamin E and selenium deficiency induces expression of the ubiquinone-dependent antioxidant system at the plasma membrane. FASEB J 12:1665–1673. 10.1096/fasebj.12.15.1665. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen HE, Danielsen V, Simesen MG, Gissel-Nielsen G, Hjarde W, Leth T, Basse A. 1979. Selenium and vitamin E deficiency in pigs. I. Influence on growth and reproduction. Acta Vet Scand 20:276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen TK, Wolstrup C, Schirmer AL, Jensen PT. 1989. Mulberry heart disease in young pigs without vitamin E and selenium deficiency. Vet Rec 124:535–537. 10.1136/vr.124.20.535. [DOI] [PubMed] [Google Scholar]
- 26.Pallarés FJ, Yaeger MJ, Janke BH, Fernandez G, Halbur PG. 2002. Vitamin E and selenium concentrations in livers of pigs diagnosed with mulberry heart disease. J Vet Diagn Invest 14:412–414. 10.1177/104063870201400509. [DOI] [PubMed] [Google Scholar]
- 27.Pinelli-Saavedra A. 2003. Vitamin E in immunity and reproductive performance in pigs. Reprod Nutr Dev 43:397–408. 10.1051/rnd:2003034. [DOI] [PubMed] [Google Scholar]
- 28.Robinson WF, Robinson NA. 2015. Cardiovascular system, p 1–101. In: Maxie MG, editor. Jubb, Kennedy, and Palmer’s pathology of domestic animals, vol 3. St Louis (MO): Elsevier. [Google Scholar]
- 29.Shen H, Thomas PR, Ensley SM, Kim WI, Loynachan AT, Halbur PG, Opriessnig T. 2011. Vitamin E and selenium levels are within normal range in pigs diagnosed with mulberry heart disease and evidence for viral involvement in the syndrome is lacking. Transbound Emerg Dis 58:483–491. 10.1111/j.1865-1682.2011.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simesen MG, Nielsen HE, Danielsen V, Gissel-Nielsen G, Hjarde W, Leth T, Basse A. 1979. Selenium and vitamin E deficiency in pigs. II. Influence on plasma selenium, vitamin E, ASAT and ALAT and on tissue selenium. Acta Vet Scand 20:289–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sims LD, Glastonbury JRW. 1996. Pathology of the pig: a diagnostic guide. Victoria (Australia): Barton, A.C.T.: Pig Research and Development. [Google Scholar]
- 32.Siddiqui A, Desai NG, Sharma SB, Aslam M, Sinha UK, Madhu SV. 2019. Association of oxidative stress and inflammatory markers with chronic stress in patients with newly diagnosed type 2 diabetes. Diabetes Metab Res Rev 35:e3147. 10.1002/dmrr.3147. [DOI] [PubMed] [Google Scholar]
- 33.Sivertsen T, Vie E, Bernhoft A, Baustad B. 2007. Vitamin E and selenium plasma concentrations in weanling pigs under field conditions in Norwegian pig herds. Acta Vet Scand 49:1–9. 10.1186/1751-0147-49-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stowe HD, Miller ER. 1985. Genetic predisposition of pigs to hypo- and hyperselenemia. J Anim Sci 60:200–211. 10.2527/jas1985.601200x. [DOI] [PubMed] [Google Scholar]
- 35.Trapp AL, Keahey KK, Whitenack DL, Whitehair CK. 1970. Vitamin E-selenium deficiency in swine: differential diagnosis and nature of field problem. J Am Vet Med Assoc 157:289–300. [PubMed] [Google Scholar]
- 36.Ullrey DE. 1981. Vitamin E for swine. J Anim Sci 53:1039–1056. 10.2527/jas1981.5341039x. [DOI] [PubMed] [Google Scholar]
- 37.University of South Carolina. [Internet]. 2016. Office of Research, University of South Carolina: SC floods project summaries. [Cited 18 June 2020]. Available at: https://www.sc.edu/about/offices_and_divisions/research/docs/sc_floods_project_summary_booklet.pdf.
- 38.Van Vleet JF. 1980. Current knowledge of selenium-vitamin E deficiency in domestic animals. J Am Vet Med Assoc 176:321–325. [PubMed] [Google Scholar]
- 39.Vleet JF Van. 1982. Comparative efficacy of five supplementation procedures to control selenium-vitamin E deficiency in swine. Am J Vet Res 43:1180–1189. [PubMed] [Google Scholar]
- 40.Van Vleet JF, Boon GD, Ferrans VJ. 1981. Induction of lesions of selenium-vitamin E deficiency in weanling swine fed silver, cobalt, tellurium, zinc, cadmium, and vanadium. Am J Vet Res 42:789–799. [PubMed] [Google Scholar]
- 41.Van Vleet JF, Carlton W, Olander HJ. 1970. Hepatosis dietetica and mulberry heart disease associated with selenium deficiency in Indiana swine. J Am Vet Med Assoc 157:1208–1219. [PubMed] [Google Scholar]
- 42.Van Vleet JF, Meyer KB, Olander HJ, Ruth GR. 1975. Efficacy and safety of selenium-vitamin E injections in newborn pigs to prevent subclinical deficiency in growing swine. Am J Vet Res 36:387–393. [PubMed] [Google Scholar]